Abstract
The majority of strains of Toxoplasma gondii belong to three distinct clonal lines known as types I, II, and III. The outcome of the immune response to infection is influenced by the parasite strain type. The goal of this study was to examine differences in the kinetics of gene expression in microglial cells infected with types I, II, or III of T. gondii. In addition, a requirement for the integrity of host Toll-like receptor (TLR) signaling in parasite-mediated changes in gene expression was evaluated. Wild type murine microglial cells infected with T. gondii displayed different kinetic patterns of pro-inflammatory cytokine expression that were dependent on the parasite strain type. In general, types II and III elicited higher sustained responses compared to type I which induced fluctuating patterns of cytokine gene expression. Contrary to this, differences in the induction of anti-apoptotic gene expression were minimal among the different type strains throughout infection. Experiments with cells lacking the TLR adaptor molecules MAL and Myd88 showed a dependency on these factors for the pro-inflammatory response but not the anti-apoptotic response. The results show that the outcome of gene expression in T. gondii-infected microglial cells is dependent on the parasite strain type in a time-dependent manner and is selective to particular subsets of genes. The induction of an anti-apoptotic response by T. gondii infection in the absence of TLR signaling reflects a complex level of modulation of host functions by the parasite.
Keywords: Toxoplasma gondii, type strain, virulence, inflammatory response, microglia, MAL, Myd88, NF-κB
1. Introduction
Toxoplasma gondii is an obligate intracellular protozoan parasite capable of establishing a life-long chronic infection in the host. The outcome of infection is greatly influenced by the parasite strain type: type I strains are highly virulent in mice (LD100 = 1) while types II and III are less virulent (LD100 > 1000) and as such are able to establish chronic infections in vivo (Sibley et al., 1992; Saeij et al., 2005; Sibley et al., 2009). Recent studies have revealed that the molecular basis of Toxoplasma virulence is highly dependent on the activity of parasite proteins localized to specialized secretory organelles termed rhoptries (Saeij et al., 2006, 2007; Taylor et al., 2006; Ong et al., 2010).
Virulence properties of different T. gondii strains are also influenced by differences in the activation of innate immune responses during infection (Robben et al., 2004; Kim et al., 2006; Lee et al., 2007; Steinfeldt et al., 2010). Similar to other pathogens, immune recognition of T. gondii by the host is mediated by the TLR family (Mun et al., 2003; Debierre-Grockiego et al., 2007; Plattner et al., 2008; Yarovinsky 2008). Signaling through the TLR pathway ultimately leads to gene activation by the NF-κB family of transcription factors (Vallabhapurapu, 2009). Previous studies have shown that the divergent virulence phenotypes observed in T. gondii infection may be explained by strain-dependent differences in TLR signaling (Kim et al., 2006; Lee et al., 2007) and extent of NF-κB activation (Robben et al., 2004; Rosowski et al., 2011). In this respect, induction of IL-12 and chemokines in macrophages by the type I RH strain was shown to be independent of the TLR adaptor molecule Myd88 as compared to types II and III (Kim et al., 2006; Lee et al., 2007). A recent study by Rosowski et al. showed that type II strains induce higher levels of NF-κB translocation and NF-κB-dependent gene expression compared to types I and III, a property dependent on the parasite dense granule protein GRA15 but independent of Myd88 (Rosowski et al., 2011).
Following the acute phase of T. gondii infection, the parasite forms cysts in response to immune pressure preferentially in the brain and establishes a chronic infection (Carruthers and Suzuki, 2007). Parasite genotype, host genetic background, and ability to modulate signaling components that regulate the immune response influence the effects of infection in the brain (Carruthers and Suzuki, 2007). Microglia are resident macrophages of the brain and spinal cord, consequently functioning as the initial responders of immune defense in the central nervous system. There are limited reports on the interactions of T. gondii with microglial cells in vitro (Chao et al., 1993a,b, 1993), particularly in cells derived from knockout mice which are not always widely available.
To gain a better understanding of the interplay between parasite genotype and host signaling components in the response of microglia to infection, the present study examined gene expression profiles induced by different strains of T. gondii in microglial cells on a temporal fashion. Special emphasis was placed on the requirement for the integrity of host TLR signaling in the regulation of expression of pro-inflammatory cytokine and anti-apoptotic genes. Experiments were focused primarily on the following NF-κB target genes: (i) the chemokines IP-10 and RANTES which have been examined as model genes to study temporal responses of NF-κB activation (Hoffmann et al., 2002); (ii) interleukin-12, a critical regulator of the immune response to T. gondii infection (Sher et al., 2003); and (iii) Bfl-1, an anti-apoptotic factor previously shown to be induced by T. gondii in an NF-κB-dependent manner (Molestina et al., 2003).
2. Methods
2.1. Cell lines
Murine microglial cells were derived from brain tissue of wild type (WT) or MAL/MyD88 double knockout (MAL−/−Myd88−/−) C57BL/6J mice. Cells were immortalized by infection with the ecotropic transforming replication-deficient retrovirus J2 using established procedures (Blasi et al., 1985, 1990). Cultures were maintained at 37°C with 5% CO2 in Dulbecco’s Modified Eagle Medium (Invitrogen™, Carlsbad, CA) supplemented with 10% fetal bovine serum (Lonza, Allendale, NJ), 100 U/ml penicillin (ATCC®, Manassas, VA), 100 µg/ml streptomycin (ATCC®), 2 mM L-glutamine, and 1 mM sodium pyruvate (Invitrogen™). Murine microglial cell lines are available at the Biodefense and Emerging Infections Research Resources Repository (BEI Resources; www.beiresources.org) under catalog numbers NR-9460 (WT) and NR-9904 (MAL−/−Myd88−/−).
2.2. Parasite strains
T. gondii GT1-FUDR3.3 (type I strain; BEI # NR-10272), ME49 B7 (type II strain; BEI # NR-10150), and CTG ARA-SYN (type III strain; BEI # NR-10151) were propagated in human foreskin fibroblasts (ATCC® CRL-1634™). Cultures were maintained at 37°C with 5% CO2 in Minimum Essential Medium Alpha (Invitrogen™) supplemented with 10% fetal bovine serum (ATCC®).
2.3. RT-PCR
Microglial cells were seeded in six-well plates at 2 × 106 cells per well and allowed to adhere overnight. Selected wells contained cells grown on sterile 12 mm coverslips for immunofluorescence assays as described in section 2.5. Microglial cells were infected with T. gondii at a multiplicity of infection (m.o.i.) of 5:1 for 2, 4, 8, 12, and 24 h. Total RNA was isolated with the RNeasy® kit (QIAGEN, Valencia, CA). cDNA was prepared from 1 µg of RNA using the ImProm-II™ RT System (Promega, Madison, WI). A 0.5 µl sample of cDNA was used as template for PCR using primers purchased from Integrated DNA Technologies (IDT®, Coralville, IA). Primers against mouse β-actin were purchased from R&D Systems® (Minneapolis, MN). A list of the target genes, primer sequences, and expected sizes of the PCR amplicons is shown in Table S1. PCR reactions were performed with the Platinum® PCR SuperMix High Fidelity kit (Invitrogen™) using the following conditions: 95°C for 5 min followed by 30 cycles of 95°C for 30 sec, 58°C for 30 sec, and 72°C for 30 sec. PCR products were resolved by agarose gel electrophoresis in the presence of SYBR® Safe DNA gel stain (Invitrogen™) and visualized with a Bio-Rad Gel Doc™ XR System (Bio-Rad, Hercules, CA).
2.4. Analysis of host gene expression
To determine differences in host gene expression after infection, densitometric analyses of DNA bands obtained in the RT-PCR experiments were performed using the ImageJ software (http://rsbweb.nih.gov/ij/). Integrated densitometric values (IDV) were normalized to β-actin and the numerical data were subjected to analysis of variance followed by the Tukey’s multiple-comparison test using the GraphPad Prism software. A P value of <0.05 was used to determine statistical significance. Data were collected from three independent experiments.
2.5. Analysis of parasite infection
The progression of T. gondii infection in microglial cells was examined by monitoring the expression of the parasite SAG1 gene by RT-PCR. SAG1 primers are indicated in Table S1 and conditions for RT-PCR were followed as indicated in section 2.3 above. Parasite growth was also examined by immunofluorescence assay. Coverslips from wells infected for 24 h were removed prior to RNA isolation and washed in 1X PBS. Cells were fixed in 3% paraformaldehyde and permeabilized in 0.1% Triton X-100 in PBS. Immunofluorescence labeling was performed with a mouse monoclonal antibody against the T. gondii SAG1 protein (Argene, Shirley, NY) diluted 1:1000 in 3% bovine serum albumin/PBS. Secondary labeling was performed with an Alexa Fluor® 594-conjugated goat anti-mouse IgG (Invitrogen™) diluted at 1:2000. Samples were examined using an Axioskop® fluorescence microscope (Zeiss, Oberkochen, Germany) at 100× magnification.
3. Results and discussion
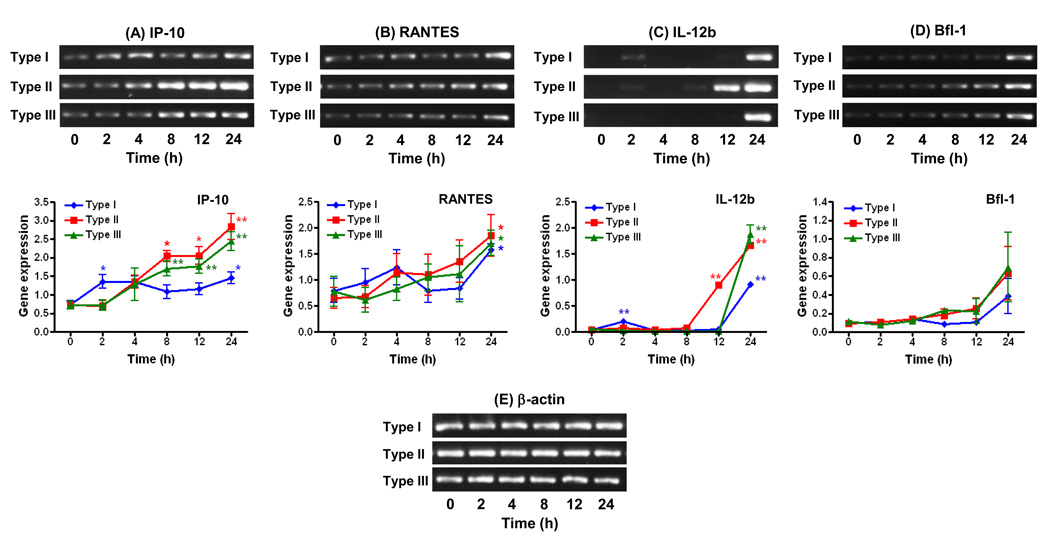
T. gondii-infected WT microglial cells displayed divergent kinetic patterns of gene expression that were influenced by the parasite genotype, most notably among the pro-inflammatory cytokines IP-10, RANTES, and IL-12b (Figure 1A–C). The expression of IP-10 in type I (GT1-FUDR3.3)-infected cells was increased at 2 h post-infection (p.i.) (Fig. 1A). This response was significantly higher than infection with the type II (ME49 B7) or type III (CTG ARA-SYN) strains (P < 0.05) which resulted in a delay in IP-10 induction (4 h, Fig. 1A). Of note, levels of IP-10 expression fluctuated during the 24 h course of infection in type I-infected cells. In contrast, increases in IP-10 expression were higher and more sustained in response to types II and type III compared to type I between 8 and 24 h of infection (Fig. 1A; P < 0.05 when comparing type I versus type II responses).
Figure. 1.
Effects of parasite strain type on the kinetics of pro-inflammatory cytokine and anti-apoptotic gene expression in WT microglial cells infected with T. gondii. Cells were infected with type I (GT1-FUDR3.3), type II (ME49 B7), or type III (CTG ARA-SYN) strains of T. gondii for 2, 4, 8, 12, and 24 h. RT-PCR amplification and analysis of gene expression of IP-10 (A), RANTES (B), IL-12b (C), Bfl-1 (D), and β-actin (E) were performed as described in section 2.3. Graphs represent levels of gene expression following normalization of the densitometric values of DNA bands to β-actin. Data points represent the means ± standard errors of the mean of three experiments. *, P < 0.05; **, P < 0.01 compared to uninfected cells (0 h time point).
Contrary to IP-10, the kinetics of RANTES expression showed only slight increases during the first 8 h of infection regardless of the strain examined (Fig. 1B). Robust expression of RANTES was not apparent until 12 h p.i. with type II and 24 h with type I and type III strains (Fig. 1B). A fluctuating pattern of RANTES expression was also observed in type I-infected WT cells, although not as marked as with IP-10.
Changes in IL-12b expression in response to T. gondii were negligible above baseline between 2 and 8 h of infection with the exception of the type I strain which induced an increase at 2 h p.i. (Fig. 1C). At 12 h, however, a sharp increase in IL-12b expression was observed in type II-infected WT cells only (P < 0.01 as compared to type I or type III). In contrast, induction of IL-12b was delayed to 24 h with type I and III strains (Fig. 1C). In general, as observed with IP-10 (Fig. 1A), type II and type III strains elicited higher IL-12b responses compared to the type I strain (P < 0.05).
As opposed to the pro-inflammatory cytokines, differences between the stimulatory activities of the three Toxoplasma strains were less pronounced with the anti-apoptotic factor Bfl-1 (Fig. 1D). Small increases with type II and III strains were apparent at 8 h of infection with both strains eliciting similar sustained responses up to 24 h. Contrary to this, the induction of Bfl-1 in response to infection with the type I strain was delayed to 24 h (Fig. 1D).
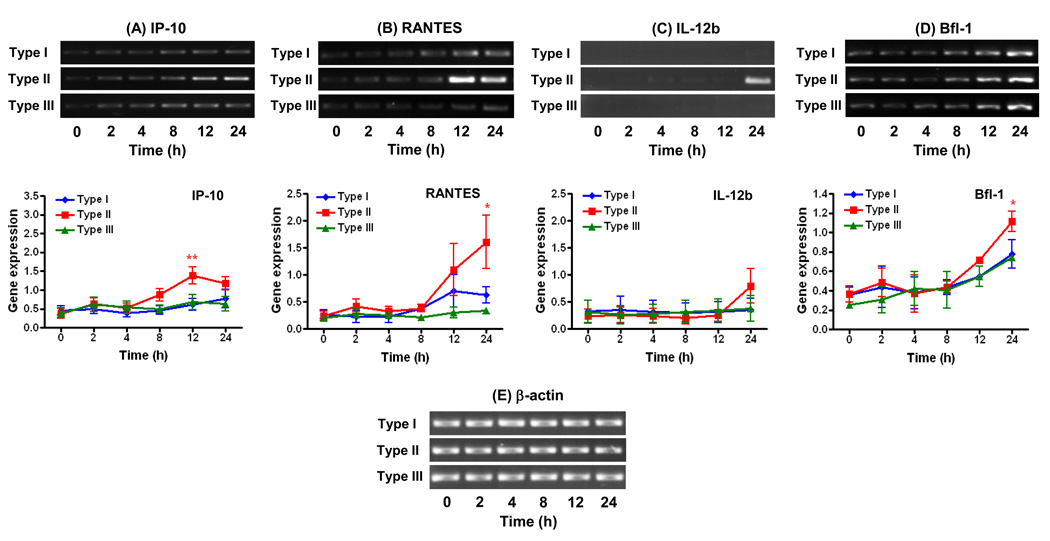
Experiments with microglial cells lacking the TLR adaptor molecules MAL and Myd88 showed profound effects in gene expression induced by T. gondii infection; however, the extent of the host response was dependent on the parasite strain type and selective to particular genes (Fig. 2). Type strains I and III were highly dependent on MAL and Myd88 compared to strain II to stimulate the expression of IP-10 (Fig. 2A), RANTES (Fig. 2B), and IL-12b (Fig. 2C). The type II strain on the other hand maintained the ability to induce expression of these genes in MAL−/−Myd88−/− cells albeit not at the same extent as in WT cells for IP-10 and IL-12b. As opposed to the pro-inflammatory cytokines, the expression of the anti-apoptotic factor Bfl-1 was unaffected by the absence of MAL/Myd88 in response to infection with type strains I, II, or III (Fig. 2D).
Figure 2.
Effects of parasite strain type on the kinetics of cytokine and anti-apoptotic gene expression in MAL−/−Myd88−/− microglial cells infected with T. gondii. Cells were infected with type I (GT1-FUDR3.3), type II (ME49 B7), or type III (CTG ARA-SYN) strains of T. gondii for 2, 4, 8, 12, and 24 h. RT-PCR amplification and analysis of gene expression of IP-10 (A), RANTES (B), IL-12b (C), Bfl-1 (D), and β-actin (E) were performed as described in section 2.3. Graphs represent levels of gene expression following normalization of the densitometric values of DNA bands to β-actin. Data points represent the means ± standard errors of the mean of three experiments. *, P < 0.05; **, P < 0.01 compared to uninfected cells (0 h time point).
Of note, both WT and MAL/Myd88 knockout microglial cells were equally capable of supporting infection of all three strains of T. gondii as determined by the kinetics of expression the parasite protein SAG1 (Fig. S1) and immunofluorescence assays performed at 24 h p.i. (Data not shown). Thus, the observed divergent gene expression profiles in the host cells could not be explained by striking differences between the growth patterns of the three strains. Overall, an m.o.i. of 5:1 resulted in the detection of parasitophorous vacuoles (PV) in 40 to 50% of cells examined by immunofluorescence, regardless of the cell line or parasite strain. Greater than 90% of infected WT or MAL/Myd88 knockout cells displayed single PV. The average percentages of PV harboring ≤ 2 tachyzoites for both cell lines were 60% for type I, 75% for type II, and 40% for the type III strain. The average percentages of PV harboring ≥ 4 tachyzoites per PV for both cell lines were 40% for type I, 25% for type II, and 60% for the type III strain.
Results from this study demonstrate that the outcome of gene expression in T. gondii-infected microglial cells is determined by the parasite strain type in a time-dependent manner and is geared towards particular subsets of genes. Strain-dependent differences in gene expression of WT microglia were more prominent among pro-inflammatory genes as compared to the anti-apoptotic factor Bfl-1. In addition, the regulation of certain pro-inflammatory genes (IP-10 and RANTES) displayed fluctuating patterns of expression over time; especially in response to a type I strain (Fig. 1A–B). Such oscillating patterns of inflammatory gene activation during the course of T. gondii infection have been reported previously and correlate with waves of activation and de-activation of NF-κB in the host cell (Molestina et al., 2005). Contrary to the type II strain, the induction of pro-inflammatory responses by types I and III were highly dependent on MAL/Myd88. The ability of the type II strain to retain partial activation of these genes in the absence of Myd88 signaling in microglial cells is likely attributed to the polymorphic parasite protein GRA15: Rosowski et al. reported that GRA15 from type II strains is a key factor promoting higher levels of NF-κB activation and resulting gene expression compared to types I and III, a phenotype which was found to be independent of host Myd88 (Rosowski et al., 2011).
As opposed to the inflammatory response, the induction of Bfl-1 in microglial cells by T. gondii infection was not dependent on MAL/Myd88. It remains to be determined whether these results reflect a role for a MAL/Myd88-independent mechanism in the establishment of an anti-apoptotic phenotype in T. gondii-infected cells. In contrast to Myd88, there are no reports describing a role of MAL in the host immune response to T. gondii to our knowledge. MAL functions as a bridging adaptor for Myd88 in the context of TLR2 and TLR4 stimulation (Yamamoto et al., 2002) and as such may participate in activation of these TLRs by T. gondii infection (Mun et al., 2003; Debierre-Grockiego et al., 2007; Yarovinsky, 2008). Similar to our findings using MAL−/−Myd88−/− microglial cells, previous studies using single knockout (Myd88−/−) murine macrophages showed a contribution for Myd88 in the activation of IL-12 and various chemokines by types II and III strains (Kim et al., 2006; Lee et al., 2007). However, macrophage responses to the highly virulent type I RH strain were found to be independent of Myd88 (Kim et al., 2006; Lee et al., 2007) which is in contrast to our observations using the type I GT1-FUDR3.3 strain (Fig. 2). In addition to the different parasite strains examined, the discrepancies between these reports and the present study may arise from a hitherto unrecognized role of MAL in the activation of gene expression by type I strains in cells lacking Myd88. Future experiments comparing single knockout MAL and Myd88 macrophages and microglial cells may help define the individual roles of these TLR adaptor molecules in the immune response to T. gondii infection.
Manipulation of host functions by Toxoplasma gondii is a multifactorial process affected by both host and parasite factors (Sinai et al., 2004; Laliberte et al., 2008; Leng et al., 2009). Knowledge of the parasite-derived molecular mechanisms that dictate Toxoplasma virulence has increased in recent years (Saeij et al., 2006; Taylor et al., 2006; Bradley et al., 2007; Ong et al., 2010). However, it is evident that more needs to be done to examine the roles of parasite factors in the temporal regulation of host gene expression using cells associated with the natural course of infection. Microglial cell lines with defined genetic defects in TLR signaling represent useful tools to discern the mechanisms of pathogenesis in Toxoplasma and other intracellular parasites that cause infections of the brain.
Supplementary Material
Expression of T. gondii SAG1 in WT and MAL−/−Myd88−/− microglial cells. Cells were infected with type I (GT1-FUDR3.3), type II (ME49 B7), or type III (CTG ARA-SYN) strains of T. gondii for 2, 4, 8, 12, and 24 h. Analysis of SAG1 (A) and human β-actin (B) expression was performed by RT-PCR as described in sections 2.3 and 2.5. Gel images are shown from a representative experiment of three performed.
Acknowledgements
This work was supported by Project Number 272201000027C-0-0-1 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blasi E, Mathieson BJ, Varesio L, Cleveland JL, Borchert PA, Rapp UR. Selective immortalization of murine macrophages from fresh bone marrow by a raf/myc recombinant murine retrovirus. Nature. 1985;318:667–670. doi: 10.1038/318667a0. [DOI] [PubMed] [Google Scholar]
- 2.Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. Journal of Neuroimmunology. 1990;27:229–237. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- 3.Bradley PJ, Sibley LD. Rhoptries: an arsenal of secreted virulence factors. Current Opinion in Microbiology. 2007;10:582–587. doi: 10.1016/j.mib.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carruthers VB, Suzuki Y. Effects of Toxoplasma gondii infection on the brain. Schizophrenia Bulletin. 2007;33:745–751. doi: 10.1093/schbul/sbm008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chao CC, Anderson WR, Hu S, Gekker G, Martella A, Peterson PK. Activated microglia inhibit multiplication of Toxoplasma gondii via a nitric oxide mechanism. Clinical Immunology and Immunopathology. 1993a;67:178–183. doi: 10.1006/clin.1993.1062. [DOI] [PubMed] [Google Scholar]
- 6.Chao CC, Hu S, Gekker G, Novick WJ, Remington JS, Peterson PK. Effects of cytokines on multiplication of Toxoplasma gondii in microglial cells. Journal of Immunology. 1993b;150:3404–3410. [PubMed] [Google Scholar]
- 7.Chao CC, Gekker G, Hu S, Peterson PK. Human microglial cell defense against Toxoplasma gondii. The role of cytokines. Journal of Immunology. 1994;152:1246–1252. [PubMed] [Google Scholar]
- 8.Debierre-Grockiego F, Campos MA, Azzouz N, Schmidt J, Bieker U, Resende MG, Mansur DS, Weingart R, Schmidt RR, Golenbock DT, Gazzinelli RT, Schwarz RT. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. Journal of Immunology. 2007;179:1129–1137. doi: 10.4049/jimmunol.179.2.1129. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IκB-NF-κB signaling module: temporal control and selective gene activation. Science. 2002;298:1241–1245. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- 10.Kim L, Butcher BA, Lee CW, Uematsu S, Akira S, Denkers EY. Toxoplasma gondii genotype determines MyD88-dependent signaling in infected macrophages. Journal of Immunology. 2006;177:2584–2591. doi: 10.4049/jimmunol.177.4.2584. [DOI] [PubMed] [Google Scholar]
- 11.Laliberte J, Carruthers VB. Host cell manipulation by the human pathogen Toxoplasma gondii. Cellular and Molecular Life Sciences. 2008;65:1900–1915. doi: 10.1007/s00018-008-7556-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee CW, Sukhumavasi W, Denkers EY. Phosphoinositide-3-kinase-dependent, MyD88-independent induction of CC-type chemokines characterizes the macrophage response to Toxoplasma gondii strains with high virulence. Infection and Immunity. 2007;75:5788–5797. doi: 10.1128/IAI.00821-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leng J, Butcher BA, Denkers EY. Dysregulation of macrophage signal transduction by Toxoplasma gondii: past progress and recent advances. Parasite Immunology. 2009;31:717–728. doi: 10.1111/j.1365-3024.2009.01122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molestina RE, Payne TM, Coppens I, Sinai AP. Activation of NF-κB by Toxoplasma gondii correlates with increased expression of antiapoptotic genes and localization of phosphorylated IκB to the parasitophorous vacuole membrane. Journal of Cell Science. 2003;116:4359–4371. doi: 10.1242/jcs.00683. [DOI] [PubMed] [Google Scholar]
- 15.Molestina RE, Sinai AP. Host and parasite-derived IKK activities direct distinct temporal phases of NF-κB activation and target gene expression following Toxoplasma gondii infection. Journal of Cell Science. 2005;118:5785–5796. doi: 10.1242/jcs.02709. [DOI] [PubMed] [Google Scholar]
- 16.Mun HS, Aosai F, Norose K, Chen M, Piao LX, Takeuchi O, Akira S, Ishikura H, Yano A. TLR2 as an essential molecule for protective immunity against Toxoplasma gondii infection. International Immunology. 2003;15:1081–1087. doi: 10.1093/intimm/dxg108. [DOI] [PubMed] [Google Scholar]
- 17.Ong YC, Reese ML, Boothroyd JC. Toxoplasma rhoptry protein 16 (ROP16) subverts host function by direct tyrosine phosphorylation of STAT6. Journal of Biological Chemistry. 2010;285:28731–28740. doi: 10.1074/jbc.M110.112359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plattner F, Yarovinsky F, Romero S, Didry D, Carlier MF, Sher A, Soldati-Favre D. Toxoplasma profilin is essential for host cell invasion and TLR11-dependent induction of an interleukin-12 response. Cell Host & Microbe. 2008;3:77–87. doi: 10.1016/j.chom.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Robben PM, Mordue DG, Truscott SM, Takeda K, Akira S, Sibley LD. Production of IL-12 by macrophages infected with Toxoplasma gondii depends on the parasite genotype. Journal of Immunology. 2004;172:3686–3694. doi: 10.4049/jimmunol.172.6.3686. [DOI] [PubMed] [Google Scholar]
- 20.Rosowski EE, Lu D, Julien L, Rodda L, Gaiser RA, Jensen KD, Saeij JP. Strain-specific activation of the NF-κB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. Journal of Experimental Medicine. 2011;208:195–212. doi: 10.1084/jem.20100717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saeij JP, Boyle JP, Boothroyd JC. Differences among the three major strains of Toxoplasma gondii and their specific interactions with the infected host. Trends in Parasitology. 2005;21:476–481. doi: 10.1016/j.pt.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Saeij JP, Boyle JP, Coller S, Taylor S, Sibley LD, Brooke-Powell ET, Ajioka JW, Boothroyd JC. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science. 2006;314:1780–1783. doi: 10.1126/science.1133690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saeij JP, Coller S, Boyle JP, Jerome ME, White MW, Boothroyd JC. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature. 2007;445:324–327. doi: 10.1038/nature05395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sher A, Collazzo C, Scanga C, Jankovic D, Yap G, Aliberti J. Induction and regulation of IL-12-dependent host resistance to Toxoplasma gondii. Immunologic Research. 2003;27:521–528. doi: 10.1385/IR:27:2-3:521. [DOI] [PubMed] [Google Scholar]
- 25.Sibley LD, Boothroyd JC. Virulent strains of Toxoplasma gondii comprise a single clonal lineage. Nature. 1992;359:82–85. doi: 10.1038/359082a0. [DOI] [PubMed] [Google Scholar]
- 26.Sibley LD, Khan A, Ajioka JW, Rosenthal BM. Genetic diversity of Toxoplasma gondii in animals and humans. Philosophical Transactions of the Royal Society of London. Series B, Biological sciences. 2009;364:2749–2761. doi: 10.1098/rstb.2009.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinai AP, Payne TM, Carmen JC, Hardi L, Watson SJ, Molestina RE. Mechanisms underlying the manipulation of host apoptotic pathways by Toxoplasma gondii. International Journal for Parasitology. 2004;34:381–391. doi: 10.1016/j.ijpara.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Steinfeldt T, Konen-Waisman S, Tong L, Pawlowski N, Lamkemeyer T, Sibley LD, Hunn JP, Howard JC. Phosphorylation of mouse immunity-related GTPase (IRG) resistance proteins is an evasion strategy for virulent Toxoplasma gondii. PLoS Biology. 2010;8:e1000576. doi: 10.1371/journal.pbio.1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor S, Barragan A, Su C, Fux B, Fentress SJ, Tang K, Beatty WL, Hajj HE, Jerome M, Behnke MS, White M, Wootton JC, Sibley LD. A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science. 2006;314:1776–1780. doi: 10.1126/science.1133643. [DOI] [PubMed] [Google Scholar]
- 30.Vallabhapurapu S, Karin M. Regulation and function of NF-κB transcription factors in the immune system. Annual Review of Immunology. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, Hoshino K, Takeuchi O, Kobayashi M, Fujita T, Takeda K, Akira S. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature. 2002;420:324–329. doi: 10.1038/nature01182. [DOI] [PubMed] [Google Scholar]
- 32.Yarovinsky F. Toll-like receptors and their role in host resistance to Toxoplasma gondii. Immunology Letters. 2008;119:17–21. doi: 10.1016/j.imlet.2008.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression of T. gondii SAG1 in WT and MAL−/−Myd88−/− microglial cells. Cells were infected with type I (GT1-FUDR3.3), type II (ME49 B7), or type III (CTG ARA-SYN) strains of T. gondii for 2, 4, 8, 12, and 24 h. Analysis of SAG1 (A) and human β-actin (B) expression was performed by RT-PCR as described in sections 2.3 and 2.5. Gel images are shown from a representative experiment of three performed.