Abstract
Three different media—Columbia agar, Wilkins-Chalgren agar, and Helicobacter pylori special peptone agar—were prepared in a diluted version and compared to the standard medium formulation in order to study a possible nutrient shock effect observed when recovering H. pylori from water by counting the number of CFU. This same parameter was subsequently used to evaluate the influence of the incubation atmosphere by using a modular atmosphere-controlled system to provide different atmospheres and by employing an established gas generation kit as a control. Both a low nutrient content of the media and a rapidly achieved microaerophilic incubation atmosphere proved to increase the numbers of environment-stressed H. pylori organisms recovered. An atmosphere of 5% CO2, 5% O2, and 3% H2 is recommended, although other atmospheres with a low oxygen concentration are also acceptable. Besides highlighting and assessing the importance of several factors in the culturability of H. pylori, this paper demonstrates the potential ability to develop an optimized technique for recovery of this pathogen from water.
Helicobacter pylori is a gram-negative bacterium with the remarkable ability to survive and replicate within the gastric mucosa of the human stomach. Infection with this pathogen can lead to several gastrointestinal diseases, such as gastritis, peptic and duodenal ulcers, gastric carcinomas, and mucosa-associated lymphoid tissue tumors. The route of transmission of H. pylori remains a controversial topic, with circumstantial evidence for infection via exposure to animals, contaminated water supplies, and oral reservoirs being reported previously (13).
In an early study, the survival ability of H. pylori in treated water systems was questioned, with the suggestion that this microorganism was very sensitive to the chlorine used in water treatment plants (9). However, Baker et al. argued later that H. pylori could tolerate disinfectants better than could the classical fecal indicator, Escherichia coli (3). Consequently, disinfected water could be free of coliforms and classified as safe but might still contain potentially infectious H. pylori that could therefore be transmitted by a waterborne route. The suspicion that water might be one of the vehicles of infection is sustained further by several recent studies that have used DNA recognition techniques such as PCR to identify the pathogen in potable water and associated biofilms (4, 8, 12, 16). However, recovery of this bacterium from water by using the present plate procedures remains elusive, and because viability is frequently associated with culturability, demonstration of the existence of viable H. pylori either in the planktonic phase or in biofilms associated with drinking water has yet to be accomplished. Two distinct explanations can be advanced for this apparent failure: either H. pylori is in fact not able to survive in potable water systems, or some of the recovery technique parameters, such as the media composition and the incubation atmosphere, are not adequate for replication and colony formation.
Several media have been assessed for the recovery of the pathogen from different environments, including infected individuals (6, 17), cattle, and beef samples (21). However, the matrices involved are all nutrient rich. In contrast, for microorganisms to survive in natural waters, they must adapt physiologically to a low-nutrient environment. As a result, the traditional recovery media may be too nutrient rich for their optimum culturability, a condition described as nutrient shock (19). Therefore, in the first part of this work, we tested the hypothesis of nutrient shock as a hampering factor in the recovery of H. pylori from water by using spreading plate procedures.
Helicobacter spp. are described as microaerophiles, requiring a low-oxygen and balanced carbon dioxide atmosphere to grow. One study has also concluded that hydrogen is an energy-yielding substrate that can facilitate the maintenance of the bacterium (15). However, the studies published so far regarding incubation atmospheric conditions concerned commercially available kits and did not make a systematic study about the influence of atmospheric concentrations (7, 23). The availability of microaerophilic cabinets with adjustable atmospheric control allows a more accurate understanding of the effects of the incubation atmosphere throughout the incubation period and permits selection of the best atmospheres needed for different microorganisms to grow. A modular atmosphere-controlled system (MACS) workstation was therefore used in the present study to determine the optimal oxygen, carbon dioxide, and hydrogen concentrations needed to recover potentially stressed H. pylori from water.
MATERIALS AND METHODS
Culture maintenance.
H. pylori NCTC 11637 was maintained on Columbia agar (Oxoid, Basingstoke, United Kingdom) supplemented with 5% (vol/vol) defibrinated horse blood (Biomérieux, Marcy l'Etoile, France) in the presence and absence of 1% (vol/vol) fetal calf serum (Merck, Darmstadt, Germany). Plates were incubated at 37°C in 2.5-liter jars (Oxoid) under microaerophilic conditions that were created by using a CampyGen gas pack (Oxoid) and streaked onto fresh plates every 2 or 3 days.
Media preparation.
Three different media—Columbia agar (CA) (Oxoid), Helicobacter pylori special peptone agar (HPSPA), and Wilkins-Chalgren agar (WCA) (Oxoid)—were compared for recovery of H. pylori from water samples. CA and WCA were prepared according to the manufacturer's instructions. HPSPA was prepared as described previously (21), i.e., by adding the agar to distilled water special peptone (Oxoid), 10 g/liter; yeast extract (Merck), 5 g/liter; beef extract (Merck), 5 g/liter; sodium chloride (Merck), 5 g/liter; pyruvic acid-sodium salt (Sigma, St. Louis, Mo.), 0.5 g/liter; and granulated agar (Merck), 15 g/liter. After allowing the three different media to cool down to 55°C following the autoclaving process, 5% (vol/vol) defibrinated horse blood was added. For each set of experiments, media were prepared and poured into plates 2 days before the experiment and stored at 4°C.
To study the effect of nutrient shock on H. pylori, the media mentioned above were prepared at half and at quarter strength by reducing the constituents two- and fourfold, respectively. The agar concentration was maintained constant. The media are described herein as half strength and quarter strength. Evaluation of the growth in different media was accomplished by counting CFU.
Induction of environmentally stressed H. pylori and subsequent recovery.
Cells from 2-day-old cultures were harvested from CA plates, suspended in 10 ml of autoclaved tap water until a concentration of 109 CFU per ml was achieved, and vortexed for 30 s. Properties of the tap water before the autoclaving procedure follow. The pH was 7.35; the turbidity was <0.4 UTN; the conductivity was 65.1 μS per cm; the total hardness was 32 mg of CaCO3 per liter; the total iron content was <0.05 mg of Fe per liter; and the free chlorine content was 0.46 mg of Cl2 per liter. This inoculum was then transferred to a sterile bioreactor containing 1,000 ml of autoclaved tap water in order to achieve a final concentration of ca. 106 CFU/ml. The bioreactor was maintained at room temperature (approximately 24 ± 2°C) and continuously stirred (120 rpm) by using a magnetic bar. Sampling was performed at different times up to 24 h. Before serial dilution (1:10) in sterile tap water, samples were vortexed for 10 s to achieve homogenization. H. pylori was enumerated in triplicate by surface plating 100 μl of the different dilutions onto the appropriate agar media. Plates were incubated at 37°C for 6 days under the same microaerophilic conditions as those used for culture maintenance.
Incubation atmosphere influence.
Using a variable atmosphere workstation (MACS VA500; Don Whitley Scientific, Yorkshire, United Kingdom), several atmospheres were tested, and results were compared with those of Campygen gas generation systems. To ensure achieving the necessary humidity within the workstation, which was set to 95%, a beaker with approximately 300 ml of water was placed inside it. On the basis of results of preliminary experiments, it was decided that only one sample taken after a relatively short time of exposure to water would reflect the differences between the two gas generation systems. Therefore, after 10 min, a sample was taken from the bioreactor and cultured on CA. To provide a better sensitivity for this method, six plates were used for each experiment.
Confirmatory procedures.
Besides checking for typical colony morphology (i.e., roundness, translucent to yellowish color, convexity, and diameter of 0.2 to 2 mm) (1), microscopic visualization with episcopic differential interference contrast microscopy (10) and hybridization with a specific-peptide nucleic acid probe (2) were performed to confirm the existence of H. pylori.
Analysis of data.
Nutrient shock results were statistically analyzed by employing a two-way analysis of variance (ANOVA) and using the Bonferroni analysis as a post hoc test. For the comparison between the variable atmosphere workstation and Campygen gas generating system, a t test was used. Computations were performed by using Statistical Program for the Social Sciences (SPSS, Inc., Chicago, Ill.). Results were considered statistically relevant if P values were ≤0.05.
RESULTS
Nutrient shock effects on H. pylori growth.
HPSPA, CA, and WCA, supplemented with 5% defibrinated horse blood, were tested and compared with their diluted versions. For all media, results demonstrated that higher recovery rates were achieved with half-strength media than with standard media (Fig. 1). A two-way ANOVA with post hoc analysis showed that these results were statistically relevant with a P value of <0.05 for CA and <0.01 for HPSPA and WCA. Half-strength CA and WCA were found to be superior to half-strength HPSPA because they consistently produced larger colonies. In addition, half-strength WCA was able to detect H. pylori for a longer period than were the standard media. Quarter-strength media failed to recover any bacteria, a result which was anticipated because H. pylori was not able to grow on these plates even when restreaked directly from the maintenance culture. Viable counts of H. pylori declined rapidly, and recovery ended after 6 h, a finding which is consistent with previous observations (20).
FIG. 1.
Comparison between recoveries obtained from diluted and standard media. ANOVA demonstrated a significant difference for all three figures between the full- and half-strength versions of the media (P < 0.05).
Influence of the incubation atmosphere.
In order to investigate whether the composition of the incubation atmosphere would also influence the recovery of H. pylori from water, several gas atmospheres were tested and compared. In initial experiments, the oxygen concentration was varied (keeping a constant composition of carbon dioxide and hydrogen) to assess potential toxic effects. The CFU result recovered was not significantly affected by the oxygen concentration between 1 and 13% (P > 0.05), but it was noted that colony size measured after 6 days of growth on normal CA medium decreased in diameter from 1 to 0.5 mm in the more extreme concentrations.
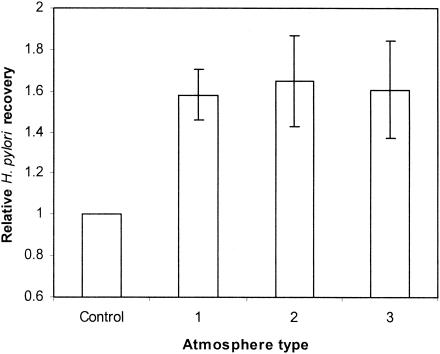
The CampyGen system achieves generation of an atmosphere of 6% oxygen and 12% carbon dioxide in approximately 30 min. In contrast, the MACS workstation environment is user defined and instantaneous. The experiments led us to conclude that the optimal oxygen concentration was 5 to 6%, which was consistent with that of the CampyGen system. The efficiency of recovery was approximately 60% higher when we used the MACS workstation than that obtained when the CampyGen gas pack (Fig. 2) was used. However, the various compositions of the oxygen-carbon dioxide-hydrogen atmospheres within the range reported were not statistically significantly different in terms of the relative levels of H. pylori recovery (P > 0.05).
FIG. 2.
Relative H. pylori recovery for different atmospheres when compared to recovery obtained by the use of Campygen sachets (control). Atmosphere 1 is 13% CO2-5% O2-3% H2; atmosphere 2 is 5% CO2-5% O2-3% H2; and atmosphere 3 is 15% CO2-6%O2-0% H2. No statistically significant difference was found between the three described atmospheres.
Interaction between nutrient shock and atmosphere influence.
To check whether the numbers of bacteria recovered by using the half-strength plates were the same as those recovered by the use of the MACS workstation, a repetition of the previous experiment was done, using both CA and half-strength CA agar plates. In the two experiments carried out, the numbers of H. pylori recovered by using half-strength medium incubated in a MACS workstation were significantly higher (P < 0.05) than those obtained by using either CA medium in the variable-atmosphere workstation or by using half-strength CA medium in a CampyGen atmosphere. This finding demonstrated a synergy between both methods.
DISCUSSION
Although evidence has linked H. pylori infection among human populations with water consumption, plate spreading techniques have so far failed to recover this pathogen from drinking water systems. The present work assesses the relative importance of different factors in the recovery of this pathogen from water in hope of improving the understanding of its behavior in those environments and clarifying the mode of transmission. Because the test strain might adapt to laboratory growth conditions, future experiments using fresh clinical isolates are recommended so as to further validate these results.
The three media selected for this study have been used for H. pylori recovery from a range of varied conditions. CA, together with Brucella agar, has been extensively used in previous studies (1), whereas HPSPA has been considered the most appropriate medium for the isolation of the pathogen from beef samples (21). WCA has been employed in the recovery of the pathogen from ready-to-eat foods (18). The finding that half-strength media gave higher recoveries than did standard media indicates that nutrient shock is indeed a hampering factor in H. pylori recovery from low-nutrient environments; it is also possible that other rich media employed in these types of systems for other types of microorganisms might be underestimating the true number of viable cells. The quarter-strength media failed to recover any bacteria. However, carbon content in the tested media was still high compared to that in R2A, the medium of choice for quantifying heterotrophic bacteria in low-nutrient environments such as water (19). This might denote that the lack of growth is due to the depletion of one or more essential growth components, other than carbon, during the growth of the colonies.
When compared to other bacteria, the number of regulatory proteins that respond to environmental stimulation of H. pylori is low, a factor which might reflect the adaptation of the bacterium to the human gastric microenvironment (11). This could also imply a lack of ability of the bacteria to adjust and survive in different niches in the environment. By showing that the direct recovery from water to a high-nutrient medium causes nutrient shock, this work suggests that the bacteria can physiologically adapt to low-nutrient environments. Moreover, a number of studies have already reported the mechanism of shape modification from spiral to coccoid as a protective mechanism against exposure to suboptimal conditions (5, 14, 22). Together, this evidence appears to demonstrate the ability of H. pylori to adapt to niches other than the human gastric environment.
Although gas packs are convenient in terms of culture maintenance, it became clear that a more precise assessment of the number of viable H. pylori recovered from the environment requires a device that allows a more stable and user-defined atmosphere during the incubation period. It appears that if oxygen concentrations are low enough, the concentrations of the other atmosphere constituents tested do not greatly affect the recovery of the pathogen. Oxygen toxicity is a detrimental factor when using CampyGen gas systems, as the generation of a suitable atmosphere takes approximately half an hour, during which time the stressed H. pylori bacteria become nonculturable cells. This means that an immediately achieved suitable incubation atmosphere is a more important factor to the recovery of the pathogen than is the composition of said atmosphere, at least between a certain range of oxygen-carbon dioxide-hydrogen concentrations.
It was also observed that the rate of recovery is improved when both methods are applied simultaneously. However, this increase might not be equal to the sum of the recoveries obtained separately, as some bacteria that can be recovered by half-strength media can also be recovered by the use of a microaerophilic cabinet.
Because the main objective in the first part of the work was to demonstrate the importance nutrient shock effect might have on H. pylori recovery from water, no attempts were made to optimize the constituents of the different media in terms of CFU and colony size. This present work can, however, be used in the future as a basis to develop a suitable medium for the recovery of the pathogen from water or other nutrient-poor environmental samples. Because it was concluded that cells can be stressed in different ways and that a good recovery process has to employ a combination of both methods, we recommend that the plates be incubated in an atmosphere of 5% CO2, 5% O2, and 3% H2, although other atmospheres with a low oxygen concentration are also acceptable.
Acknowledgments
This work was supported by the Fundação para a Ciência e Tecnologia (Ph.D. grant SFRH/BD/4705/2001 and project POCTI/35849/ESP/2000) and EC contract no. EVK1-CT-2002-00108.
REFERENCES
- 1.Anderson, L. P., and T. Wadstrom. 2001. Basic bacteriology and culture, p. 27-38. In H. L. T. Mobley, G. L. Mendz, and S. L. Hazell (ed.), Helicobacter pylori: physiology and genetics. ASM Press, Washington, D.C.
- 2.Azevedo, N. F., M. J. Vieira, and C. W. Keevil. 2003. Establishment of a continuous model system to study Helicobacter pylori survival in potable water biofilms. Water Sci. Technol. 47:155-160. [PubMed] [Google Scholar]
- 3.Baker, K. H., J. P. Hegarty, B. Redmond, N. A. Reed, and D. S. Herson. 2002. Effect of oxidizing disinfectants (chlorine, monochloramine, and ozone) on Helicobacter pylori. Appl. Environ. Microbiol. 68:981-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunn, J. E., W. G. MacKay, J. E. Thomas, D. C. Reid, and L. T. Weaver. 2002. Detection of Helicobacter pylori DNA in drinking water biofilms: implications for transmission in early life. Lett. Appl. Microbiol. 34:450-454. [DOI] [PubMed] [Google Scholar]
- 5.Cole, S. P., V. F. Kharitonov, and D. G. Guiney. 1999. Effect of nitric oxide on Helicobacter pylori morphology. J. Infect. Dis. 180:1713-1717. [DOI] [PubMed] [Google Scholar]
- 6.Hachem, C. Y., J. E. Clarridge, D. G. Evans, and D. Y. Graham. 1995. Comparison of agar-based media for primary isolation of Helicobacter pylori. J. Clin. Pathol. 48:714-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henriksen, T. H., A. Lia, R. Schoyen, T. Thoresen, and A. Berstad. 2000. Assessment of optimal atmospheric conditions for growth of Helicobacter pylori. Eur. J. Clin. Microbiol. Infect. Dis. 19:718-720. [DOI] [PubMed] [Google Scholar]
- 8.Horiuchi, T., T. Ohkusa, M. Watanabe, D. Kobayashi, H. Miwa, and Y. Eishi. 2001. Helicobacter pylori DNA in drinking water in Japan. Microbiol. Immunol. 45:515-519. [DOI] [PubMed] [Google Scholar]
- 9.Johnson, C. H., E. W. Rice, and D. J. Reasoner. 1997. Inactivation of Helicobacter pylori by chlorination. Appl. Environ. Microbiol. 63:4969-4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keevil, C. W. 2003. Rapid detection of biofilms and adherent pathogens using scanning confocal laser microscopy and episcopic differential interference contrast microscopy. Water Sci. Technol. 47:105-116. [PubMed] [Google Scholar]
- 11.Marais, A., G. L. Mendz, S. L. Hazell, and F. Megraud. 1999. Metabolism and genetics of Helicobacter pylori: the genome era. Microbiol. Mol. Biol. Rev. 63:642-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazari-Hiriart, M., Y. Lopez-Vidal, and J. J. Calva. 2001. Helicobacter pylori in water systems for human use in Mexico City. Water Sci. Technol. 43:93-98. [PubMed] [Google Scholar]
- 13.Mitchell, H., and F. Megraud. 2002. Epidemiology and diagnosis of Helicobacter pylori infection. Helicobacter 7(Suppl. 1):8-16. [DOI] [PubMed] [Google Scholar]
- 14.Nilsson, H. O., J. Blom, W. Abu-Al-Soud, A. A. Ljungh, L. P. Andersen, and T. Wadstrom. 2002. Effect of cold starvation, acid stress, and nutrients on metabolic activity of Helicobacter pylori. Appl. Environ. Microbiol. 68:11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olson, J. W., and R. J. Maier. 2002. Molecular hydrogen as an energy source for Helicobacter pylori. Science 298:1788-1790. [DOI] [PubMed] [Google Scholar]
- 16.Park, S. R., W. G. Mackay, and D. C. Reid. 2001. Helicobacter sp. recovered from drinking water biofilm sampled from a water distribution system. Water Res. 35:1624-1626. [DOI] [PubMed] [Google Scholar]
- 17.Piccolomini, R., G. DeBonaventura, D. Festi, G. Catamo, F. Laterza, and M. Neri. 1997. Optimal combination of media for primary isolation of Helicobacter pylori from gastric biopsy specimens. J. Clin. Microbiol. 35:1541-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poms, R. E., and S. R. Tatini. 2001. Survival of Helicobacter pylori in ready-to-eat foods at 4°C. Int. J. Food Microbiol. 63:281-286. [DOI] [PubMed] [Google Scholar]
- 19.Reasoner, D. J., and E. E. Geldreich. 1985. A new medium for the enumeration and subculture of bacteria from potable water. Appl. Environ. Microbiol. 49:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shahamat, M., U. Mai, C. Paszkokolva, M. Kessel, and R. R. Colwell. 1993. Use of autoradiography to assess viability of Helicobacter pylori in water. Appl. Environ. Microbiol. 59:1231-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stevenson, T. H., L. M. Lucia, and G. R. Acuff. 2000. Development of a selective medium for isolation of Helicobacter pylori from cattle and beef samples. Appl. Environ. Microbiol. 66:723-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tominaga, K., N. Hamasaki, T. Watanabe, T. Uchida, Y. Fujiwara, O. Takaishi, K. Higuchi, T. Arakawa, E. Ishii, K. Kobayashi, I. Yano, and T. Kuroki. 1999. Effect of culture conditions on morphological changes of Helicobacter pylori. J. Gastroenterol. 34(Suppl. 11):28-31. [PubMed] [Google Scholar]
- 23.Van Horn, K., and C. Toth. 1999. Evaluation of the AnaeroPack Campylo system for growth of microaerophilic bacteria. J. Clin. Microbiol. 37:2376-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]