Abstract
The Riley–Day syndrome is the most common of the hereditary sensory and autonomic neuropathies (Type III). Among the well-recognized clinical features are reduced pain and temperature sensation, absent deep tendon reflexes and a progressively ataxic gait. To explain the latter we tested the hypothesis that muscle spindles, or their afferents, are absent in hereditary sensory and autonomic neuropathy III by attempting to record from muscle spindle afferents from a nerve supplying the leg in 10 patients. For comparison we also recorded muscle spindles from 15 healthy subjects and from two patients with hereditary sensory and autonomic neuropathy IV, who have profound sensory disturbances but no ataxia. Tungsten microelectrodes were inserted percutaneously into fascicles of the common peroneal nerve at the fibular head. Intraneural stimulation within muscle fascicles evoked twitches at normal stimulus currents (10–30 µA), and deep pain (which often referred) at high intensities (1 mA). Microneurographic recordings from muscle fascicles revealed a complete absence of spontaneously active muscle spindles in patients with hereditary sensory and autonomic neuropathy III; moreover, responses to passive muscle stretch could not be observed. Conversely, muscle spindles appeared normal in patients with hereditary sensory and autonomic neuropathy IV, with mean firing rates of spontaneously active endings being similar to those recorded from healthy controls. Intraneural stimulation within cutaneous fascicles evoked paraesthesiae in the fascicular innervation territory at normal stimulus intensities, but cutaneous pain was never reported during high-intensity stimulation in any of the patients. Microneurographic recordings from cutaneous fascicles revealed the presence of normal large-diameter cutaneous mechanoreceptors in hereditary sensory and autonomic neuropathy III. Our results suggest that the complete absence of functional muscle spindles in these patients explains their loss of deep tendon reflexes. Moreover, we suggest that their ataxic gait is sensory in origin, due to the loss of functional muscle spindles and hence a compromised sensorimotor control of locomotion.
Keywords: congenital insensitivity to pain, familial dysautonomia, HSAN, microneurography, muscle spindles, peripheral nerve, Riley–Day syndrome
Introduction
The hereditary sensory and autonomic neuropathies (HSANs) are a group of rare neurological disorders caused by different genetic mutations. All forms of HSANs have absent axon-reflex flare responses to intradermal application of histamine, and do not generate sensations of pain or itch, and each is associated with varying degrees of disturbed somatosensory, sensorimotor, and autonomic function (Dyck, 1993; Axelrod, 2002). HSAN III, also known as the Riley–Day syndrome, is the most common of the HSAN disorders and is largely limited to individuals of Eastern European (Ashkenazi) Jewish descent. It is caused by a single point mutation on chromosome 9q (Blumenfeld et al., 1993) that affects gene IKBKAP, responsible for the production of IκB kinase complex-associated protein (Slaugenhaupt et al., 2001). Also termed familial dysautonomia, the disorder is expressed at birth and becomes apparent in infancy. Characteristic features are deficient lacrimation and hypertension associated with anxiety (Riley et al., 1949; Norcliffe-Kaufmann et al., 2010), but it has long been recognized that there are significant sensory disturbances: pain and temperature thresholds are greatly elevated and patients report a relative indifference to pain (Hilz and Axelrod, 2000). In addition, deep tendon and H-reflexes are absent (Aguayo et al., 1971) and the gait is markedly ataxic: patients adopt a wide stance, hold their arms in a high guard position, veer into walls and are remarkably unsteady on turning; without vision, patients stagger and fall. The ataxia progressively worsens over time. Data from our laboratory indicate that by the age of 20 years, 3% of patients require walking aids; the need increases linearly so that by the age of 30 years this increases to 14%, by the age of 40 years to 27% and by the age of 50 years some 49% of patients require assistance walking.
Ataxia may be the result of cerebellar or proprioceptive deficits. Clinically, the Romberg sign, i.e. the marked increase in postural sway when closing the eyes, has been used to distinguish cerebellar from proprioceptive ataxia. In patients with cerebellar ataxia, postural sway worsens with eye closure but it is already present with the eyes open, thus patients have a ‘negative Romberg sign’. For example, the abnormally increased postural sway and ataxic gait seen in chronic alcoholics, assessed both with the eyes open or closed, is associated with atrophy of the cerebellar vermis (Sullivan et al., 2006). Moreover, the postural sway is inversely correlated to the volume of the cerebellar vermis; the greater the cerebellar loss, the greater the postural sway (Sullivan et al., 2006). Conversely, in patients with HSAN III there is little evidence of gross cerebellar atrophy (Cohen and Solomon, 1955; Brown et al., 1964; Yatsu and Zussman, 1964; Solitare and Cohen, 1965). However, MRI has recently suggested possible white matter loss in the middle cerebral peduncle, as assessed by fractional anisotropy (Axelrod et al., 2010). While the latter study did consider the cerebellar changes as a likely candidate for the postural ataxia seen in HSAN III, it did not report atrophy of the cerebellar vermis which, based on the above, one might expect. This leads us to be circumspect about the significance of the white matter loss in the middle cerebellar peduncles as a cause for the gait ataxia, given the overall lack of evidence of cerebellar damage. Furthermore, patients with HSAN III are fairly stable with their eyes open but, when deprived of vision, their postural sway worsens and movements can lead to falling; thus patients are said to have a ‘positive Romberg sign’. These observations lead us to hypothesize that the ataxia in HSAN III is of sensory, rather than cerebellar origin. As for the afferents implicated in the sensory loss, the most likely candidates are the muscle spindles. As noted above, we know that deep tendon and H-reflexes—which depend on mechanical activation of the muscle spindles, or electrical stimulation of their afferents, respectively—are absent in HSAN III. What we do not know is whether this is due to a reduced excitability of the spinal motoneurons, a reduced efficacy of the spinal reflex or absent (or ineffective) muscle spindle afferent input to the spinal motoneurons. Moreover, as post-mortem examinations are difficult to obtain, we do not know whether the muscle spindles are absent or present, yet functionally ineffective as stretch receptors.
The spontaneous firing of individual muscle spindle afferents can readily be recorded in awake human subjects via tungsten microelectrodes inserted percutaneously into muscle fascicles of accessible peripheral nerves (microneurography). This spontaneous discharge is dependent on the resting length of the muscle, and can be increased by applying pressure to the tendon or muscle belly or by rotating the joint in the direction that increases muscle stretch (Vallbo, 1974; Burke et al., 1988). The purpose of the present study was to use this approach to test the hypothesis that muscle spindles are functionally absent in patients with HSAN III. For comparison, we also recorded the spontaneous discharge of muscle spindles in healthy subjects and studied two patients with HSAN IV. Also known as congenital insensitivity to pain with anhidrosis, HSAN IV is a very rare form of inherited neuropathy, caused by mutations of the TrkA/NGF gene (Indo et al., 1996; Toscano and Andria, 2001). Sensory insensitivity is much more profound in this condition; there is a complete absence of small-diameter nerves in the skin (Goebel et al., 1980) and pain and temperature sensibilities are lost, the loss of nociception leading to unrecognized injuries and Charcot joints. However, deep tendon and H-reflexes are intact and there is no ataxia in these patients. Accordingly, we predicted that functional muscle spindle afferents would be found in HSAN IV, but not in HSAN III.
Materials and methods
Experiments were performed on 13 patients with HSAN. Molecular confirmation of diagnosis of HSAN III (n = 10) and HSAN IV (n = 3) was available for all patients. All patients were recruited from the database of the Dysautonomia Center at New York University Medical Center and gave informed consent to the procedures that were approved by the Institutional Review Board of the New York University Medical Center. Recordings from control subjects were obtained in the laboratory of the corresponding author in Sydney, under approval of the Human Research Ethics Committee of the University of New South Wales. All studies were performed in accordance with the Declaration of Helsinki. The participants were seated in a semi-reclined posture in a comfortable chair with the legs supported in the extended position; in three experiments the patients were supine. The common peroneal nerve at the fibular head was located by external stimulation (0.2 ms pulses, 1 Hz, 2.5–5.5 mA; Stimulus Isolator; ADInstruments). In one of the three patients with HSAN IV, we could not locate the common peroneal nerve because of gross abnormality of the knee joints; accordingly, this patient was only included to indicate the similarity of the clinical findings in this group, but no nerve recordings were obtained from this patient. Intraneural recordings were made from muscle and cutaneous fascicles of the common peroneal nerve via tungsten microelectrodes (FHC) inserted percutaneously at the level of the fibular head. Muscle fascicles were identified as supplying either the peroneal muscles, extensor digitorum longus, extensor hallucis longus or tibialis anterior muscles according to the muscle twitches produced by intraneural stimulation at 10–20 µA. We know from experience that such currents indicate that the microelectrode tip is located within the fascicle. Cutaneous fascicles were identified by the absence of muscle twitches and reports of paraesthesiae projecting down the lateral aspect of the leg or on the dorsum of the foot. Neural activity was amplified (gain 20 000 ×, bandpass 0.3–5.0 kHz) using an isolated amplifier (NeuroAmp EX, ADInstruments) and stored on computer (10 kHz sampling) using a computer-based data acquisition and analysis system (PowerLab 16SP hardware and LabChart 7 software; ADInstruments). Afferent activity was discriminated using Spike Histogram software and instantaneous frequencies calculated (Spike Histogram, ADInstruments). In addition to the above experimental procedures, standard clinical neurophysiological assessments included motor nerve conduction of the common peroneal and ulnar nerves, F-waves, H-reflex and sensory nerve conduction of the sural and ulnar nerves. Compound muscle action potentials were recorded with Ag–AgCl electrodes placed over the extensor digitorum brevis muscle (peroneal nerve) or the abductor digiti minimi muscle (ulnar nerve); compound sensory action potentials were recorded antidromically over the sural nerve at the ankle or the digital nerves of the little finger. F-waves were recorded from the extensor digitorum brevis muscle after distal stimulation of the peroneal nerve at the ankle. H-reflexes were recorded from the soleus muscle after stimulation of the tibialis posterior nerve at the popliteal fossa. All recordings were performed on a Nicolet Viking EMG machine following standardized procedures. Statistical analyses were performed using Prism 5 for Macintosh (GraphPad Software Inc.).
Results
Clinical features
Thirteen patients with HSAN were studied. Seven female and three male patients (age 27 ± 3 years) were diagnosed with HSAN Type III (Riley–Day syndrome; more commonly referred to as familial dysautonomia). One female and two male brothers (age 19 ± 1 years), were diagnosed with HSAN Type IV. All patients were followed at the Dysautonomia Center, where the initial diagnoses had been performed in infancy or early childhood. With the exception of the three patients with HSAN Type IV, all patients had an ataxic, broad-based gait and frequent falling; two required a walker and three used a wheelchair. All had a positive Romberg sign, impaired proprioception in the toes, as well as atrophy of the leg muscles and absence of deep tendon reflexes. Characteristically, all patients with HSAN III had markedly elevated thresholds to noxious stimulation of the skin and elevated temperature thresholds; these patients expressed a relative indifference to pain. For the three patients with HSAN IV, noxious and temperature sensibility in the skin was absent. Clinical data are presented in Table 1. Temperature thresholds were abnormal in all patients with HSAN III; they were also abnormal in the patients with HSAN IV. However, vibration thresholds for the lower limb were abnormal in the patients with HSAN III but normal in HSAN IV, which fits with the preserved proprioception, lack of ataxia and negative Romberg sign in HSAN IV. The abnormal values for vibratory thresholds in the left lower extremity of two patients with HSAN III can be explained by peripheral nerve damage due to trauma/surgery in that limb.
Table 1.
Clinical and electrophysiological data for the 10 patients with HSAN III and 3 patients with HSAN IV
| Case | Sex | Age (years) | HSAN | Gait | Prop | Romberg sign | Stretch reflexes | H-reflexes | Cold (°C) | Warm (°C) | Vib UR | Vib UL | Vib LR | Vib LL | Peroneal motor CV (m/s) | Ulnar motor CV (m/s) | Ulnar sensory CV (m/s) | Sural sensory CV (m/s) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 16 | III | Ataxic | Reduced | Positive | Absent | Absent | 28 | 38.4 | 15 | 12 | 16 | 18 | 41.4 | 55.5 | 47.6 | |
| 2 | F | 18 | III | Ataxic | Reduced | Positive | Absent | 26.9 | 39 | 8 | 9 | 12 | 12 | |||||
| 3 | F | 19 | III | Ataxic | Reduced | Positive | Absent | Absent | 23 | 37.5 | 10 | 10 | 10 | 11 | 43.3 | 56.0 | 56.8 | 37.5 |
| 4 | F | 22 | III | Ataxic | Reduced | Positive | Absent | Absent | 10.6 | 44 | 24 | 20 | 26 | 28 | 44.7 | 40.0 | 48.5 | 32.9 |
| 5 | M | 22 | III | Ataxic | Reduced | Positive | Absent | Absent | 15 | 44.4 | 8 | 10 | 8 | 8 | 43.4 | 50.0 | ||
| 6 | F | 25 | III | Ataxic | Reduced | Positive | Absent | 27.8 | 36.6 | 18 | 19 | 26 | 29 | |||||
| 7 | M | 29 | III | Ataxic | Reduced | Positive | Absent | 26.5 | 46.7 | 15 | 15 | 22 | 28 | |||||
| 8 | M | 31 | III | Ataxic | Reduced | Positive | Absent | 19 | 41.3 | 14 | 16 | 18 | 20 | 42.6 | 51.0 | 36.4 | ||
| 9 | F | 34 | III | Ataxic | Reduced | Positive | Absent | Absent | 18.4 | 43.2 | 23 | 27 | 25 | 26 | 44.2 | 46.5 | 37.3 | |
| 10 | F | 53 | III | Ataxic | Reduced | Positive | Absent | 13 | 46.1 | 5 | 23 | 36 | 38 | |||||
| 11 | M | 18 | IV | Normal | Normal | Negative | Present | 17.7 | 46 | 5 | 7 | 8 | 8 | |||||
| 12 | M | 19 | IV | Normal | Normal | Negative | Present | 14.2 | 41.8 | 5 | 5 | 5 | 20 | |||||
| 13 | F | 21 | IV | Normal | Normal | Negative | Present | Present | 17.7 | 46 | 5 | 5 | 5 | 11 | 49.3 | 45.1 |
Cold = cold threshold measured over the left thenar eminence; CV = conduction velocity; LL = left lower limbs; LR = right lower limbs; Prop = proprioceptive acuity; UL = left upper limbs; UR = right upper limbs; Warm = warm threshold measured over the left thenar eminence; Vib = vibration thresholds.
Tables 2–4 provide electrophysiological data from a larger sample of patients with HSAN III (n = 26; age 26 ± 12 years) examined in the laboratory and a group of controls (n = 25; age 32 ± 16 years). Compared with controls, patients with HSAN III had mildly, but significantly, increased distal motor latencies and reduced motor conduction velocities of both the peroneal and ulnar nerves (Table 2). There were no differences between patients and controls in proximal versus distal compound muscle action potential changes and compound muscle action potential duration. There was no correlation between compound muscle action potential amplitude and either nerve conduction velocities or distal latencies. Patients with HSAN III had significantly reduced sensory conduction velocities and reduced sensory nerve action potential amplitudes in both peroneal and ulnar nerves (Table 3). Distal latency was significantly increased in the sural but not in the ulnar nerve; sensory nerve action potential duration was similar in the patients with HSANs and controls. Two patients with HSAN III had absent sural sensory nerve action potentials and three patients had absent ulnar sensory nerve action potentials. A positive correlation was observed between sensory nerve action potential amplitude and sensory conduction velocity in the sural nerve, but not in the ulnar nerve, for the patients. No double peak morphology of sensory nerve action potentials were observed in any of the patients with HSAN III.
Table 2.
Motor nerve conduction parameters for the common peroneal and ulnar nerves
| Group | Distal latency (ms) | CMAP duration (ms) | CMAP amplitude (mV) | CMAP area (mv/s) | Proximal–distal (% amp) | Proximal–distal (% area) | CV (m/s) |
|---|---|---|---|---|---|---|---|
| Peroneal | |||||||
| HSAN III (n = 16) | 5.1 ± 1.5 | 6.1 ± 1.8 | 5.1 ± 3.2 | 11.6 ± 7.8 | −11.1 ± 19.0 | −9.3 ± 12.0 | 45.8 ± 8.0 |
| Control (n = 23) | 4.3 ± 0.6* | 6.4 ± 1.0 (NS) | 8.0 ± 3.7 ** | 19.1 ± 7.8*** | −11.2 ± 9.0 (NS) | −6.3 ± 9.5 (NS) | 51.3 ± 5.4* |
| Ulnar | |||||||
| HSAN III (n = 18) | 3.0 ± 0.5 | 6.2 ± 1.7 | 15.8 ± 3.9 | 36.3 ± 11.6 | −11.0 ± 10.3 | −3.2 ± 6.6 | 49.4 ± 6.4 |
| Control (n = 24) | 2.8 ± 0.3* | 6.2 ± 1.1 (NS) | 14.3 ± 2.6 (NS) | 31.8 ± 6.1 (NS) | −7.0 ± 5.8 (NS) | −1.7 ± 8.0 (NS) | 59.5 ± 6.5*** |
*P < 0.05; **P < 0.02; ***P < 0.001.
CMAP = compound muscle action potential; CV = conduction velocity; NS = not significantly different between patients and controls.
Table 3.
Sensory nerve conduction parameters for the common peroneal and ulnar nerves
| Group | Distal latency (ms) | SNAP duration (ms) | SNAP amplitude (mV) | CV (m/s) |
|---|---|---|---|---|
| Sural | ||||
| HSAN III (n = 16) | 2.4 ± 0.4 | 1.3 ± 0.3 | 13.6 ± 7.1 | 41.6 ± 7.3 |
| Control (n = 24) | 2.1 ± 0.2*** | 1.3 ± 0.3 (NS) | 28.4 ± 10.4*** | 47.4 ± 4.3*** |
| Ulnar | ||||
| HSAN III (n = 12) | 2.1 ± 0.4 | 1.6 ± 0.8 | 11.1 ± 7.2 | 46.5 ± 9.1 |
| Control (n = 24) | 1.9 ± 0.2 (NS) | 1.2 ± 0.6 (NS) | 22.7 ± 9.1*** | 51.7 ± 5.0* |
*P < 0.05; ***P < 0.001.
CV = conduction velocity; ns = not significantly different between patients and controls; SNAP = sensory nerve action potential.
Table 4.
F-wave parameters for the common peroneal nerve
| Group | CMAP latency (ms) | CMAP amplitude (mV) | F-wave latency (ms) | F-wave amplitude (mV) | F-wave persistence (%) |
|---|---|---|---|---|---|
| HSAN III (n = 6) | 5.1 ± 1.1 | 4.8 ± 3.9 | 45.1 ± 6.1 | 0.3 ± 0.2 | 74.3 ± 27.8 |
| Control (n = 13) | 4.6 ± 0.7 (NS) | 6.7 ± 3.1 (NS) | 46.7 ± 4.7 (NS) | 0.3 ± 0.1 (NS) | 68.1 ± 24.1 (NS) |
CMAP = compound muscle action potential; ns = not significantly different between patients and controls.
F-waves were evaluated in the peroneal nerve of 12 patients and in all the controls. Five patients and five controls showed no F-responses. No differences between groups were observed for any of the posterior tibialis compound muscle action potential and F-wave parameters (Table 4). H-responses were evaluated in the tibialis posterior nerve of 16 patients and in all the controls. H-responses were absent in all 16 patients and in two controls. No differences between groups were observed for the posterior tibialis compound muscle action potential latency and amplitude. Patients with HSAN III reached a supramaximal compound muscle action potential response with a significantly lower intensity than controls.
Responses to intraneural electrical stimulation
Electrical stimulation of the common peroneal nerve at the fibular head was possible in all patients, with the exception of one patient with HSAN IV in whom gross abnormality of the knees made it difficult to locate the nerve. Stimulation currents required to evoke muscle twitches or paraesthesiae with a 1-mm surface probe over the nerve were normal: 2.5–5.5 mA (0.2 ms pulses). Moreover, by stimulating through the microelectrode it was possible to evoke muscle twitches at very low stimulation currents (10–20 µA), intensities we know from experience indicate that the electrode tip had entered a motor fascicle. Paraesthesiae could likewise be induced by intraneural stimulation within cutaneous fascicles at similar current intensities, and the projected sensations matched the distributions of the fascicles supplying either the dorsum of the foot or the lateral aspect of the leg. However, sites within the nerves at which motor or sensory responses could be induced were very sparse, which fits with the known atrophy of peripheral nerves in HSANs. Interestingly, although subjects were defined as having insensitivity to pain, in some subjects high-intensity (1 mA) intraneural stimulation within muscle fascicles could induce pain that was dull and aching in character, and could refer to the ankle, but no subjects reported sharp or burning pain following intraneural stimulation of cutaneous fascicles. Apart from these distinct noxious sensations, subjects occasionally reported aberrant sensations that were difficult to define: ‘it feels like something swimming in there’ or ‘I feel something but I don't know how to describe it’. Such sensations were never reported by the healthy individuals, who reported distinct sensations in the innervation territory, both with respect to quality and location.
Intraneural recordings from muscle fascicles
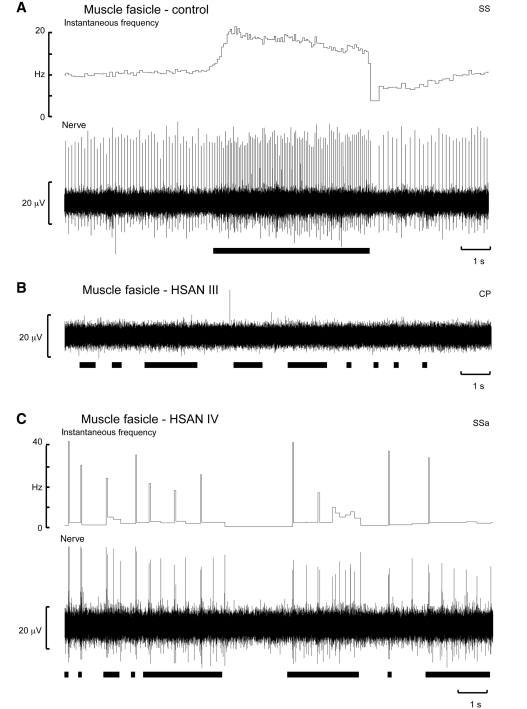
Muscle spindle afferent activity could readily be recorded in the 15 control subjects and, through discrete manipulation of the microelectrode, single afferent units could be isolated. A unitary recording from one such muscle spindle afferent, which supplied a secondary ending in the extensor hallucis longus muscle, is shown in Fig. 2A. This afferent was spontaneously active at rest and increased its firing during palpation of the muscle belly or tendon or, as shown in Fig. 2A, during passive plantar flexion of the big toe and hence stretch of the receptor-bearing muscle. Nine of the afferents were provisionally classified as supplying primary endings; the remainder innervated secondary endings. Typically, in our hands, a recording session in a healthy subject would yield between two and four identified muscle spindle afferents, each of which could be held for 30–60 min, depending on the type of experiment we were doing and for how long a given unit needed to be studied. Of course, if we were simply counting the number of muscle spindle afferents encountered within a muscle fascicle we would find many more; the purpose of this study was not to perform such a quantitative survey but to assess whether functional muscle spindles were present in patients with HSAN III. Indeed, the rich muscle spindle afferent activity seen in the control subjects was sharply contrasted with what we found in the patients with HSAN III; on entering a muscle fascicle in each of these patients there was a striking absence of spontaneous muscle afferent activity. Moreover, stretch-evoked activity could not be detected during muscle palpation or joint rotation. Normally, spontaneous or evoked activity in muscle spindles is readily encountered and this activity provides a means of navigating the microelectrode tip within a muscle fascicle of the nerve. Yet, despite extensive intrafascicular searching in each patient, no spontaneously active muscle spindle afferents could be detected in any of the 10 patients with HSAN III. Moreover, there were no afferent responses to percussion over the tendons or belly of the parent muscle, implying a complete absence of functional muscle spindles in these patients. Table 5 indicates the fascicles of the common peroneal nerve explored in each patient.
Figure 2.
Mean firing rates of 15 spontaneously active muscle spindles recorded from healthy controls and of five spontaneously active muscle spindles recorded from the two patients with HSAN IV. There were no significant differences in mean firing rates. No spontaneous (or stretch-evoked) activity could be recorded in the 10 patients with HSAN III.
Table 5.
Microneurographic data for the 10 patients with HSAN III and two of three patients with HSAN IV (the nerve could not be entered in one)
| Case | Sex | Age | HSAN | Nerve | Fascicle 1 | Fascicle 2 | Fascicle 3 | Fascicle 4 |
|---|---|---|---|---|---|---|---|---|
| 1 | F | 16 | III | Right peroneal | TA (18) | CUT D (42) | ||
| 2 | F | 18 | III | Right peroneal | PER (23) | PER (18) | ||
| 3 | F | 19 | III | Right peroneal | CUT D (73) | EDL (19) | ||
| 4 | F | 22 | III | Right peroneal | PER (50) | |||
| 5 | M | 22 | III | Right peroneal | PER (24) | PER (13) | ||
| 6 | F | 25 | III | Right peroneal | PER (25) | PER (8) | CUT D (9) | PER (11) |
| 7 | M | 29 | III | Left peroneal | EDL (50) | EDL (8) | EDL (16) | |
| 8 | M | 31 | III | Right peroneal | EDL (45) | TA (32) | ||
| 9 | F | 34 | III | Right peroneal | EDL (62) | |||
| 10 | F | 53 | III | Right peroneal | TA (16) | CUT L (12) | ||
| 11 | M | 18 | IV | – | ||||
| 12 | M | 19 | IV | Left peroneal | PER (34) | EDL (60) | EDL (16) | |
| 13 | F | 21 | IV | Right peroneal | EDL (23) |
The numbers in parentheses refer to the exploration time (in minutes) of each fascicle.
CUT D = cutaneous fascicle supplying the dorsum of the foot; CUT L = cutaneous fascicle supplying the lateral aspect of the leg; EDL = muscle fascicle supplying extensor digitorum longus; PER = muscle fascicle supplying the peronei; TA = muscle fascicle supplying tibialis anterior.
An intraneural recording from a muscle fascicle in one patient with HSAN III is shown in Fig. 1B. Despite evidence that the microelectrode tip was located well within the muscle fascicle—good muscle twitches could be induced during intraneural stimulation at 10 µA (which we know means that the microelectrode tip was located close to motor axons and hence muscle afferents)—extensive searching failed to uncover any spontaneous or stretch-evoked muscle spindle afferent activity. Conversely, spindle afferent activity could be recorded from each of the two patients with HSAN Type IV in whom we could enter the nerve, although the spindle activity was still rather sparse compared with that observed in healthy individuals. Experimental records from one of these patients are shown in Fig. 1C. This was a single-unit recording from a muscle spindle primary afferent within the fascicle supplying extensor digitorum longus. Typical of primary endings this afferent responded to brisk muscle stretch induced by passive plantar flexion of the toes. Five muscle spindle afferents, three provisionally classified as innervating primary endings and two supplying secondary endings, were recorded in these two patients. As shown in Fig. 2, there were no differences in spontaneous firing rates at rest for these afferents (7.6 ± 0.8 Hz) and for the 15 afferents recorded in the control subjects (7.7 ± 0.8 Hz).
Figure 1.
Intraneural recordings from muscle fascicles of the common peroneal nerve during muscle stretch in a control subject (A), a patient with HSAN III (B) and a patient with HSAN IV (C). (A) A single-unit recording from a muscle spindle secondary ending located in the extensor hallucis longus muscle; the afferent responded during passive plantar-flexions of the big toe. (B) A complete absence of muscle spindle afferent activity in the peronei fascicle during muscle stretch induced by passive inversion of the foot. (C) A single-unit recording from a muscle spindle primary ending located in the extensor digitorum longus muscle; the afferent responded dynamically during passive plantar-flexions of the toes. The black horizontal bars indicate periods of passive muscle stretch.
Intraneural recordings from cutaneous fascicles
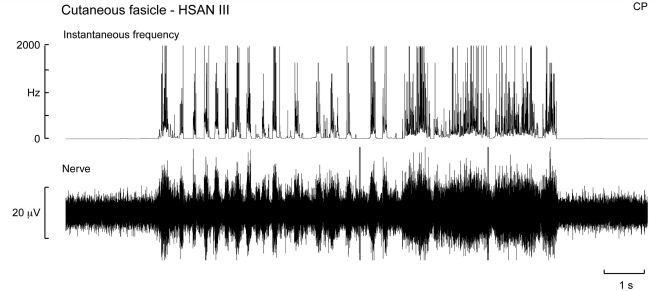
Unlike the afferent silence we encountered when exploring muscle fascicles, in four patients in whom cutaneous fascicles were explored afferent activity from large myelinated axons could readily be found by stroking the skin of the fascicular innervation territory. A multi-unit recording of cutaneous afferent activity from a patient with HSAN Type III is shown in Fig. 3—the same subject illustrated in Fig. 1B, in whom we could not detect any muscle spindles. The presence of cutaneous mechanoreceptors is not surprising, given that all patients had intact cutaneous sensibility; indeed, many reported being highly sensitive to tactile stimulation.
Figure 3.
Intraneural recording from a cutaneous fascicle of the common peroneal nerve in a patient with HSAN III. The sensory innervation of the skin appeared normal, as illustrated by this multi-unit recording from the cutaneous fascicle supplying the dorsum of the foot. Cutaneous mechanoreceptors were excited during stroking of the skin within the fascicular innervation territory.
Discussion
The aim of this study was to assess whether functional muscle spindle afferents were present in patients with HSAN III, as their absence could explain the loss of deep tendon reflexes, H-reflexes, proprioceptive acuity and the characteristic ataxic gait seen in these patients. Our use of intraneural microelectrodes to record from the common peroneal nerve allowed us to undertake ‘functional in vivo biopsy’ of the territories supplied by the nerve. In this way, we have documented, for the first time that patients with HSAN III are, apparently, devoid of functional muscle spindles, though these are still present in a related neuropathy, HSAN Type IV. Conversely, in both HSAN Types III and IV, large myelinated cutaneous afferents are still present and tactile sensation is preserved.
Electrophysiological features in hereditary sensory and autonomic neuropathy III
Patients with HSAN III had significant motor slowing, as shown by the increased distal motor latencies and decreased motor conduction velocities in the upper and lower limbs. Distal and proximal segments of the nerves were equally affected, indicating diffuse rather than focal slowing. This conduction slowing could be due to demyelination of motor axons or loss of fast conducting axons; although both abnormalities could occur in HSAN III, several lines of evidence indicate that axonal loss is more likely. First, the intensity of the slowing was mild, unlike the profound slowing in patients with other hereditary neuropathies with primary demyelination. Second, we did not find clear-cut electrodiagnostic signs of peripheral demyelination, such as increased duration, partial conduction block or increased F-latencies. Although we could not demonstrate a correlation between reduced compound muscle action potential amplitude and decreased motor conduction velocity, it seems likely that the loss of faster conducting motor axons is contributing to the observed motor conduction slowing. The decrease in amplitude and area of compound muscle action potentials, in the absence of prominent features of demyelination, is an unequivocal sign of motor axon loss. Moreover, reduced compound muscle action potentials in the peroneal but not in the ulnar nerve suggest that motor axonal degeneration in patients with HSAN III is also a length-dependent process. The preservation of all F-wave parameters demonstrates that the proximal segments of motor nerves, roots and plexuses are spared, with the spinal motoneurons having normal excitability. These findings are congruent with a relative preservation of the motor efferent pathways. Slight, but significant, slowing of motor conduction velocities in the median, ulnar and peroneal nerves, but not the tibial nerve, have been documented previously in patients with HSAN III (Axelrod et al., 1981), but there is no evidence of peripheral denervation of the muscle (Aguayo et al., 1971) and normal motor end-plates are present (Pearson et al., 1971, 1974).
Patients with HSAN III had mild to moderate slowing in peripheral sensory fibres, as shown by the decreased sensory conduction velocities in the upper and lower limbs and the increased distal latency for the sural nerve. The lower magnitude of sensory slowing of the ulnar than the sural nerve, together with preserved sensory latencies in the ulnar nerve, indicates that the sensory deficits in the lower limbs were worse than in the upper limbs. The absence of double peak potentials and increased sensory nerve action potential durations, the mild slowing and the relative preservation of ulnar distal latency suggest that patients with HSAN III do not have severe demyelination of sensory axons. The severe decrement in the amplitude of the sensory nerve action potentials recorded from the ulnar and sural nerves was the most notable electrophysiological abnormality in HSAN III. Sensory nerve action potentials were reduced to 50% of control values in both nerves, suggesting diffuse and severe nerve abnormalities; a reduction in sensory nerve action potential amplitude, in the absence of prominent signs of demyelination, is an unequivocal sign of sensory axonal loss.
Sensorimotor disturbances in hereditary sensory and autonomic neuropathy III
While only one case has been examined, the motor nerve to the extensor digitorum brevis muscle clearly has a greatly reduced number of large myelinated axons (Dyck et al., 1978). Given that motor strength is essentially normal in HSAN III, and the diameters of the large ventral horn neurons and ventral root axons are not changed in HSAN III (Dyck et al., 1978), the reduction in myelinated axons in the nerve supplying extensor digitorum brevis must reflect a loss of large myelinated afferents—i.e. muscle spindles and Golgi tendon organs. This fits with the reduced size of the dorsal roots and reduced diameters of the cell bodies in the dorsal root ganglia in HSAN III (Fogelson et al., 1967; Pearson et al., 1971, 1974; Dyck et al., 1978), and the reduction in area and focal demyelination of the dorsal columns in this disorder (Fogelson et al., 1967). Moreover, ventral horn neurons of intermediate size, believed to be the gamma motoneurons, are absent (Dyck et al., 1978). However, there have been no histological studies of the muscle spindles themselves; it is possible that they are present but are not functional or, and this is perhaps more likely, they failed to develop. Because of the scarcity of autopsy material among these patients it is difficult to undertake histological studies of muscle, particularly given that one needs a large sample of muscle in order to perform an adequate survey.
What can account for this apparent lack of (functional) muscle spindles in HSAN III? We know that the embryological development of muscle spindles depends on the migration of neural crest cells that occurs several days after the first migration. The first neurons from the dorsal root ganglia to migrate are large and end up supplying tactile afferents in the skin and endings responsible for visceral sensation (Hamburger and Levi-Montalcini, 1949). The second neurons to migrate are small and subserve pain and temperature sensibility, whereas others become large and innervate the muscle spindles (Hamburger and Levi-Montalcini, 1949). It would appear that the preservation of tactile mechanoreceptor inputs accounts for the normal tactile sensibility in HSAN III and IV, whereas failure of the second wave of migration from the dorsal root ganglion accounts for the loss of temperature and pain sensitivity in the skin and the loss of muscle spindles, as proposed by Aguayo et al. (1971). Importantly, if the muscle spindles do not develop, the gamma motoneurons—which supply the intrafusal muscle fibres—also fail to develop; muscle spindles release a specific glial cell line-derived nerve growth factor essential for the outgrowth of gamma motoneurons (Schneider et al., 2009). Interestingly, mice deficient in the transcription factor EGR3 show a complete absence of muscle spindles but normal extrafusal muscle fibres (Tourtelotte and Milbrandt, 1998). These mutant mice showed a 75% loss of large myelinated axons in the dorsal roots, which fits with the loss of muscle spindle afferents, and also showed a 90% reduction in the number of small myelinated axons in the ventral roots—i.e. a loss of gamma motoneurons (Tourtelotte and Milbrandt, 1998). As noted above, these same changes have been observed in HSAN III (Dyck et al., 1978). Moreover, mice that have spindle agenesis exhibit a waddling, uncoordinated gait and abnormal positioning of the limbs (Tourtelotte and Milbrandt, 1998). Interestingly, these mice develop scoliosis—also a common feature in HSAN III (Riley et al., 1954, Yoslow et al., 1971). Another mutant mouse model associated with loss of muscle spindles is produced by a deletion in the cytoplasmic dynein heavy chain 1 gene (Dync1h1); this animal expresses a pure proprioceptive sensory neuropathy, without motor involvement or loss of cutaneous nociception, and has absent H-reflexes (Chen et al., 2007). Moreover, like the Egr3-deficient mice, these animals walk with an unsteady ‘jerky and wobbling gait’ and splay their hindlimbs. Interestingly, although motor conduction velocities and compound muscle action potentials are not affected, grip strength in the hind limbs is reduced—presumably this is due to the loss of facilitation of motor output provided by muscle spindles (see below).
Functionally, the loss of muscle spindle afferents in HSAN III fits with the loss of deep tendon reflexes and H-reflexes. In addition, the loss of functional stretch receptors in the muscles means that the CNS is deprived of its most important source of proprioceptive (kinaesthetic) information—the muscle spindles (Burke et al., 1988; Proske and Gandevia, 2009). We believe the loss of muscle spindles may account for the characteristic hypotonicity of the muscles at birth, the reported (yet inconsistent) loss of proprioceptive acuity and poor reaching skills, and the ataxic gait seen in HSAN III. Indeed, we posit that the ataxia can be explained by the loss of muscle spindles, as can a Romberg sign and poor performance in the finger-to-nose pointing task. The wide base of the feet, and the slow cadence of the gait during walking, is similar to that seen in patients with complete large-fibre sensory neuropathy, who are critically dependent on vision to control the positions of their limbs in space (Cole and Sedgwick, 1992; Lajoie et al., 1996). However, unlike the latter (extremely rare) condition, in which large-diameter cutaneous as well as muscle afferents are lost but small-diameter afferents are preserved, patients with HSAN II or III do have access to mechanoreceptor inputs from the skin. Histological studies have shown that in HSAN III the sural nerve has a profoundly reduced complement of unmyelinated axons, a reduced number of myelinated axons and a reduced cross-sectional area of the nerve (Aguayo et al., 1971; Pearson et al., 1975). Nevertheless, histological studies have confirmed the presence of normal Meissner and Pacinian corpuscles in the skin of the fingers and toes (Winkelmann et al., 1966; Pearson et al., 1971), and we could record apparently normal tactile afferent activity from cutaneous fascicles in these patients in the present investigation.
It is known that cutaneous afferents and, to a much smaller extent joint receptors, can encode joint movements (Burke et al., 1987) and that proprioceptive cues can be obtained from cutaneous afferents in the absence of changes in muscle (or joint) afferent input (Edin and Johansson, 1995; Collins et al., 2005). We also know that muscle spindle afferents exert a facilitatory influence on alpha motoneurons, effectively providing amplification of descending motor command at the spinal cord, but that experimental block of muscle afferent input in human subjects does not affect the capacity to recruit and grade the discharge of individual alpha motoneurons (Gandevia et al., 1990, 1993; Macefield et al., 1993). Moreover, we know that tactile afferents can facilitate motor output in the complete absence of muscle afferent input, allowing voluntary commands to be focused on the target muscle (Gandevia et al., 1990, 1994; Macefield et al., 1993). Clearly, by taking advantage of movement-related or position-related signals originating in the skin there is sufficient redundancy in the system to allow patients with HSAN III to control their limbs without vision, albeit with a compromised sensorimotor control.
Sensory ataxia has also been observed in individuals with arthrogryposis multiplex, in whom deep tendon and H-reflexes are absent (Shibasaki et al., 2004). Somatosensory evoked potentials to ‘proprioceptive stimulation’—muscle stretch induced by brisk passive flexion of the proximal interphalangeal joint (which is not really a selective stimulus for muscle spindles)—are also absent in this condition, yet cutaneous somatosensory evoked potentials are normal (Shibasaki et al., 2004). Indeed, we predict that somatosensory evoked potentials to selective stimulation of large-diameter muscle afferents from the foot, by intramuscular electrical stimulation at the motor point of abductor hallucis (Macefield et al., 1989a), will be absent in HSAN III, and that afferent volleys to selective stimulation of these afferents (Macefield et al., 1989b) will not be able to be recorded within peripheral nerves in HSAN III. As noted above, patients with HSAN IV do not suffer the same sensorimotor problems as our patients with HSAN III, other than those caused by joint deformities; they do have access to the proprioceptive information provided by muscle spindle afferents—as confirmed in the present study—as well as sensory signals from cutaneous afferents.
Pain induced by intraneural stimulation in hereditary sensory and autonomic neuropathy
Small-diameter cutaneous afferents are reduced or absent in all types of HSANs, such that cutaneous pain thresholds are elevated (HSAN III) or absent (HSAN IV) and patients exhibit a relative indifference to pain. None of our patients reported sharp or burning pain during high-intensity intraneural stimulation within cutaneous fascicles at levels that would be painful in healthy individuals, but always reported paraesthesiae that were projected to the innervation territory of the fascicle; the paraesthesiae are due to electrical stimulation of large-diameter cutaneous afferents. Interestingly, patients did report noxious sensations evoked by intraneural stimulation within motor fascicles. These were felt as diffuse, poorly localized sensations in the muscle belly. In addition, patients often reported a dull ache that projected to the ankle, indicating that the sensation had referred, a feature typical of muscle pain. Indeed, it is known that in intact subjects the pain evoked by intraneural stimulation of muscle fascicles is often referred to sites remote from the muscles innervated by the fascicles (Torebjork et al., 1984). This suggests that, although large-diameter muscle afferents are absent, some small-diameter afferents may remain. And given that stimulation of both small-diameter myelinated (Group III) afferents and unmyelinated (Group IV) afferents evoke the same noxious sensations (Marchettini et al., 1996), it may be that the muscle pain evoked by intraneural stimulation in our patients reflects activation of Group III muscle afferents.
Limitations
Perhaps the greatest criticism that can be levelled at this study is that, with the exception of the electrophysiological data, it is largely qualitative. While this is true, the consistency of our findings warrants reporting. Indeed, our exploration of muscle fascicles in patients with HSAN III consistently revealed a complete absence of muscle spindles, and this fits the neurological profiles of these patients. Moreover, it is worth emphasizing that an absence of tendon or H-reflexes does not necessarily mean that muscle spindles or their afferents are absent—it could be that spinal excitability is so low that reflex responses cannot be generated. We have now demonstrated that functional muscle spindles do indeed appear to be absent in HSAN III. We should also emphasize that we have very limited observations in the patients with HSAN IV (n = 2), whereas 10 patients with HSAN III were studied. Nevertheless, the fact that we could record from muscle spindles in the two patients with HSAN IV, and that their spontaneous firing rates were comparable with those recorded from spindles in healthy individuals, fits with the clinical picture of these patients—they have intact tendon reflexes and H-reflexes.
It is worth pointing out that these were very difficult experiments, and we typically spent 3–4 h exploring a common peroneal nerve in each patient. Although we could readily locate the common peroneal nerve by surface stimulation at the fibular head, and evoke muscle twitches and paraesthesiae at intensities comparable with those used in healthy subjects (2.5–5.5 mA, 0.2 ms, 1 Hz), our microneurographic exploration of the nerve was by no means easy. For instance, we could generate muscle twitches from specific muscles at currents we know from experience indicate that we were stimulating from within the muscle fascicle (<20 µA), yet when we switched to the amplifier and listened to the intrafascicular recording we were greeted by silence; there was no spontaneous nerve activity, and none could be evoked by stretching or palpating the parent muscle or by percussing its tendon. We continued to search by making small adjustments of the microelectrode while mechanically stimulating the muscle, and in each patient we explored two or three muscle fascicles—intermittently confirming that we were still within a muscle fascicle by electrically stimulating through the microelectrode—yet found no signs of muscle afferent activity in the patients with HSAN III. Conversely, cutaneous fascicles were rich with evoked tactile afferent activity. By comparison, attempts to record from the common peroneal nerve in subjects with spinal cord injury presented different problems: the electrical thresholds required to stimulate the nerve were often 10 times higher than the levels required in intact subjects, and frequently the nerve could not be stimulated at all (Lin et al., 2007). Moreover, patients with spinal cord injury could not report cutaneous sensations. However, when we did enter a muscle or cutaneous fascicle in spinal patients we could record from spontaneously active muscle spindles in muscle fascicles, or activate tactile afferents in cutaneous fascicles. Accordingly, despite comparably difficult experiments, we are confident in our claim that we have adequately explored the common peroneal nerve in HSANs and stand by our conclusions that functional muscle spindles are absent in HSAN III.
Conclusion
Our observations of a lack of functional muscle spindles afferents in patients with HSAN III explains the lack of tendon and H-reflexes seen in these patients. Moreover, for the reasons pointed out above, we believe that the most parsimonious explanation for the ataxia is the loss of proprioceptive feedback provided by the muscle spindles. However, we cannot fully discount alternative interpretations. Indeed, an earlier small study of vestibular function in HSAN III revealed an absence of vestibulo-occular reflexes (Siggers et al., 1975), though whether this extends to disturbed vestibulospinal control is unknown. Finally, it is worth pointing out that there is evidence of axonal loss in the middle cerebellar peduncle, as well as in the optic radiation, and evidence of atrophy in the prefrontal cortex in these patients; given that the middle cerebellar peduncle plays an important role in relaying proprioceptive and vestibular information to the cerebellum, it has been argued that a reduction in the number of axons within this structure could contribute to their ataxia (Axelrod et al., 2010), as could the frontal lobe atrophy. To answer our initial question, we believe that loss of functional muscle spindles can account for the ataxia seen in HSAN III, but it may not be the only cause.
Funding
This work was supported by the National Institutes of Health (U54NS065736 to H.K. and L.N.-K.); Food and Drug Administration (FD-R-3731-01 to H.K. and L.N.-K.); Dysautonomia Foundation, Inc. (to F.B.A. and H.K.); The University of Western Sydney (to V.G.M.).
Acknowledgements
We are very grateful to the assistance provided by Clinical Fellow Dr Ishan Adhikari and Paediatric Nurse Practitioner Joseph Reyes, and the patients and their parents and carers.
Glossary
Abbreviations
- HSAN
hereditary sensory and autonomic neuropathy
References
- Aguayo A, Nair CPV, Bray GM. Peripheral nerve abnormalities in the Riley Day syndrome. Arch Neurol. 1971;24:106–16. doi: 10.1001/archneur.1971.00480320034003. [DOI] [PubMed] [Google Scholar]
- Axelrod FB. Hereditary sensory and autonomic neuropathies. Familial dysautonomia and other HSANs. Clin Auton Res. 2002;12(Suppl 1):I2–14. doi: 10.1007/s102860200014. [DOI] [PubMed] [Google Scholar]
- Axelrod FB, Hilz MJ, Berlin D, Yau PL, Javier D, Sweat V, et al. Neuroimaging supports central pathology in familial dysautonomia. J Neurol. 2010;257:198–206. doi: 10.1007/s00415-009-5293-1. [DOI] [PubMed] [Google Scholar]
- Axelrod FB, Iyer K, Fish I, Pearson J, Sein ME, Spielholz N. Progressive sensory loss in familial dysautonomia. Pediatrics. 1981;67:517–22. [PubMed] [Google Scholar]
- Blumenfeld A, Slaugenhaupt SA, Axelrod FB, Lucente DE, Maayan CH, Lieberg CB, et al. Localization of the gene for familial dysautonomia on chromosome 9 and definition of DNA markers for genetic diagnosis. Nat Genet. 1993;4:160–4. doi: 10.1038/ng0693-160. [DOI] [PubMed] [Google Scholar]
- Brown WJ, Beauchemin JA, Linde LM. A neuropathological study of familial dysautonomia (Riley-Day syndrome) in siblings. J Neurol Neurosurg Psychiatry. 1964;27:131–9. doi: 10.1136/jnnp.27.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Gandevia SC, Macefield G. Responses to passive movement of receptors in joint, skin and muscle of the human hand. J Physiol. 1988;402:347–61. doi: 10.1113/jphysiol.1988.sp017208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XJ, Levedakou EN, Millen KJ, Wollmann RL, Soliven B, Popko B. Proprioceptive sensory neuropathy in mice with a mutation in the cytoplasmic dynein heavy chain 1 gene. J Neurosci. 2007;27:14515–24. doi: 10.1523/JNEUROSCI.4338-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Solomon NH. Familial dysautonomia; case report with autopsy. J Pediatr. 1955;46:663–70. doi: 10.1016/s0022-3476(55)80171-0. [DOI] [PubMed] [Google Scholar]
- Cole JD, Sedgwick EM. The perceptions of force and of movement in a man without large myelinated sensory afferents below the neck. J Physiol. 1992;449:503–15. doi: 10.1113/jphysiol.1992.sp019099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DF, Refshauge KM, Todd G, Gandevia SC. Cutaneous receptors contribute to kinesthesia at the index finger, elbow, and knee. J Neurophysiol. 2005;94:1699–706. doi: 10.1152/jn.00191.2005. [DOI] [PubMed] [Google Scholar]
- Dyck PJ. Neuronal atrophy and degeneration predominantly affecting peripheral sensory and autonomic neurons. In: Dyck PJ, Thomas PK, Griffin JW, Low PA, Podulso JF, editors. Peripheral neuropathy. Vol. 2. Philadelphia: W.B. Saunders; 1993. pp. 1065–93. [Google Scholar]
- Dyck PJ, Kawamura Y, Low PA, Shimono M, Solovy JS. The number and sizes of reconstructed peripheral autonomic, sensory and motor neurons in a case of dysautonomia. J Neuropathol Exp Neurol. 1978;37:741–55. doi: 10.1097/00005072-197811000-00003. [DOI] [PubMed] [Google Scholar]
- Edin BB, Johansson N. Skin strain patterns provide kinaesthetic information to the human central nervous system. J Physiol. 1995;487:243–51. doi: 10.1113/jphysiol.1995.sp020875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelson MH, Rorke LB, Kaye R. Spinal cord changes in familial dysautonomia. Arch Neurol. 1967;17:103–8. doi: 10.1001/archneur.1967.00470250107012. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Macefield G, Burke D, McKenzie DK. Voluntary activation of human motor axons in the absence of muscle afferent feedback: the control of the deafferented hand. Brain. 1990;113:1563–82. doi: 10.1093/brain/113.5.1563. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Macefield VG, Bigland-Ritchie B, Gorman R, Burke D. Motoneuronal output and gradation of effort in attempts to contract acutely paralyzed leg muscles in man. J Physiol. 1993;447:411–27. doi: 10.1113/jphysiol.1993.sp019907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel HH, Veit S, Dyck PJ. Confirmation of virtual unmyelinated fiber absence in hereditary sensory neuropathy type IV. J Neuropathol Exp Neurol. 1980;39:670–5. doi: 10.1097/00005072-198011000-00005. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Levi-Montalcini R. Proliferation, differentiation and degeneration in the spinal ganglia of the chick embryo under normal and experimental conditions. J Exp Zool. 1949;111:457–501. doi: 10.1002/jez.1401110308. [DOI] [PubMed] [Google Scholar]
- Hilz MJ, Axelrod FB. Quantative sensory testing of thermal and vibratory perception in familial dysautonomia. Clin Autonom Res. 2000;10:177–83. doi: 10.1007/BF02291353. [DOI] [PubMed] [Google Scholar]
- Indo Y, Tsuruta M, Hayashida Y, Karim MA, Ohta K, Kawano T, et al. Mutations in the TRKA/NGF receptor gene in patients with congenital insensitivity to pain with anhidrosis. Nat Genet. 1996;13:485–8. doi: 10.1038/ng0896-485. [DOI] [PubMed] [Google Scholar]
- Lajoie Y, Teasdale N, Cole JD, Burnett M, Bard C, Fleury M, et al. Gait of a deafferented subject without large myelinated sensory fibers below the neck. Neurology. 1996;47:109–15. doi: 10.1212/wnl.47.1.109. [DOI] [PubMed] [Google Scholar]
- Lin CS, Macefield VG, Elam M, Wallin BG, Engel S, Kiernan MC. Axonal changes in spinal cord injured patients distal to the site of injury. Brain. 2007;130:985–94. doi: 10.1093/brain/awl339. [DOI] [PubMed] [Google Scholar]
- Macefield G, Burke D, Gandevia SC. The cortical distribution of muscle and cutaneous afferent projections from the human foot. Electroencephal Clin Neurophysiol. 1989a;72:518–28. doi: 10.1016/0013-4694(89)90229-0. [DOI] [PubMed] [Google Scholar]
- Macefield G, Gandevia SC, Burke D. Conduction velocities of muscle and cutaneous afferents in the upper and lower limbs of human subjects. Brain. 1989b;112:1519–32. doi: 10.1093/brain/112.6.1519. [DOI] [PubMed] [Google Scholar]
- Macefield VG, Gandevia SC, Bigland-Ritchie B, Gorman R, Burke D. The firing rates of human motoneurones voluntarily activated in the absence of muscle afferent feedback. J Physiol. 1993;447:429–43. doi: 10.1113/jphysiol.1993.sp019908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchettini P, Simone DA, Caputi G, Ochoa JL. Pain from excitation of identified muscle nociceptors in humans. Brain Res. 1996;740:109–16. doi: 10.1016/s0006-8993(96)00851-7. [DOI] [PubMed] [Google Scholar]
- Norcliffe-Kaufmann LJ, Axelrod FB, Kaufmann H. Afferent baroreflex failure in familial dysautonomia. Neurology. 2010;75:1904–11. doi: 10.1212/WNL.0b013e3181feb283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J, Axelrod F, Dancis J. Current concepts of dysautonomia: neuropathological defects. Ann NY Acad Sci. 1974;228:288–300. doi: 10.1111/j.1749-6632.1974.tb20517.x. [DOI] [PubMed] [Google Scholar]
- Pearson J, Budzilovich G, Finegold MJ. Sensory, motor and autonomic dysfunction: the nervous system in familial dysautonomia. Neurol. 1971;21:84–8. doi: 10.1212/wnl.21.5.486. [DOI] [PubMed] [Google Scholar]
- Pearson J, Dancis J, Axelrod F, Grover N. The sural nerve in familial dysautonomia. J Neuropathol Exp Neurol. 1975;34:413–24. doi: 10.1097/00005072-197509000-00004. [DOI] [PubMed] [Google Scholar]
- Proske U, Gandevia SC. The kinaesthetic senses. J Physiol. 2009;587:4139–46. doi: 10.1113/jphysiol.2009.175372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley CM, Day RL, Greely DMcL, Langford WS. Central autonomic dysfunction with defective lacrimation. Pediatrics. 1949;3:468–77. [PubMed] [Google Scholar]
- Riley CM, Freedman AM, Langford WS. Further observations on familial dysautonomia. Pediatrics. 1954;14:475–80. [PubMed] [Google Scholar]
- Shibasaki H, Hitomi T, Mezaki T, Kihara T, Tomimoto H, Ikeda A, et al. A new form of congenital proprioceptive sensory neuropathy associated with arthrogryposis multiplex. J Neurol. 2004;251:1340–4. doi: 10.1007/s00415-004-0539-4. [DOI] [PubMed] [Google Scholar]
- Schneider NA, Brown MN, Smith CA, Pickel J, Alvarez FJ. Gamma motor neurons express distinct genetic markers at birth and require muscle spindle-derived GDNF for postnatal survival. Neural Dev. 2009;4:42. doi: 10.1186/1749-8104-4-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggers DC, Haciska DT, McKusick VA. Vestibular dysfunction in familial dysautonomia: the Riley-Day syndrome. Arch Dis Child. 1975;50:890–3. doi: 10.1136/adc.50.11.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaugenhaupt SA, Blumenfeld A, Gill SP, Leyne M, Mull J, CmicroAjungco MP, et al. Tissue-specific expression of a splicing mutation in the IKBKAP gene causes familial dysautonomia. Am J Hum Genet. 2001;68:598–605. doi: 10.1086/318810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solitare GB, Cohen GS. Peripheral autonomic nervous system lesions in congenital or familial dysautonomia. Riley-Day syndrome. Neurology. 1965;15:321–7. doi: 10.1212/wnl.15.4.321. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rose J, Pfefferbaum A. Effect of vision, touch and stance on cerebellar vermian-related sway and tremor: a quantitative physiological and MRI study. Cereb Cortex. 2006;16:1077–86. doi: 10.1093/cercor/bhj048. [DOI] [PubMed] [Google Scholar]
- Torebjork HE, Ochoa JL, Schady W. Referred pain from intraneural stimulation of muscle fascicles in the median nerve. Pain. 1984;18:145–56. doi: 10.1016/0304-3959(84)90882-0. [DOI] [PubMed] [Google Scholar]
- Tourtelotte WG, Milbrandt J. Sensory ataxia and muscle spindle agenesis in mice lacking the transcription factor Egr3. Nat Genet. 1998;20:87–91. doi: 10.1038/1757. [DOI] [PubMed] [Google Scholar]
- Toscano E, Andria G. Congenital insensitivity to pain with anhidrosis: an NGF/TrkA related disorder. Am J Med Genet. 2001;99:164–5. doi: 10.1002/1096-8628(2000)9999:999<00::aid-ajmg1125>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Vallbo AB. Afferent discharge from human muscle spindles in non-contracting muscles. Steady state impulse frequency as a function of joint angle. Acta Physiol Scand. 1974;90:303–18. doi: 10.1111/j.1748-1716.1974.tb05593.x. [DOI] [PubMed] [Google Scholar]
- Winkelmann RK, Bourlond A, Smith AA. Nerves in the skin of a patient with familial dysautonomia (Riley-Day syndrome) Pediatrics. 1966;38:1060–2. [PubMed] [Google Scholar]
- Yatsu F, Zussman W. Familial dysautonomia (Riley-Day syndrome). Case report with postmortem findings of a patient at age 31. Arch Neurol. 1964;10:459–63. doi: 10.1001/archneur.1964.00460170029004. [DOI] [PubMed] [Google Scholar]
- Yoslow W, Becker MH, Bartles J, Thompson WAL. Orthopaedic defects in familial dysautonomia: a review of 65 cases. J Bone Joint Surg Am. 1971;53:1541–50. [PubMed] [Google Scholar]