Abstract
Several potential reduced exposure products (PREPs) for smokeless tobacco (SLT) users are marketed in the United States, though their effects are largely unknown. These products include some that are low in tobacco-specific nitrosamines (TSNs), like Stonewall, a pressed tobacco tablet, and General snus, a moist snuff product produced in Sweden. Methodology assessing the toxicant exposure and effects of cigarette-like PREPs for smokers has been developed, and might be modified for use in evaluating PREPs for SLT users. This report describes two studies examining the toxicant exposure and effects of two PREPs for SLT users. Study 1 (n = 13) consisted of four 4.5-hr laboratory sessions where SLT products (own brand, Stonewall, General snus, and tobacco-free placebo) were used for four 30-min episodes and nicotine exposure and tobacco/nicotine abstinence symptoms were measured. Study 2 (n = 19) consisted of four 5-day ad libitum use periods when participants used own brand, Stonewall, General snus, or no SLT and urinary levels of metabolites of nicotine (cotinine) and the TSN 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNAL) and abstinence symptoms were measured. Compared with own brand, Stonewall was associated with lower levels of cotinine and NNAL, while General snus was associated with similar levels of cotinine and lower levels of NNAL. Abstinence symptoms generally did not differ across tobacco conditions. These results show that clinical laboratory methods can be used to evaluate the toxicant exposure and abstinence symptom suppression associated with PREPs for SLT users.
Introduction
Tobacco use is responsible for over 440,000 deaths annually in the United States (Centers for Disease Control and Prevention [CDC], 2002). Most of these deaths are attributable to cigarette smoking, though smokeless tobacco (SLT) use is also associated with tobacco-related mortality (USDHHS, 1986). Like cigarette smoke, SLT contains a variety of toxicants and its use is linked to cancer (e.g., head/neck, larynx, esophagus, and pancreas) and cardiovascular disease, as well as oral cavity disorders such as leukoplakia, tooth loss, and gum recession (Henley, Thun, Connell, & Calle, 2005; USDHHS, 1986). In particular, SLT-related cancers are likely related to user exposure to known carcinogens such as tobacco-specific nitrosamines (TSNs; Hoffman & Hoffman, 1997). SLT users are also exposed to the dependence-producing drug nicotine at levels that are similar or higher than cigarette smokers, and SLT use episodes are longer than cigarette use episodes, leading to more prolonged nicotine exposure for SLT users (e.g., Benowitz, Porchet, Sheiner, & Jacob, 1988; Fant, Henningfield, Nelson, & Pickworth, 1999). As with cigarettes, SLT use supports tobacco/nicotine dependence, as evidenced by abstinence-induced symptoms that are suppressed by subsequent tobacco or pharmaceutical nicotine administration (e.g., Ebbert et al., 2007; Hatsukami & Severson, 1999; Hatsukami et al., 2000).
Recently the tobacco industry has begun marketing SLT products with the stated intent of reducing users’ exposure to some tobacco-related toxicants. For example, Star Scientific markets Stonewall, a tobacco tablet that is low in at least one TSN (i.e., 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, or NNK; Stepanov, Jensen, Hatsukami, & Hecht, 2006) and Swedish Match markets a range of “snus” products (e.g., General snus, also low in some TSNs; Stepanov et al., 2006). For cigarette-like PREPs, laboratory methods have been developed to test marketing claims regarding toxicant exposure (Breland, Acosta, & Eissenberg, 2003; Breland, Buchhalter, Evans & Eissenberg, 2002; Breland, Evans, Buchhalter, & Eissenberg, 2002). However, these methods have not been adapted for PREPs for SLT users. Indeed, only two published studies have examined the toxicant exposure associated with PREPS for SLT users. In one, Stonewall delivered nicotine and suppressed craving (Kotlyar et al., 2007). In the other, switching from normally marketed SLT brands to General snus reduced NNK exposure significantly (as indexed by urinary levels of NNAL, an NNK metabolite; Hatsukami et al., 2004). The purpose of the current report is to adapt efficient and reliable methods used to examine the withdrawal suppression and toxicant exposure associated with cigarette-like PREPs to examine the short- and longer-term effects of PREPs for SLT users.
Study 1
Study 1 used methods adapted from short-term evaluations of the nicotine exposure and withdrawal suppression associated with cigarette-like PREP use (e.g., Buchhalter & Eissenberg, 2000; Buchhalter, Schrinel, & Eissenberg, 2001; Breland, Buchhalter, et al., 2002; Breland, Evans, et al., 2002).
Method
Participants
Participants in this IRB-approved study were recruited by advertisement and word-of-mouth. Sessions took place at Virginia Commonwealth University’s Clinical Behavioral Pharmacology Laboratory. A telephone interview preceded an in-person screening visit, where informed consent was obtained.
Participants (n = 13; 1 female, 0 nonwhite) were included if they were between ages 18 and 50 years (M = 29.2 years, SD = 9.8 years), generally healthy by self-report, reported using five or fewer smoked tobacco products in the last six months, and reported current use of SLT on a daily basis for the last 12 months (M = 4.6 uses/day, SD = 2.6 for a mean of 6.3 years, SD = 5.4). Participants reported their usual brands as Skoal (n = 8), Kodiak (n = 3), and Copenhagen (n = 2). Current SLT use was verified with a combination of low CO (<7 ppm; M = 2.4 ppm, SD = 1.3; Vitalograph Breath CO, Lenexa, KS) and urinary cotinine levels indicative of current tobacco use (≥200 ng/ml, as indexed by a value ≥4 of 6 possible on a semi-quantitative immunoassay test, NicAlert™, NYMOX Corporation; M = 5.9, SD = 0.3). Participants were excluded if they reported any of the following: history of chronic health or psychiatric conditions, history of or active cardiovascular disease, current pregnancy (nonpregnancy was also confirmed via urinalysis), current breastfeeding, low or high blood pressure, seizures, or regular use of prescription medication (other than vitamins or birth control).
Procedure
Each qualified participant completed four, Latin-square ordered, approximately 4-hr conditions that differed by the SLT product used: own brand, Bacc-off (nontobacco placebo smokeless product; Bacc-off, Selma, AL), Stonewall (compressed tobacco tablet; Star Scientific, Chester, VA), or General snus (loose moist snuff product; Swedish Match, Stockholm, Sweden). Each condition was separated by at least 48 hr. If a breath sample contained CO ≤ 7 ppm, and participants reported no SLT use in the previous 10 hr, a catheter was inserted in a forearm vein followed by a 30-min rest period. After the rest period, blood was sampled and subjective measures were administered. The participant then received 2 g of product (or 1 Stonewall tablet), to use ad libitum for 30 min. After 30 min, participants removed any remaining product from their mouth, blood was sampled, and subjective measures were administered. The same pattern (30-min rest period, blood sampling, subjective measures, product use, blood sampling, subjective measures) was repeated three more times for a total of four product use periods within each session. Participants were paid $40 after the first session, $60 after the second session, $80 after the third session, and $100 after the fourth session, for a total compensation of $280.
Outcome measures
All blood samples were centrifuged immediately and plasma separated and stored at −70°C for later analysis of nicotine level using high-performance liquid chromatography (HPLC) and liquid chromatography mass spectrometry (modified from Naidong, Shou, Chen & Jiang, 2001; Breland, Kleykamp, & Eissenberg, 2006). The limit of quantitation (LOQ) was 2.0 ng/ml.
Three computerized subjective measures were used to assess the effects of each condition. Two of the measures (taken from Gire & Eissenberg, 2000) were presented as visual analogue scales (VAS) that consisted of an item above a horizontal line that had anchors on the left (“not at all”) and right (“extremely”). Subjects moved a mouse controlled cursor and clicked to produce a vertical mark on the horizontal line. The score was the distance of the vertical mark from the left anchor, expressed as a percentage of line length. The first VAS measure (Withdrawal scale) consisted of 11 tobacco withdrawal-related items: “Urges to dip,” “Irritability/frustration/anger,” “Anxious,” “Difficulty concentrating,” “Restlessness,” “Hunger,” “Impatient,” “Craving a dip/nicotine,” “Drowsiness,” “Depression/feeling blue,” and “Desire for sweets.” The second VAS measure (Direct Effects scale) consisted of thirteen VAS items: “Overall, how strong is the tobacco?,” “What amount of the tobacco have you swallowed?,” “How well does the tobacco pack?,” “Has your salivation (spit) increased?,” “Does the tobacco produce any burning sensations?,” “Do you feel any tingling in your mouth when using the tobacco?,” “Do you feel any nausea when using the tobacco?,” “Does your heart race when using the tobacco?,” “Do you feel a head rush when using the tobacco?,” “Does the tobacco help you relax?,” “Do you like the way the tobacco makes you feel?,” “Do you like the way the tobacco tastes?,” and “How alert does the tobacco make you feel?” A third subjective measure was adapted from the Questionnaire of Smoking Urges QSU; (Tiffany and Drobes, 1991) by substituting the phrase “dip/tobacco” for “cigarette” or “dipping/using tobacco” for “smoking.” Participants rated items on a 7-point scale ranging from 0 (Strongly disagree) to 6 (Strongly agree). This adapted version of the QSU has not been validated empirically, but we adopted the two-factor structure reported for the original measure (Tiffany & Drobes, 1991).
Heart rate and blood pressure were also assessed regularly, but results from these secondary outcome measures are not reported here.
Data analyses
Because of the small number of participants who completed this study, we chose to simplify our analysis by analyzing only episodes 1 and 4. Plasma nicotine and subjective data were entered into a within-subject ANOVA with condition (Own Brand, Placebo, Stonewall, and General), episode (1st and 4th), and time (pre- and post-SLT use) as the within-subject factors, with the exception of items on the Direct Effects questionnaire, which were analyzed by condition and episode only. For plasma nicotine, values below the LOQ (2.0 ng/ml) were replaced with the LOQ. Huynh–Feldt corrections were used to correct for violations of the sphericity assumption (Keppel & Wickens, 2004). Statistical testing of differences between select pairs of means, using planned comparisons (i.e., dependent t-tests) was conducted only when significant interactions or main effects were observed (pre- and post-use within conditions, and post-use across conditions compared with Own Brand only). Note that two participants were missing a total of two plasma nicotine data points. The mean of the data from the other 12 participants at that time point was substituted and, when the data were analyzed with and without these two participants, the pattern of results was unchanged. Thus all results are reported with n = 13.
Results
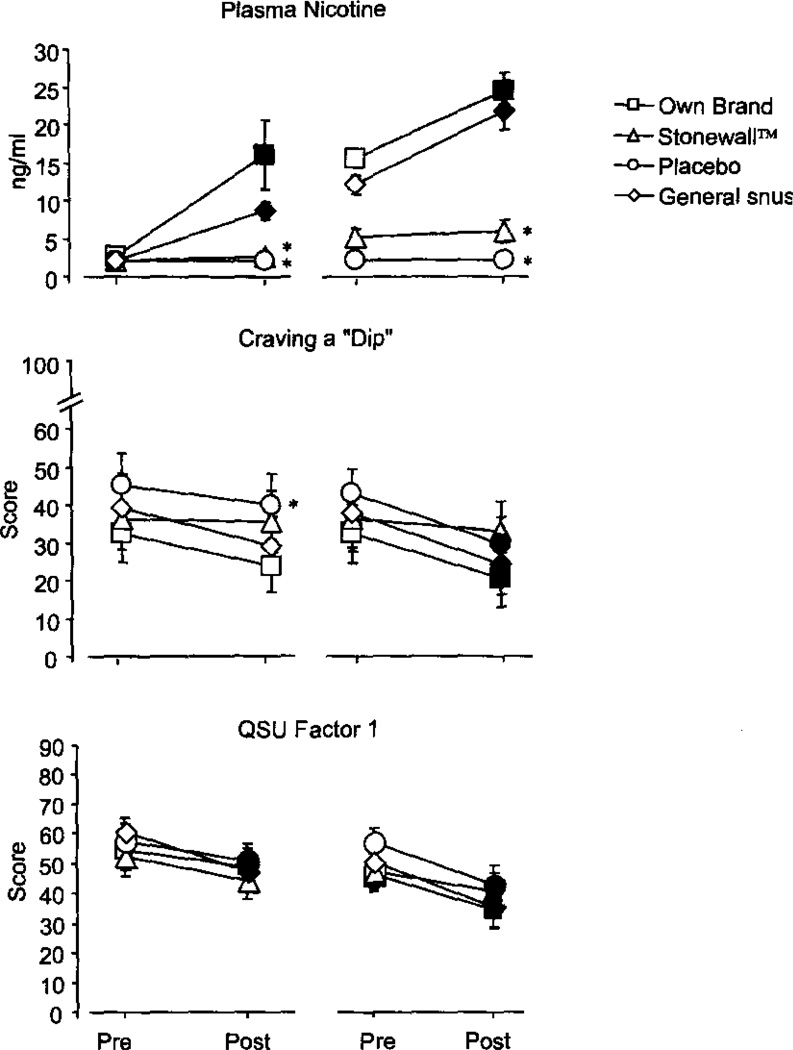
For plasma nicotine, significant interactions of condition by episode [F(3,36) = 10.9, p<.01] and condition by time [F(3, 36) = 21.2, p<.001] were observed (Figure 1). Across all conditions, presession nicotine levels were consistent with 8–10 hr tobacco abstinence (mean pre-use episode 1 = 2.2 ng/ml, SEM = 0.15). In the Own Brand and General snus conditions, SLT use increased plasma nicotine level significantly in both the first and fourth use episodes. For example, for the first Own Brand use episode, plasma nicotine increased from a mean of 2.6 ng/ml (SEM = 0.6) to a mean of 16.1 ng/ml (SEM = 4.6), and for the first use episode of the General snus condition, plasma nicotine increased from a mean of 2.0 ng/ml (SEM = 0.0) to a mean of 8.7 ng/ml (SEM = 1.1). Neither Stonewall nor the Placebo condition produced significant increases in plasma nicotine at any use episode.
Figure 1.
Plasma nicotine, Withdrawal scale item “Craving a dip”, and QSU Factor 1 for episodes 1 (left side of each panel) and 4 (right side of each panel) from Study 1. Averaged data (± 1 SEM) from 13 participants using Own Brand, Stonewall, Placebo, or General snus. Filled symbols indicate a significant difference from “Pre” value; asterisks (*) indicate a significant difference from Own Brand (all p<.05, dependent t-test).
Significant main effects of time were observed for three items of the Withdrawal scale (“Urges to Dip,” “Craving a dip,” and “Drowsiness”) and both factors of the QSU [F(1,12)>5.4, p<.05]. As shown in Figure 1, significant decreases from pre to post-use were observed for “Craving a dip” in episode 4 only. Specifically, in episode 4, the mean score decreased significantly in the Own Brand [from 32.6 (SEM = 7.4) to 20.2 (SEM = 6.8)], General snus [from 37.8 (SEM = 7.2) to 24.3 (SEM = 7.1)], and the Placebo conditions [from 43.1 (SEM = 6.2) to 29.5 (SEM = 7.2) all p<.05], but not in the Stonewall condition. For “Urges to Dip” a similar pattern was observed, though a significant increase was observed in the General snus condition after the first use episode. For “Drowsiness,” the only significant decrease was observed in the General snus condition after the first use episode.
Scores for QSU Factor 1 are also shown in Figure 1; mean scores decreased significantly after the first use of Placebo [from 57.8 (SEM = 5.3) to 50.8 (SEM = 5.8)] and General snus [from 60.8 (SEM = 4.5) to 47.2 (SEM = 5.9)], and after the fourth use of Own Brand [from 45.9 (SEM = 5.4) to 34.1 (SEM = 5.9), Stonewall [from 47.2 (SEM = 5.8) to 40.4 (SEM = 5.9)], Placebo [from 56.5 (SEM = 4.9) to 42.7 (SEM = 6.6)], and General snus [from 50.3 (SEM = 4.2) to 35.3 (SEM = 6.5); all p<.05]. For QSU Factor 2, significant decreases were observed in the Own Brand condition after the fourth use episode, and in the General snus condition after the first and fourth use episodes.
Only one significant interaction involving the condition factor was observed: a significant interaction of condition by time for the item “Irritability/Frustration/Anger” [F(3,36) = 3.5, p<.05]. In the first episode of the Placebo condition, post-use score (M = 20.2; SEM = 4.0) was significantly higher than pre-use score (M = 14.2; SEM = 4.4). Compared with Own Brand (M = 12.6; SEM = 4.0), post-use scores in episode 1 were significantly higher for the Placebo condition (M = 20.2; SEM = 4.0) and for General snus (M = 17.6; SEM = 4.9).
For the Direct Effects VAS, a significant main effect of condition was observed for the items assessing “heart race,” “head rush,” “tobacco pack,” “tobacco strength,” “amount swallowed,” “like feel,” “like taste,” “relax” and “alert,” (F>3.2, p<.05). For virtually every item, Own Brand means were generally greater than means for other conditions. For example, for the item “How well does the tobacco pack?” participants rated Own Brand as 59.4 (SEM = 6.0), significantly higher than Stonewall (M = 23.8; SEM = 7.7), Placebo (M = 25.3; SEM = 6.0), and General snus (M = 16.0; SEM = 5.0). For the item “Do you like the way the tobacco tastes?” participants rated Own Brand as 60.5 (SEM = 7.0), significantly higher than Stonewall (M = 35.9; SEM = 7.9), Placebo (M = 20.2; SEM = 6.0), and General snus (M = 13.7; SEM = 5.2). One exception to this pattern is the “What amount of the tobacco have you swallowed?” item where the mean for Stonewall (55.9, SEM = 10.3) was significantly greater than that for Own Brand (12.0; SEM = 7.4).
Study 2
Study 2 used methods adapted from longer-term evaluations of the nicotine and carcinogen exposure and withdrawal suppression associated with cigarette-like PREP use (e.g., Breland et al., 2003; Breland et al., 2006).
Method
Participants
Inclusion/exclusion criteria were as described for Study 1. Participants (n = 19, 0 female, 1 nonwhite) were, on average, 24.0 years old (SD = 12.2), and reported an average of 5.2 uses/day of SLT (SD = 3.4) for an average of 8.1 years, (SD = 6.8). Participants reported their usual brands as Skoal (n = 11), Copenhagen (n = 4), Kodiak (n = 3), and Hawken (n = 1). Mean CO level at screening was 2.6 ppm (SD = 1.1), and mean semi-quantitative urine cotinine score was 5.7 (SD = 0.5).
Procedure and outcome measures
Each participant completed four, Latin-square ordered, 5-day conditions (i.e., Monday–Friday) that differed by SLT product: own brand, Stonewall, General or No SLT. The No SLT condition was included to compare the nicotine and carcinogen exposure associated with longer-term PREP use with the ideal condition (complete cessation of tobacco use) as in previous work (e.g., Breland et al., 2006). Each condition was separated by at least 72 hr (i.e., Saturday–Sunday) during which participants could use own brand SLT. On days 1–5, participants attended the laboratory for approximately 30 min when CO level was recorded and participants completed the Withdrawal scale and the modified QSU (heart rate and blood pressure were also assessed, but results from these secondary outcome measures are not reported here). Also, the Direct Effects scale was administered on days 2–5. If the condition for the week involved tobacco use, on each of days 1–4 the participant was given approximately 45 g of own brand or General snus, or 20 Stonewall tablets for use over the next 24 hr; participants were instructed that these products could be used ad libitum. Participants were reminded to use only the product provided to them (or no tobacco/nicotine-containing products in the No SLT condition). In addition, on days 1, 3, and 5, a urine sample was obtained and stored at −70°C for later analysis for metabolites of the TSN NNK: 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol and [4-(methylnitrosamino)-1-(3-pyridyl)but-1-yl]-β-O-d-glucosiduronic acid (hereafter NNAL-Total, as in Breland et al., 2006). NNK is one of the most carcinogenic tobacco toxicants (Hecht, 1998). These urine samples were also used to measure the nicotine metabolite cotinine (as in Breland et al., 2006). The sample was also assessed for cotinine level immediately using semi-quantitative test strips, and these data were used, in combination with expired air CO, to assess compliance with tobacco use restrictions (as in Breland et al., 2006). Participants who demonstrated compliance on day 3 were paid $30 and day 5 were paid $70, with an additional $100 on day 5 of the final condition.
Data analysis
All data were analyzed using a condition (Own Brand, Stonewall, General, No SLT) by day (days 1, 3, and 5 for NNAL analysis; days 1–5 for subjective measurements) within-subject ANOVA. For urine cotinine, values below the LOQ (2.0 ng/ml) were replaced with the LOQ, and for urine NNAL, values below the LOQ (50 pg/mL) were replaced with the LOQ. In other respects the data analysis was as reported for Study 1. Note that two participants were missing a total of three urine NNAL data points. The mean of the data from the other participants at that time point was substituted and, when the data were analyzed with and without these two participants, the pattern of results was unchanged. Thus all results are reported with n = I9.
Results
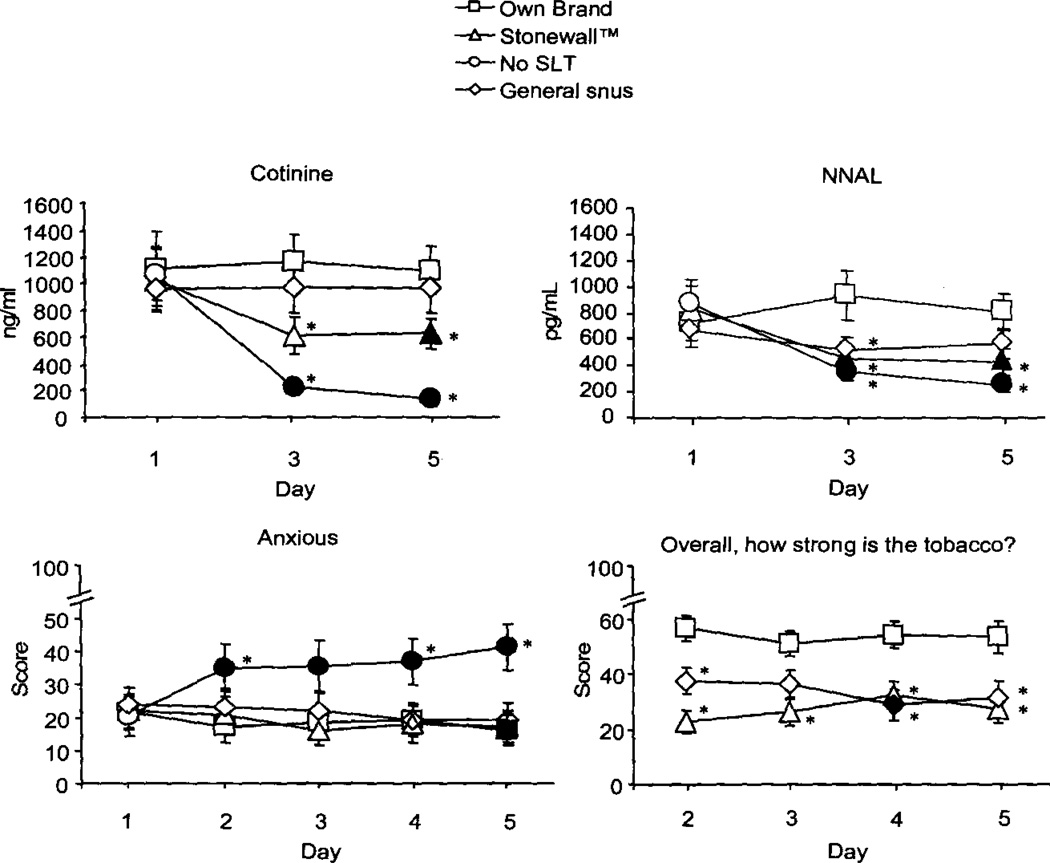
For urine cotinine, a significant condition by day interaction was observed [F(6, 108) = 5.9, p<.0.1]. As Figure 2 shows, urine cotinine levels were similar on day 1 (mean, collapsed across condition = 1041.5 ng/ml, SEM = 217.0) but, in the No SLT condition, dropped significantly by day 3 (M = 222.5 ng/ml, SEM = 48.6) and remained low on day 5 (M = 143.0 ng/ml, SEM = 58.4). Cotinine levels also decreased significantly by Stonewall day 5 (M = 628.1 ng/ml, SEM = 112.1), but did not differ across days for Own Brand and General.
Figure 2.
Urinary cotinine; Urinary NNAL; Withdrawal scale item: “Anxious”; Direct Effects Scale: “Overall, how strong is the tobacco?” from Study 2. Averaged data (± 1 SEM) for 19 participants using Own Brand, Stonewall, No SLT, or General snus. Filled symbols indicate a significant difference from initial assessment; asterisks (*) indicate a significant difference from Own Brand (all p<.05, dependent t-test).
A significant condition by day interaction was observed for NNAL [F(6, 108) = 3.8, p<.05]. As Figure 2 shows, NNAL levels were similar between conditions on day 1 (mean, collapsed across condition = 770.6 pg/ml, SEM = 156.1) but, for Stonewall and No SLT, decreased significantly on days 3 and 5 (day 3, No SLT M = 347.8 pg/ml, SEM = 57.2; Stonewall 451.4 pg/ml, SEM = 74.5; day 5, No SLT M = 254.3 pg/ml, SEM = 49.7; Stonewall 426.0 pg/ml, SEM = 99.9). Across conditions, significantly lower levels of NNAL were observed in the Stonewall, No SLT, and General snus conditions on day 3, compared with Own Brand. By day 5, significantly lower levels of NNAL were observed in the Stonewall and No SLT conditions only.
For the Withdrawal scale, a condition by day interaction [F(12, 216)>2.2, p<.05] was observed for the items “Anxious,” “Restlessness,” and “Difficulty concentrating.” Generally, mean scores were similar across conditions on day 1, and increased on subsequent days for the No SLT condition only. For example, for the “Anxious” item, mean score in the No SLT condition on day 1 was 18.1 (SEM = 5.9) and was significantly elevated across days—by day 5, the mean score was 39.8, SEM = 7.2 (p<.05). A significant decrease was also observed in the Own Brand condition; by day 5 scores were significantly lower than on day 1 (day 1 M = 21.6, SEM = 5.2; day 5 M = 16.0, SEM = 4.8). Across conditions, significant differences were observed between the Own Brand condition and No SLT condition on some days, A similar pattern of results was observed for items assessing “Restlessness” and “Difficulty Concentrating,” and also for both factors of the modified QSU.
Significant effects of condition and/or day were observed for other items of the Hughes–Hatsukami scale. Main effects of condition were observed for: “Urges to dip”, “Irritability/Frustration/Anger”, “Impatient”, “Craving a dip/nicotine”, “Insomnia/disturbed sleep”, “Increased eating”, and “Desire for sweets,” and both factors of the QSU. Generally, higher scores were seen for the No SLT condition compared with the other conditions.
Significant condition by day interactions were observed for items assessing “tobacco strength” and “tobacco pack” [F(9, 162)>2.1, p<.05]. Figure 2 displays results for the item “Overall, how strong is this tobacco?” Mean scores were similar across each study day for Own Brand, and lower on most days for Stonewall and General snus. A similar pattern was observed for the item “How well does this tobacco pack?”
Main effects of condition were observed for nine items of the Direct Effects scale, including “Do you like the way the tobacco tastes?” (the item with the largest condition F value). Collapsed across day, mean scores for this item were significantly higher for Own Brand (M = 74.2, SEM = 4.1) than for General snus (M = 10.8, SEM = 2.3), but not for Stonewall (M = 43.9, SEM = 6.3). For most of the other items, the pattern of results was similar (Own Brand higher than the other two product-containing conditions), with the exception of items assessing amount of tobacco swallowed, salivation increase, and nausea. For the items assessing amount swallowed and salivation, Stonewall was rated the highest, followed, by Own Brand, and then General snus. For the item assessing “Nausea,” General snus was rated highest, followed by Stonewall, and then Own brand.
Discussion
The purpose of these studies was to adapt models used to evaluate cigarette-like PREPs for smokers for use in the evaluation of the toxicant exposure and effects of oral PREPs for SLT users. With respect to particular products, in Study 1 we observed that General snus, but not Stonewall, approximated Own Brand nicotine exposure (consistent with Hatsukami et al., 2004), though Stonewall and General snus both suppressed withdrawal acutely (as did the nontobacco placebo; Gire & Eissenberg, 2000). Study 1 results with respect to nicotine exposure and withdrawal suppression were also consistent with data from Study 2. Urine cotinine levels for General snus and Own Brand did not differ across days, though they decreased when participants used Stonewall, and withdrawal was suppressed in all tobacco conditions. Withdrawal suppression is important, as PREPs that fail to suppress withdrawal may not substitute for products that deliver maximum tobacco toxicant exposure (e.g., Breland et al., 2003; Hughes and Keely, 2004). Study 2 also demonstrated that PREP-induced decreases in NNK exposure could be measured (as indexed by urinary NNAL). Taken together, these results highlight the reliability of laboratory methods for evaluating the toxicant exposure and withdrawal suppressing effects of PREPs for SLT users. While other methods might also be used (e.g., Hatsukami et al., 2004) those reported here are relatively brief, cost-efficient, and, because of the within-subject design, statistically powerful.
Of course, the methods used here also have some limitations, including compliance assessment and uncontrolled product use. In Study 2, participants left the laboratory setting to use PREPs in their natural environment. With this design, own brand SLT use during a PREP condition may be undetectable and would confound measures of toxicant exposure and withdrawal suppression. Sequestering participants in an inpatient research unit, which can be costly, may be the only method of ensuring exclusive PREP use (e.g., Eissenberg et al., 1996; Hart et al., 2002). Also, in both studies, topography of use was uncontrolled. For example, in Study 1, some participants may have absorbed more nicotine than others if they kept the product close to the buccal mucosa more consistently. In studies of cigarette-like PREPs topography is often measured to control for similar potential confounds (e.g., Breland et al., 2006), but no methods have been developed to measure SLT use topography. Also, in Study 2, participants used products ad libitum, which led to differential use across conditions: when amount used each day is expressed as percentage of product provided, participants used significantly less General snus (M = 31.7%, SEM = 3.8) compared with Own Brand (M = 49.2%, SEM = 5.4) and Stonewall (M = 45.3%, SEM = 5.1; p<.01, dependent t-test; no differences were observed across days within each condition). Such differences highlight the advantages of standardizing product delivery (as in Study 1). Finally, another potential limitation is that the study primarily involved participants who were white and male. However, in the United States at least, this sample likely reflects the population of SLT users (SAMSHA, 2006).
In sum, PREP proliferation is driving the need for comprehensive evaluation strategies that include epidemiological, preclinical, and clinical research (Hatsukami et al., 2005; Stratton, Shetty, Wallace, & Bondurant, 2001). These evaluation strategies should be applied to all PREPs for tobacco users, whether cigarette-like, SLT-like, or novel products (e.g., Buchhalter and Eissenberg, 2000). Ideally, these evaluation strategies will be standardized, will include reliable and efficient laboratory methodology, will be performed prior to PREP release to the consumer, and will be overseen by a regulatory body that also controls PREP availability and marketing. These approaches of standardization, premarket evaluation, and strong regulation will be important components of meaningful harm reduction for tobacco users (e.g., Stratton et al., 2000).
Acknowledgments
This research was supported by US Public Health Service grant CA103827;
Footnotes
the authors have no potential conflicts of interest to report.
Some of the data presented here were also presented at the 13th Annual Meeting of the Society for Research on Nicotine and Tobacco.
References
- Benowitz NL, Porchet H, Sheiner L, Jacob P., 3rd Nicotine absorption and cardiovascular effects with smokeless tobacco use: Comparison with cigarettes and nicotine gum. Clinical Pharmacology and Therapeutics. 1988;44(1):23–28. doi: 10.1038/clpt.1988.107. [DOI] [PubMed] [Google Scholar]
- Breland AB, Acosta MC, Eissenberg T. Tobacco specific nitrosamines and potential reduced exposure products for smokers: A preliminary evaluation of Advance™. Tobacco Control. 2003;12(3):317–321. doi: 10.1136/tc.12.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breland AB, Buchhalter AR, Evans SE, Eissenberg T. Evaluating acute effects of potential reduced-exposure products for smokers: Clinical laboratory methodology. Nicotine & Tobacco Research. 2002;4 Suppl 2:S131–S140. doi: 10.1080/1462220021000032780. [DOI] [PubMed] [Google Scholar]
- Breland AB, Evans SE, Buchhalter AR, Eissenberg T. Acute effects of Advance™: A potential reduced exposure product for smokers. Tobacco Control. 2002;11(4):376–378. doi: 10.1136/tc.11.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breland AB, Kleykamp BA, Eissenberg T. Clinical laboratory evaluation of potential reduced exposure products for smokers. Nicotine & Tobacco Research. 2006;8(6):727–738. doi: 10.1080/14622200600789585. [DOI] [PubMed] [Google Scholar]
- Buchhalter AR, Eissenberg T. Preliminary evaluation of a novel smoking system: Effects on subjective and physiological measures and on smoking behavior. Nicotine & Tobacco Research. 2000;2(1):39–43. doi: 10.1080/14622200050011286. [DOI] [PubMed] [Google Scholar]
- Buchhalter AR, Schrinel L, Eissenberg T. Withdrawal-suppressing effects of a novel smoking system: Comparison with own brand, not own brand, and de-nicotinized cigarettes. Nicotine & Tobacco Research. 2001;3(2):111–118. doi: 10.1080/14622200110042636. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Annual Smoking-Attributable Mortality, Years of Potential Life Lost, and Economic Costs–United States, 1995–1999. Morbidity and Mortality Weekly Report. 2002;51(14):300–303. [PubMed] [Google Scholar]
- Ebbert JO, Dale LC, Severson H, Croghan IT, Rasmussen DF, Schroeder DR, Vander Weg MW, Hurt RD. Nicotine lozenges for the treatment of smokeless tobacco use. Nicotine & Tobacco Research. 2007;9(2):233–240. doi: 10.1080/14622200601080349. [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Greenwald MK, Johnson RE, Liebson IA, Bigelow GE, Stitzer ML. Buprenorphine’s physical dependence potential: Antagonist-precipitated withdrawal in humans. Journal of Pharmacology and Experimental Therapeutics. 1996;276(2):449–459. [PubMed] [Google Scholar]
- Fant RV, Henningfield JE, Nelson RA, Pickworth WB. Pharmacokinetics and pharmacodynamics of moist snuff in humans. Tobacco Control. 1999;8(4):387–392. doi: 10.1136/tc.8.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gire JT, Eissenberg T. Placebo control study of acute smokeless tobacco abstinence in young adult men. Psychology of Addictive Behaviors. 2000;14(4):356–366. [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Comer SD, Foltin RW, Fischman MW. Comparison of smoked marijuana and oral Delta(9)-tetrahydrocannabinol in humans. Psychopharmacology (Berl) 2002;164(4):407–4l5. doi: 10.1007/s00213-002-1231-y. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Giovino GA, Eissenberg T, Clark PI, Lawrence D, Leischow S. Methods to assess potential reduced exposure products. Nicotine & Tobacco Research. 2005;7(6):827–844. doi: 10.1080/14622200500266015. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Severson HH. Oral spit tobacco: Addiction, prevention and treatment. Nicotine & Tobacco Research. 1999;1(1):21–44. doi: 10.1080/14622299050011131. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Grillo M, Boyle R, Allen S, Jensen J, Bliss R, Brown S. Treatment of spit tobacco users with transdermal nicotine system and mint snuff. Journal of Consulting and Clinical Psychology. 2000;68(2):241–249. [PubMed] [Google Scholar]
- Hatsukami DK, Lemmonds C, Zhang Y, Murphy SE, Le C, Carmella SG, Hecht SS. Evaluation of carcinogen exposure in people who used “reduced exposure” tobacco products. Journal of the National Cancer Institute. 2004;96(11):844–852. doi: 10.1093/jnci/djh163. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chemical Research in Toxicology. 1998;11(6):559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- Henley SJ, Thun MJ, Connell C, Calle EE. Two large prospective studies of mortality among men who use snuff or chewing tobacco (United States) Cancer Causes Control. 2005;16(4):347–358. doi: 10.1007/s10552-004-5519-6. [DOI] [PubMed] [Google Scholar]
- Hoffmann D, Hoffmann I. The changing cigarette, 1950–1995. Journal of Toxicology and Environmental Health. 1997;50(4):307–364. doi: 10.1080/009841097160393. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP. The effect of a novel smoking system—Accord®—on ongoing smoking and toxin exposure. Nicotine & Tobacco Research. 2004;6(6):1021–1027. doi: 10.1080/14622200412331296011. [DOI] [PubMed] [Google Scholar]
- Keppel G, Wickens TD. Design and analysis: A researcher’s handbook. 4th edition. New Jersey: Pearson Prentice Hall; 2004. [Google Scholar]
- Kotlyar M, Mendoza-Baumgart MI, Li ZZ, Pentel PR, Barnett BC, Feuer RM, Smith EA, Hatsukami DK. Nicotine pharmacokinetics and subjective effects of three potential reduced exposure products, moist snuff and nicotine lozenge. Tobacco Control. 2007;16(2):138–142. doi: 10.1136/tc.2006.018440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidong W, Shou W, Chen YL, Jiang X. Novel liquid chromatographic-tandem mass spectrometric methods using silica columns and aqueous-organic mobile phases for quantitative analysis of polar ionic analytes in biological fluids. Journal of Chromatography. B, Biomedical Sciences and Applications. 2001;754(2):387–399. doi: 10.1016/s0378-4347(01)00021-4. [DOI] [PubMed] [Google Scholar]
- Stepanov I, Jensen J, Hatsukami D, Hecht SS. Tobacco-specific nitrosamines in new tobacco products. Nicotine & Tobacco Research. 2006;8(2):309–313. doi: 10.1080/14622200500490151. [DOI] [PubMed] [Google Scholar]
- Stratton K, Shetty P, Wallace R, Bondurant S, editors. Clearing the smoke: The science base for tobacco harm reduction. Washington, DC: National Academy Press; 2001. Committee to assess the science base for tobacco harm reduction, Board on Health Promotion and Disease Prevention. [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Rockville, MD: Substance Abuse and Mental Health Services Administration, Office of Applied Studies; 2006. Results From the 2005 National Survey on Drug Use and Health: Detailed Tables. (PDF–58KB) Available from http://oas.samhsa.gov/NSDUH/2k5nsduh/tabs/Sect2peTabs37to41.pdf. [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. British Journal of Addiction. 1991;86(11):1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- United States Department of Health and Human Services. Bethesda, MD: United States Department of Health and Human Services, Public Health Service; 1986. The health consequences of using smokeless tobacco: A report of the advisory committee to the Surgeon General. [Google Scholar]