Highlights
► Leoligin significantly activated CETP in human plasma at 100 pM. ► Leoligin concentrations of 1 mM inhibited CETP activity. ► There was no short-term toxicity apparent in mice treated with leoligin.
Keywords: Cholesterol metabolism, High-density lipoproteins, Cholesteryl ester transfer protein
Abstract
Objective
Cholesteryl ester transfer protein (CETP) plays a central role in the metabolism of high-density lipoprotein particles. Therefore, we searched for new drugs that bind to CETP and modulate its activity.
Methods
A preliminary pharmacophore-based parallel screening approach indicated that leoligin, a major lignan of Edelweiss (Leontopodium alpinum Cass.), might bind to CETP. Therefore we incubated leoligin ex vivo at different concentrations with human (n = 20) and rabbit plasma (n = 3), and quantified the CETP activity by fluorimeter. Probucol served as positive control. Furthermore, we dosed CETP transgenic mice with leoligin and vehicle control by oral gavage for 7 days and measured subsequently the in vivo modulation of CETP activity (n = 5 for each treatment group).
Results
In vitro, leoligin significantly activated CETP in human plasma at 100 pM (p = 0.023) and 1 nM (p = 0.042), respectively, whereas leoligin concentrations of 1 mM inhibited CETP activity (p = 0.012). The observed CETP activation was not species specific, as it was similar in magnitude for rabbit CETP. In vivo, there was also a higher CETP activity after oral dosage of CETP transgenic mice with leoligin (p = 0.015). There was no short-term toxicity apparent in mice treated with leoligin.
Conclusion
CETP agonism by leoligin appears to be safe and effective, and may prove to be a useful modality to alter high-density lipoprotein metabolism.
1. Introduction
Cardiovascular diseases remain to be the leading cause of death in the developed world. The inverse relation between high-density lipoprotein cholesterol (HDL-C) and coronary artery disease (CAD) has brought attention to pharmacological interventions increasing plasma HDL-C [1,2]. Cholesteryl ester transfer protein (CETP) exchanges cholesteryl esters of high-density lipoproteins (HDL) for triglycerides of apolipoprotein B containing lipoproteins. In the presence of CETP, large HDL2 particles lose their cholesteryl esters and shrink in size, whereas triglyceride-rich very low density lipoproteins (VLDL) transform into cholesteryl ester rich intermediate density lipoproteins [2–4]. CETP, thus, plays a central role in lipoprotein metabolism and was pursued for several years as a prime target for pharmacological intervention in order to treat dyslipidemia and to prevent atherosclerosis [5–8].
Animal studies suggested that rodents lacking plasma CETP activity or getting a CETP inhibitor held elevated HDL-C levels and showed resistance to diet-induced atherosclerosis. Furthermore, patients with CETP mutations tended to have increased HDL-C plasma concentrations and less CAD [4]. Thus, the pharmacological inhibition of CETP seemed to be a promising strategy to fight CAD. First pilot studies with CETP inhibitors showed indeed higher HDL-C and minor low-density lipoprotein cholesterol (LDL-C) levels without serious adverse events [5,6]. However, the first large multicenter randomized controlled clinical trial with the CETP inhibitor torcetrapib was disrupted prior to schedule because of an excess of cardiovascular and overall mortality in the active treatment group [7]. Moreover, recent work from Vasan et al. and us revealed that low endogenous CETP plasma levels per se were associated with increased cardiovascular and all-cause mortality in the Framingham, the LURIC, and the KAROLA population [9–11].
Having focussed on CETP inhibitors up to now, there is much less information on potential benefits of a pharmacological increase of CETP activity. Hitherto, the only known CETP-activator was probucol [12]. Probucol was initially developed as an anti-oxidant to be used in the manufacturing of tires, but was found to have cholesterol-lowering properties and thus marketed for a number of years as hypolipidemic agent. A remarkable property of probucol is its ability to lower cholesterol in patients with homozygous familial hypercholesterolemia [13]. In such patients, probucol causes a dramatic decrease in tendon and planar xanthomas, seemingly out of proportion to the degree of cholesterol lowering. Probucol increases both the amount and the activity of CETP, and enhances the reverse cholesterol transport [12,14]. Unfortunately, probucol has severe untoward effects including prolongation of the QTc interval. Therefore, the substance was retracted from the market in the USA and Europe, but is still in use in Japan [15].
As the role of CETP in the pathogenesis of atherosclerosis is still open to debate [16], we looked for new biological compounds to alter CETP activity. Our data from in silico screenings indicated that leoligin, the major lignan of the alpine flower Edelweiss (Leontopodium alpinum Cass.), may bind to CETP. This natural lignan belongs to the class of lariciresinol derivatives and was shown to inhibit intimal hyperplasia of venous bypass grafts [17], as well as the in vitro leukotriene biosynthesis [18]. In the present study, we examined the effects on blood lipids of this unique compound providing clear evidence that leoligin enhances CETP activity ex vivo and in vivo.
2. Materials and methods
2.1. Plant material, isolation, and purification of leoligin
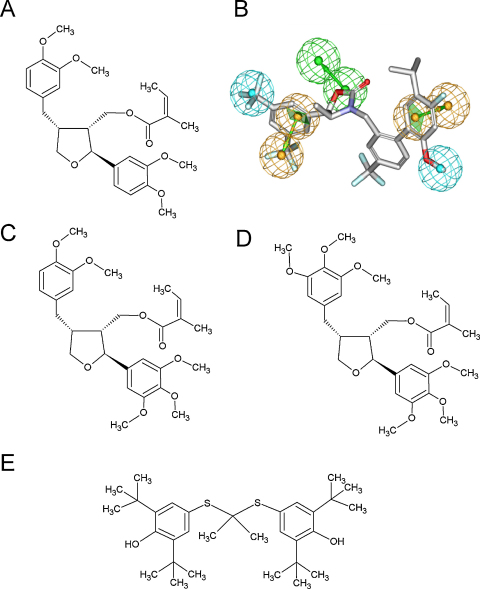
Leoligin (Fig. 1A) was isolated as described previously from 5.3 kg sub-aerial parts of Edelweiss (L. alpinum Cass.) which were obtained from Swiss horticultures [17]. The purity of leoligin according to LC-DAD/MS- and NMR examination was >98%. Furthermore, a voucher specimen (CH 5002) has been deposited at the herbarium of the Institute of Pharmacy/Pharmacognosy, University of Innsbruck. In addition, two further natural derivatives of leoligin 5-methoxyleoligin (=[(2S,3R,4R)-4-(3,4-dimethoxybenzyl)-2-(3,4,5-trimethoxyphenyl)tetrahydro-furan-3-yl]methyl-(2Z)-2-methylbut-2-en-oate and 5,5′-dimethoxyleoligin (=[(2S,3R,4R)-4-(3,4,5-trimethoxybenzyl)-2-(3,4,5-trimethoxyphenyl)tetrahydro-furan-3-yl]methyl-(2Z)-2-methylbut-2-en-oate were isolated as described in [18] and used for testing. The purity of both compounds was comparable to that of leoligin.
Fig. 1.
Chemical structure of leoligin, leoligin derivatives, and probucol, and the pharmacophore model for CETP. Leoligin (A) is a lignan, which was isolated from the roots of Edelweiss (Leontopodium alpinum Cass.). The IUPAC name for leoligin is: [(2S,3R,4R)-4-(3,4-dimethoxybenzyl)-2-(3,4-dimethoxyphenyl)tetrahydrofuran-3-yl]methyl (2Z)-2-methylbut-2-enoat. (B) The pharmacophore model for CETP shows in its vectorized feature consisting of two green spheres a hydrogen bond acceptor, the two orange vectorized features represent aromatic interactions, and the two blue spheres display hydrophobic interactions. The figure was generated applying the DS Visualizer software. There are two natural derivatives of leoligin, namely 5-methoxyleoligin (=[(2S,3R,4R)-4-(3,4-dimethoxybenzyl)-2-(3,4,5-trimethoxyphenyl)tetrahydro-furan-3-yl]methyl-(2Z)-2-methylbut-2-en-oate (C), and 5,5′-dimethoxyleoligin (=[(2S,3R,4R)-4-(3,4,5-trimethoxybenzyl)-2-(3,4,5-trimethoxyphenyl)tetrahydro-furan-3-yl]methyl-(2Z)-2-methylbut-2-en-oate (D). Probucol (4,4′-[propane-2, 2-diylbis(thio)]bis(2,6-di-tert-butylphenol)) (E) is a diphenolic substance with cholesterol-lowering and anti-inflammatory properties. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
2.2. In silico screening
We performed the pharmacophore-based parallel screening as previously described [19]. In brief, each pharmacological target is represented by one or more pharmacophore models. A pharmacophore model is an abstract representation of the lock-and-key hypothesis of protein–ligand interactions. It consists of the steric and electronic features that are necessary to ensure the optimal supramolecular interactions with a specific biological target and to trigger or block its biological response. A pharmacophore is composed of chemical features that describe types of protein–ligand interactions such as hydrogen bonds, charged groups, aromatic structures and hydrophobic areas. The technology to perform simultaneous, parallel screening of one compound against a multitude of pharmacophore models is available as a Pipeline Pilot-based program protocol in Discovery Studio 2.01 (Accelrys Inc., San Diego, CA, USA). Together with this software, the Inte:Ligand (www.inteligand.com) pharmacophore model collection of 2208 in-house generated pharmacophore models was used for the virtual screening. The CETP model from the InteLigand pharmacophore model database (www.inteligand.com/pharmdb/) and validation studies were performed using the CATALYST software package (Accelrys Inc., San Diego, CA, USA). Anacetrapib, a CETP inhibitor (IC50 = 13 nM) that recently proceeded to phase III clinical trials, was selected as template for ligand-based pharmacophore elucidation [8]. Applying the feature mapping algorithm of CATALYST, several models were generated by placing pharmacophoric features on the chemical moieties of the energetically minimized 3D structure of anacetrapib. The ability of the generated models to enrich CETP ligands among biologically inactive compounds was validated by virtually screening them against a test set. The test set comprised 14 CETP ligands and 32 compounds that did not show biological activity for CETP below 100 μM (Suppl. Table 1). The latter were chosen as inactive ligands for model validation. The best model was kept for virtual activity profiling within the InteLigand pharmacophore model collection. The CETP model comprised a hydrogen bond acceptor fitted to the oxazolidin-2-one moiety of anacetrapib, as well as two aromatic and two hydrophobic interactions. The model retrieved 13 test set inhibitors (93%) and two inactive compounds (6%). The enrichment of actives compared to a random selection was measured using the enrichment factor (EF) calculated by the following equation [19]: EF = (TP/n)/(A/N). TP is the number of actives retrieved by the model, n is the number of actives and inactive compounds retrieved by the model, A is the number of actives in the test set, and N is the number of all compounds in the test set. The EF determined for the CETP model was 2.85. The maximum EF for this dataset, where a model would find only active compounds, would have been 3.29. The high EF of this model indicated high quality and restrictivity.
2.3. Lipidiological analyses
The study protocol was approved by the university ethics review board (# AN4143 294/4.1) and complies with the Declaration of Helsinki. All participants gave written informed consent before the trial and filled standardized questionnaires for baseline information. Blood samples were collected from 20 healthy volunteers after an overnight fast. Total plasma cholesterol, triglycerides, and HDL-C were measured in whole plasma using Roche Diagnostics commercial kits (Roche Diagnostics, Mannheim, Germany). Due to the small volume size of available plasma samples from CETP transgenic mice, single measurements of HDL- and LDL-cholesterol for each animal were not possible. As a surrogate, we used FPLC lipid profiles from pooled murine plasma samples to differentiate between the high-density and low-density lipoprotein cholesterol fractions in the different treatment arms. The murine plasma samples were subjected to FPLC fractionation analysis with two tandem superose 6 columns (GE Healthcare, Vienna, Austria), as described previously [20]. CETP activity was measured using a commercial CETP activity assay kit (BioVision, Mountain View, CA, USA). Briefly, CETP activity was determined in microplates by a fluorescent method using a donor molecule containing a fluorescent self-quenched neutral lipid that is transferred to an acceptor molecule in the presence of CETP. Three μL of human plasma were used per well and the plate was incubated for 30 min at 37 °C. CETP-mediated transfer of the fluorescent neutral lipid to the acceptor molecule results in an increase in fluorescence which was measured with the fluorimeter Tecan infinite M200 and the appropriate software i-control 1.6 (Tecan Group, Maennedorf, Switzerland). CETP plasma levels were determined using an enzyme-linked immunosorbent assay (ELISA) employing a CETP-specific recombinant single-chain antibody as coating antibody and an affinity-purified polyclonal rabbit anti-CETP antibody as detection antibody, respectively [21]. The TaqIB polymorphism in the human CETP gene was determined by restriction fragment length polymorphism (RFLP) analysis as well as by real time polymerase chain (PCR) reaction with TaqMan-MGB primers and probes. For RFLP analysis, a 991-bp fragment of intron 1 of the CETP gene was amplified using the following primer pair: 5′-CAG GGG TCT TTT CAT GGA CAC-3′ (forward) and 5′-CAC TTG TGC AAC CCA TAC TTG ACT-3′ (reverse). The resulting PCR-product was digested with TaqI (New England BioLabs, Ipswich, MA, USA). For real time analyses we used the forward primer sequence 5′-CCC CTA ACC TGG CTC AGA TC-3′, the reverse primer sequence 5′-GCC AGG TAT AGG GAT TTG TGT TGT T-3′, and the TaqMan-MGB probes FAM-CCC TAA CTT GAA CCC and HEX-CCC TAA CTC GAA CCC [22]. TaqMan Real Time PCR reactions were performed on a MX4000H Multiplex Quantitative PCR System (Stratagene, Amsterdam, Netherlands).
2.4. Animal studies
All animals were handled in strict accordance with good animal practice as defined by the Austrian Authorities and the European Commission Directive 86/609/EEC, and all animal work was approved by the Austrian Animal Care and Use Committee (# BMWF-66.011/017-II/3b/2010). Animals were maintained in a virus/antibody-free central animal facility of the Innsbruck Medical University. CETP transgenic C57BL/6-Tg(CETP)UCTP20Pnu/J mice expressing human CETP under the control of its own promoter and other major regulatory elements, were kindly provided by Miranda van Eck [23]. The mice were fed a standard chow diet (Ssniff, Soest, Germany). Both, diet and water were provided ad libitum. After 4 weeks of acclimatization, the CETP transgenic mice were divided into two treatment groups: a leoligin-treated, and a control group with vehicle alone. Leoligin was dissolved in a stock solution of dimethylsulfoxide (Sigma, St. Louis, MI, USA), and subsequently diluted in 0.5% methylcellulose (Carl Roth, Karlsruhe, Germany). The daily dosage of leoligin was 0.14 mg/d. It was applied orally once daily using an infusion canule with a bulb end (Acufirm Ernst Kratz Nadelfabrik, Dreieich, Germany) [24]. After 7 days of treatment, animals were fasted for 6 h and blood samples were taken for lipidological analyses.
2.5. Molecular docking
In order to gain more insight into the mechanism of action of leoligin, it was docked into the X-ray crystal structure of CETP (Protein Data Bank entry 2obd, www.pdb.org PDB). The software GOLD 3.1 (Cambridge Crystallographic Data Center – CCDC, Cambridge, UK; www.ccdc.cam.ac.uk/products/life_sciences/gold/) was used in order to perform these docking experiments [25]. This software employs a genetic algorithm that allows the prediction of possible binding modes of small molecules to the ligand binding site of a protein. GOLD creates several docking solutions which subsequently are ranked by scoring functions. For docking experiments, the default parameters of GOLD were applied. The ligand binding site was defined by choosing all four ligands (two cholesteryl esters and two phospholipids), and all atoms within 10 Å were considered for docking. Crystallized water molecules were preserved. Leoligin preparation for GOLD was carried out using Corina 3.0. Driver options were set to preserve stereochemistry, write hydrogens, and remove additional 2D info from output files.
2.6. Statistical analysis
Results are presented as mean ± SEM. When comparing two groups, the Student's t-test was performed for data with normal distribution and the nonparametric Mann–Whitney U test was performed for data without normal distribution, respectively. A p-value <0.05 was considered significant. When comparing three groups, we performed the Kruskal–Wallis test. When significances were detected, groups were compared by Mann–Whitney U test. The level of significance was corrected to the number of groups, and a p-value <0.025 was considered significant. All statistical analyses were performed using SPSS 15.1 for Windows (SPSS, Inc., Chicago, IL, USA).
3. Experimental results
Edelweiss (L. alpinum Cass.) is a popular alpine plant that has been used for centuries in folk medicine to treat indigestion, fever, and ‘abdominal aches’. In order to analyze the potential therapeutic significance of this plant, its phytochemical constituents were isolated, characterized and screened for potential pharmacological targets using pharmacophore-based activity profiling. Our preliminary in silico studies revealed a possible interaction of leoligin (Fig. 1A), the major lignan of Edelweiss, with the pharmacophore model for cholesteryl ester transfer protein (Fig. 1B). Here, we specifically analyzed this interaction and found leoligin to activate human CETP in vitro and in vivo.
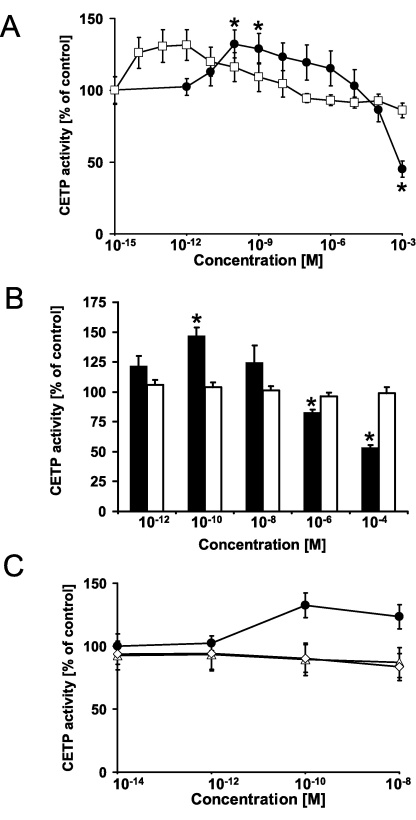
First, we determined the in vitro activity of CETP after incubation with different concentrations of leoligin and its two natural derivatives (Fig. 1C and D). Probucol is an activator of human CETP and served as positive control (Fig. 1E). The clinical and biochemical characteristics of the study population (n = 20) are given in the Suppl. Table 2 In human plasma, leoligin significantly increased CETP activity by 32.3% at a concentration of 100 pM (p = 0.023), and by 29.0% at a concentration of 1 nM (p = 0.042) (Fig. 2A). Higher leoligin doses (1 mM) significantly inhibited CETP activity to 44.8% of control (p = 0.012). The activation of CETP by leoligin was similar in size as that observed for probucol. Probucol significantly increased CETP activity in vitro at an even lower concentration of 1 pM up to 31.5% (p = 0.040). The modulation of CETP by leoligin was not restricted to the human CETP isoform. We also tested the interaction of leoligin with the rabbit CETP isoform. Leoligin significantly increased rabbit CETP activity to 146.9 ± 6.9% of control (n = 3; p = 0.047) in a concentration of 100 pM (p = 0.047), and it decreased rabbit CETP activity in a higher concentration of 100 μM to 53.4% ± 1.9% of control (n = 5; p = 0.00006), respectively (Fig. 2B). Thus, there was a similar pharmacological fingerprint of leoligin with an activation of CETP activity in the nanomolar range and an inhibition of CETP activity in the millimolar range both in human and in rabbit plasma. In order to prove the specificity of this interaction of leoligin with CETP, we purified human CETP from plasma in an immunosorbent microplate coated with CETP-specific recombinant antibodies. After several washing steps and spiking with different leoligin concentrations, CETP activity was measured and showed again a clear activation at concentrations of 100 pM to 1 nM (p < 0.05). We also tested the two natural leoligin derivatives 5-methoxyleoligin and 5,5′-dimethoxyleoligin in our experimental setting (Fig. 1C and D). As shown in Fig. 2C, the leoligin derivatives 5-methoxyleoligin and 5,5′-dimethoxyleoligin were not effective in inducing CETP activity, respectively (n = 10 for each leoligin derivative).
Fig. 2.
Leoligin enhances the activity of human and rabbit cholesteryl ester transfer protein. (A) The activity of the cholesteryl ester transfer protein (CETP) was determined in human plasma after incubation with different concentrations of leoligin (closed circles) and probucol (open squares), respectively. Leoligin significantly increased CETP activity at concentrations of 100 pM (n = 20; p = 0.023) and 1 nM (p = 0.042). At higher leoligin concentrations of 1 mM, CETP activity was significantly inhibited (p = 0.012). Probucol (open squares) increased the CETP activity at even lower concentrations of 1 pM (n = 20; p = 0.040). (B) Leoligin (black bars) significantly increased rabbit CETP activity in a concentration of 100 pM (n = 3; p = 0.047), and inhibited rabbit CETP activity at higher concentrations of 1 μM (n = 5; p = 0.019), and 100 μM (n = 5; p = 0.00006), respectively. Probucol (white bars) did not alter rabbit CETP activity. Data are given as means ± SEM. Significant differences were calculated by Student's t-test for unpaired data. * indicates p-value <0.05. (C) Leoligin-derivatives did not significantly regulate the activity of human cholesteryl ester transfer protein (n = 10). The activity of the cholesteryl ester transfer protein (CETP) was determined in human plasma after incubation with different concentrations of leoligin (closed circles), 5,5′-dimethoxyleoligin (open diamonds), and 5-methoxyleoligin (open triangles), respectively. Data are given as means ± SEM.
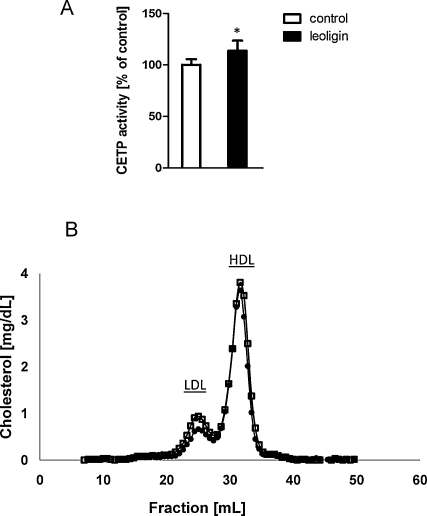
Next, we tested whether leoligin activated CETP in vivo. For this purpose we used CETP transgenic mice. The daily dosages of leoligin were applied by oral gavage over 7 days. As shown in Fig. 3A, there was a higher in vivo CETP activity in leoligin treated mice when compared to controls. Moreover, there was a trend to lower LDL-cholesterol levels in leoligin treated animals, when compared to controls (Fig. 3B). In order to prove that the in vivo treatment with leoligin was both safe and effective, we also made a blood cell count, and analyzed liver and kidney parameters after 7 days of treatment. As shown in Table 1, there was no significant difference in these biochemical markers between the control and the leoligin-treated group.
Fig. 3.
Leoligin activates cholesteryl ester transfer protein in vivo. CETP transgenic mice (n = 5 in each group) were dosed orally with equal daily amounts of leoligin (black bars), or vehicle control (white bars) for 7 days. (A) The CETP activity was measured after these 7 days. There was a higher in vivo CETP activity in leoligin treated mice when compared to controls (p = 0.015). (B) The lipid profiles showed a trend to lower LDL-cholesterol levels in the leoligin treated animals (closed circles), when compared to controls (open squares). * indicates p-value <0.05.
Table 1.
Biochemical characteristics of CETP transgenic mice treated with leoligin and vehicle control.
| Control | Leoligin | p-Value | |
|---|---|---|---|
| Body weight (g) | 24.0 ± 1.5 | 23.7 ± 1.4 | 0.902 |
| Cholesterol (mg/dL) | 68 ± 7 | 76 ± 4 | 0.404 |
| Triglycerides (mg/dL) | 80 ± 10 | 92 ± 4 | 0.307 |
| CETP mass (μg/mL) | 1.40 ± 0.10 | 1.22 ± 0.07 | 0.240 |
| PLTP (pmol/μL/h) | 20.5 ± 3.7 | 17.5 ± 2.7 | 0.560 |
| Urea (mg/dL) | 53.9 ± 3.5 | 49.9 ± 1.2 | 0.418 |
| Alanine transaminase (U/L) | 30.3 ± 2.2 | 35.7 ± 3.1 | 0.321 |
| Aspartate transaminase (U/L) | 66.0 ± 7.8 | 80.7 ± 13.1 | 0.476 |
| Leukocytes (G/L) | 5.8 ± 0.2 | 6.3 ± 0.5 | 0.537 |
| Erythrocytes (T/L) | 7.7 ± 0.2 | 7.5 ± 0.2 | 0.490 |
| Hemoglobin (g/dL) | 14.6 ± 0.5 | 14.1 ± 0.2 | 0.401 |
| Hematocrit (%) | 40.1 ± 1.2 | 38.5 ± 0.3 | 0.296 |
| MCV (fL) | 52.0 ± 0.5 | 51.3 ± 0.7 | 0.492 |
| MCH (pg) | 18.9 ± 0.1 | 19.0 ± 0.2 | 0.722 |
Abbreviations: PLTP, phospholipid transfer protein; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin.
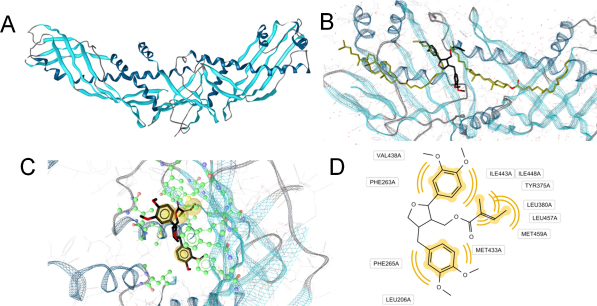
Ultimately, we performed docking experiments to characterize the putative molecular binding mode for leoligin to CETP. In general, CETP is a boomerang-shaped protein with a long, continuous tunnel (Fig. 4A). Within this tunnel two cholesteryl ester and two phospholipid molecules can bind at the same time. In the center of this tunnel, the so-called neck, its diameter narrows. In the docking studies, leoligin bound near the neck region forming extensive hydrophobic contacts (Fig. 4B–D).
Fig. 4.
Proposed molecular binding mode of leoligin next to the CETP neck region. CETP (A) is a boomerang-shaped protein forming a long, hydrophobic tunnel. In the X-ray crystal structure (B), this tunnel contains two cholesteryl ester molecules (gold). Leoligin (black) was predicted to bind in an area just between these two ligands, next to the CETP neck region. (C) Docking mode of leoligin within the hydrophobic CETP channel. Amino acids involved in direct protein–ligand interactions are colored in green. Substituents of leoligin forming hydrophobic contacts with CETP are highlighted in yellow spheres. (D) 2D representation of leoligin–CETP interactions. Respective interactions are indicated by yellow curves. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
4. Discussion
In the present study, we clearly demonstrate that leoligin, a major lignan from the roots of Edelweiss (L. alpinum Cass.), activated CETP both ex vivo and in vivo. This pharmacological interaction was not restricted to the human isoform of CETP, but was also true for the rabbit CETP, and occurred at nanomolar concentrations. Beside its effectiveness, CETP agonism by leoligin seemed safe in the short term, as there was no overt toxicity observed in our experimental setting.
The pharamcophore model for CETP was designed using established CETP inhibitors. While searching for CETP inhibitors, we found a CETP activator. This paradoxical finding might indicate that leoligin is an allosteric activator of cholesteryl ester transfer at nanomolar concentrations. Moreover, it is not surprising for the hydrophobic substance leoligin to inhibit CETP activity at millimolar concentration, as it may accumulate in the hydrophobic tunnel of CETP and thus inhibit cholesteryl ester and phospholipid transport by CETP at excessively high concentrations.
Cholesteryl ester transfer protein antagonism has been pursued for several years as a prime target for pharmacological intervention in an effort to increase high-density lipoprotein cholesterol and to prevent atherosclerosis. However, the clinical outcome of these trials addressing CETP inhibition has been equivocal [26–28]. So far, the only clinically relevant CETP activator has been probucol, which remarkably lowers cholesterol in patients with homozygous familial hypercholesterolemia [29]. At the moment, we cannot anticipate whether leoligin is just a new pharmacological tool to study high-density lipoprotein metabolism or whether it may also prove a useful modality to treat selected patient populations. Leoligin seems to differ from probucol, as leoligin did not to increase CETP mass in CETP transgenic mice, whereas probucol increases both human CETP plasma levels and CETP activity [12,14]. This difference may be due to genuine pharmacodynamic characteristics of the tested drugs, but could also be caused by the completely different experimental settings. We used a short-term dosing of CETP transgenic mice over 7 days and have not yet analyzed long-term effects of leoligin treatment in our animal model. Moreover, CETP transgenic mice do carry the human CETP gene under the control of its own promoter, but can obviously not substitute for human clinical trials. Therefore, further analyses are needed to study the long-term effects of leoligin on CETP expression in different tissues and organisms.
Taken all experimental evidence together, it appears that the clinical impact of CETP modulation on atherosclerosis most likely depends on the actual lipoprotein distribution in a given clinical setting. Therefore, future clinical trials addressing CETP modulation will have to carefully select patients with well characterized plasma lipids and lipoproteins, if they are to find out what kind of patients, if any, will benefit from such interventions.
Conflict of interest
None.
Acknowledgements
This work was supported by the Fonds zur Foerderung der wissenschaftlichen Forschung (P1999-B05 and NFN S10702). We thank Inte:Ligand for generously providing the pharmacophore model collection for this study.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.atherosclerosis.2011.07.023.
Appendix A. Supplementary data
References
- 1.Khera A.V., Cuchel M., de la Llera-Moya M. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patsch J.R., Prasad S., Gotto A.M., Jr., Patsch W. High density lipoprotein 2. Relationship of the plasma levels of this lipoprotein species to its composition, to the magnitude of postprandial lipemia, and to the activities of lipoprotein lipase and hepatic lipase. J Clin Invest. 1987;80:341–347. doi: 10.1172/JCI113078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tall A.R. Plasma cholesteryl ester transfer protein. J Lipid Res. 1993;34:1255–1274. [PubMed] [Google Scholar]
- 4.Inazu A., Brown M.L., Hesler C.B. Increased high-density lipoprotein levels caused by a common cholesteryl-ester transfer protein gene mutation. N Engl J Med. 1990;323:1234–1238. doi: 10.1056/NEJM199011013231803. [DOI] [PubMed] [Google Scholar]
- 5.Brousseau M.E., Schaefer E.J., Wolfe M.L. Effects of an inhibitor of cholesteryl ester transfer protein on HDL cholesterol. N Engl J Med. 2004;350:1505–1515. doi: 10.1056/NEJMoa031766. [DOI] [PubMed] [Google Scholar]
- 6.Kastelein J.J., van Leuven S.I., Burgess L. Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N Engl J Med. 2007;356:1620–1630. doi: 10.1056/NEJMoa071359. [DOI] [PubMed] [Google Scholar]
- 7.Barter P.J., Caulfield M., Eriksson M. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 8.Cannon C.P., Shah S., Dansky H.M. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N Engl J Med. 2010;363:2406–2415. doi: 10.1056/NEJMoa1009744. [DOI] [PubMed] [Google Scholar]
- 9.Duwensee K., Breitling L.P., Tancevski I. Cholesteryl ester transfer protein in patients with coronary heart disease. Eur J Clin Invest. 2010;40:616–622. doi: 10.1111/j.1365-2362.2010.02313.x. [DOI] [PubMed] [Google Scholar]
- 10.Ritsch A., Scharnagl H., Eller P. Cholesteryl ester transfer protein and mortality in patients undergoing coronary angiography: the Ludwigshafen Risk and Cardiovascular Health study. Circulation. 2010;121:366–374. doi: 10.1161/CIRCULATIONAHA.109.875013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vasan R.S., Pencina M.J., Robins S.J. Association of circulating cholesteryl ester transfer protein activity with incidence of cardiovascular disease in the community. Circulation. 2009;120:2414–2420. doi: 10.1161/CIRCULATIONAHA.109.872705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franceschini G., Sirtori M., Vaccarino V. Mechanisms of HDL reduction after probucol. Changes in HDL subfractions and increased reverse cholesteryl ester transfer. Arteriosclerosis. 1989;9:462–469. doi: 10.1161/01.atv.9.4.462. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto A., Matsuzawa Y., Yokoyama S. Effects of probucol on xanthomata regression in familial hypercholesterolemia. Am J Cardiol. 1986;57:29H–35H. doi: 10.1016/0002-9149(86)90434-0. [DOI] [PubMed] [Google Scholar]
- 14.Quinet E.M., Huerta P., Nancoo D. Adipose tissue cholesteryl ester transfer protein mRNA in response to probucol treatment: cholesterol and species dependence. J Lipid Res. 1993;34:845–852. [PubMed] [Google Scholar]
- 15.Yamashita S., Matsuzawa Y. Where are we with probucol: a new life for an old drug? Atherosclerosis. 2009;207:16–23. doi: 10.1016/j.atherosclerosis.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 16.von Eckardstein A. Mulling over the odds of CETP inhibition. Eur Heart J. 2010;31:390–393. doi: 10.1093/eurheartj/ehp394. [DOI] [PubMed] [Google Scholar]
- 17.Reisinger U., Schwaiger S., Zeller I. Leoligin, the major lignan from Edelweiss, inhibits intimal hyperplasia of venous bypass grafts. Cardiovasc Res. 2009;82:542–549. doi: 10.1093/cvr/cvp059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwaiger S., Adams M., Seger C. New constituents of Leontopodium alpinum and their in vitro leukotriene biosynthesis inhibitory activity. Planta Med. 2004;70:978–985. doi: 10.1055/s-2004-832625. [DOI] [PubMed] [Google Scholar]
- 19.Schuster D. 3D Pharmacophores as tools for activity profiling. Drug Discov Today Technol. 2010;7:205–211. doi: 10.1016/j.ddtec.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Tancevski I., Frank S., Massoner P. Increased plasma levels of LDL cholesterol in rabbits after adenoviral over expression of human scavenger receptor class B type I. J Mol Med. 2005;83:927–932. doi: 10.1007/s00109-005-0695-8. [DOI] [PubMed] [Google Scholar]
- 21.Ritsch A., Auer B., Foger B. Polyclonal antibody-based immunoradiometric assay for quantification of cholesteryl ester transfer protein. J Lipid Res. 1993;34:673–679. [PubMed] [Google Scholar]
- 22.Borggreve S.E., Hillege H.L., Wolffenbuttel B.H. The effect of cholesteryl ester transfer protein -629C-> A promoter polymorphism on high-density lipoprotein cholesterol is dependent on serum triglycerides. J Clin Endocrinol Metab. 2005;90:4198–4204. doi: 10.1210/jc.2005-0182. [DOI] [PubMed] [Google Scholar]
- 23.Hoekstra M., Ye D., Hildebrand R.B., Zhao Y. Scavenger receptor class B type I-mediated uptake of serum cholesterol is essential for optimal adrenal glucocorticoid production. J Lipid Res. 2009;50:1039–1046. doi: 10.1194/jlr.M800410-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eller P., Eller K., Wolf A.M., Reinstadler S.J. Atorvastatin attenuates murine anti-glomerular basement membrane glomerulonephritis. Kidney Int. 2010;77:428–435. doi: 10.1038/ki.2009.478. [DOI] [PubMed] [Google Scholar]
- 25.Qiu X., Mistry A., Ammirati M.J. Crystal structure of cholesteryl ester transfer protein reveals a long tunnel and four bound lipid molecules. Nat Struct Mol Biol. 2007;14:106–113. doi: 10.1038/nsmb1197. [DOI] [PubMed] [Google Scholar]
- 26.Sirtori C.R., Mombelli G. Cholesteryl ester transfer protein antagonism by drugs – a poor choice. Clin Chem. 2010;56:1550–1553. doi: 10.1373/clinchem.2010.147389. [DOI] [PubMed] [Google Scholar]
- 27.Quintao E.C., Cazita P.M. Lipid transfer proteins: past, present and perspectives. Atherosclerosis. 2010;209:1–9. doi: 10.1016/j.atherosclerosis.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Heinecke J. HDL and cardiovascular-disease risk – time for a new approach? N Engl J Med. 2011;364:170–171. doi: 10.1056/NEJMe1012520. [DOI] [PubMed] [Google Scholar]
- 29.Sawayama Y., Shimizu C., Maeda N. Effects of probucol and pravastatin on common carotid atherosclerosis in patients with asymptomatic hypercholesterolemia. Fukuoka Atherosclerosis Trial (FAST) J Am Coll Cardiol. 2002;39:610–616. doi: 10.1016/s0735-1097(01)01783-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.