Abstract
Food and drugs can activate brain dopamine systems and sensitivity to the effects of drugs acting on those systems is influenced by amount and content of food consumed. This study examined the effects of drinking sucrose on behavioral effects of the directly-acting dopamine receptor agonist quinpirole. Male Sprague-Dawley rats (n=6/group) had free access to water or 10% sucrose and quinpirole dose-response curves (yawning and hypothermia) were generated weekly for 8 weeks. Subsequently, all rats drank water for 8 weeks with quinpirole dose-response curves determined on weeks 9, 10, and 16. In rats drinking sucrose, the ascending (D3 receptor-mediated), but not descending (D2 receptor-mediated), limb of the yawning dose-response curve shifted leftward. The D3 receptor-selective antagonist PG01037 shifted the ascending limb of the dose-response curve to the right in all rats. When rats that previously drank sucrose drank water, their sensitivity to quinpirole did not return to normal. Quinpirole-induced hypothermia was not different between groups. These data show that drinking sucrose increases sensitivity to a dopamine D3, but not D2, receptor-mediated effect and that this change is long lasting. Dopamine receptors mediate the effects of many drugs and the actions of those drugs are likely impacted by dietary factors.
Keywords: quinpirole, yawning, sucrose, dopamine receptor, hypothermia, rat
Introduction
Some drugs of abuse, as well as consumption of certain foods, can activate common dopamine pathways (Hernandez and Hoebel, 1988a; Wise and Rompre, 1989) and elevate levels of extracellular dopamine in brain (Di Chiara and Imperato, 1988; Hernandez and Hoebel, 1988b). Dopamine receptors mediate the effects of some drugs of abuse (e.g. indirectly-acting dopamine-receptor agonists; Caine and Koob, 1993; Sinnott et al., 1999) and previous studies have examined how drugs (e.g., cocaine) and dietary conditions (e.g., consumption of sucrose) impact dopamine systems (Kleven et al., 1990; Georges et al., 1999; Letchworth et al., 1999; Rada et al., 2005; Rada et al., 2010). For example, dopamine D1 receptor binding (Colantuoni et al., 2001), dopamine D3 receptor mRNA (Spangler et al., 2004), and extracellular dopamine concentrations (Rada et al., 2005) are increased in rats that have intermittent access to a sucrose solution. Given a choice between cocaine and a sweetened solution, rats respond more for sucrose than for cocaine (Lenoir et al., 2007) and rats with access to sucrose drink less amphetamine solution as compared to rats with no access to sucrose (Kanarek et al., 1996). Collectively, these data suggest that access to a sweetened solution impacts the effects of drugs acting indirectly on dopamine receptors. Less is known on whether access to sucrose or other highly palatable substances modifies sensitivity to drugs acting directly at dopamine receptors (Foley et al., 2006), although recent studies show that sensitivity to the directly-acting dopamine receptor agonist quinpirole is increased in rats eating high fat food (Baladi and France, 2009, 2010).
The current study examined whether drinking a 10% sucrose solution alters the sensitivity of rats to quinpirole-induced yawning and hypothermia. It is well established that D3 receptors mediate the induction (ascending limb of dose-response curve) while D2 receptors mediate the inhibition (descending limb) of dopamine receptor agonist (e.g., quinpirole)-induced yawning (Collins et al., 2005; Baladi et al., 2010), whereas hypothermia produced by the same drugs is thought to be mediated by dopamine D2 receptors (Nunes et al., 1991; Chaperon et al., 2003; Collins et al., 2007; Baladi et al., 2010). Based on results obtained in rats eating another highly palatable substance (high fat chow), it was hypothesized that sensitivity to quinpirole-induced yawning would increase in rats drinking sucrose, as compared with rats drinking water. In addition, if a leftward shift in the ascending limb of the quinpirole dose-response curve in rats drinking sucrose reflects increased sensitivity at D3 receptors, then a D3 receptor-selective antagonist should shift the ascending limb of the curve back to the right, without affecting the descending limb of the curve. Finally, if drinking sucrose selectively increases sensitivity at D3 (i.e., not at D2) receptors, then drinking sucrose should not affect either the descending limb of the yawning dose-response curve or the hypothermic effects of quinpirole.
Methods
Subjects
Twelve male Sprague Dawley rats (Harlan, Indianapolis, Indiana, USA), weighing 250–300 g upon arrival, were housed individually in an environmentally controlled room (24 ± 1 °C, 50 ± 10% relative humidity) under a 12/12 h light/dark cycle with free access to water and standard laboratory chow (for some rats, access to water was replaced with a 10% sucrose solution; see below). Animals were maintained and experiments were conducted in accordance with the Institutional Animal Care and Use Committee, the University of Texas Health Science Center at San Antonio, and with the 1996 Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, the National Research Council, and the National Academy of Sciences).
Drinking Conditions
For the first 8 weeks of the study (i.e., 56 days), 6 rats had free access to water in the home cage whereas the other 6 rats had free access to 10% sucrose in water. For the final 8 weeks of the 16-week study, all rats had free access to water only. Body weight and fluid intake were recorded daily.
Yawning
Yawning was defined as an opening and closing of the mouth such that the lower incisors were completely visible (Sevak et al., 2008; Baladi and France, 2009). On test days, rats were transferred from their home cage to a test cage (same dimensions as the home cage but with no food, water, or bedding) and allowed to habituate for 15 min. All experiments began with assessment of yawning after injection of vehicle. Dose–response curves were generated for cumulative doses of quinpirole (0.0032, 0.01, 0.032, 0.1, 0.32 mg/kg i.p.) administered every 30 min. Beginning 20 min after each injection, the total number of yawns was observed for 10 min and recorded. Dose-response curves were generated once per week for 8 weeks while rats drank either water or sucrose. Next, all rats had free access to water and quinpirole dose-response curves were determined in weeks 9, 10, and 16 of the study.
During week 7, rats received an injection of the D3 dopamine receptor-selective antagonist PG01037 (56.0 mg/kg s.c.) 30 min prior to the first dose of a quinpirole dose-response determination. This dose of PG01037 in this strain of rat and by the s.c. route of administration has antagonist actions at D3 dopamine receptors (Collins et al., 2005; Baladi et al., 2010).
Body Temperature
Rectal body temperature was measured in a temperature controlled room (24 ± 1 °C and 50 ± 10% relative humidity) by inserting a lubricated thermal probe attached to a thermometer 3 cm into the rectum. Animals were adapted to the procedure by measuring body temperature on several occasions prior to beginning the study. During yawning experiments, body temperature was measured after each 10 min observation period and prior to the next injection.
Data Analyses
Results are expressed as the average (± SEM) number of yawns during a 10-min observation period and the average change in body temperature (°C), plotted as a function of dose. Data are also presented as average body weight in g and average fluid intake per 24 h in ml, both plotted as a function of day. Body weight and fluid intake data were analyzed with either a one-way (time) or two-way (time and drinking condition) ANOVA for repeated measures. A post-hoc Bonferroni test was used to examine significant differences between drinking conditions with significance set at p< 0.05. For each group, differences between quinpirole dose–response curves for yawning (separately for the ascending and descending limbs) and hypothermia were analyzed by simultaneously fitting straight lines to the linear portion of the dose-response curves by means of GraphPad Prism (GraphPad, San Diego, California, USA). The linear portion included doses that spanned the 50% level of effect and included not more than one dose producing greater than 75% effect and not more than one dose producing less than 25% effect. Differences between the slopes and intercepts of the curves were analyzed with the F-ratio test (GraphPad Prism) as detailed elsewhere (Koek et al., 2006). The common slope values calculated by GraphPad Prism were used to constrain the fit of the parallel line assay. Differences between quinpirole dose-response curves in the presence and absence of antagonist were analyzed by line analysis methods described above. Using a maximum effect that was determined for individual rats, ED50 values were calculated using linear regression; 95% confidence limits (CL) were calculated from ED50 values averaged among rats.
Drugs
Quinpirole dihydrochloride was purchased from Sigma-Aldrich (St. Louis, MO) and PG01037 (N-{4-[4-(2,3-dichlorophenyl)-piperazin-1-yl]-trans-but-2-enyl}-4-pyridine-2-yl benzamide HCl) was synthesized by Jianjing Cao (Medicinal Chemistry Section, National Institute on Drug Abuse, Baltimore, MD) using methods reported previously (Grundt et al., 2005). Quinpirole was dissolved in sterile 0.9% saline and PG01037 was dissolved in 0.9% saline and 10% β-cyclodextrin. PG01037 and quinpirole were administered s.c. and i.p., respectively, in a volume of 1 ml/kg.
Results
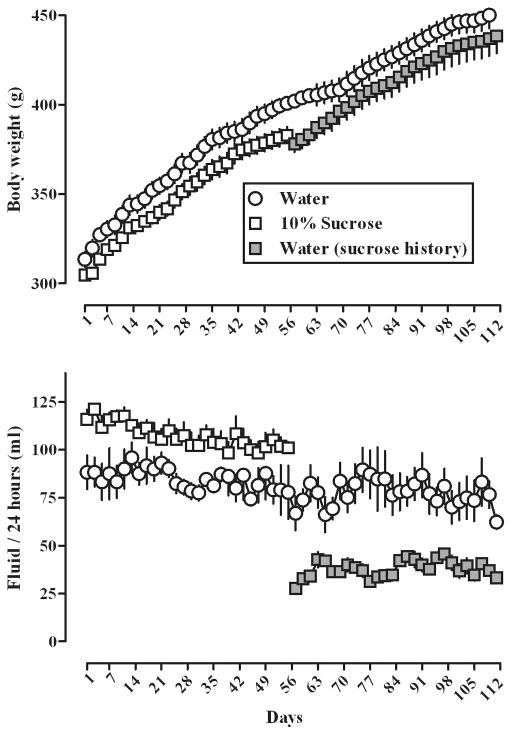
After 56 days (8 weeks) of free access to either water or 10% sucrose solution, body weight increased an average of 87 ± 2 (mean ± SEM) and 78 ± 4 g, respectively (Fig 1, upper panel, open circles and squares). For the remaining 56 days (8 weeks) of the study, when all rats had free access to water, the body weight of rats that previously drank sucrose (closed squares) initially decreased (−5 ± 2 g) then recovered with those rats gaining, on average, an additional 60 ± 8 g by the end of the study. Rats that drank only water gained an additional 50 ± 5 g during the last 56 days of the 112-day (16-week) study (Fig 1, upper panel).
Fig 1.
Body weight (upper panel) and fluid intake (lower panel) in two groups of rats over the 16-week (112-day) study. One group had free access to water throughout the study (open circles) while the other group had free access to a 10% sucrose solution for 8 weeks (56 days; open squares) followed by free access to water for 8 weeks (gray squares). Each symbol represents the mean (± SEM) of 6 rats. Horizonal axes: day of study. Vertical axes: mean (± SEM) body weight in g (upper panel) and mean (± SEM) fluid intake in ml in a 24-hour period (lower panel).
Fluid intake during the initial 56 days of the study remained constant within each group of rats with no significant difference across days; moreover, while there was a trend for more drinking of sucrose as compared with water, this difference was not statistically significant (Fig 1, lower panel). When rats that previously drank sucrose had access to water only (closed squares, lower panel, Fig 1), fluid intake decreased significantly (p <.05) compared with daily fluid intake when the same rats drank sucrose and compared with intake in rats that drank only water throughout the study. There was no significant difference in fluid intake within each group for the last 56 days of the study.
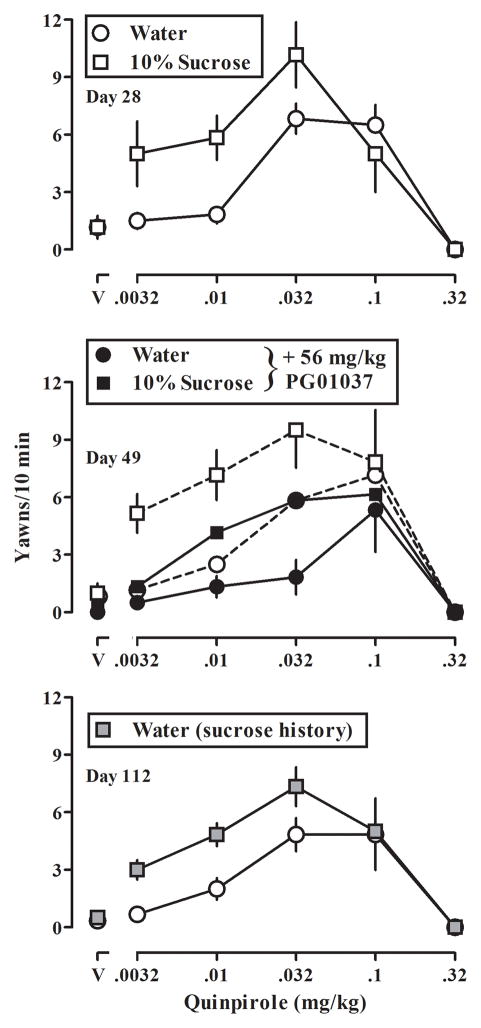
In all rats, small doses of quinpirole increased yawning, which decreased with the administration of larger doses, resulting in an inverted U-shaped dose-response curve (Fig 2). The quinpirole dose-response curves were not different between rats that drank water or a 10% sucrose solution for 21 days (Table 1); however, after 28 days the ascending limb, but not the descending limb, of the quinpirole dose-response curve was shifted significantly to the left (p<0.05) in rats drinking sucrose, as compared with rats drinking water (Fig 2, upper panel). That is, the linear portion corresponding to the ascending limb of the dose-response curve in rats with free access to either 10% sucrose solution or water could be fitted with a common slope but different x-intercepts, indicating that drinking sucrose shifted the curve to the left. Furthermore, in rats drinking sucrose the ascending limb of the quinpirole dose-response curve remained shifted significantly to the left (p<0.05) for the next two weeks (Table 1).
Fig 2.
Quinpirole-induced yawning in two groups of rats on days 28, 49, and 112 of the study (upper, middle, and lower panels, respectively). Data shown by symbols and dashed lines in the middle panel are the effects of quinpirole determined on day 42 (one week prior to the antagonism study with PG 01037 on day 49) in rats drinking either water or 10% sucrose. Horizonal axes: dose in milligrams per kilogram of body weight; “V” = vehicle. Vertical axes: mean (± SEM) number of yawns in 10 min. See Fig 1 for other details.
Table 1.
ED50 values for the ascending and descending limbs of the quinpirole dose-response curve for yawning
| Day | Ascending | Descending | Ascending | Descending |
|---|---|---|---|---|
| Water | Sucrose | |||
| 7 | 0.020a (0.008–0.049) | 0.156b (0.116–0.211) | 0.010 (0.003–0.031) | 0.123 (0.067–0.228) |
| 14 | 0.018 (0.006–0.049) | 0.151 (0.114–0.200) | 0.014 (0.022–0.009) | 0.139 (0.088–0.219) |
| 21 | 0.015 (0.008–0.031) | 0.123 (0.075–0.201) | 0.011 (0.004–0.027) | 0.135 (0.085–0.213) |
| 28 | 0.013 (0.008–0.023) | 0.142 (0.111–0.182) | 0.007 (0.005–0.011) | 0.091 (0.054–0.154) |
| 35 | 0.011 (0.008–0.016) | 0.140 (0.108–0.181) | 0.007 (0.002–0.021) | 0.098 (0.046–0.211) |
| 42 | 0.013 (0.006–0.028) | 0.160 (0.128–0.199) | 0.004 (0.003–0.007) | 0.107 (0.058–0.199) |
| 49 | (Antagonist experiment) | |||
| 56 | 0.013 (0.009–0.019) | 0.127 (0.090–0.182) | 0.006 (0.003–0.013) | 0.083 (0.045–0.156) |
|
| ||||
| Water | Water (sucrose history) | |||
| 63 | 0.011 (0.006–0.019) | 0.112 (0.072–0.175) | 0.006 (0.003–0.012) | 0.096 (0.050–0.183) |
| 70 | 0.015 (0.009–0.025) | 0.106 (0.071–0.156) | 0.009 (0.005–0.015) | 0.087 (0.050–0.152) |
| 112 | 0.011 (0.005–0.022) | 0.118 (0.075–0.184) | 0.006 (0.003–0.010) | 0.100 (0.060–0.168) |
ED50 values (95% CL) for the ascending limb of the quinpirole dose-response curve for yawning in 6 rats
ED50 values (95% CL) for the descending limb of the quinpirole dose-response curve for yawning in 6 rats
A dose of 56 mg/kg of the dopamine D3 receptor selective antagonist PG01037 shifted the ascending limb of the quinpirole yawning dose-response curve significantly to the right (p<0.05), both in rats drinking water and in rats drinking sucrose (Fig 2, middle panel), without affecting the descending limb of the dose-response curve in either group. In rats drinking either sucrose or water, the linear portion corresponding to the ascending limb of the dose-response curve in the presence and absence of the antagonist could be fitting with a common slope but different x-intercepts. Furthermore, for each group, quinpirole yawning dose-response curves determined before (i.e. day 42) and after (i.e. day 56) the antagonist experiment were not different, indicating that antagonist treatment had no long-lasting effect (Table 1).
Rats that previously drank sucrose subsequently drank water only while other rats continued drinking water; dose-response curves for quinpirole were determined in all rats during weeks 9, 10 and 16 (i.e., 7, 14, and 56 days after termination of access to sucrose in 6 of the rats). At 7, 14, and 56 days after termination of access to sucrose the ascending limb of the yawning dose-response curve remained shifted significantly to the left (p<0.05) in rats that previously drank sucrose (Fig 2, lower panel; Table 1). However, the descending limb of the quinpirole dose-response curve was not changed and was not different between groups.
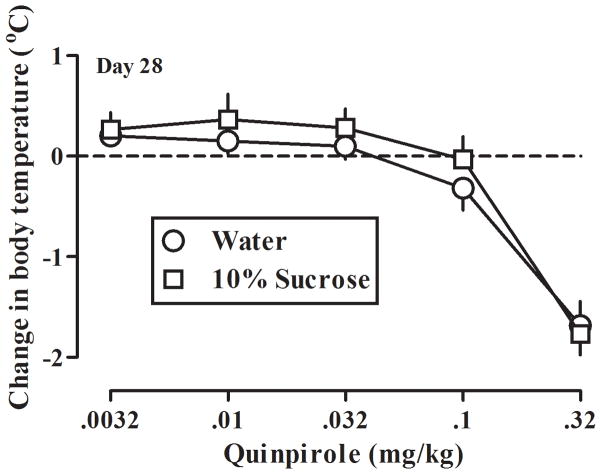
Dose-response curves for quinpirole-induced hypothermia were determined once per week for the first 8 weeks (56 days) of the study when different groups of rats drank either water or sucrose and also during weeks 9, 10, and 16 (i.e., study days 63, 70, and 112) when all rats drank water. Regardless of drinking condition, baseline (i.e., in the absence of drug) body temperature and dose-response curves for quinpirole-induced hypothermia were not different between groups. For example, in rats drinking either water or 10% sucrose solution for 28 days, the control body temperature (mean ± SEM) after vehicle injection was 36.9 ± 0.16°C and 36.9 ± 0.17°C, respectively. The hypothermic effects of quinpirole were not different between rats drinking water and rats that drank a 10% sucrose solution for 28 days (Fig 3) as indicated by common slopes and x-intercepts.
Fig 3.
Quinpirole-induced hypothermia in two groups of rats on day 28 of the study. Vertical axis: mean (± SEM) change in body temperature in °C. See Fig 1 for other details
Discussion
This study shows that rats drinking 10% sucrose solution are more sensitive than rats drinking water to quinpirole-induced yawning. Specifically, the ascending limb of the yawning dose-response curve, which is known to be mediated by agonist activity at dopamine D3 receptors (Baladi et al., 2010; Collins et al., 2005), was shifted to the left in rats that drank sucrose for 4 weeks. Furthermore, the dopamine D3 receptor-selective antagonist PG01037 shifted the ascending limb of the dose-response curve to the right in all rats, indicating that D3 receptors mediate the induction of yawning, both in rats drinking sucrose (i.e., when the curve is shifted leftward) and in rats drinking water. Results from this study also suggest that increased sensitivity to quinpirole-induced yawning persists after rats no longer have access to sucrose. Changes in sensitivity to quinpirole were selective for D3 and not D2 receptor mediated effects, insofar as the descending limb of the yawning dose-response curve as well as the dose-response curve for quinpirole-induced hypothermia were not different between rats drinking sucrose and those drinking water.
The mechanisms mediating changes in sensitivity to dopamine receptor agonists under different nutritional conditions are not well established. A previous study reported that consumption of sucrose increased dopamine D3 receptor mRNA levels and decreased dopamine D2 receptor mRNA levels (Spangler et al., 2004); however, in that study rats had intermittent access to sucrose and food. In the current study, where D3 but not D2 receptor-mediated effects were altered by consumption of sucrose, rats had unlimited access to sucrose (or water) and food. There are other examples where both the pattern of consumption (e.g., access) and the type of consumption (e.g., food, drink) affected dopamine systems and the actions of drugs acting on those systems; moreover, D3 and D2 receptor mediated effects are sometimes altered in a manner that is qualitatively and quantitatively different. For example, eating high fat chow increases the sensitivity of rats to D3 and D2 receptor mediated effects of quinpirole (Baladi and France, 2009; Baladi et al., 2011); similar to the current study, altered sensitivity to quinpirole was not apparent until after 28 days of eating high fat chow (Baladi et al., 2011). On the other hand, food restriction, regardless of chow type, increases the sensitivity of rats to D2, but not D3, receptor mediated effects of direct-acting dopamine receptor agonists (Collins et al., 2008; Baladi et al., 2011) and this effect develops quickly (within 7 days). In the current study, drinking sucrose increased sensitivity to D3 and not D2 receptor mediated effects of quinpirole. Together with other studies, it is clear that body weight, access conditions, and the type of substance consumed can significantly impact behavior and neurochemistry (Avena et al., 2008; Sevak et al., 2008).
Although there was no significant difference in body weight between the two groups of rats throughout this study, there was a trend for rats drinking sucrose to weigh less than rats drinking water, even when rats that previously drank sucrose were given free access to water. Others have shown that with more calories being consumed from drinking a sucrose solution there is a reduction in the amount of calories consumed from eating chow (Spector and Smith, 1984). Similarly, in the current study rats drinking sucrose did not gain weight faster than rats drinking water, presumably due to differences in food intake between groups. There was a trend for rats with access to a sucrose solution to drink more (volume) as compared with rats with access to water, although the difference was not statistically significant. The concentration-response curve for sucrose intake is an inverted U-shape (Collier and Bolles, 1968; Spector and Smith, 1983), whereby consumption of sucrose solution increases to a maximum volume with increasing concentration; however, further increases in concentration result in decreased consumption. In the current study, when rats that previously drank sucrose were given access to water, fluid intake decreased significantly as compared with intake when the same rats drank sucrose and compared with rats that drank water throughout the study. Other studies have shown that in rats with intermittent access to palatable chow, the consumption of standard chow decreases as compared with rats eating only standard chow (Cottone et al., 2008). Thus, a history of drinking sucrose might result in long-term changes both in sensitivity to drugs and in fluid intake.
Among many factors (e.g., age, sex, and drug history) that can affect sensitivity to drugs, dietary conditions appear to significantly impact both brain neurochemistry and the behavioral effects of drugs acting on dopamine systems. It is well established that the same brain circuitry can be activated by drugs and by non-drug reinforcers (e.g. food, sucrose) and the current study extends the generality of those findings to the consumption of sucrose impacting the behavioral effects of a direct-acting dopamine receptor agonist. Given that many drugs (therapeutics and drugs of abuse) act on dopamine systems and that consumption of highly palatable foods (sucrose and high fat) has increased worldwide (Malik et al., 2006; Popkin and Nielsen, 2003), there is a need to better understand the short and long term consequences of eating certain foods on sensitivity to drugs.
Acknowledgments
CPF is supported by a Senior Scientist Award (KO5 DA17918)
References
- Avena NM, Rada P, Hoebel BG. Underweight rats have enhanced dopamine release and blunted acetylcholine response in the nucleus accumbens while bingeing on sucrose. Neurosci. 2008;156:865–871. doi: 10.1016/j.neuroscience.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, France CP. High fat diet and food restriction differentially modify the behavioral effects of quinpirole and raclopride in rats. Eur J Pharmacol. 2009;610:55–60. doi: 10.1016/j.ejphar.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, France CP. Eating high-fat chow increases the sensitivity of rats to quinpirole-induced discriminative stimulus effects and yawning. Behav Pharmacol. 2010;21:615–620. doi: 10.1097/FBP.0b013e32833e7e5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, Newman AH, France CP. Dopamine D3 receptors mediate the discriminative stimulus effects of quinpirole in free-feeding rats. J Pharmacol Exp Ther. 2010;332:308–315. doi: 10.1124/jpet.109.158394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baladi MG, Newman AH, France CP. Influence of body weight and type of chow on the sensitivity of rats to the behavioral effects of the direct-acting dopamine-receptor agonist quinpirole. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2320-6. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB, Koob GF. Modulation of cocaine self-administration in the rat through D-3 dopamine receptors. Science. 1993;260:1814–1816. doi: 10.1126/science.8099761. [DOI] [PubMed] [Google Scholar]
- Chaperon F, Tricklebank MD, Unger L, Neijt HC. Evidence for regulation of body temperature in rats by dopamine D2 receptor and possible influence of D1 but not D3 and D4 receptors. Neuropharmacology. 2003;44:1047–1053. doi: 10.1016/s0028-3908(03)00113-8. [DOI] [PubMed] [Google Scholar]
- Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, Schwartz GJ, Moran TH, Hoebel BG. Excessive sugar intake alters binding to dopamine and mu-opioid receptors in the brain. Neuroreport. 2001;12:3549–3552. doi: 10.1097/00001756-200111160-00035. [DOI] [PubMed] [Google Scholar]
- Collier G, Bolles R. Some determinants of intake of sucrose solutions. J Comp Physiol Psychol. 1968;65:379–383. doi: 10.1037/h0025824. [DOI] [PubMed] [Google Scholar]
- Collins GT, Witkin JM, Newman AH, Svensson KA, Grundt P, Cao J, Woods JH. Dopamine agonist-induced yawning in rats: a dopamine D3 receptor-mediated behavior. J Pharmacol Exp Ther. 2005;314:310–319. doi: 10.1124/jpet.105.085472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Calinski DM, Newman AH, Grundt P, Woods JH. Food restriction alters N′-propyl-4,5,6,7-tetrahydrobenzothiazole-2,6-diamine dihydrochloride (pramipexole)-induced yawning, hypothermia, and locomotor activity in rats: evidence for sensitization of dopamine D2 receptor-mediated effects. J Pharmacol Exp Ther. 2008;325:691–697. doi: 10.1124/jpet.107.133181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Newman AH, Grundt P, Rice KC, Husbands SM, Chauvignac C, Chen J, Wang S, Woods JH. Yawning and hypothermia in rats: effects of dopamine D3 and D2 agonists and antagonists. Psychopharmacology (Berl) 2007;193:159–170. doi: 10.1007/s00213-007-0766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. Intermittent access to preferred food reduces the reinforcing efficacy of chow in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1066–R1076. doi: 10.1152/ajpregu.90309.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley KA, Fudge MA, Kavaliers M, Ossenkopp KP. Quinpirole-induced behavioral sensitization is enhanced by prior scheduled exposure to sucrose: A multi-variable examination of locomotor activity. Behav Brain Res. 2006;167:49–56. doi: 10.1016/j.bbr.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Georges F, Stinus L, Bloch B, Le Moine C. Chronic morphine exposure and spontaneous withdrawal are associated with modifications of dopamine receptor and neuropeptide gene expression in the rat striatum. Eur J Neurosci. 1999;11:481–490. doi: 10.1046/j.1460-9568.1999.00462.x. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Feeding and hypothalamic stimulation increase dopamine turnover in the accumbens. Physiol Behav. 1988a;44:599–606. doi: 10.1016/0031-9384(88)90324-1. [DOI] [PubMed] [Google Scholar]
- Hernandez L, Hoebel BG. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci. 1988b;42:1705–1712. doi: 10.1016/0024-3205(88)90036-7. [DOI] [PubMed] [Google Scholar]
- Kanarek RB, Mathes WF, Przypek J. Intake of dietary sucrose or fat reduces amphetamine drinking in rats. Pharmacol Biochem Behav. 1996;54:719–723. doi: 10.1016/0091-3057(96)00012-3. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Perry BD, Woolverton WL, Seiden LS. Effects of repeated injections of cocaine on D1 and D2 dopamine receptors in rat brain. Brain Res. 1990;532:265–270. doi: 10.1016/0006-8993(90)91768-c. [DOI] [PubMed] [Google Scholar]
- Koek W, Carter LP, Wu H, Coop A, France CP. Discriminative stimulus effects of flumazenil: perceptual masking by baclofen, and lack of substitution with gamma-hydroxybutyrate and its precursors 1,4-butanediol and gamma-butyrolactone. Behav Pharmacol. 2006;17:239–247. doi: 10.1097/00008877-200605000-00005. [DOI] [PubMed] [Google Scholar]
- Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PLoS One. 2007;2:e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letchworth SR, Sexton T, Childers SR, Vrana KE, Vaughan RA, Davies HM, Porrino LJ. Regulation of rat dopamine transporter mRNA and protein by chronic cocaine administration. J Neurochem. 1999;73:1982–1989. [PubMed] [Google Scholar]
- Lévesque D, Diaz J, Pilon C, Martres MP, Giros B, Souil E, Schott D, Morgat JL, Schwartz JC, Sokoloff P. Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxyl-N,N-di-n-propyl-2-aminotetralin. Proc Natl Acad Sci USA. 1992;89:8155–8159. doi: 10.1073/pnas.89.17.8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84:274–288. doi: 10.1093/ajcn/84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes JL, Sharif NA, Michel AD, Whiting RL. Dopamine D2-receptors mediate hypothermia in mice: ICV and IP effects of agonists and antagonists. Neurochem Res. 1991;16:1167–1174. doi: 10.1007/BF00966597. [DOI] [PubMed] [Google Scholar]
- Popkin BM, Nielsen SJ. The sweetening of the world’s diet. Obes Res. 2003;11:1325–1332. doi: 10.1038/oby.2003.179. [DOI] [PubMed] [Google Scholar]
- Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Rada P, Bocarsly ME, Barson JR, Hoebel BG, Leibowitz SF. Reduced accumbens dopamine in Sprague-Dawley rats prone to overeating a fat-rich diet. Physiol Behav. 2010;101:394–400. doi: 10.1016/j.physbeh.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevak RJ, Koek W, Owens WA, Galli A, Daws LC, France CP. Feeding conditions differentially affect the neurochemical and behavioral effects of dopaminergic drugs in male rats. Eur J Pharmacol. 2008;592:109–115. doi: 10.1016/j.ejphar.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnott RS, Mach RH, Nader MA. Dopamine D2/D3 receptors modulate cocaine’s reinforcing and discriminative stimulus effects in rhesus monkeys. Drug Alcohol Depend. 1999;54:97–110. doi: 10.1016/s0376-8716(98)00162-8. [DOI] [PubMed] [Google Scholar]
- Spangler R, Wittkowski KM, Goddard NL, Avena NM, Hoebel BG, Leibowitz SF. Opiate-like effects of sugar on gene expression in reward areas of the rat brain. Brain Res Mol Brain Res. 2004;124:134–142. doi: 10.1016/j.molbrainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Spector AC, Smith JC. A detailed analysis of sucrose drinking in the rat. Physiol Behav. 1984;33:127–136. doi: 10.1016/0031-9384(84)90023-4. [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annu Rev Psychol. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]