Abstract
Adult hippocampal neurogenesis (AHN) of laboratory rodents is enhanced by physical exercise in a running wheel. However, little is known about modulation of AHN in wild-living rodent species. The finding that AHN cannot be modulated by voluntary exercise in wild wood mice suggests that AHN may be regulated differently under natural conditions than in laboratory adapted animals. In order to minimize genetic influences, we aimed to investigate the genetically closest wild-living relatives of laboratory mice. Here, C57BL/6 mice and F1 offspring of wild house mice (Mus musculus domesticus) were tested in two different running paradigms: voluntary running and running-for-food - a condition in which mice had to run for their daily allowance of food. In house mice, we found a non-significant trend towards increased numbers of proliferating cells and doublecortin-positive immature neurons in both voluntary runners and runners-for-food. Voluntary running in C57BL/6 mice resulted in a 30% increase in cell proliferation and a pronounced 70% increase in doublecortin-positive cells. C57BL/6 runners-for-food ran as much as voluntary runners, but they showed no enhancement of cell proliferation, a small increase in the number of doublecortin-positive cells and more pyknotic cells compared to controls. Taken together, these findings suggest that motivational aspects of running are critical determinants of the increased cell proliferation in C57BL/6 mice. In contrast, running has smaller and context-independent effects in house mice. The findings imply a difference in the regulation of AHN in C57BL/6 mice and their wild-derived conspecifics.
Keywords: exercise, motivation, context, strain, comparative, optical fractionator
1. Introduction
In laboratory mice and rats, the most investigated positive modulator of adult hippocampal neurogenesis (AHN) is physical exercise. Exercise robustly promotes cell proliferation and neuronal differentiation [1–4], which also correlates with improved spatial learning abilities [5–7]. However, this correlation may not reflect a causal relationship, since AHN is not necessary to fulfill cognitive demands placed on the hippocampus during all (Microchiroptera, [8]) or part (Shrews, [9]) of the adult life of some mammals.
In view of the positive connotations associated with exercise, extrapolation to humans seems intuitive and has generated much attention to this field. Although exercise may ameliorate deficits associated with psychiatric and neurodegenerative disorders [10–12], it is uncertain if effects are mediated by a modulation of AHN [13, 14]. Although AHN has been described in humans [15], it is not clear if behaviors that modulate AHN in laboratory rodents should do so in humans because of differences in both genetics and motivation for running. In laboratory mice, an increase in performance in expectation of a reward is not associated with an increase in AHN [2], suggesting that psychological states during exercise and the underlying motivation are factors in the regulation of AHN. This idea is supported by the finding that positive effects of forced treadmill running on AHN are lost if the speed or duration is increased beyond a certain level [16]. This level is, in terms of both speed and duration, far lower than that required to enhance neurogenesis in voluntary running mice, suggesting that a positive effects of running on AHN may relate more to the perception and motivation of the running mouse than to the level of performance.
Species-specific effects of running suggest that genetics may play a strong role in response to running. Even on a close phylogenetic scale, AHN in wild wood mice (Apodemus sylvaticus), which are near relatives of laboratory mice [17, 18], show no modulation of AHN by exercise [19]. This lack of modulation of AHN in wood mice may be genetic or the consequence of natural living conditions. With an increasing complexity of the environment and associated variety of external influences on AHN, the importance of any single stimulus may decrease. Whether stable AHN is a complexity-related or a species-specific phenomenon only seen in wood mice can best be tested by investigating genetically closely related wild-derived and laboratory rodents. In this study, Western house mice (Mus musculus domesticus), which are the closest relatives of common laboratory inbred strains [20], and C57BL/6 mice were tested in voluntary running conditions and under a running paradigm that required mice to run for their daily allowance of food. We investigated differences in running performance, cell proliferation, cell death and the number of doublecortin-positive young neurons between these groups.
2. Materials and Methods
2.1 Animals
2.1.1. Experiment 1: C57BL/6 mice
We used 36 twelve week old female C57BL/6 mice (Charles River Laboratories, Germany). All animals were habituated to an inverted light-dark cycle over a period of one week before they were administered to the experimental groups. Habituation was performed in the same room in which the experiment took place.
2.1.2 Experiment 2: house mice
We tested 22 twelve week old first generation (F1) offspring of wild-caught western European house mice (Mus musculus domesticus; 17 males and 5 females) kindly provided by the Department of Animal Behavior, University of Zurich. All animals were habituated to an inverted light-dark cycle over a period of one week before they were randomly distributed to the experimental groups. Each experimental group contained at least one female. Habituation was performed in the same room in which the experiment took place.
We will refer to ‘strains’ when both C57BL/6 and house mice are meant. Animal husbandry was identical for the two strains, with the exception that the animals were kept in different facilities and that C57BL/6 and house mice were weaned at the ages of 21 and 24 days respectively. The slightly different ages at weaning are unlikely to have an impact on the outcomes in that proliferation and neuronal differentiation were indistinguishable in adults of two strains of mice subjected to early postnatal stress in the form of maternal separation [21].
2.2 Exercise
The experimental design was the same for both C57BL/6 and house mice. At the beginning of the experiment, mice were individually housed in either standard cages (controls), standard cages equipped with a running wheel (voluntary running group) or cages in which the wheel was connected to a feeding system (runners-for-food group). All runners had free access to the running wheel throughout the experimental period of two weeks. Their performance was recorded in one-hour bins by a controller system (AMS Software & Electronic GmbH, Flensburg, Germany).
In a pilot experiment, age-matched C57BL/6 mice were housed individually for one week in running wheel-equipped standard cages and their average daily food consumption was recorded. In the main experiment the running and control mice were fed a daily amount of 5 g standard lab food (Provimi Kliba AG, Switzerland), which corresponded to the average food intake of the pilot animals.
Runners-for-food had to run for their daily allowance of chow. This was achieved by connecting a feeder to the controller. After performing a preset number of revolutions, the feeder released ~100 mg of chow. To satisfy the daily need of 5 g the feeder had to dispense 50 portions. The preset number of revolutions that dispensed one portion of food was set daily to 1/50th of the performance of the animal on the previous day to ensure that all animals received on average 50 portions. This approach allowed the animals to exercise at their individual level without being overfed or food deprived. Control and voluntary running mice were fed with 5 g of fine grained chow in cages furnished identically to those of the runners-for-food.
During the whole experimental period, animals were disturbed only once a day to read and adjust the controllers as well as to feed the control and voluntary running animals.
2.3 Histology and Immunohistochemistry
After 14 days all animals were overdosed with Pentobarbital (50 mg/kg) in the middle of their active phase and perfused transcardially with cold phosphate buffered saline (PBS) and cold 4% paraformaldehyde with 15% saturated picric acid (PFA). The brains were removed and separated into left and right hemispheres before they were postfixed in 4% PFA for 12 hours and cryoprotected in 30% sucrose. The right hemispheres were cut into 40 μm sagittal sections, stored at −20°C in a cryoprotectant, and processed as described below.
2.3.1 Ki67 and DCX
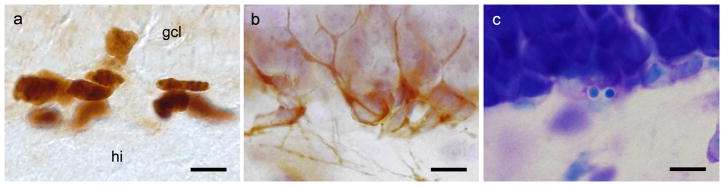
Proliferating cells were immunohistochemically stained for endogenous Ki67 nucleoprotein (Fig. 1a) expressed during the cell cycle [22, 23]. To identify newly generated cells of neuronal lineage, we used an antibody against Doublecortin (DCX; Fig 1b), a microtubule-associated protein expressed during the migration of neurons and the growth of their dendrites [24–26].
Fig. 1. Visualization of proliferating cells, DCX-positive and pyknotic cells.
(a) Immunohistochemical staining of proliferating cells with Ki67 antibody. Scale bar: 10 μm; hi: hilus, gcl: granule cell layer). (b) DCX-positive cells of neuronal lineage, counterstained with haematoxylin; scale bar: 10 μm. (c) Condensed chromatin of a Giemsa-stained pyknotic cell, forming two distinct nuclear bodies; scale bar: 10 μm.
For Ki67 epitope retrieval, we incubated free floating sections for 40 min in citrate buffer, pH 6.0, at 95°C. Thereafter, sections were preincubated in 2% normal goat serum (NGS), 0.1% bovine serum albumin (BSA) and 0.25% Triton in Tris-buffered saline (TBS), for 60 min at room temperature (RT) and then incubated overnight with the primary Ki67 antibody (polyclonal rabbit NCL-Ki67p, Novocastra, 1:5000 in preincubation solution). A two-hour incubation with the secondary antibody (biotinylated goat anti-rabbit IgG 1:1000 + 2% NGS + 0.1% BSA in TBS) was followed by incubation with a streptavidin-biotin complex (Vectastain Elite ABC kit, Vector Laboratories, Burlingame, CA) and stained with diaminobenzidine (DAB) as chromogen.
For DCX immunocytochemistry, endogenous peroxidase was blocked with 0.6% H2O2 in TBS-Triton. Subsequently, sections were preincubated at RT with 2% normal rabbit serum (NRS), 0.1% BSA and 0.25% Triton in TBS. Thereafter the sections were incubated overnight with the primary DCX antibody (polyclonal goat IgG, Santa Cruz Biotechnology, 1:1000 in preincubation solution) at 4°C. After the incubation with secondary antibody (rabbit anti-goat IgG, Vectastain Elite ABS Kit, 1:1000 + 2% NRS + 0.1% BSA in TBS) and streptavidin-biotin-complex (Vectastain Elite ABC kit), the sections were stained with DAB and counterstained with Ehrlich’s haematoxylin. Until incubation with primary antibody, all rinses were in TBS-Triton, afterwards with TBS alone. Sections were mounted, dehydrated with alcohol, cleared and coverslipped.
2.3.2 Giemsa
Pyknotic cells were used as indicators of neuronal death [27, 28]. The staining was performed according to the protocol of Iñiguez [29] (Fig. 1c). Mounted sections were incubated in filtered Giemsa staining solution (Giemsa stock solution 1.09204.0500, Merck, Darmstadt, Germany) diluted 1: 10 in buffer (67 mmol KH2PO4) at RT for 40 min, rinsed in buffer for 90 s and differentiated in 3 × 1 minute in 99% alcohol before they were cleared and coverslipped.
2.4 Cell counts
2.4.1 Total DCX positive cells
The total numbers of DCX positive cells were estimated in every fifth section with the optical fractionator [30] using the StereoInvestigator software (MicroBrightField Inc. Williston, USA) with a 100× oil-immersion lens (N.A.1.30). Cells were counted in a frame of 30 × 30 μm with an x, y-step size of 125 μm between sampling locations. Cells in the top focal plane were excluded from the counts. Total cell numbers (N) were calculated with the formula N = ΣQ− × (1/asf) × 1/ssf, (Q− = total number of cells counted, asf = area sampling fraction = a(frame)/a(x, y step) and ssf = section sampling fraction).
2.4.2 Total Ki67 positive- and pyknotic cells
Ki67 positive- and pyknotic cells were counted manually in every fifth section with a 100× oil-immersion lens (N.A.1.30). Pyknotic cells were identified by their strongly stained nuclei whose chromatin condensed into peripherally (C or doughnut shape), solid or multiple cell bodies [27, 28]. Again, cells in the top focal plane of the section were not counted. Total cell numbers (N) were calculated by multiplying the cell counts by the section sampling fraction (five).
2.5 Statistics
For statistical analyses SPSS 17.0 statistical software (SPSS Inc, Chicago, Illinois) was used. First, number estimates of Ki67- and DCX-positive cells as well as pyknotic cells between experimental groups were tested with a general linear model (GLM) using one-way ANOVA for each strain separately. In addition for house mice, a two-way ANOVA was used to test for gender and gender*experiment interaction effects. For the comparison of experimental effect sizes, we performed a one-way ANOVA on normalized cell numbers (numbers in house mouse or C57BL/6 running groups as percent of the house mouse or C57BL/6 controls) across all six groups. We used normalized data because of the species-specific differences in basal neurogenesis rate. A p<0.05 was considered significant. In case of a significant main effect, group differences were tested using Fisher’s PLSD post-hoc test. Correlation analyses were performed with paired two-group Spearman rank correlations. The precision of cell number estimates was calculated using the approach of Gundersen and Jensen [31, 32] using a smoothness factor of 0 [33].
3. Results
3.1 Performance
In neither strain did we observe differences in body weight between runners and runners-for-food before or after the experiment (p > 0.05 for all comparisons), indicating that caloric demands were equally well satisfied and that the motivation to perform based on caloric demand was similar in both strains.
3.1.1 Experiment 1: C57BL/6 mice
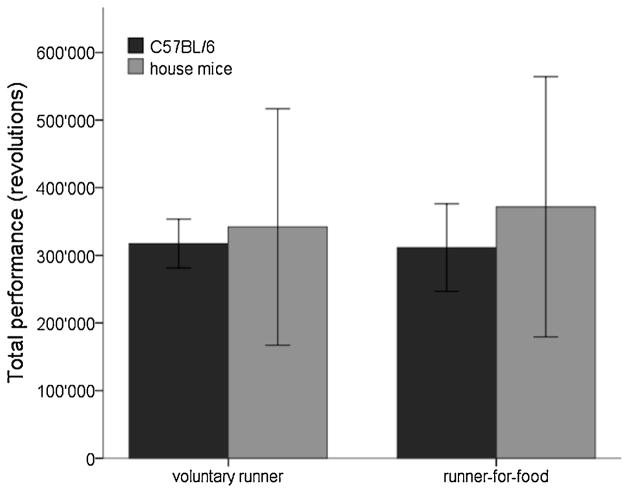
We observed an inter-individual variability in performance, ranging from 13’000 to 29’000 revolutions per day (or 5.7 to 12.8 km). The daily average performances were 22’391 revolutions (9.9 km) and 22’248 revolutions (9.8 km) for voluntary runners and runners-for-food respectively (p=0.93; Tab. 1, Fig. 2). In both treatment groups performance increased significantly in the second week of the experiment (voluntary runners: 27%, p<0.001; runners-for-food: 28%, p<0.001), but the increases did not differ between the groups (p=0.96).
Table 1.
Mean daily performance and total cell numbers. Standard deviations are given in parenthesis.
| controls | runners | runners-for-food | coefficient error (CE) | Counted sections 2) | ||
|---|---|---|---|---|---|---|
| Ø running week 1 1) | C57BL/6 | 19997 (3495) | 20317 (5551) | |||
| house mice | 21125 (10680) | 24419 (13038) | ||||
|
| ||||||
| Ø running week 2 1) | C57BL/6 | 24785 (2872) | 24178 (4716) | |||
| house mice | 27733 (14648) | 28687 (16039) | ||||
|
| ||||||
| Ø running | C57BL/6 | 22391 (2647) | 22248 (4633) | |||
| house mice | 24429 (12485) | 26553 (13742) | ||||
|
| ||||||
| Ki67 | C57BL/6 | 7987 (3619) | 10554 (5163) | 7899 (2947) | 0.089 | 13.4 (12–15) |
| house mice | 1623 (266) | 2113 (505) | 2152 (621) | 0.077 | 13.5 (12–16) | |
|
| ||||||
| DCX | C57BL/6 | 21882 (6143) | 36712 (9491) | 30006 (10529) | 0.059 | 14.1 (12–17) |
| house mice | 10900 (2166) | 14579 (3986) | 14269 (3578) | 0.084 | 13.7 (11–16) | |
|
| ||||||
| Pyknotic cells | C57BL/6 | 249 (43) | 261 (35) | 294 (57) | 0.063 | 14.5 (13–17) |
| house mice | 290 (81) | 286 (104) | 262 (90) | 0.072 | 13.7 (12–16) | |
data presented in revolutions;
mean values with ranges in parenthesis
Fig. 2. Performance in the running wheel does not differ between voluntary and running-for-food mice.
In C57BL/6− and house mice total performance does not differ between voluntary runners and runners-for-food. No significant difference between C57BL/6− and house mice is found. Bars represent mean standard deviation (SD).
3.1.2 Experiment 2: house mice
Average performances of the house mice did not differ from C57BL/6 mice (p=0.25), but we found a larger inter-individual variability. Performances ranged from 520 to 49’000 revolutions per day (or 0.2 to 21.5 km). Performance did not differ between the two house mouse running groups (p=0.76; Fig. 2). The average daily performances were 24’429 (10.7 km) revolutions for voluntary runners and 26’553 (11.7 km; Tab. 1) revolutions for runners-for-food. Both groups ran more during the second week of the experiment (voluntary runners: 31%, p=0.002; runners-for-food: 17%, p=0.001). Increases did not differ between groups (p=0.25) or between house mice and C57BL/6 mice (p=0.25).
3.2 Effects on AHN
3.2.1 Experiment 1: C57BL/6 mice
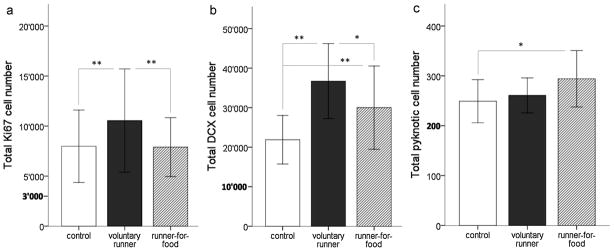
One-way ANOVA within C57BL/6 mice showed that voluntary runners displayed a 32% increase in the number of Ki67 positive cells compared to controls (p=0.002; Fig. 3a) and a 34% increase compared to runners-for-food (p=0.001; Fig. 3a). Runners-for-food did not differ from controls (p=0.91; Fig. 3a). Also for DCX, we found the highest number of positively stained cells in the voluntary runners (Fig. 3b). This group showed 68% more DCX positive cells than control animals (p<0.001; Fig; 3c) and 22% more DCX positive cells than the runners-for-food (p=0.01; Fig. 3b). Runners-for-food showed a significant increase (37%) in the number of DCX-positive cells when compared to controls (p=0.004, Fig. 3b). We found significantly more pyknotic cells in the runners-for-food compared to the control (p=0.02; Fig. 3c) and a trend towards an increased number of pyknotic cells in runners-for-food compared to voluntary runners (p=0.08; Fig. 3c).
Fig. 3. Cell numbers in C57BL/6.
(a) Cell proliferation is significantly increased in voluntary running C57BL/6 mice but not in runners-for-food. (b) The number of DCX-positive cells is significantly increased in voluntary runners and runners-for-food, but the increase is smaller in runners-for-food. (c) C57BL/6 runners-for-food display an enhanced number of pyknotic cells compared to controls. *: p<0.05, **: p<0.01. Bars = SD.
3.2.2 Experiment 2: house mice
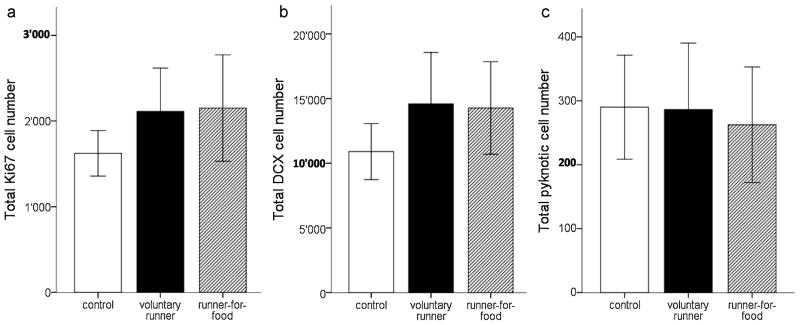
One-way ANOVA within house mice showed no main effect for Ki67 and DCX positive cells (pKi67=0.1; pDCX=0.1; Fig. 4a/b). However, both running groups displayed a consistent trend towards increased numbers of Ki67− and DCX positive cells when compared to controls (voluntary runners: pKi67=0.08; pDCX=0.05; runners-for-food: pKi67=0.05, pDCX=0.07; Fig. 4a/b). Comparison of the number of pyknotic cells showed no significant differences between the groups (p =0.82; Fig. 4c). Of note is that n=22 for house mice was lower than the n=36 for C57BL/6 mice.
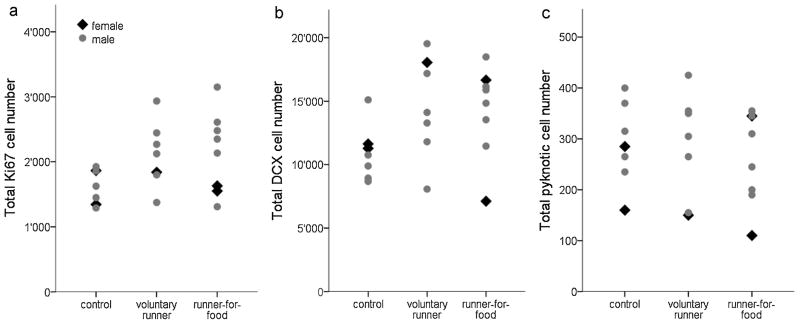
Fig. 4. Cell numbers in house mice.
In house mice, (a) we find no significant running-induced increase in proliferation and (b) in the number of DCX-positive cells, although there is a consistent 30% trend of an increased number of proliferating cells and cells of the neuronal lineage. (c) No group differences are found in the number of pyknotic cells. Note also that proliferation and neuronal differentiation in house mice is overall lower than in C57BL/6. Bars = SD.
3.2.3 Gender Effects
Animals of both genders were only tested for the house mice. Aside from a trend towards lower pyknotic cell numbers in females (ppyknosis=0.07), we did not observe gender effects (pKi67=0.25; pDCX=0.81) or gender x group interactions (pKi67=0.45; pDCX =0.3; ppyknosis =0.65). Due to the small number of females only large effects would have been detectable. However, the distribution of the female data points within the experimental groups were mostly within the bounds defined by male data points (Fig. 5a-c), and gender effects would be unlikely to reach significance also in larger group sizes. The few female data points outside the bounds set by males were all lower than the male data points.
Fig. 5. Equal distribution of cell numbers in female and male house mice.
(a) No gender differences are found in the numbers of proliferating cells, (b) DCX-positive cells and (c) pyknotic cells in house mice. The distribution of female data points within the groups suggests that there would be no gender differences even with a larger number of females.
3.2.4 Effect sizes
The analyses of values normalized to the mean of the control group of the corresponding strain allowed the statistical comparison of effect sizes across strains. The main difference between strains was found in the effect of the experiment on DCX positive cells, where voluntary running C57BL/6 mice differed significantly from that in voluntary running house mice (p=0.007). Ki67 positive cells revealed a difference between house mice and C57BL/6 runners-for-food (p=0.047). The effect size of running-for-food on pyknotic cells also differed between strains (p=0.01).
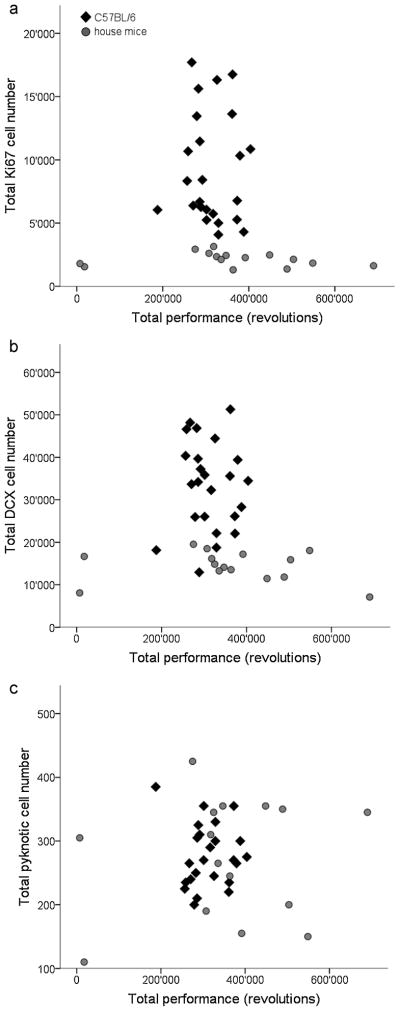
3.3 Correlations between performance and Ki67, DCX or pyknosis
In both, C57BL/6 and house mice we did not find a correlation between the individual performance and Ki67− (C57BL/6: r=−0.179, p=0.4; house mice: r=−0.286, p=0.3; Fig. 6a), DCX− (C57BL/6: r=−0.152, p=0.48; house mice: r=−0.275, p=0.32; Fig. 6b) and pyknotic cell numbers (C57/BL/6: r=0.220, p=0.30; house mice: r=0.011, p=0.97; Fig. 6c).
Fig. 6. Missing correlation between cell counts and performance.
No correlations between individual running performance and (a) proliferation, (b) neuronal differentiation or (c) pyknosis are found in either C57BL/6 or house mice. Larger inter-individual variability is observed in the number of proliferating and DCX-positive cells in C57BL/6 mice, whereas house mice show small variability on an overall lower level. However, house mice show larger variability in performance than C57BL/6. Data points represent total values (estimated cell numbers and wheel revolutions in the two weeks of the experiment) of the individual mice.
4. Discussion
In this study we show that cell proliferation and the number of young neurons in C57BL/6 mice are up-regulated by voluntary wheel running. However, if the mice have to run for their food, cell proliferation remains at control level and the increase immature neurons is smaller. Western wild house mice show a consistent but non-significant trend towards a running-induced increase in proliferating cells and immature neurons, which, in contrast to C57BL/6 mice, does not differ between voluntary runners and runners-for-food. The increase in the number of immature neurons is much higher in C57BL/6 mice than in house mice. Consistent with previous studies [2, 19], no correlations were found between AHN and performance.
4.1 Voluntary running affects neurogenesis differently in C57BL/6 and in house mice
Voluntary exercise in laboratory rodents increases spine density [34, 35], alters synaptic plasticity [36, 37], and enhances AHN [2–4]. Exercise also improves cognitive abilities of the animals in spatial- and non-spatial learning tasks [6, 38–40]. In the present study, C57BL/6 mice displayed the expected increase in AHN after voluntary exercise. Regulatory mechanisms of AHN differ depending on the genetic background of laboratory strains [41–43], but the enhancement of cell proliferation by voluntary exercise is common and robust [44, 45]. While we found a similar proportional increase in cell proliferation in both C57BL/6 and house mice (32% and 30%, respectively), the effect of running on the number of young neurons was much stronger in C57BL/6 than in house mice (68% versus 34%), despite their comparable running performance. This increase in house mice is also much smaller than those reported in other laboratory animals [4, 46].
4.2 Constant versus dynamic AHN: Benefits in a complex environment
Running and environmental changes have no effect on neurogenesis in wild-caught wood mice [19]. Here we used Western house mice (Mus musculus domesticus), which is the subspecies most closely related to common laboratory inbred strains. C57BL/6 mice share 92% of their genome with M. m. domesticus [20]. Western house mice are active, explorative and omnivorous animals that live commensally with humans where food is available [47]. House mice experience a complex social environment, with many aggressive interactions [47], but also many social contacts [48]. Reproductive physiology is influenced by season and pheromones [47]. Environmental and social changes, hormonal fluctuations and extended locomotor activity are among the factors that influence neurogenesis and to which house mice are constantly exposed. A critical comment concerning the behavioral significance of AHN is that changes in AHN are preemptive, i.e. new neurons can only improve cognitive function after the delay necessitated by their integration into hippocampal circuitry [49]. A strategy for long-term regulation of neurogenesis in a natural environment subject to constant changes could be the maintenance of a relatively constant pool of dividing and differentiating neurons. Likewise, a dynamic AHN in animals which normally are not challenged seems an appropriate adaptation. Back crosses of C57BL/6 and DBA mice have shown large differences in cell proliferation, neuronal differentiation, survival and in the relations between these aspects of AHN [41]. This finding highlights the potential genetic variability of these processes, which is likely to include the possibility to react differently to stimuli. Exercise in laboratory rats induces changes in gene expression that are related to enhanced AHN [50]. Small changes in the expression pattern of these genes can change the regulation of AHN. The absence of exercise effects on AHN in wild wood mice [19] and the small increase of AHN in house mice suggest that during the course of speciation and domestication different alleles were selected for the regulation of AHN.
The modulation of AHN by running in female and male C57BL/6 is similar [2, 51]. Likewise, C57BL/6 females and males do not differ significantly in running performance [52], AHN [53] or aged-dependent regulation thereof [54]. This suggests that, although we did not test a large number of female house mice due to limited availability, they would show, like the male house mice, a limited modulation of AHN by running relative to C57BL/6 mice. Considering the statistical trend towards lower pyknotic cell numbers in females and the fact that female data points outside the range defined by male data points are exclusively lower than male data points, the inclusion of additional females might have further strengthened the observed differences between C57/BL/6 mice and house mice in line with the current interpretation of the experimental outcomes.
4.3 Differences in inter-individual variability
The idea of a relatively constant pool of young neurons in wild species would also explain why we find low inter-individual variability in Ki67 and DCX cell counts in house mice relative to C57BL/6 mice. This difference in variability is particularly interesting in light of the presumably high genetic variability in house mice compared with the inbred C57BL/6 line, suggesting that experiential factors may be more important than genetic factors in controlling AHN. The variety of stimuli capable of altering AHN in C57BL/6 might be reflected in the major distribution of AHN between individuals. In contrast, we find large individual differences of performance in house mice, and even larger differences in wild wood mice [19]. Dohm et al. found significant differences in running behavior between wild- and laboratory mice [55]. The genetic homogeneity of laboratory strains may thus be responsible for a more consistent behavioral performance in C57BL/6 mice compared to house mice.
4.4 Context sensitivity of neurogenesis in C57BL/6− but not house mice
C57BL/6 runners-for-food do not show an increase in cell proliferation, which contrasts sharply with the voluntary runners, while cell death in the runners-for-food group is significantly increased compared to control mice. Ki67 and cell death can only be detected in short time windows, while DCX expression lasts about three weeks [56]. A slow impact of the running-for-food context on the cell parameters, i.e. changing from most likely voluntary perception to context dependency, could explain why we still find an enhanced number of young neurons.
Alternatively and not considering expression times of the markers, the running-for-food context may impose a stress on the animals comparable to that introduced by treadmill running [16]. Stress in our context may have counteracted the running-induced enhancement of proliferation but not cell survival. This idea is supported by previous studies, which have generally found that stress inhibits cell proliferation [57–60] but does not affect, or even increases, survival of new granule cells [46]. An interaction between stress and running effects, as previously described in individually housed rats [61], also appears to be consistent with our data. Runners-for-food showed no increase in cell proliferation but a 37% increase in the number of DCX positive cells, which may reflect increased cell survival. In voluntary runners, subtracting the 32% enhancement of proliferation from the 68% increase in young neurons, which reflects a combination of cell birth and cell survival effects, leaves a survival effect (36%) that is nearly identical to that seen in runners for food. Together this may suggest that running produced a stress- or context-sensitive 32% increase in cell proliferation and a stress-insensitive ~37% increase in cell survival.
Interestingly, wild mice did not show differences between voluntary running and running-for-food. House mice typically live where food is readily available, searching for better conditions if it becomes scarce [47]. They are naturally highly active and flexible in behavioral strategies, and running-for-food may therefore not impose a stress. Tolerance for context-dependent stress would have to be tested further, e.g. by forced exposure of subordinate to dominant males, a condition that house mice naturally avoid. In laboratory male mice, this experiment increases proliferation in subordinate and dominant males [62].
Our results indicate that exercise effects on AHN in C57BL/6 mice are context-sensitive. Part of the interest in exercise as a modulator of AHN is derived from the intuitive ease with which findings seemingly can be translated to humans. A straightforward extrapolation is challenged by our finding that a running regimen, even one that only requires as much running as mice would choose under voluntary conditions, significantly diminishes the positive effects on AHN. Moreover, exercise had much smaller, and context-independent effects on AHN in laboratory-bred offspring of wild house mice, indicating that even within a species, genetic background may determine if and how AHN is modulated.
Acknowledgments
We gratefully acknowledge the technical laboratory assistance of Sarah Nötzli and the support for the wild mouse experiment by Barbara König. This work was supported by the NCCR ‘Neural Plasticity and Repair’ (F.K.) and the Swiss National Science Foundation (3100A0_122589/1) (H-P.L. and I.A.) and the Intramural Program of the National Institutes of Health, National Institute of Mental Health, 1ZIAMH002784 (H.A.C.).
References
- 1.Holmes MM, Galea LA, Mistlberger RE, Kempermann G. Adult hippocampal neurogenesis and voluntary running activity: circadian and dose-dependent effects. J Neurosci Res. 2004;76:216–22. doi: 10.1002/jnr.20039. [DOI] [PubMed] [Google Scholar]
- 2.Klaus F, Hauser T, Slomianka L, Lipp HP, Amrein I. A reward increases running-wheel performance without changing cell proliferation, neuronal differentiation or cell death in the dentate gyrus of C57BL/6 mice. Behav Brain Res. 2009;204:175–81. doi: 10.1016/j.bbr.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Olson AK, Eadie BD, Ernst C, Christie BR. Environmental enrichment and voluntary exercise massively increase neurogenesis in the adult hippocampus via dissociable pathways. Hippocampus. 2006;16:250–60. doi: 10.1002/hipo.20157. [DOI] [PubMed] [Google Scholar]
- 4.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neurosci. 1999;2:266–70. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 5.Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. J Appl Physiol. 2006;101:1237–42. doi: 10.1152/japplphysiol.00500.2006. [DOI] [PubMed] [Google Scholar]
- 6.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96:13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–5. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amrein I, Dechmann DK, Winter Y, Lipp HP. Absent or low rate of adult neurogenesis in the hippocampus of bats (Chiroptera) PLoS ONE. 2007;2:e455. doi: 10.1371/journal.pone.0000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartkowska K, Djavadian RL, Taylor JRE, Turlejski K. Generation recruitment and death of brain cells throughout the life cycle of Sorex shrews (Lipotyphla) Eur J Neurosci. 2008;27:1710–21. doi: 10.1111/j.1460-9568.2008.06133.x. [DOI] [PubMed] [Google Scholar]
- 10.Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:4217–21. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pietropaolo S, Sun Y, Li RX, Brana C, Feldon J, Yee BK. The impact of voluntary exercise on mental health in rodents: A neuroplasticity perspective. Behav Brain Res. 2008;192:42–60. doi: 10.1016/j.bbr.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Solberg LC, Horton TH, Turek FW. Circadian rhythms and depression: effects of exercise in an animal model. Am J Physiol Regul Integr Comp Physiol. 1999;276:R152–R61. doi: 10.1152/ajpregu.1999.276.1.R152. [DOI] [PubMed] [Google Scholar]
- 13.Lucassen PJ, Stumpel MW, Wang Q, Aronica E. Decreased numbers of progenitor cells but no response to antidepressant drugs in the hippocampus of elderly depressed patients. Neuropharmacology. 2010;58:940–9. doi: 10.1016/j.neuropharm.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Marlatt MW, Lucassen PJ, van Praag H. Comparison of neurogenic effects of fluoxetine, duloxetine and running in mice. Brain Res. 2010;1341:93–9. doi: 10.1016/j.brainres.2010.03.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nature Med. 1998;4:1313–7. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 16.Kim YP, Kim HB, Jang MH, Lim BV, Kim YJ, Kim H, et al. Magnitude- and time-dependence of the effect of treadmill exercise on cell proliferation in the dentate gyrus of rats. Int J Sports Med. 2003;24:114–7. doi: 10.1055/s-2003-38202. [DOI] [PubMed] [Google Scholar]
- 17.Michaux JR, Chevret P, Filippucci MG, Macholan M. Phylogeny of the genus Apodemus with a special emphasis on the subgenus Sylvaemus using the nuclear IRBP gene and two mitochondrial markers: cytochrome b and 12S rRNA. Mol Phylogenet Evol. 2002;23:123–36. doi: 10.1016/S1055-7903(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 18.Steppan SJ, Adkins RM, Spinks PQ, Hale C. Multigene phylogeny of the Old World mice, Murinae, reveals distinct geographic lineages and the declining utility of mitochondrial genes compared to nuclear genes. Mol Phylogenet Evol. 2005;37:370–88. doi: 10.1016/j.ympev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Hauser T, Klaus F, Lipp H-P, Amrein I. No effect of running and laboratory housing on adult hippocampal neurogenesis in wild caught long-tailed wood mouse. BMC Neurosci. 2009;10:43. doi: 10.1186/1471-2202-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang H, Bell TA, Churchill GA, de Villena FPM. On the subspecific origin of the laboratory mouse. Nature Genetics. 2007;39:1100–7. doi: 10.1038/ng2087. [DOI] [PubMed] [Google Scholar]
- 21.Navailles S, Hof PR, Schmauss C. Antidepressant drug-induced stimulation of mouse hippocampal neurogenesis is age-dependent and altered by early life stress. J Comp Neurol. 2008;509:372–81. doi: 10.1002/cne.21775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eisch AJ, Mandyam CD. Adult neurogenesis: can analysis of cell cycle proteins move us “Beyond BrdU”? Curr Pharm Biotechnol. 2007;8:147–65. doi: 10.2174/138920107780906540. [DOI] [PubMed] [Google Scholar]
- 23.Starborg M, Gell K, Brundell E, Höög C. The murine Ki-67 cell proliferation antigen accumulates in the nucleolar and heterochromatic regions of interphase cells and at the periphery of the mitotic chromosomes in a process essential for cell cycle progression. J Cell Sci. 1996;109:143–53. doi: 10.1242/jcs.109.1.143. [DOI] [PubMed] [Google Scholar]
- 24.Francis F, Koulakoff A, Boucher D, Chafey P, Schaar B, Vinet MC, et al. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron. 1999;23:247–56. doi: 10.1016/s0896-6273(00)80777-1. [DOI] [PubMed] [Google Scholar]
- 25.Koizumi H, Higginbotham H, Poon T, Tanaka T, Brinkman BC, Gleeson JG. Doublecortin maintains bipolar shape and nuclear translocation during migration in the adult forebrain. Nature Neurosci. 2006;9:779–86. doi: 10.1038/nn1704. [DOI] [PubMed] [Google Scholar]
- 26.Koizumi H, Tanaka T, Gleeson JG. doublecortin-like kinase functions with doublecortin to mediate fiber tract decussation and neuronal migration. Neuron. 2006;49:55–66. doi: 10.1016/j.neuron.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 27.Amrein I, Slomianka L, Lipp HP. Granule cell number, cell death and cell proliferation in the dentate gyrus of wild-living rodents. Eur J Neurosci. 2004;20:3342–50. doi: 10.1111/j.1460-9568.2004.03795.x. [DOI] [PubMed] [Google Scholar]
- 28.Heine VM, Maslam S, Joels M, Lucassen PJ. Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus, in absence of an age-related hypothalamus-pituitary-adrenal axis activation. Neurobiol Aging. 2004;25:361–75. doi: 10.1016/S0197-4580(03)00090-3. [DOI] [PubMed] [Google Scholar]
- 29.Iniguez C, Gayoso MJ, Carreres J. A versatile and simple method for staining nervous tissue using Giemsa dye. J Neurosci Meth. 1985;13:77–86. doi: 10.1016/0165-0270(85)90045-7. [DOI] [PubMed] [Google Scholar]
- 30.West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–97. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- 31.Gundersen HJG, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microscopy. 1987;147:229–63. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 32.Gundersen HJG, Jensen EBV, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology – reconsidered. J Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- 33.Slomianka L, West MJ. Estimators of the precision of stereological estimates: an example based on the CA1 pyramidal cell layer of rats. Neuroscience. 2005;136:757–67. doi: 10.1016/j.neuroscience.2005.06.086. [DOI] [PubMed] [Google Scholar]
- 34.Redila VA, Christie BR. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience. 2006;137:1299–307. doi: 10.1016/j.neuroscience.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 35.Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–9. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 37.O’Callaghan RM, Ohle R, Kelly AM. The effects of forced exercise on hippocampal plasticity in the rat: A comparison of LTP, spatial- and non-spatial learning. Behav Brain Res. 2007;176:362–6. doi: 10.1016/j.bbr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 38.Adlard PA, Perreau VM, Engesser-Cesar C, Cotman CW. The timecourse of induction of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus following voluntary exercise. Neurosci Lett. 2004;363:43–8. doi: 10.1016/j.neulet.2004.03.058. [DOI] [PubMed] [Google Scholar]
- 39.Eisenstein SA, Holmes PV. Chronic and voluntary exercise enhances learning of conditioned place preference to morphine in rats. Pharmacol Biochem Behav. 2007;86:607–15. doi: 10.1016/j.pbb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Samorajski T, Delaney C, Durham L, Ordy JM, Johnson JA, Dunlap WP. Effect of exercise on longevity, body-weight, locomotor performance, and passive-avoidance memory of C57bl/6j mice. Neurobiol Aging. 1985;6:17–24. doi: 10.1016/0197-4580(85)90066-1. [DOI] [PubMed] [Google Scholar]
- 41.Kempermann G, Gage FH. Genetic determinants of adult hippocampal neurogenesis correlate with acquisition, but not probe trial performance, in the water maze task. Eur J Neurosci. 2002;16:129–36. doi: 10.1046/j.1460-9568.2002.02042.x. [DOI] [PubMed] [Google Scholar]
- 42.Kempermann G, Gage FH. Genetic influence on phenotypic differentiation in adult hippocampal neurogenesis. Brain Res Dev Brain Res. 2002;134:1–12. doi: 10.1016/s0165-3806(01)00224-3. [DOI] [PubMed] [Google Scholar]
- 43.Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci USA. 1997;94:10409–14. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clark PJ, Brzezinska WJ, Puchalski EK, Krone DA, Rhodes JS. Functional analysis of neurovascular adaptations to exercise in the dentate gyrus of young adult mice associated with cognitive gain. Hippocampus. 2009;19:937–50. doi: 10.1002/hipo.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thuret S, Toni N, Aigner S, Yeo GW, Gage FH. Hippocampus-dependent learning is associated with adult neurogenesis in MRL/MpJ mice. Hippocampus. 2009;19:658–69. doi: 10.1002/hipo.20550. [DOI] [PubMed] [Google Scholar]
- 46.Snyder JS, Glover LR, Sanzone KM, Kamhi JF, Cameron AH. The effects of exercise and stress on the survival and maturation of adult-generated granule cells. Hippocampus. 2009;19:898–906. doi: 10.1002/hipo.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berry RJ, Tattersall FH, Hurst J. Genus Mus. In: Harris S, Yalden DW, editors. Mammals of the British Isles: Handbook. 4. Southhampton, U.K: The Mammal Society; 2008. pp. 141–9. [Google Scholar]
- 48.Weidt A, Hofmann SE, Konig B. Not only mate choice matters: fitness consequences of social partner choice in female house mice. Anim Behav. 2008;75:801–8. [Google Scholar]
- 49.Kempermann G. Why new neurons? Possible functions for adult hippocampal neurogenesis. J Neurosci. 2002;22:635–8. doi: 10.1523/JNEUROSCI.22-03-00635.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tong LQ, Shen H, Perreau VM, Balazs R, Cotman CW. Effects of exercise on gene-expression profile in the rat hippocampus. Neurobiol Disease. 2001;8:1046–56. doi: 10.1006/nbdi.2001.0427. [DOI] [PubMed] [Google Scholar]
- 51.Clark PJ, Brzezinska WJ, Thomas MW, Ryzhenko NA, Toshkov SA, Rhodes JS. Intact neurogenesis is required for benefits of exercise on spatial memory but not motor performance or contextual fear conditioning in C57BL/6J mice. Neuroscience. 2008;155:1048–58. doi: 10.1016/j.neuroscience.2008.06.051. [DOI] [PubMed] [Google Scholar]
- 52.Lightfoot JT, Leamy L, Pomp D, Turner MJ, Fodor AA, Knab A, et al. Strain screen and haplotype association mapping of wheel running in inbred mouse strains. J Appl Physiol. 2010;109:623–34. doi: 10.1152/japplphysiol.00525.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lagace DC, Fischer SJ, Eisch AJ. Gender and endogenous levels of estradiol do not influence adult hippocampal neurogenesis in mice. Hippocampus. 2007;17:175–80. doi: 10.1002/hipo.20265. [DOI] [PubMed] [Google Scholar]
- 54.Ben Abdallah NMB, Slomianka L, Vyssotski AL, Lipp H-P. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol Aging. 2010;31:151–61. doi: 10.1016/j.neurobiolaging.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 55.Dohm MR, Richardson CS, Garland T. Exercise physiology of wild and random-bred laboratory house mice and their reciprocal hybrids. Am J Physiol Regul Integr Comp Physiol. 1994;36:R1098–R108. doi: 10.1152/ajpregu.1994.267.4.R1098. [DOI] [PubMed] [Google Scholar]
- 56.Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, et al. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J Neurosci. 2009;29:14484–95. doi: 10.1523/JNEUROSCI.1768-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–8. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Heine VM, Maslam S, Zareno J, Joels M, Lucassen PJ. Suppressed proliferation and apoptotic changes in the rat dentate gyrus after acute and chronic stress are reversible. Eur J Neurosci. 2004;19:131–44. doi: 10.1046/j.1460-9568.2003.03100.x. [DOI] [PubMed] [Google Scholar]
- 59.Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–71. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- 60.Tanapat P, Hastings NB, Rydel TA, Galea LA, Gould E. Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. J Comp Neurol. 2001;437:496–504. doi: 10.1002/cne.1297. [DOI] [PubMed] [Google Scholar]
- 61.Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci. 2006;9:526–33. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fiore M, Amedola T, Triaca V, Alleva E, Aloe L. Fighting in the aged male mouse increases the expression of TrkA and TrkB in the subventricular zone and in the hippocampus. Behav Brain Res. 2005;157:351–62. doi: 10.1016/j.bbr.2004.08.024. [DOI] [PubMed] [Google Scholar]