Abstract
Although bone marrow-derived mononuclear cells (BMNC) have been extensively used in cell therapy for cardiac diseases, little mechanistic information is available to support reports of their efficacy. To address this shortcoming, we compared structural and functional recovery and associated global gene expression profiles in post-ischaemic myocardium treated with BMNC transplantation. BMNC suspensions were injected into cardiac scar tissue 10 days after experimental myocardial infarction. Six weeks later, mice undergoing BMNC therapy were found to have normalized antibody repertoire and improved cardiac performance measured by ECG, treadmill exercise time and echocardiography. After functional testing, gene expression profiles in cardiac tissue were evaluated using high-density oligonucleotide arrays. Expression of more than 18% of the 11981 quantified unigenes was significantly altered in the infarcted hearts. BMNC therapy restored expression of 2099 (96.2%) of the genes that were altered by infarction but led to altered expression of 286 other genes, considered to be a side effect of the treatment. Transcriptional therapeutic efficacy, a metric calculated using a formula that incorporates both recovery and side effect of treatment, was 73%. In conclusion, our results confirm a beneficial role for bone marrow-derived cell therapy and provide new information on molecular mechanisms operating after BMNC transplantation on post ischemic heart failure in mice.
Keywords: Heart failure, Microarray analysis, Immune-inflammatory response, Cardiac function
Introduction
Ischaemic heart disease is a leading cause of morbidity and mortality worldwide. In the United States, the estimated incidence of myocardial infarction is approximately 600 000 new attacks and ~300 000 recurrent attacks each year [1]. Currently, survival rates are dismal and treatment options limited. The ability to heal diseased heart tissue by cell therapy is therefore a tantalizing prospect. Bone marrow-derived stem cell (BMSC) therapy has been under investigation for treatment of myocardial infarction and heart failure for almost a decade. Early studies in animal models provided striking evidence that BMSC transplantation has the potential to improve cardiac function and survival through repair and regeneration of infarcted myocardium [2, 3]. Clinical studies using BMSCs for the treatment of myocardial infarction showed similar beneficial results [4, 5]. Recent meta-analyses of human trials suggest that BMSC transplantation is associated with modest, but significant, levels of cardiac rehabilitation following acute myocardial infarction and chronic ischaemic heart disease [6, 7]. While this notion is not without controversy [8–10], the potential efficacy of BMSC therapy cannot be ignored.
At present, however, mechanistic understanding of stem cell treatment and cardiac repair and regeneration is poor. A fundamental point of conflict is the disproportionate improvement in heart function given an almost negligible degree of BMSC engraftment. Typically, human imaging studies document that fewer than ~5% of transplanted BMSCs are detectable in infarcted myocardium after 24 h. Thus, in addition to conventional roles in tissue regeneration through direct BMSC differentiation [2, 3, 11] attention is now also focused on their potential function in rescue and repair of damaged tissue through alternative mechanisms [2, 11–17]. These include, among other possibilities, the secretion of paracrine factors that mobilize endogenous stem cell populations and ameliorate deleterious remodeling processes while enhancing beneficial mechanisms.
In a previous study, we characterized the systemic immune-inflammatory response in chronically ischaemic myocardium following acute myocardial infarction, and profiled associated transcriptomic changes [18]. We discovered significant alterations to the anti-heart reactive antibody repertoire and to levels of circulating inflammatory cytokines. Concordant with these findings, genes involved in complement activation and immune-inflammatory response were significantly upregulated amidst widespread transcriptomic changes. Together, these data indicated that post-ischaemic heart remodeling is accompanied by immune-mediated mechanisms that act both systemically and locally. The goals of the present study were to evaluate the therapeutic potency of bone marrow mononuclear cell (BMNC) transplantation, and assess the extent to which therapy augments pathophysiological remodeling in the post-ischaemic heart with regards to cardiac gene expression and the immune-inflammatory response. BMNC contain small numbers of hematopoietic stem cells, endothelial cell precursors and mesenchymal stem cells, and are the most common cell population used in clinical studies [7, 19]. Our findings demonstrate that BMNC therapy completely reversed the majority of infarction-related changes to gene expression with little cell engraftment, thereby supporting a paracrine role for BMNC in the prevention of disease-associated remodeling of gene expression.
Materials and Methods
Animals
Male and female C57BL/6 mice, aged from 8–10 weeks (20.5–25.5 g) were obtained from the Animal Facility of the Federal University of Rio de Janeiro. Mice were housed at controlled temperature (23°C) with a 12:12 h light–dark cycle and received standard mouse chow and water ad libitum. This investigation conformed to the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Research, Commission on Life Science, National Research Council, USA) and was approved by the Institutional Animal Committee at the Federal University of Rio de Janeiro.
Post-ischaemic Heart Failure
Mice underwent infarction by surgical ligation of the descending branch of the left coronary artery as described previously [20–22]. Post-surgical procedures to assess myocardial damage were performed as described previously [18]. To ascertain that myocardial infarction was properly induced, electrocardiograms (ECG) were recorded 24 h after MI in conscious mice with a subcutaneous electrode implanted 1 day before surgery. In addition, blood samples obtained from caudal vein 3 days after MI were used to determine levels of the myocardial damage marker cardiac troponin I (cTnI). Only animals demonstrating pathological Q waves in ECG and elevation of cTnI levels above 0.3 μg/dl were included in the infarcted group.
Functional Assessment
Electrical function was evaluated by electrocardiogram 24 h after MI, 25 and 45 days after cell injection. Echocardiograms were obtained before, 22 and 37 days after MI in anesthetized mice. Treadmill exercise testing [23] was performed just before (day 0) and 7, 23, 44 and 54 days post MI, in all groups. The last three time points correspond to 13, 34 and 44 days after cell therapy or placebo, which was applied at 10 days post MI. All anatomical and functional parameters were calculated using standard methods [24] as described previously [18].
Bone Marrow Mononuclear Cell Transplantation
Mononuclear bone marrow cell isolation was performed as described by Barbash et al. [25]. Briefly, femurs and tibia of 2 month old wild-type or GFP mice were excised under sterile conditions, with special care to remove all connective tissue attached to the bones. Bone marrow was extracted by flushing the cavity with sterile phosphate-buffered saline (PBS). Bone marrow aspirates were subject to Ficoll density gradient centrifugation to eliminate unwanted cell types and to select the mononuclear bone marrow cell fraction. Cells were collected by centrifugation (1500 rpm for 5 min) and re-suspended in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Grand Island, New York, USA) supplemented with 10% Fetal Bovine Serum, 100 U/ml penicillin and 100 mg/ml streptomycin. Matrigel (BD Biosciences) diluted 1:2 with medium or with medium containing 1.5×106 BMNC was directly injected using a 10 μl syringe into 3 different regions (30 μl total) at the borders of cardiac scar tissue 10 days after experimental infarction.
Fluorescence Imaging
GFP-expressing BMNC were injected as described above and the animals were sacrificed 1, 2 and 7 weeks after cell injection. The hearts were excised, fixed with formaldehyde (4% [w/v] prepared fresh from paraformaldehyde powder) overnight at 4° C and embedded in OCT (Tissue Tek), before sectioning (5 μm) under freezing conditions. Sections were inspected by epifluorescence microscopy (Axiovert 100, Carl Zeiss, Germany) to evaluate the presence of GFP-expressing cells.
Microarray Analysis
Whole heart RNA samples were profiled and compared using AECOM mouse 32 K microarrays spotted with Operon version 3.0 70-mer oligonucleotides (description of this platform may be found at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GPL5371). Samples were extracted from MI animals treated with BMNC or vehicle for comparison with samples extracted from control animals (4 replicas were prepared for each experimental and control group). The hybridization protocol, slide type and scanner settings were uniform throughout the entire experiment to minimize technical noise [18, 26, 27]. All target genes with statistically significant changes (p<0.05) of at least 1.5-fold were considered to be differentially expressed. GenMapp [28] and MappFinder (www.genmapp.org) software and associated databases were used to identify the most affected GO (Gene Ontology) categories. Experiments were performed according to MIAME standards [29] and all results deposited at http://www.ncbi.nlm.nih.gov/sites/entrez?db=geo as series GSE 29769. In order to determine whether genes differentially expressed in untreated and treated infarcted hearts with respect to control hearts were disproportionately affected in specific pathways, we used GenMAPP and MappFinder software (Gladstone Institute; San Fransciso: www.genmapp.org) to provide the statistics of affected GO categories. GO sets with fewer than 10 analyzed members were excluded from this analysis. The transcriptomic recovery efficacy (TRE) was computed as:
where: D, U, X indicate whether the gene was down-, up- or not-regulated in the untreated (first position) or treated (second position) infarcted hearts.
Reactive Antibody Repertoire
Serum IgM and IgG concentrations were determined by ELISA, using anti-mouse isotype specific reagents (Southern Biotechnology Associates, Birmingham, AL). A semiquantitative immunoblot analysis of cardiac antibody reactive repertoire was performed 49 days after treatment as previously described [30].
Cytokine Analysis
Plasma levels of cytokines and chemokines, including IL-1β, IL-4, IL-6, KC (IL-8), IL-10, IL-12 (p40), IFN-γ, TNF-α and monocyte chemoattractant protein (MCP-1) were measured before, 1 day after MI and 49 days after cell treatment using an immunofluorescence assay (Luminex Inc. Austin, TX, USA) with multiplex cytokine reagents supplied by Biosource International, (Camarillo, CA, USA) as previously described [31].
Statistical Analysis
All values are expressed as mean ± SD. Differences between groups were evaluated by two-way ANOVA for treadmill exercise test, and unpaired t-test for other variables. Statistical significance was defined by p<0.05.
Results
BMNC Transplantation Prevents Functional Remodeling of the Post-ischaemic Heart
To evaluate the therapeutic potential of BMNC therapy in chronic ischaemic heart disease, acute myocardial infarction was induced in mice prior to intramyocardial injection with BMNC or control vehicle. Surgical mortality was 22% (11/50 mice). Non-infarcted mice were separated and ischemic injury animals were stratified by cTnI levels into BMNC and vehicle-treated groups. Following injection, immediate mortality was 41% (16/39 mice). Remodeling of heart function was assessed using electrocardiograms and echocardiography. Quantification of electrocardiogram and echocardiograph parameters obtained in BMNC and vehicle-treated groups 45 days after treatment are provided in Tables 1 and 2 respectively. Infarcted mice treated with BMNC demonstrated electrocardiographic improvement 25 days after cell therapy, evidenced by the absence of pathological Q wave. In contrast, the vehicle-injected group displayed a persistent pathological Q wave throughout the study. Moreover, cell therapy improved systolic performance and prevented ventricular dilation. However, both groups displayed equal thinning of the anterior wall, compared to the posterior wall. Together these data indicate that BMNC therapy prevents the electrical and anatomical remodeling normally associated with chronic ischaemic heart disease following acute myocardial infarction.
Table 1.
Electrocardiographic analysis 45 days post-myocardial infarction in mice treated with vehicle or BMNC
| Myocardial Infarction+Vehicle (n=11) | Myocardial Infarction+BMNC (n=12) | |
|---|---|---|
| Heart Rate (bpm) | 532±77 | 683±70 |
| Amplitude | ||
| P wave (mV) | 0.15±0.07 | 0.10±0.05 |
| Q wave (mV) | −0.368±0.269 | −0.060±0.106* |
| QRS complex (mV) | 0.36±0.31 | 0.20±0.15 |
| P (ms) | 0.014±0.002 | 0.011±0.002 |
| ST segment (mV) | 0.397±0.363 | 0.206±0.182 |
| T wave (mV) | −0.14±0.27 | −0.12±0.14 |
| Duration | ||
| PR (ms) | 0.037±0.006 | 0.018±0.003 |
| QRS (ms) | 0.015±0.002 | 0.013±0.003 |
| QT (ms) | 0.068±0.049 | 0.049±0.008 |
| QTc | 0.201±0.143 | 0.163±0.023 |
Abbreviation: BMNC, Bone Marrow Mononuclear Cells. Data are reported as mean ± SD. QT corrected interval (QTc) calculated by Bazett formula. Statistical significance was determined at p<0.05 (*) by Student’st-test.
Table 2.
Echocardiographic analysis 37 days post-myocardial infarction in mice treated with vehicle or BMNC
| Myocardial Infarction+ Vehicle (n=11) |
Myocardial Infarction+ BMNC (n=12) |
|
|---|---|---|
| Heart Rate (bpm) |
249±41 | 311±86 |
| Diameters | ||
| Awthd (cm) | 0.06±0.01 | 0.06±0.01 |
| Pwthd (cm) | 0.08±0.04 | 0.08±0.03 |
| LVDd (cm) | 0.40±0.05 | 0.32±0.12* |
| LVDs (cm) | 0.32±0.05 | 0.22±0.12* |
| Fractional | 22±7 | 34±14* |
| Shortening (%) |
Abbreviations: BMNC, Bone Marrow Mononuclear Cells; Awthd, diastolic Anterior wall thickness; Pwthd, diastolic Posterior wall thickness; LVDd, diastolic Left Ventricular Dimension; LVDs, systolic Left Ventricular Dimension. Data are reported as mean ± SD. Statistical significance was determined at p<0.05 (*) by Student’st-test.
Heart function was further assessed by treadmill exercise test and oxygen consumption (Fig. 1). One week after MI surgery, both experimental groups displayed similar decrease in oxygen consumption during exercise test. However, while the vehicle-treated group showed a subsequent, progressive drop in oxygen consumption, treatment with BMNC arrested this change and prevented further decrease in oxygen consumption until the end of the study. Although this difference between infracted hearts with and without BMNC treatment was significant, therapy did not completely restore the parameters to pre-infarction levels.
Fig. 1.
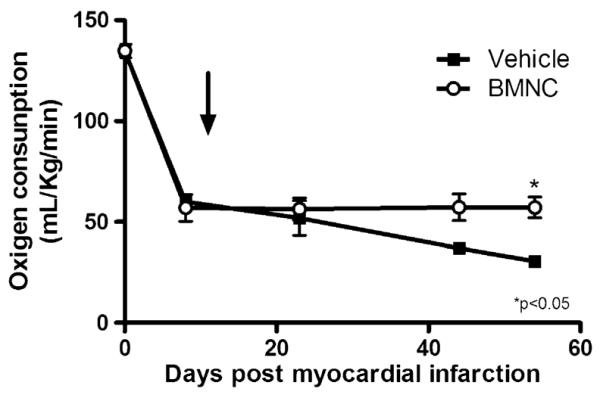
Functional evaluation of post-ischaemic heart failure. Treadmill exercise test on myocardial infarction mice treated with mononuclear cells (BMNC) or vehicle (VEH). Data are reported as means ± SD. Arrow indicates treatment day with vehicle or bone marrow-derived mononuclear stem cells (10 days post myocardial infarction). p<0.05 (*) vs VEH (two-way ANOVA)
Functional Improvement Disproportionately Exceeds Numbers of Transplanted BMNC Retained in Damaged Myocardium
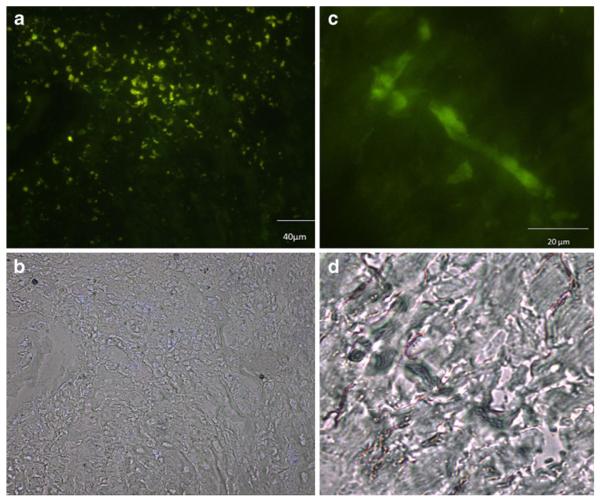
To investigate the extent of cell engraftment after intramyocardial injection, GFP-expressing BMNC were injected into infarcted hearts (Fig. 2). One week after injection, large numbers of GFP-positive cells were identified at the injection site, near the healed infarct border zone (Fig. 2a). Two weeks after injection only a few cells with an elongated morphology could be identified at the injection site (Fig. 2b), none being detected at remote areas. After 7 weeks no GFP-positive cells were detected at the site of injection (results not shown).
Fig. 2.
Histological assessment of GPF/BMNC engraftment. (a and b) 7 days and (c and d) 14 days after GFP-BMNC injection into the hearts of post-infarction mice fluorescent cells were still detectable. (a and c) fluorescence and (b and d) bright field
Stem Cell Therapy Reverses Disease-Associated Alterations to Cardiac Gene Expression
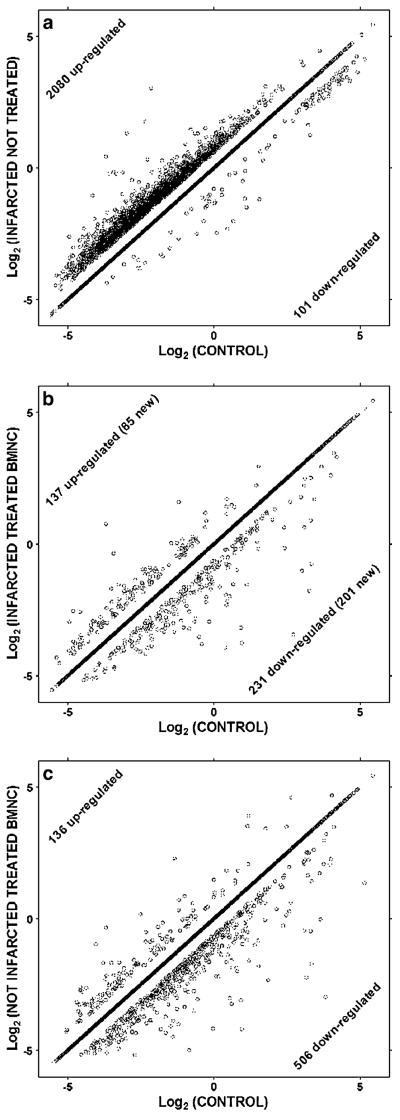
Whole heart tissues from mice were profiled by microarray analyses to examine changes in gene expression associated with ischaemic heart disease and response to reparative cell therapy. As shown in Fig. 3, ischaemic heart disease following acute myocardial infarction is characterized by extensive remodeling of cardiac gene expression. 11981 distinct genes were probed in all samples. Of these, a total of 2181 genes were significantly regulated; 2080 (17.4%) were upregulated and 101 (0.8%) were downregulated in post-ischemic hearts treated with vehicle alone (A). In striking contrast, however, few of these changes were observed in post-ischaemic hearts treated with BMNC injection. BMNC therapy normalized the expression of 2099 (96.2%) of these genes altered by disease (B). Interestingly, the recovery was so strong for 23 genes that their expression changed from upregulated in the untreated infarcted heart to downregulated after BMNC therapy (Table 3). In addition, a further 286 genes had their expression modified by the cell injection as shown in Fig. 3b. Overall, cell therapy with MNC resulted in the remarkable reestablishment of expression of genes altered in infarction to control levels, so that transcriptomic modification after myocardial infarction and BMNC injection was only 3.4%, compared to 18.2% in MI alone. Therapeutic efficacy, a novel metric that quantifies transcriptomic recovery by taking into account both restorative and side effects of treatment was calculated as 73,4%. When BMNC therapy was applied to a normal non-infarcted heart gene expression was also altered. Over 500 genes were down-regulated while 136 were up-regulated as shown in Fig. 3c. Growth factors, cell adhesion, signal transduction and G protein coupled receptors were the main up-regulated categories, while cell cycle was the main down-regulated process.
Fig. 3.
BMNC therapy reverses disease-associated changes to cardiac gene expression profiles. Significantly regulated genes in untreated (a) and treated (b) infarcted hearts with respect to controls. Note the substantial reduction of the number of regulated genes in treated hearts. In normal hearts treated with BMNC the majority of genes is down-regulated (c). Genes on the diagonal were not significantly regulated
Table 3.
Significant reversal of expression patterns of genes altered in post-ischaemic mice treated with BMNC or vehicle
| Name | Symbol | GeneID | VEH | BMNC |
|---|---|---|---|---|
| Ankyrin repeat domain 1 (cardiac muscle) | Ankrd1 | 107765 | 2.09 | −2.29 |
| CCAAT/enhancer binding protein (C/EBP), gamma | Cebpg | 12611 | 1.75 | −1.60 |
| Centromere protein C1 | Cenpc1 | 12617 | 1.59 | −1.72 |
| Cytochrome b5 reductase-like | Cyb5rl | 230582 | 1.61 | −1.59 |
| Epidermal growth factor receptor pathway substrate 15 | Eps15 | 13858 | 2.11 | −2.96 |
| GTPase activating Rap/RanGAP domain-like 1 | Garnl1 | 56785 | 1.61 | −2.10 |
| Interferon (alpha and beta) receptor 1 | Ifnar1 | 15975 | 1.51 | −3.56 |
| Kinesin family member 21A | Kif21a | 16564 | 1.58 | −1.52 |
| Matrix metallopeptidase 23 | Mmp23 | 26561 | 1.83 | −1.56 |
| Membrane protein, palmitoylated 6 (MAGUK p55 subfamily member 6) | Mpp6 | 56524 | 1,57 | −1.57 |
| Methyltransferase like 1 | Mettl1 | 17299 | 1,62 | −1.56 |
| Paired related homeobox 1 | Prrx1 | 18933 | 1,91 | −1.66 |
| Phosphatase, orphan 2 | Phospho2 | 73373 | 1,54 | −2.73 |
| Ras-related GTP binding D | Rragd | 52187 | 1.52 | −1.56 |
| RUN and FYVE domain-containing 2 | Rufy2 | 70432 | 1.96 | −2.42 |
| Sorting nexin 5 | Snx5 | 69178 | 1.90 | −8.07 |
| Structural maintenance of chromosomes 6 | Smc6 | 67241 | 1.84 | −1.95 |
| Tetraspanin 7 | Tspan7 | 21912 | 1.62 | −6.67 |
| Tetratricopeptide repeat domain 14 | Ttc14 | 67120 | 1.56 | −2.64 |
| Translocase of outer mitochondrial membrane 70 homolog A (yeast) | Tomm70a | 28185 | 1.51 | −1.66 |
| Transmembrane protein 176A | Tmem176a | 66058 | 1.76 | −4.57 |
| WD repeat domain 4 | Wdr4 | 57773 | 1.73 | −1.56 |
| Zinc finger, CCHC domain containing 10 | Zcchc10 | 67966 | 1.74 | −1.72 |
Fold difference in expression (negative for down-regulation) are compared between vehicle treated animals (VEH) and BMNC treated animals (BMNC).
Using GenMapp software to categorize Gene Ontology (GO) terms, we examined whether certain cellular pathways encoded by altered genes were prominently affected in the infarcted vehicle and BMNC treated groups. In the infarcted and vehicle treated group prominently upregulated pathways were those involved in regulation of programmed cell death and apoptosis including DNA fragmentation, mitochondrial cytochrome c release, and activation of protein kinase C and RAS components. Angiogenesis and vessel morphogenesis components were similarly upregulated (Angpt1, Bmp4, Clgalt1, Casp8, Fgfr1, Hif1a, Narg1, Pnp1a6, Pten, Rbpj, Rtin4, Tnfrsf12a1, Vegfa, Vegfc, Gna13, Sema5a, Tgfbr2, Htatip2, Rnh1, Serpin\e1, Tek, Nfq, Stab1 and Sphk1), as were immune system process/acute phase response/complement activation (Bnip3, Egln2, Ep300, Hif1a, Nf1, Rnase4 and Sod3), regulation of striated muscle development (Bmp4, Hdac9, Luc71 and Mkl2), and cation channel activity (Snc7a, Trpm7, Cnga3, Kcnc4, Itpr2, Pkd2 and Tpcn1). In addition, a disproportionate number of genes involved in circadian rhythm were up-regulated (Arnt1, Clock, Per1, Per3, Timeless and Npas2).
As shown in the Supplementary Table, genes encoding a number of pathways were primary targets of cell therapy after myocardial infarction. Following treatment with mononuclear cells, upregulated categories included processes related to angiogenesis and blood vessel development and particularly genes related to embryonic epithelium morphogenesis and keratinocyte differentiation (e.g., Apaf1, Aldh1a1, and Psen2), and negative regulation of the acute inflammatory response. Interestingly, different apoptotic genes were more highly expressed with than without BMNC treatment (Aldh1a1, Erecb and Spn),. Because such a small number of genes were downregulated as compared with upregulation, the GenMapp analysis consists of too few genes to provide reliable Z-scores. However, it is worth noting that many of the downregulated genes represented mitochondrion function and respiratory chain function, including glucose metabolism and acetyl-coA biosynthesis; additionally, neuron regeneration and dendritic spine formation genes were decreased.
BMNC Transplantation Diminishes the Immune-Inflammatory Response to Chronic Ischaemic Heart Disease
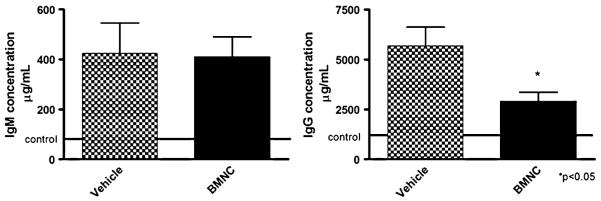
In order to assess the impact of cell therapy on the inflammatory response to ischaemia we compared the reactive antibody repertoires and circulating cytokine profiles of both experimental groups. Sera of post-ischaemic mice treated with BMNC or vehicle were analyzed for total IgM and IgG levels by quantitative ELISA tests. An increase in both immunoglobulin isotypes was identified in the sera of all vehicle treated mice 49 days post-ischaemia (control levels are represented by the dotted line in Fig. 4). Levels of IgM did not reach significant differences between vehicle and cell treated groups (A) but IgG levels decreased in the cell treated group (B). To evaluate further the impact of chronic ischemic myocardial injury on the serum levels of anti-heart reactive antibodies we performed semi-quantitative immunoblot analyses. Both BMNC and vehicle treated mouse groups exhibited comparable anti-heart serum IgM and IgG repertoires, characterized by natural antibody repertoire reactive to heart antigens with no significant difference between groups (data not shown).
Fig. 4.
Immunoglobulin detection. Increased levels of serum IgM and IgG in mice with post-ischemic heart failure reverted by MNC therapy. Serum IgM (left) and IgG (right) concentrations in post-ischemic heart failure treated with mononuclear cells (black column) or with vehicle (white column) were tested by ELISA 7 weeks after myocardial infarction. Solid line represents data for non-manipulated age-matched control C57BL/10 mice. Data are reported as means ± SD for at least 5 mice per group. *P=0.0183 vs MI (unpaired Student’sT test)
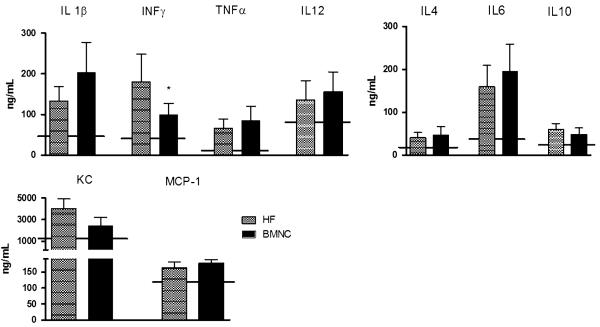
The two groups exhibited persistently elevated circulating levels of IL-1β,IL-12 andTNF-α that were not altered by cell therapy. Moreover, cell therapy did not increase the levels of anti-inflammatory cytokines (IL4, IL6and IL10)orswitchimmune responsetoa Th2 type (data not shown). Infarction induced persistent increase of INF-γ, a typical T helper Th1 response mediator, and treatment with BMNC attenuated it (Fig. 5). Additionally, the use of MNC did not affect circulating levels of KC or MCP-1 (Fig. 5).
Fig. 5.
Serum cytokines in mice with post-ischemic heart failure treated with vehicle (MI) or mononuclear (MNC). Real-time multiplex of Th1-related cytokines IL-1β (a), IFN-γ (b), TNF-α (c) and IL-12 (d) in circulating plasma from heart failure mice treated with mononuclear cells (MNC) or vehicle (MI) 7 weeks after surgery. Solid line represents normal plasma measurement. Data are reported as means ± SD of least 5 mice per group. *P<0.05 vs MI (unpaired Student’sT test)
Discussion
Despite the proven efficacy of bone marrow-derived stem cell transplantation in the treatment of ischaemic heart disease [7, 8], the underlying mechanisms are poorly understood. Original reports hinted that transplanted cell populations regenerated damaged tissue directly through differentiation and formation of de novo myocardium and blood vessels [2, 3]. However, current thinking favors the hypothesis that exogenous stem cell therapy promotes and propagates endogenous repair and regeneration mechanisms [10], (1) e.g. the recruitment of existing, autologous stem cell pools and other beneficial paracrine effects [13, 32]. Therefore, in order to evaluate the potential paracrine influence of stem cell therapy on the overall health of post-ischaemic myocardium, we investigated the functional and transcriptomic consequences of intramyocardial injection with bone marrow mononuclear cells in a setting of chronic ischaemic heart disease following acute myocardial infarction.
We found that BMNC therapy either arrested or completely prevented remodeling of cardiac function. Electrocardiograms revealed normal physiological parameters. Evidence from echocardiography demonstrated that therapy prevented left ventricular dilatation. Together, these findings suggest that cell therapy preserved the excitatory and contractile properties of ventricular myocardium. However, comparable anterior wall thinning was detected in both experimental groups, suggesting that injected BMNC were unable to regenerate damaged tissue. This loss of muscle mass is a likely reason for the decreased cardiac output recorded in vehicle and BMNC treated animals, as evidenced by the results of the treadmill exercise test. Both groups suffered a precipitous drop in oxygen consumption following myocardial infarction. In vehicle treated animals, a subsequent, progressive decline was also observed throughout the remainder of the study. This was not the case for BMNC treated animals. While the performance of BMNC-treated animals did not recover to pre-infarction levels, neither did it decline further in the same manner observed in vehicle-treated animals. Thus, the emergent picture is that while injected BMNC are not able to regenerate tissue damaged by the initial ischaemic insult, it appears possible to prevent further disease-related structural and functional remodeling in the post-ischaemic heart.
These findings accord well with current thinking that the underlying mechanism is based on paracrine influence of transplanted BMNC on surrounding tissue. This hypothesis is supported by our observations that very few transplanted cells were retained within infarcted myocardium in the short-term, and their long-term presence was undetectable, a common finding in such studies [8]. The paracrine influence may manifest in a number of ways, including mobilization of resident progenitor cells, promoting secretion of multiple factors favorable to repair, or likely, a combination of both [12–14, 16, 17, 33]. In the present study we did not examine the potential recruitment, differentiation and proliferation of endogenous stem cell pools. Instead, we focused on the downstream consequences of BMNC therapy to the overall homeostatic health of ventricular myocardium by examining global gene expression profiles of treated hearts. Recent work by Richard Lee’s group, using a double transgenic mouse model, shows that endogenous repair of the heart is attained when c-kit positive bone marrow-derived cells are injected immediately after the infarction. Injected cells do not differentiate or fuse with resident cardiomyocytes and cannot be found 4 weeks after cell therapy, supporting a paracrine mechanism of action [32].
Gene expression profiles were determined 59 days after induction of myocardial infarction and 49 days after cell therapy of the infarcted hearts. Although no cells could be found in the injected hearts, gene expression was significantly altered in the cell treated group. This finding indicates that BMNC treatment produces a change in gene expression that long outlasts their presence in the tissue. The analysis showed that a vast number of genes that were upregulated by myocardial infarction were restored to normal by cell therapy, 23 of them being even downregulated. Among these last genes we found some intimately related to important processes involved in the remodeling of the infarcted heart, such as cell cycle regulation (Ankrd1), cell adhesion (tetraspanin 7), matrix degradation (Mmp23) and inflammatory response (Ifnar1). We also found genes that were not altered by myocardial infarction and that were up and downregulated by cell therapy in the infarcted hearts. These included genes related to cell cycle and energy metabolism, respectively.
The significant number of up and mainly down-regulated genes after BMNC injection into normal hearts may reflect just the presence of a new cell population in the organ, since we could not detect alterations in organ physiology, by electro and echocardiogram. Nonetheless, a detrimental effect of cell therapy cannot be discarded, since cell cycle processes were strongly down-regulated by cell injection. Furthermore, apoptosis related genes were up-regulated in the infarct and cell treated hearts. The apoptosis related genes that were up-regulated after cell therapy in the infarcted hearts were distinct and in smaller number than the ones up-regulated by infarction alone, and we speculate that they could be related to inflammatory cell apoptosis, as observed during BMNC therapy in the chagasic mouse model [34], where we have also observed substantial transcriptomic recovery [35].
In that regard, a notable finding of our previous gene expression studies was that approximately 40% of all sampled genes related to immune-inflammatory-defense response e.g. chemokines, interferon receptors, interleukin receptors and tumor necrosis factor receptors, were significantly upregulated in post-ischaemic heart. These elevations indicate pro-inflammatory properties within chronically injured myocardial tissue [18]. The expression of almost all of these genes was also normalized by BMNC therapy, suggesting a deactivation of a pro-inflammatory environment, and a return to an almost normal, physiological homeostatic environment.
Although cell therapy did not increase serum anti-inflammatory cytokines levels (IL4, IL6 and IL10) or switch immune response to a Th2 type, it was able to attenuate the infarction-induced increase in INF-γ, a typical T helper type 1 (Th1) response mediator (Fig. 5). These results indicate that systemic inflammatory responses are not significantly altered, but downregulation of immune-inflammatory cardiac receptor genes in the arrays suggest that local cardiac inflammatory responses may in fact be modulated by cell therapy.
BMNC are an attractive cell source for MI therapy because they induce significant improvement in cardiac function, they are able to modulate gene reprogramming in an infarcted model, they are easily isolated from the bone marrow and they require minimal manipulation. Moreover, BMNC induced an astonishing reprogramming of ~95% of the genes altered by infarction even 7 weeks after cell engraftment, a time at which BMNC were no longer detectable.
Supplementary Material
Acknowledgments
Support CAPES-MEC, FAPERJ, CNPq, Decit/MS and NIH (RO1 HL73732-01).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s12015-011-9282-2) contains supplementary material, which is available to authorized users.
Disclosure of potential conflicts of interest The authors indicate no potential conflicts of interest.
Contributor Information
Stephan Lachtermacher, Instituto de Biofísica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro, Bloco G, Ilha do Fundão 21949–900, Rio de Janeiro, RJ, Brazil.
Bruno L. B. Esporcatte, Instituto de Biofísica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro, Bloco G, Ilha do Fundão 21949–900, Rio de Janeiro, RJ, Brazil
Fábio da Silva de Azevedo Fortes, Instituto de Biofísica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro, Bloco G, Ilha do Fundão 21949–900, Rio de Janeiro, RJ, Brazil.
Nazareth Novaes Rocha, Instituto de Biofísica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro, Bloco G, Ilha do Fundão 21949–900, Rio de Janeiro, RJ, Brazil; Departamento de Fisiologia e Farmacologia, Universidade Federal Fluminense, Niterói, Brazil.
Fabrício Montalvão, Instituto de Biofísica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro, Bloco G, Ilha do Fundão 21949–900, Rio de Janeiro, RJ, Brazil.
Patricia C. Costa, Instituto de Biofísica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro, Bloco G, Ilha do Fundão 21949–900, Rio de Janeiro, RJ, Brazil
Luciano Belem, PROCEP-Centro de Ensino e Pesquisa do Hospital Pró-Cardíaco, Rio de Janeiro, RJ, Brazil.
Arnaldo Rabischoffisky, PROCEP-Centro de Ensino e Pesquisa do Hospital Pró-Cardíaco, Rio de Janeiro, RJ, Brazil.
Hugo C. C. Faria Neto, Laboratório de Imunofarmacologia, Instituto Oswaldo Cruz, Fiocruz, Rio de Janeiro, Brazil.
Rita Vasconcellos, Departamento de Imunobiologia, Universidade Federal Fluminense, Niterói, Brazil.
Dumitru A. Iacobas, Albert Einstein College of Medicine, Bronx, USA
Sanda Iacobas, Albert Einstein College of Medicine, Bronx, USA.
David C. Spray, Albert Einstein College of Medicine, Bronx, USA
Neil M. Thomas, Albert Einstein College of Medicine, Bronx, USA
Regina C. S. Goldenberg, Instituto de Biofísica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro, Bloco G, Ilha do Fundão 21949–900, Rio de Janeiro, RJ, Brazil
Antonio C. Campos de Carvalho, Instituto de Biofísica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro, Bloco G, Ilha do Fundão 21949–900, Rio de Janeiro, RJ, Brazil; Albert Einstein College of Medicine, Bronx, USA.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics-2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Jackson KA, Majka SM, Wang H, Pocius J, Hartley CJ, Majesky MW, et al. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. The Journal of Clinical Investigation. 2001;107(11):1395–1402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410(6829):701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 4.Losordo DW, Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease: part II: cell-based therapies. Circulation. 2004;109(22):2692–2697. doi: 10.1161/01.CIR.0000128596.49339.05. [DOI] [PubMed] [Google Scholar]
- 5.Wollert KC, Drexler H. Clinical applications of stem cells for the heart. Circulation Research. 2005;96(2):151–163. doi: 10.1161/01.RES.0000155333.69009.63. [DOI] [PubMed] [Google Scholar]
- 6.Singh S, Arora R, Handa K, Khraisat A, Nagajothi N, Molnar J, et al. Stem cells improve left ventricular function in acute myocardial infarction. Clinical Cardiology. 2009;32(4):176–180. doi: 10.1002/clc.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Archives of Internal Medicine. 2007;167(10):989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 8.Menasche P. Cardiac cell therapy: Lessons from clinical trials. Journal of Molecular and Cellular Cardiology. 2010;50(2):258–265. doi: 10.1016/j.yjmcc.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428(6983):664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 10.Sussman MA, Murry CE. Bones of contention: marrow-derived cells in myocardial regeneration. Journal of Molecular and Cellular Cardiology. 2008;44(6):950–953. doi: 10.1016/j.yjmcc.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428(6983):668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 12.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circulation Research. 2010;107(7):913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao R, Pfister O, Jain M, Mouquet F. The bone marrow-cardiac axis of myocardial regeneration. Progress in Cardiovascular Diseases. 2007;50(1):18–30. doi: 10.1016/j.pcad.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mouquet F, Pfister O, Jain M, Oikonomopoulos A, Ngoy S, Summer R, et al. Restoration of cardiac progenitor cells after myocardial infarction by self-proliferation and selective homing of bone marrow-derived stem cells. Circulation Research. 2005;97(11):1090–1092. doi: 10.1161/01.RES.0000194330.66545.f5. [DOI] [PubMed] [Google Scholar]
- 15.Nasef A, Ashammakhi N, Fouillard L. Immuno-modulatory effect of mesenchymal stromal cells: possible mechanisms. Regenerative Medicine. 2008;3(4):531–546. doi: 10.2217/17460751.3.4.531. [DOI] [PubMed] [Google Scholar]
- 16.Rota M, Kajstura J, Hosoda T, Bearzi C, Vitale S, Esposito G, et al. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(45):17783–17788. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoon YS, Wecker A, Heyd L, Park JS, Tkebuchava T, Kusano K, et al. Clonally expanded novel multipotent stem cells from human bone marrow regenerate myocardium after myocardial infarction. The Journal of Clinical Investigation. 2005;115(2):326–338. doi: 10.1172/JCI22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lachtermacher S, Esporcatte BL, Montalvao F, Costa PC, Rodrigues DC, Belem L, et al. Cardiac gene expression and systemic cytokine profile are complementary in a murine model of post-ischemic heart failure. Brazilian Journal of Medical and Biological Research. 2010;43(4):377–389. doi: 10.1590/s0100-879x2010007500014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin-Rendon E, Brunskill SJ, Hyde CJ, Stanworth SJ, Mathur A, Watt SM. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. European Heart Journal. 2008;29(15):1807–1818. doi: 10.1093/eurheartj/ehn220. [DOI] [PubMed] [Google Scholar]
- 20.Ahn D, Cheng L, Moon C, Spurgeon H, Lakatta EG, Talan MI. Induction of myocardial infarcts of a predictable size and location by branch pattern probability-assisted coronary ligation in C57BL/6 mice. American Journal of Physiology. Heart and Circulatory Physiology. 2004;286(3):H1201–H1207. doi: 10.1152/ajpheart.00862.2003. [DOI] [PubMed] [Google Scholar]
- 21.Patten RD, Aronovitz MJ, Deras-Mejia L, Pandian NG, Hanak GG, Smith JJ, et al. Ventricular remodeling in a mouse model of myocardial infarction. The American Journal of Physiology. 1998;274(5 Pt 2):H1812–H1820. doi: 10.1152/ajpheart.1998.274.5.H1812. [DOI] [PubMed] [Google Scholar]
- 22.Salto-Tellez M, Yung LS, El Oakley RM, Tang TP, ALmsherqi ZA, Lim SK. Myocardial infarction in the C57BL/6J mouse: a quantifiable and highly reproducible experimental model. Cardiovascular Pathology. 2004;13(2):91–97. doi: 10.1016/S1054-8807(03)00129-7. [DOI] [PubMed] [Google Scholar]
- 23.Bayat H, Swaney JS, Ander AN, Dalton N, Kennedy BP, Hammond HK, et al. Progressive heart failure after myocardial infarction in mice. Basic Research in Cardiology. 2002;97(3):206–213. doi: 10.1007/s003950200013. [DOI] [PubMed] [Google Scholar]
- 24.Gao XM, Dart AM, Dewar E, Jennings G, Du XJ. Serial echocardiographic assessment of left ventricular dimensions and function after myocardial infarction in mice. Cardiovascular Research. 2000;45(2):330–338. doi: 10.1016/s0008-6363(99)00274-6. [DOI] [PubMed] [Google Scholar]
- 25.Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, et al. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108(7):863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 26.Iacobas DA, Iacobas S, Urban-Maldonado M, Spray DC. Sensitivity of the brain transcriptome to connexin ablation. Biochimica et Biophysica Acta. 2005;1711(2):183–196. doi: 10.1016/j.bbamem.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Iacobas DA, Iacobas S, Li WE, Zoidl G, Dermietzel R, Spray DC. Genes controlling multiple functional pathways are transcriptionally regulated in connexin43 null mouse heart. Physiological Genomics. 2005;20(3):211–223. doi: 10.1152/physiolgenomics.00229.2003. [DOI] [PubMed] [Google Scholar]
- 28.Dahlquist KD, Salomonis N, Vranizan K, Lawlor SC, Conklin BR. GenMAPP, a new tool for viewing and analyzing microarray data on biological pathways. Nature Genetics. 2002;31(1):19–20. doi: 10.1038/ng0502-19. [DOI] [PubMed] [Google Scholar]
- 29.Brazma A, Hingamp P, Quackenbush J, Sherlock G, Spellman P, Stoeckert C, et al. Minimum information about a microarray experiment (MIAME)-toward standards for microarray data. Nature Genetics. 2001;29(4):365–371. doi: 10.1038/ng1201-365. [DOI] [PubMed] [Google Scholar]
- 30.Malanchere E, Marcos MA, Nobrega A, Coutinho A. Studies on the T cell dependence of natural IgM and IgG antibody repertoires in adult mice. European Journal of Immunology. 1995;25(5):1358–1365. doi: 10.1002/eji.1830250534. [DOI] [PubMed] [Google Scholar]
- 31.Szodoray P, Alex P, Brun JG, Centola M, Jonsson R. Circulating cytokines in primary Sjogren’s syndrome determined by a multiplex cytokine array system. Scandinavian Journal of Immunology. 2004;59(6):592–599. doi: 10.1111/j.0300-9475.2004.01432.x. [DOI] [PubMed] [Google Scholar]
- 32.Loffredo FS, Steinhauser ML, Gannon J, Lee RT. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8(4):389–398. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoon YS, Lee N, Scadova H. Myocardial regeneration with bone-marrow-derived stem cells. Biology of the Cell. 2005;97(4):253–263. doi: 10.1042/BC20040099. [DOI] [PubMed] [Google Scholar]
- 34.Soares MBP, Lima RS, Rocha LL, Takyia CM, Pontes-de-Carvalho L, de Carvalho ACC, et al. Transplanted bone marrow cells repair heart tissue and reduce myocarditis in chronic chagasic mice. The American Journal of Pathology. 2004;164(2):441–447. doi: 10.1016/s0002-9440(10)63134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soares MBP, Lima RS, Souza BSF, Vasconcelos JF, Rocha LL, dos Santos R. Ribeiro, et al. Reversion of gene expression alterations in hearts of mice with chronic chagasic cardiomyopathy after transplantation of bone marrow cells. Cell Cycle. 2011;10(9):1448–1455. doi: 10.4161/cc.10.9.15487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.