Abstract
A new strain isolated from soil utilizes cyclopropanecarboxylate as the sole source of carbon and energy and was identified as Rhodococcus rhodochrous (H. Nishihara, Y. Ochi, H. Nakano, M. Ando, and T. Toraya, J. Ferment. Bioeng. 80:400-402, 1995). A novel pathway for the utilization of cyclopropanecarboxylate, a highly strained compound, by this bacterium was investigated. Cyclopropanecarboxylate-dependent reduction of NAD+ in cell extracts of cyclopropanecarboxylate-grown cells was observed. When intermediates accumulated in vitro in the absence of NAD+ were trapped as hydroxamic acids by reaction with hydroxylamine, cyclopropanecarboxohydroxamic acid and 3-hydroxybutyrohydroxamic acid were formed. Cyclopropanecarboxyl-coenzyme A (CoA), 3-hydroxybutyryl-CoA, and crotonyl-CoA were oxidized with NAD+ in cell extracts, whereas methacrylyl-CoA and 3-hydroxyisobutyryl-CoA were not. When both CoA and ATP were added, organic acids corresponding to the former three CoA thioesters were also oxidized in vitro by NAD+, while methacrylate, 3-hydroxyisobutyrate, and 2-hydroxybutyrate were not. Therefore, it was concluded that cyclopropanecarboxylate undergoes oxidative degradation through cyclopropanecarboxyl-CoA and 3-hydroxybutyryl-CoA. The enzymes catalyzing formation and ring opening of cyclopropanecarboxyl-CoA were shown to be inducible, while other enzymes involved in the degradation were constitutive.
In general, degradation of synthetic compounds by living organisms is difficult. Therefore, these compounds cause pollution of the environment when released to nature. To study microbial metabolisms of artificial organic compounds is thus important to protect the natural environment from pollution. We are interested in biodegradation of synthetic compounds having a cyclopropane ring, because such molecules are highly strained aliphatic compounds exceptionally reactive toward bromine or other electrophilic reagents and this reactivity leads to cleavage of the three-membered ring. Some miticides, herbicides, and drugs are synthetic cyclopropane derivatives. A cyclopropane ring is also found in natural products, such as pyrethrins, hypoglycins, and cyclopropane fatty acids in the bacterial lipids. In plants, 1-aminocyclopropane-1-carboxylate undergoes fragmentation into the plant hormone ethylene, carbon dioxide, and hydrogen cyanide (1, 13, 29). Pirrung and McGeehan have proposed a radical mechanism for this degradation catalyzed by 1-aminocyclopropane-1-carboxylate oxidase (20, 21). In bacteria and yeast, 1-aminocyclopropane-1-carboxylate is decomposed into α-ketobutyrate and ammonia by 1-aminocyclopropane-1-carboxylate deaminase, a pyridoxal 5′-phosphate-dependent enzyme (11, 30). However, few papers have appeared so far dealing with the microbial metabolism of cyclopropanecarboxylate (9, 19, 23, 24) or cyclopropane ring-containing fatty acids (16, 28). Furthermore, enzymes involved in the ring-opening reactions in these metabolisms have not yet been characterized. Higher animals seem to be incapable of metabolizing a cyclopropane ring, although they can shorten the chain of cyclopropane fatty acids (31).
To investigate the mechanism of enzymatic cleavage of a cyclopropane ring and compare it with chemical reactions, we isolated a cyclopropanecarboxylate-utilizing bacterium (strain CPC-1) from soil and identified it as Rhodococcus rhodochrous (18). In the present paper, we report a novel degradation pathway for cyclopropanecarboxylate in R. rhodochrous. It has been reported that cyclohexanecarboxylate and cyclopentanecarboxylate are metabolized by bacteria through a pathway involving β-oxidation of coenzyme A (CoA) intermediates (4, 22).
MATERIALS AND METHODS
Organic acids.
Cyclopropanecarboxylic acid and the other organic acids used in this study, except dl-3-hydroxyisobutyric acid, were reagent-grade commercial products. dl-3-Hydroxyisobutyric acid was obtained by alkaline hydrolysis of racemic methyl 3-hydroxyisobutyrate, followed by treatment with Dowex 50 (H+ form).
Hydroxamic acids.
Cyclopropanecarboxohydroxamic acid and dl-3-hydroxybutyrohydroxamic acid were synthesized by reaction of hydroxylamine with cyclopropanecarbonyl chloride and β-butyrolactone, respectively, as described by Hauser and Renfrow (10) for the synthesis of benzohydroxamic acid. Data for dl-3-hydroxybutyrohydroxamic acid were as follows: nuclear magnetic resonance (NMR) [200 MHz, D2O, 3-(trimethylsilyl)propanesulfonic acid sodium salt (TSS)], δ 1.22 (d, 3H, J = 6.3 Hz, C4-H), 2.30 (d, 2H, J = 6.6 Hz, C2-H), 4.18 (m, 1H, J = 6.3, 6.6 Hz, C3-H) ppm. Methacrylohydroxamic acid and dl-3-hydroxyisobutyrohydroxamic acid were similarly obtained by reaction of hydroxylamine with methacrylic anhydride and racemic methyl 3-hydroxyisobutyrate, respectively. Methacrylic anhydride was prepared by reaction of pyridinium methacrylate with methacrylyl chloride, according to the general directions of Allen et al. (2). Data for methacrylic anhydride were as follows: infrared (neat), 2950, 2925, 1780, 1720, 1635, 1450, 1295, 1035, 940 cm−1. Methacrylyl chloride was obtained by reaction of methacrylic acid with thionyl chloride.
CoA thioesters.
Cyclopropanecarboxyl-CoA was synthesized from cyclopropanecarboxylic acid, 1,1′-carbonyldiimidazole, and CoA according to the general procedure of Kawaguchi et al. (12). Crotonyl-CoA, methacrylyl-CoA, and dl-3-hydroxybutyryl-CoA were prepared by reaction of CoA with crotonic anhydride, methacrylic anhydride, and β-butyrolactone, respectively, as described by Simon and Shemin (25) for the synthesis of succinyl-CoA. dl-3-Hydroxyisobutyryl-CoA was obtained by the mixed-anhydride method of Beck et al. (3) as described for the synthesis of methylmalonyl-CoA. These CoA thioesters were purified to homogeneity by high-performance liquid chromatography on a reverse-phase column (Cosmosil 5C18; Nacalai Tesque) as described by Clough et al. (5). Solvents used were 30 to 35% methanol/5 mM potassium phosphate buffer (pH 4.0)/0.1% (vol/vol) 2-mercaptoethanol for cyclopropanecarboxyl-CoA, crotonyl-CoA, and methacrylyl-CoA and 20% methanol/5 mM potassium phosphate buffer (pH 4.0)/0.1% (vol/vol) 2-mercaptoethanol for dl-3-hydroxybutyryl-CoA and dl-3-hydroxyisobutyryl-CoA. The fractions containing each CoA thioester were concentrated to a small volume and subjected to column chromatography on Sephadex G-10 to separate desired CoA thioesters from inorganic phosphate.
N-Acetylcysteamine and cyclopropanecarbonyl-N-acetylcysteamine.
N-Acetylcysteamine was obtained by reaction of cysteamine with acetic anhydride, as described by Martin et al. (17). Cyclopropanecarboxyl-N-acetylcysteamine was synthesized by reaction of N-acetylcysteamine with cyclopropanecarbonyl chloride, according to the method of Endo et al. (7) for the synthesis of 2,3-decadienoyl-N-acetylcysteamine. The desired product was purified by preparative thin-layer chromatography (TLC) on silica gel by using ethyl acetate as a solvent. Data for cyclopropanecarboxyl-N-acetylcysteamine were as follows: NMR (200 Hz, C6D6, C6D5H = 7.15), δ 0.38-1.04 (m, 4H, cyclopropyl-3-CH2), 1.44 (s, 3H, CO-CH3), 1.60 (m, 1H, cyclopropyl-2-CH), 2.79 (t, 2H, 1′-CH2), 3.18 (m, 2H, 2′-CH2), 4.67 (m, 1H, N-H) ppm.
Microorganisms and cultivation.
The cyclopropanecarboxylate-utilizing R. rhodochrous (strain CPC-1) isolated from soil in Takamatsu, Japan (18), was precultured aerobically at 37°C for 30 h on a reciprocal shaker (120 strokes/min) in CPC-minimal medium [0.1% (NH4)2SO4, 0.01% KH2PO4, 0.05% MgSO4 · 7H2O, 0.01% CaCl2, and 0.1% cyclopropanecarboxylic acid (adjusted to pH 7.0 with NaOH)]. The cells were then transferred to the fresh CPC-minimal medium supplemented with 0.8% Casamino Acids and 0.3% cyclopropanecarboxylate (adjusted to pH 7.0). The inoculum size was 1%. The bacterium was grown aerobically at 37°C on a reciprocal shaker (120 strokes/min) and harvested by centrifugation in the late logarithmic phase. The cells were washed twice with 0.05 M potassium phosphate buffer (pH 7.2) and stored at −80°C.
Cell extracts.
About 1 g of wet cells grown on cyclopropanecarboxylate or citrate was suspended in 5 ml of 0.05 M potassium phosphate buffer (pH 7.2). Cells were disrupted by intermittent sonication (20 kHz and 240 W) for 20 min. Cell extracts were obtained by centrifugation at 15,000 × g for 30 min.
Oxidation of various substrates in cell extracts.
The rate of oxidation of an organic acid in cell extract was determined from an initial velocity of NADH formation. When cyclopropanecarboxylate or other organic acids were used as substrates, the reaction mixtures contained 1 mM CoA, 1 mM ATP, 1 mM MgCl2, 1 mM NAD+, 1 mM dithiothreitol (DTT), an appropriate amount of cell extract, 25 mM potassium phosphate buffer (pH 7.2), and 10 mM (each) substrates in a total volume of 1.0 ml. The enzyme reaction was started by adding a substrate, and the rate of reduction of NAD+ at 37°C was measured from the increase in absorbance at 340 nm. A molar extinction coefficient at 340 nm of 6.22 × 103 M−1 cm−1 for NADH was used.
When a CoA thioester was used as a substrate, the rate of its oxidation in cell extract was determined by the same procedure except that each CoA thioester was added to a concentration of more than 200 μM instead of an organic acid and that CoA, ATP, and MgCl2 were omitted from the reaction mixtures.
Other analytical procedures.
1H-NMR spectra were obtained on a Varian VXR-200 NMR spectrometer operating in the Fourier transform mode. Infrared spectra were measured on a JASCO IRA-1 spectrometer. Protein concentration was determined by the method of Lowry et al. (15) by using bovine serum albumin as the standard.
The concentration of a CoA thioester was determined colorimetrically after the CoA thioester was converted into the corresponding hydroxamic acid by incubation at 37°C for 30 min with 1 M hydroxylamine. After the addition of 0.4% FeCl3 in 0.3 N HCl, the absorbance at 500 to 540 nm was measured. Calibration curves were obtained with synthetic hydroxamic acids. A concentration of crotonyl-CoA was determined using its molar extinction coefficient of 22.6 × 103 M−1 cm−1 at 260 nm (26).
TLC was carried out using Merck precoated silica gel plates. Hydroxamic acids were located on TLC plates by spraying 1.25% FeCl3 in 1 N HCl (8). Thioesters were visualized by spraying p-anisaldehyde-acetic acid-ethanol-concentrated H2SO4 (9.3:3.8:340:12.5, vol/vol) and heating at 100°C for 10 min (27). Reducing compounds were detected on TLC plates by spraying 5 to 10% ethanolic solution of phosphomolybdic acid (P2O5 · 24MoO3 · xH2O) and heating at 120°C until spot formation was attained (14).
RESULTS
Utilization of organic acids as growth substrates by the bacterium.
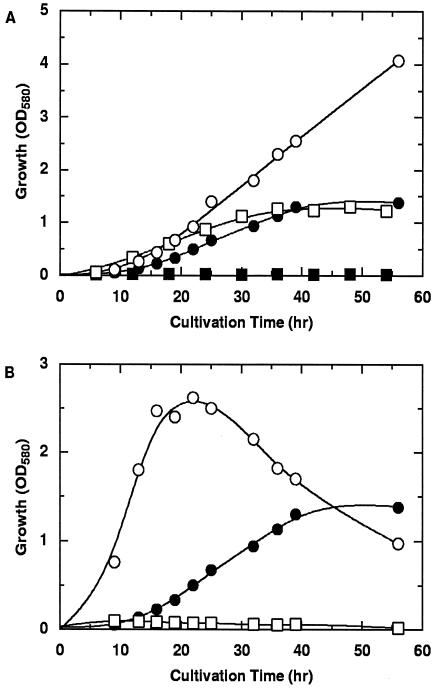
Several organic acids were examined for effectiveness as growth substrates when supplied instead of cyclopropanecarboxylate in the minimal medium. As illustrated in Fig. 1, R. rhodochrous was able to grow on dl-3-hydroxybutyrate and crotonate in addition to cyclopropanecarboxylate but not on dl-2-hydroxybutyrate. Higher concentrations (more than 0.4%) of cyclopropanecarboxylate and dl-3-hydroxybutyrate were inhibitory for growth in minimal media. Citrate was a good growth substrate for the bacterium, while glucose was a poor substrate.
FIG. 1.
Growth of cyclopropanecarboxylate-utilizing R. rhodochrous in minimal media containing various carbon sources. (A) Growth on 0.1% cyclopropanecarboxylate (•), 0.4% crotonate (○), 0.1% dl-3-hydroxybutyrate (□), or 0.1% dl-2-hydroxybutyrate (▪). (B) Growth on 0.1% cyclopropanecarboxylate (•), 0.1% citrate (○), or 0.1% glucose (□). OD580, optical density at 580 nm.
Oxidation of cyclopropanecarboxylate in cell extracts and cofactor requirements.
Cell extracts prepared from cells grown aerobically on cyclopropanecarboxylate were tested for their ability to catalyze in vitro oxidation of cyclopropanecarboxylate with NAD+. An increase in absorbance at 340 nm was observed when cyclopropanecarboxylate was added to a mixture of CoA, ATP, Mg2+, NAD+, and an appropriate amount of cell extract (data not shown). This result demonstrated that the reduction of NAD+ into NADH is coupled with oxidation of cyclopropanecarboxylate in cell extracts.
Cofactor requirements for the reaction are shown in Table 1. The reduction of NAD+ was strictly dependent on cyclopropanecarboxylate, CoA, ATP, and cell extract. It is therefore evident that the reduction of NAD+ with cyclopropanecarboxylate is catalyzed by the enzymes included in the cell extract. DTT was not required for catalysis, but it was routinely added for stabilization of enzymes. The requirements of CoA and ATP suggest that, for the oxidation of cyclopropanecarboxylate with NAD+ in the cell extract, its conversion into the CoA thioester is an obligatory initial step.
TABLE 1.
Cofactor requirements for NAD+ reduction in cell extract of cyclopropanecarboxylate-grown R. rhodochrous cells
| Missing componenta | Amt of NADH formed (nmol/min/mg of protein) |
|---|---|
| None | 3.0 |
| Cyclopropanecarboxylate | 0.0 |
| CoA | 0.1 |
| ATP | 0.1 |
| NAD+ | 0.0 |
| Extract | 0.0 |
The complete system contained 10 mM cyclopropanecarboxylate, 1 mM CoA, 1 mM ATP, 1 mM MgCl2, 1 mM NAD, 25 mM potassium phosphate buffer (pH 7.2), and 12 mg of protein in the extract in a total volume of 1.0 ml. The rate of NADH formation was determined as described in the text.
Trapping of metabolic intermediates as hydroxamic acid derivatives and their identification.
To accumulate and trap CoA thioester intermediates as hydroxamic acid derivatives, large-scale incubation without NAD+ was carried out—that is, cell extract containing 18 mg of protein was incubated at 37°C for 1 day with 12 mM cyclopropanecarboxylate in the presence of 0.4 mM CoA, 3.4 mM ATP, 1.2 mM DTT, 15 mM hydroxylamine, and 0.03 M potassium phosphate buffer (pH 7.2) in a total volume of 500 ml. When the reaction mixture was analyzed by TLC on silica gel in water-saturated 2-butanol, two hydroxamic acid derivatives were detected by spraying the FeCl3-HCl reagent. Their Rf values were 0.75 (hydroxamic acid I; purple developed) and 0.59 (hydroxamic acid II; red developed). The ratio of amounts of hydroxamic acids I and II formed was roughly estimated by TLC to be 1:3 in this experiment. As shown in Table 2, hydroxamic acid I comigrated with synthetic cyclopropanecarboxohydroxamic acid upon TLC in three different solvent systems. Hydroxamic acid II comigrated with synthetic 3-hydroxybutyrohydroxamic acid but not with synthetic cyclopropanecarboxohydroxamic acid, methacrylohydroxamic acid, or 3-hydroxyisobutyrohydroxamic acid.
TABLE 2.
TLC analyses of the metabolic intermediates trapped as hydroxamic acid derivativea
| Hydroxamic acid |
Rf value in solvent:
|
||
|---|---|---|---|
| A | B | C | |
| Hydroxamic acid I | 0.75 | 0.59 | 0.69 |
| Hydroxamic acid II | 0.59 | 0.31 | 0.42 |
| Cyclopropanecarboxohydroxamic acid | 0.76 | 0.59 | 0.68 |
| 3-Hydroxybutyrohydroxamic acid | 0.60 | 0.33 | 0.43 |
| Methacrylohydroxamic acid | 0.79 | ||
| dl-3-Hydroxyisobutyrohydroxamic acid | 0.64 | 0.42 | 0.49 |
Experimental details are described in the text. Solvent systems used for silica gel TLC were as follows: A, water-saturated 2-butanol; B, 2-butanol containing 1% CH3COOH; and C, 2-butanol containing 1% NH4OH.
To isolate hydroxamic acid II, the reaction mixture was concentrated to a small volume and applied to a column (5 by 10 cm) of silica gel, followed by elution with methanol. The eluate was evaporated to dryness, taken up into a small amount of water, and applied to a column of Dowex 1 (adjusted to pH 12.0). After the column was washed with 0.5 mM NH4Cl, the adsorbed hydroxamic acids were eluted with 0.85 mM NH4Cl. Hydroxamic acid II was isolated by high-performance liquid chromatography on a reverse-phase (octadecyl silane) column using 1% acetic acid as the eluting agent. The 200-MHz 1H-NMR spectrum of the isolated hydroxamic acid II in D2O was taken with TSS as an internal standard. Three signals were observed at δ 1.22 (d, 3H, J = 6.2 Hz), 2.30 (d, 2H, J = 6.5 Hz), and 4.18 (m, 1H, J = 6.2, 6.5 Hz) ppm. This spectrum also indicated that hydroxamic acid II is 3-hydroxybutyrohydroxamic acid. Synthetic dl-3-hydroxybutyrohydroxamic acid gave an identical NMR spectrum, as described in Materials and Methods. From these lines of evidence, hydroxamic acid II obtained by the enzymatic reaction was identified as 3-hydroxybutyrohydroxamic acid. Therefore, it is strongly suggested that cyclopropanecarboxylate is metabolized through cyclopropanecarboxyl-CoA and 3-hydroxybutyryl-CoA.
Oxidation of plausible CoA thioester intermediates and corresponding organic acids in cell extracts.
A hypothetical degradation pathway for cyclopropanecarboxylate can be drawn as follows: cyclopropanecarboxylate→cyclopropanecarboxyl-CoA→3-hydroxybutyryl-CoA→acetoacetyl-CoA→ · · · . Totest this pathway, putative CoA thioester intermediates and other CoA thioesters were synthesized and examined for their effectiveness as substrates for the oxidation system in the cell extracts. As shown in Table 3, both of the putative intermediates, i.e., cyclopropanecarboxyl-CoA and 3-hydroxybutyryl-CoA, as well as crotonyl-CoA were oxidized with NAD+ in the extract in the absence of CoA and ATP. Methacrylyl-CoA and 3-hydroxyisobutyryl-CoA, neither of which is considered a metabolic intermediate in the above pathway, did not undergo oxidation at a significant rate at a concentration of more than 200 μM. Synthetic S-cyclopropanecarboxyl-N-acetylcysteamine did not serve as a substrate at all, even at a concentration of 6 mM, when it was used as a substrate instead of the corresponding CoA thioester (data not shown). Therefore, it became clear that the oxidation system for cyclopropanecarboxylate is specific for CoA thioesters.
TABLE 3.
Reduction of NAD+ with CoA thioesters and corresponding carboxylic acids in cell extract of cyclopropanecarboxylate-grown cellsa
| Substrate | Amt of NADH formed (nmol/min/mg of protein)
|
|
|---|---|---|
| Without CoA and ATP | With CoA and ATP | |
| None | 0 | 0 |
| Cyclopropanecarboxyl-CoA | 3.3 | |
| 3-Hydroxybutyryl-CoA | 26.6 | |
| Crotonyl-CoA | 8.1 | |
| Methacrylyl-CoA | 0.0 | |
| 3-Hydroxyisobutyryl-CoA | 0.1 | |
| Cyclopropanecarboxylate | 2.6 | |
| 3-Hydroxybutyrate | 8.8 | |
| Crotonate | 27.3 | |
| Methacrylate | 0.1 | |
| 3-Hydroxyisobutyrate | 0.1 | |
| 2-Hydroxybutyrate | 0.0 | |
Specificities for organic acid substrates and CoA thioester substrates were determined in the presence and absence of 1 mM CoA plus 1 mM ATP, respectively.
Oxidation of several organic acids with NAD+ in cell extracts was also examined in the presence of supplemented CoA and ATP. As shown in Table 3, the two carboxylic acids corresponding to the putative intermediates, i.e., cyclopropanecarboxylate and 3-hydroxybutyrate, were oxidized with NAD+ in the extract. Crotonate was also oxidized, whereas methacrylate, 3-hydroxyisobutyrate, and 2-hydroxybutyrate were not. Therefore, the CoA thioesters corresponding to the latter three acids seemed not to be involved in the degradation of cyclopropanecarboxylate. These results also provide additional evidence that cyclopropanecarboxyl-CoA and 3-hydroxybutyryl-CoA are metabolic intermediates in this order in the dissimilation of cyclopropanecarboxylate. Although crotonate and crotonyl-CoA were also oxidizable in the extract in the presence and absence of CoA plus ATP, respectively, it remains to be elucidated whether cyclopropanecarboxylate is oxidized via crotonyl-CoA or not.
Inducibility of substrate-oxidizing enzyme activities.
The extract prepared from cells grown on citrate was tested for activity to oxidize various substrates with NAD+. As shown in Table 4, 3-hydroxybutyryl-CoA and crotonyl-CoA were oxidized with NAD+ in the extract, whereas cyclopropanecarboxyl-CoA was not. This result indicated that the enzyme responsible for the ring opening of cyclopropanecarboxyl-CoA is not constitutive but inducible when cells are grown on cyclopropanecarboxylate. The extract of citrate-grown cells catalyzed the oxidation of 3-hydroxybutyrate and crotonate with NAD+ in the presence of CoA plus ATP but not the oxidation of cyclopropanecarboxylate at a significant rate. Unlike the results with the extract of cyclopropanecarboxylate-grown cells (Table 3), the rates of oxidation of crotonate and 3-hydroxybutyrate were much slower than those of the corresponding CoA thioesters (Table 4). Therefore, it is likely that the enzyme catalyzing formation of CoA thioesters from cyclopropanecarboxylate and these substrates is also inducible. There may be a possibility that there is also an inducible enzyme(s) in addition to the constitutive enzymes for the oxidation of 3-hydroxybutyryl-CoA, because the activity of this CoA thioester in extract from cyclopropanecarboxylate-grown cells is much higher than that in extract from citrate-grown cells.
TABLE 4.
Reduction of NAD+ by CoA thioesters and corresponding carboxylic acids in cell extract of citrate-grown cellsa
| Substrate | Amt of NADH formed (nmol/min/mg of protein)
|
|
|---|---|---|
| Without CoA and ATP | With CoA and ATP | |
| None | 0 | 0 |
| Cyclopropanecarboxyl-CoA | 0.0 | |
| 3-Hydroxybutyryl-CoA | 4.8 | |
| Crotonyl-CoA | 4.7 | |
| Cyclopropanecarboxylate | 0.0 | |
| 3-Hydroxybutyrate | 0.3 | |
| Crotonate | 1.0 | |
Assay conditions were identical to those described in Table 3, footnote a.
DISCUSSION
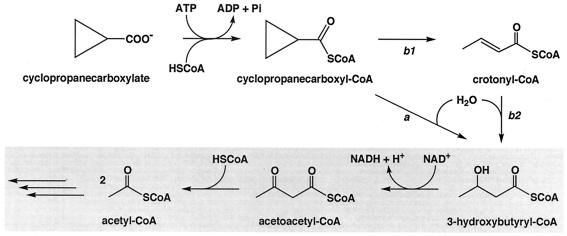
From the data presented in this paper, a plausible pathway for the oxidative degradation of cyclopropanecarboxylate by R. rhodochrous can be drawn (Fig. 2). When cyclopropanecarboxylate is taken up by the cell, it is first converted into its CoA thioester with the consumption of free energy liberated by ATP hydrolysis. Cyclopropanecarboxyl-CoA then undergoes the ring-opening reaction prior to oxidation by a hitherto unknown enzyme. Crotonate was a growth substrate for the bacterium, and crotonate and crotonyl-CoA were oxidized with NAD+ in the cell extract in the presence and absence of CoA plus ATP, respectively. However, it is not clear at present whether crotonyl-CoA is involved as a metabolic intermediate in the conversion of cyclopropanecarboxyl-CoA into 3-hydroxybutyryl-CoA. Further characterization of the enzyme responsible for the ring fission of cyclopropanecarboxyl-CoA would be necessary to solve this important problem. 3-Hydroxybutyryl-CoA is probably dehydrogenated into acetoacetyl-CoA by NAD+-linked β-hydroxyacyl-CoA dehydrogenase, followed by thiolytic cleavage to acetyl-CoA by acetoacetyl-CoA thiolase. The presence of the latter two enzymes in the extracts was confirmed (data not shown).
FIG. 2.
Postulated pathways for oxidative degradation of cyclopropanecarboxylate by R. rhodochrous. Possible pathways for the conversion of cyclopropanecarboxyl-CoA into 3-hydroxybutyryl-CoA are shown. The β-oxidation system for oxidative degradation is shadowed.
Since the extract of citrate-grown cells was incapable of oxidizing cyclopropanecarboxyl-CoA, the putative ring-opening enzyme for cyclopropanecarboxyl-CoA seems to be an inducible enzyme. Since 3-hydroxybutyrate and crotonate were oxidized in the same extract much more slowly than the corresponding CoA thioesters, it was postulated that at least one of the acyl-CoA synthetases was an inducible enzyme. Other enzymes involved in the oxidative degradation of cyclopropanecarboxylate were shown to be constitutive, although there may be an inducible enzyme(s) in addition to the constitutive enzymes for the oxidation of 3-hydroxybutyryl-CoA. Therefore, it can be concluded that cyclopropanecarboxylate is first converted into 3-hydroxybutyryl-CoA by cyclopropanecarboxyl-CoA through the unknown, nonoxidative pathway and then enters the constitutive β-oxidation system for oxidative degradation. Two possible pathways for the conversion of cyclopropanecarboxyl-CoA into 3-hydroxybutyryl-CoA are shown in Fig. 2. Pathway a is the direct conversion, but this does not rule out a pathway involving an unstable intermediate(s), such as cyclopropenecarboxyl-CoA. Pathway b is the indirect conversion involving crotonyl-CoA as a metabolic intermediate. To decide which pathway is more likely, we have to await the purification of the cyclopropane ring-opening enzyme.
Tipton and Al-Shatir reported that cyclopropane ring-containing, long-chain fatty acids are degraded by the protozoa Ochromonas danica (28) and Tetrahymena pyriformis (31). They proposed that these fatty acids are metabolized by the latter organism through a minor modification of β-oxidation (31). Schiller and Chung reported that cyclopropanecarboxylate is converted into 4-hydroxybutyrate by the fungus Fusarium oxysporum through the direct addition of water to an intermediate across one of its C-C bonds and then degraded by a known pathway (23, 24). Guilbert and Chung proposed that the activated intermediate is cyclopropanecarboxylcarnitine (9). Duncombe and Rising found that incubation of rat and guinea pig liver mitochondria with cyclopropanecarboxylate results in formation of a metabolite which cochromatographs with cyclopropanecarboxylcarnitine (6). Patterson and Hegeman reported that cyclopropanecarboxylate is degraded by a Corynebacterium sp. with isobutyryl-CoA, methacrylyl-CoA, and 3-hydroxyisobutyryl-CoA as intermediates (19). It is therefore evident that the pathway reported in this paper for the degradation of cyclopropanecarboxylate by R. rhodochrous is quite different from those proposed by other investigators. Characterization of a novel cyclopropane ring-opening enzyme for cyclopropanecarboxyl-CoA is under current investigation.
Acknowledgments
We thank Yukiko Kurimoto and Ruriko Ogawa for assistance in the manuscript preparation.
REFERENCES
- 1.Adams, D. O., and S. F. Yang. 1979. Ethylene biosynthesis: identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc. Natl. Acad. Sci. USA 76:170-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, C. F. H., C. J. Kibler, D. M. McLachlin, and C. V. Wilson. 1955. Acid anhydrides. Org. Synth. Collect. Vol. 3:28-30. [Google Scholar]
- 3.Beck, W. S., M. Flavin, and S. Ochoa. 1957. Metabolism of propionic acid in animal tissues. III. Formation of succinate. J. Biol. Chem. 229:997-1010. [PubMed] [Google Scholar]
- 4.Blakley, E. R. 1978. The microbial degradation of cyclohexanecarboxylic acid by a β-oxidation pathway with simultaneous induction to the utilization of benzoate. Can. J. Microbiol. 24:847-855. [DOI] [PubMed] [Google Scholar]
- 5.Clough, R. C., S. R. Barnum, and J. G. Jaworski. 1989. Synthesis of radiolabeled acetyl-coenzyme A from sodium acetate. Anal. Biochem. 176:82-84. [DOI] [PubMed] [Google Scholar]
- 6.Duncombe, W. G., and T. J. Rising. 1972. Studies on the hypoglycaemic compound cyclopropanecarboxylic acid. Effects on fatty acid oxidation in vitro. Biochem. Pharmacol. 21:1075-1088. [DOI] [PubMed] [Google Scholar]
- 7.Endo, K., G. M. Helmkamp, Jr., and K. Bloch. 1970. Mode of inhibition of β-hydroxydecanoyl thioester dehydrase by 3-decynoyl-N-acetylcysteamine. J. Biol. Chem. 245:4293-4296. [PubMed] [Google Scholar]
- 8.Fink, K., and R. M. Fink. 1949. Application of filter paper partition chromatography to qualitative analysis of volatile and non-volatile organic acids. Proc. Soc. Exp. Biol. Med. 70:654-656. [DOI] [PubMed] [Google Scholar]
- 9.Guilbert, C. C., and A. E. Chung. 1974. Metabolism of cyclopropanecarboxylic acid. A new role for carnitine. J. Biol. Chem. 249:1026-1030. [PubMed] [Google Scholar]
- 10.Hauser, C. R., and W. B. Renfrow, Jr. 1955. Benzohydroxamic acid. Org. Synth. Collect. Vol. 3:67-68. [Google Scholar]
- 11.Honma, M., and T. Shimomura. 1978. Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric. Biol. Chem. 42:1825-1831. [Google Scholar]
- 12.Kawaguchi, A., T. Yoshimura, and S. Okuda. 1981. A new method for the preparation of acyl-CoA thioesters. J. Biochem. 89:337-339. [DOI] [PubMed] [Google Scholar]
- 13.Kitagawa, H., R. Iwaki, B. Yanagi, and T. Sato. 1956. Syntheses and hypnotic actions of alkylcoumarin derivatives. J. Pharm. Soc. Jpn. 76:186-189. [Google Scholar]
- 14.Kritchevsky, D., and M. R. Kirk. 1952. Detection of steroids in paper chromatography. Arch. Biochem. Biophys. 35:346-351. [DOI] [PubMed] [Google Scholar]
- 15.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 16.Magat, W. J., and C. L. Tipton. 1970. Oxidation of cyclopropane fatty acids by Ochromonas danica. Arch. Biochem. Biophys. 141:353-355. [DOI] [PubMed] [Google Scholar]
- 17.Martin, R. B., S. Lowey, E. L. Elson, and J. T. Edsall. 1959. Hydrolysis of 2-methyl-Δ2-thioazoline and its formation from N-acetyl-β-mercaptoethylamine. Observations on an N-S acyl shift. J. Am. Chem. Soc. 81:5089-5095. [Google Scholar]
- 18.Nishihara, H., Y. Ochi, H. Nakano, M. Ando, and T. Toraya. 1995. Isolation and identification of a cyclopropanecarboxylate-utilizing bacterium. J. Ferment. Bioeng. 80:400-402. [Google Scholar]
- 19.Patterson, C. O., and G. D. Hegeman. 1978. Metabolism of cyclopropane carboxylic acid by bacteria. Microb. Degrad. Pollut. Mar. Environ. 1978:89-104. [Google Scholar]
- 20.Pirrung, M. C., and G. M. McGeehan. 1983. Ethylene biosynthesis. 1. A model for two reactive intermediates. J. Org. Chem. 48:5143-5144. [Google Scholar]
- 21.Pirrung, M. C., and G. M. McGeehan. 1985. Ethylene biosynthesis. 4. Cyclopropyl-substituted aminocyclopropanecarboxylic acid. A study of the mechanism of ethylene biosynthesis. Angew. Chem. 97:1074-1075. [Google Scholar]
- 22.Rho, E. M., and W. C. Evans. 1975. The aerobic metabolism of cyclohexanecarboxylic acid by Acinetobacter anitratum. Biochem. J. 148:11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schiller, J., and A. E. Chung. 1970. Mechanism of cyclopropane ring cleavage in cyclopropanecarboxylic acid. J. Biol. Chem. 245:6553-6557. [PubMed] [Google Scholar]
- 24.Schiller, J., and A. E. Chung. 1970. The metabolism of cyclopropanecarboxylic acid. J. Biol. Chem. 245:5857-5864. [PubMed] [Google Scholar]
- 25.Simon, E. J., and D. Shemin. 1953. The preparation of S-succinyl coenzyme A. J. Am. Chem. Soc. 75:2520. [Google Scholar]
- 26.Stadtman, E. R. 1957. Preparation and assay of acylcoenzyme A and other thiol esters; use of hydroxylamine. Methods Enzymol. 3:931-941. [Google Scholar]
- 27.Stahl, E., and U. Kaltenbach. 1961. Dünnschicht-chromatographie. VI. Mitteilung. Spurenanalyse von zuckergemischen auf kieselgur G-schichten. J. Chromatogr. 5:351-355. [Google Scholar]
- 28.Tipton, C. L., and N. M. Al-Shatir. 1974. The metabolism of cyclopropane fatty acids by Tetrahymena pyriformis. J. Biol. Chem. 249:886-889. [PubMed] [Google Scholar]
- 29.von Lauger, P., H. Martin, and P. Muller. 1944. Constitution and toxic action of natural and new synthetic insecticides. Helv. Chim. Acta 27:892-928. [Google Scholar]
- 30.Walsh, C., R. A. Pascal, M. Johnston, R. Raines, D. Dikshit, A. Krantz, and M. Honma. 1981. Mechanistic studies on the pyridoxal phosphate enzyme 1-aminocyclopropane-1-carboxylate deaminase from Pseudomonas sp. Biochemistry 20:7509-7519. [DOI] [PubMed] [Google Scholar]
- 31.Wood, R., and R. Reiser. 1965. Cyclopropane fatty acid metabolism: physical and chemical identification of propane ring metabolic products in the adipose tissue. J. Am. Oil Chem. Soc. 42:315-320. [DOI] [PubMed] [Google Scholar]