Abstract
Patients with corticobasal degeneration can present with several different clinical syndromes, making ante-mortem diagnosis a challenge. Corticobasal syndrome is the clinical phenotype originally described for corticobasal degeneration, characterized by asymmetric rigidity and apraxia, cortical sensory deficits, dystonia and myoclonus. Some patients do not develop these features, but instead have clinical features consistent with the Richardson syndrome presentation of progressive supranuclear palsy, characterized by postural instability, early unexplained falls, vertical supranuclear gaze palsy, symmetric motor disability and dysphagia. The aim of this study was to identify differences in corticobasal degeneration presenting with corticobasal syndrome (n = 11) or Richardson syndrome (n = 15) with respect to demographic, clinical and neuropathological features. Corticobasal degeneration cases were also compared with patients with pathologically proven progressive supranuclear palsy with Richardson syndrome (n = 15). Cases with corticobasal degeneration, regardless of presentation, shared histopathological and tau biochemical characteristics, but they had differing densities of tau pathology in neuroanatomical regions that correlated with their clinical presentation. In particular, those with corticobasal syndrome had greater tau pathology in the primary motor and somatosensory cortices and putamen, while those with Richardson syndrome had greater tau pathology in limbic and hindbrain structures. Compared with progressive supranuclear palsy, patients with corticobasal degeneration and Richardson syndrome had less neuronal loss in the subthalamic nucleus, but more severe neuronal loss in the medial substantia nigra and greater atrophy of the anterior corpus callosum. Clinically, they had more cognitive impairment and frontal behavioural dysfunction. The results suggest that Richardson syndrome can be a clinicopathological presentation of corticobasal degeneration. Atrophy of anterior corpus callosum may be a potential neuroimaging marker to differentiate corticobasal degeneration from progressive supranuclear palsy in patients with Richardson syndrome.
Keywords: pathology, immunocytochemistry, progressive supranuclear palsy, tau protein, corticobasal degeneration
Introduction
Corticobasal degeneration is a progressive neurodegenerative disorder with distinct pathological features. There is usually focal cortical atrophy affecting parasagittal superior frontoparietal regions with relative sparing of the temporal and occipital lobes. Neuropathological diagnostic criteria for corticobasal degeneration require tau inclusions in neurons and glia, with astrocytic plaques and extensive thread-like pathology in both white matter and grey matter having differential diagnostic significance (Dickson et al., 2002). Tau-positive globose neurofibrillary tangles or ‘corticobasal bodies’ are common in the substantia nigra and locus coeruleus, while ballooned or achromatic neurons are common in affected cortical regions.
The classic clinical presentation of corticobasal degeneration, corticobasal syndrome, was first described in three cases by Rebeiz and co-workers (1968). Core features of corticobasal syndrome include levodopa-unresponsive parkinsonism, asymmetric akinesia and rigidity, accompanied by evidence of cortical and basal ganglia dysfunction, such as limb and oculomotor apraxia, cortical sensory deficits, dystonic posturing of a limb, myoclonus and alien limb phenomenon (Gibb et al., 1990; Riley et al., 1990). Clinicopathological studies have highlighted clinical heterogeneity of corticobasal degeneration, causing frequent ante-mortem clinical misdiagnosis (Wadia and Lang, 2007; Kouri et al., 2011). Corticobasal syndrome is the clinical presentation in ∼50% of cases with corticobasal degeneration (Litvan et al., 2000), with other presentations including progressive non-fluent aphasia (Kertesz et al., 2005; Josephs et al., 2006), severe cognitive dysfunction and dementia (Bergeron et al., 1996; Grimes et al., 1999; Kertesz et al., 2005; Murray et al., 2007), executive dysfunction with behavioural disturbances resembling frontotemporal dementia (Rinne et al., 1994; Boeve et al., 2003; Hassan et al., 2010) and a clinical syndrome indistinguishable from the Richardson syndrome presentation of progressive supranuclear palsy (Shiozawa et al., 2000; Josephs et al., 2006; Ling et al., 2010). Due to the rarity of corticobasal degeneration, the relative frequency of these various clinical presentations in pathologically confirmed corticobasal degeneration is unknown.
Progressive supranuclear palsy and corticobasal degeneration have overlapping tau pathology (Feany et al., 1996), and both are characterized by accumulation of insoluble tau enriched in the four-repeat (4R tau) isoform (Buee and Delacourte, 1999). Corticobasal degeneration typically has greater cortical involvement, whereas progressive supranuclear palsy has greater subcortical tau pathology (Dickson, 1999). The most distinguishing histopathological feature of corticobasal degeneration and progressive supranuclear palsy is the different astrocytic lesions—astrocytic plaques in corticobasal degeneration (Feany and Dickson, 1995) and tufted astrocytes in progressive supranuclear palsy (Yamada et al., 1992). There is also evidence to suggest that tau protein profiles may differ between corticobasal degeneration and progressive supranuclear palsy with respect to lower molecular weight species (Arai et al., 2004). Although corticobasal degeneration and progressive supranuclear palsy have considerable clinical and pathological overlap, most cases represent distinct clinicopathological entities.
In this study, we compared demographic, clinical and neuropathological features in corticobasal degeneration presenting as one of two different clinical syndromes—corticobasal syndrome or Richardson syndrome. For comparison purposes, we included similar studies in patients with progressive supranuclear palsy clinically presenting as Richardson syndrome. For each case we assessed anatomical distribution of tau pathology with state of the art digital imaging methods, semi-quantitative neuronal loss in key basal ganglia nuclei and anterior corpus callosum width in an effort to account for the distinct clinical presentations of corticobasal syndrome and Richardson syndrome in corticobasal degeneration and to identify clinical and pathological features that may be useful in differentiating corticobasal degeneration and progressive supranuclear palsy pathology in patients with Richardson syndrome.
Materials and methods
Subject selection
Patients with a neuropathological diagnosis of corticobasal degeneration (Dickson et al., 2002) were identified from the neuropathology files of the Mayo Clinic Jacksonville brain bank between 1999 and 2009. From 108 cases with autopsy-confirmed corticobasal degeneration, 41 had clinical features consistent with Richardson syndrome (CBD-RS) (Williams et al., 2008) and 21 had clinical features consistent with corticobasal syndrome (CBD-CBS) (Litvan et al., 2000), with the remainder having a range of other clinical presentations. A subset of patients with corticobasal syndrome (n = 11) and Richardson syndrome (n = 15) were selected for further study based upon the quality and completeness of the medical records. A subset of neuropathologically typical progressive supranuclear palsy (Hauw et al., 1994) (n = 15) with clinical presentation as Richardson syndrome (PSP-RS) were selected as a comparison group from over 600 autopsy-confirmed patients with progressive supranuclear palsy in the CurePSP/Society for PSP Brain Bank. Cases with PSP-RS were selected based on the quality and completeness of the medical records, and they were matched to the 26 patients with corticobasal degeneration for demographic features, including age of onset and sex. Of the final 41 patients, 34% were followed at the Mayo Clinic, while 66% were referral cases to the CurePSP/Society for PSP Brain Bank. There was no significant difference (χ2 = 4.0; P = 0.41) in the proportion of patients referred to the PSP Brain Bank compared with other referral sources for the three groups (i.e. CBD-CBS, CBD-RS and PSP-RS). Most patients (56%) were evaluated by a movement disorder specialist, with the remaining followed by a behavioural neurologist (12%) or a general practice neurologist (32%) There was no significant difference in the proportion of patients followed by specialists compared with general practice neurologists for the three groups (i.e. CBD-CBS, CBD-RS and PSP-RS) (χ2 = 3.7; P = 0.45). All but one of the 41 patients were Caucasian; one was African-American.
Clinical classification
The demographics and clinical information abstracted from medical records included age of onset, age of death, sex and race, as well as symptoms and signs arising during the course of the disease. Initial symptoms reported by the subject or caregiver and evidence of signs reported in the first neurological examination were recorded for each patient. The following clinical features were recorded as present or absent: history of falls, handwriting difficulty, depression, cognitive and memory impairment, urinary incontinence, dysphagia and dysarthria. Information about neurological findings included: bradykinesia, tremor, postural instability, rigidity (appendicular and axial), limb apraxia, dystonia (limb, cervical and blepharospasm), myoclonus, alien limb phenomenon, pyramidal signs, frontal release signs, cortical sensory loss, vertical supranuclear gaze palsy and aphasia. Behavioural and cognitive measures included: impairments in learning and memory; impairments in executive abilities; perseveration; and personality disorder of either abulic/apathetic or disinhibited types. If a symptom or sign was not specifically mentioned in the medical records, it was so noted and not considered to be absent, given the retrospective nature of the study. Disease duration was defined as the difference between the age at onset of the first sign or symptom and the age at death.
Although there are no autopsy-validated, clinical diagnostic criteria for corticobasal syndrome, we used generally accepted diagnostic criteria for corticobasal syndrome for inclusion in this study (Litvan et al., 2000; Riley and Lang, 2000), including presence of progressive asymmetric rigidity and apraxia, accompanied by signs of cortical or basal ganglia dysfunction, such as, cortical sensory loss, myoclonus and dystonia. Because patients in this study were not part of a prospective study with standardized collection of clinical data and since medical records from a variety of clinical sources were reviewed retrospectively, we used Richardson syndrome criteria as proposed by Williams et al. (2008) rather than more stringent National Institute of Neurological Disorders and Stroke – Society for Progressive Supranuclear Palsy (NINDS-SPSP) research criteria for clinically probable progressive supranuclear palsy (Litvan et al., 1996). Cases with Richardson syndrome as operationalized by Williams and colleagues (2008) had ‘gradual onset of postural instability and falls within the first 2 years of disease, along with vertical supranuclear gaze palsy, a frontal dysexecutive syndrome, and rigidity and bradykinesia that is unresponsive to levodopa’ (Williams et al., 2008). For analysis purposes, we also considered the number of patients in each group who also met NINDS-SPSP research criteria for clinically probable progressive supranuclear palsy.
Tissue sampling and pathological assessment
Pathological assessment and diagnosis was according to research criteria for progressive supranuclear palsy and corticobasal degeneration as previously described (Hauw et al., 1994; Dickson et al., 2002). In all cases, neuropathological evaluation was on right or left hemi-brains, the other side frozen for biochemical studies. Given that most of the brains included in this study were from referral sources, it was impossible to control which hemi-brain was evaluated for each case. Nevertheless, we recorded the side evaluated for neuropathological studies with respect to the clinical signs and took this into consideration with respect to analysis of tau burden (Supplementary Table 1). In all cases, primary and association cortices, basal ganglia, diencephalon, brainstem and cerebellum were evaluated with tau immunohistochemistry as previously described (Ahmed et al., 2008). Thioflavin S fluorescent microscopy was used to assess Alzheimer-type pathology (Barker et al., 2002), and haematoxylin and eosin-stained sections were used to evaluate neuronal loss and gliosis. Cases were also assessed for the presence or absence of argyrophilic grain disease with tau immunohistochemistry (Togo et al., 2002).
For tau immunohistochemistry, sections were processed using a DAKO autostainer (Universal Staining System) using 3,3-diaminobenzidine (DAB) as the chromogen and a phospho-tau antibody (CP13, mouse IgG1, 1 : 1000, kind gift from Dr Peter Davies, Albert Einstein College of Medicine, Bronx, NY, USA). After immunostaining, the sections were counterstained with haematoxylin.
Stained sections were scanned on the Aperio ScanScope XT slide scanner and converted to high-resolution digital images (Supplementary Fig. 1). Regions of interest were chosen based on their known involvement in corticobasal degeneration and progressive supranuclear palsy (Ishizawa and Dickson, 2001). Additional regions throughout the brain were also examined in order to fully assess the distribution and severity of tau pathology. A total of 25 regions of interest were analysed in all 41 cases using ImageScope software. The regions of interest included: grey and white matter of the primary motor and somatosensory, superior frontal and inferior temporal cortices, CA2 hippocampal subfield, dentate gyrus, amygdala, putamen, globus pallidus, anterior and ventrolateral thalamic nuclei, subthalamic nucleus, substantia nigra, red nucleus, superior colliculus, cerebral peduncle, pontine nuclei, tegmentum of the medulla, inferior olivary nucleus, corpus callosum and cerebellar white matter.
Digital image analysis with Aperio ImageScope has the advantage of quantifying histological features with a continuous variable that displays a dynamic range of values. Additionally, this technique effectively limits the interrater variability because the entire anatomical region of interest is sampled rather than much smaller and select microscopic fields that vary from observer to observer. Regions of interest were outlined, and immunostained pixels were counted with a colour deconvolution algorithm. The algorithm was specifically designed to detect chromogen-positive structures and expressed as the number of positive pixels to the total pixels for the entire region of interest as a per cent burden. During image analysis procedures, investigators were blinded to the clinical status.
Given that atrophy of the corpus callosum is common in corticobasal degeneration (Yamauchi et al., 1998; Groschel et al., 2004), we also measured the thickness of the corpus callosum. These measurements were made on digital images of 5-μm thick paraffin-embedded tissue sections that included the anterior cingulate gyrus, obtained from coronal brain slices at the level of nucleus accumbens. The ImageScope ruler feature was used to measure the thickness (expressed in millimetres) at a consistent location in all cases (Supplementary Fig. 2).
The degree of neuronal loss in the subthalamic nucleus and substantia nigra was assessed semi-quantitatively using the following scheme: 0 = none; 1 = mild; 2 = moderate; 3 = severe; 4 = almost complete. The subthalamic nucleus was assessed in both medial (limbic) and lateral (sensorimotor) regions of the nucleus, separately. The substantia nigra neuronal population was recorded for both the ventrolateral cell groups (A9) (Dickson et al., 2009) and the medial neuronal cell groups or ventral tegmental area (A10). During assessment of neuronal loss, the neuropathologist was blind to the clinical status.
Tau biochemistry
Brain tissue from eight cases (two CBD-CBS, three CBD-RS and three PSP-RS) was homogenized in 10 volumes of Tris-buffered saline containing protease and phosphatase inhibitors (50 mM Tris base, pH 8.0, 274 mM sodium chloride, 5 mM potassium chloride–TE buffer) and centrifuged at 26 300g for 20 min at 4°C. Supernatants were collected as S1 fractions, and the pellets (P1) were re-homogenized in five volumes of salt/sucrose buffer (10 mM Tris, pH 7.4, 800 mM sodium chloride, 10% sucrose, 1 mM ethylene glycol tetraacetic acid, 1 mM phenylmethylsulphonyl fluoride) and centrifuged as before. The resulting supernatants were incubated with 1% sarkosyl for 1 h at 37°C and centrifuged at 150 000g for 1 h at 4°C. The resulting pellet (P3) was resuspended in TE buffer (10 mM Tris, pH 8.0, 1 mM ethylenediaminetetraacetic acid).
Electrophoresis and immunoblotting were performed as previously described (Sahara et al., 2002). The samples were diluted with an equivalent volume of 2× Novex Tris–glycine sodium dodecyl sulphate sample buffer (Invitrogen), and equal volumes were run on 10% sodium dodecyl sulphate–polyacrylamide gels (Invitrogen), transferred to Immobilon membranes (Millipore), and immunoblotted. The primary antibody used against phosphorylated tau was PHF-1 (1 : 1000; kind gift of Dr Peter Davies) and anti-mouse IgG secondary antibody (1 : 5000).
Statistical analyses
All statistical analyses were performed with the statistical package in SigmaPlot 11.0 (Systat Software, Inc.) with significance set at P < 0.05. The Student's t-test was used to compare continuous variables (age at onset, age at death and disease duration) between CBD-CBS and CBD-RS. Chi-square or Fisher's exact tests were used to compare categorical variables (initial presenting signs and symptoms, presence of clinical features, referral source and neurologist type) of the three study groups. Tau burden per cent and neuronal loss scores were compared with the Mann–Whitney rank sum test. The effect of the side of brain evaluated on tau burden was tested with two-way ANOVA for corticobasal degeneration cases with either corticobasal syndrome or Richardson syndrome presentations, adjusting for side examined (contralateral side of brain considered 1 and ipsilateral side of brain considered 0 with respect to predominant side affected clinically).
Results
Demographic and initial presentation
Eleven patients with corticobasal syndrome (seven males, four females) and 15 Richardson syndrome patients (seven males, eight females) with a pathological diagnosis of corticobasal degeneration met inclusion and exclusion criteria (Table 1). Fifteen patients with progressive supranuclear palsy (eight males, seven females) were selected who had typical clinical features of Richardson syndrome (Williams et al., 2005, 2008). The mean age at onset, disease duration and age at death did not differ between CBD-RS and CBD-CBS. The mean disease duration for PSP-RS was 7.7 years, which was slightly longer compared with both corticobasal degeneration groups. CBD-CBS and CBD-RS did not differ with respect to presence of concomitant Alzheimer-type pathology—Braak neurofibrillary tangle stage [median (25th, 75th percentile)]: CBD-CBS 1.5 (1, 2) versus CBD-RS 2 (1, 2.5); P = 0.96 and presence of argyrophilic grain disease: CBD-CBS 64% versus CBD-RS 53%; P = 0.70.
Table 1.
Demographics and final clinical diagnosis
| Demographic feature | CBD-CBS | CBD-RS | PSP-RS |
|---|---|---|---|
| Cases, n | 11 | 15 | 15 |
| Sex (M/F) | 7/4 | 7/8 | 8/7 |
| Age at onset (years) | 64.3 ± 7.8 (53–79) | 62.9 ± 5.6 (55–74) | 62.1 ± 7.5 (47–75) |
| Disease duration (years) | 5.8 ± 2.9 (2–11) | 6.1 ± 2.5 (3–11) | 7.7 ± 2.6 (5–13) |
| Age of death (years) | 70.2 ± 9.8 (57–87) | 68.9 ± 6.6 (60–82) | 69.0 ± 7.6 (52–83) |
Data are mean ± standard deviation (with range); corticobasal syndrome = clinical corticobasal syndrome; Richardson syndrome = clinical Richardson syndrome.
Initial symptoms and signs are detailed in Table 2. At initial presentation, nine (60%) of the patients with CBD-RS and 10 (67%) of the patients with PSP-RS had unexplained falls and gait disturbance, whereas motor complaints of patients with CBD-CBS were related most commonly to having difficulty using an upper limb. Memory complaints were noted in 40% of patients with CBD-RS as indicated by caregivers and from information obtained from medical records. Early behavioural changes were noted in 20% of both patients with CBD-RS and patients with PSP-RS, which was not observed in patients with CBD-CBS. Similarly, initial symptoms of depression were noted in one-third of patients with CBD-RS, 27% with PSP-RS and none of the patients with CBD-CBS.
Table 2.
Initial clinical presenting signs and symptoms
| Clinical feature | CBD-CBS (n = 11), n (%) | CBD-RS (n = 15), n (%) | PSP-RS (n = 15), n (%) |
|---|---|---|---|
| Symptoms | |||
| Unilateral limb dysfunction | 7 (64) | 0 | 0 |
| Falls/gait difficulty | 0 | 9 (60) | 10 (67) |
| Eye movement abnormalities | 0 | 1 (7) | 4 (27) |
| Behavioural changes | 0 | 3 (20) | 3 (20) |
| Memory problems | 0 | 6 (40) | 0 |
| Depression | 0 | 5 (33) | 4 (27) |
| Signs | |||
| Bradykinesia | 1 (9) | 1 (7) | 2 (13) |
| Dysarthria | 0 | 0 | 3 (20) |
| Aphasia | 1 (9) | 1 (7) | 0 |
| Astereognosis | 1 (9) | 0 | 0 |
| Action tremor | 1 (9) | 1 (7) | 0 |
Clinical features of corticobasal degeneration and progressive supranuclear palsy
Clinical assessment of symptoms and signs are summarized in Table 3. Given that the groups were included if they fitted accepted clinical criteria for corticobasal syndrome and Richardson syndrome, the majority of differences between CBD-CBS and CBD-RS are expected. For example, patients with CBD-CBS more often exhibited asymmetric limb rigidity and apraxia, focal limb dystonia, myoclonus and cortical sensory deficits than patients with CBD-RS. They had less early unexplained falls, vertical gaze palsy and dysphagia. Limb apraxia was not a common feature in either CBD-RS or PSP-RS, but when present, was bilateral; which contrasted with the marked asymmetry of limb apraxia in patients with CBD-CBS. Pyramidal and extrapyramidal signs were noted at similar frequencies across the three groups.
Table 3.
Symptoms/signs in pathologically confirmed corticobasal degeneration and progressive supranuclear palsy
| Symptoms/signs | CBD-CBS (n = 11), n (%) |
CBD-RS (n = 15), n (%) |
PSP-RS (n = 15), n (%) |
|||
|---|---|---|---|---|---|---|
| Present | Absent | Present | Absent | Present | Absent | |
| Corticobasal syndrome features | ||||||
| Appendicular rigidity | 11 (100) | 0 | 15 (100) | 0 | 14 (93) | 0 |
| Asymmetric | 11 (100)* | 0 | 3 (20) | 4 | 0 | 4 |
| Limb apraxia | 10 (91)* | 0 | 3 (20) | 3 | 2 (13) | 1 |
| Asymmetric | 10 (91)* | 0 | 0 | 0 | 1 (7) | 0 |
| Limb dystonia | 9 (82)* | 0 | 1 (7) | 5 | 1 (7) | 6 |
| Myoclonus | 7 (64)* | 2 | 0 | 3 | 0 | 4 |
| Cortical sensory | 8 (73)* | 3 | 3 (20) | 10 | 0 | 7 |
| Alien limb | 4 (36)* | 0 | 0 | 0 | 0 | 0 |
| Aphasia | 4 (36)* | 3 | 1 (7) | 5 | 0 | 2 |
| Richardson syndrome features | ||||||
| Vertical gaze palsy | 2 (18)* | 7 | 14 (93) | 1 | 15 (100) | 0 |
| Dysphagia | 3 (27)* | 7 | 12 (80) | 2 | 11 (73) | 2 |
| Falls/gait difficulty | 9 (82) | 1 | 15 (100) | 0 | 15 (100) | 0 |
| Falls (within 1 year of onset) | 2 (18) | 1 | 4 (27) | 0 | 7 (47) | 0 |
| Falls (within 2 year of onset) | 3 (27) | 1 | 8 (47) | 0 | 12 (80) | 0 |
| Axial rigidity | 5 (45) | 3 | 12 (80) | 1 | 15 (100) | 0 |
| Cognitive or behavioural disorder | ||||||
| Behavioural abnormality | 5 (45) | 4 | 14 (93)*,† | 0 | 7 (47) | 5 |
| Memory impairment | 2 (18)* | 5 | 10 (67) | 1 | 4 (27) | 1 |
| Frontal release signs | 5 (45) | 0 | 6 (40) | 1 | 6 (40) | 0 |
| Movement disorder | ||||||
| Bradykinesia | 11 (100) | 0 | 15 (100) | 0 | 15 (100) | 0 |
| Postural instability | 8 (73) | 3 | 15 (100) | 0 | 15 (100) | 0 |
| Dysarthria | 11 (100) | 0 | 12 (80) | 2 | 14 (93) | 0 |
| Rest tremor | 1 (9) | 7 | 4 (27) | 11 | 0 | 15 |
| Other tremor | 7 (64) | 2 | 9 (60) | 2 | 6 (40) | 9 |
| Handwriting difficulty | 7 (64) | 0 | 7 (47) | 0 | 10 (67) | 0 |
| Cervical dystonia | 0 | 0 | 2 (13) | 0 | 1 (7) | 0 |
| Blepharospasm | 0 | 0 | 3 (20) | 0 | 1 (7) | 0 |
| Pyramidal signs | 7 (64) | 4 | 7 (47) | 5 | 7 (47) | 1 |
| Other | ||||||
| Urinary incontinence | 1 (9) | 8 | 11 (73)*,† | 1 | 3 (20) | 5 |
| Depression | 5 (45) | 4 | 10 (67) | 1 | 8 (53) | 1 |
*P < 0.05 comparing CBD-RS and CBD-CBS using chi-square test; †P < 0.05 comparing CBD-RS and PSP-RS.
Patients with CBD-RS could not be distinguished from patients with PSP-RS based on the presence of core features of Richardson syndrome. Unexplained falls within the first year of disease onset, which is a key diagnostic feature of the NINDS-SPSP research criteria for progressive supranuclear palsy (Litvan et al., 1996), were similar in frequency in patients with PSP-RS (47%) and CBD-RS (27%) (P = 0.45). Vertical gaze palsy and dysphagia were common in both patients with CBD-RS and patients with PSP-RS.
There were, however, some unexpected clinical differences between patients with CBD-RS and PSP-RS. For example, all but one patient with CBD-RS had executive and behavioural abnormalities during the disease course, which was significantly more common than in patients with PSP-RS (P = 0.014). The behavioural features often noted included disinhibition, social withdrawal, apathy, agitation and impatience. In addition, urinary incontinence was reported in 73% of patients with CBD-RS, but this was noted in only three (20%) patients with PSP-RS (P = 0.01).
Pathological findings
Neuronal loss in subthalamic nucleus and substantia nigra
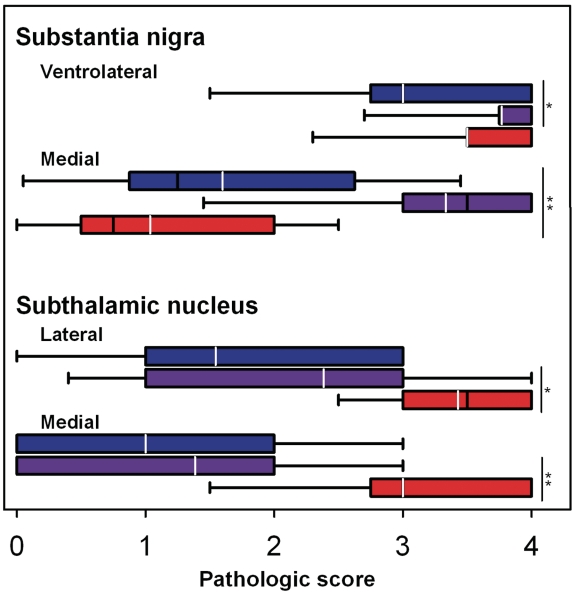
Neuronal loss was assessed semi-quantitatively in the substantia nigra and subthalamic nucleus blinded to clinical and pathological diagnosis (Fig. 1). The subthalamic nucleus had similar neuronal loss in medial and lateral parts in patients with CBD-CBS and CBD-RS, but more severe neuronal loss in PSP-RS, which fits with the known selective vulnerability of the subthalamic nucleus in progressive supranuclear palsy (Hauw et al., 1994). The ventrolateral cell group of the substantia nigra, which is selectively affected in Parkinsonian disorders (Dickson et al., 2009), was severely affected in both patients with CBD-RS and PSP-RS, but less affected in patients with CBD-CBS. In contrast, the medial cell group of the substantia nigra, which forms part of the mesolimbic ventral tegmental area, was selectively decreased in patients with CBD-RS compared with both CBD-CBS and PSP-RS.
Figure 1.
Semi-quantitative assessment of neuronal loss in the substantia nigra and subthalamic nucleus between CBD-CBS (blue), CBD-RS (purple) and PSP-RS (red). The lateral portion of the substantia nigra refers to the ventrolateral neuronal population (A9) and the medial substantia nigra corresponds to the ventral tegmental area (A10). Pathological scoring scheme: 0 = none; 1 = mild; 2 = moderate; 3 = severe; 4 = almost complete; Black line = median pathological score; white line = mean; *P < 0.05; **P < 0.001.
Atrophy of the corpus callosum
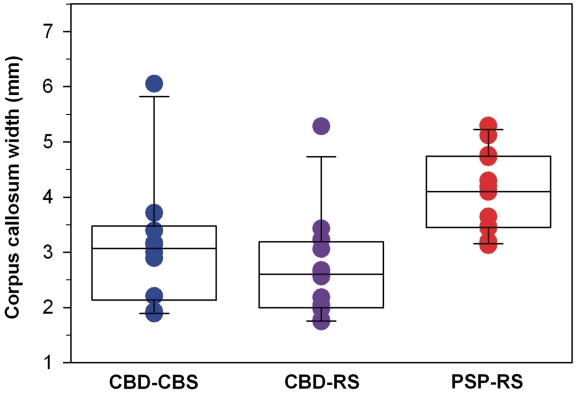
Callosal width (in millimetres) was measured with ImageScope on digital images of 5-μm thick tissue sections of the cingulate gyrus taken at the level of the anterior cingulate gyrus (Fig. 2). Both patients with CBD-CBS (median) and CBD-RS (median) had marked atrophy of the anterior corpus callosum compared with patients with PSP-RS (median values: CBD-CBS, 3.1 mm; CBD-RS, 2.6 mm; PSP-RS, 4.1 mm; P = 0.0008). In contrast, the width of the corpus callosum was not different between patients with CBD-CBS and CBD-RS (P = 0.31).
Figure 2.
Anterior corpus callosum measurements differentiate corticobasal degeneration and progressive supranuclear palsy. Both CBD-CBS (P = 0.008) and CBD-RS (P = 0.0008) have significantly more callosal atrophy compared with PSP-RS. Measurements were taken on coronal sections immediately adjacent to the cingulate gyrus. Callosal widths are in millimetres; black line = median width.
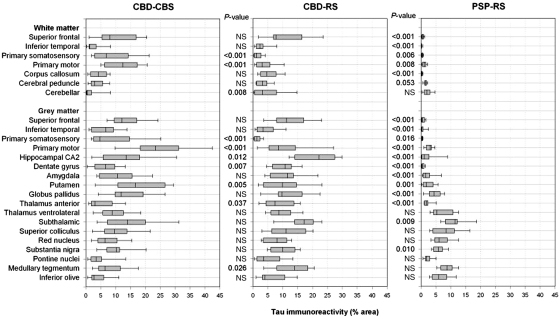
Distribution and severity of tau immunoreactivity
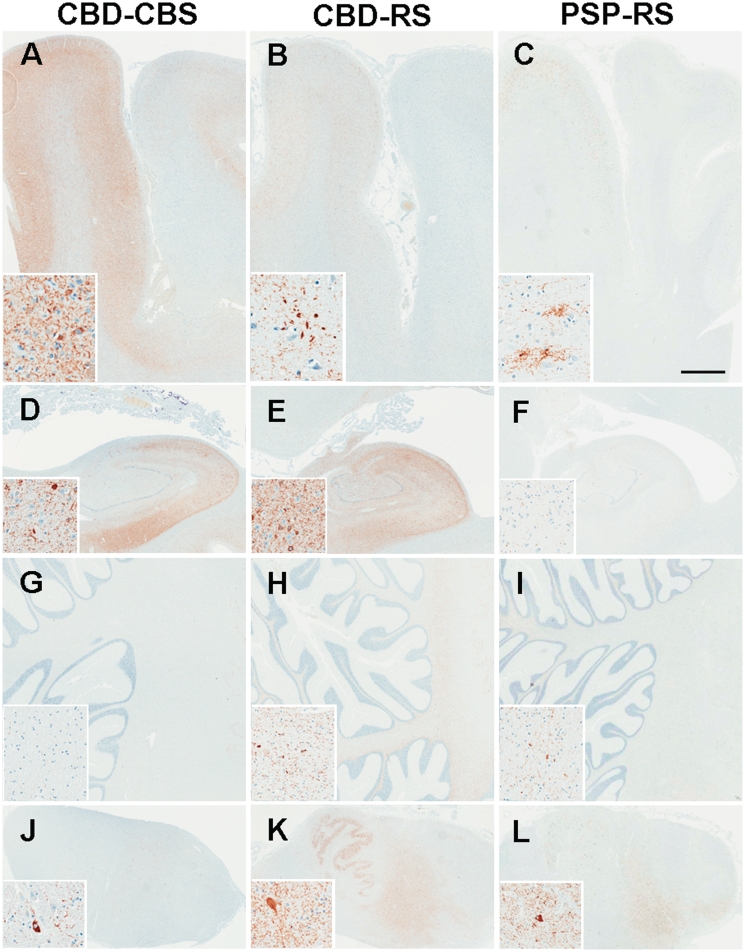
Tau burden was quantified by digital image analysis in 25 anatomical regions in the neocortex, basal ganglia, brainstem and cerebellum. Figure 3 illustrates tau pathology in representative brain regions, and Fig. 4 summarizes quantification of tau pathology by brain region in the 26 cases with corticobasal degeneration and 15 cases with progressive supranuclear palsy. In patients with CBD-CBS the regions with the most severe tau pathology, mostly in the form of neuronal pretangles and thread-like processes, were the primary motor cortex, putamen, globus pallidus externa and subthalamic nucleus. Primary motor and somatosensory cortices had significantly greater tau burden in both grey and white matter in patients with CBD-CBS compared with CBD-RS (all P < 0.001), whereas both groups had similar tau burden in the superior frontal cortex. Patients with CBD-CBS also had increased tau pathology in the putamen (P = 0.005) compared with CBD-RS.
Figure 3.
Tau immunohistochemistry in representative brain regions. CBD-RS has relative sparing of motor and somatosensory cortices (A–C; inset = primary motor cortex) and greater tau pathology in the hippocampus (D–F; inset = CA2) compared with CBD-CBS. CBD-RS tau burden in the cerebellar white matter (G–I) and medulla (J–L; inset = tegmentum) is more comparable with PSP-RS. Bar in panel C represents 3 mm in all the panels and 60 µm in all the insets.
Figure 4.
Regional distribution of tau immunoreactivity as assessed by image analysis in CBD-CBS, CBD-RS and PSP-RS. The Mann–Whitney rank sum test was used to compare tau burden in CBD-RS with either CBD-CBS or PSP-RS. NS = not significant.
Grey matter of the superior frontal cortex was the most severely affected cortical region in patients with CBD-RS; however, the greatest burden of tau pathology was in the hippocampus, subthalamic nucleus, amygdala and the white matter tracts in the brainstem (e.g. the tegmentum of the medulla and superior colliculus). The hippocampal CA2 (P = 0.012), dentate gyrus (P = 0.007) and anterior nucleus of the thalamus (P = 0.037), which are components of the extended limbic system, and the tegmentum of the medulla (P = 0.026) and cerebellar white matter (P = 0.008) had greater tau pathology in patients with CBD-RS compared with CBD-CBS.
The overall severity of tau pathology in both clinical subtypes of pathologically confirmed corticobasal degeneration was much greater than in patients with PSP-RS. The most affected regions in corticobasal degeneration ranged from ∼10 to 20% tau burden, while in PSP-RS the regions with the highest percentage of tau immunoreactivity ranged from 5 to 10%. As expected, the brunt of tau pathology in patients with PSP-RS was in brainstem regions, followed by the basal ganglia. Other than a mild involvement of the primary motor cortex, there was little cortical tau pathology in patients with PSP-RS. The greatest cortical burden in PSP-RS was grey (3% mean tau burden) and white matter (1% mean tau burden) of the primary motor cortex. All other cortical regions assessed in patients with PSP-RS had <0.5% mean tau burden.
For cases with CBD-CBS, the hemisphere opposite the side of greater clinical involvement was evaluated in 5 of 11 cases. When side was taken into consideration for the regions that were significantly different between patients with CBD-CBS and CBD-RS using a two-way ANOVA adjusting for side, there was no significant effect on tau burden in any of these regions except for grey and white matter of somatosensory cortex and for the putamen (Supplementary Table 1), consistent with less parietal tau burden for cases in which the ipsilateral cortex was evaluated and greater putaminal tau burden for cases in which the ipsilateral basal ganglia was evaluated.
Tau biochemistry
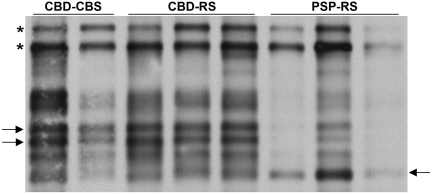
Homogenates of the frontal cortices of six cases were prepared. Since soluble tau species in corticobasal degeneration and progressive supranuclear palsy do not differ (Arai et al., 2004), their sarkosyl-insoluble fractions were analysed by immunoblot using the phosphorylation-dependent PHF-1 antibody (Fig. 5). The typical doublet at 64 and 68 kDa was seen in all cases; however, the lower molecular weight tau species differed between corticobasal degeneration and progressive supranuclear palsy. Cases with CBD-CBS and CBD-RS both had predominantly two closely associated bands ∼37 kDa, whereas the major lower molecular weight species in PSP-RS was at 33 kDa.
Figure 5.
Immunoblot analysis of the sarkosyl-insoluble fraction from frontal cortex homogenates of CBD-CBS, CBD-RS and PSP-RS. The characteristic doublet at 64 and 68 kDa (asterisks), CBD-CBS and CBD-RS have a lower molecular weight doublet at ∼37 kDa (two arrows, left) and PSP-RS has a single band at ∼33 kDa (arrow, right).
Discussion
This study investigated two distinct clinical phenotypes (corticobasal syndrome and Richardson syndrome) of pathologically confirmed corticobasal degeneration and found regional differences in tau burden using state of the art digital imaging methods, which correlated with the two clinical presentations. All patients, regardless of clinical presentation, met neuropathological criteria for corticobasal degeneration (Dickson, 1999), including presence of pathognomonic astrocytic plaques (Komori et al., 1998) and numerous thread-like processes in grey and white matter of cortical and subcortical regions. In addition, they had characteristic low molecular weight tau fragments in sarkosyl-insoluble fractions of brain, a molecular fingerprint that differentiates corticobasal degeneration from progressive supranuclear palsy (Arai et al., 2004).
Corticobasal degeneration is clinically characterized by both motor and cognitive disturbances that can present with a variety of syndromes, including corticobasal syndrome, progressive non-fluent aphasia, behavioural variant frontotemporal dementia and Richardson syndrome (Kouri et al., 2011). Due to the rarity of autopsy-confirmed corticobasal degeneration, the exact proportion of the various clinical presentations is unknown. When the 26 patients with corticobasal degeneration in the present study are included with four large clinicopathological studies of corticobasal degeneration (Kouri et al., 2011), for a total of 98 autopsy-confirmed patients with corticobasal degeneration, 36% presented with corticobasal syndrome and 27% with Richardson syndrome. This, of course, may not reflect the actual proportion of all patients with corticobasal degeneration given the inevitable bias of autopsy studies in parkinsonian disorders (Bower et al., 2002).
The present study adds to increasing evidence that corticobasal degeneration can be the pathological substrate for Richardson syndrome (Josephs and Dickson, 2003; Wadia and Lang, 2007; Ling et al., 2010) and for the first time, has specifically identified the neuropathological features that explain differences in clinical presentation. By definition, patients with CBD-RS had early unexplained falls, early cognitive dysfunction, vertical supranuclear gaze palsy and postural instability. Since this was not a longitudinal, prospective research study and clinical information was collected by retrospective review of medical records, we chose to use more lenient clinical criteria for Richardson syndrome proposed by Williams et al. (2008) rather than NINDS-SPSP research criteria for probable progressive supranuclear palsy (Litvan et al., 1996). A recent study that used the same criteria reviewed the clinical features of pathologically confirmed corticobasal degeneration presenting with either corticobasal syndrome or Richardson syndrome (Ling et al., 2010). In that study, patients with CBD-RS had an earlier age at symptom onset (62.8 versus 69.0 years) compared with patients with CBD-CBS (Ling et al., 2010). In the present series, we noted a similar trend, with patients with CBD-RS being slightly younger than patients with CBD-CBS (62.3 versus 64.3 years). They also found a delayed onset of vertical gaze palsy and infrequent occurrence of predominant downgaze palsy in their cases with CBD-RS (Ling et al., 2010). In a meta-analysis of pathologically confirmed corticobasal degeneration, Litvan et al. (2000) suggested that eye movement problems in corticobasal degeneration are more often characterized by oculomotor apraxia preceding vertical gaze palsy and typically have equal severity of both vertical and horizontal gaze palsy, whereas patients with progressive supranuclear palsy have greater involvement of vertical gaze without oculomotor apraxia. The presence or absence of oculomotor apraxia and the degree of severity of upward compared with downward vertical gaze palsy were not well documented in the medical records of all the cases in the current retrospective analysis. It remains to be determined if eye movement abnormalities can differentiate patients with CBD-RS from patients with PSP-RS.
Corticobasal degeneration: pathological basis for clinical heterogeneity
The anatomical distribution of brain atrophy, which reflects neuronal loss and tau pathology, determines the clinical syndrome in corticobasal degeneration, as is true for other neurodegenerative disorders (Boxer et al., 2006; Josephs et al., 2008; Whitwell et al., 2010). In the present study, distribution of corticobasal degeneration-related tau pathology differed between cases with CBD-CBS compared with CBD-RS. In cases with CBD-CBS, tau pathology was marked in forebrain structures, including motor and somatosensory cortices and the putamen. In patients with CBD-RS, limbic structures, including the hippocampal subfields, dentate gyrus and the anterior nucleus of the thalamus, as well as the tegmentum of the medulla and cerebellar white matter, had more tau pathology compared with patients with CBD-CBS. The greater tau burden in the lower brainstem and cerebellum in cases with CBD-RS may explain the clinical overlap with cases with PSP-RS, whereas the relative sparing of the cortex may explain the absence of hallmark cortical signs of corticobasal syndrome.
Involvement of the frontal and especially parietal cortices has been emphasized in corticobasal degeneration since the original report (Rebeiz et al., 1968), and these cortical regions were markedly affected in patients with CBD-CBS, with a 3-fold greater tau burden in the primary motor cortex compared with that in patients with CBD-RS. In the present series, tau pathology in the primary somatosensory cortex in cases with CBD-CBS was 10-fold greater in white matter and 6-fold greater in grey matter compared with cases with CBD-RS, which had mild tau pathology in the parietal lobe. This minimal involvement of parietal lobe in CBD-RS may correlate with absence of cortical signs, such as limb apraxia and cortical sensory loss, in this corticobasal degeneration phenotype.
A highly characteristic clinical feature of corticobasal syndrome is asymmetry (Litvan et al., 1999), but symmetrical clinical presentations of corticobasal degeneration are increasingly recognized (Hassan et al., 2010). Most of the patients with CBD-CBS in the present series had clinical asymmetry. The inability to document laterality of tau pathology, given that only one hemi-brain was studied in each case, is a limitation of the present study. Neuropathological analyses were on the side predicted to be more severely affected (i.e. contralateral to clinically most affected side) in 5 of the 11 cases with CBD-CBS. Had the ‘correct’ hemisphere been evaluated, one might predict that differences in cortical tau burdens would have been even greater between cases with CBD-CBS and CBD-RS. Support for this contention was strengthened with a two-way ANOVA adjusting for side of the brain evaluated, with a significant effect of side detected for parietal lobe grey and white matter (Supplementary Table 1). Unexpectedly, the opposite effect was noted for burden of tau in the putamen.
Symmetry of cortical involvement, although not specifically addressed in this study, may also have contributed to the Richardson syndrome clinical phenotype. Of note is the fact that symmetrical corticobasal degeneration may have clinical features that overlap with Richardson syndrome (Hassan et al., 2010). Also, behavioural and cognitive deficits are clinically more significant in symmetrical corticobasal degeneration (Hassan et al., 2010). It is thus of interest that cognitive and behavioural changes were also more frequent in our patients with CBD-RS. One might speculate that this is due to greater tau burden in limbic structures, including the hippocampus and anterior thalamus, in patients with CBD-RS compared with CBD-CBS.
In addition to differences in anatomical distribution of tau pathology, cases with CBD-CBS and CBD-RS had distinct patterns of neuronal loss in the subthalamic nucleus and substantia nigra. Severe neuronal loss and gliosis in the subthalamic nucleus is a defining neuropathological feature of progressive supranuclear palsy (Hauw et al., 1994), while there is less consistent neuronal loss in the subthalamic nucleus in corticobasal degeneration. It was therefore not surprising that both patients with CBD-CBS and CBD-RS had less neuronal loss in the subthalamic nucleus than patients with PSP-RS. The substantia nigra has variable neuronal loss and gliosis in corticobasal degeneration (Dickson et al., 2002). Neuronal loss affecting the medial portion of the substantia nigra has been shown to correlate with dementia in parkinsonian disorders (Rinne et al., 1989). In accord with this observation, patients with CBD-RS had more severe neuronal loss in medial substantia nigra than patients with CBD-CBS. This correlates with the observed increased frequency of cognitive and frontal behavioural deficits in CBD-RS compared with CBD-CBS. Both patients with PSP-RS and CBD-RS had severe neuronal loss in the ventrolateral cell groups of the substantia nigra, which is known to correlate with extrapyramidal features in parkinsonism (Fearnley and Lees, 1991; Dickson et al., 2009). In contrast, patients with CBD-CBS had less neuronal loss in the ventrolateral substantia nigra.
Richardson syndrome: pathological heterogeneity
Richardson syndrome is the most common presentation of progressive supranuclear palsy, but there is increasing recognition of clinical heterogeneity of progressive supranuclear palsy, with some patients presenting with pure akinesia with gait freezing, progressive non-fluent aphasia or even Parkinson's disease (Williams et al., 2005, 2007; Ahmed et al., 2008). Moreover, there are other pathological processes that can produce the Richardson syndrome clinical phenotype, including corticobasal degeneration, Lewy body disease, multiple system atrophy and cerebrovascular disease (Hughes et al., 2002; Josephs and Dickson, 2003; Williams and Lees, 2010). Results of the present study suggest that patients with Richardson syndrome who also have severe cognitive impairment may have underlying pathology of corticobasal degeneration, but confirmation requires additional longitudinal studies of Richardson syndrome coupled with post-mortem examination.
Despite considerable overlap in clinical features of CBD-RS and PSP-RS, there were significant differences with respect to distribution and severity of tau pathology, morphology of tau lesions, lower molecular weight sarkosyl-insoluble tau species and pattern of neuronal loss in the subthalamic nucleus and substantia nigra. There were also significant differences between CBD-RS and PSP-RS with respect to degree of corpus callosum atrophy. Previous clinical studies of corticobasal syndrome have noted atrophy of corpus callosum (Yamauchi et al., 1998; Groschel et al., 2004), but this may not be specific since it can also be detected in frontotemporal lobar degeneration (Yamauchi et al., 2000; Josephs et al., 2004). On the other hand, few of the available studies have been on pathologically verified cases (Josephs et al., 2004). It thus remains a possibility that in patients presenting with Richardson syndrome, presence of corpus callosum atrophy may assist in differential diagnosis of underlying pathology; however, confirmation awaits longitudinal studies of Richardson syndrome with post-mortem neuropathology.
Conclusion
Both corticobasal degeneration and progressive supranuclear palsy are 4R tauopathies with a range of clinical presentations that correlate with differences in distribution of atrophy and tau pathology. The explanation for selective vulnerability in 4R tauopathies is unknown, but contribution of genetic background can be hypothesized. Further studies are needed to determine genetic variants that might contribute to these differences. Clinicopathological studies suggest that corticobasal degeneration and progressive supranuclear palsy represent a disease spectrum, and the results of the present study support this concept. In particular, the present study indicates that corticobasal degeneration can be the pathological substrate of Richardson syndrome, just as previous studies have shown that progressive supranuclear palsy can be the pathological substrate of corticobasal syndrome (Boeve et al., 1999). Although there is clinical and pathological overlap in corticobasal degeneration and progressive supranuclear palsy (Feany et al., 1996; Wakabayashi and Takahashi, 2004), most cases have distinct pathological, biochemical and clinical features that warrant retention of the current classification of 4R tauopathies. If future disease-modifying therapies are discovered that target specifically 4R tau dysfunction, there would be little significance in differentiating CBD-RS from PSP-RS. On the other hand, if the factors that drive cellular and anatomical specificity in corticobasal degeneration differ meaningfully from progressive supranuclear palsy, making this distinction may eventually be of more than academic interest. To that end, additional research is needed to determine if corticobasal degeneration and progressive supranuclear palsy have the same pathogenesis.
Funding
Mayo Foundation (Jacoby Professorship of Alzheimer Research); National Institutes of Health (NIH; grants P50-AG16574, P50-NS72187 and P01-AG017216); CurePSP (for The Society for Progressive Supranuclear Palsy Brain Bank); NIH (grants RC2-NS070276, R01-NS057567, P50-NS072187 to Z.K.W.); Mayo Clinic Florida (MCF) Research Committee CR programs (MCF #90052018 and MCF #90052030 to Z.K.W.); gift from Carl Edward Bolch, Jr and Susan Bass Bolch (MCF #90052031/PAU #90052 to Z.K.W.); NIH (grants R01-AG037491 and R01-DC010367, to K.A.J.); Peebler PSP Research Foundation (to R.R.); NIH (grant R01-AG024040, to I.L.).
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
The authors would like to thank all patients and their families for support of this research. We would also like to thank Audrey Strongosky, Jennifer Lash and Beth Marten for logistic and administrative assistance in collecting material for this study. Ashley Cannon provided assistance with immunoblot analyses. The authors acknowledge Monica Castanedes Casey, Linda Rousseau and Virginia Phillips for histology and immunohistochemistry.
Glossary
Abbreviations
- CBD-CBS
corticobasal degeneration presenting as corticobasal syndrome
- CBD-RS
corticobasal degeneration presenting as Richardson syndrome
- PSP-RS
progressive supranuclear pasly presenting as Richardson syndrome
References
- Ahmed Z, Josephs KA, Gonzalez J, DelleDonne A, Dickson DW. Clinical and neuropathologic features of progressive supranuclear palsy with severe pallido-nigro-luysial degeneration and axonal dystrophy. Brain. 2008;131:460–72. doi: 10.1093/brain/awm301. [DOI] [PubMed] [Google Scholar]
- Arai T, Ikeda K, Akiyama H, Nonaka T, Hasegawa M, Ishiguro K, et al. Identification of amino-terminally cleaved tau fragments that distinguish progressive supranuclear palsy from corticobasal degeneration. Ann Neurol. 2004;55:72–9. doi: 10.1002/ana.10793. [DOI] [PubMed] [Google Scholar]
- Barker WW, Luis CA, Kashuba A, Luis M, Harwood DG, Loewenstein D, et al. Relative frequencies of Alzheimer disease, Lewy body, vascular and frontotemporal dementia, and hippocampal sclerosis in the State of Florida Brain Bank. Alzheimer Dis Assoc Disord. 2002;16:203–12. doi: 10.1097/00002093-200210000-00001. [DOI] [PubMed] [Google Scholar]
- Bergeron C, Pollanen MS, Weyer L, Black SE, Lang AE. Unusual clinical presentations of cortical-basal ganglionic degeneration. Ann Neurol. 1996;40:893–900. doi: 10.1002/ana.410400611. [DOI] [PubMed] [Google Scholar]
- Boeve BF, Maraganore DM, Parisi JE, Ahlskog JE, Graff-Radford N, Caselli RJ, et al. Pathologic heterogeneity in clinically diagnosed corticobasal degeneration. Neurology. 1999;53:795–800. doi: 10.1212/wnl.53.4.795. [DOI] [PubMed] [Google Scholar]
- Boeve BF, Lang AE, Litvan I. Corticobasal degeneration and its relationship to progressive supranuclear palsy and frontotemporal dementia. Ann Neurol. 2003;54(Suppl. 5):S15–9. doi: 10.1002/ana.10570. [DOI] [PubMed] [Google Scholar]
- Bower JH, Dickson DW, Taylor L, Maraganore DM, Rocca WA. Clinical correlates of the pathology underlying parkinsonism: a population perspective. Mov Disord. 2002;17:910–6. doi: 10.1002/mds.10202. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Geschwind MD, Belfor N, Gorno-Tempini ML, Schauer GF, Miller BL, et al. Patterns of brain atrophy that differentiate corticobasal degeneration syndrome from progressive supranuclear palsy. Arch Neurol. 2006;63:81–6. doi: 10.1001/archneur.63.1.81. [DOI] [PubMed] [Google Scholar]
- Buee L, Delacourte A. Comparative biochemistry of tau in progressive supranuclear palsy, corticobasal degeneration, FTDP-17 and Pick's disease. Brain Pathol. 1999;9:681–93. doi: 10.1111/j.1750-3639.1999.tb00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson DW. Neuropathologic differentiation of progressive supranuclear palsy and corticobasal degeneration. Journal of Neurology. 1999;246(Suppl. 2):II6–15. doi: 10.1007/BF03161076. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Bergeron C, Chin SS, Duyckaerts C, Horoupian D, Ikeda K, et al. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol. 2002;61:935–46. doi: 10.1093/jnen/61.11.935. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Braak H, Duda JE, Duyckaerts C, Gasser T, Halliday GM, et al. Neuropathological assessment of Parkinson's disease: refining the diagnostic criteria. Lancet Neurol. 2009;8:1150–7. doi: 10.1016/S1474-4422(09)70238-8. [DOI] [PubMed] [Google Scholar]
- Feany MB, Dickson DW. Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am J Pathol. 1995;146:1388–96. [PMC free article] [PubMed] [Google Scholar]
- Feany MB, Mattiace LA, Dickson DW. Neuropathologic overlap of progressive supranuclear palsy, Pick's disease and corticobasal degeneration. J Neuropathol Exp Neurol. 1996;55:53–67. doi: 10.1097/00005072-199601000-00006. [DOI] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Gibb WR, Luthert PJ, Marsden CD. Clinical and pathological features of corticobasal degeneration. Adv Neurol. 1990;53:51–4. [PubMed] [Google Scholar]
- Grimes DA, Lang AE, Bergeron CB. Dementia as the most common presentation of cortical-basal ganglionic degeneration. Neurology. 1999;53:1969–74. doi: 10.1212/wnl.53.9.1969. [DOI] [PubMed] [Google Scholar]
- Groschel K, Hauser TK, Luft A, Patronas N, Dichgans J, Litvan I, et al. Magnetic resonance imaging-based volumetry differentiates progressive supranuclear palsy from corticobasal degeneration. Neuroimage. 2004;21:714–24. doi: 10.1016/j.neuroimage.2003.09.070. [DOI] [PubMed] [Google Scholar]
- Hassan A, Whitwell JL, Boeve BF, Jack CR, Jr, Parisi JE, Dickson DW, et al. Symmetric corticobasal degeneration (S-CBD) Parkinsonism Relat Disord. 2010;16:208–14. doi: 10.1016/j.parkreldis.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, et al. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy) Neurology. 1994;44:2015–9. doi: 10.1212/wnl.44.11.2015. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain. 2002;125:861–70. doi: 10.1093/brain/awf080. [DOI] [PubMed] [Google Scholar]
- Ishizawa K, Dickson DW. Microglial activation parallels system degeneration in progressive supranuclear palsy and corticobasal degeneration. J Neuropathol Exp Neurol. 2001;60:647–57. doi: 10.1093/jnen/60.6.647. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Dickson DW. Diagnostic accuracy of progressive supranuclear palsy in the Society for Progressive Supranuclear Palsy brain bank. Mov Disord. 2003;18:1018–26. doi: 10.1002/mds.10488. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Tang-Wai DF, Edland SD, Knopman DS, Dickson DW, Parisi JE, et al. Correlation between antemortem magnetic resonance imaging findings and pathologically confirmed corticobasal degeneration. Arch Neurol. 2004;61:1881–4. doi: 10.1001/archneur.61.12.1881. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Petersen RC, Knopman DS, Boeve BF, Whitwell JL, Duffy JR, et al. Clinicopathologic analysis of frontotemporal and corticobasal degenerations and PSP. Neurology. 2006;66:41–8. doi: 10.1212/01.wnl.0000191307.69661.c3. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Whitwell JL, Dickson DW, Boeve BF, Knopman DS, Petersen RC, et al. Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Aging. 2008;29:280–9. doi: 10.1016/j.neurobiolaging.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A, McMonagle P, Blair M, Davidson W, Munoz DG. The evolution and pathology of frontotemporal dementia. Brain. 2005;128:1996–2005. doi: 10.1093/brain/awh598. [DOI] [PubMed] [Google Scholar]
- Komori T, Arai N, Oda M, Nakayama H, Mori H, Yagishita S, et al. Astrocytic plaques and tufts of abnormal fibers do not coexist in corticobasal degeneration and progressive supranuclear palsy. Acta Neuropathol. 1998;96:401–8. doi: 10.1007/s004010050911. [DOI] [PubMed] [Google Scholar]
- Kouri N, Whitwell JL, Josephs KA, Rademakers R, Dickson DW. Corticobasal degeneration: a pathologically distinct 4R tauopathy. Nat Rev Neurol. 2011;7:263–72. doi: 10.1038/nrneurol.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling H, O'Sullivan SS, Holton JL, Revesz T, Massey LA, Williams DR, et al. Does corticobasal degeneration exist? A clinicopathological re-evaluation. Brain. 2010;133:2045–57. doi: 10.1093/brain/awq123. [DOI] [PubMed] [Google Scholar]
- Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- Litvan I, Grimes DA, Lang AE, Jankovic J, McKee A, Verny M, et al. Clinical features differentiating patients with postmortem confirmed progressive supranuclear palsy and corticobasal degeneration. J Neurol. 1999;246(Suppl. 2):II1–5. doi: 10.1007/BF03161075. [DOI] [PubMed] [Google Scholar]
- Litvan I, Grimes DA, Lang AE. Phenotypes and prognosis: clinicopathologic studies of corticobasal degeneration. Adv Neurol. 2000;82:183–96. [PubMed] [Google Scholar]
- Murray R, Neumann M, Forman MS, Farmer J, Massimo L, Rice A, et al. Cognitive and motor assessment in autopsy-proven corticobasal degeneration. Neurology. 2007;68:1274–83. doi: 10.1212/01.wnl.0000259519.78480.c3. [DOI] [PubMed] [Google Scholar]
- Rebeiz JJ, Kolodny EH, Richardson EP., Jr Corticodentatonigral degeneration with neuronal achromasia. Arch Neurol. 1968;18:20–33. doi: 10.1001/archneur.1968.00470310034003. [DOI] [PubMed] [Google Scholar]
- Riley DE, Lang AE, Lewis A, Resch L, Ashby P, Hornykiewicz O, et al. Cortical-basal ganglionic degeneration. Neurology. 1990;40:1203–12. doi: 10.1212/wnl.40.8.1203. [DOI] [PubMed] [Google Scholar]
- Riley DE, Lang AE. Clinical diagnostic criteria. Adv Neurol. 2000;82:29–34. [PubMed] [Google Scholar]
- Rinne JO, Rummukainen J, Paljarvi L, Rinne UK. Dementia in Parkinson's disease is related to neuronal loss in the medial substantia nigra. Ann Neurol. 1989;26:47–50. doi: 10.1002/ana.410260107. [DOI] [PubMed] [Google Scholar]
- Rinne JO, Lee MS, Thompson PD, Marsden CD. Corticobasal degeneration. A clinical study of 36 cases. Brain. 1994;117(Pt 5):1183–96. doi: 10.1093/brain/117.5.1183. [DOI] [PubMed] [Google Scholar]
- Sahara N, Lewis J, DeTure M, McGowan E, Dickson DW, Hutton M, et al. Assembly of tau in transgenic animals expressing P301L tau: alteration of phosphorylation and solubility. J Neurochem. 2002;83:1498–508. doi: 10.1046/j.1471-4159.2002.01241.x. [DOI] [PubMed] [Google Scholar]
- Shiozawa M, Fukutani Y, Sasaki K, Isaki K, Hamano T, Hirayama M, et al. Corticobasal degeneration: an autopsy case clinically diagnosed as progressive supranuclear palsy. Clin Neuropathol. 2000;19:192–9. [PubMed] [Google Scholar]
- Togo T, Cookson N, Dickson DW. Argyrophilic grain disease: neuropathology, frequency in a dementia brain bank and lack of relationship with apolipoprotein E. Brain Pathol. 2002;12:45–52. doi: 10.1111/j.1750-3639.2002.tb00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadia PM, Lang AE. The many faces of corticobasal degeneration. Parkinsonism Relat Disord. 2007;13(Suppl. 3):S336–40. doi: 10.1016/S1353-8020(08)70027-0. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Takahashi H. Pathological heterogeneity in progressive supranuclear palsy and corticobasal degeneration. Neuropathology. 2004;24:79–86. doi: 10.1111/j.1440-1789.2003.00543.x. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR, Jr, Boeve BF, Parisi JE, Ahlskog JE, Drubach DA, et al. Imaging correlates of pathology in corticobasal syndrome. Neurology. 2010;75:1879–87. doi: 10.1212/WNL.0b013e3181feb2e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DR, de Silva R, Paviour DC, Pittman A, Watt HC, Kilford L, et al. Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson's syndrome and PSP-parkinsonism. Brain. 2005;128:1247–58. doi: 10.1093/brain/awh488. [DOI] [PubMed] [Google Scholar]
- Williams DR, Holton JL, Strand K, Revesz T, Lees AJ. Pure akinesia with gait freezing: a third clinical phenotype of progressive supranuclear palsy. Mov Disord. 2007;22:2235–41. doi: 10.1002/mds.21698. [DOI] [PubMed] [Google Scholar]
- Williams DR, Lees AJ, Wherrett JR, Steele JC. J. Clifford Richardson and 50 years of progressive supranuclear palsy. Neurology. 2008;70:566–73. doi: 10.1212/01.wnl.0000286938.39473.0e. [DOI] [PubMed] [Google Scholar]
- Williams DR, Lees AJ. What features improve the accuracy of the clinical diagnosis of progressive supranuclear palsy-parkinsonism (PSP-P)? Mov Disord. 2010;25:357–62. doi: 10.1002/mds.22977. [DOI] [PubMed] [Google Scholar]
- Yamada T, McGeer PL, McGeer EG. Appearance of paired nucleated, Tau-positive glia in patients with progressive supranuclear palsy brain tissue. Neurosci Lett. 1992;135:99–102. doi: 10.1016/0304-3940(92)90145-w. [DOI] [PubMed] [Google Scholar]
- Yamauchi H, Fukuyama H, Nagahama Y, Katsumi Y, Dong Y, Hayashi T, et al. Atrophy of the corpus callosum, cortical hypometabolism, and cognitive impairment in corticobasal degeneration. Arch Neurol. 1998;55:609–14. doi: 10.1001/archneur.55.5.609. [DOI] [PubMed] [Google Scholar]
- Yamauchi H, Fukuyama H, Nagahama Y, Katsumi Y, Hayashi T, Oyanagi C, et al. Comparison of the pattern of atrophy of the corpus callosum in frontotemporal dementia, progressive supranuclear palsy, and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2000;69:623–9. doi: 10.1136/jnnp.69.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.