Abstract
Distal sensory polyneuropathy is a frequent complication of lentivirus infections of the peripheral nervous system including both human immunodeficiency virus and feline immunodeficiency virus. Proteinase-activated receptors are G protein-coupled receptors implicated in the pathogenesis of neuroinflammation and neurodegeneration. Proteinase-activated receptor-1 is expressed on different cell types within the nervous system including neurons and glia, but little is known about its role in the pathogenesis of inflammatory peripheral nerve diseases, particularly lentivirus-related distal sensory polyneuropathy. Herein, the expression and functions of proteinase-activated receptor-1 in the peripheral nervous system during human immunodeficiency virus and feline immunodeficiency virus infections were investigated. Proteinase-activated receptor-1 expression was most evident in autopsied dorsal root ganglion neurons from subjects infected with human immunodeficiency virus, compared with the dorsal root ganglia of uninfected subjects. Human immunodeficiency virus or feline immunodeficiency virus infection of cultured human or feline dorsal root ganglia caused upregulation of interleukin-1β and proteinase-activated receptor-1 expression. In the human immunodeficiency virus- or feline immunodeficiency virus-infected dorsal root ganglia, interleukin-1β activation was principally detected in macrophages, while neurons showed induction of proteinase-activated receptor-1. Binding of proteinase-activated receptor-1 by the selective proteinase-activated receptor-1-activating peptide resulted in neurite retraction and soma atrophy in conjunction with cytosolic calcium activation in human dorsal root ganglion neurons. Interleukin-1β exposure to feline or human dorsal root ganglia caused upregulation of proteinase-activated receptor-1 in neurons. Exposure of feline immunodeficiency virus-infected dorsal root ganglia to the interleukin-1 receptor antagonist prevented proteinase-activated receptor-1 induction and neurite retraction. In vivo feline immunodeficiency virus infection was associated with increased proteinase-activated receptor-1 expression on neurons and interleukin-1β induction in macrophages. Moreover, feline immunodeficiency virus infection caused hyposensitivity to mechanical stimulation. These data indicated that activation and upregulation of proteinase-activated receptor-1 by interleukin-1β contributed to dorsal root ganglion neuronal damage during lentivirus infections leading to the development of distal sensory polyneuropathy and might also provide new targets for future therapeutic interventions.
Keywords: PAR1, HIV, FIV, dorsal root ganglion, IL-1β
Introduction
Proteinase-activated receptors (PARs) are seven transmembrane G protein-coupled receptors activated by serine proteases (Hansen et al., 2008). These receptors contain an extracellular N-terminal domain, which can be cleaved proteolytically, exposing a new, previously cryptic N-terminal sequence. This ensuing peptide sequence acts as a ‘tethered’ receptor-activating ligand, which after proteolytic cleavage, binds to the receptor and activates it (Hansen et al., 2008; Soh et al., 2010). There are four known members of the PAR family: PAR1,2,3,4. PAR1,3,4 are activated by thrombin while trypsin cleaves PAR2 (Hollenberg and Houle, 2005). PARs can also be activated with synthetic peptides, which have the corresponding tethered ligand sequence, e.g. the activating peptide sequence for PAR1 is TFLLR-NH2 (Ramachandran and Hollenberg, 2008). All four PARs have been detected in the PNS, particularly in the dorsal root ganglia. Numerous studies have shown the involvement of PAR-mediated modulation of neuroinflammation and pain through putative actions in sensory neurons (Steinhoff et al., 2000; Garavilla et al., 2001; Augé et al., 2009).
PAR1 is present on small nociceptive as well as on large myelinated dorsal root ganglion neurons (Steinhoff et al., 2000). In contrast, PAR2 is predominantly expressed on nociceptive neurons, PAR3 on CGRP-positive neurons (Steinhoff et al., 2000; Garavilla et al., 2001,) and PAR4 is present on the non-neuronal cells surrounding neurons (Zhu et al., 2005). Activation of PAR2 has been shown to induce neuropathic pain in sensory neurons of dorsal root ganglia (Alier et al., 2008; Helyes et al., 2010); however, the role of PAR1 in peripheral nerve degeneration is less clear. Intraplantar injection of a selective PAR1-activating peptide causes mechanical and thermal analgesia (Asfaha et al., 2002). Conversely, PAR1 activation increases intracellular calcium [Cai] levels in cultured rat dorsal root ganglion neurons and injection of the PAR1-activating peptide into the rat paw also mediates marked and sustained oedema, suggestive of local inflammation (Knecht et al., 2007).
Distal sensory polyneuropathy is the most common neurological disorder in patients with HIV infection (Vivithanaporn et al., 2010). The signs and symptoms of distal sensory polyneuropathy include hyposensitivity, paraesthesiae, hyperalgesia, allodynia and ataxia with sensory loss in the feet as a common early sign (Power et al., 2009). The mechanisms underlying HIV-associated distal sensory polyneuropathy are uncertain; however, several lines of evidence point to changes in the host immune milieu including cytokine activation in response to viral infection, which mediate neuronal injury leading to the signs and symptoms of distal sensory polyneuropathy (Pardo et al., 2001).
Feline immunodeficiency virus (FIV) is a naturally occurring lentivirus causing the acquired immunodeficiency syndrome (AIDS) in domestic cats and shares similar viral properties and pathogenic features with HIV (Elder et al., 2010). In vivo FIV infection causes distal sensory polyneuropathy defined by loss of sensory axons and reduced innervation of the skin, all in conjunction with neuroinflammation within the dorsal root ganglion and nerve and has been established as a model for HIV-distal sensory polyneuropathy (Kennedy et al., 2004; Zhu et al., 2007). The working hypothesis in the present study was that lentivirus infections upregulate PAR1 in the PNS contributing to the neurodegenerative aspects of distal sensory polyneuropathy. A multi-platform experimental strategy was pursued in which human clinical samples, ex vivo human and feline dorsal root ganglion cultures and an in vivo animal model of HIV infections were investigated.
Materials and methods
Tissue samples
Human dorsal root ganglia were obtained at autopsy with consent (approved by Johns Hopkins University Ethics Committee) from HIV-1 seropositive [with (n = 5) and without (n = 5) distal sensory polyneuropathy] and seronegative (n = 5) subjects.
Human dorsal root ganglion cultures
Human dorsal root ganglion cultures were prepared from 15- to 19-week foetuses from therapeutic abortions with consent (approved by the University of Alberta Ethics Committee), as described previously (Acharjee et al., 2010). The dorsal root ganglia were aseptically harvested from all spinal segments in Dulbecco's modified Eagle medium (Gibco), enzymatically treated for 40 min with 0.125% type IV collagenase (Sigma) and 0.2 mg/ml DNase (Roche), followed by 0.25% trypsin (Invitrogen). Trypsin was inactivated by the addition of equal volume of Dulbecco's modified Eagle medium supplemented with 10% foetal bovine serum (Gibco), 1% l-glutamine (Gibco), 1% non-essential amino acids (Gibco), 1% sodium pyruvate (Gibco), 1% dextrose (Gibco), 1% pencillin and streptomycin (Gibco), 20 μg/ml gentamycin (Gibco) and 0.5 μg/ml fungizone (Gibco) (hereafter referred to as neuronal medium). Dorsal root ganglia were then mechanically dissociated by trituration, filtered with a 70 -μm mesh and centrifuged at 1000 r.p.m. for 10 min. The pellet was resuspended in neuronal medium and plated in matrigel (BD Sciences)-coated plates.
Feline dorsal root ganglion cultures
Dorsal root ganglia from healthy adult cats (12-weeks old) were removed aseptically and enzymatically softened in digestion medium (0.5 mg/ml trypsin, 1 mg/ml collagenase type IA, and 0.1 mg/ml DNase type I in Dulbecco's modified Eagle medium) for 30 min at 37°C. Digestion media were removed by centrifugation at 1500 r.p.m. for 5 min, and cells were washed twice with culture medium [Dulbecco's modified Eagle medium containing 10% heat-inactivated foetal bovine serum, 5% horse serum (Life Technologies), 2 mM L-glutamine and 1% N-2 supplement (Gibco), 0.1 mg/ml penicillin/streptomycin (Gibco) and 5% L929 cell-conditioned medium (as a source of macrophage CSF-1)]. The tissue solution was triturated using a sterile plastic pipette until a homogeneous cell suspension was obtained and adjusted to a concentration of 0.5 × 106 cells/ml. Cells were seeded into a 24-well plate (1 ml/well) and incubated at 37°C, 5% CO2. The medium was changed on the following day, and every third day thereafter.
Human and feline macrophage cultures
Human peripheral blood mononuclear cells were purified from healthy donors with histopaque (Sigma), and maintained in RPMI 1640 (Gibco) medium with 15% foetal calf serum (Gibco) per ml. Human primary monocyte-derived macrophages were isolated from peripheral blood mononuclear cells by plastic adherence and cultured in RPMI medium (Zhang et al., 2003). Feline macrophages were isolated from the pelvic and femoral bone marrow of healthy specific pathogen-free cats, as reported previously (Zhu et al., 2005). The cells were cultured in Dulbecco's modified Eagle medium containing 2 mM l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 10% heat-inactivated foetal bovine serum and 10% L929 cell derived-conditioned medium as a source of CSF-1 in 10% CO2 in plastic dishes permitting cellular differentiation, resulting in monolayer cultures that were >95% pure macrophages.
Feline Schwann cell cultures
Sciatic nerves from healthy 12-week-old cats were harvested and chopped finely in L-15 media containing 1% pencillin and streptomycin, enzymatically digested with 1% collagenase followed by 0.25% trypsin for 30 min at 37°C. L-15 media were added to the reaction. An equal volume of D-10 media with 1% pencillin and streptomycin was added to inactivate the trypsin. The preparation was centrifuged at 1500 r.p.m. for 10 min, and then the pellet was resuspended in D-10/1% pencillin and streptomycin media and plated in 24-well plates.
HIV and FIV infections
Human dorsal root ganglion cultures were infected with HIV-1 SF162, as described previously (Jones et al., 2005). Briefly, supernatants derived from HIV-1 SF162-infected human peripheral blood mononuclear cells (104 TCID50/ml) were added to the dorsal root ganglion cultures for 6–8 h. For mock (control) infections, the supernatants from uninfected human peripheral blood mononuclear cells were applied. For FIV or mock infections in and ex vivo, a neurovirulent FIV strain, FIV-Ch was used, as described previously (Johnston et al., 2002; Kennedy et al., 2004; Zhu et al., 2006).
Peptides and cytokines
The receptor-activating peptide (PAR1-activating peptide) corresponding to the tethered ligand domain of PAR1, TFLLR-NH2, which can activate this receptor in many species, including the cat, together with inactive, control peptides, RLLFT-NH2 which do not activate PAR1, were synthesized by the Peptide Synthesis Facility at the University of Calgary, Canada (peplab@ucalgary.ca). Peptides were prepared in 25 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer, pH 7.4, and standardized by quantitative amino acid analysis and mass spectrometry to confirm peptide concentration and purity. Recombinant human IL-1β and IL-1 receptor antagonist were obtained from R&D Biosystems.
Calcium imaging
Human dorsal root ganglion cultures were plated in 35 mm Nunclon tissue culture dishes (VWR). The 2- to 5-day cultures loaded with 5 µM Fluo-8 acetoxymethyl ester (Fluo-8 AM) (ABD Bioquest) for 30 min beforehand were imaged as previously described (Ruangkittisakul et al., 2006; Lu et al., 2009). The membrane-permeant fluorescent calcium indicator Fluo-8 AM was used for imaging of relative dynamic changes of [Cai]. An increase in fluorescence intensity of Fluo-8 AM corresponds to an increase in [Cai]. Changes in Fluo-8 AM -fluorescence intensity with an elevated concentration of potassium chloride (KCl: 35 mM, 60 s application), PAR1-activating peptide or control peptides were measured using a confocal microscope, equipped with an argon (488 nm) laser and filters (×20 XLUMPlanF1, NA 0.95 objective; Olympus FV300). Full frame images (512 × 512 pixels) were acquired at a scanning time of 3 s per frame. Selected regions of interest were drawn around distinct cell bodies and time course traces of change in fluorescence intensity were generated with FluoView v.4.3 (Olympus).
Experimental animals and tissue collection
Specific pathogen-free neonatal (Day 1) cats were infected with 0.2 ml of infectious (104 TCID50/ml) or heat-inactivated FIV (mock-infected cats) in accordance with Canadian Council on Animal Care guidelines, as described previously (Johnston et al., 2002). L4-L5 dorsal root ganglia were harvested and stored at −80°C immediately for RNA collection or fixed in 4% phosphate buffered saline–buffered paraformaldehyde for at least 1 week at 4°C for immunofluorescence.
Neurobehavioural testing
All behavioural testing was done by an experimenter blinded to specific experimental groups. Fifteen-week-old cats (infected at Day 1) were placed on an elevated wire mesh and allowed to acclimatize for 15–20 min to the testing apparatus before the testing began. For tactile sensation, von Frey monofilaments were used to assess the response (lifting, guarding) to mechanical stimuli. Calibrated von Frey hair filaments were applied to the plantar surface of each hind paw in ascending order of bending force (range: 0.2–100 g). Each hair was applied five times per rear paw, and the number of times the paw was withdrawn was recorded. A score of 1 was assigned to withdrawal of one rear paw and a score of 2 for withdrawal of both rear paws. Scores were reported as an average of all test animals in each group (FIV+, n = 5; FIV−, n = 4) for each von Frey hair diameter.
Real-time reverse transcriptase polymerase chain reaction
RNeasy® Mini Kit (Qiagen) was used for RNA isolation from tissues and cells after lysis with TRIzol® (Invitrogen) using the manufacturer's guidelines. For the human and feline dorsal root ganglion cultures, the cells were washed in phosphate-buffered saline and RNA was extracted in a similar fashion. RNA was dissolved in RNase-free water and was subsequently used for complementary DNA synthesis. The primers used in real-time polymerase chain reaction are listed in Table 1. Semi-quantitative analyses were performed by monitoring the increase in fluorescence of SYBR-green dye (Invitrogen) in real-time on a Bio-Rad (Hercules) i-Cycler. Real-time fluorescence measurements were performed, and a threshold cycle value for each gene of interest was determined as reported previously (Jones et al., 2007). All data were normalized to GAPDH messenger RNA levels for human and cats. Agarose gels were used to observe the pol transcripts after polymerase chain reaction amplification of complementary DNA from dorsal root ganglion cultures.
Table 1.
Primers used in real time polymerase chain reaction
| Gene | Organism | Primer | Sequence |
|---|---|---|---|
| PAR1 | Human | Forward | 5′-CGC AGA GCC CGG GAC AAT GG-3′ |
| Reverse | 5′-CGG TGC GGG CAG ACA ACA-3′ | ||
| PAR1 | Feline | Forward | 5′-TTC CGG GGC TCA ACA TCA CCA-3′ |
| Reverse | 5′-GGC GGC GGA CAG GAA CAA AG-3′ | ||
| IL-1β | Human, Feline | Forward | 5′-CCA AAG AAG AAG ATG GAA AAG CG-3′ |
| Reverse | 5′-GGT GCT GAT GTA CCA GTT GGG-3′ | ||
| GAPDH | Human, Feline | Forward | 5′-AGC CTT CTC CAT GGT GGT GAA GAC-3′ |
| Reverse | 5′-CGG AGT CAA CGG ATT TGG TCG-3′ |
Immunohistochemistry and immunofluorescence
Rabbit anti-human PAR1 antiserum, generated against a peptide (ORNNATLDPR/SFLLRNPNDKY-AMIDE) representing the cleavage/activation sequence of human PAR1, with an N-terminal ornithine added for N-terminal coupling as a hapten to keyhole limpet haemocyanin, was used in accordance with the previously described studies (Boven et al., 2003). Dorsal root ganglion cultures or autopsied tissues were immunolabelled with rabbit anti-ionized calcium binding adapter (Iba-1) protein (1:500, Wako), rabbit polyclonal human PAR1 antibody (1:500), mouse anti-HIV p24 (1:100, Dako Cytomation), anti-human IL-1 β antibody (Exaplha Biologicals) and mouse anti-MAP-2 protein (1:200, Sigma). For dorsal root ganglion cultures, immunolabelling with Cy3 conjugated goat anti-rabbit (Invitrogen) or Alexa 488 goat anti-mouse Alexa 488 secondary antibodies were performed. The sections were secondarily immunolabelled with alkaline phosphate-conjugated secondary antibodies followed by 5-bromo-4-chlor-indolyl-phosphate (BCIP) and then washed and mounted in acrytol (Zhu et al., 2009).
Measurement of neurite length and soma diameter in cultured human dorsal root ganglion neurons
After completion of immunolabelling with anti-MAP-2 antibody, slides were imaged for subsequent measurements of the neuronal soma area, maximal neurite length per neuron, using a minimum of 25–50 neurons per individual experimental treatment from three separate wells by an examiner unaware of the slide identity. Neurite length and soma diameter were measured from the same neuron. Using ScionImage image analysis software (Scion), each parameter was assessed, as reported previously (Jones et al., 2005). All experiments were repeated at least twice and performed in triplicate.
Statistical analysis
Statistical analyses were performed using GraphPad InStat version 3.0 (GraphPad Software), using ANOVA, for messenger RNA alteration and neurite retraction. Unpaired t-tests were used for calcium imaging and behavioural analyses. Values of P < 0.05 were considered significant.
Results
PAR1 expression in human dorsal root ganglia
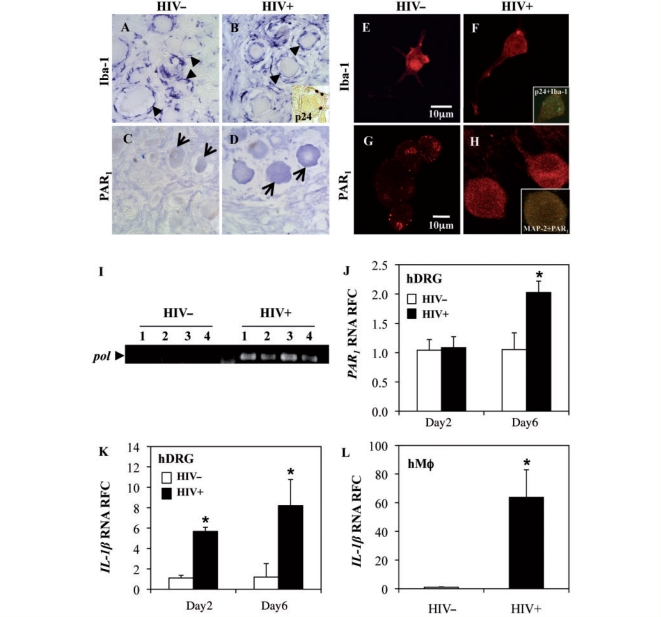
Although previous studies demonstrated upregulation of PAR1 in the brains of patients with HIV encephalitis (Boven et al., 2003), little is known about its expression in the PNS during viral infections. Human dorsal root ganglia are composed of neurons, macrophages, Schwann cells and satellite cells. Of these, only macrophages appear permissive to HIV infection (Cosenza et al., 2002; Jones et al., 2005). Immunocytochemical studies of autopsied dorsal root ganglia from HIV-infected (HIV+) and uninfected (HIV−) patients were performed. Macrophages, identified by Iba-1 immunoreactivity, surrounded the neuronal cell bodies in both HIV− (Fig. 1A) and HIV+ (Fig. 1B) patients’ dorsal root ganglia, although they appeared more abundant and hypertrophied in HIV+ samples. HIV-1 p24 immunoreactivity was detected in cells resembling macrophages, proximal to neurons (Fig. 1B, inset). PAR1 immunoreactivity was observed chiefly in the neurons of both HIV− (Fig. 1C) and HIV+ (Fig. 1D) tissues with increased immunopositivity in dorsal root ganglia from HIV+ patients. Human dorsal root ganglion cultures were infected with HIV-1 (HIV+) and compared with mock-infected cultures (HIV−) at Day 2 and Day 6 post-infection. Macrophages in dorsal root ganglion cultures were detected using an anti-Iba-1 antibody and were present in both HIV− (Fig. 1E) and HIV+ (Fig. 1F) dorsal root ganglion cultures. Double immunolabelling with p24 and Iba-1 revealed co-localization of p24 with the Iba-1 only in the HIV+ cultures (Fig 1F, inset); HIV− dorsal root ganglion cultures did not display HIV-1 p24 immunoreactivity (data not shown). PAR1 immunodetection revealed numerous positive cells resembling neurons (Fig. 1G and H), albeit with greater expression in the HIV+ cultures (Fig. 1H). PAR1 immunoreactivity was co-localized with MAP-2, a neuronal protein (Fig. 1H). Transcript analysis of HIV+ and HIV− dorsal root ganglion cultures showed the presence of HIV pol in all HIV+ cultures (Fig. 1I), confirming infection. PAR1 expression in HIV+ human dorsal root ganglia was investigated relative to HIV− cultures, revealing that PAR1 transcript levels were increased at Day 6 post-infection in HIV+ dorsal root ganglia (Fig. 1J). Since IL-1β has been implicated in peripheral nerve injury (Hucho and Levine, 2007; Binshtok et al., 2008), changes in IL-1β transcript expression were studied and found to be upregulated in HIV-infected dorsal root ganglia at both Day 2 and Day 6 post-infection (Fig. 1K). To determine if macrophages were involved in the disease process, human macrophages were infected with HIV-1 and analysed for IL-1β (Fig. 1L). While IL-1β transcript levels were increased, PAR1 expression showed similar levels in HIV− and HIV+ macrophage cultures (data not shown). These latter studies highlighted HIV infection-mediated upregulation of PAR1 and IL-1β expression in human neurons and macrophages, respectively, within dorsal root ganglia.
Figure 1.
PAR1 expression in human dorsal root ganglia. (A and B) Iba-1 immunoreactivity in macrophages (arrowheads) was observed in the dorsal root ganglia (DRG) of both HIV− (A) and HIV+ (B) patients. p24 immunostaining was detected only in HIV+ dorsal root ganglia in macrophage-resembling cells (B, inset). (C and D) PAR1 immunoreactivity was detectable in neurons of both HIV− (C) and HIV+ (D) patients (arrows) although PAR1 immunolabelling appeared to be increased in the HIV-infected tissues (original magnification ×400). (E–H) Immunofluorescence in mock (HIV−) and HIV (HIV+) infected human dorsal root ganglion cultures. Iba-1 immunolabelling of macrophages was observed in HIV− (E) and HIV+ (F) cultures, which was co-localized with p24 immunoreactivity in HIV+ cultures (F, inset). PAR1 immunolabelling was observed in both HIV− (G) and HIV+ (H) cultures, albeit at higher intensity in HIV+ cultures, which was co-localized with MAP-2 immunoreactivity (J, inset). (I) Ethidium bromide-stained agarose gel showed HIV-pol transcripts in human dorsal root ganglion cultures infected with HIV-1 but not in mock-infected (HIV−) dorsal root ganglion cultures. (J and K) HIV+ dorsal root ganglion cultures showed increased PAR1 (J) and IL-1β (K) transcript levels at Day 6 post-infection compared with the mock-infected cultures. (L) IL-1β transcript abundance was increased in HIV-infected human macrophages. Bars represent mean ± SEM (Students t-test, *P < 0.05) (original magnification: G–J ×600). RFC = Relative Fold Change.
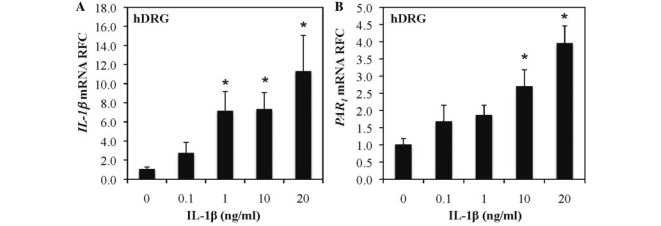
To investigate whether IL-1β induction directly caused PAR1 upregulation, human dorsal root ganglia were exposed to different concentrations of IL-1β for 48 h (Fig. 2). IL-1β (Fig. 2A) and PAR1 (Fig. 2B) transcript analysis showed that both genes were induced in an IL-1β concentration-dependent manner. We also investigated the IL-1β dependence for PAR1 induction in another human neuronal cell line, SKN-SH (Supplementary Fig. 1) and found that IL-1β also induced PAR1 expression in this cell line. Thus, IL-1β directly activated PAR1 expression in neurons, recapitulating our findings of PAR1 and IL-1β co-induction during HIV infection.
Figure 2.
IL-1β induces PAR1 expression in human dorsal root ganglia. Human dorsal root ganglia (hDRG) were exposed to different concentrations of IL-1β for 48 h, and RNA was collected from the cells. IL-1β (A) and PAR1 (B) transcript expression was increased in a concentration-dependent manner.*P < 0.05 (Bonferroni post hoc analysis). RFC = Relative Fold Change.
PAR1 activation increases cytosolic calcium in human dorsal root ganglion neurons and causes neuronal injury
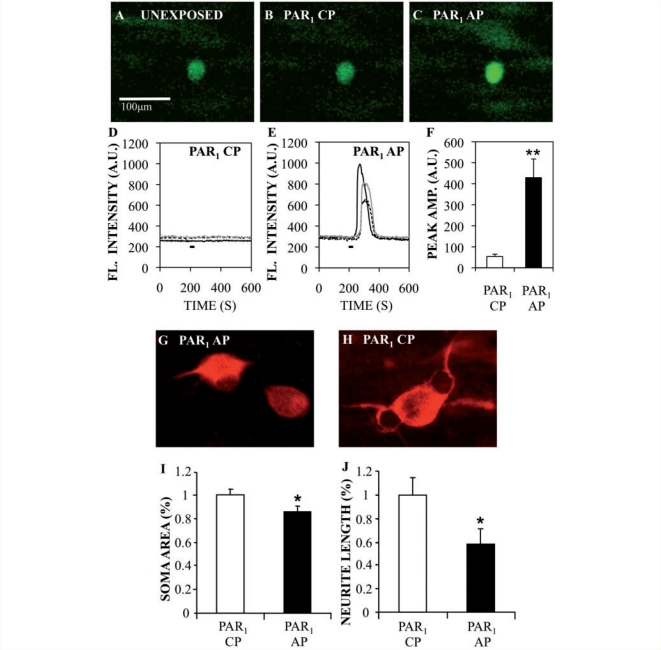
PAR1 upregulation and activation have several potential downstream effects on cellular signalling. Previous studies demonstrated that PAR1 activation can mediate rises in intracellular calcium concentrations [Cai] (Knecht et al., 2007) in rat dorsal root ganglion neurons and that changes in neuronal [Cai] can lead to neuronal injury. The effect of PAR1 activation on calcium-induced Fluo-8 AM fluorescence was investigated in human dorsal root ganglion neurons. Exposure of Fluo-8 AM-loaded human dorsal root ganglion cultures to PAR1 activating peptide (50 μM) prompted transient but robust increases in fluorescence intensity in neurons within seconds of application (Fig. 3C and E), whereas PAR1 control peptide (50 μM) did not evoke any response (Fig 3B and D). Exposure of human dorsal root ganglion neurons to PAR1-activating peptide (n = 27) for 30 s increased fluorescent intensity by 5-fold compared with PAR1 control peptides (n = 19) (Fig. 3F), indicating that PAR1 activation increased calcium load in the cytosol. Changes in Fluo-8 AM-fluorescence evoked by depolarizing cells with a high concentration of potassium chloride (35 mM KCl, 60 s application) were used as ‘positive control’ for calcium responses and data were only collected from cells in the plane of focus, which responded reversibly to a high KCl challenge (Supplementary Fig. 2). To extend this analysis, human neurons were exposed to PAR1-activating peptide or control peptides for 48 h, then immunolabelled with MAP-2; neurite length and neuronal soma area were measured to assess viability. Neurons exposed to PAR1-activating peptide (Fig. 3G) displayed shorter processes than neurons exposed to PAR1 control peptides (Fig. 3H). These latter observations were confirmed by quantitative analyses of neuronal soma area (Fig. 3I) and neurite length (Fig. 3J), which indicated significant reductions in PAR1-activating peptide-exposed cultures for both measurements, compared with PAR1 control peptide-exposed cultures. These findings emphasized the cytotoxic effects of PAR1 activation on neurons involving calcium activation.
Figure 3.
PAR1-activating peptide increases cytosolic Ca2+ in human dorsal root ganglion cultures and causes neuronal damage. (A–C) Confocal imaging of Fluo-8 AM labelled human dorsal root ganglion neurons before (A) and during PAR1 control peptides (CPs) (B) and PAR1-activating peptide (AP) (C), showed an increase in intracellular fluorescence after PAR1-activating peptide exposure. (D and E) Time courses of fluorescent intensity changes in dorsal root ganglion neurons in response to PAR1 control peptides (50 µM) (D) and PAR1-activating peptide (50 µM) application (E). (F) Graphic representation of average fluorescence peak amplitude in human dorsal root ganglion neurons. (G and H) Human dorsal root ganglion cultures showed MAP-2 immunoreactivity in neurons exposed to PAR1-activating peptide (G) or PAR1 control peptides (H). (I and J) Mean neuronal soma area (I) and neurite length (J) were significantly reduced after exposure to PAR1-activating peptide. The black line in D and E represents the time during which the peptide was applied. Bar graphs represents mean ± SEM (Student's t-test, *P < 0.05; **P < 0.01). (Original magnification: ×200 for A–C and ×600 for G and H).
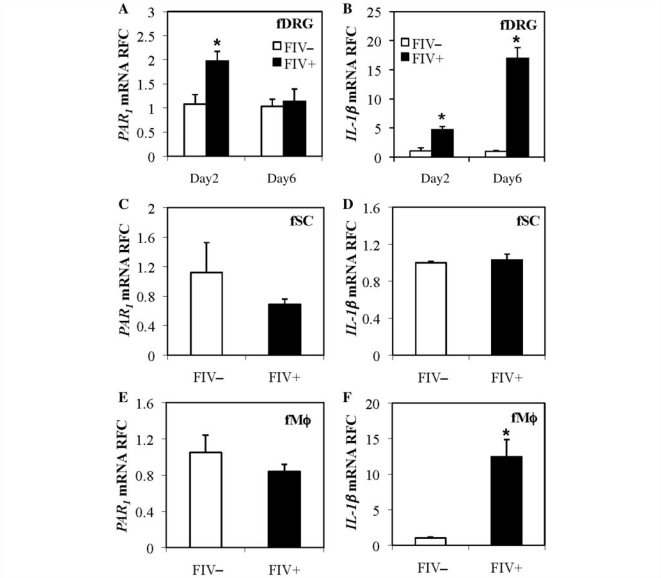
FIV infection of feline dorsal root ganglion and monocyte-derived macrophage cultures
FIV infection of cats causes distal sensory polyneuropathy with dorsal root ganglion neuronal damage and immune activation (Kennedy et al., 2004; Zhu et al., 2005). Like human dorsal root ganglia, feline dorsal root ganglia contain neurons, Schwann cells and macrophages although only macrophages are permissive to FIV infection (Zhu et al., 2005). To investigate whether FIV infection influenced PAR1 and IL-1β expression in dorsal root ganglia, as observed during HIV-1 infection, feline dorsal root ganglion cultures were infected with a neurovirulent strain of FIV (FIV+) or were mock-infected (FIV−). RNA was harvested subsequently from the cultures at Day 2 or Day 6 post-infection. PAR1 (Fig. 4A) was upregulated transiently at Day 2 post-infection while IL-1β (Fig. 4B) transcripts were consistently induced at Day 2 and Day 6 post-infection in FIV+ cultures compared with FIV− cultures. In feline Schwann cells, PAR1 (Fig. 4C) and IL-1β (Fig. 4D) were not upregulated following FIV infection. FIV infection of feline macrophages induced expression of IL-1β (Fig. 4F) but not PAR1 (Fig. 4E), similar to findings in HIV-1 infection (Fig. 1). These observations indicated that while there was no change in PAR1 transcript levels in macrophages and glial cells, the overall PAR1 transcript expression increased in dorsal root ganglion cultures, implying that PAR1 induction chiefly occurred in the neurons. In contrast, IL-1β induction occurred principally in macrophages following FIV infection, recapitulating findings in HIV-infected human dorsal root ganglion cultures.
Figure 4.
FIV infection of feline dorsal root ganglion, macrophage and Schwann cell cultures caused induction of neuronal PAR1 and macrophage IL-1 β. Feline dorsal root ganglia (fDRG), macrophages (MΦ) and Schwann cells (fSC) were infected by FIV-Ch (FIV+) or mock (FIV−) controls. (A and B) Bar graphs showing increased PAR1 transcript levels (A) at Day 2 post-infection and increased IL-1β (B) at Day 2 and Day 6 post-infection in FIV+ dorsal root ganglion cultures. (C and D) Schwann cells showed no change in PAR1 or IL-1β transcript expression following infection with FIV. (E and F) Macrophages did not show any change in PAR1 levels (C) while IL-1β expression increased (D) at Day 2 post-infection (Students t-test, *P < 0.05). RFC = Relative Fold Change.
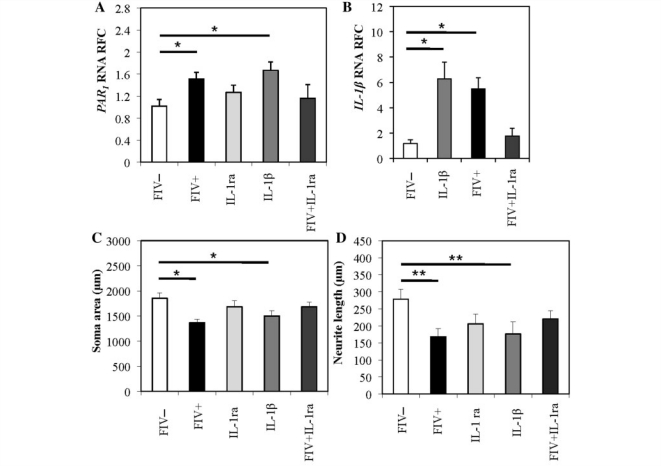
IL-1β receptor antagonist blocks FIV-induced upregulation of PAR1 and neuronal injury
As both HIV and FIV infections in dorsal root ganglia concurrently induced both PAR1 and IL-1β; the contribution of IL-1β to PAR1 induction during lentivirus infection was investigated. Exposure of dorsal root ganglion cultures to IL-1β induced PAR1 (Fig. 5A) and IL-1β (Fig. 5B), similar to FIV infection. When IL-1β receptor antagonist (20 ng/ml) was applied to dorsal root ganglia during FIV infection, induction of PAR1 and IL-1β was not observed. To determine if IL-1 receptor antagonist could also reverse FIV-associated neuronal damage, IL-1 receptor antagonist was added to cultures during FIV infection. Under this condition, neuronal soma atrophy (Fig. 5C) and neurite retraction (Fig. 5D) were prevented while IL-1β (20 ng/ml) exposure alone caused neuronal soma atrophy (Fig. 5C) and neurite retraction (Fig. 5D). Addition of IL-1 receptor antagonist alone to uninfected cultures did not cause any change in soma size, neurite length or PAR1 transcript levels (Fig. 5A, C and D). These results indicated that IL-1β induced PAR1 expression and neuronal injury while FIV-induced PAR1 expression was blocked by IL-1 receptor antagonist.
Figure 5.
IL-1β receptor agonist (IL-1ra) prevents the FIV-induced upregulation of PAR1 transcripts and neuronal injury. Graphic representation of PAR1 (A) and IL-1β (B) transcripts after FIV infection or by treatment with IL-1β (20 ng/ml). FIV-induced neuronal injury was measured by neuronal soma size (C) and neurite length (D) and was prevented by exposure to IL-1β receptor agonist (IL-1ra) (mean ± SEM) (ANOVA with Bonferroni's post hoc test, *P < 0.05, **P < 0.01).
FIV infection induced PAR1 expression in dorsal root ganglion in vivo and tactile hyposensitivity
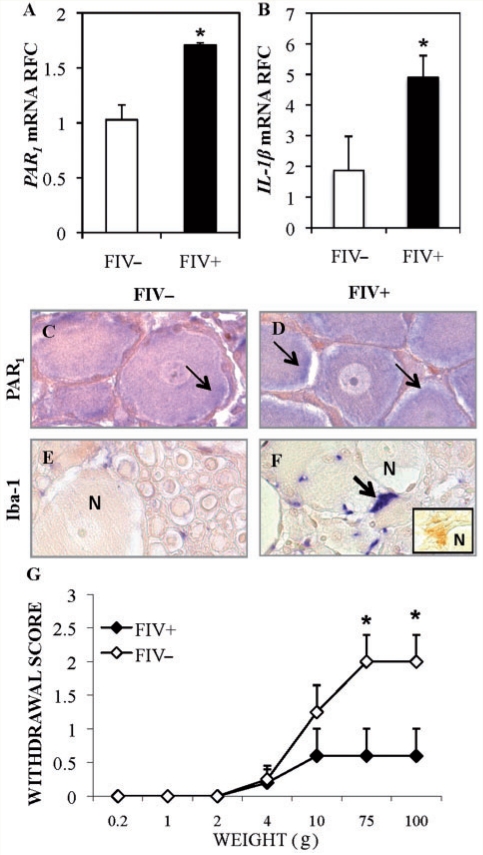
Although ex vivo HIV-1 and FIV infections of cultured dorsal root ganglion caused PAR1 and IL-1β upregulation, the in vivo effects of lentivirus infection on these genes were not known. To this end, an animal model of HIV-distal sensory polyneuropathy was used (Kennedy et al., 2004; Zhu et al., 2007) in which cats were infected with FIV-Ch (FIV+) or mock virus (FIV−). Our previous findings indicate that FIV infection suppresses CD4+ T-lymphocyte levels in blood. Similarly, plasma viral copy numbers were high in FIV-infected animals, but not detected in FIV-uninfected animals (Kennedy et al., 2004; Maingat et al., 2009). Analysis of dorsal root ganglia derived from FIV+ or FIV− animals showed increased expression of PAR1 (Fig. 6A) and IL-1β (Fig. 6B) transcripts at Week 15 post-infection in FIV+ cats. Immunohistochemical analyses of the dorsal root ganglia revealed PAR1 immunoreactivity in both FIV− (Fig. 6C) and FIV+ (Fig. 6D) dorsal root ganglion neurons albeit at a higher level in FIV+ dorsal root ganglia (Fig. 6D). Iba-1 immunopositive cells were apparent in both FIV− (Fig. 6E) and FIV+ dorsal root ganglia (Fig. 6F) with increased immunoreactivity in FIV+ dorsal root ganglia in close proximity to neurons. Detectable IL-1β immunoreactivity in cells abutting neurons was also observed in FIV+ samples (Fig. 6F). To determine if FIV infection affected mechanical sensation, sensitivity to tactile stimuli was assessed in FIV− and FIV+ cats; stimulation of the hind paws of FIV− or FIV+ animals with a range of von Frey hair filaments revealed significant tactile hyposensitivity among the FIV+ cats (Fig. 6G). Thus, FIV infection resulted in increased neuronal PAR1 and macrophage IL-1β expression in the PNS in conjunction with tactile hyposensitivity.
Figure 6.
In vivo FIV infection induced PAR1 and IL-1β expression in dorsal root ganglion and tactile hyposensitivity. (A and B) PAR1 (A) and IL-1β (B) transcript levels in dorsal root ganglia from 12-week-old FIV+ animals were upregulated compared with uninfected animals. (C–F) Immunostaining of dorsal root ganglia from FIV− and FIV+ animals revealed increased PAR1 (C and D) and Iba-1 (E and F) immunoreactivity in FIV+ dorsal root ganglion specimens; PAR-1 immunorectivity was apparent in both the cytoplasm and on the plasma membrane of neurons (C and D, N = neuron). IL-1 β immunoreactivity was detectable in FIV+ samples only (F, inset). (G) Stimulation of the rear paws of FIV− (n = 4) and FIV+ cats (n = 5) with increasing weights of Von Frey hairs revealed tactile hyposensitivity among FIV+ animals. (Students t-test, *P < 0.05). RFC = Relative Fold Change.
Discussion
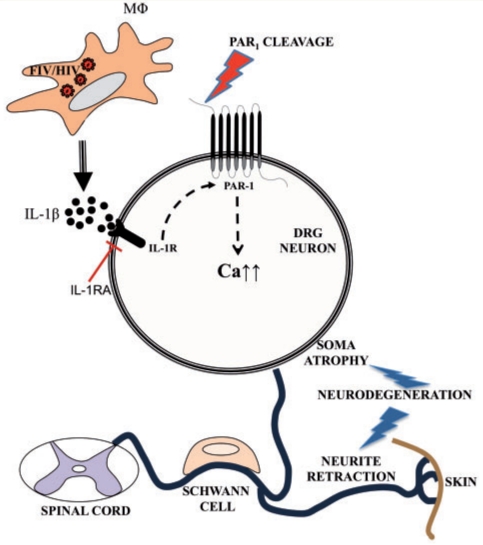
Although PAR1 has been reported to be present on dorsal root ganglion neurons (Garavilla et al., 2001), the current studies represent the first analyses of PAR1's expression and actions in the context of a viral infection of the PNS. These studies capitalized on a multi-platform experimental approach including clinical samples, ex vivo human dorsal root ganglia and an established animal model, displaying distal sensory polyneuropathy. Dorsal root ganglion neuronal cell body PAR1 expression was upregulated during lentivirus infection and its activation by the PAR1-activating peptide caused a rise in [Cai] with ensuing human neuronal injury. PAR1 was detected on dorsal root ganglion neuronal plasma membranes as well as within the cytoplasm, and was induced by IL-1β. However, IL-1 receptor antagonist blocked PAR1 induction and dorsal root ganglion neuronal injury. The present study also revealed that PAR1 was induced in dorsal root ganglion neurons both in vivo and ex vivo, during lentivirus infections, which was mediated by increased IL-1β expression in macrophages. These findings implicated PAR1 expression and activation in the pathogenesis of peripheral neuropathy during lentivirus infections (Fig. 7).
Figure 7.
Lentivirus infections mediate PAR1 upregulation and activation with ensuing neuronal damage. HIV and FIV infections of dorsal root ganglion (DRG) macrophages cause increased IL-1β expression, which induces of PAR1 in the dorsal root ganglion neurons. IL-1 receptor agonist (IL-1ra) blocks FIV-induced PAR1 over-expression. PAR1 activation elevates cytosolic calcium levels, which in turn, initiates an intracellular signalling resulting in neuronal damage.
PAR1 and IL-1β interactions were consistent across different ex vivo models and an in vivo model of lentivirus infections, underscoring the general applicability of the current observations. Several cytokines are upregulated in dorsal root ganglia during HIV infection (Acharjee et al., 2010) but given the prominent role of IL-1β in peripheral neuropathy (Rothman and Winkelstein, 2010; Rothman et al., 2011), we chose to focus on IL-1β. There was an apparent mismatch between PAR1 and IL-1β upregulation in FIV-infected dorsal root ganglion cultures at Day 6 post-infection (Fig. 4A). Both PAR1 and IL-1β are upregulated at Day 2 (Fig. 4A and B and Fig. 5A and B); however, at Day 6 post-infection, PAR1 is not upregulated although IL-1β is. One possible explanation for this is that the neurons are severely injured in Day 6 and that affects the transcriptional machinery.
FIV infection causes distal sensory polyneuropathy in vivo defined by sensory loss, loss of sensory axons and reduced innervation of the skin, all in conjunction with neuroinflammation and viral replication within the dorsal root ganglion and nerve (Kennedy et al., 2004). Moreover, both FIV and HIV infections of dorsal root ganglion cultures also result in neuronal injury (Jones et al., 2005; Zhu et al., 2006). The pathogenic mechanisms underlying the development of distal sensory polyneuropathy in lentivirus infections remain uncertain, but distal sensory polyneuropathy is apparent in HIV, FIV and likely Simian Immunodeficiency Virus infections in both adults and adolescents with immunosuppression as a requisite feature in all infections (Pardo et al., 2001; Zink et al., 2006; Laast et al., 2007). There are compelling data to indicate the PNS is directly infected by lentiviruses with myeloid/monocytoid (macrophage) cells as the principal target cells of infection, while neurons and perhaps Schwann cells are injured as bystanders (Jones and Power, 2006). However, the extent to which neurons are lost or undergo cell death is unclear; previous in vivo studies of both HIV and FIV infections have failed to show significant dorsal root ganglion neuronal cell body loss despite marked losses in axons, particularly small diameter fibres (Bradley et al., 1998; Kennedy et al., 2004). It is plausible that select dorsal root ganglion neuronal populations are lost for which current investigative tools are insufficiently sensitive to detect at present. This point is supported by clinical findings that distal sensory polyneuropathy is a heterogeneous disorder defined by sensory loss, neuropathic pain and loss of deep tendon reflexes with the severity of each of these signs varying from patient-to-patient and with the disease stage (Kolson and Gonzalez-Scarano, 2001; Cornblath and Hoke, 2006). Nonetheless, other dorsal root ganglion cells including Schwann cells, macrophages (infected and/or activated) and, perhaps, satellite cells influence neuronal viability and function in a complex manner (Cornblath and Hoke, 2006; Hahn et al., 2008). Hence, the multiple mechanisms underlying distal sensory polyneuropathy likely involve interactions between neurons and surrounding cells with the pathogenesis representing a molecular cascade beginning with lentivirus infection of dorsal root ganglion macrophages and the subsequent release of immune molecules such as IL-1β. These events induce neuronal expression of PAR1 and its ligation to cause a rise in [Cai] and eventual axonal process retraction, as observed herein (Fig. 7).
PAR2 has been reported to be pro-nociceptive in same models (Alier et al., 2008; Helyes et al., 2010). However, PAR2 expression was not increased in the present in vivo and in vitro models (data not shown). In contrast to PAR2, PAR1 activation might exert pro-analgesic effects depending on the model system (Martin et al., 2009). This dichotomy in the PARs’ actions is evident herein; PAR1 induction and activation in the in vivo FIV model resulted in neurodegenerative consequences (neurite growth interruption and tactile hyposensitivity) but without evidence of mechanical allodynia, similar to previous studies of this model investigating temperature sensitivity (Kennedy et al., 2004). The actions of PAR1 activation on dorsal root ganglion neurons might reflect the long-recognized ability of thrombin, presumably acting through PAR1, to cause neurite retraction (Cunningham and Gurwitz, 1989; Gill et al., 1998; Suidan et al., 1992). Thus, PAR1 activation might mediate neurodegeneration while PAR2 modulates neuropathic pain in a peripheral neuropathy like distal sensory polyneuropathy. Indeed, this hypothesis is congruent with the findings that combination anti-retroviral therapy, which contributes to the suppression of viral replication and neuroinflammation, often improves the severity of distal sensory polyneuropathy-associated symptomatic neuropathic pain, but the clinical signs of mechanical hyposensitivity and deep tendon reflex loss usually persist without recovery in the long term (Martin et al., 2003).
PAR1 expression has been shown to be induced by IL-1β exposure in human astrocytes, but PAR1 ligation also diminished CNS neuronal viability with a concurrent increase in [Cai] (Boven et al., 2003; Fang et al., 2003). Earlier studies have implicated increased nitric oxide synthase 2 (NOS2) expression in Schwann cells during distal sensory polyneuropathy with the production of NO as a mediator of neuronal injury; the NOS2 inhibitor, aminoguanidine, prevented lentivirus-induced dorsal root ganglion neuronal injury (Zhu et al., 2005). Similarly, CD8+ T cells infiltrating the dorsal root ganglion were also found to be neurotoxic, probably by activating neurons (and macrophages) through a CD40–CD153 interaction (Zhu et al., 2006). These reports can be reconciled with the present findings as PAR1 induction and activation on neurons can be viewed as a trigger for neurotoxicity. In fact, infiltrating CD8+ T cells might also release proteases such as granzymes, which cleave PAR1 with ensuing activation (Steinhoff et al., 2005). Satellite glial cells, which envelope the dorsal root ganglion neurons, have distinct functions and are thought to be important constituents in controlling microenvironment including secretion of cytokines (Watkins and Maier, 2002; Hanani, 2005). These cells lose their characteristics in the long-term culture (>1 day), during which time the satellite cells migrate away from the neurons and become indistinguishable from the Schwann cells (Hanani, 2005). Hence, we have not investigated the role of satellite cells in this study. Collectively, the present findings together with past reports of PAR1 expression and actions in the CNS serve to emphasize the potential impact of PAR1 on neural tissues.
In the current study, multiple models, viruses and host species were investigated. This multi-platform approach might be construed as a criticism; however, all the findings pointed to the same outcome: lentivirus infection induces IL-1β-dependent upregulation of PAR1 on dorsal root ganglion neurons resulting in neuronal injury (Fig. 7). The pathways by which PAR1 determine neuronal viability remains uncertain. Identification of the critical downstream mechanisms might provide insights into future treatment options for distal sensory polyneuropathy, but prevention of PAR1 activation represents a promising approach to treating distal sensory polyneuropathy and other sensory neuropathies.
Funding
National Institutes of Health (NIH 5R01NS062670-03); Canadian Foundation for Innovation-Alberta Science and Research Investment Program (202623) and Canadian Institutes of Health Research (G118160525).
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
The authors thank Krista Nelles for assistance with manuscript preparation and Dr Robert Campenot for helpful discussions.
Glossary
Abbreviations
- FIV
feline immunodeficiency virus
- HIV
human immunodeficiency virus
- PAR
proteinase-activated receptor
References
- Acharjee S, Noorbakhsh F, Stemkowski PL, Olechowski C, Cohen EA, Ballanyi K, et al. HIV-1 viral protein R causes peripheral nervous system injury associated with in vivo neuropathic pain. FASEB J. 2010;24:4343–53. doi: 10.1096/fj.10-162313. [DOI] [PubMed] [Google Scholar]
- Alier KA, Endicott JA, Stemkowski PL, Cenac N, Cellars L, Chapman K, et al. Intrathecal administration of proteinase-activated receptor-2 agonists produces hyperalgesia by exciting the cell bodies of primary sensory neurons. J Pharmacol Exp Ther. 2008;324:224–33. doi: 10.1124/jpet.107.129171. [DOI] [PubMed] [Google Scholar]
- Asfaha S, Brussee V, Chapman K, Zochodne DW, Vergnolle N. Proteinase-activated receptor-1 agonists attenuate nociception in response to noxious stimuli. Br J Pharmacol. 2002;135:1101–6. doi: 10.1038/sj.bjp.0704568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augé C, Balz-hara D, Steinhoff M, Vergnolle N, Cenac N. Protease-activated receptor-4 (PAR4): a role as inhibitor of visceral pain and hypersensitivity. Neurogastroenterol Motility. 2009;21:1189–e107. doi: 10.1111/j.1365-2982.2009.01310.x. [DOI] [PubMed] [Google Scholar]
- Binshtok AM, Wang H, Zimmermann K, Amaya F, Vardeh D, Shi L, et al. Nociceptors are interleukin-1beta sensors. J Neurosci. 2008;28:14062–73. doi: 10.1523/JNEUROSCI.3795-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boven LA, Vergnolle N, Henry SD, Silva C, Imai Y, Holden J, et al. Up-regulation of proteinase-activated receptor 1 expression in astrocytes during HIV encephalitis. J Immunol. 2003;170:2638–46. doi: 10.4049/jimmunol.170.5.2638. [DOI] [PubMed] [Google Scholar]
- Bradley WG, Shapshak P, Delgado S, Nagano I, Stewart R, Rocha B. Morphometric analysis of the peripheral neuropathy of AIDS. Muscle Nerve. 1998;21:1188–95. doi: 10.1002/(sici)1097-4598(199809)21:9<1188::aid-mus10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Cornblath DR, Hoke A. Recent advances in HIV neuropathy. Curr Opin Neurol. 2006;19:446–50. doi: 10.1097/01.wco.0000245366.59446.57. [DOI] [PubMed] [Google Scholar]
- Cosenza M, Zhao M, Si Q, Lee S. Human brain parenchymal microglia express CD14 and CD45 and are productively infected by HIV-1 in HIV-1 encephalitis. Brain Pathol. 2002;12:442–55. doi: 10.1111/j.1750-3639.2002.tb00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham D, Gurwitz D. Proteolytic regulation of neurite outgrowth from neuroblastoma cells by thrombin and protease nexin-1. J Cell Biochem. 1989;39:55–64. doi: 10.1002/jcb.240390107. [DOI] [PubMed] [Google Scholar]
- de Garavilla L, Vergnolle N, Young SH, Ennes H, Steinhoff M, Ossovskaya VS, et al. Agonists of proteinase-activated receptor 1 induce plasma extravasation by a neurogenic mechanism. Br J Pharmacol. 2001;133:975–87. doi: 10.1038/sj.bjp.0704152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J, Lin Y, Fink E, Grant C. Feline immunodeficiency virus (FIV) as a model for study of lentivirus infections: parallels with HIV. Curr HIV Res. 2010;8:73–80. doi: 10.2174/157016210790416389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Kovács KJ, Fisher LL, Larson AA. Thrombin inhibits NMDA-mediated nociceptive activity in the mouse: possible mediation by endothelin. J Physiol. 2003;549:903–17. doi: 10.1113/jphysiol.2002.036384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JS, Pitts K, Rusnak FM, Owen WG, Windebank AJ. Thrombin induced inhibition of neurite outgrowth from dorsal root ganglion neurons. Brain Res. 1998;797:321–7. doi: 10.1016/s0006-8993(98)00344-8. [DOI] [PubMed] [Google Scholar]
- Hahn K, Robinson B, Anderson C, Li W, Pardo CA, Morgello S, et al. Differential effects of HIV infected macrophages on dorsal root ganglia neurons and axons. Exp Neurol. 2008;210:30–40. doi: 10.1016/j.expneurol.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res Reviews. 2005;48:457–76. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Hansen K, Oikonomopoulou K, Li Y, Hollenberg M. Proteinases, proteinase-activated receptors (PARs) and the pathophysiology of cancer and diseases of the cardiovascular, musculoskeletal, nervous and gastrointestinal systems. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:377–92. doi: 10.1007/s00210-007-0194-2. [DOI] [PubMed] [Google Scholar]
- Helyes Z, Sándor K, Borbély É, Tékus V, Pintér E, Elekes K, et al. Involvement of transient receptor potential vanilloid 1 receptors in protease-activated receptor-2-induced joint inflammation and nociception. Eur J Pain. 2010;14:351–8. doi: 10.1016/j.ejpain.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Hollenberg MD, Houle S. Proteinases as hormone-like signal messengers. Swiss Med Wkly. 2005;135:425–36. doi: 10.4414/smw.2005.11037. [DOI] [PubMed] [Google Scholar]
- Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron. 2007;55:365–76. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Johnston JB, Silva C, Power C. Envelope gene-mediated neurovirulence in feline immunodeficiency virus infection: induction of matrix metalloproteinases and neuronal injury. J Virol. 2002;76:2622–33. doi: 10.1128/JVI.76.6.2622-2633.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G, Power C. Regulation of neural cell survival by HIV-1 infection. Neurobiol Dis. 2006;21:1–17. doi: 10.1016/j.nbd.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Jones GJ, Barsby NL, Cohen EA, Holden J, Harris K, Dickie P, et al. HIV-1 Vpr causes neuronal apoptosis and in vivo neurodegeneration. J Neurosci. 2007;27:3703–11. doi: 10.1523/JNEUROSCI.5522-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G, Zhu Y, Silva C, Tsutsui S, Pardo CA, Keppler OT, et al. Peripheral nerve-derived HIV-1 is predominantly CCR5-dependent and causes neuronal degeneration and neuroinflammation. Virology. 2005;334:178–93. doi: 10.1016/j.virol.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Kennedy JM, Hoke A, Zhu Y, Johnston JB, van Marle G, Silva C, et al. Peripheral neuropathy in lentivirus infection: evidence of inflammation and axonal injury. AIDS. 2004;18:1241–50. doi: 10.1097/00002030-200406180-00002. [DOI] [PubMed] [Google Scholar]
- Knecht W, Cottrell GS, Amadesi S, Mohlin J, Skåregärde A, Gedda K, et al. Trypsin IV or mesotrypsin and p23 cleave protease-activated receptors 1 and 2 to induce inflammation and hyperalgesia. J Biol Chem. 2007;282:26089–100. doi: 10.1074/jbc.M703840200. [DOI] [PubMed] [Google Scholar]
- Kolson DL, Gonzalez-Scarano F. HIV-associated neuropathies: role of HIV-1, CMV, and other viruses. J Peripher Nerv Syst. 2001;6:2–7. doi: 10.1046/j.1529-8027.2001.006001002.x. [DOI] [PubMed] [Google Scholar]
- Laast VA, Pardo CA, Tarwater PM, Queen SE, Reinhart TA, Ghosh MP, et al. Pathogenesis of simian immunodeficiency virus-induced alterations in macaque trigeminal ganglia. J Neuropathol Exp Neurol. 2007;66:26–34. doi: 10.1097/nen.0b013e31802c398d. [DOI] [PubMed] [Google Scholar]
- Lu VB, Biggs JE, Stebbing MJ, Balasubramanyan S, Todd KG, Lai AY, et al. Brain-derived neurotrophic factor drives the changes in excitatory synaptic transmission in the rat superficial dorsal horn that follow sciatic nerve injury. J Physiol. 2009;587:1013–32. doi: 10.1113/jphysiol.2008.166306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingat F, Vivithanaporn P, Zhu Y, Taylor A, Baker G, Pearson K, et al. Neurobehavioral performance in feline immunodeficiency virus infection: integrated analysis of viral burden, neuroinflammation, and neuronal injury in cortex. J Neurosci. 2009;29:8429–37. doi: 10.1523/JNEUROSCI.5818-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L, Augé C, Boué J, Buresi MC, Chapman K, Asfaha S, et al. Thrombin receptor: an endogenous inhibitor of inflammatory pain, activating opioid pathways. Pain. 2009;146:121–9. doi: 10.1016/j.pain.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Martin C, Solders G, Sönnerborg A, Hansson P. Painful and non-painful neuropathy in HIV-infected patients: an analysis of somatosensory nerve function. Eur J Pain. 2003;7:23–31. doi: 10.1016/s1090-3801(02)00053-8. [DOI] [PubMed] [Google Scholar]
- Pardo CA, McArthur JC, Griffin J. HIV neuropathy: Insights in the pathology of HIV peripheral nerve disease. J Peripher Nerv Sys. 2001;6:21–7. doi: 10.1046/j.1529-8027.2001.006001021.x. [DOI] [PubMed] [Google Scholar]
- Power C, Boissé L, Rourke S, Gill MJ. NeuroAIDS: an evolving epidemic. Can J Neurol Sci. 2009;36:285–95. doi: 10.1017/s0317167100007009. [DOI] [PubMed] [Google Scholar]
- Ramachandran R, Hollenberg M. Proteinases and signalling: pathophysiological and therapeutic implications via PARs and more. Br J Pharmacol. 2008;153(Suppl 1):S263–82. doi: 10.1038/sj.bjp.0707507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman SM, Ma LH, Whiteside GT, Winkelstein BA. Inflammatory cytokine and chemokine expression is differentially modulated acutely in the dorsal root ganglion in response to different nerve root compressions. Spine. 2011;36:197–202. doi: 10.1097/BRS.0b013e3181ce4f4d. [DOI] [PubMed] [Google Scholar]
- Rothman S, Winkelstein B. Cytokine antagonism reduces pain and modulates spinal astrocytic reactivity after cervical nerve root compression. Ann Biomed Eng. 2010;38:2563–76. doi: 10.1007/s10439-010-0012-8. [DOI] [PubMed] [Google Scholar]
- Ruangkittisakul A, Schwarzacher SW, Secchia L, Poon Betty Y, Ma Y, Funk GD, et al. High sensitivity to neuromodulator-activated signaling pathways at physiological [K+] of confocally imaged respiratory center neurons in on-line-calibrated newborn rat brainstem slices. J Neurosci. 2006;26:11870–80. doi: 10.1523/JNEUROSCI.3357-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh U, Dores M, Chen B, Trejo J. Signal transduction by protease-activated receptors. Br J Pharmacol. 2010;160:191–203. doi: 10.1111/j.1476-5381.2010.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff M, Buddenkotte J, Shpacovitch V, Rattenholl A, Moormann C, Vergnolle N, et al. Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocr Rev. 2005;26:1–43. doi: 10.1210/er.2003-0025. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Vergnolle N, Young SH, Tognetto M, Amadesi S, Ennes HS, et al. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6:151–8. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- Suidan HS, Stone SR, Hemmings BA, Monard D. Thrombin causes neurite retraction in neuronal cells through activation of cell surface receptors. Neuron. 1992;8:363–75. doi: 10.1016/0896-6273(92)90302-t. [DOI] [PubMed] [Google Scholar]
- Vivithanaporn P, Heo G, Gamble J, Krentz HB, Hoke A, Gill MJ, et al. Neurologic disease burden in treated HIV/AIDS predicts survival. Neurology. 2010;75:1150–8. doi: 10.1212/WNL.0b013e3181f4d5bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol Rev. 2002;82:981–1011. doi: 10.1152/physrev.00011.2002. [DOI] [PubMed] [Google Scholar]
- Zhang K, Rana F, Silva C, Ethier J, Wehrly K, Chesebro B, et al. Human immunodeficiency virus type 1 envelope-mediated neuronal death: uncoupling of viral replication and neurotoxicity. J Virol. 2003;77:6899–912. doi: 10.1128/JVI.77.12.6899-6912.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Antony J, Liu S, Martinez JA, Giuliani F, Zochodne D, et al. CD8+ lymphocyte-mediated injury of dorsal root ganglion neurons during lentivirus infection: CD154-dependent cell contact neurotoxicity. J Neurosci. 2006;26:3396–403. doi: 10.1523/JNEUROSCI.4767-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Antony JM, Martinez JA, Glerum DM, Brussee V, Hoke A, et al. Didanosine causes sensory neuropathy in an HIV/AIDS animal model: impaired mitochondrial and neurotrophic factor gene expression. Brain. 2007;130:2011–23. doi: 10.1093/brain/awm148. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Vergote D, Pardo C, Noorbakhsh F, McArthur JC, Hollenberg MD, et al. CXCR3 activation by lentivirus infection suppresses neuronal autophagy: neuroprotective effects of antiretroviral therapy. FASEB J. 2009;23:2928–41. doi: 10.1096/fj.08-128819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W-J, Yamanaka H, Obata K, Dai Y, Kobayashi K, Kozai T, et al. Expression of mRNA for four subtypes of the proteinase-activated receptor in rat dorsal root ganglia. Brain Res. 2005;1041:205–11. doi: 10.1016/j.brainres.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Zink M, Laast V, Helke K, Brice A, Barber S, Clements J, et al. From mice to macaques–animal models of HIV nervous system disease. Curr HIV Res. 2006;4:293–305. doi: 10.2174/157016206777709410. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.