Abstract
Biofilms are a preferred mode of survival for many microorganisms including Vibrio cholerae, the causative agent of the severe secretory diarrhoeal disease cholera. The ability of the facultative human pathogen V. cholerae to form biofilms is a key factor for persistence in aquatic ecosystems and biofilms act as a source for new outbreaks. Thus, a better understanding of biofilm formation and transmission of V. cholerae is an important target to control the disease. So far the Vibrio exopolysaccharide was the only known constituent of the biofilm matrix. In this study we identify and characterize extracellular DNA as a component of the Vibrio biofilm matrix. Furthermore, we show that extracellular DNA is modulated and controlled by the two extracellular nucleases Dns and Xds. Our results indicate that extracellular DNA and the extracellular nucleases are involved in diverse processes including the development of a typical biofilm architecture, nutrient acquisition, detachment from biofilms and the colonization fitness of biofilm clumps after ingestion by the host. This study provides new insights into biofilm development and transmission of biofilm-derived V. cholerae.
Introduction
The causative agent of cholera is the Gram-negative bacterium Vibrio cholerae (Koch, 1884). The intestinal disease is characterized by profuse secretory diarrhoea and vomiting that rapidly leads to dehydration and death by hypovolaemic shock. The recent outbreak of cholera in Haiti has drawn public attention, but it should be emphasized that cholera is currently endemic in approximately 50 countries and V. cholerae infects several million individuals globally each year (Sack et al., 2004; WHO, 2009; Ryan, 2011). Hallmarks of the life cycle of the clinically relevant V. cholerae strains are the transitions between two dissimilar habitats: as a natural inhabitant of the aquatic ecosystems and as a pathogen in the human gastrointestinal tract (Reidl and Klose, 2002; Schild et al., 2008a; Nelson et al., 2009).
Finally, a better understanding of the environmental persistence and survival of V. cholerae has become a goal for possible control of the spread of the disease (Islam et al., 1993; Colwell, 1996; Sack et al., 2004). One key factor for environmental survival and transmission of bacteria is the ability to form matrix-enclosed surface-associated communities, also called biofilms. In the aquatic environment V. cholerae is believed to form biofilms on surfaces provided by plants, algae, zooplankton, crustaceans and insects (Huq et al., 1990; Tamplin et al., 1990; Colwell, 1996). In particular chitin, one of the most abundant biopolymers in the aquatic environments, is an important substrate for V. cholerae and has impact on its physiology including the utilization as a carbon and nitrogen source as well as an inducer of natural competence (Meibom et al., 2004; 2005). Upon oral ingestion by its human host, V. cholerae cells associated in biofilms might be protected from digestive enzymes, acidic pH and antimicrobial substances, thereby enhancing colonization and facilitating transmission of the disease (Zhu and Mekalanos, 2003; Huq et al., 2008). However, it has been shown that even if the natural biofilm structure is mechanically dispersed the resulting V. cholerae cells greatly out-compete planktonically grown cells in the infant mouse model (Tamayo et al., 2010). Thus, biofilm formation seems to play a major role in the physiology, ecology and epidemiology of V. cholerae.
Recent studies analysed structural prerequisites for V. cholerae biofilm formation including flagella, pili and exopolysaccharide biosynthesis (Watnick et al., 1999; 2001; Watnick and Kolter, 1999; Chiavelli et al., 2001; Lauriano et al., 2004; Meibom et al., 2004; Moorthy and Watnick, 2004; 2005; Reguera and Kolter, 2005; Yang et al., 2009). In addition, signalling mechanisms including two-component systems, quorum sensing and c-di-GMP involved in regulation of biofilm formation have been elucidated (Tischler and Camilli, 2004; 2005; Houot et al., 2010; Krasteva et al., 2010). So far, the V. cholerae exopolysaccharide (VPS) is the only characterized matrix component required for biofilm formation in most V. cholerae isolates analysed (Yildiz and Schoolnik, 1999; Kierek and Watnick, 2003; Yildiz et al., 2004). The genes encoding proteins for VPS synthesis and secretion are arranged in two clusters vpsA-K (VC0917-27) and vpsL-Q (VC0934-9). The UhpA family regulator VpsT and the response regulator VpsR positively regulate transcription of vps genes (Yildiz et al., 2001; Casper-Lindley and Yildiz, 2004). Furthermore, HapR, a key regulator of the quorum sensing cascade in V. cholerae, acts as a negative regulator of biofilm formation by repression of vps genes including vpsT and vpsR (Yildiz et al., 2004; Beyhan et al., 2007; Waters et al., 2008). Because transcription of hapR is controlled by quorum sensing and RpoS, biofilm formation seems to be regulated by central physiological signals, like cell density or carbon concentration (Hammer and Bassler, 2003; Zhu and Mekalanos, 2003; Yildiz et al., 2004).
In general, the matrix of bacterial biofilms primarily consists of exopolysaccharides, but compounds like proteins, lipids and nucleic acids can be found in biofilms of microorganisms and serve important functions (Goller and Romeo, 2008; Karatan and Watnick, 2009; Flemming and Wingender, 2010). Recently, extracellular DNA (eDNA) was shown to be required for biofilm formation in Pseudomonas aeruginosa (Whitchurch et al., 2002). Since then, eDNA has been found in biofilms of several Gram-positive and Gram-negative bacteria such as Streptococcus ssp., Enterococcus faecalis, Listeria monocytogenes, Neisseria mengitidis and Helicobacter pylori (Moscoso et al., 2006; Thomas et al., 2008; Grande et al., 2010; Harmsen et al., 2010; Lappann et al., 2010). In almost every one of these studies a different role of eDNA in biofilms has been reported. Extracellular DNA is implicated to mediate initial attachment, act as a structural component stabilizing the biofilm matrix or is used as a nutrient source. To the contrary, in Caulobacter crescentus eDNA can bind and mask the polar holdfast of swarmer cells thereby inhibiting their attachment to biofilms (Berne et al., 2010). In Bordetella eDNA is important for maintaining biofilm integrity in vitro and in vivo (Conover et al., 2011). Thus, the physiological roles of eDNA in biofilms seem to be versatile, although still poorly characterized how it is regulated as a component of the biofilm.
Vibrio cholerae encodes two extracellular nucleases Dns (VC0470) and Xds (VC2621) that are secreted into the culture supernatant (Newland et al., 1985; Focareta and Manning, 1987; 1991a,b). Deletion of both extracellular nucleases, especially Dns, increases the transformation efficiency regardless of whether the competence of V. cholerae is induced by growth on chitin or CaCl2-treatment (Focareta and Manning, 1991a; Blokesch and Schoolnik, 2008). This is probably due to increased stability of exogenous DNA in the absence of the nucleases. Xds is a 100 kDa polypeptide and is assigned by computational analysis to the protein family PF03372, which includes a large number of Mg2+-dependent endonucleases and exonucleases (Mol et al., 1995; Dlakic, 2000). Additionally, a recent study identified xds as a gene induced at a late stage of infection in the infant mouse small intestine (Schild et al., 2007). Dns, also known as VcEndA, has been crystallized and comprehensively analysed for its biochemical properties (Altermark et al., 2007; Niiranen et al., 2008). It belongs to the endonuclease I superfamily and consequently should cleave nucleic acids at non-specific internal sites. Recently, it has been demonstrated that dns is repressed by HapR and therefore co-regulated with the vps genes (Blokesch and Schoolnik, 2008).
In this study we show that deletion of Xds and Dns results in increased biofilm formation. Intrigued by this observation, we identified and characterized eDNA as a constituent of V. cholerae biofilms. Our data demonstrate that the extracellular nucleases control the level of eDNA and are involved in multiple processes including the development of a typical three-dimensional biofilm structure, detachment from a mature biofilm and utilization of eDNA as a nutrient source. Infection studies indicate that the dissolution of biofilms driven by the activity of the nucleases is a crucial step for the colonization fitness of V. cholerae.
Results
Deletion of dns and/or xds results in an increase of biofilm formation
To determine whether nucleases play a role in V. cholerae biofilm formation, in-frame deletion mutants of dns and xds as well as a double deletion mutant were generated. Their biofilm formation capacity was investigated after 12, 24, 40, 48 and 72 h using the static biofilm assay with crystal violet staining (Fig. 1). The VPS-deficient deletion mutant ΔvpsA served as negative control, as it is incapable of biofilm formation under static conditions (Yildiz and Schoolnik, 1999; Fong et al., 2010). At 12 h no significant difference between wild type and mutants was observed (Fig. 1A). After 24 h the ΔdnsΔxds mutant showed a significant twofold increase in biofilm formation compared with the wild type (Fig. 1B). Within the next days the biofilm amount of the double mutant further increased, while the wild type biofilm remained at an almost constant level (Fig. 1A–E). Accordingly, the differences in biofilm production were even more pronounced after 48 and 72 h where the ΔdnsΔxds mutant exhibited sixfold higher biofilm levels compared with the wild type (Fig. 1D and E). A single deletion of xds did not result in enhanced biofilm formation within the first 48 h, whereas deletion of dns showed a small, but significant increase in biofilm mass compared with the wild type within this period (Fig. 1A–D). At 72 h both single mutants exhibited significantly higher biofilm levels than the wild type (Fig. 1E). However, even at 72 h neither of the single mutants produced such large biofilm amounts as the double mutant. These data show that deletion of one extracellular nuclease can be partially compensated by the remaining nuclease in these biofilm assays. The growth rates of planktonic cells of Δdns, Δxds and ΔdnsΔxds mutants were similar to that of wild type (Fig. S1A). Thus, the increased biofilm formation capacity of the mutants is not the result of altered growth rates.
Fig. 1.
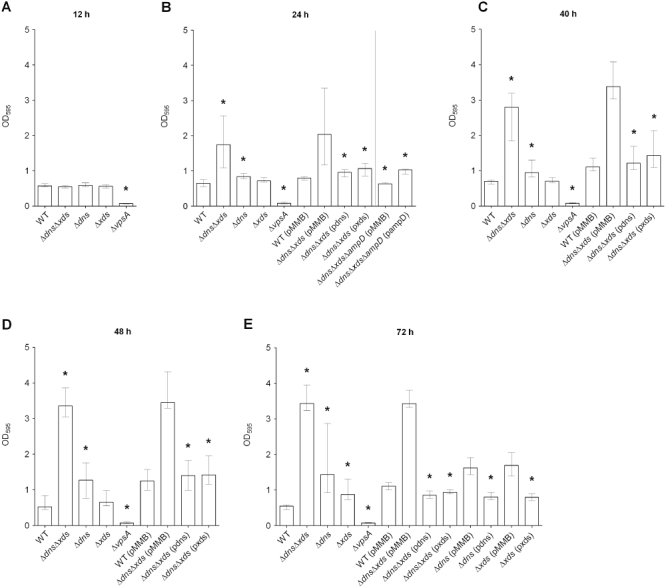
Deletion of dns and xds results in an increase of biofilm formation. Biofilms of the wild type (WT), deletion mutants, mutants and WT with empty pMMB plasmid or complemented mutants, as indicated, were quantified after 12 h (A), 24 h (B), 40 h (C), 48 h (D) and 72 h (E). The time points are indicated on top of each panel. The biofilm formation capacity was assayed under static conditions by crystal violet staining and subsequent determination of the OD595. Shown are the medians from at least eight independent measurements. The error bars indicate the interquartile range. Significant differences (*P < 0.05) are indicated for the following comparisons: deletion mutants with wild type; ΔdnsΔxds (pdns), ΔdnsΔxds (pxds) and ΔdnsΔxdsΔampD (pMMB) with ΔdnsΔxds (pMMB); ΔdnsΔxdsΔampD (pMMB) with ΔdnsΔxdsΔampD (pampD); Δdns (pdns) with Δdns (pMMB); and Δxds (pxds) with Δxds (pMMB).
Wild type biofilm levels could be restored in the ΔdnsΔxds mutant for all relevant time points by the expression of dns or xds in trans, but not by the plasmid vector alone (Fig. 1B–E). Complementation was also achieved for the single mutants at 72 h by the expression of dns or xds in trans respectively (Fig. 1E). It should be noted, that the presence of the expression vector at later time points generally resulted in a slight increase of biofilm production. Similar observations have been previously reported for conjugative plasmids (Ghigo, 2001; Reisner et al., 2006). Because this was true for wild type as well as for the mutants it has to be a general effect and we therefore included vector control groups for all complementation analyses in this study. Taken together, these results demonstrate that absence of the two extracellular nucleases Dns and Xds results in increased amounts of the biofilm mass.
The dns and xds genes are important for the development of the biofilm structure
We hypothesized that the increase of biofilm levels in the double mutant might also affect a natural biofilm development and the biofilm architecture. Therefore, the three-dimensional structure of wild type and mutant biofilms were analysed using a flow cell system. Initial attachment was investigated after incubation for 2 h in Luria–Bertani (LB) or 50-fold diluted LB (2%), while flow cell biofilms were grown for 9 or 24 h using undiluted LB or 2% LB broth respectively. Bacteria were stained with the green fluorescent stain SYTO 9 and visualized by confocal laser scanning microscopy (Figs 2, S2 and S3). Quantitative analysis of these biofilm images was performed with COMSTAT (http://www.comstat.dk) (Heydorn et al., 2000; M. Vorregaard et al., pers. comm.). At the early 2 h time point no difference in the surface coverage of the wild type and the ΔdnsΔxds mutant was observed for both nutrient conditions investigated (Fig. S2). Thus, the ΔdnsΔxds mutant showed no obvious change during initial attachment. At the later time point, the wild type biofilm showed the characteristic architecture of a mature three-dimensional biofilm with pillars of cells separated by fluid-filled channels as previously described by others (Fig. 2) (Watnick and Kolter, 1999; Watnick et al., 2001; Yildiz et al., 2004; Yildiz and Visick, 2009). In contrast, the biofilm of the ΔdnsΔxds mutant appeared to be very thick and compact, covering the entire surface of the flow cell without any visible fluid-filled channels (Fig. 2). The differences in the structural parameters of the biofilms were also confirmed by quantitative analysis using COMSTAT, which revealed a significant threefold increase in biomass as well as a significant 30% greater maximum thickness of the ΔdnsΔxds mutant biofilms compared with the wild type (Fig. 2C). Additionally, the roughness and average diffusion distance were analysed (Fig. 2C). The biofilm roughness provides a measure for the thickness variation of the biofilm and is an indicator for biofilm heterogeneity (Heydorn et al., 2000). The average diffusion distance indicates the shortest average distance from a pixel containing biomass to a pixel without biomass. This is an indicator for distances over which nutrients and substrate components have to diffuse to the bacteria (Yang et al., 2000). The ΔdnsΔxds mutant biofilms exhibited a significant lower roughness, but a significant higher average diffusion distance compared with the wild type. Similar results were obtained using 2% LB broth (Fig. S3). These results confirmed that the biofilm architecture of the ΔdnsΔxds mutant showed less heterogeneity in combination with higher density. Hence, the double mutant biofilm lacked the typical structural details present in wild type biofilms. The biofilm morphology and structural parameters of the single mutants ranged between the wild type and the double mutant, strengthening the idea that the loss of one extracellular nuclease can be partially compensated by the remaining nuclease (Figs 2 and S3). Overall, these data suggest that the deletion of dns and xds leads to an uncoordinated accumulation of large amounts of biomass and formation of an unstructured biofilm.
Fig. 2.
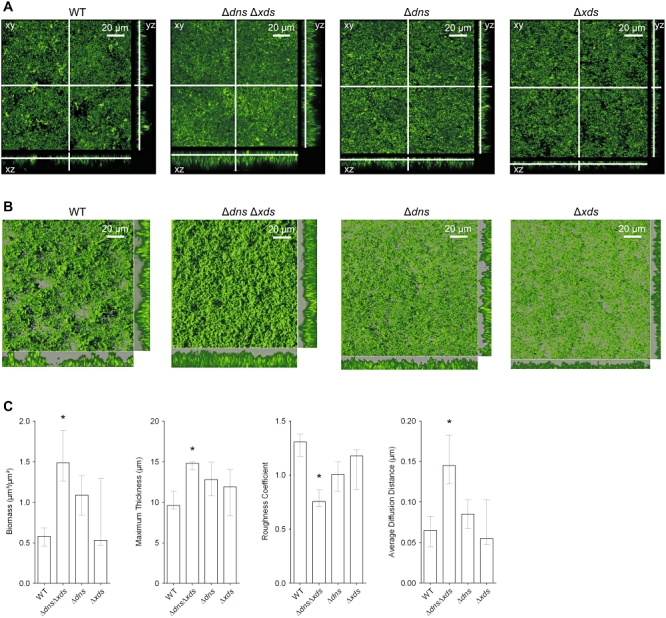
Absence of extracellular nucleases results in alterations of the biofilm architecture.
A. Shown are confocal laser scanning microscopy images of the wild type, ΔdnsΔxds, Δdns and Δxds mutant biofilms as horizontal (xy) and vertical (xz and yz) projections (large and side panels, respectively). Biofilms were allowed to form for 9 h in flow cell chambers supplied with LB and stained with SYTO 9 fluorescent nucleic acid stain. Large panels represent selected single optical sections through the acquired three-dimensional data sets.
B. Micrographs represent three-dimensional images of the wild type, ΔdnsΔxds, Δdns and Δxds mutant biofilms analysed by the IMARIS software package using same data sets as in panel (A). The large images are three-dimensional top-down images of the biofilms, and the small images to the right of and below the large images are side views of sections. Movies of the wild type and ΔdnsΔxds mutant biofilms, which allow views from different angles, are provided in the supporting information (bf_wt.mov and bf_mut.mov).
C. Image stacks of the wild type and mutant biofilms were analysed for the biomass, the maximum thickness, the roughness coefficient and the average diffusion distance using the COMSTAT software. Shown are the medians of at least six image stacks from three independent experiments for each strain. The error bars indicate the interquartile range. Significant differences (*P < 0.05) of structural parameters are indicated for the multiple comparisons of the deletion mutants with the wild type.
The increase of biofilm formation of the ΔdnsΔxds mutant is not related to vps expression
Vibrio cholerae mutants exhibiting increased biofilm formation compared with the wild type have been reported previously. However, these phenotypes correlated with enhanced vps gene expression and increased VPS production, as demonstrated for hapR mutants, rugose phase variants or mutants with increased c-di-GMP levels (Yildiz et al., 2001; Bomchil et al., 2003; Zhu and Mekalanos, 2003; Tischler and Camilli, 2004). To reveal whether the enhanced biofilm formation observed in the ΔdnsΔxds mutant was dependent on vps expression, chromosomal transcriptional fusions of a promoterless phoA reporter gene to vpsA, the first gene in the vps-I locus, were constructed in the wild type, the ΔdnsΔxds and the ΔhapR mutant. Thus, the measured PhoA activity reflects the transcription levels of vpsA in the respective strains. Because HapR acts as a negative regulator on vps genes, the ΔhapR mutants served as control for high vps gene expression (Hammer and Bassler, 2003; Zhu and Mekalanos, 2003; Yildiz et al., 2004). As expected, the ΔhapR mutant showed a significant fourfold increase in vpsA-phoA expression compared with the wild type (Fig. 3A). In contrast, ΔdnsΔxds mutant and wild type exhibited comparable levels of PhoA activity indicating similar transcription levels of vpsA in these strains. Consequently, vps transcription is not altered in ΔdnsΔxds mutant and the increased biofilm formation of ΔdnsΔxds cannot be simply explained by higher vps expression. Additionally, Δdns, Δxds and ΔdnsΔxds mutants showed no obvious changes in colony morphology, which are frequently observed in mutants with enhanced VPS production (Yildiz and Schoolnik, 1999; Watnick et al., 2001; Lauriano et al., 2004).
Fig. 3.
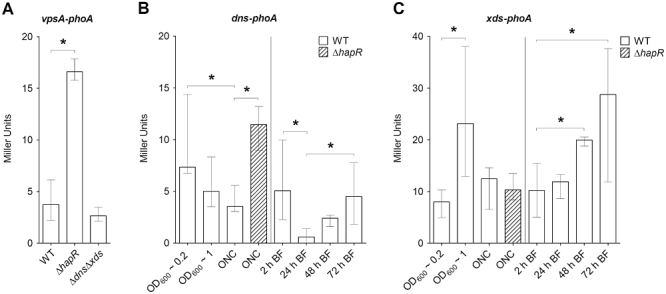
vpsA transcription is not altered in ΔdnsΔxds mutant and analysis of the xds and dns expression patterns.
A. Alkaline phosphatase activities (in Miller Units) were measured from overnight cultures of wild type, ΔhapR mutant and ΔdnsΔxds mutant with a chromosomal vpsA-phoA transcriptional fusion. Shown are the medians from at least eight independent measurements. The error bars indicate the interquartile range. The activity in the ΔhapR mutant compared with the wild type is significantly different (*P < 0.05).
B and C. Shown are the alkaline phosphatase activities (in Miller Units) of wild type (open bars) and ΔhapR mutant (shaded bars) with either a chromosomal dns-phoA (B) or xds-phoA (C) transcriptional fusion. Activities were measured from cultures grown with aeration until they reached an OD600 of ∼0.2 or ∼1, from overnight cultures (ONC) or from biofilms (BF) grown under static conditions at the indicated time points. Results in panels (B) and (C) are the medians from at least eight independent measurements. The error bars indicate the interquartile range. Significant differences (*P < 0.05) of the alkaline phosphatase activities are indicated for the respective single or multiple comparisons: Activities in the overnight cultures of the wild type and ΔhapR mutant carrying the dns-phoA or xds-phoA fusion; as well as biofilms of the wild type carrying the dns-phoA or xds-phoA at the different time points.
dns and xds exhibit different expression patterns
To analyse the expression of dns and xds, chromosomal transcriptional fusions of a promoterless phoA reporter gene to xds and dns were constructed. PhoA activities during planktonic growth were measured from wild type carrying the dns-phoA or xds-phoA transcriptional fusion grown in LB with aeration until the culture reached an OD600 of ∼ 0.2 or ∼ 1 as well as from overnight cultures respectively (Fig. 3B and C). These time points reflect the early and late exponential phase as well as late stationary phase of a culture. As shown in Fig. 3B the highest activity for dns-phoA was detected directly in the early stage of the culture at the lowest cell density (OD600 ∼ 0.2). From there the expression steadily declined, which inversely correlates with the increase in cell density and accumulation of quorum sensing signals. This is consistent with a previous report, demonstrating that the quorum sensing regulator HapR is not only important for the repression of vps genes, but additionally acts as a negative regulator for dns transcription (Blokesch and Schoolnik, 2008). We verified that by measuring the activities of the dns-phoA fusion in a ΔhapR mutant. As expected, the expression of dns-phoA in overnight cultures is higher in the ΔhapR mutant indicated by the threefold increase of PhoA activity compared with the respective activity of the wild type (Fig. 3B).
In contrast, xds revealed a different expression pattern. The PhoA activities of the wild type carrying the xds-phoA transcriptional fusion peaked at an OD600 of ∼ 1, with lower levels at earlier and later stages of the culture (Fig. 3C). This already argues that regulation of xds is not strongly linked to the cell density and quorum sensing. To validate if xds expression is not influenced by HapR, the transcriptional phoA-fusion to xds was also constructed in a ΔhapR strain. Again, PhoA activities were measured from overnight cultures to allow accumulation of quorum sensing signals. In contrast to dns-phoA, comparable levels of PhoA activity in overnight cultures of the wild type and ΔhapR mutant carrying the xds-phoA transcriptional fusion were observed. Thus, expression of xds is independent of HapR and not co-regulated with dns via the quorum sensing cascade.
Furthermore, we used the wild type carrying the dns-phoA or xds-phoA transcriptional fusion to analyse the expression of both nucleases at different time points along the maturation of static biofilms (Fig. 3B and C). Expression of dns-phoA was relatively high at the 2 and 72 h time point reflecting very early and late stages of the biofilm development. In between expression levels first significantly dropped and then significantly increased again with the minimum level at 24 h. In contrast, expression of xds-phoA showed a steady increase in expression levels along the biofilm development, with the activities of the early 2 h and the late 48 and 72 h time points being significantly different. Thus, dns and xds exhibit different temporal expression patterns during biofilm formation. Furthermore, these results suggest that expression of xds and dns in biofilms and in the planktonic stage are not completely comparable and diverse environmental signals might influence the regulation of the extracellular nucleases depending on the respective condition.
Dns and Xds exhibit distinct nuclease activities
Nuclease assays have been used to analyse and confirm the extracellular nuclease activity of Xds and Dns as described previously (Newland et al., 1985; Focareta and Manning, 1987; 1991a,b; Blokesch and Schoolnik, 2008). The wild type, the ΔdnsΔxds mutant and the ΔdnsΔxds mutant complemented with either dns or xds were analysed on DNase test agar plates (Fig. S4A). Degradation of DNA embedded in the agar is visualized by the zones of clearing for the wild type as well as for the ΔdnsΔxds mutant expressing Xds or Dns in trans respectively. In contrast, the ΔdnsΔxds mutant showed no detectable degradation of DNA. Furthermore, incubation of V. cholerae chromosomal DNA with culture supernatants from the respective strains and subsequent analysis of the degradation allowed visualization of the extracellular nuclease activities (Fig. S4B). Consistently, degradation of the chromosomal DNA was observed for the wild type and the ΔdnsΔxds mutant expressing Xds or Dns in trans, but not for the ΔdnsΔxds mutant with the vector control. It should be noted, that the impact of the two extracellular nucleases differs between the assays. Xds exhibits strong activity on the DNase test agar (Fig. S4A), while Dns seems to be the dominant enzyme in the second nuclease assay (Fig. S4B).
To investigate the activities of Dns and Xds in more detail, we tested the nuclease activities of culture supernatants via degradation of circular or linearized DNA of the plasmid pBAD24 over 30 min (Fig. 4A–C). Degradation of linearized DNA by the wild type supernatant was already visible after 1 min, while the degradation for the circular DNA was delayed by a few minutes (Fig. 4A–C, lanes 1 and 6). Circular plasmid DNA was almost completely degraded after 30 min incubation (Fig. 4C, lane 1). In case of the LB control or the supernatant of the ΔdnsΔxds mutant no degradation was observable throughout the experiment regardless what type of DNA was used (Fig. 4A–C, lanes 5 and 10 or 2 and 7, respectively). Thus, the observed nuclease activity of the wild type supernatant correlates with presence of Dns and Xds, allowing degradation of circular or linearized DNA. This also indicates that Xds is not surface associated but rather secreted, as has already been shown for Dns by others (Focareta and Manning, 1987; Blokesch and Schoolnik, 2008).
Fig. 4.
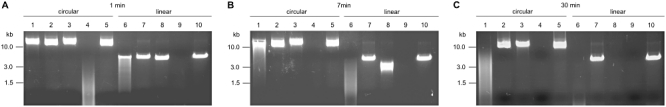
Xds and Dns exhibit different extracellular nuclease activities. Supernatants derived from bacterial cultures were assayed for their DNase activity by adding either circular (lanes 1 to 5) or linearized plasmid DNA (lanes 6 to 10). The supernatants were derived from following bacterial cultures: wild type (pMMB) (lane 1 and 6), ΔdnsΔxds (pMMB) (lane 2 and 7), ΔdnsΔxds (pxds) (lane 3 and 8), ΔdnsΔxds (pdns) (lane 4 and 9), LB broth control (lane 5 and 10). After incubation for 1, 7 and 30 min (panels A, B and C, respectively) the quality of the DNA was visualized on agarose gels. The incubation time is indicated on top of each panel.
By expression of each nuclease in trans in the ΔdnsΔxds mutant background the activities of Xds and Dns could be analysed separately. Rapid degradation of circular and linearized plasmid DNA was observed in the presence of Dns (Fig. 4A, lanes 4 and 9). In contrast, Xds was only capable of degrading linearized DNA (Fig. 4B/C, lanes 3 and 8). Complete degradation of linear DNA by Xds took notably longer compared with Dns. Furthermore, degradation of the linearized DNA by Xds occurred via an intermediate of defined decreased length, indicated by a relative distinct degradation band (Fig. 4B, lane 8), which migrates faster compared with the control (Fig. 4B, lane 10). In contrast, degradation by Dns resulted in a typical smear of the DNA. These assays allowed the discrimination of the enzymatic activities and suggest that Xds degrades only linearized DNA from its ends and thus is an exonuclease, whereas Dns degrades circular and linearized DNA into different sized products and thus is an endonuclease.
Extracellular DNA is a component of the V. cholerae biofilm matrix
The extracellular nuclease activities of Xds and Dns and the altered biofilm formation in absence of both enzymes suggest that eDNA is a, so far uncharacterized, matrix component in V. cholerae biofilms. To test this hypothesis, we first investigated whether V. cholerae biofilms are sensitive to endo- and exonuclease treatment at an early and late time point. In case of the early time point, wild type and ΔdnsΔxds mutant were allowed to form biofilms for 21 h before the supernatant was removed and biofilms were incubated for an additional 3 h with DNase I, λExonuclease or a combination of both nucleases (Fig. 5A). Thus, the biofilms were allowed to form for a total time period of 24 h, which reflects the earliest time point with a significant difference in biofilm formation of the ΔdnsΔxds mutant compared with the wild type (Fig. 1B). Addition of DNase I or λExonuclease to V. cholerae cultures did not reduce growth or the viability of the cells as confirmed by measurements of the OD600 and cfu counts by plating (data not shown). Incubation with nuclease buffer alone was used as control condition reflecting biofilms without treatment. Nuclease sensitivity was determined by quantification of the residual biofilm after 3 h incubation by crystal violet staining (Fig. 5A). Treatment with DNase I or λExonuclease as well as with the combination of both enzymes significantly reduced the biofilm amount of the ΔdnsΔxds mutant to wild type levels and even lower. Interestingly, nuclease treatment of the wild type biofilm also decreased the biofilm amount with a significant twofold reduction in case of the combined treatment with both nucleases. These results demonstrate that wild type and the ΔdnsΔxds mutant biofilms are nuclease-sensitive at the early stages of biofilm development. We also investigated the nuclease sensitivity of the wild type and the ΔdnsΔxds, mutant at a late time point (72 h). However, at such late stages even a combined and prolonged treatment with DNase I and λExonuclease resulted in no significant decrease of the biofilm amount for all strains tested (Fig. S5). We speculate that biofilms at late stages are either too compact to allow externally added nuclease to be effective or other matrix components have accumulated and can compensate for the degradation of eDNA.
Fig. 5.
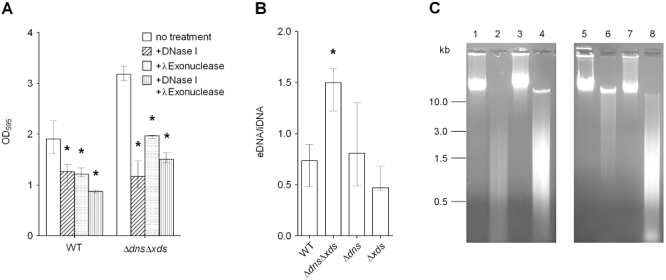
Extracellular DNA is a component of the V. cholerae biofilm matrix.
A. Analysis of nuclease sensitivity of wild type and ΔdnsΔxds mutant biofilms. Biofilms grown for 21 h under static conditions were treated 3 h with DNase I (diagonally shaded bars), λExonuclease (dotted bars) or with a combination of DNase I and λExonuclease (horizontally shaded bars). Biofilm mass remaining after nuclease treatment was quantified by crystal violet staining. Significant differences compared with untreated biofilms of the wild type or ΔdnsΔxds mutant are indicated (*P < 0.05).
B. Quantification of eDNA from wild type, ΔdnsΔxds, Δdns and Δxds mutant biofilms grown under static conditions. The bars represent the ratio eDNA to intracellular DNA (iDNA), which is significantly increased for the ΔdnsΔxds mutant compared with the wild type (*P < 0.05). Results of panel (A) and (B) are the medians from at least six independent measurements. The error bars indicate the interquartile range.
C. Visualization of the quality of eDNA and iDNA derived from wild type, ΔdnsΔxds, Δdns and Δxds mutant biofilms by agarose gel analysis. Shown are representative samples of iDNA derived from wild type biofilm (lane 1), eDNA derived from wild type biofilm (lane 2), iDNA derived from ΔdnsΔxds mutant biofilm (lane 3), eDNA derived from ΔdnsΔxds mutant biofilm (lane 4), iDNA derived from Δdns mutant biofilm (lane 5), eDNA derived from Δdns mutant biofilm (lane 6), iDNA derived from Δxds mutant biofilm (lane 7) and eDNA derived from Δxds mutant biofilm (lane 8).
To visualize the eDNA, 9 h old flow cell biofilms were stained with SYTO 9 and BOBO-3 (Fig. 6). The membrane-permeant green fluorescent nucleic acid stain SYTO 9 generally labels all bacteria within the biofilm, while the red fluorescent nucleic acid dye BOBO-3 cannot penetrate through intact membranes and consequently only stains nucleic acids outside of the bacteria or membrane-compromised bacteria. Control experiments assessing the bacterial viability in the biofilm by live/dead staining using SYTO 9 and propidium iodide nucleic acid stains, the later of which only enters non-viable cells, indicate that only a few membrane-compromised bacteria are present at this time point (Fig. S6). As shown in Fig. 6, eDNA can be visualized in wild type and ΔdnsΔxds biofilms. In both cases, highest eDNA levels seem to be detected close to the cell surface decorating the bacterial cells. The eDNA in the wild type biofilm seemed to be more evenly distributed, whereas in the ΔdnsΔxds biofilm some hot spots with higher eDNA amounts can be detected. This indicates that Dns and Xds might be important for a homogenous distribution of the eDNA in biofilms, but makes it difficult to compare the overall amount of eDNA present. Therefore, statically grown 48 h old biofilms were dispersed, eDNA and intracellular DNA (iDNA) were separated from each other and the levels of eDNA and iDNA were quantified. At this time point the maximal difference in biofilm mass between wild type and ΔdnsΔxds was observed (Fig. 1D). The ratios of eDNA to iDNA for the wild type, ΔdnsΔxds, Δdns and Δxds mutant biofilms are shown in Fig. 5B. The ratios of the single mutants are not significantly altered compared with wild type. These results corroborate the hypothesis that deletion of one nuclease can be compensated by the remaining nuclease. The significant higher ratio in the ΔdnsΔxds mutant clearly indicates higher levels of eDNA in the biofilm of the double mutant compared with the wild type. Thus, the increased biofilm formation capacity of the ΔdnsΔxds mutant correlates with higher amounts of eDNA in the mutant biofilm.
Fig. 6.
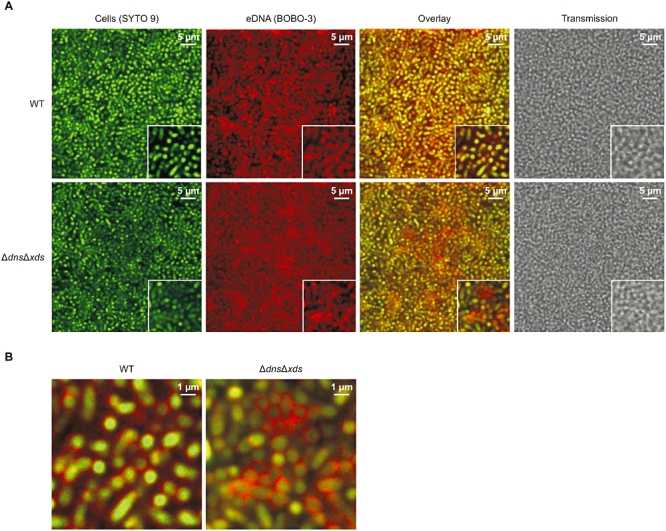
Visualization of eDNA in wild type and ΔdnsΔxds mutant biofilms.
A. Shown are two-dimensional confocal laser scanning microscopy images of biofilms grown for 9 h in flow cell chambers and subsequently stained with SYTO 9 (green) and BOBO-3 (red). Bacterial cells appear in green and the eDNA in red. Representative zoomed images are shown in the bottom right corner. Images represent single optical sections acquired in comparable focal planes of the three-dimensional data sets.
B. Shown are enlarged images of the overlay presented in panel (A) in the bottom right corner.
Subsequently, the quality of iDNA and eDNA of the wild type, ΔdnsΔxds, Δdns and Δxds mutant biofilms was analysed on agarose gels (Fig. 5C). As expected, iDNA of all strains consisted of high molecular weight molecules migrating above 10 kb (Fig. 5C, lane 1, 3, 5 and 7). Extracellular DNA of the wild type was degraded to smaller fragments with the majority between 0.5 and 3 kb (Fig. 5C, lane 2). No high molecular weight eDNA was visible in the wild type sample. In contrast, the eDNA samples of the Δdns and ΔdnsΔxds mutants contained a considerable amount of high molecular weight eDNA (Fig. 5C, lane 4 and 6). The Δxds mutant sample revealed an intermediate phenotype with the intensity of high molecular weight DNA ranging between the wild type and the ΔdnsΔxds mutant (Fig. 5C, lane 8). A notable amount of smaller fragments was also visible in the eDNA samples of the mutants, especially for ΔdnsΔxds and Δxds. The lack of smaller fragments in the Δdns might be a result of the remaining Xds activity, which efficiently degrades these fragments down to the nucleotide level. As shown above, the supernatants of the ΔdnsΔxds mutant exhibit no detectable nuclease activity. Thus, the observed smaller-sized fragments most likely result from degradation of DNA by cytoplasmic nucleases probably originating from cell lysis. We doubt that nucleases released during the DNA preparation significantly contribute to the observed degradation, as dispersion of the biofilm had no detectable effect on cell viability confirmed via cfu measurements by plating and direct counts by microscopy (see Experimental procedures).
From these data, we speculate that the visible degradation of eDNA in the ΔdnsΔxds mutant originates from cytoplasmic nucleases as a consequence of spontaneous autolysis during biofilm growth, which could also be an important mechanism for eDNA release. Indeed, proteins that are involved in cell septation, cell wall recycling and autolysis have been recently linked to DNA release at early time points in N. meningitidis biofilms (Lappann et al., 2010). The authors observed the most prominent decrease of eDNA for mutants exhibiting reduced autolysis, i.e. an ampD mutant, encoding a N-acetylmuramyl-L-alanine amidase involved in cell wall recycling (Tipper, 1969; Huff et al., 1970; Gilpin et al., 1972; Singer et al., 1972; Oshida et al., 1995; Park, 1995; Firczuk and Bochtler, 2007; Vollmer et al., 2008). To test whether a deletion of ampD can also decrease eDNA levels in V. cholerae a ΔdnsΔxdsΔampD mutant was constructed. The biofilm formation capacity of this triple mutant was analysed after 24 h using the static biofilm assay with crystal violet staining (Fig. 1B). The 24 h time point was chosen, as AmpD of N. meningitidis has been shown to impact eDNA release especially at early time points (Lappann et al.). Additionally, we observed growth defects of ΔampD mutants in V. cholerae beyond 32 h, but not within 24 h (Fig. S1A). The triple mutant exhibited significantly reduced biofilm levels compared with the extracellular nuclease double mutant. A similar tendency, although not as pronounced, was also observed for a ΔampD single mutant showing median biofilms levels lower than the wild type (Fig. S7). Expression of ampD in trans could partially restore biofilm production in the triple mutant resulting in a slight increase, but the levels of the ΔdnsΔxds mutant could not be reached (Fig. 1B). It has to be noted that expression of ampD in trans resulted in a slight growth defect that could also impact biofilm formation (Fig. S1A). Overall, these results strengthen the idea that at least one source of eDNA in bacterial biofilms is due to autolysis of cells.
The two extracellular nucleases are essential for utilization of eDNA as phosphate source
Besides the presence in the bacterial biofilm matrices, eDNA is also an abundant polymer in the aquatic ecosystem (Paul et al., 1987; Trevors, 1996; Dell'Anno and Danovaro, 2005). Therefore, eDNA could represent an important nutrient source for V. cholerae in this environment. For P. aeruginosa it has been recently shown that an extracellular nuclease is required for utilization of DNA as sole source for carbon, nitrogen and phosphate (Mulcahy et al., 2010). In that study the most pronounced growth phenotypes were observed using conditions with DNA as the sole source of phosphate. Thus, we determined whether V. cholerae can utilize DNA using Xds and Dns by growth assays under conditions of phosphate limitation. The growth of the wild type, ΔdnsΔxds, Δdns and Δxds mutants was monitored for 55 h in glucose minimal medium either containing inorganic phosphate and no DNA, or lacking inorganic phosphate but supplemented with herring sperm DNA (Fig. 7). In medium containing inorganic phosphate the wild type and all mutants showed similar growth abilities. Under conditions with DNA as the sole phosphate source growth was observed for the wild type after a long lag-phase. No growth was detected for the double mutant. Both single mutants were able to use DNA as the sole phosphate source, but exhibited a pronounced delay in growth compared with the wild type. Expression of dns and xds in trans using the IPTG-inducible vector pMMB could not be achieved in these experiments, as glucose served as a carbon source resulting in catabolite repression. We have tried to perform the growth assays under phosphate limiting conditions with different carbon sources (i.e. glycerol or maltose). Unfortunately, either no significant growth could be observed or precipitations occurred after 24 h, which tremendously affected the OD600 measurements. To overcome this limitation of the complementation analysis, we incubated DNA solutions with supernatants harbouring Dns or Xds activity, before their use as the sole source of phosphate in growth experiments with the double mutant (Fig. S1C). DNA incubated with a supernatant lacking Dns or Xds activity served as a control. In these growth experiments pre-incubation of the DNA with supernatants harbouring Dns or Xds activity allowed faster growth to higher optical densities of the double mutant compared with the control condition (Fig. S1C). The observable growth of the double mutant under control conditions is most likely due to phosphate added together with the DNA solution, which contained culture supernatants with 6.5 mM phosphate. Overall, these data are consistent with the results of growth kinetics obtained for the single mutants in Fig. 7. In summary, presence of each nuclease alone already allows V. cholerae to utilize DNA as a phosphate source, but presence of both activities is required to obtain the best growth under these conditions. Absence of both phosphate sources, inorganic phosphate and DNA, did not allow any growth for wild type and mutant strains (data not shown), indicating that phosphate was the limiting factor in all growth assays.
Fig. 7.
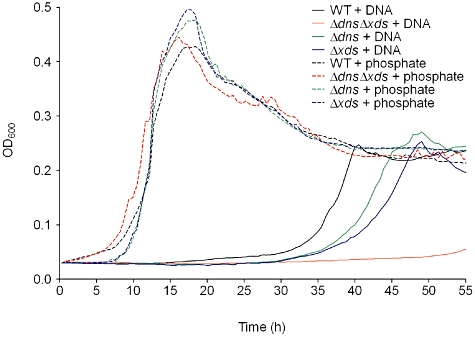
The two extracellular nucleases are important for utilization of DNA as phosphate source. Shown are the growth kinetics of wild type (black), ΔdnsΔxds (red), Δdns (green) and Δxds (blue) mutant monitored over 55 h in either M9 Tris glucose with inorganic phosphate (dashed lines) or M9 Tris glucose supplemented with herring sperm DNA (2.5 mg ml−1) as the sole source of phosphate (solid lines). Values represent medians from at least six independent measurements.
We also performed growth experiments using DNA as a sole source of carbon or nitrogen. However, even the wild type showed no visible growth using final DNA concentrations of up to 10 mg ml−1. We did not pursue these growth experiments, because addition of such high amounts of DNA resulted in a general growth defect in glucose minimal medium (Fig. S1B).
Based on these results, we investigated if dns and xds are expressed at higher levels under conditions of phosphate limitation. PhoA activities during planktonic growth were measured from wild type carrying the dns-phoA or xds-phoA transcriptional fusion grown in minimal medium containing high (65 mM) or low (6.5 mM) concentrations of inorganic phosphate (Fig. S8A). A 10-fold reduction of inorganic phosphate to 6.5 mM resulted in a severe growth delay of the wild type (Fig. S8B) and was the lowest phosphate concentration, which still allowed enough growth to acquire a substantial amount of cell density to measure PhoA activity. As shown in Fig. S8A a three- to five-fold higher activity for dns-phoA and xds-phoA was observed under conditions of phosphate limitation.
In summary, these results demonstrate that DNA can serve at least as a phosphate source allowing growth of V. cholerae under phosphate limiting conditions and that the two extracellular nucleases play a crucial role in this process. Accordingly, both nucleases are induced under phosphate limiting conditions.
Impaired detachment from biofilms results in reduced colonization fitness of the ΔdnsΔxds mutant
Based on the high amounts of compact biofilm produced by the ΔdnsΔxds mutant, we speculated that the mutant might be impaired for detachment from biofilms. To test whether this is true, wild type, ΔdnsΔxds, Δdns and Δxds mutants were allowed to form static biofilms for 40 h before the detachment rate within a 3 h time period was determined (Fig. 8). This time point was chosen, because the biofilm mass of the wild type increased steadily until 40 h in the static biofilm assay, but then remained almost at the same level (Fig. 1). The detachment rate in the ΔdnsΔxds mutant biofilm was significantly reduced compared with levels observed for the wild type. Although statistically not significant, a tendency towards decreased detachment was also observable for the dns and xds single mutants.
Fig. 8.
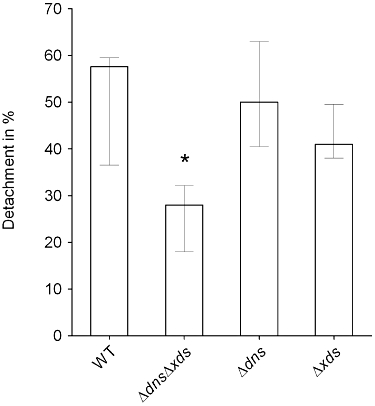
Deletion of dns and xds reduces the detachment in V. cholerae biofilms. Static biofilms were allowed to form for 40 h, subsequently the planktonic cell supernatant was replaced by cell free spent LB. The number of cells detached from the biofilm was determined after 3 h. The bars represent the percentage of detached cells to the overall cell number in the biofilm. Shown are the medians of at least nine independent measurements. The error bars indicate the interquartile range. The detachment rate of the ΔdnsΔxds mutant is significantly different compared with the wild type (*P < 0.05).
Biofilms are a likely form in which V. cholerae enters the human host, but after passage through the gastric barrier the bacteria have to detach from the biofilm to adhere and penetrate through the mucosal layer aided by motility (Freter and Jones, 1976; Freter and O'Brien, 1981; Huo et al., 1996; Colwell et al., 2003; Zhu and Mekalanos, 2003; Butler and Camilli, 2005). The low detachment rate of the ΔdnsΔxds mutant in vitro could also affect the dissolution of biofilm clumps in vivo resulting in reduced colonization fitness. Previous reports already demonstrated that inactivation of one or both extracellular nucleases do not reduce virulence of planktonic V. cholerae (Focareta and Manning, 1991a). To test whether the colonization fitness changes by using biofilms as inoculum, we performed competition experiments comparing the colonization efficiency with differentially marked biofilm clumps and planktonic cells of either the wild type or the ΔdnsΔxds mutant. Presence of uniform pieces of biofilm clumps in the inoculum for in vivo experiments, with approximately 50 to 100 cells for wild type and double mutant, was confirmed by microscopy (Fig. S9). The original output cfu of the planktonic and biofilm derived cells of the wild type and ΔdnsΔxds mutant obtained in these experiments are provided separately in Fig. S10. Based on the results, the planktonic cells of the wild type and the ΔdnsΔxds mutant exhibit a comparable colonization fitness. Thus, consistent with the data from Focareta et al. a direct colonization defect of planktonic cells of the ΔdnsΔxds mutant can be excluded [(Focareta and Manning, 1991a) and Fig. S10]. The competition indices resembling the ratio of biofilm derived to planktonic cells of the wild type or the ΔdnsΔxds mutant are shown in Fig. 9. Wild type biofilms out-competed wild-type planktonic cells about fivefold in the colonization assay, whereas wild type biofilms and planktonic cells showed comparable growth in vitro. This result is consistent with a previous report demonstrating that growth in biofilms induces a hyperinfectious phenotype in V. cholerae (Tamayo et al., 2010). In contrast, when inocula composed of the ΔdnsΔxds mutant were used, the biofilms showed a reduced colonization compared with planktonic cells. As is evident from the results shown in Fig. 8, the ΔdnsΔxds mutant detaches at low rates from its biofilms. Thus, the observed colonization defect of ΔdnsΔxds mutant biofilms is most likely due to inefficient dissolution of biofilm clumps within the lumen of the small intestine.
Fig. 9.
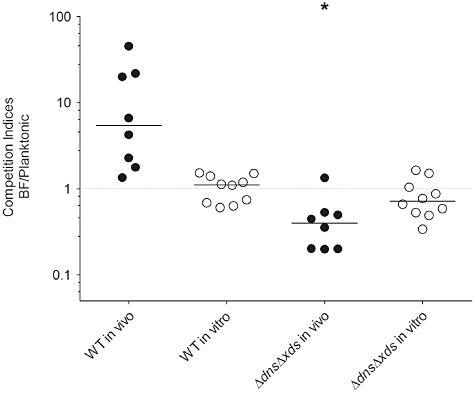
Extracellular nucleases are important for the colonization fitness of V. cholerae biofilms in vivo. Planktonic ΔdnsΔxds mutant cells out-compete their biofilm-derived counterparts (BF) in the small intestine of mice, whereas the opposite is true for wild type. In both tested cases the planktonic cells were lacZ negative and the biofilm-derived cells lacZ positive. Results are shown as the competitive indices (CI) in vivo using the infant mouse model (filled circles) and in vitro in LB shaking cultures (open circles). Each circle represents the CI from a single assay. Horizontal lines indicate medians for each data set. The in vivo CI of the ΔdnsΔxds mutant is significantly different compared with the wild type (*P < 0.05).
Discussion
The facultative pathogen V. cholerae transits between the pathogenic lifestyle in the gastrointestinal tract of the human host and the persistence as a natural inhabitant of aquatic ecosystems. Thereby the aquatic environment serves as an environmental reservoir for V. cholerae between the seasonal outbreaks (Faruque et al., 1998; Alam et al., 2007). Within aquatic ecosystems biofilms on chitinous surfaces are likely to be a preferred survival mode of V. cholerae. The biofilm formation capacity of V. cholerae is well documented and numerous studies have investigated structural factors including flagella, pili and exopolysaccharide synthesis, as well as the regulatory processes that control their expression.
In this study we demonstrated that the activity of the two extracellular deoxyribonucleases Dns and Xds is required for the development of a normal three-dimensional architecture of the biofilm. Absence of the nucleases causes a massive and unco-ordinated accumulation of the biofilm mass finally resulting in compact, thick and unstructured biofilms. Dns and Xds are solely responsible for the extracellular nuclease activity of V. cholerae, as no detectable extracellular nuclease activity was observed for the ΔdnsΔxds mutant in any assay described in this study. Detection of both activities in the supernatants also indicates that both nucleases are secreted rather than surface associated, which had previously only been reported for Dns (Focareta and Manning, 1987; Blokesch and Schoolnik, 2008). Although single mutants of the extracellular nucleases already showed increased biofilm amounts, and slightly reduced detachment rates compared with the wild type, the most prominent effects throughout the study were observed when both nucleases were deleted. These data show that Dns and Xds can at least partially compensate for each other and may act somewhat synergistically.
This study revealed two distinct differences between the nucleases regarding their enzymatic activity and transcriptional regulation. In order to discriminate the enzymatic activities we analysed the degradation of circular or linearized DNA added to culture supernatants containing either Dns or Xds. Dns was able to degrade both types of DNA and can therefore be classified as an endonuclease as previously suggested (Altermark et al., 2007; Blokesch and Schoolnik, 2008; Niiranen et al., 2008). In contrast, Xds exhibits an exonuclease activity, as it only targeted linearized DNA. Based on the observed temporal degradation pattern of linearized DNA the overall DNase activity of Dns seemed to be higher compared with Xds. This is consistent with previous reports that Dns hinders natural transformation by degradation of free eDNA, while Xds only contributes marginally to this effect (Blokesch and Schoolnik, 2008).
Consistent with a previous report we confirmed that expression of dns is regulated via HapR acting as a repressor (Blokesch and Schoolnik, 2008). In contrast, our results also demonstrate that xds transcription is independent of HapR. Consequently, dns is co-regulated with vps genes (Hammer and Bassler, 2003; Blokesch and Schoolnik, 2008). Furthermore, the temporal expression of dns and xds along the biofilm development was investigated and revealed different expression patterns for the two nucleases. The expression of dns was relatively high at the early and late stages of the biofilm, with a significant decrease in between. In contrast, xds expression increased steadily with the highest levels at the late time point. The expression patterns in combination with the other results of this study suggest that under low cell density, for example during initial stages of biofilm formation, highly expressed vps genes drive the production of VPS and Dns degrades available eDNA into smaller sized fragments. Xds may participate in the degradation of eDNA at this stage of biofilm formation, but our results imply that Dns is the dominant nuclease showing very efficient DNA degradation. This could also explain that throughout the study the deletion of dns had slightly more pronounced effects on biofilm formation and morphology especially at earlier time points compared with the deletion of xds. As the biofilm matures, accumulation of autoinducers and increase of cell density then results in repression of vps and dns transcription, while xds remains expressed. Thus, at these stages of the biofilm Xds might become the dominant nuclease resulting in further degradation of eDNA down to the nucleotide level. At the latest time point high expression of both nucleases was observed, indicating that the nucleases are induced in late stages of mature biofilms. One signal for induction of both nucleases at these late stages might be nutrient limitation. As demonstrated in this study, both nucleases are induced under conditions of phosphate limitation. Because both nucleases are also involved in the detachment process, the induction of both nucleases upon phosphate limitation could also trigger the dissolution of biofilms. Degradation of eDNA might not only yield nucleotides as a source of phosphate, but could also generate free nucleosides. Indeed, Vibrio encodes genes involved in nucleoside catabolism, which are controlled by the repressor CytR. Interestingly, in V. cholerae CytR represses also biofilm development and exopolysaccharide synthesis (Haugo and Watnick, 2002). Thus, genes encoding for nucleoside catabolism might be derepressed during biofilm formation in the aquatic environment and nucleosides could serve as a nutrient source for V. cholerae under these nutrient-limiting conditions.
Besides VPS, no other constituent of the V. cholerae biofilm matrix has been described so far. Based on the observations that the ΔdnsΔxds mutant shows increased biofilm formation without changing VPS production and that biofilms of V. cholerae are sensitive to nuclease treatment, we hypothesized that eDNA is an uncharacterized component of the V. cholerae biofilm matrix. We confirm the presence of eDNA by its visualization in biofilms using fluorescence microscopy and its isolation from the biofilm matrix. The enhanced biofilm production of the ΔdnsΔxds mutant compared with the wild type is likely due to the elevated levels of eDNA found in the extracellular nuclease mutant biofilm matrix. Such increased eDNA levels might allow enhanced attachment and recruit more cells to the biofilm. Another explanation could be that bacteria as well as VPS are trapped and retain in the biofilm. The correlation between eDNA and biofilm levels might also explain the observed increase in biofilm production upon presence of a plasmid. Because plasmid DNA could also contribute to eDNA in the biofilm, the presence of a plasmid could result in more eDNA and consequently more biofilm. The mechanisms of the eDNA release are still poorly understood, but autolysis is hypothesized to be a major source. In E. faecalis two extracellular proteases and in Staphylococcus aureus the cid/ lrg operons are involved in an autolysis-dependent eDNA release (Thomas et al., 2008; Mann et al., 2009). In N. meningitidis, where eDNA is important for the initiation of biofilm formation, ampD mutants with reduced autolysis also exhibit significantly diminished biofilm formation (Lappann et al., 2010). Consistent with this report, deletion of ampD also reduced biofilm formation in V. cholerae, indicating that autolysis is at least one origin of eDNA. A second source might be outer membrane vesicles (OMVs), which are naturally secreted by a variety of Gram-negative bacteria including V. cholerae (Chatterjee and Das, 1967; Kondo et al., 1993; Schild et al., 2008b). DNA within OMVs was previously observed for N. gonorrhoeae, P. aeruginosa and E. coli (Dorward et al., 1989; Yaron et al., 2000; Renelli et al., 2004). Future studies have to investigate whether OMVs derived from V. cholerae also contain DNA and are secreted during biofilm formation.
Extracellular DNA has been recently found in biofilms of other bacteria and shown to perform different roles in the biofilm development of the respective microorganisms. By analysing not only eDNA, but also its degradative enzymes Dns and Xds, we were able to characterize multiple roles for eDNA in the V. cholerae biofilm. We found that eDNA and the extracellular nucleases Dns and Xds are essential for the development of the normal three-dimensional biofilm structure with holes, pillars and fluid-filled channels. Thus, eDNA in V. cholerae not only facilitates initial attachment as described for Pseudomonas and Neisseria, but is also an important structural factor in biofilms.
Extracellular DNA is not only a ubiquitous component of the soil, but also of marine and freshwater habitats where it can reach concentrations of 88 µg l−1 (Vlassov et al., 2007). In addition, DNA is also a significant component of the small-intestinal mucus (Ferencz et al., 1980). Because V. cholerae transits between both environments along its life cycle, it might have evolved systems to utilize eDNA as a carbon, nitrogen and phosphate source. Acquisition of nutrients is crucial for persistence in the nutrient-poor aquatic ecosystem. Thus, V. cholerae can for example utilize chitin as a carbon and nitrogen source (Meibom et al., 2004). However, free phosphate is an especially limiting factor for survival of most organisms including V. cholerae in the aquatic reservoirs (Pratt et al., 2009). A recent study revealed that inorganic phosphate declines 1600-fold from 160 ppm in cholera patient stool to 0.1 ppm in pond water (Nelson et al., 2008). Hence, V. cholerae faces a severe drop in free inorganic phosphate during the transition from the host into the aquatic environment. Thus, acquisition of phosphate is crucial upon entry into the aquatic lifestyle. As demonstrated in this study, V. cholerae is capable of utilizing DNA as a phosphate source due to the activity of the extracellular nucleases Dns and Xds. Recently, xds was identified as a late in vivo induced gene (Schild et al., 2007). Strikingly, a number of such genes induced late during infection have been demonstrated to increase fitness of V. cholerae after release into the environment. As the formation of biofilms is thought to play a key role in environmental survival, Xds and Dns could be important for nutrient acquisition in the host as well as in the aquatic environment due to degradation of DNA.
Extracellular DNA represents also a dynamic gene pool from which bacteria can obtain genetic information by horizontal gene transfer (Vlassov et al., 2007). The mosaic-structured genome of V. cholerae implicates the importance of horizontal gene transfer during evolution (Heidelberg et al., 2000). Virulence of V. cholerae is most likely the result of a series of horizontal gene transfer events that allowed a benign marine bacterium to evolve into a human pathogen (Reidl and Klose, 2002). The newly emerged serogroup O139 has evolved from an O1 El Tor ancestor through the acquisition of the genes encoding for the O139 antigen and capsule (Bik et al., 1995). Interestingly, V. cholerae induces natural competence while growing on chitin (Meibom et al., 2005). This implicates the possibility of horizontal gene transfer while V. cholerae is forming biofilms on natural chitinous material in the aquatic environment. A successful serogroup conversion of an O1 recipient by an O139 donor and transfer of classical biotype cholera toxin genes from V. cholerae serotype O141 to O1 biotype El Tor were recently demonstrated by co-culturing both strains on chitin surfaces (Blokesch and Schoolnik, 2007; Udden et al., 2008). The presence of eDNA in V. cholerae biofilms, as demonstrated in this study, indicates that V. cholerae, like other bacteria, contributes to the pool of extracellular genetic material available for different strains and species and strengthens the current model of the O1-to-O139 conversion and acquisition of virulence factors in the evolution of V. cholerae.
Vibrio cholerae biofilm clumps formed in the aquatic reservoir are an important source for new cholera outbreaks (Huo et al., 1996; Hall-Stoodley and Stoodley, 2005; Pruzzo et al., 2008). This is highlighted by the observation that filtration of water reducing the concentration of particles greater than 20 µm in size can reduce cholera incidence rates in endemic areas by 48% (Colwell et al., 2003). Biofilm-associated V. cholerae are better protected against acids or bile salts and biofilms most likely allow a higher number of bacteria to reach the small intestine (Nalin et al., 1978; 1979; Zhu and Mekalanos, 2003; Hartley et al., 2006). However, at the primary site of colonization V. cholerae has to detach from the biofilm and use flagellar motility to attach and penetrate through the mucosal layer as well as to induce full virulence (Freter and Jones, 1976; Freter et al., 1981; Butler and Camilli, 2004; 2005; Lauriano et al., 2004; Liu et al., 2008; Moisi et al., 2009). It has been previously demonstrated that the unregulated VPS production of hapR mutants results in lower detachment rates, which correlates with a reduced colonization fitness of hapR mutant biofilms (Zhu and Mekalanos, 2003; Liu et al., 2007). As shown in this study, extracellular nucleases contribute to an efficient detachment from the mature biofilm. Consistently, a colonization defect for biofilms of the ΔdnsΔxds mutant compared with planktonic cells was observed. In contrast, wild type biofilms out-competed planktonic cells, which is consistent with a recent report demonstrating that growth in a biofilm induces a hyperinfectious phenotype in V. cholerae (Tamayo et al., 2010). Because the biofilms of the mutant and wild type were dispersed into clumps of similar size with approximately 50–100 cells, we doubt that the thick and compact structure of the mutant biofilm by itself hinders the bacteria to efficiently colonize the small intestine. Along with the observed nuclease sensitivity of biofilms and the results of the detachment assay, we rather speculate that Dns and Xds are involved in the dissolution step of biofilm-derived cells in vivo via degradation of eDNA. To our knowledge, this is the first report of enzymes that are directly involved in the detachment process by degrading material of the V. cholerae biofilm matrix.
In summary, we identified eDNA as an important, versatile matrix component of V. cholerae biofilms. Extracellular DNA, together with the corresponding modulatory extracellular nucleases Dns and Xds, are involved in several processes of V. cholerae during life in a biofilm, including the development of a typical biofilm architecture, detachment from biofilms, nutrient acquisition and the in vivo colonization fitness of biofilm clumps after ingestion by the host.
Experimental procedures
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the ‘Bundesgesetzblatt fuer die Republik Oesterreich’ and the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the University of Graz as well as the Austrian Federal Ministry for Science and Research BM.W-F (Permit Number: 39/158 ex2000/10). Intragastric infections were performed under isoflurane anaesthesia and all efforts were made to minimize suffering.
Bacterial strains and culture conditions
Bacterial strains and plasmids used in this study are listed in Table 1. The clinical isolate V. cholerae O1 El Tor C6709 was used as wild type (Roberts et al., 1992). E. coli DH5αλpir was used for maintenance of plasmids and SM10λpir to deliver plasmids to V. cholerae by conjugation (Kolter et al., 1978; Hanahan, 1983; Miller and Mekalanos, 1988). Unless stated otherwise strains were grown in LB broth with aeration at 37°C or for biofilm experiments under static conditions at room temperature (RT). Antibiotics and other supplements were used in the following final concentrations: streptomycin (Sm), 100 µg ml−1; ampicillin (Ap), 100 µg ml−1 or 50 µg ml−1 in combination with other antibiotics; kanamycin (Km), 50 µg ml−1; isopropyl-β-thiogalactopyranoside (IPTG), 0.5 mM; glucose (Gluc), 0.2%; sucrose (Suc), 10%, 30 µg ml−1 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (Xgal).
Table 1.
Strains used in this study
| Strain/plasmid | Description | Reference |
|---|---|---|
| E. coli | ||
| DH5αλpir | F-Φ80ΔlacZΔM15Δ(argF lac)U169 deoR recA1 endA1 hsdR17 (rK-mK+) supE44 thi-1 gyrA69 relA1, λpirR6K, Apr | Hanahan (1983) |
| SM10λpir | thi thr leu tonA lacY supE recA::RPA-2-Te::Mu λpirR6K, Kmr | Miller and Mekalanos (1988) |
| V. cholerae | ||
| C6709 | WT, O1 El Tor Inaba, clinical isolate, 1991 Peru, tcpA+ctx+hapR+, spontaneous Smr | Roberts et al. (1992) |
| C6709lacZ | C6709, lacZ::res-neo-sacB-res | Tamayo et al. (2008) |
| Δdns | Deletion of dns in C6709, Smr | This study |
| ΔhapR | Deletion of hapR in C6709, Smr | This study |
| ΔvpsA | Deletion of vpsA in C6709, Smr | This study |
| Δxds | Deletion of xds in C6709, Smr | This study |
| ΔdnsΔxds | Deletion of xds in Δdns, Smr | This study |
| ΔdnsΔxdsΔampD | Deletion of in ampD in ΔdnsΔxds, Smr | This study |
| ΔdnsΔxds lacZ | ΔdnsΔxds, lacZ::res-neo-sacB-res | This study |
| C6709dns::pGPphoA | Insertion of pGPphoAdns in dns of C6709, Smr, Apr | This study |
| ΔhapRdns::pGPphoA | Insertion of pGPphoAdns in dns of ΔhapR, Smr, Apr | This study |
| C6709vpsA::pGPphoA | Insertion of pGPphoAvpsA in vpsA of C6709 Smr, Apr | This study |
| ΔhapRvpsA::pGPphoA | Insertion of pGPphoAvpsA in vpsA of ΔhapR, Smr, Apr | This study |
| C6709xds::pGPphoA | Insertion of pGPphoAxds in xds of C6709, Smr, Apr | This study |
| ΔhapRxds::pGPphoA | Insertion of pGPphoAxds in xds of ΔhapR, Smr, Apr | This study |
| Plasmids | ||
| pCVD442 | oriR6K mobRP4 sacB, Apr | Donnenberg and Kaper (1991) |
| pGPphoA | pGP704 with promotorless phoA of SM10λpir, Apr | Moisi et al. (2009) |
| pMMB | pMMB67EH, IncQ broad-host-range low-copy-number cloning vector, IPTG inducible, Apr | Morales et al. (1991) |
| pBAD24 | araBADp cloning vector, Apr | Guzman et al. (1995) |
| pCVD442ΔampD | pCVD442::ΔampD, Apr | This study |
| pCVD442Δdns | pCVD442::Δdns, Apr | This study |
| pCVD442ΔhapR | pCVD442::ΔhapR, Apr | This study |
| pCVD442ΔvpsA | pCVD442::ΔvpsA, Apr | This study |
| pCVD442Δxds | pCVD442::Δxds, Apr | This study |
| pGPphoAdns | pGPphoA with ‘dns’ fragment of C6709, Apr | This study |
| pGPphoAvpsA | pGPphoA with ‘vpsA’ fragment of C6709, Apr | This study |
| pGPphoAxds | pGPphoA with ‘xds’ fragment of C6709, Apr | This study |
| pampD | ampD of C6709 in pMMB, Apr | This study |
| pdns | dns of C6709 in pMMB, Apr | This study |
| pxds | xds of C6709 in pMMB, Apr | This study |
Construction of suicide plasmids, deletion mutants and expression plasmids
Chromosomal DNA was isolated using the method described by Grimberg et al. (Grimberg et al., 1989). For phenol extraction phase lock gel tubes (Eppendorf) were used and the chromosomal DNA was obtained by ethanol and salt precipitation. Plasmids, digested plasmids and PCR products were purified using the QIAquick PCR purification, the QIAquick gel extraction, or the QIAprep Spin Mini Kit (Qiagen). PCRs for subcloning were performed with Phusion polymerase (New England Biolabs), for all other reactions Taq DNA polymerase (New England Biolabs) was used. Oligonucleotides used in this study are listed in Table 2.
Table 2.
Oligonucleotides used in this study
| Oligonucleotides | Sequence (5′ to 3′) |
|---|---|
| VC0470_XbaI_1 | CGTCTAGATAGCCAAGATCGCCGAAAa |
| VC0470_2 | CGTCTTTTATGTGATGGGCAGAATCTCACC |
| VC0470_3 | CTGCCCATCACATAAAAGACGTAGATAAGTAGGTTTTT |
| VC0470_XbaI_4 | CGTCTAGAGGTATGGCTGATCGTTGTGA |
| VC0583_SacI_1 | TTTGAGCTCTGCGCGTAGTCGATACCG |
| VC0583_EcoRI_2 | TTTGAATTCCATAGGGGTATATCCTTGCCA |
| VC0583_EcoRI_3 | TTTGAATTCTAGTTTCTTGGGCAGCACAAA |
| VC0583_XbaI_4 | TTTTCTAGATACGCGTCATACCGGAAA |
| VC0917_XbaI_1 | TTTTCTAGATTATTTTACGCGATAAGA |
| VC0917_EcoRI_2 | TTTGAATTCCACTTCCCCACATCCTCTT |
| VC0917_EcoRI_3 | TTTGAATTCTAGACTCATCAGGGGATGACA |
| VC0917_SacI_4 | TTTGAGCTCACACGAGGATGGCGGTT |
| VC2621_SacI_1 | TTAGAGCTCGAATACCAACTGCATTTCAT |
| VC2621_SphI_2 | TTTGCATGCCATGATGTACCTTCTCCTCCCT |
| VC2621_SphI_3 | TTTGCATGCTAGAAAGTGGCATTTTTGATA |
| VC2621_XbaI_4 | TTATCTAGAAGAGCTTGGCAAAATGGGCA |
| ampD_SacI_1 | TTTGAGCTCGAGCAATCGGCACTGG |
| ampD_EcoRI_2 | AAAGAATTCCATCCTTACTCCTTGCTTATA |
| ampD_EcoRI_3 | AATGAATTCTAGGTTTTAGTTGGTGCGAAA |
| ampD_XbaI_4 | AATTCTAGATGAAGATTGGTCTATTTTATG |
| dns_SacI | TTTGAGCTCACCTTTGCCGCCCCC |
| dns_KpnI | TTTGGTACCTCAGTTCGGGCATTGCTCACG |
| vpsA_SacI | AAAGAGCTCTGTCAAGACAATCGTTTT |
| vpsA_KpnI | TTTGGTACCGACTAATTTCACCGT |
| xds_SacI | TTTGAGCTCCGCGATAAAGTGGTGAAGCTG |
| xds_KpnI | TTTGGTACCTTTCTAGCGACGGCGACG |
| VC0470_SacI_5′ | TTTGAGCTCCTACTTATCTACGTCTTTTTAG |
| VC0470_XbaI_3′ | AAATCTAGATTCTGCCCATCAGTTCGG |
| VC2621_SacI_5′ | TTTGAGCTCAAATAGGGAGGAGAAGGTAC |
| VC2621_XbaI_3′ | TTTTCTAGACTTTCTAGCGACGGCGACG |
| ampD_SacI_5′ | AAAGAGCTCTATAAGCAAGGAGTAAGGATG |
| ampD_XbaI_3′ | AAATCTAGAGGGAACTCAACCAAGC |
Restriction sites are underlined.
Constructions of in-frame deletion mutants were carried out as described by Donnenberg and Kaper (Donnenberg and Kaper, 1991). PCR fragments of approximately 800 bp upstream and downstream of the gene of interest were PCR-amplified using the oligonucleotide pairs A_B_1 and A_B_2 or A_B_3 and A_B_4, in which A stands for the gene and B for the restriction site used. In-frame deletion of dns was obtained by splicing overlap extension PCR (Horton et al., 1989) using oligonucleotide pairs VC0470_XbaI_1 and VC0470_2 as well as VC0470_3 and VC0470_XbaI_4. After digestion of the PCR fragments with the appropriate restriction enzyme (New England Biolabs) indicated by the name of the oligonucleotide, they were ligated into the SacI/XbaI-digested pCVD442 or XbaI-digested pCVD442 respectively.
Derivatives of pGPphoA were constructed to obtain chromosomal transcriptional fusions of phoA to respective gene transcripts. Gene fragments of dns, xds and vpsA, containing the translational stop codon of the respective gene, were amplified by PCR using oligonucleotide pairs dns_SacI and dns_KpnI, xds_SacI and xds_KpnI or vpsA_SacI and vpsA_KpnI. PCR products were digested with SacI and KpnI and ligated into the pGPphoA vector that had been digested with the same enzymes.
For the expression plasmids the respective gene was PCR-amplified using the oligonucleotide pairs designated in the way A_B_5′ and A_B_3′, in which A stands for the gene and B for the restriction site used. The PCR fragment was digested with SacI and XbaI, and ligated in the similar digested pMMB expression vector. After transformation of the ligation products in DH5αλpir, Apr colonies were characterized by PCR and/ or restriction analysis (data not shown).
In case of the suicide plasmid derivatives the correct constructs were transformed into SM10λpir and mobilized into V. cholerae by conjugation, which was achieved by cross-streaking donor and recipient on LB agar plates followed by incubation for 6 h on 37°C. V. cholerae conjugants were purified via selection for Smr and Apr colonies. In the case of pCVD442 derivatives sucrose selection was used to obtain Aps colonies. Chromosomal insertions or deletions were confirmed by PCR (data not shown).
Static biofilm assay with crystal violet staining
Static biofilm assays were performed as previously published (Watnick and Kolter, 1999) with following modifications. An overnight culture of the respective strain was diluted 1:150 in LB broth. One hundred and fifty microlitres of this dilution was placed into sterile polystyrene 96 well U bottom microtiter plate wells (Sterilin) and biofilm was grown for a time period of 12, 24, 40, 48 or 72 h at RT. Wells were subsequently rinsed six times with 200 µl dH2O with a mircoplate washer (LP41) and adhered bacteria were stained with 180 µl crystal violet solution (0.1%) for 10 min. The wells were again washed four times and the crystal violet stained biofilm was solubilized in 250 µl ethanol (96%). Wells loaded with LB broth only were included in every experiment as a negative control and showed no detectable crystal violet staining. Biofilm formation was then quantified by measuring an OD595 using a microplate reader (Biorad).
Alkaline phosphatase (PhoA) assay
To determine the enzymatic activities for transcriptional phoA fusions, alkaline phosphatase assays were performed of the respective strains as described previously using cultures grown to an OD600 of ∼0.2 or ∼1, overnight cultures or cells derived from statically grown biofilms at the indicated time points (Schild et al., 2005; 2009). The activities are expressed in Miller Units, calculated as following: (A405 × 1000)/(A600 × ml × min).
Flow cell biofilm experiments
For visualization of biofilms they were formed in three-channel flow cells with a modified version of the biofilm setup described by Sternberg et al. (Sternberg et al., 1999; Sternberg and Tolker-Nielsen, 2006). A coverslip 24 × 50 mm (Menzel-Glaeser) was used as substratum for biofilm growth. The respective overnight cultures were adjusted to OD600 = 0.1 using either LB or 50-fold diluted LB (2%) as described by Yildiz et al. (2004). Approximately 250 µl of the dilutions was inoculated per channel. After static incubation for 2 h at RT, flow of LB or 2% LB was initiated at a constant rate of 3 ml h−1 with the use of a Watson Marlow 205S. Biofilm was allowed to form at RT for a time period of 9 h using LB or 24 h using 2% LB. Images of attached bacteria or biofilms were recorded by confocal laser scanning microscopy.
Fluorescent staining
To show biofilm morphology, biofilms were grown in flow cells and stained with SYTO 9. SYTO 9 from the Live/Dead BacLight Bacterial Viability kit (Invitrogen) was freshly diluted 400-fold in LB broth and approximately 250 µl of the dilution was injected into the flow cell channel. Biofilm was stained at RT for 20 min and images were recorded by confocal laser scanning microscopy.
For visualization of eDNA, flow cell biofilms were stained with a mixture of SYTO 9 and BOBO-3 (Invitrogen). SYTO 9 allows visualization of cells, whereas BOBO-3 is membrane impermeable and therefore specifically stains eDNA (Schleheck et al., 2009). 0.4 µl of each fluorescent dye was added to 1500 µl LB and approximately 250 µl of this mix was injected into the flow cell channel. Biofilm was stained at RT for 30 min and images were recorded by confocal laser scanning microscopy.
Confocal laser scanning microscopy
Microscopy was performed using a Leica SP5 confocal microscope (Leica Microsystems, Mannheim, Germany) with spectral detection and a Leica HCX PL APO CS 40 × oil immersion objective (NA 1.25). Optical sectioning was performed in 0.2 µm steps.
SYTO 9 (Invitrogen) was excited at 488 nm and fluorescence emission was detected between 500–530 nm. Propidium Iodide (Invitrogen) was also excited with the 488 nm argon laser line and fluorescence emission was detected between 570–630 nm. BOBO-3 (Invitrogen) was excited at 561 nm and fluorescence emission was detected between 570–620 nm. Fluorescence signal of double labelled specimens and transmission images were acquired simultaneously. Images were recorded without differential interference contrast (DIC) optics. For visualization and processing of three-dimensional image data the Leica LAF and IMARIS software (volume rendering with shadow projection) was used. Quantitative analysis of the image stacks was performed using computer program COMSTAT (http://www.comstat.dk) (Heydorn et al., 2000; M. Vorregaard et al., pers. comm.). At least six image stacks from three independent experiments were used for the analysis.
DNase activity assays
A DNase activity assay using plasmid or chromosomal DNA as substrate was performed essentially as described previously (Blokesch and Schoolnik, 2008; Mulcahy et al., 2010). Cells from overnight cultures of the respective V. cholerae strains were pelleted by centrifugation at 5000 g for 10 min and the supernatants were withdrawn. Twenty microlitres of the filter-sterilized supernatant was incubated at RT with 1 µg of purified circular plasmid DNA (pBAD24), linearized plasmid DNA (pBAD24 digested with EcoRI) or chromosomal DNA derived from V. cholerae wild type. Finally, samples were visualized on agarose gels (0.8%).
Nuclease treatment of biofilms
Static biofilms were cultivated as described above. At the respective time points the wells were rinsed four times with spent LB (obtained from a 24 or 72 h old biofilm culture of the respective strain) before DNase I (AppliChem), λExonuclease (New England Biolabs) or in combination were added to a final concentration of 133 Kunitz units per ml. Addition of spent LB with nuclease buffer (50 mM Tris/HCl pH 7.5, 10 mM MgCl2, 50 µg µl−1 BSA, 5 mM CaCl2) served as a control. The biofilm was incubated with the respective solution for 3 or 7 h at RT respectively. Afterwards the wells were rinsed four times with distilled water and the remaining biofilm was stained with crystal violet and quantified as described above.
eDNA and iDNA quantification
The eDNA and iDNA assay was performed essentially as previously published (Steinberger and Holden, 2005; Nakamura et al., 2008; Kreth et al., 2009; Harmsen et al., 2010). Briefly, an overnight culture of the respective strain was diluted 1:150 in LB broth. A borosilicate glass tube was inoculated with 1 ml of the resulting dilution and biofilm was allowed to form for 48 h under static conditions. Afterwards the biofilm was dispersed by treatment in an ultrasonic water bath for 1 min. Vortexing and treatment in the ultrasonic water bath was confirmed not to lyse cells or affect cell viability by several means including cfu measurements by plating and direct counts by microscopy. These controls have been conducted as described previously (Tamayo et al., 2010). Cells were removed by centrifugation for 10 min at 5000 g. The cell pellet was used for isolation of iDNA performing a standard chromosomal DNA preparation as described above. For the eDNA preparation the supernatant was transferred to a phase lock gel tube (Eppendorf) and DNA was isolated by phenol extraction followed by ethanol precipitation. Precipitated iDNA and eDNA pellets were suspended in 100 µl ddH2O (Fresenius). DNA concentrations were measured using a NanoDrop 1000 (Thermo Scientific), which allowed calculation of the amount of eDNA and iDNA in the biofilm. The results are given by the ratio of eDNA to iDNA. Samples of eDNA and iDNA were also separated and visualized on agarose gels (0.8%).
Growth kinetics
Growth kinetics were carried out in transparent 24-well plates (Greiner) in 1 ml culture volume using M9 minimal medium supplemented with glucose (0.2%) and Tris/HCl pH 8.0 (100 mM), to remain the buffer capacity even under conditions without inorganic phosphate (von Kruger et al., 1999). The respective strains were grown in a pre-culture for 24 h in M9 Tris glucose with aeration and shaking at 37°C. Cells derived from the pre-cultures were washed with M9 Tris glucose without inorganic phosphate and adjusted to OD600 = 0.03 with either M9 Tris glucose or M9 Tris glucose without inorganic phosphate (0 mM) supplemented with 2.5 mg ml−1 herring sperm DNA as phosphate source. OD600 was monitored every 30 min in the FLUOstar OMEGA plate reader (BMG Labtech) at 37°C with shaking.
Detachment assay
The detachment assay was performed as previously published (Zhu and Mekalanos, 2003; Liu et al., 2007), but with following modifications. An overnight culture of the respective strain was diluted 1:150 in LB broth. A borosilicate glass tube was inoculated with 1.5 ml of the resulting dilution and biofilm was allowed to form for 40 h under static conditions at RT. Glass tubes were subsequently rinsed two times with LB and 1.6 ml spent LB (obtained from a 40 h old biofilm culture of the respective strain) was added. One hundred microlitres was directly taken as sample To reflecting the original cfu in the supernatant, which result either from residual planktonic cells or mechanical disturbance of the biofilm due to addition of spent LB. After 3 h incubation at RT another sample from the supernatant was taken reflecting time point Td, which contains the cells from To and additionally cells that have detached during the 3 h incubation. The remaining biofilm was dispersed as described above in 2 ml fresh LB broth. This sample, called Tb, reflects the cells remaining in the biofilm.
Appropriate dilutions were plated on LB plates and incubated at 37°C over night. Before plating, each sample was rigorously mixed by vortexing and treated in an ultrasonic water bath to disperse biofilms to avoid biofilm clumps masking the real cfu titre. Complete dispersion was confirmed by microscopy. The detachment rate was defined by the percentage of detached cells within the 3 h period compared with the whole cfu present in the original biofilm and calculated as follows: (cfu Td – cfu To)/(cfu Td + cfu To + cfu Tb) × 100.
Competition experiments
Competition experiments and mouse experiments were performed as previously described (Schild et al., 2007; Moisi et al., 2009). For competition experiments 5- to 6-day-old C57Bl/6 mice were used as well as LB broth as an in vitro control. Mice were separated from their dams 1 h before infection. Subsequently, they were anaesthetized by inhalation of isoflurane gas and then inoculated by oral gavage with 50 µl of the respective inoculum. To prepare the inocula, the respective biofilm-derived cells (lacZ+) and planktonic cells (lacZ-) were mixed at 1:1 ratios in LB broth before inoculation. To determine the input ratios and bacterial titres the inocula were plated on LB plates containing Xgal. Before plating each sample was rigorously mixed by vortexing and treated in an ultrasonic water bath to disperse biofilms to avoid biofilm clumps masking the real cfu as described above. Complete dispersion was confirmed by microscopy. The final titres were approximately 105 cfu per mouse. The inoculated mice were kept away from their dams for 24 h. At 24 h post inoculation, mice were euthanized, the small bowel from each mouse was removed by dissection and mechanically homogenized in 1 ml LB broth with 30% glycerol. Appropriate dilutions starting with 100 µl of the homogenate were plated on LB/Sm/Xgal plates. The competition index is calculated as the blue/white ratio of the output normalized to the blue/white ratio of the input. Additionally the recovered cfu per small bowel of the planktonic and biofilm derived cells of the wild type and ΔdnsΔxds mutant are shown separately in Fig. S10.
To obtain biofilm-derived cell samples, biofilms were allowed to form under static conditions in 1.5 ml LB broth in borosilicate glass tubes for 48 h at RT. Biofilms were rinsed with LB broth, removed mechanically from the glass tubes and adjusted in 1.6 ml fresh LB. For all competition experiments, three independently grown biofilms were formed in parallel and pooled. Presence of uniform pieces of biofilm clumps with approximately 50–100 cells was confirmed by microscopy (Fig. S9). For planktonic cell sample preparation the respective strain was grown over night (16 h) in LB Sm broth and then adjusted to the desired concentration.
Statistical analysis
Data were analysed using the Mann–Whitney U-test in the case of single comparisons or by a Kruskal–Wallis test followed by post hoc Dunn's multiple comparisons. Differences were considered significant for P values of < 0.05.
Acknowledgments
This work was supported by Dr Heinrich Joerg-Stiftung (Karl-Franzens Universitaet, Graz, Austria) to A.S., by the Austrian Science Fund (FWF) grants: P22986 to S.S. and W901 (DK Molecular Enzymology) to A.S., V.H.I.F., S.R., S.D.K., J.R. and S.S., by the Austrian Federal Ministry for Science and Research (Project GOLD, in the framework of the Austrian Genomics Program GEN-AU) to S.D.K. and H.W. and by NIH Grant AI055058 to A.C. A.C. is a Howard Hughes Medical Institute Investigator.
Supporting information
Additional supporting information may be found in the online version of this article.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Alam M, Sultana M, Nair GB, Siddique AK, Hasan NA, Sack RB, et al. Viable but nonculturable Vibrio cholerae O1 in biofilms in the aquatic environment and their role in cholera transmission. Proc Natl Acad Sci USA. 2007;104:17801–17806. doi: 10.1073/pnas.0705599104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altermark B, Niiranen L, Willassen NP, Smalas AO, Moe E. Comparative studies of endonuclease I from cold-adapted Vibrio salmonicida and mesophilic Vibrio cholerae. FEBS J. 2007;274:252–263. doi: 10.1111/j.1742-4658.2006.05580.x. [DOI] [PubMed] [Google Scholar]
- Berne C, Kysela DT, Brun YV. A bacterial extracellular DNA inhibits settling of motile progeny cells within a biofilm. Mol Microbiol. 2010;77:815–829. doi: 10.1111/j.1365-2958.2010.07267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyhan S, Bilecen K, Salama SR, Casper-Lindley C, Yildiz FH. Regulation of rugosity and biofilm formation in Vibrio cholerae: comparison of VpsT and VpsR regulons and epistasis analysis of vpsT, vpsR, and hapR. J Bacteriol. 2007;189:388–402. doi: 10.1128/JB.00981-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bik EM, Bunschoten AE, Gouw RD, Mooi FR. Genesis of the novel epidemic Vibrio cholerae O139 strain: evidence for horizontal transfer of genes involved in polysaccharide synthesis. EMBO J. 1995;14:209–216. doi: 10.1002/j.1460-2075.1995.tb06993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokesch M, Schoolnik GK. Serogroup conversion of Vibrio cholerae in aquatic reservoirs. PLoS Pathog. 2007;3:e81. doi: 10.1371/journal.ppat.0030081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokesch M, Schoolnik GK. The extracellular nuclease Dns and its role in natural transformation of Vibrio cholerae. J Bacteriol. 2008;190:7232–7240. doi: 10.1128/JB.00959-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomchil N, Watnick P, Kolter R. Identification and characterization of a Vibrio cholerae gene, mbaA, involved in maintenance of biofilm architecture. J Bacteriol. 2003;185:1384–1390. doi: 10.1128/JB.185.4.1384-1390.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SM, Camilli A. Both chemotaxis and net motility greatly influence the infectivity of Vibrio cholerae. Proc Natl Acad Sci USA. 2004;101:5018–5023. doi: 10.1073/pnas.0308052101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SM, Camilli A. Going against the grain: chemotaxis and infection in Vibrio cholerae. Nat Rev Microbiol. 2005;3:611–620. doi: 10.1038/nrmicro1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper-Lindley C, Yildiz FH. VpsT is a transcriptional regulator required for expression of vps biosynthesis genes and the development of rugose colonial morphology in Vibrio cholerae O1 El Tor. J Bacteriol. 2004;186:1574–1578. doi: 10.1128/JB.186.5.1574-1578.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee SN, Das J. Electron microscopic observations on the excretion of cell-wall material by Vibrio cholerae. J Gen Microbiol. 1967;49:1–11. doi: 10.1099/00221287-49-1-1. [DOI] [PubMed] [Google Scholar]
- Chiavelli DA, Marsh JW, Taylor RK. The mannose-sensitive hemagglutinin of Vibrio cholerae promotes adherence to zooplankton. Appl Environ Microbiol. 2001;67:3220–3225. doi: 10.1128/AEM.67.7.3220-3225.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell RR. Global climate and infectious disease: the cholera paradigm. Science. 1996;274:2025–2031. doi: 10.1126/science.274.5295.2025. [DOI] [PubMed] [Google Scholar]
- Colwell RR, Huq A, Islam MS, Aziz KM, Yunus M, Khan NH, et al. Reduction of cholera in Bangladeshi villages by simple filtration. Proc Natl Acad Sci USA. 2003;100:1051–1055. doi: 10.1073/pnas.0237386100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover MS, Mishra M, Deora R. Extracellular DNA is essential for maintaining Bordetella biofilm integrity on abiotic surfaces and in the upper respiratory tract of mice. PLoS ONE. 2011;6:e16861. doi: 10.1371/journal.pone.0016861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Anno A, Danovaro R. Extracellular DNA plays a key role in deep-sea ecosystem functioning. Science. 2005;309:2179. doi: 10.1126/science.1117475. [DOI] [PubMed] [Google Scholar]
- Dlakic M. Functionally unrelated signalling proteins contain a fold similar to Mg2+-dependent endonucleases. Trends Biochem Sci. 2000;25:272–273. doi: 10.1016/s0968-0004(00)01582-6. [DOI] [PubMed] [Google Scholar]
- Donnenberg MS, Kaper JB. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorward DW, Garon CF, Judd RC. Export and intercellular transfer of DNA via membrane blebs of Neisseria gonorrhoeae. J Bacteriol. 1989;171:2499–2505. doi: 10.1128/jb.171.5.2499-2505.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faruque SM, Albert MJ, Mekalanos JJ. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol Mol Biol Rev. 1998;62:1301–1314. doi: 10.1128/mmbr.62.4.1301-1314.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferencz A, Orskov I, Orskov F, Klemm P. Deoxyribonucleic acid is a significant component of the small-intestinal mucus. Acta Pathol Microbiol Scand B. 1980;88:347–348. doi: 10.1111/j.1699-0463.1980.tb02654.x. [DOI] [PubMed] [Google Scholar]
- Firczuk M, Bochtler M. Folds and activities of peptidoglycan amidases. FEMS Microbiol Rev. 2007;31:676–691. doi: 10.1111/j.1574-6976.2007.00084.x. [DOI] [PubMed] [Google Scholar]
- Flemming HC, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- Focareta T, Manning PA. Extracellular proteins of Vibrio cholerae: molecular cloning, nucleotide sequence and characterization of the deoxyribonuclease (DNase) together with its periplasmic localization in Escherichia coli K-12. Gene. 1987;53:31–40. doi: 10.1016/0378-1119(87)90090-4. [DOI] [PubMed] [Google Scholar]
- Focareta T, Manning PA. Distinguishing between the extracellular DNases of Vibrio cholerae and development of a transformation system. Mol Microbiol. 1991a;5:2547–2555. doi: 10.1111/j.1365-2958.1991.tb02101.x. [DOI] [PubMed] [Google Scholar]
- Focareta T, Manning PA. Genetic analysis of the export of an extracellular DNase of Vibrio cholerae using DNase-beta-lactamase fusions. Gene. 1991b;108:31–37. doi: 10.1016/0378-1119(91)90484-s. [DOI] [PubMed] [Google Scholar]
- Fong JC, Syed KA, Klose KE, Yildiz FH. Role of Vibrio polysaccharide (vps) genes in VPS production, biofilm formation and Vibrio cholerae pathogenesis. Microbiology. 2010;156:2757–2769. doi: 10.1099/mic.0.040196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R, Jones GW. Adhesive properties of Vibrio cholerae: nature of the interaction with intact mucosal surfaces. Infect Immun. 1976;14:246–256. doi: 10.1128/iai.14.1.246-256.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R, O'Brien PC. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: chemotactic responses of Vibrio cholerae and description of motile nonchemotactic mutants. Infect Immun. 1981;34:215–221. doi: 10.1128/iai.34.1.215-221.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R, O'Brian PC, Macsai MS. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vivo studies. Infect Immun. 1981;34:234–240. doi: 10.1128/iai.34.1.234-240.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghigo JM. Natural conjugative plasmids induce bacterial biofilm development. Nature. 2001;412:442–445. doi: 10.1038/35086581. [DOI] [PubMed] [Google Scholar]
- Gilpin RW, Chatterjee AN, Young FE. Autolysis of microbial cells: salt activation of autolytic enzymes in a mutant of Staphylococcus aureus. J Bacteriol. 1972;111:272–283. doi: 10.1128/jb.111.1.272-283.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goller CC, Romeo T. Environmental influences on biofilm development. Curr Top Microbiol Immunol. 2008;322:37–66. doi: 10.1007/978-3-540-75418-3_3. [DOI] [PubMed] [Google Scholar]
- Grande R, Di Giulio M, Bessa LJ, Di Campli E, Baffoni M, Guarnieri S, Cellini L. Extracellular DNA in Helicobacter pylori biofilm: a backstairs rumour. J Appl Microbiol. 2010;110:490–498. doi: 10.1111/j.1365-2672.2010.04911.x. [DOI] [PubMed] [Google Scholar]
- Grimberg J, Maguire S, Belluscio L. A simple method for the preparation of plasmid and chromosomal E. coli DNA. Nucleic Acids Res. 1989;17:8893. doi: 10.1093/nar/17.21.8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman L-M, Beblin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose pBAD promotor. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Stoodley L, Stoodley P. Biofilm formation and dispersal and the transmission of human pathogens. Trends Microbiol. 2005;13:7–10. doi: 10.1016/j.tim.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Hammer BK, Bassler BL. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol Microbiol. 2003;50:101–104. doi: 10.1046/j.1365-2958.2003.03688.x. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Harmsen M, Lappann M, Knochel S, Molin S. Role of extracellular DNA during biofilm formation by Listeria monocytogenes. Appl Environ Microbiol. 2010;76:2271–2279. doi: 10.1128/AEM.02361-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley DM, Morris JG, Jr, Smith DL. Hyperinfectivity: a critical element in the ability of V. cholerae to cause epidemics? PLoS Med. 2006;3:e7. doi: 10.1371/journal.pmed.0030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugo AJ, Watnick PI. Vibrio cholerae CytR is a repressor of biofilm development. Mol Microbiol. 2002;45:471–483. doi: 10.1046/j.1365-2958.2002.03023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidelberg JF, Eisen JA, Nelson WC, Clayton RA, Gwinn ML, Dodson RJ, et al. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature. 2000;406:477–483. doi: 10.1038/35020000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersboll BK, Molin S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology. 2000;146:2395–2407. doi: 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]
- Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- Houot L, Chang S, Pickering BS, Absalon C, Watnick PI. The phosphoenolpyruvate phosphotransferase system regulates Vibrio cholerae biofilm formation through multiple independent pathways. J Bacteriol. 2010;192:3055–3067. doi: 10.1128/JB.00213-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff E, Silverman CS, Adams NJ, Awkard WS. Extracellular cell wall lytic enzyme from Staphylococcus aureus: purification and partial characterization. J Bacteriol. 1970;103:761–769. doi: 10.1128/jb.103.3.761-769.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo A, Xu B, Chowdhury MA, Islam MS, Montilla R, Colwell RR. A simple filtration method to remove plankton-associated Vibrio cholerae in raw water supplies in developing countries. Appl Environ Microbiol. 1996;62:2508–2512. doi: 10.1128/aem.62.7.2508-2512.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq A, Colwell RR, Rahman R, Ali A, Chowdhury MA, Parveen S, et al. Detection of Vibrio cholerae O1 in the aquatic environment by fluorescent-monoclonal antibody and culture methods. Appl Environ Microbiol. 1990;56:2370–2373. doi: 10.1128/aem.56.8.2370-2373.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huq A, Whitehouse CA, Grim CJ, Alam M, Colwell RR. Biofilms in water, its role and impact in human disease transmission. Curr Opin Biotechnol. 2008;19:244–247. doi: 10.1016/j.copbio.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Islam MS, Drasar BS, Sack RB. The aquatic environment as a reservoir of Vibrio cholerae: a review. J Diarrhoeal Dis Res. 1993;11:197–206. [PubMed] [Google Scholar]
- Karatan E, Watnick P. Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol Mol Biol Rev. 2009;73:310–347. doi: 10.1128/MMBR.00041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierek K, Watnick PI. Environmental determinants of Vibrio cholerae biofilm development. Appl Environ Microbiol. 2003;69:5079–5088. doi: 10.1128/AEM.69.9.5079-5088.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch R. An address on cholera and its bacillus. Br Med J. 1884;2:403–407. doi: 10.1136/bmj.2.1235.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolter R, Inuzuka M, Helinski DR. Trans-complementation-dependent replication of a low molecular weight origin fragment from plasmid R6K. Cell. 1978;15:1199–1208. doi: 10.1016/0092-8674(78)90046-6. [DOI] [PubMed] [Google Scholar]
- Kondo K, Takade A, Amako K. Release of the outer membrane vesicles from Vibrio cholerae and Vibrio parahaemolyticus. Microbiol Immunol. 1993;37:149–152. doi: 10.1111/j.1348-0421.1993.tb03192.x. [DOI] [PubMed] [Google Scholar]
- Krasteva PV, Fong JC, Shikuma NJ, Beyhan S, Navarro MV, Yildiz FH, Sondermann H. Vibrio cholerae VpsT regulates matrix production and motility by directly sensing cyclic di-GMP. Science. 2010;327:866–868. doi: 10.1126/science.1181185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreth J, Vu H, Zhang Y, Herzberg MC. Characterization of hydrogen peroxide-induced DNA release by Streptococcus sanguinis and Streptococcus gordonii. J Bacteriol. 2009;191:6281–6291. doi: 10.1128/JB.00906-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kruger WM, Humphreys S, Ketley JM. A role for the PhoBR regulatory system homologue in the Vibrio cholerae phosphate-limitation response and intestinal colonization. Microbiology. 1999;145:2463–2475. doi: 10.1099/00221287-145-9-2463. [DOI] [PubMed] [Google Scholar]
- Lappann M, Claus H, van Alen T, Harmsen M, Elias J, Molin S, Vogel U. A dual role of extracellular DNA during biofilm formation of Neisseria meningitidis. Mol Microbiol. 2010;75:1355–1371. doi: 10.1111/j.1365-2958.2010.07054.x. [DOI] [PubMed] [Google Scholar]
- Lauriano CM, Ghosh C, Correa NE, Klose KE. The sodium-driven flagellar motor controls exopolysaccharide expression in Vibrio cholerae. J Bacteriol. 2004;186:4864–4874. doi: 10.1128/JB.186.15.4864-4874.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Stirling FR, Zhu J. Temporal quorum-sensing induction regulates Vibrio cholerae biofilm architecture. Infect Immun. 2007;75:122–126. doi: 10.1128/IAI.01190-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Miyashiro T, Tsou A, Hsiao A, Goulian M, Zhu J. Mucosal penetration primes Vibrio cholerae for host colonization by repressing quorum sensing. Proc Natl Acad Sci USA. 2008;105:9769–9774. doi: 10.1073/pnas.0802241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann EE, Rice KC, Boles BR, Endres JL, Ranjit D, Chandramohan L, et al. Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS ONE. 2009;4:e5822. doi: 10.1371/journal.pone.0005822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meibom KL, Li XB, Nielsen AT, Wu CY, Roseman S, Schoolnik GK. The Vibrio cholerae chitin utilization program. Proc Natl Acad Sci USA. 2004;101:2524–2529. doi: 10.1073/pnas.0308707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meibom KL, Blokesch M, Dolganov NA, Wu CY, Schoolnik GK. Chitin induces natural competence in Vibrio cholerae. Science. 2005;310:1824–1827. doi: 10.1126/science.1120096. [DOI] [PubMed] [Google Scholar]
- Miller VL, Mekalanos JJ. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisi M, Jenul C, Butler SM, New A, Tutz S, Reidl J, et al. A novel regulatory protein involved in motility of Vibrio cholerae. J Bacteriol. 2009;191:7027–7038. doi: 10.1128/JB.00948-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol CD, Kuo CF, Thayer MM, Cunningham RP, Tainer JA. Structure and function of the multifunctional DNA-repair enzyme exonuclease III. Nature. 1995;374:381–386. doi: 10.1038/374381a0. [DOI] [PubMed] [Google Scholar]
- Moorthy S, Watnick PI. Genetic evidence that the Vibrio cholerae monolayer is a distinct stage in biofilm development. Mol Microbiol. 2004;52:573–587. doi: 10.1111/j.1365-2958.2004.04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorthy S, Watnick PI. Identification of novel stage-specific genetic requirements through whole genome transcription profiling of Vibrio cholerae biofilm development. Mol Microbiol. 2005;57:1623–1635. doi: 10.1111/j.1365-2958.2005.04797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales VM, Backman A, Bagdasarian M. A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene. 1991;97:39–47. doi: 10.1016/0378-1119(91)90007-x. [DOI] [PubMed] [Google Scholar]
- Moscoso M, Garcia E, Lopez R. Biofilm formation by Streptococcus pneumoniae: role of choline, extracellular DNA, and capsular polysaccharide in microbial accretion. J Bacteriol. 2006;188:7785–7795. doi: 10.1128/JB.00673-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulcahy H, Charron-Mazenod L, Lewenza S. Pseudomonas aeruginosa produces an extracellular deoxyribonuclease that is required for utilization of DNA as a nutrient source. Environ Microbiol. 2010;12:1621–1629. doi: 10.1111/j.1462-2920.2010.02208.x. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Higashiyama Y, Izumikawa K, Seki M, Kakeya H, Yamamoto Y, et al. The roles of the quorum-sensing system in the release of extracellular DNA, lipopolysaccharide, and membrane vesicles from Pseudomonas aeruginosa. Jpn J Infect Dis. 2008;61:375–378. [PubMed] [Google Scholar]
- Nalin DR, Levine RJ, Levine MM, Hoover D, Bergquist E, McLaughlin J, et al. Cholera, non-vibrio cholera, and stomach acid. Lancet. 1978;2:856–859. doi: 10.1016/s0140-6736(78)91568-4. [DOI] [PubMed] [Google Scholar]
- Nalin DR, Daya V, Reid A, Levine MM, Cisneros L. Adsorption and growth of Vibrio cholerae on chitin. Infect Immun. 1979;25:768–770. doi: 10.1128/iai.25.2.768-770.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EJ, Chowdhury A, Flynn J, Schild S, Bourassa L, Shao Y, et al. Transmission of Vibrio cholerae is antagonized by lytic phage and entry into the aquatic environment. PLoS Pathog. 2008;4:e1000187. doi: 10.1371/journal.ppat.1000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EJ, Harris JB, Morris JG, Jr, Calderwood SB, Camilli A. Cholera transmission: the host, pathogen and bacteriophage dynamic. Nat Rev Microbiol. 2009;7:693–702. doi: 10.1038/nrmicro2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland JW, Green BA, Foulds J, Holmes RK. Cloning of extracellular DNase and construction of a DNase-negative strain of Vibrio cholerae. Infect Immun. 1985;47:691–696. doi: 10.1128/iai.47.3.691-696.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niiranen L, Altermark B, Brandsdal BO, Leiros HK, Helland R, Smalas AO, Willassen NP. Effects of salt on the kinetics and thermodynamic stability of endonuclease I from Vibrio salmonicida and Vibrio cholerae. FEBS J. 2008;275:1593–1605. doi: 10.1111/j.1742-4658.2008.06317.x. [DOI] [PubMed] [Google Scholar]
- Oshida T, Sugai M, Komatsuzawa H, Hong YM, Suginaka H, Tomasz A. A Staphylococcus aureus autolysin that has an N-acetylmuramoyl-L-alanine amidase domain and an endo-beta-N-acetylglucosaminidase domain: cloning, sequence analysis, and characterization. Proc Natl Acad Sci USA. 1995;92:285–289. doi: 10.1073/pnas.92.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JT. Why does Escherichia coli recycle its cell wall peptides? Mol Microbiol. 1995;17:421–426. doi: 10.1111/j.1365-2958.1995.mmi_17030421.x. [DOI] [PubMed] [Google Scholar]
- Paul JH, Jeffrey WH, DeFlaun MF. Dynamics of extracellular DNA in the marine environment. Appl Environ Microbiol. 1987;53:170–179. doi: 10.1128/aem.53.1.170-179.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt JT, McDonough E, Camilli A. PhoB regulates motility, biofilms, and cyclic di-GMP in Vibrio cholerae. J Bacteriol. 2009;191:6632–6642. doi: 10.1128/JB.00708-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzzo C, Vezzulli L, Colwell RR. Global impact of Vibrio cholerae interactions with chitin. Environ Microbiol. 2008;10:1400–1410. doi: 10.1111/j.1462-2920.2007.01559.x. [DOI] [PubMed] [Google Scholar]
- Reguera G, Kolter R. Virulence and the environment: a novel role for Vibrio cholerae toxin-coregulated pili in biofilm formation on chitin. J Bacteriol. 2005;187:3551–3555. doi: 10.1128/JB.187.10.3551-3555.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidl J, Klose KE. Vibrio cholerae and cholera: out of the water and into the host. FEMS Microbiol Rev. 2002;26:125–139. doi: 10.1111/j.1574-6976.2002.tb00605.x. [DOI] [PubMed] [Google Scholar]
- Reisner A, Holler BM, Molin S, Zechner EL. Synergistic effects in mixed Escherichia coli biofilms: conjugative plasmid transfer drives biofilm expansion. J Bacteriol. 2006;188:3582–3588. doi: 10.1128/JB.188.10.3582-3588.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renelli M, Matias V, Lo RY, Beveridge TJ. DNA-containing membrane vesicles of Pseudomonas aeruginosa PAO1 and their genetic transformation potential. Microbiology. 2004;150:2161–2169. doi: 10.1099/mic.0.26841-0. [DOI] [PubMed] [Google Scholar]
- Roberts A, Pearson GD, Mekalanos JJ. Cholera vaccines strains derived from a 1991 Peruvian isolate of Vibrio cholerae and other El Tor strains, pp. 43–47. Proc. 28th Joint Conf. U.S.-Jpn. Coop. Med- Sci. Program Cholera Relat. Diarrh. Dis. 1992 [Google Scholar]
- Ryan ET. The cholera pandemic, still with us after half a century: time to rethink. PLoS Negl Trop Dis. 2011;5:e1003. doi: 10.1371/journal.pntd.0001003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack DA, Sack RB, Nair GB, Siddique AK. Cholera. Lancet. 2004;363:223–233. doi: 10.1016/s0140-6736(03)15328-7. [DOI] [PubMed] [Google Scholar]
- Schild S, Lamprecht AK, Reidl J. Molecular and functional characterization of O antigen transfer in Vibrio cholerae. J Biol Chem. 2005;280:25936–25947. doi: 10.1074/jbc.M501259200. [DOI] [PubMed] [Google Scholar]
- Schild S, Tamayo R, Nelson EJ, Qadri F, Calderwood SB, Camilli A. Genes induced late in infection increase fitness of Vibrio cholerae after release into the environment. Cell Host Microbe. 2007;2:264–277. doi: 10.1016/j.chom.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild S, Bishop AL, Camilli A. Ins and Outs of Vibrio cholerae. Microbe. 2008a;3:131–136. [Google Scholar]
- Schild S, Nelson EJ, Camilli A. Immunization with Vibrio cholerae outer membrane vesicles induces protective immunity in mice. Infect Immun. 2008b;76:4554–4563. doi: 10.1128/IAI.00532-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild S, Nelson EJ, Bishop AL, Camilli A. Characterization of Vibrio cholerae outer membrane vesicles as a candidate vaccine for cholera. Infect Immun. 2009;77:472–484. doi: 10.1128/IAI.01139-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleheck D, Barraud N, Klebensberger J, Webb JS, McDougald D, Rice SA, Kjelleberg S. Pseudomonas aeruginosa PAO1 preferentially grows as aggregates in liquid batch cultures and disperses upon starvation. PLoS ONE. 2009;4:e5513. doi: 10.1371/journal.pone.0005513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer HJ, Wise EM, Jr, Park JT. Properties and purification of N-acetylmuramyl-L-alanine amidase from Staphylococcus aureus H. J Bacteriol. 1972;112:932–939. doi: 10.1128/jb.112.2.932-939.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberger RE, Holden PA. Extracellular DNA in single- and multiple-species unsaturated biofilms. Appl Environ Microbiol. 2005;71:5404–5410. doi: 10.1128/AEM.71.9.5404-5410.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg C, Tolker-Nielsen T. Growing and analyzing biofilms in flow cells. Curr Protoc Microbiol. 2006 doi: 10.1002/9780471729259.mc01b02s00. Chapter 1: Unit 1B.2. [DOI] [PubMed] [Google Scholar]
- Sternberg C, Christensen BB, Johansen T, Toftgaard Nielsen A, Andersen JB, Givskov M, Molin S. Distribution of bacterial growth activity in flow-chamber biofilms. Appl Environ Microbiol. 1999;65:4108–4117. doi: 10.1128/aem.65.9.4108-4117.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamayo R, Schild S, Pratt JT, Camilli A. Role of cyclic Di-GMP during el tor biotype Vibrio cholerae infection: characterization of the in vivo-induced cyclic Di-GMP phosphodiesterase CdpA. Infect Immun. 2008;76:1617–1627. doi: 10.1128/IAI.01337-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamayo R, Patimalla B, Camilli A. Growth in a biofilm induces a hyperinfectious phenotype in Vibrio cholerae. Infect Immun. 2010;78:3560–3569. doi: 10.1128/IAI.00048-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamplin ML, Gauzens AL, Huq A, Sack DA, Colwell RR. Attachment of Vibrio cholerae serogroup O1 to zooplankton and phytoplankton of Bangladesh waters. Appl Environ Microbiol. 1990;56:1977–1980. doi: 10.1128/aem.56.6.1977-1980.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas VC, Thurlow LR, Boyle D, Hancock LE. Regulation of autolysis-dependent extracellular DNA release by Enterococcus faecalis extracellular proteases influences biofilm development. J Bacteriol. 2008;190:5690–5698. doi: 10.1128/JB.00314-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper DJ. Mechanism of autolysis of isolated cell walls of Staphylococcus aureus. J Bacteriol. 1969;97:837–847. doi: 10.1128/jb.97.2.837-847.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischler AD, Camilli A. Cyclic diguanylate (c-di-GMP) regulates Vibrio cholerae biofilm formation. Mol Microbiol. 2004;53:857–869. doi: 10.1111/j.1365-2958.2004.04155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischler AD, Camilli A. Cyclic diguanylate regulates Vibrio cholerae virulence gene expression. Infect Immun. 2005;73:5873–5882. doi: 10.1128/IAI.73.9.5873-5882.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevors JT. DNA in soil: adsorption, genetic transformation, molecular evolution and genetic microchip. Antonie Van Leeuwenhoek. 1996;70:1–10. doi: 10.1007/BF00393564. [DOI] [PubMed] [Google Scholar]
- Udden SM, Zahid MS, Biswas K, Ahmad QS, Cravioto A, Nair GB, et al. Acquisition of classical CTX prophage from Vibrio cholerae O141 by El Tor strains aided by lytic phages and chitin-induced competence. Proc Natl Acad Sci USA. 2008;105:11951–11956. doi: 10.1073/pnas.0805560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassov VV, Laktionov PP, Rykova EY. Extracellular nucleic acids. Bioessays. 2007;29:654–667. doi: 10.1002/bies.20604. [DOI] [PubMed] [Google Scholar]
- Vollmer W, Joris B, Charlier P, Foster S. Bacterial peptidoglycan (murein) hydrolases. FEMS Microbiol Rev. 2008;32:259–286. doi: 10.1111/j.1574-6976.2007.00099.x. [DOI] [PubMed] [Google Scholar]
- Waters CM, Lu W, Rabinowitz JD, Bassler BL. Quorum sensing controls biofilm formation in Vibrio cholerae through modulation of cyclic di-GMP levels and repression of vpsT. J Bacteriol. 2008;190:2527–2536. doi: 10.1128/JB.01756-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watnick P, Kolter R. Steps in the develompent of a Vibrio cholerae El Tor biofilm. Mol Microbiol. 1999;34:586–595. doi: 10.1046/j.1365-2958.1999.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watnick PI, Fullner KJ, Kolter R. A Role for the Mannose-Sensitive Hemagglutinin in Biofilm Formation by Vibrio cholerae El Tor. J Bacteriol. 1999;181:3606–3609. doi: 10.1128/jb.181.11.3606-3609.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watnick PI, Lauriano CM, Klose KE, Croal L, Kolter R. The absence of a flagellum leads to altered colony morphology, biofilm development and virulence in Vibrio cholerae O139. Mol Microbiol. 2001;39:223–235. doi: 10.1046/j.1365-2958.2001.02195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. Extracellular DNA required for bacterial biofilm formation. Science. 2002;295:1487. doi: 10.1126/science.295.5559.1487. [DOI] [PubMed] [Google Scholar]
- WHO. Cholera, 2008. Wkly Epidemiol Rec. 2009;31:309–324. [WWW document]. URL http://www.who.int/wer. [PubMed] [Google Scholar]
- Yang M, Frey EM, Liu Z, Bishar R, Zhu J. The virulence transcriptional activator AphA enhances biofilm formation of Vibrio cholerae by activating the expression of the biofilm regulator VpsT. Infect Immun. 2009;78:697–703. doi: 10.1128/IAI.00429-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Beyenal H, Harkin G, Lewandowski Z. Quantifying biofilm structure using image analysis. J Microbiol Methods. 2000;39:109–119. doi: 10.1016/s0167-7012(99)00097-4. [DOI] [PubMed] [Google Scholar]
- Yaron S, Kolling GL, Simon L, Matthews KR. Vesicle-mediated transfer of virulence genes from Escherichia coli O157:H7 to other enteric bacteria. Appl Environ Microbiol. 2000;66:4414–4420. doi: 10.1128/aem.66.10.4414-4420.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz FH, Schoolnik GK. Vibrio cholerae O1 El Tor: Identification of a gene cluster required for the rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proc Natl Acad Sci USA. 1999;96:4028–4033. doi: 10.1073/pnas.96.7.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz FH, Visick KL. Vibrio biofilms: so much the same yet so different. Trends Microbiol. 2009;17:109–118. doi: 10.1016/j.tim.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz FH, Dolganov NA, Schoolnik GK. VpsR, a Member of the Response Regulators of the Two-Component Regulatory Systems, Is Required for Expression of vps Biosynthesis Genes and EPS(ETr)-Associated Phenotypes in Vibrio cholerae O1 El Tor. J Bacteriol. 2001;183:1716–1726. doi: 10.1128/JB.183.5.1716-1726.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildiz FH, Liu XS, Heydorn A, Schoolnik GK. Molecular analysis of rugosity in a Vibrio cholerae O1 El Tor phase variant. Mol Microbiol. 2004;53:497–515. doi: 10.1111/j.1365-2958.2004.04154.x. [DOI] [PubMed] [Google Scholar]
- Zhu J, Mekalanos JJ. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Dev Cell. 2003;5:647–656. doi: 10.1016/s1534-5807(03)00295-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


