Abstract
The effects of cadmium (Cd; 0.1–1000 μM) and fusicoccin (FC) on growth, Cd2+ content, and membrane potential (E m) in maize coleoptile segments were studied. In addition, the E m changes and accumulation of Cd and calcium (Ca) in coleoptile segments treated with Cd2+ combined with 1 μM FC or 30 mM tetraethylammonium (TEA) chloride (K+-channel blocker) were also determined. In this study, the effects of Ca2+-channel blockers [lanthanum (La) and verapamil (Ver)] on growth and content of Cd2+ and Ca2+ in coleoptile segments were also investigated. It was found that Cd at high concentrations (100 and 1000 μM) significantly inhibited endogenous growth of coleoptile segments and simultaneously measured proton extrusion. FC combined with Cd2+ counteracted the toxic effect of Cd2+ on endogenous growth and significantly decreased Cd2+ content (not the case for Cd2+ at the highest concentration) in coleoptile segments. Addition of Cd to the control medium caused depolarization of E m, the extent of which was dependent on Cd concentration and time of treatment with Cd2+. Hyperpolarization of E m induced by FC was suppressed in the presence of Cd2+ at 1000 μM but not Cd2+ at 100 μM. It was also found that treatment of maize coleoptile segments with 30 mM TEA chloride caused hyperpolarization of E m and decreased Cd2+ content in coleoptile segments, suggesting that, in the same way as for FC, accumulation of Cd2+ was dependent on plasma membrane (PM) hyperpolarization. Similar to FC, TEA chloride also decreased Ca2+ content in coleoptile segments. La and Ver combined with Cd2+ (100 μM) significantly decreased Cd content in maize coleoptile segments, but only La completely abolished the toxic effect of Cd2+ on endogenous growth and growth in the presence of FC. Taken together, these results suggest that the mechanism by which FC counteracts the toxic effect of Cd2+ (except at 1000 μM Cd2+) on the growth of maize coleoptile segments involves both stimulation of PM H+-ATPase activity by FC as well as Cd2+-permeable, voltage-dependent Ca channels, which are blocked by FC and TEA chloride-induced PM hyperpolarization.
Cadmium (Cd) is widely acknowledged as being one of the most phytotoxic agents (reviewed in Das et al. 1997; Sanità di Toppi and Gabbrielli 1999; Seregin and Ivanov 2001; Pál et al. 2006) inhibiting plant growth when present in excess (Sandalio et al. 2001; Wójcik and Tukiendorf 2005; Nouairi et al. 2006; Karcz and Kurtyka 2007; Kurtyka et al. 2008). Because Cd is one of the most readily absorbed and most rapidly translocated heavy metals in plants, it exerts strong toxicity (Di Cagno et al. 1999; Pál et al. 2006). Among other detrimental effects of Cd2+, membrane damage and changes in enzyme activities affecting uptake and transport of mineral nutrients have been reported (Lindberg and Wingstrand 1985; Ros et al. 1990; Fodor et al. 1995; Llamas et al. 2000; Gonçalves et al. 2009). Several reports have shown that Cd2+ caused a decrease of K+ content in various plant materials (Rubio et al. 1994; Llamas et al. 2000; Kurtyka et al. 2008; Gonçalves et al. 2009).
Furthermore, it has also been shown that Cd induces depolarization of membrane potential (E m) Kennedy and Gonsalvez 1987; Aidid and Okamoto 1992; Llamas et al. 2000; Pavlovkin et al. 2006; Karcz and Kurtyka 2007), which probably results from decreased plasma membrane (PM) H+-ATPase activity (Ros et al. 1992; Fodor et al. 1995; Astolfi et al. 2003; Kabała et al. 2008), leading to decreased H+ extrusion (Astolfi et al. 2003; Karcz and Kurtyka 2007). In contrast to Cd2+, fusicoccin (FC), the fungal toxin produced by Fusicoccum amygdale Del., enhances elongation growth by stimulating H+ efflux driven by PM H+-ATPase (Marré 1979; Kutschera and Schopfer 1985; Lüthen et al. 1990; Hager et al. 1991; Karcz et al. 1995; Palmgren 1998; Karcz and Burdach 2002; Hager 2003). Simultaneously with FC-induced proton extrusion, hyperpolarization of the PM is observed (Cleland et al. 1977; Karcz and Burdach 2007), which in turn activates an inwardly rectifying K+ channel (K+in) (Philippar et al. 1999; Tode and Lüthen 2001). It is also establishes that an FC-binding site arises from interaction of the 14-3-3 protein dimer with the C-terminal autoinhibitory domain of the H+-ATPase and that FC stabilizes this complex (Jahn et al. 1997; Baunsgaard et al. 1998; Fuglsang et al. 1999; Oecking and Hagemann 1999; Würtele et al. 2003). Taking into account that FC causes effects opposite to those produced by Cd, it was interesting to study whether this phytotoxin is able to counteract the toxic effect of Cd on the growth of maize coleoptile segments. This experimental design can provide new data on the toxic effects of Cd on plant growth.
The main goal of our experiments was to study the mechanisms of Cd-induced inhibition of elongation growth and the mechanism of Cd uptake. This goal was realized by: (1) studying the effects of Cd2+ on growth in the presence or absence of FC while simultaneously measuring growth-medium pH changes; (2) establishing E m changes in parenchymal cells treated with Cd2+ or Cd2+ applied together with FC and tetraethylammonium (TEA) chloride; (3) determining Cd2+ and Ca2+ content in maize coleoptile segments incubated in medium containing Cd2+ or Cd2+ together with FC and TEA chloride; (4) studying the effects of Cd2+ on both growth and content of Cd2+ and Ca2+ in maize coleoptile segments incubated in the presence of FC combined with Ca2+-channel blockers [lanthanum chloride (La) and verapamil (Ver)].
Materials and Methods
Plant Material
Seeds of maize (Zea mays L. cv. K33 × F2) were soaked in tap water for 2 h, sown on wet wood in plastic boxes, and placed in a growth chamber at 27 ± 1°C. The experiments were performed with 10 mm-long coleoptile segments cut from 96 h old etiolated maize seedlings. Intact coleoptile segments, with the first leaves removed, were excised 3 mm below the tip and incubated in a control medium made of 1000 μM KCl, 100 μM NaCl, and 100 μM CaCl2.
Chemicals
FC (Sigma, USA) was dissolved in ethanol and added to the incubation medium at a final concentration of 1 μM. The maximal ethanol concentration of 0.2% did not affect growth of coleoptile segments (data not shown). CdCl2·2.5 H2O (Fluka, Switzerland) was dissolved in control medium. TEA chloride (Sigma, USA), La (Sigma, USA), and Ver (Sigma, USA) were dissolved in deionized water and used at a final concentration of 30 mM, 5 mM, and 50 μM, respectively. Stock solutions of TEA chloride, La, and Ver were prepared in concentrations that were 100-fold greater compared with those used in the experiments.
Growth and pH Measurements
Growth experiments were performed using an apparatus that allowed simultaneous measurement of elongation growth and pH of the incubation medium from the same tissue sample (Karcz et al. 1990; Karcz and Burdach 2002, 2007). The optical system used for growth measurement (shadow graph methods) permitted recording of the longitudinal extension of a stack of 21 segments. In this setup, the segments, after their excision, were incubated in 6.3 cm3 (0.3 cm3 segment−1) of an intensively aerated control medium (1000 μM KCl, 100 μM NaCl, and 100 μM CaCl2). As described previously by Karcz et al. (1995) and by Karcz and Burdach (2002), in this system the incubation medium also flowed through the lumen of the coleoptile cylinders. This feature permitted treatment solutions to be in direct contact with the interior of the segments, which significantly enhanced both the elongation growth of coleoptile segments as well as proton secretion (Karcz et al. 1995).
All manipulations and growth experiments were conducted under dim green light. The temperature of all solutions in the elongation and pH-measuring system was thermostatically controlled at 25 ± 0.5°C. pH measurements were performed with a pH meter (type CI-316; Elmetron, Poland) and pH electrode (OSH 10-10; Metron, Poland). Cd, FC, and calcium (Ca)-channel blockers (La or Ver) were introduced into the incubation medium at 120 min into the experiment. The initial pH of the incubation solution was adjusted to 5.8–6.0 with either 0.1 M NaOH or 0.1 M HCl.
Electrophysiology
The electrophysiological experiments were carried out with intact, 10 mm-long coleoptile segments that were prepared the same as for the growth experiments. E m was measured by recording the voltage between a 3 M KCl-filled glass micropipette inserted into the parenchymal cells and a reference electrode in the bathing medium containing the same composition as used in the growth experiments. For electrophysiological experiments, the segments were preincubated for 2 h in an intensively aerated bathing medium, whereupon the segments were transferred into a perfusion Plexiglass chamber mounted on a vertically placed microscope stage. The microelectrodes were inserted into cells under microscope by means of micromanipulator (Hugo Sachs Electronik; March-Hugstteten, Germany). Medium changes were performed by means of a peristaltic pump (Type Peri-Star PRO; World Precision Instruments, USA), which allowed changing of the bathing medium in the chamber (usually fourfold within <2 min). Micropipettes were pulled on a vertical pipette puller (model L/M-3P-A; List-Medical, Germany) from borosilicate glass capillaries (type 1B150F-3; World Precision Instruments, USA). Tip diameters measured <1 μm. Cd, FC, and TEA chloride were introduced into the control medium after stabilization (<10 min) of E m.
Determination of Cd and Ca Content
Cd and Ca concentrations in maize coleoptile segments were determined by emission spectrometry with excitation by way of an argon inductively coupled plasma technique by means of a Spektroflame-M spectrophotometer (Spectro Analytical Instruments, Germany). Before chemical analysis, 110 intact coleoptile segments were split along the long axis and preincubated for 2 h in an intensively aerated growth medium (control). The composition and volume (0.3 cm3 segment−1) of the incubation medium were the same as that used in the growth experiments. After preincubation of the coleoptile segments, Cd (0.1–1000 μM), Cd together with FC (1 μM), or TEA chloride (30 mM) were introduced into the incubation medium for 5 h. The variant in which these three components (Cd2+, FC, and TEA chloride) were combined was also studied. Moreover, Cd and Ca content in maize coleoptile segments exposed (for 5 h) to 100 μM Cd or Cd combined with (1 μM) FC or/and Ca-channel blockers (La and Ver) was also determined. In this case, the segments were first preincubated for 2 h in control medium, whereupon Cd or Cd with FC or/and Ca-channel blockers were added. After each treatment, the halves of the segments were removed from the solution and washed three times with deionized water, whereupon they were dried at 80°C to determine dry weight. For Cd and Ca analyses, dry plant tissue was mineralized. Each sample (approximately 0.2 g dry matter) was treated with 5 ml ultrapure concentrated nitric acid (Merck, Germany) and left for 24 h. Next, the samples were digested until complete mineralization was achieved. After mineralization, the samples were diluted with redistilled water to a volume of 10 ml. Concentrations of Cd and Ca were measured by inductively coupled plasma–atomic emission spectroscopy (frequency 27.12 MHz; power 1.0 kW; plasma gas 14.0 l/min; auxiliary gas 0.5 l/min; carrier gas 1 l/min; and analytical line Cd2+ 228.802 nm and Ca2+ 422.673 nm). As standards for control of the elemental analysis, Virginia tobacco leaves (CTA-VTL-2) were used; the results fit the range of certified recommended values. All experiments concerning the accumulation of Cd2+ and Ca2+ were replicated at least four times, and results are expressed as means ± SEs.
Statistical Analysis
Data were analyzed using computer software Statistical for Windows (StatSoft 2008; STATISTICA data analysis software system, version 8.0. http://www.statsoft.com, USA). Differences between individual treatments and control were analyzed using one-way analysis of variance and Fisher’s least significant difference (LSD) test. Statistical significance was defined at P < 0.05.
Results
Effect of Cd2+ on Endogenous and FC-Induced Growth of Coleoptile Segments Incubated With or Without Ca-Channel Blockers
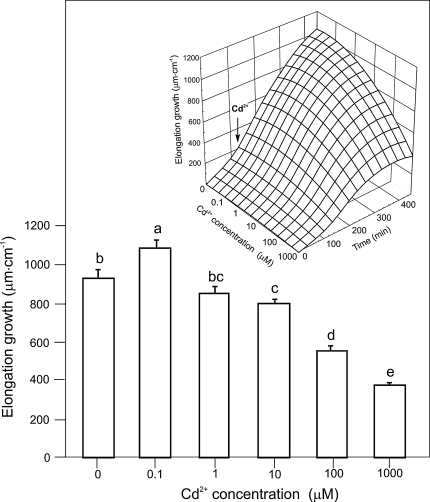
The effect of Cd2+ (0.1–1000 μM) on the growth of maize coleoptile segments incubated in control medium (endogenous growth) is shown in Fig. 1. As can be seen in this figure, Cd2+ applied to the medium (after 2 h; preincubation of segments in control medium) at concentrations of 10, 100, and 1000 μM decreased endogenous growth of maize coleoptile segments by 14, 40, and 60%, respectively. However, Cd at 1 μM did not cause any significant changes in endogenous growth (856.8 ± 32.6 μm cm−1) compared with the control (935.0 ± 43.9 μm cm−1). Even the lowest Cd concentration (0.1 μM) used stimulated (by 16%) the growth of maize coleoptile segments. The inset in Fig. 1 shows the time courses of the endogenous growth of maize coleoptile segments as a function of Cd2+ concentration.
Fig. 1.
Effect of Cd (0.1–1000 μM) on the endogenous growth (μm cm−1) of maize coleoptile segments. The elongation of a stack of 21 segments was measured as described in Materials and Methods. The growth of maize coleoptile segments after 7 h is shown (2 h of preincubation in control medium plus 5 h of incubation with Cd). The inset shows Cd dependence of endogenous growth as a function of time. After preincubation (2 h) of coleoptile segments in control medium, Cd was added (arrow). Values are means of ten independent experiments. Bars indicate means ± SEs. Statistical analysis (using software Statistica) showed that differences between values of elongation growth for control and 1.0 μM Cd2+ were statistically not significant at 420 min (LSD test P < 0.05)
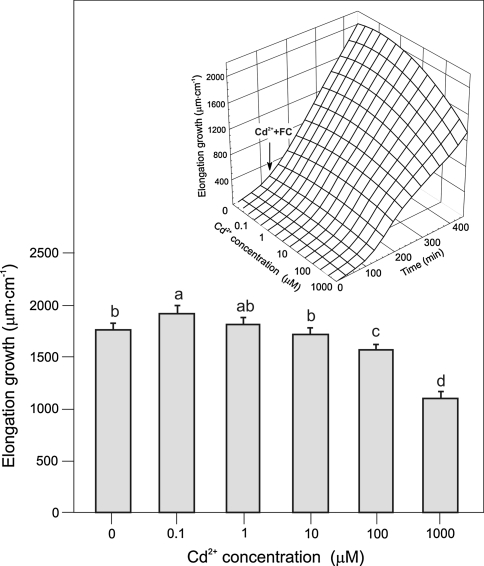
FC added to the control medium at 120 min rapidly enhanced endogenous growth of maize coleoptile segments (Fig. 2). It was found that FC-induced growth of maize coleoptile segments was enhanced approximately twofold compared with growth seen in control medium. Application of FC together with Cd2+ counteracted the toxic effect of Cd on endogenous growth, which means that growth of coleoptile segments in the presence of both substances was always greater than endogenous growth (935.0 ± 43.9 μm cm−1). In contrast, Cd2+ at 100 and 1000 μM inhibited FC-induced growth by 10 and 30%, respectively (Fig. 2). Moreover, the data presented here (Fig. 2) show that the growth of coleoptile segments treated with FC combined with 0.1 μM Cd2+ was greater than FC-induced growth (1764.5 ± 67 μm cm−1).
Fig. 2.
Effect of Cd (0.1–1000 μM) on growth (μm cm−1) of maize coleoptile segments incubated in the presence of 1 μM FC. The elongation of a stack of 21 segments was measured as described in Materials and Methods. The growth of maize coleoptile segments after 7 h is shown (2 h of preincubation in control medium plus 5 h of incubation with Cd combined with FC). The inset shows Cd dependence of growth in the presence of FC as a function of time. After preincubation (2 h) of coleoptile segments in the control medium, Cd combined with FC (1 μM) was added (arrow). Values are means of ten independent experiments. Bars indicate means ± SEs. Means followed by the same letter are not significantly different from each other (LSD test P < 0.05)
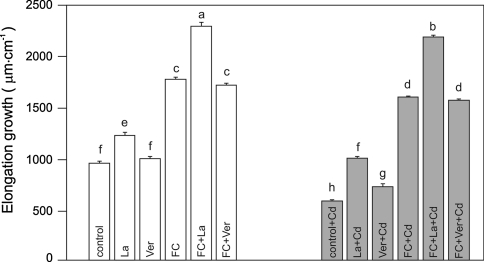
When Ver was added to the control medium at 50 μM, it did not change either endogenous or FC-induced growth of maize coleoptile segments (Fig. 3). However, La at 5 mM significantly stimulated (by 30%) both endogenous and FC-induced growth. As indicated in Fig. 3, La combined with Cd2+ abolished the toxic effect of Cd on both endogenous growth and growth in the presence of FC. Compared with La, Ver did not abolish the toxic effect of Cd2+ on FC-induced growth, and it only partly diminished the inhibitory effect of Cd2+ on endogenous growth (Fig. 3).
Fig. 3.
Effect of 100 μM Cd on endogenous and FC-induced growth (μm cm−1) of maize coleoptile segments incubated in the presence of Ca-channel blockers (La and Ver). Values are means of five independent experiments. Bars indicate means ± SEs. Means followed by the same letter are not significantly different from each other (LSD test P < 0.05)
Effect of Cd2+ on pH Changes of the Incubation Medium
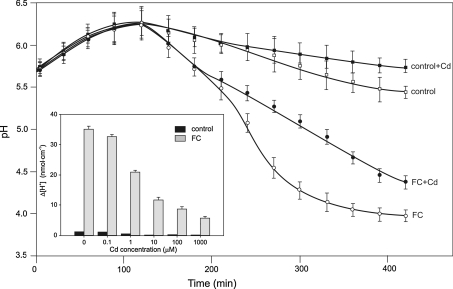
pH changes of the incubation medium were measured simultaneously with the elongation growth of coleoptile segments. The data in Fig. 4 indicate that coleoptile segments incubated in control medium characteristically changed the medium’s pH; usually within the first 2 h an increase in pH to 6.0–6.5 was observed, followed by a slow decrease in pH to approximately 5.5 after 7 h. When FC was added (after 2 h of preincubation) to the control medium, an additional decrease in pH to 4.0 was observed. However, when Cd2+ alone (at concentrations >0.1 μM) or Cd2+ together with FC was added at 120 min, significant suppression of medium acidification was found (Fig. 4 inset). For example, the H+ concentration in the medium found in the presence of Cd2+ (100 μM) combined with FC was approximately fourfold lower than with FC alone (Fig. 4 inset).
Fig. 4.
Effect of 100 μM Cd on pH changes of the incubation medium measured simultaneously (using the same tissue sample) with endogenous growth and growth in the presence of FC (1 μM). The segments were first preincubated (2 h) in control medium, whereupon FC, Cd, or Cd together with FC were added (arrow). Values for pH are means of ten independent experiments. Bars indicate means ± SEs. To avoid illegibility of the figure, only some curves have been shown. The inset shows Cd dependence of medium pH expressed as Δ [H+], where Δ [H+] indicates the difference between H+ concentration [H+] at 420 and 120 min. The differences between pH values for control medium, FC, Cd, or Cd combined with FC were statistically significant at 420 min (LSD test P < 0.05)
Effect of Cd2+, FC, and TEA Chloride on Em
Cd2+ ions administered into the control medium caused depolarization of E m, the extent of which was dependent on Cd2+ concentration and time after its addition (Table 1). For example, treatment of parenchymal cells of maize coleoptile segments with 100 or 1000 μM Cd2+ caused depolarization of E m by 55 and 67 mV, respectively. In turn, addition of FC to the control medium caused immediate hyperpolarization of E m by 23 mV (from −121 ± 4.1 to −144 ± 6.2 mV). This hyperpolarization of E m was suppressed in the presence of Cd2+ at 1000 μM but not with Cd2+ at 100 μM (Table 1). Cd2+ at 1000 μM not only suppressed FC-induced hyperpolarization of the E m but also caused additional membrane depolarization.
Table 1.
Em changes of parenchymal coleoptile cells with addition of Cd (100 and 1000 μM) and Cd together with FC (1 μM) and/or 30 mM TEA chloride
| Treatments | Em (mV) | |||
|---|---|---|---|---|
| Em after stabilization in control medium (time 0) | Em after 20 min | Em after 40 min | Em after 60 min | |
| FC | −121.0 ± 4.1 | −137.0 ± 5.5 | −144.2 ± 5.1 | −144.0 ± 6.2 |
| TEA chloride | −116.1 ± 4.6 | −123.8 ± 5.7 | −152.3 ± 4.8 | −149.3 ± 4.0 |
| TEA chloride + FC | −117.0 ± 6.2 | −129.1 ± 5.5 | −146.2 ± 11.7 | −146.2 ± 11.9 |
| Cd (100 μM) | −119.5 ± 8.4 | −112.8 ± 5.3 | −68.0 ± 3.2 | −64.4 ± 3.3 |
| FC + Cd | −118.8 ± 4.3 | −134.2 ± 7.5 | −145.3 ± 7.2 | −142.0 ± 8.5 |
| TEA chloride + Cd | −114.5 ± 6.3 | −127.7 ± 6.1 | −149.7 ± 6.8 | −143.4 ± 7.3 |
| TEA chloride + FC + Cd | −115.4 ± 5.0 | −121.6 ± 4.7 | −144.1 ± 6.3 | −146.0 ± 7.9 |
| Cd (1000 μM) | −122.0 ± 7.7 | −102.6 ± 4.2 | −55.9 ± 1.8 | −55.1 ± 2.6 |
| FC + Cd | −120.6 ± 8.1 | −117.8 ± 4.5 | −72.5 ± 3.8 | −60.5 ± 3.5 |
| TEA chloride + Cd | −119.2 ± 6.6 | −128.6 ± 8.5 | −136.3 ± 7.5 | −125.9 ± 6.3 |
| TEA chloride + FC + Cd | −121.4 ± 8.4 | −130.7 ± 8.2 | −134.9 ± 8.2 | −128.7 ± 6.9 |
At time 0, the control medium was changed for a new one, at the same salt composition, containing Cd, FC, TEA chloride, or Cd together with FC or/and TEA chloride. Em was measured continuously after 2 h of segment preincubation in control medium. Data are means of at least five independent experiments. Error indicates means ± SEs
TEA chloride applied to the control medium caused hyperpolarization of E m, during which E m became 33.2 ± 4.0 mV more negative than the original potential (−116.1 ± 4.6 mV) (Table 1). TEA chloride combined with Cd2+ counteracted this Cd2+-induced depolarization of E m (Table 1). Interestingly, Cd2+ at the highest concentration (1000 μM), but not Cd2+ at 100 μM, suppressed hyperpolarization of E m induced by TEA chloride. When FC and TEA chloride were combined, they did not cause any additional effect on E m compared with that induced by each substance separately. In turn, combined FC, TEA chloride, and Cd2+ counteracted depolarization of E m induced by Cd2+ (Table 1). This was similar to TEA chloride applied only with Cd2+, but it was not the case for FC applied with Cd2+ at 1000 μM.
Cd and Ca Content in Maize Coleoptile Segments
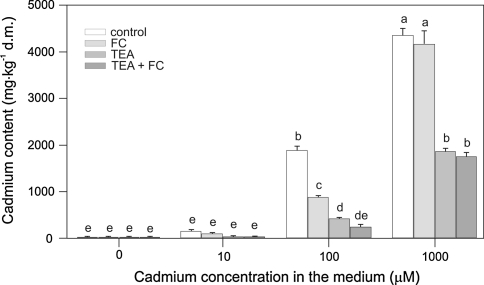
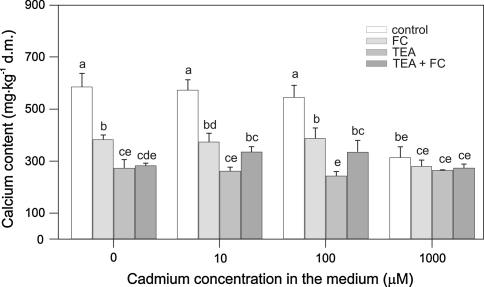
Cd and Ca content in maize coleoptile segments was also determined (Figs. 5, 6, 7). It was found that Cd content in maize coleoptile segments depended on Cd concentration in the control medium. An increase in Cd concentration from 10 to 100 μM caused an approximately 11-fold increase of its accumulation in maize coleoptile segments, whereas an increase in Cd2+ concentration from 100 to 1000 μM caused only a 2.3-fold increase of Cd2+ tissue content (Fig. 5). When FC was combined with Cd2+ at 100 μM, a 50% decrease of the Cd accumulation in coleoptile segments [from 1901 ± 103 to 895 ± 27 mg kg−1 dry mass (dm)] was observed compared with the segments treated with Cd2+ alone. FC did not change the content of Cd2+ in coleoptile segments treated with Cd2+ at 1000 μM. Furthermore, the effect of TEA chloride on Cd2+ and Ca2+ accumulation in segments of maize coleoptiles was also studied. These results indicate that TEA chloride decreased accumulation of both elements (Figs. 5, 6). In segments treated with TEA chloride combined with 100 or 1000 μM Cd2+, Cd2+ content was relatively lower compared with segments treated with Cd alone. As illustrated in Fig. 6, a total Ca2+ content of 573.32 ± 49.9 mg kg−1 (dm) was decreased approximately twofold after TEA chloride application. The application of TEA chloride together with FC or/and Cd2+ to the control medium led to significant decrease (P < 0.05) in Cd (Fig. 5) and Ca concentrations (Fig. 6).
Fig. 5.
Cd content in maize coleoptile segments exposed (5 h) to either 10, 100, or 1000 μM Cd, Cd combined with FC (1 μM), and/or 30 mM TEA. The segments were first preincubated (2 h) in control medium, whereupon Cd or Cd with FC or/and TEA were added. Results are the means of four independent experiments. Bars indicate means ± SEs. Means followed by the same letter are not significantly different from each other (LSD test P < 0.05)
Fig. 6.
Ca content in maize coleoptile segments exposed (5 h) to either 10, 100, or 1000 μM Cd, Cd combined with FC (1 μM), and/or 30 mM TEA. The segments were first preincubated (2 h) in control medium, whereupon Cd or Cd with FC or/and TEA were added. Results are the means of four independent experiments. Bars indicate means ± SEs. Means followed by the same letter are not significantly different from each other (LSD test P < 0.05)
Fig. 7.
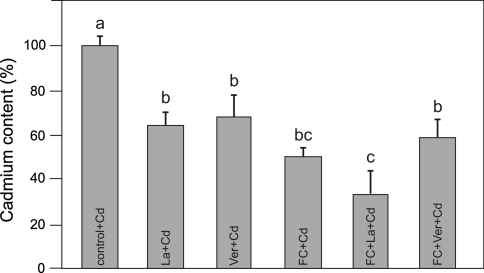
Cd content in maize coleoptile segments exposed (5 h) to either 100 μM Cd, Cd combined with FC (1 μM), and/or Ca-channel blockers (La and Ver). The segments were first preincubated (2 h) in control medium, whereupon Cd or Cd with FC or/and Ca-channel blockers (La and Ver) were added. Results are the means of four independent experiments. Bars indicate means ± SEs. Means followed by the same letter are not significantly different from each other (LSD test P < 0.05)
Interestingly, FC alone or FC combined with Cd2+ decreased Ca content in maize coleoptile segments (Fig. 6). In addition, Cd2+ at the highest concentration (1000 μM) decreased Ca content by 50%, probably as a result of Cd competitively inhibiting Ca uptake. Both FC and Ca-channel blockers (La and Ver) significantly decreased (LSD test P < 0.05) Cd content in maize coleoptile segments (Fig. 7). La and Ver practically did not change Ca content in maize coleoptile segments (data not shown).
Discussion
Despite there being much research on Cd toxicity, our knowledge concerning the effect of this heavy metal on plant growth is still limited. The main goal of this work was to determine the mechanisms of Cd-induced inhibition of elongation growth as well as the mechanism of Cd uptake.
It is currently well established that an FC-binding site arises from the interaction of 14-3-3 protein dimer with the C-terminal autoinhibitory domain of PM H+-ATPase, and FC stabilizes this complex, thus causing an increase in proton pump activity (Jahn et al. 1997; Baunsgaard et al. 1998; Fuglsang et al. 1999; Oecking and Hagemann 1999; Würtele et al. 2003). The data presented in this article, i.e., that FC causes (1) acceleration of elongation growth compared with endogenous growth (Figs. 1, 2), (2) enhancement of proton extrusion compared with FC-free medium (Fig. 4), and (3) immediate hyperpolarization of the PM (Table 1), are in good agreement with the results obtained by other investigators (Cleland et al. 1977; Kutschera and Schopfer 1985; Felle 1989; Lüthen et al. 1990; Rück et al. 1993; Karcz and Burdach 2002; Hager 2003; Karcz and Burdach 2007).
The simultaneous measurements of elongation growth and pH level of the incubation medium showed that in maize coleoptile segments, Cd2+ at >1 μM produced a significant inhibition of endogenous growth (Fig. 1) as well as a decrease in proton extrusion (Fig. 4). Cd2+ also caused depolarization of E m (Table 1). These findings match strikingly with Cd2+ content in maize coleoptile segments (Figs. 5, 7) and support the results of our previous experiments (Karcz and Kurtyka 2007). It should be pointed out that the large range of Cd concentrations used in our experiments showed that concentrations effectively producing growth inhibition are high compared with concentrations usually found in soil solutions. This discrepancy, among others, results from the fact that in the case of environmental studies, durations of growth experiments are usually significantly longer compared with studies using a model system, such as coleoptile segments, which, as our experiments showed, need greater Cd concentration. The inhibition of proton extrusion and depolarization of E m observed here in the presence of Cd2+ are in line with the findings of other investigators, who reported an inhibition of PM H+-ATPase (Ros et al. 1992; Fodor et al. 1995; Astolfi et al. 2003) and depolarization of E m by Cd2+ (Kennedy and Gonsalvez 1987; Aidid and Okamoto 1992; Llamas et al. 2000; Pavlovkin et al. 2006).
Addition of FC together with Cd2+ counteracted the toxic effect of Cd on endogenous growth (growth in control) of maize coleoptile segments, although Cd2+ at concentrations of 100 and 1000 μM inhibited FC-induced growth by 10 and 30%, respectively (Fig. 2). The first phenomenon is probably due to stimulation of the segment elongation by FC, which, according to the “acid growth theory,” enhances growth by stimulating proton extrusion through PM H+-ATPase. It is suggested that this proton extrusion is large enough to overcome the toxic effect of Cd2+ on endogenous growth (Fig. 4). In contrast, Cd2+ at concentrations of 100 and 1000 μM diminished FC-induced growth and proton extrusion, probably as a result of PM H+-ATPase inhibition. This last suggestion is also supported by the experiments in which FC-induced E m hyperpolarization was suppressed by Cd2+ at a concentration of 1000 μM. Until now, there has been no doubt that FC-induced PM hyperpolarization is a consequence of a stimulated proton extrusion through PM H+-ATPase (Cleland et al. 1977; Marré 1979; Kutschera and Schopfer 1985; Karcz et al. 1995; Karcz and Burdach 2007).
The results presented here also show that FC combined with Cd2+ at 100 μM decreased (by 50%) Cd content in maize coleoptile segments (Figs. 5, 7). This lower Cd2+ concentration could also decrease the toxic effect of Cd2+ (at least for Cd2+ at 100 μM) on elongation growth of coleoptile segments. In the case of greater Cd2+ concentration (1000 μM), FC (but not TEA chloride) was not effective in either recovering Cd2+-induced membrane depolarization or decreasing Cd content in maize coleoptile cells. This finding is probably due to irreversible inhibition of PM H+-ATPase by such high Cd concentrations. In trying to explain the effect of FC on Cd2+ accumulation in maize coleoptile segments, we assumed that Cd enters plant cells by way of Ca channels. Such a possibility has previously been described in plants and animals (Perfus-Barbeoch et al. 2002 and literature therein). It is also known that activation of PM Ca channels and resulting Ca2+ influx depends on the E m (for review see White 2000).
Taking into account that FC combined with Cd2+ at 100 μM hyperpolarized E m potential of the parenchymal cells (Table 1), it could be suggested that the Ca channels (probably depolarization-activated Ca2+ channels) are closed, thus decreasing Cd2+ influx into the cell. These findings are also supported by the fact that FC alone, or FC combined with Cd2+, decreased Ca content cells of coleoptile segments (Fig. 6). To prove our hypothesis that Cd2+ enters plant cells by way of Ca channels, we performed experiments with Ca-channel blockers (La and Ver) and also with TEA chloride, which blocks potassium channels (Thiel et al. 1996; Claussen et al. 1997; White 2000; Tode and Lüthen 2001; Osawa and Matsumoto 2002; Lindberg et al. 2004; Shishova and Lindberg 2004) as well as Ca channels (White 2000; White et al. 2002). As indicated in Fig. 7, both La and Ver significantly decreased Cd2+ content in maize coleoptile segments. This finding is probably due to the blocking of Ca channels, by which Cd2+ ions enter cells. TEA chloride was not used in growth experiments because it inhibits FC-induced growth in maize coleoptile segments, probably as a result of K+ inward rectifying (ZMK1) channel inhibition (Philippar et al. 1999; Tode and Lüthen 2001; Siemieniuk 2006). However, it was found that TEA chloride was much more effective than FC in (1) PM hyperpolarization (Table 1), (2) decreasing Cd and Ca content in coleoptile segments treated with Cd2+ (Figs. 5, 6), and (3) recovery of E m after its depolarization induced by the highest Cd2+ concentration (Table 1). Our data showing the effects of TEA chloride on E m of plant cells are consistent with results obtained by Olivetti et al. (1995) and Siemieniuk (2006), who showed that TEA chloride hyperpolarized the E m of root cap cells of Phaseolus vulgaris and parenchymal cells of Zea mays coleoptile segments, respectively. It also has been shown previously (Pavlovkin et al. 2006) that FC added to Cd2+-treated (1 mM) cortical cells in maize roots accelerated recovery of E m.
The results reported by Lindberg et al. (2004) with protoplasts isolated from wheat seedlings should also be mentioned. These investigators showed that Cd2+ uptake into the cytosol of wheat leaves and root protoplasts was inhibited by Ca and potassium chloride as well as by Ver and TEA chloride (inhibitors of Ca and potassium channels, respectively). They suggested that Cd uptake into the cytosol of wheat protoplasts is mediated in part by channels permeable to Ca and potassium and is dependent on E m. Lindberg et al. (2004) suggested that Cd2+ can be partly taken up by K+-channels because TEA chloride inhibits Cd2+ uptake. However TEA chloride could also inhibit PM Ca2+-channels (White 2000; White et al. 2002) excluding a role of K+-channels in decreasing Cd2+ uptake. Our results with TEA chloride also support this hypothesis because TEA chloride significantly diminished uptake of Cd2+ and Ca2. Because TEA chloride inhibits FC-induced growth (Tode and Lüthen 2001; Siemieniuk 2006), probably as a result of ZMK1 channel inhibition, it is unlikely that Cd2+ enters the cells by way of K+-channels. We showed that hyperpolarization of PM, rather than its depolarization observed in the presence of metabolic inhibitors and TEA chloride (Lindberg et al. 2004), is responsible for decreasing Cd2+ uptake by coleoptile segments.
The results presented here are generally in agreement with data obtained by Perfus-Barbeoch et al. (2002), who showed in patch-clamp experiments with Vicia faba guard cell protoplasts that PM K+-channels were insensitive to external Cd2+ application, whereas Ca2+-channels were found to be permeable to Cd2+. They also showed that Cd2+ enters cells by way of hyperpolarization-activated Ca2+-channels. In contrast to Perfus-Barbeoch et al. (2002), we suggest that Cd2+ enters the cells of maize coleoptile segments through depolarization-activated Ca2+-channels. Such channels have been characterized from suspension cultures of mesophyll and root cells (Thuleau et al. 1994a, b; Pineros and Tester 1997; White 2000). All of them are activated at voltages less negative than −140 mV.
In conclusion, the results presented in this article demonstrate that the mechanism by which FC counteracts the toxic effects of Cd2+ (except for 1000 μM Cd2+) on the growth of maize coleoptile segments involves both stimulation of PM H+-ATPase activity by FC and Cd2+-permeable, voltage-dependent Ca channels, which are blocked by FC- and TEA chloride-induced PM hyperpolarization.
Acknowledgments
We thank Iza K. Radecka and Terence J. Bartlett (University of Wolverhampton, School of Applied Sciences, Wolverhampton, GB) for critical reading of this manuscript and correcting the English text.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Aidid SB, Okamoto H. Effects of lead, cadmium and zinc on the electric membrane potential at the xylem/symplast interface and cell elongation of Impatiens balsamina. Environ Exp Bot. 1992;32:439–448. doi: 10.1016/0098-8472(92)90056-8. [DOI] [Google Scholar]
- Astolfi S, Zuchi S, Chiani A, Passera C. In vivo and in vitro effects of cadmium on H+ATPase activity of plasma membrane vesicles from oat (Avena sativa L.) roots. J Plant Physiol. 2003;160:387–393. doi: 10.1078/0176-1617-00832. [DOI] [PubMed] [Google Scholar]
- Baunsgaard L, Fuglsang AT, Jahn T, Korthout HAAJ, De Boer AH, Palmgren MG. The 14-3-3 proteins associate with the plasma membrane H+-ATPase to generate a fusicoccin binding complex and a fusicoccin responsive system. Plant J. 1998;13:661–671. doi: 10.1046/j.1365-313X.1998.00083.x. [DOI] [PubMed] [Google Scholar]
- Claussen M, Lüthen H, Blatt M, Böttger M. Auxin-induced growth and its linkage to potassium channels. Planta. 1997;201:227–234. doi: 10.1007/BF01007708. [DOI] [Google Scholar]
- Cleland RE, Prins HBA, Harper JR, Higinbotham W. Rapid hormone-induced hyperpolarization of the oat coleoptile transmembrane potential. Plant Physiol. 1977;59:395–397. doi: 10.1104/pp.59.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P, Samantaray S, Rout GR. Studies on cadmium toxicity in plant: a review. Environ Pollut. 1997;98:29–36. doi: 10.1016/S0269-7491(97)00110-3. [DOI] [PubMed] [Google Scholar]
- Di Cagno R, Guidi L, Stefani A, Soldatini GF. Effects of cadmium on growth of Helianthus annuus seedlings: physiological aspects. New Phytol. 1999;144:65–71. doi: 10.1046/j.1469-8137.1999.00497.x. [DOI] [Google Scholar]
- Felle H. pH as a second messenger in plants. In: Boss WF, Morre DJ, editors. Second messengers in plant growth and development. New York: Liss; 1989. pp. 145–166. [Google Scholar]
- Fodor E, Szabó-Nagy A, Erdei L. The effects of cadmium on the fluidity and H+-ATPase activity of plasma membrane from sunflower and wheat. J Plant Physiol. 1995;147:87–92. [Google Scholar]
- Fuglsang AT, Visconti S, Drumm K, Jahn T, Stensballe A, Mattei B, et al. Binding of 14-3-3 protein to the plasma membrane H+-ATPase AHA2 involves the three C-terminal residues Tyr(946)-thr-Val and requires phosphorylation of Thr(947) J Biol Chem. 1999;274:36774–36780. doi: 10.1074/jbc.274.51.36774. [DOI] [PubMed] [Google Scholar]
- Gonçalves JF, Goldschmidt FA, Maldaner J, Pereira LB, Tabaldi LA, Rauber R, et al. Cadmium and mineral nutrient accumulation in potato plantlets growth under cadmium stress in two different experimental culture conditions. Plant Physiol Biochem. 2009;47:814–821. doi: 10.1016/j.plaphy.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Hager A. Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: historical and new aspects. J Plant Res. 2003;116:483–505. doi: 10.1007/s10265-003-0110-x. [DOI] [PubMed] [Google Scholar]
- Hager A, Debus G, Edel HG, Stransky H, Serrano R. Auxin induces exocitosis and the rapid synthesis of a high turnover pool of plasma membrane H+-ATPase. Planta. 1991;185:527–537. doi: 10.1007/BF00202963. [DOI] [PubMed] [Google Scholar]
- Jahn T, Fuglesang AT, Olsson A, Brϋntrup IM, Collinge DB, Volkmann D, et al. The 14-3-3 protein interacts directly with the C-terminal region of the plant plasma membrane H+-ATPase. Plant Cell. 1997;9:1805–1814. doi: 10.1105/tpc.9.10.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabała K, Janicka-Russak M, Burzyński M, Kłobus G. Comparison of heavy metal effect on the proton pumps of plasma membrane and tonoplast in cucumber root cells J. Plant Physiol. 2008;165:278–288. doi: 10.1016/j.jplph.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Karcz W, Burdach Z. A comparison of the effects of IAA and 4-Cl-IAA on growth, proton secretion and membrane potential in maize coleoptile segments. J Exp Bot. 2002;53:1089–1098. doi: 10.1093/jexbot/53.371.1089. [DOI] [PubMed] [Google Scholar]
- Karcz W, Burdach Z. Effects of temperature on growth, proton extrusion and membrane potential in maize (Zea mays L) coleoptile segments. Plant Growth Regul. 2007;52:141–150. doi: 10.1007/s10725-007-9184-0. [DOI] [Google Scholar]
- Karcz W, Kurtyka R. Effect of cadmium on growth, proton extrusion and membrane potential in maize coleoptile segments. Biol Plantarum. 2007;51:713–719. doi: 10.1007/s10535-007-0147-0. [DOI] [Google Scholar]
- Karcz W, Stolarek J, Pietruszka M, Małkowski E. The dose-response curves for IAA induced elongation growth and acidification of the incubation medium of Zea mays L. coleoptile segments. Physiol Plant. 1990;80:257–261. doi: 10.1111/j.1399-3054.1990.tb04405.x. [DOI] [Google Scholar]
- Karcz W, Stolarek J, Lekacz H, Kurtyka R, Burdach Z. Comparative investigation of auxin and fusicoccin-induced growth and H+-extrusion in coleoptile segments of Zea mays L. Acta Physiol Plant. 1995;17:3–8. [Google Scholar]
- Kennedy CD, Gonsalvez FAN. The action of divalent zinc, cadmium, mercury, copper and lead on the trans-root potential and H+ efflux of excised roots. J Exp Bot. 1987;38:800–817. doi: 10.1093/jxb/38.5.800. [DOI] [Google Scholar]
- Kurtyka R, Małkowski E, Kita A, Karcz W. Effect of calcium and cadmium on growth and accumulation of cadmium, calcium, potassium and sodium in maize seedlings. Pol J Environ Stud. 2008;17:51–56. [Google Scholar]
- Kutschera U, Schopfer P. Evidence for the acid-growth theory of fusiccocin action. Planta. 1985;163:494–499. doi: 10.1007/BF00392706. [DOI] [PubMed] [Google Scholar]
- Lindberg S, Wingstrand G. Mechanism for Cd2+ inhibition of (K+ + Mg2+) ATPase activity and K+ (86Rb+) uptake in roots of sugar beet (Beta vulgaris) Physiol Plant. 1985;63:181–186. doi: 10.1111/j.1399-3054.1985.tb01900.x. [DOI] [Google Scholar]
- Lindberg S, Landberg T, Greger M. A new method to detect cadmium uptake in protoplasts. Planta. 2004;219:526–532. doi: 10.1007/s00425-004-1256-z. [DOI] [PubMed] [Google Scholar]
- Llamas A, Ullrich CI, Sanz A. Cd2+ effects on transmembrane electrical potential difference, respiration and membrane permeability of rice (Oryza sativa L.) roots. Plant Soil. 2000;219:21–28. doi: 10.1023/A:1004753521646. [DOI] [Google Scholar]
- Lüthen H, Bigdon M, Böttger M. Reexamination of the acid growth theory of auxin action. Plant Physiol. 1990;93:931–939. doi: 10.1104/pp.93.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marré E. Fusicoccin: a tool in plant physiology. Annu Rev Plant Physiol. 1979;30:273–288. doi: 10.1146/annurev.pp.30.060179.001421. [DOI] [Google Scholar]
- Nouairi I, Ammar WB, Youssef NB, Daoud DBM, Ghorbal MH, Zarrouk M. Comparative study of cadmium effects on membrane lipid composition of Brassica juncea and Brassica napus leaves. Plant Sci. 2006;170:511–519. doi: 10.1016/j.plantsci.2005.10.003. [DOI] [Google Scholar]
- Oecking C, Hagemann K. Association of 14-3-3 proteins with the C-terminal autoinhibitory domain of the plant plasma membrane H+-ATPase generates a fusicoccin-binding complex. Planta. 1999;207:480–482. doi: 10.1007/s004250050507. [DOI] [Google Scholar]
- Olivetti GP, Cumming JR, Etherton B. Membrane potential depolarization of root cap cells precedes aluminum tolerance in snapbean. Plant Physiol. 1995;109:123–129. [Google Scholar]
- Osawa H, Matsumoto H. Aluminium triggers malate-independent potassium release via ion channel from the root apex in wheat. Planta. 2002;215:405–412. doi: 10.1007/s00425-002-0767-8. [DOI] [PubMed] [Google Scholar]
- Pál M, Horváth E, Janda T, Páldi E, Szalai G. Physiological changes and defense mechanisms induced by cadmium stress in maize. J Plant Nutr Soil Sci. 2006;169:1–8. doi: 10.1002/jpln.200520573. [DOI] [Google Scholar]
- Palmgren MG. Proton gradients and plant growth: role of the plasma membrane H+-ATPase. Adv Bot Res. 1998;28:1–70. doi: 10.1016/S0065-2296(08)60293-1. [DOI] [Google Scholar]
- Pavlovkin J, Luxová M, Mistríková I, Mistrík I. Short- and long-term effects of cadmium on transmembrane electric potential (Em) in maize roots. Biol Bratislava. 2006;61:109–114. doi: 10.2478/s11756-006-0016-x. [DOI] [Google Scholar]
- Perfus-Barbeoch L, Leonhardt N, Vavasseur A, Forestier C. Heavy metal toxicity: cadmium permeates through calcium channels and disturbs the plant water status. Plant J. 2002;32:539–548. doi: 10.1046/j.1365-313X.2002.01442.x. [DOI] [PubMed] [Google Scholar]
- Philippar K, Fuchs I, Luthen H, Hoth S, Bauer CS, Haga K, et al. Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. PNAS. 1999;96:12186–12191. doi: 10.1073/pnas.96.21.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineros M, Tester M. Calcium channels in greater plant cells: selectivity, regulation and pharmacology. J Exp Bot. 1997;48:551–577. doi: 10.1093/jxb/48.Special_Issue.551. [DOI] [PubMed] [Google Scholar]
- Ros R, Cooke DT, Burden RS, James CS. Effects of the herbicide MCPA, and the heavy metals, cadmium and nickel on the lipid composition, Mg2+-ATPase activity and fluidity of plasma membranes from rice, Oryza sativa (cv. Bahia) shoots. J Exp Bot. 1990;41:457–462. doi: 10.1093/jxb/41.4.457. [DOI] [Google Scholar]
- Ros R, Morales A, Segura J, Picazo I. In vivo and in vitro effects of nickel and cadmium on the plasmalemma ATPase from rice (Oryza sativa L.) shoots and roots. Plant Sci. 1992;83:1–6. doi: 10.1016/0168-9452(92)90055-Q. [DOI] [Google Scholar]
- Rubio MI, Escrig I, Martinez-Cortina C, López-Benet FJ, Sanz A. Cadmium and nickel accumulation in rice plants. Effects on mineral nutrition and possible interactions of abscisic and gibberellic acids. Plant Growth Regul. 1994;14:151–157. doi: 10.1007/BF00025217. [DOI] [Google Scholar]
- Rück A, Palme K, Venis MA, Napier RM, Felle H. Patch-clamp analysis establishes a role for an auxin binding protein in the auxin stimulation of plasma membrane current in Zea mays protoplasts. Plant J. 1993;4:41–46. doi: 10.1046/j.1365-313X.1993.04010041.x. [DOI] [Google Scholar]
- Sandalio LM, Dalurzo HC, Gómez M, Romero-Puertas MC, del Río LA. Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot. 2001;52:2115–2126. doi: 10.1093/jexbot/52.364.2115. [DOI] [PubMed] [Google Scholar]
- Sanità di Toppi L, Gabbrielli R. Response to cadmium in higher plans. Environ Exp Bot. 1999;41:105–130. doi: 10.1016/S0098-8472(98)00058-6. [DOI] [Google Scholar]
- Seregin IV, Ivanov VB. Physiological aspects of cadmium and lead toxic effects on higher plants. Russ J Plant Physiol. 2001;48:523–544. doi: 10.1023/A:1016719901147. [DOI] [Google Scholar]
- Shishova M, Lindberg S. Auxin induces an increase of Ca2+ concentration in the cytosol of wheat leaf protoplasts. J Plant Physiol. 2004;161:937–945. doi: 10.1016/j.jplph.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Siemieniuk A (2006) Doctoral thesis, The effect of potassium and calcium on IAA and FC-induced elongation growth of maize (Zea mays L.) coleoptile segments. University of Silesia, Katowice, Poland
- Thiel G, Brüdern A, Gradmann D. Small inward rectifying K+ channels in coleoptiles: inhibition by external Ca2+ and function in cell elongation. J Membr Biol. 1996;149:9–20. doi: 10.1007/s002329900002. [DOI] [PubMed] [Google Scholar]
- Thuleau P, Moreau M, Schroeder JI, Ranjeva R. Recruitment of plasma membrane voltage-dependent calcium-permeable channels in carrot cells. EMBO J. 1994;13:5843–5847. doi: 10.1002/j.1460-2075.1994.tb06928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuleau P, Ward JM, Ranjeva R, Schroeder JI. Voltage-dependent calcium-permeable channels in the plasma membrane of a higher plant cells. EMBO J. 1994;13:2970–2975. doi: 10.1002/j.1460-2075.1994.tb06595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tode K, Lüthen H. Fusicoccin- and IAA-induced elongation growth share the same pattern of K+ dependence. J Exp Bot. 2001;52:251–255. doi: 10.1093/jexbot/52.355.251. [DOI] [PubMed] [Google Scholar]
- White PJ. Calcium channels in higher plant. Biochim Biophys Acta. 2000;1465:171–189. doi: 10.1016/S0005-2736(00)00137-1. [DOI] [PubMed] [Google Scholar]
- White PJ, Bowen HC, Demidchik V, Nichols C, Davies JM. Genes for calcium-permeable channels in the plasma membrane of plant root cells. Biochim Biophys Acta. 2002;1564:299–309. doi: 10.1016/S0005-2736(02)00509-6. [DOI] [PubMed] [Google Scholar]
- Wójcik M, Tukiendorf A. Cadmium uptake, localization and detoxification in Zea mays. Biol Plantarum. 2005;49:237–245. doi: 10.1007/s10535-005-7245-7. [DOI] [Google Scholar]
- Würtele M, Jelich-Ottmann Ch, Wittinghofer A, Oecking C. Structural view of a fungal toxin acting on a 14-3-3 regulatory complex. EMBO J. 2003;22:987–994. doi: 10.1093/emboj/cdg104. [DOI] [PMC free article] [PubMed] [Google Scholar]