Abstract
Aortic stiffness increases with age and vascular risk factor exposure and is associated with increased risk for structural and functional abnormalities in the brain. High ambient flow and low impedance are thought to sensitize the cerebral microcirculation to harmful effects of excessive pressure and flow pulsatility. However, haemodynamic mechanisms contributing to structural brain lesions and cognitive impairment in the presence of high aortic stiffness remain unclear. We hypothesized that disproportionate stiffening of the proximal aorta as compared with the carotid arteries reduces wave reflection at this important interface and thereby facilitates transmission of excessive pulsatile energy into the cerebral microcirculation, leading to microvascular damage and impaired function. To assess this hypothesis, we evaluated carotid pressure and flow, carotid–femoral pulse wave velocity, brain magnetic resonance images and cognitive scores in participants in the community-based Age, Gene/Environment Susceptibility – Reykjavik study who had no history of stroke, transient ischaemic attack or dementia (n = 668, 378 females, 69–93 years of age). Aortic characteristic impedance was assessed in a random subset (n = 422) and the reflection coefficient at the aorta–carotid interface was computed. Carotid flow pulsatility index was negatively related to the aorta–carotid reflection coefficient (R = −0.66, P<0.001). Carotid pulse pressure, pulsatility index and carotid–femoral pulse wave velocity were each associated with increased risk for silent subcortical infarcts (hazard ratios of 1.62–1.71 per standard deviation, P<0.002). Carotid–femoral pulse wave velocity was associated with higher white matter hyperintensity volume (0.108 ± 0.045 SD/SD, P = 0.018). Pulsatility index was associated with lower whole brain (−0.127 ± 0.037 SD/SD, P<0.001), grey matter (−0.079 ± 0.038 SD/SD, P = 0.038) and white matter (−0.128 ± 0.039 SD/SD, P<0.001) volumes. Carotid–femoral pulse wave velocity (−0.095 ± 0.043 SD/SD, P = 0.028) and carotid pulse pressure (−0.114 ± 0.045 SD/SD, P = 0.013) were associated with lower memory scores. Pulsatility index was associated with lower memory scores (−0.165 ± 0.039 SD/SD, P<0.001), slower processing speed (−0.118 ± 0.033 SD/SD, P<0.001) and worse performance on tests assessing executive function (−0.155 ± 0.041 SD/SD, P<0.001). When magnetic resonance imaging measures (grey and white matter volumes, white matter hyperintensity volumes and prevalent subcortical infarcts) were included in cognitive models, haemodynamic associations were attenuated or no longer significant, consistent with the hypothesis that increased aortic stiffness and excessive flow pulsatility damage the microcirculation, leading to quantifiable tissue damage and reduced cognitive performance. Marked stiffening of the aorta is associated with reduced wave reflection at the interface between carotid and aorta, transmission of excessive flow pulsatility into the brain, microvascular structural brain damage and lower scores in various cognitive domains.
Keywords: haemodynamics, aortic stiffness, magnetic resonance imaging, brain structure, cognitive function
Introduction
Aortic stiffness increases dramatically with advancing age and is associated with increased risk for major cardiovascular disease events (Laurent et al., 2001, 2003; Meaume et al., 2001; Boutouyrie et al., 2002; Sutton-Tyrrell et al., 2005; Mattace-Raso et al., 2006; Willum-Hansen et al., 2006; Mitchell et al., 2010a), including clinically recognized stroke (Laurent et al., 2003). In young, healthy adults, the aorta is highly compliant and first-generation arteries are relatively stiff. This abrupt transition from the compliant (low impedance) aorta to the stiff (high impedance) branch vessels represents impedance mismatch. When a traveling wave encounters such a discontinuity, a portion of the pulsatile energy stored in that wave is reflected and therefore is not transmitted into the distal vasculature. Wave reflection at the junction between the normally compliant aorta and relatively stiff muscular arteries represents a protective mechanism that limits transmission of excessive pulsatility into the microcirculation. The magnitude of the reflection coefficient at such a boundary depends on the degree of impedance mismatch between proximal and distal vessels, with a greater difference in impedance producing a larger reflection. A disproportionate increase in aortic impedance with little change or a decrease in muscular artery impedance with advancing age and in the presence of various vascular risk factors leads to progressive impedance matching between aorta and peripheral arteries. Impedance matching reduces the reflection coefficient and hence the amount of wave reflection at the interface between aorta and branch vessels and therefore increases transmission of excessive pulsatile energy into the periphery where it may cause damage (Mitchell et al., 2004a; Mitchell, 2008).
High flow organs such as the brain and kidneys are particularly sensitive to excessive pressure and flow pulsatility (Mitchell, 2008). High local blood flow is associated with low microvascular impedance, which facilitates penetration of excessive pulsatile energy into the microvascular bed. Aortic stiffening is associated with microcirculatory remodelling that may serve to limit capillary exposure to excessive pulsatility and also impairs reactivity (Mitchell et al., 2005), potentially contributing to repeated episodes of microvascular ischaemia and tissue damage. Microvascular ischaemia in the brain manifests as white matter hyperintensities and small, clinically unrecognized focal brain infarcts that ultimately manifest as cognitive impairment and frank dementia. Thus, microvascular damage and remodelling may represent a mechanistic link between aortic stiffening, brain lesions and cognitive impairment. We hypothesized that disproportionate stiffening of the proximal aorta as compared with the carotid arteries reduces wave reflection at this important interface and therefore facilitates transmission of excessive pulsatile energy into the cerebral microcirculation, leading to microvascular damage and impaired function.
Several studies have evaluated relations between arterial stiffness and various measures of brain structure and function in selected (Scuteri et al., 2007; Henskens et al., 2008; Brandts et al., 2009; Kearney-Schwartz et al., 2009; van Elderen et al., 2010) and community-based samples (Poels et al., 2007; Hashimoto et al., 2008; Waldstein et al., 2008; Elias et al., 2009). These studies have demonstrated relations between aortic pulse wave velocity or pulse pressure and white matter hyperintensity volume, lacunar infarcts or cognitive function; however, a comprehensive evaluation of relations of pulse wave velocity and pressure and flow pulsatility with segmented brain volumes, subclinical infarcts and cognitive function in an adequately powered community-based sample has not been performed. Therefore, we analysed relations of carotid–femoral pulse wave velocity (CFPWV), central pulse pressure (CPP) and carotid flow pulsatility index with brain structure and function in the community-based Age, Gene/Environment Susceptibility – Reykjavik (AGES-Reykjavik) study.
Patients and methods
Study participants
The rationale and design of the AGES-Reykjavik study have been presented (Harris et al., 2007). AGES-Reykjavik originates from the Reykjavik Study, a community-based cohort established in 1967 to prospectively study cardiovascular disease in Iceland. Between 2002 and 2006, a total of 5764 males and females participated in detailed evaluations of cardiovascular, neurocognitive, musculoskeletal and metabolic phenotypes. AGES-Reykjavik was approved by the National Bioethics Committee in Iceland, which acts as the institutional review board for the Icelandic Heart Association (approval number VSN-00-063), and by the National Institute on Ageing Intramural Institutional Review Board. All participants gave written informed consent.
Arterial tonometry was added to the study protocol for all participants beginning January 12, 2005, and continuing through to the end of the examination cycle. Tonometry and carotid blood flow measurements were attempted in 1082 (90%) of the 1204 participants who presented for examination during this time period. Complete tonometry and carotid flow data were obtained in 898 (83%) of the 1082 participants in whom measurements were attempted. From this initial sample of 898 participants, we further excluded cases for the following non-exclusive reasons: missing MRI data (n = 102) or cognitive data (n = 56), or a history of stroke (n = 65), transient ischaemic attack (n = 46) or dementia (n = 25). These exclusions resulted in a final sample of 668 participants who were included in all analyses except as noted in the text.
The study examination was carried out over the course of three clinic visits (Harris et al., 2007). Cognitive testing was performed during the first clinic visit, whereas tonometry and brain imaging were performed during the second clinic visit. A third clinic visit acquired a number of additional phenotypes and included a formal dementia assessment if indicated by the results of earlier testing. The entire series of examinations was completed in a 4–6 week time interval.
Haemodynamic data acquisition
The haemodynamic protocol has been described (Mitchell et al., 2008). In brief, following 15–20 min of supine posture, auscultatory brachial blood pressure was assessed by using a computer-controlled device that automatically inflated the cuff to a user preset maximum pressure and then precisely controlled deflation at 2 mmHg/s. This device digitized and recorded cuff pressure, electrocardiogram (1000 Hz) and a cuff microphone channel (12 kHz) throughout the inflation and deflation sequence so that all blood pressure measurements could be over-read by the core lab (Cardiovascular Engineering, Inc.). Arterial tonometry with electrocardiogram was obtained from the brachial, radial, femoral and carotid arteries using a custom transducer (Cardiovascular Engineering, Inc.). Body surface measurements from suprasternal notch to pulse recording sites were obtained by using a fibreglass tape measure for carotid, brachial and radial sites and a caliper for the femoral site. Bilateral common carotid artery images and flows were assessed with an Acuson Sequoia ultrasound system using a duplex linear array probe with an 8.0 MHz imaging frequency and a 4.0 MHz Doppler carrier frequency. In a random subset of approximately half of the participants examined each day, a limited echocardiogram was performed as previously described in order to measure aortic inflow and compute aortic input impedance (Mitchell et al., 2008).
Brain magnetic resonance imaging
All eligible participants were offered a high-resolution brain MRI acquired on a study-dedicated 1.5-T Signa Twinspeed system (General Electric Medical Systems). The imaging protocol has been described (Harris et al., 2007; Scher et al., 2009) and included 3D spoiled-gradient recalled T1-weighted, fast spin echo proton density/T2-weighted, fluid-attenuated inversion recovery (FLAIR) and echo-planar imaging gradient echo T2*-weighted sequences. All images were acquired to give full brain coverage with slices angled parallel to the anterior commissure–posterior commissure line in order to give reproducible image views in the oblique-axial plane.
Cognitive function testing
The neuropsychologic battery consisted of nine tests: a modified version of the California Verbal Learning Test (Delis et al., 1987); Digits Forward and Backwards (Wechsler, 1955); Digit Symbol Substitution Test (Wechsler, 1955); Figure Comparison (Salthouse and Babcock, 1991); Stroop Test, Parts I–III (Stroop, 1935); a short version of the Cambridge Neuropsychological Test Automated Battery Spatial Working Memory test; and the Mini-Mental State Examination (Folstein et al., 1975). To assess robust measures of key cognitive domains, we created composite scores based on previous theoretical and empirical work in population-based samples (Schaie et al., 1998; Wilson et al., 1999; De Groot et al., 2000). The memory composite includes the California Verbal Learning Test immediate and delayed recall. The processing speed composite includes the Digit Symbol Substitution Test, Figure Comparison and the Stroop Test, Parts I (word naming) and II (colour naming). The executive function composite includes Digits Backward, the Cambridge Neuropsychological Test Automated Battery spatial working memory test and the Stroop Test, Part III (word–colour interference). All tests were normally distributed in the cohort and inter-rater reliability was excellent (Spearman's correlations for specific cognitive tests range from 0.96 to 0.99). Composite measures were computed for each test by converting raw scores to standardized z-scores and averaging them across the tests in each composite. A confirmatory factor analysis, previously reported, showed that the fit of the composites was adequate (Saczynski et al., 2008).
Haemodynamic data analysis
Tonometry waveforms were signal-averaged using the electrocardiogram R-wave as a fiducial point. Systolic and diastolic brachial cuff pressures were used to calibrate peak and trough of the signal-averaged brachial waveform. Diastolic and integrated mean brachial pressures were then used to calibrate carotid, radial and femoral waveforms (Kelly and Fitchett, 1992). CFPWV and aortic input impedance were calculated as previously described (Mitchell et al., 2002). Peripheral vascular resistance was calculated by dividing mean arterial pressure by cardiac output. Characteristic impedance was computed in the time domain as the ratio of change in pressure divided by change in flow as assessed from the foot of the respective waveforms to the time when flow reached 95% of peak flow. Characteristic impedance was also expressed as normalized impedance by dividing characteristic impedance by peripheral resistance. Augmentation index was assessed from the carotid pressure waveform (Murgo et al., 1980).
Carotid artery flows were analysed from digitized Doppler audio data using a semi-automated signal-averaging approach as detailed previously (Mitchell et al., 2004b). Flow spectra were signal-averaged (1000 Hz resolution) using the electrocardiogram as a fiducial point. Flow velocities were multiplied by carotid artery cross-sectional area to obtain volume flows. Carotid pulsatility index was computed for each carotid by dividing the flow pulse amplitude (peak flow minus flow at the onset of the systolic upstroke) by mean flow. Values for the right and left carotid were averaged. Carotid vascular resistance was calculated by dividing mean arterial pressure by bilateral mean carotid volume flow. Carotid characteristic impedance was calculated by dividing the peak derivative of pressure by the peak derivative of flow (Dujardin and Stone, 1981). Carotid characteristic impedance was also expressed as normalized impedance by dividing by carotid vascular resistance. A carotid reflection coefficient was computed by modelling bilateral carotid impedance and distal aortic impedance as a bifurcation of the proximal aorta. The reflection coefficient at this junction may be expressed as follows: reflection coefficient = (Aproximal – Acarortid – Adistal) / (Aproximal + Acarotid + Adistal), where A is admittance (the reciprocal of impedance) and proximal and distal denote the aorta proximal and distal to the carotid origin, respectively. To estimate distal aortic impedance, we assumed local pulse wave velocity and mean flow velocity were constant in the aorta proximal and distal to the origin of the carotid arteries. Under these conditions, Zdistal = Zproximal x (Qproximal/Qdistal), where Z denotes impedance and Q denotes mean volume flow.
Brain image analysis
Total brain, white and grey matter, and white matter hyperintensity volumes were computed automatically with an algorithm based on the Montreal Neurological Institute pipeline (Zijdenbos et al., 2002). The AGES-Reykjavik/Montreal Neurological Institute pipeline has been modified to accommodate full brain coverage including cerebellum and brainstem, multispectral images (T1-weighted 3D spoiled-gradient recalled sequence, FLAIR and proton density/T2-weighted fast spin echo sequences), high throughput and minimal editing. White matter hyperintensities were considered present in regions where signal intensity was higher than that of normal white and grey matter on both T2-weighted and FLAIR images. Total brain volume (an indicator of atrophy) was derived as the percentage of the total tissue volume relative to the intracranial volume. The other brain tissue classes were similarly expressed as a percentage of total intracranial volume.
Infarcts were evaluated as described previously (Saczynski et al., 2009; Scher et al., 2009) and defined as a defect of the brain parenchyma with a signal intensity equal to cerebrospinal fluid on all pulse sequences (FLAIR, T2-weighted, proton density-weighted). Cortical infarct-like lesions were defined as parenchymal defects involving or limited to the cortical ribbon and surrounded by an area of high signal intensity on FLAIR images. Defects in the subcortical area without a rim or area of high signal intensity on FLAIR, and without evidence of haemosiderin on the T2*-weighted echo-planar imaging gradient echo scan were labelled as large Virchow–Robin spaces; these were excluded from the definition of subcortical infarcts. Defects in the subcortical area with evidence of haemosiderin on the T2*-weighted echo-planar imaging gradient echo scan were labelled as resorbed haematomas and were also excluded from the definition of subcortical infarcts. Lesions 4 mm or larger were recorded as infarcts except for those in the cerebellum, for which there was no size criterion. Infarcts that spanned two areas were assigned to the location containing the largest measured diameter of the defect regardless of orientation. Quality control procedures included assessments of intraobserver variability every 6 months and assessments for interobserver differences every 3 months. The intraobserver weighted κ statistics were 0.89 for global white matter lesions and 0.92 for parenchymal defects; the interobserver weighted κ statistics were 0.71 for global white matter lesions and 0.66 for parenchymal defects. In addition, a 5% random sample was reread by a trained neuroradiologist at Leiden University Medical Centre and differences discussed.
Statistical analysis
Sample characteristics and haemodynamic variables were tabulated separately by sex. Multivariable linear regression analysis was used to assess relations between arterial stiffness variables and continuous measures of brain structure and function. Logistic regression analysis was used to assess relations between arterial stiffness variables and presence of subclinical infarcts. All models adjusted for correlates of arterial function in this sample (Supplementary Table 1), including age, height, weight, heart rate, mean arterial pressure, high density lipoprotein cholesterol, glucose, hypertension treatment and statin use. In linear regression models, exposure and outcome variables were standardized by sex. In logistic models, sex was included as an indicator variable. Models assessing measures of cognitive function were further adjusted for education level (elementary, secondary, junior college, university) and score on the Geriatric Depression Scale short form (Sheikh and Yesavage, 1986; Vidal et al., 2010).
CPP, carotid pulsatility index and augmentation index were natural log transformed and CFPWV was inverted (1000/CFPWV, ms/m) in order to normalize the distributions of these skewed variables prior to regression analysis. Exposure variables and covariates were entered as sex-specific z-scores in all models. The z-score for inverse CFPWV was multiplied by −1 in order to restore the directionality between increasing z-score and increasing arterial stiffness. Values are presented as mean ± SD except as noted. A two-sided P<0.05 was considered significant.
Results
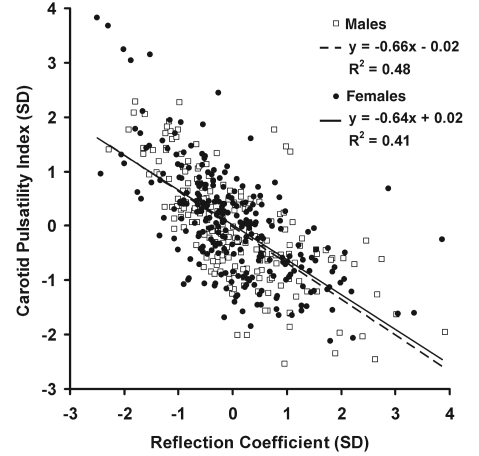
Clinical characteristics of this older (69–93 years of age), community-based sample are presented in Table 1 and haemodynamic variables are presented in Table 2. Pulse pressure was high, particularly in females, and mean CFPWV was >12 m/s, a level associated with markedly increased cardiovascular disease risk (Mitchell et al., 2010a). Characteristic impedance of the aorta was high, particularly in females, consistent with their elevated pulse pressure. Carotid characteristic impedance was much higher than aortic because of the smaller size of the carotids. However, normalized carotid impedance remained twice that of the aorta, indicating higher relative impedance to pulsatile flow in the carotids even after accounting for differences in size of the vascular bed. Mismatch between carotid and aortic impedance was associated with a positive but highly variable carotid reflection coefficient (Table 2), with 95% of the values falling between 0% and 15%. Because of the mismatch between aortic and carotid impedance, carotid pulsatility index was less than half that of the aorta (Table 2). Carotid pulsatility index had a strong negative relation with carotid reflection coefficient (Fig. 1). In contrast, the age-adjusted relations between pulsatility index and CPP (R = 0.33, P<0.001) or carotid vascular resistance (R = 0.26, P<0.001) were relatively modest, indicating that factors other than pressure pulsatility and local vascular resistance contributed to excessive flow pulsatility. Relations between key arterial measures and vascular risk factors are presented in Supplementary Table 1. Each of the vascular stiffness measures was related to age, mean arterial pressure, heart rate and fasting glucose level, with variable relations to other vascular risk factors. Brain MRI results are summarized in Table 3. Cognitive composites were standardized by sex (mean score 0.0 ± 1.0).
Table 1.
Characteristics of the sample
| Variables | Females | Males |
|---|---|---|
| n | 378 | 290 |
| Age (years) | 75 ± 4 | 76 ± 4 |
| Height (cm) | 162 ± 5 | 176 ± 6 |
| Weight (kg) | 70 ± 12 | 83 ± 13 |
| Waist (cm) | 98 ± 12 | 102 ± 10 |
| Body mass index (kg/m2) | 27 ± 4 | 27 ± 4 |
| Cholesterol (mmol/l) | 5.7 ± 1.0 | 5.0 ± 1.0 |
| HDL (mmol/l) | 1.8 ± 0.4 | 1.5 ± 0.4 |
| Triglycerides (mmol/l) | 1.2 ± 0.6 | 1.1 ± 0.6 |
| Glucose (mmol/l) | 5.6 ± 1.1 | 5.8 ± 1.3 |
| Medical history, n (%) | ||
| Coronary heart disease | 27 (7) | 86 (30) |
| Diabetes | 29 (8) | 38 (13) |
| Treated hypertension | 240 (63) | 156 (54) |
| Statin use | 69 (18) | 90 (31) |
| Current smoker | 44 (12) | 32 (11) |
| Education level, n (%) | ||
| Elementary | 103 (27) | 38 (13) |
| Secondary | 188 (50) | 166 (57) |
| Junior college | 59 (16) | 39 (13) |
| University | 28 (7) | 47 (16) |
| GDS score, median (25%, 75%) | 2 (1, 3) | 2 (1, 3) |
| MMSE, median (25%, 75%) | 27 (26, 29) | 27 (26, 28) |
GDS = Geriatric depression scale; HDL = high-density lipoprotein; MMSE = Mini-Mental State Examination score; TIA = transient ischaemic attack.
Table 2.
Haemodynamic variables
| Variables | Females | Males |
|---|---|---|
| Blood pressure (mmHg) | ||
| Systolic | 141 ± 20 | 137 ± 18 |
| Diastolic | 67 ± 9 | 67 ± 10 |
| Mean | 96 ± 12 | 93 ± 12 |
| Peripheral pulse pressure | 74 ± 19 | 70 ± 17 |
| Central pulse pressure | 72 ± 22 | 65 ± 20 |
| Forward wave | 56 ± 18 | 55 ± 17 |
| Carotid–femoral PWV (m/s) | 12.2 ± 3.7 | 13.4 ± 4.4 |
| iCFPWV (ms/m) | 88 ± 21 | 82 ± 23 |
| Augmentation index (%) | 17 ± 17 | 12 ± 13 |
| Heart rate (beats/min) | 64 ± 9 | 60 ± 10 |
| Carotid pressure flow measures | ||
| Mean flow (ml/s) | 12.9 ± 2.8 | 14.5 ± 3.2 |
| Resistance, DSC | 10,454 ± 2,758 | 9,035 ± 2,436 |
| Characteristic impedance, DSC | 2,758 ± 880 | 2,020 ± 640 |
| Normalized impedance, ratio | 0.27 ± 0.07 | 0.23 ± 0.07 |
| Pulsatility index, ratio | 1.7 ± 0.4 | 2.0 ± 0.5 |
| Aortic pressure flow measuresa | ||
| Mean flow (ml/s) | 62.4 ± 14.7 | 64.9 ± 15.5 |
| Resistance, DSC | 2,147 ± 554 | 2,010 ± 539 |
| Characteristic impedance, DSC | 272 ± 95 | 235 ± 88 |
| Normalized impedance, ratio | 0.13 ± 0.04 | 0.12 ± 0.04 |
| Pulsatility index, ratio | 4.3 ± 0.5 | 4.8 ± 0.6 |
| Carotid reflection coefficient (%) | 6.0 ± 3.0 | 6.2 ± 3.8 |
a In a subset of 230 females and 192 males with echocardiography data.
DSC = dyne × s/cm5; iCFPWV = inverse carotid–femoral pulse wave velocity; PWV = pulse wave velocity.
Figure 1.
Relation between carotid pulsatility index and the reflection coefficient at the origin of the carotid arteries, modelled as an effective bifurcation of the aorta into the anterior head circulation (both common carotids) and the rest of the body. As carotid reflection coefficient decreases, representing increased matching between aortic and carotid impedances, carotid flow pulsatility increases steeply. Pulsatility index was natural log transformed. Both variables were centered and standardized by sex (mean = 0 and SD = 1).
Table 3.
Brain magnetic resonance imaging data
| Variables | Females | Males |
|---|---|---|
| Total brain parenchyma, % ICV | 74 ± 4 | 71 ± 4 |
| Grey matter, % ICV | 47 ± 3 | 45 ± 3 |
| White matter, % ICV | 26 ± 2 | 26 ± 2 |
| Cerebrospinal fluid, % ICV | 26 ± 4 | 29 ± 4 |
| WMH, ln(% ICV) | −4.9 ± 1.0 | −4.9 ± 0.9 |
| WMH volume, median (25–75%) (ml) | 10 (6–21) | 12 (7–23) |
| Infarcts, n (%) | ||
| Cortical | 16 (4) | 32 (11) |
| Subcortical | 36 (10) | 52 (18) |
| Cerebellar | 39 (10) | 43 (15) |
ICV = intracranial volume; WMH = white matter hyperintensity volume.
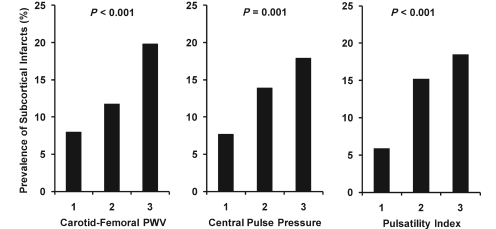
The prevalence of subcortical infarcts increased steeply across tertiles of each of the arterial measures (Fig. 2). In multivariable adjusted models, 1 SD increments in arterial measures were associated with 62–71% excess risk for prevalent subcortical infarct (Table 4). In contrast, none of the arterial measures was associated with prevalent cortical infarcts. Augmentation index was not related to prevalent cortical or subcortical infarcts (P > 0.5). Since carotid pulsatility index is a ratio, we evaluated the mean and pulsatile components together in a model and found that carotid flow pulse amplitude was associated with doubling of the risk for prevalent subcortical infarction, whereas mean flow was not associated with risk (Table 4). In a stepwise model that offered all three arterial measures, CFPWV and carotid pulsatility index entered together, suggesting that these related but distinct measures of vascular function contribute separately to the prevalence of subcortical infarcts (Table 4).
Figure 2.
Prevalence of subcortical infarctions according to sex-specific tertiles of aortic stiffness measures in participants with no prior history of clinical stroke or transient ischaemic attack. PWV = pulse wave velocity.
Table 4.
Multivariable adjusted relations between measures of arterial function and presence of subcortical infarctions
| Variables | Hazard ratio | Lower CI | Upper CI | P-value |
|---|---|---|---|---|
| Carotid–femoral PWVa | 1.62 | 1.20 | 2.20 | 0.002 |
| Central pulse pressureb | 1.71 | 1.24 | 2.36 | 0.001 |
| Carotid pulsatility indexb | 1.63 | 1.25 | 2.12 | <0.001 |
| Dual carotid flow components | ||||
| Carotid flow pulse amplitude | 2.08 | 1.51 | 2.88 | <0.001 |
| Carotid mean flow | 0.78 | 0.58 | 1.04 | 0.10 |
| Dual stiffness measures | ||||
| Carotid–femoral PWVa | 1.44 | 1.06 | 1.98 | 0.021 |
| Carotid pulsatility indexb | 1.50 | 1.14 | 1.98 | 0.004 |
All models adjusted for age, sex, height, weight, heart rate, mean arterial pressure, high-density lipoprotein cholesterol, glucose, hypertension treatment and statin use.
a Inverse transformed, standardized by sex and multiplied by −1.
b Natural log transformed and standardized by sex; CI = 95% confidence intervals.
PWV = pulse wave velocity.
Relations between quantitative measures of brain structure and vascular measures are presented in the upper section of Table 5. Higher CFPWV was associated with higher white matter hyperintensity volume. Higher carotid pulsatility index was associated with lower total brain parenchyma, grey and particularly white matter volumes but was not related to white matter hyperintensity volume. Augmentation index was not associated with segmented brain volumes (Supplementary Table 2). When mean and pulsatile components of carotid flow were considered jointly, total brain parenchyma and grey matter volumes were related only to mean flow, whereas white matter volume was related to both flow components (Supplementary Table 2).
Table 5.
Multivariable adjusted relations between arterial measures and brain structure and function
| Variables | Carotid–femoral PWVa |
Central pulse pressureb |
Carotid pulsatility indexb |
|||
|---|---|---|---|---|---|---|
| β ± SE | P-value | β ± SE | P-value | β ± SE | P-value | |
| Brain structure | ||||||
| Total brain parenchyma | −0.044 ± 0.042 | 0.3 | −0.006 ± 0.044 | 0.9 | −0.127 ± 0.037 | 0.001 |
| Grey matter | −0.064 ± 0.042 | 0.1 | 0.027 ± 0.044 | 0.5 | −0.079 ± 0.038 | 0.038 |
| White matter | −0.039 ± 0.044 | 0.4 | −0.065 ± 0.046 | 0.2 | −0.128 ± 0.039 | <0.001 |
| White matter hyperintensities | 0.108 ± 0.045 | 0.018 | −0.021 ± 0.048 | 0.7 | 0.017 ± 0.041 | 0.7 |
| Cognitive functionc | ||||||
| Memory | −0.095 ± 0.043 | 0.028 | −0.114 ± 0.045 | 0.013 | −0.165 ± 0.039 | <0.001 |
| Speed | 0.027 ± 0.037 | 0.5 | −0.018 ± 0.039 | 0.6 | −0.118 ± 0.033 | <0.001 |
| Executive function | −0.076 ± 0.045 | 0.09 | −0.094 ± 0.048 | 0.05 | −0.155 ± 0.041 | <0.001 |
All models adjusted for age, height, weight, heart rate, mean arterial pressure, high-density lipoprotein cholesterol, glucose, hypertension treatment and statin use.
a Inverse transformed, standardized by sex and multiplied by −1.
b Natural log transformed and standardized by sex.
c Further adjusted for education level and Geriatric Depression Scale score.
Relations between cognitive scores and vascular measures are presented in the lower section of Table 5. CFPWV and CPP were associated with lower memory scores only while carotid pulsatility index was strongly associated with reduced function in all three cognitive domains (Table 5). Augmentation index was not related to cognitive measures (Supplementary Table 2). In a model that included mean and pulsatile components of carotid flow together, both components were related to scores in each of the cognitive domains (Supplementary Table 2). When measures of brain structure (grey and white matter volumes, white matter hyperintensity volumes and prevalent subcortical infarcts) were included in memory models, associations with CPP (P = 0.028) and the carotid flow pulse amplitude (P = 0.001) remained significant, whereas CFPWV was no longer significant (P = 0.1). When MRI measures of brain structure were included in models for executive function and speed, the association of carotid flow pulse amplitude with executive function (P = 0.001) and speed (P = 0.018) were attenuated but remained significant. In secondary analyses, we included indicator variables for diabetes and obesity (body mass index ≥ 30 kg/m2) and sex-standardized, log-transformed cholesterol and triglyceride levels in the brain structure and function models. Associations between arterial measures and brain measures were not substantively altered by inclusion of these additional covariates.
Discussion
This study provides a detailed assessment of relations between arterial structure and function and brain structure and function in a large community-based sample of older males and females. Primary arterial measures that were assessed included pressure and flow pulsatility and CFPWV. Brain structural measures included grey and white matter and white matter hyperintensity volumes and infarcts assessed by MRI. We also performed comprehensive quantitative cognitive testing and derived summary measures for three major cognitive domains (memory, speed and executive function). We found that higher levels of pressure and flow pulsatility and CFPWV were associated with diffuse microvascular brain lesions, including subcortical infarcts and greater white matter hyperintensity volume, and reduced scores in multiple cognitive domains. We evaluated relations between arterial stiffness and carotid flow pulsatility and showed that disproportionately higher aortic stiffness was associated with progressive impedance matching between aorta and carotids. Resulting loss of the normal impedance mismatch at this interface reduced the carotid reflection coefficient and facilitated transmission of excessive flow pulsatility into the carotid system. Our findings are consistent with the hypothesis that marked proximal aortic stiffening in older people facilitates transmission of excessive pressure and flow pulsatility into the carotid circulation where these abnormal physical forces trigger microvascular damage and remodelling that limits flow or flow reserve, leading to microvascular ischaemia, quantifiable tissue damage and reduced cognitive performance.
As in prior studies, we found relations of higher CFPWV, the current gold standard for assessing aortic stiffness, and higher central pulse pressure with increased prevalence of brain lesions and lower cognitive scores (Hanon et al., 2005; Poels et al., 2007; Scuteri et al., 2007; Waldstein et al., 2008; Elias et al., 2009; Kearney-Schwartz et al., 2009). Relations between memory scores and CFPWV were no longer significant after adjusting for MRI measures of brain structure, suggesting that increased CFPWV affects memory by contributing to quantifiable structural brain damage, including increased white matter hyperintensity volume and higher prevalence of subcortical infarctions. In memory models that offered CFPWV, central pulse pressure, augmentation index and flow pulsatility index together, only pulsatility index was significant, suggesting that the adverse effects of stiffness and wave reflection are mediated by transmission of excessive flow pulsatility into the cerebral circulation. Furthermore, excessive flow pulsatility is transmitted at high pulse pressure in participants with a stiff aorta, which has a multiplicative effect on the amount of pulsatile energy transmitted into and dissipated within the cerebral microvasculature. Prior studies have shown that increased aortic stiffness and excessive pressure pulsatility are associated with increased resting microvascular resistance and markedly impaired reactivity in response to ischaemic stress in the forearm (Mitchell et al., 2005). Our observations are consistent with the hypothesis that excessive central pressure and flow pulsatility are also associated with microvascular dysfunction and damage in the brain, leading to parenchymal damage and cognitive impairment. In light of the body of literature demonstrating relations between the arterial measures that we have used and brain structure and function, future observational or interventional studies of cognitive function should consider including CFPWV, central pulse pressure and carotid pulsatility index as part of the clinical evaluation.
Higher carotid pulsatility index is often considered an indicator of increased cerebrovascular resistance. However, we have found that relations between pulsatility index, aortic stiffness and cerebrovascular function are more complex than this simple paradigm suggests. Relations between pulsatility index and pulse pressure or cerebrovascular resistance were relatively modest. In contrast, we have shown that impedance matching between aorta and carotids, as assessed by the carotid reflection coefficient, is closely related to flow pulsatility in the carotid vasculature (Fig. 1). Excessive pressure and flow pulsatility is associated with microvascular remodelling that increases resting resistance and limits hyperaemic reserve (Mitchell et al., 2005). The concordant increase in flow pulsatility and cerebrovascular resistance, which limits mean flow, results in additive effects on pulsatility index. These opposite effects of aortic stiffening on pulsatile and mean flow may account for the robust associations between pulsatility index and brain measures, including cognitive function.
Wave reflection in the arterial system is often portrayed as deleterious. However, it is important to recall that wave reflection at the interface between a compliant aorta and much stiffer peripheral arteries limits transmission of excessive pulsatile energy into the periphery. We have shown that a disproportionate increase in aortic characteristic impedance as compared with carotid input impedance markedly reduces the amount of wave reflection at this proximal interface and facilitates transmission of increased pulsatility into the carotid circulation. An increase in CFPWV contributes to earlier return of the reflected wave and increased augmentation in younger people with modest elevations of CFPWV (Mitchell et al., 2010b). However, in older people, marked stiffening of the aorta may reduce wave reflection at the interface between aorta and proximal large arteries throughout the body, similar to the reduction we have demonstrated at the interface between carotids and aorta. The resulting reductions in local and global wave reflection may contribute to the dissociation between augmentation index and measures of aortic stiffness or carotid flow pulsatility and a lack of association between augmentation index and measures of brain structure and function. Thus, in older people, the adverse effects of aortic stiffening are not captured by a measure of global wave reflection, such as augmentation index. Furthermore, loss of proximal wave reflection and increased distal transmission of flow pulsatility may play a major role in target organ damage associated with excessive aortic stiffness.
In our study, CFPWV was related to white matter hyperintensity volume, whereas CPP and pulsatility index were not. Prior studies have shown a relation between aortic PWV and white matter hyperintensity (Henskens et al., 2008; van Elderen et al., 2010). Increased CFPWV is associated with collagen deposition and fibrosis in the aortic wall (Najjar et al., 2005). This raises the possibility that the association between CFPWV and white matter hyperintensity may relate, in part, to a general response to tissue injury and repair characterized by excessive fibrosis in the arterial wall and in the white matter of the brain. Alternatively, regions of the brain that are prone to develop white matter hyperintensity may be particularly susceptible to the nature of the haemodynamic insult produced by excessive waveform momentum present when pulse wave velocity is markedly elevated. White matter hyperintensities typically accumulate in regions that are supplied by directly penetrating branches of the deep cerebral circulation (Pantoni and Garcia, 1997). These vessels differ markedly from the long, circuitous pial network that supplies the cortex and outer white matter. The latter pial vessels dampen pulsatility and decelerate the advancing waveform and hence may provide the cerebral cortex with an additional level of protection from excessive central pressure and flow pulsatility and waveform momentum.
There are limitations of our study that need to be considered. We studied an exclusively older sample with a minimum age of 69 years. In light of known non-linearities between arterial measures and age, additional studies in younger cohorts will be required in order to establish whether associations observed in our older cohort apply in younger samples. Our study employed a cross-sectional design and therefore examined associations; additional work will be required to ascertain whether these associations represent causal relations. We have demonstrated significant and variable wave reflection at the interface between aorta and carotids. Since we measured carotid pressure distal and aortic flow proximal to this interface, we may have variably overestimated aortic characteristic impedance by an average of ∼6%, which is small relative to the marked increase in characteristic impedance that is seen after midlife. The strengths of our study include a large community-based sample with routine ascertainment of detailed measures of arterial and brain structure and function.
Perspective
The prevalence of abnormal aortic stiffness increases dramatically with age from midlife (Mitchell et al., 2007, 2010b) and is associated with a markedly increased risk for major cardiovascular disease events (Mitchell et al., 2010a). Aortic stiffening increases exposure of the microcirculation to abnormal physical forces and is associated with microvascular damage, remodelling and impaired reactivity. We have shown that increased aortic stiffness is associated with excessive carotid pressure and flow pulsatility, microvascular brain lesions and reduced performance in multiple cognitive domains. In light of the rapid ageing of the world population, our findings suggest that the burden of arterial stiffness-related disorders, such as cognitive impairment and dementia, may increase substantially over the next decade unless measures are identified and implemented that limit or reduce aortic stiffening with advancing age.
Funding
National Institutes of Health (contract N01-AG-12100); National Institute on Ageing Intramural Research Programme; Hjartavernd (the Icelandic Heart Association); Althingi (the Icelandic Parliament); National Institutes of Health, National Heart, Lung and Blood Institute (HL094898).
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviations
- AGES-Reykjavik
Age, Gene/Environment Susceptibility – Reykjavik
- CFPWV
carotid–femoral pulse wave velocity
- CPP
central pulse pressure
- FLAIR
fluid-attenuated inversion recovery
References
- Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–5. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]
- Brandts A, van Elderen SG, Westenberg JJ, van der GJ, van Buchem MA, Huisman MV, et al. Association of aortic arch pulse wave velocity with left ventricular mass and lacunar brain infarcts in hypertensive patients: assessment with MR imaging. Radiology. 2009;253:681–8. doi: 10.1148/radiol.2533082264. [DOI] [PubMed] [Google Scholar]
- De Groot JC, de Leeuw FE, Oudkerk M, van Gijn J, Hofman A, Jolles J, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol. 2000;47:145–51. doi: 10.1002/1531-8249(200002)47:2<145::aid-ana3>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test manual – adult version (research edition) New York: The Psychological Corporation; 1987. [Google Scholar]
- Dujardin JP, Stone DN. Characteristic impedance of the proximal aorta determined in the time and frequency domain: a comparison. Med Biol Eng Comput. 1981;19:565–8. doi: 10.1007/BF02442770. [DOI] [PubMed] [Google Scholar]
- Elias MF, Robbins MA, Budge MM, Abhayaratna WP, Dore GA, Elias PK. Arterial pulse wave velocity and cognition with advancing age. Hypertension. 2009;53:668–73. doi: 10.1161/HYPERTENSIONAHA.108.126342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Hanon O, Haulon S, Lenoir H, Seux ML, Rigaud AS, Safar M, et al. Relationship between arterial stiffness and cognitive function in elderly subjects with complaints of memory loss. Stroke. 2005;36:2193–7. doi: 10.1161/01.STR.0000181771.82518.1c. [DOI] [PubMed] [Google Scholar]
- Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–87. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto J, Aikawa T, Imai Y. Large artery stiffening as a link between cerebral lacunar infarction and renal albuminuria. Am J Hypertens. 2008;21:1304–9. doi: 10.1038/ajh.2008.291. [DOI] [PubMed] [Google Scholar]
- Henskens LH, Kroon AA, van Oostenbrugge RJ, Gronenschild EH, Fuss-Lejeune MM, Hofman PA, et al. Increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension. 2008;52:1120–6. doi: 10.1161/HYPERTENSIONAHA.108.119024. [DOI] [PubMed] [Google Scholar]
- Kearney-Schwartz A, Rossignol P, Bracard S, Felblinger J, Fay R, Boivin JM, et al. Vascular structure and function is correlated to cognitive performance and white matter hyperintensities in older hypertensive patients with subjective memory complaints. Stroke. 2009;40:1229–36. doi: 10.1161/STROKEAHA.108.532853. [DOI] [PubMed] [Google Scholar]
- Kelly R, Fitchett D. Noninvasive determination of aortic input impedance and external left ventricular power output: a validation and repeatability study of a new technique. J Am Coll Cardiol. 1992;20:952–63. doi: 10.1016/0735-1097(92)90198-v. [DOI] [PubMed] [Google Scholar]
- Laurent S, Boutouyrie P, Asmar R, Gautier I, Laloux B, Guize L, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37:1236–41. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- Laurent S, Katsahian S, Fassot C, Tropeano AI, Gautier I, Laloux B, et al. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 2003;34:1203–6. doi: 10.1161/01.STR.0000065428.03209.64. [DOI] [PubMed] [Google Scholar]
- Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–63. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- Meaume S, Benetos A, Henry OF, Rudnichi A, Safar ME. Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of age. Arterioscler Thromb Vasc Biol. 2001;21:2046–50. doi: 10.1161/hq1201.100226. [DOI] [PubMed] [Google Scholar]
- Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol. 2008;105:1652–60. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GF, Gudnason V, Launer LJ, Aspelund T, Harris TB. Hemodynamics of increased pulse pressure in older women in the community-based Age, Gene/Environment Susceptibility-Reykjavik Study. Hypertension. 2008;51:1123–8. doi: 10.1161/HYPERTENSIONAHA.107.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GF, Guo CY, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, et al. Cross-sectional correlates of increased aortic stiffness in the community: the Framingham Heart Study. Circulation. 2007;115:2628–36. doi: 10.1161/CIRCULATIONAHA.106.667733. [DOI] [PubMed] [Google Scholar]
- Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010a;121:505–11. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GF, Izzo JL, Jr, Lacourciere Y, Ouellet JP, Neutel J, Qian C, et al. Omapatrilat reduces pulse pressure and proximal aortic stiffness in patients with systolic hypertension: results of the conduit hemodynamics of omapatrilat international research study. Circulation. 2002;105:2955–61. doi: 10.1161/01.cir.0000020500.77568.3c. [DOI] [PubMed] [Google Scholar]
- Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004a;43:1239–45. doi: 10.1161/01.HYP.0000128420.01881.aa. [DOI] [PubMed] [Google Scholar]
- Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF, Jr, et al. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension. 2004b;44:134–9. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- Mitchell GF, Vita JA, Larson MG, Parise H, Keyes MJ, Warner E, et al. Cross-sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness: the Framingham Heart Study. Circulation. 2005;112:3722–8. doi: 10.1161/CIRCULATIONAHA.105.551168. [DOI] [PubMed] [Google Scholar]
- Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, et al. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation. 2010b;122:1379–86. doi: 10.1161/CIRCULATIONAHA.109.914507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgo JP, Westerhof N, Giolma JP, Altobelli SA. Aortic input impedance in normal man: relationship to pressure wave forms. Circulation. 1980;62:105–16. doi: 10.1161/01.cir.62.1.105. [DOI] [PubMed] [Google Scholar]
- Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46:454–62. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997;28:652–9. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- Poels MM, van Oijen M, Mattace-Raso FU, Hofman A, Koudstaal PJ, Witteman JC, et al. Arterial stiffness, cognitive decline, and risk of dementia: the Rotterdam study. Stroke. 2007;38:888–92. doi: 10.1161/01.STR.0000257998.33768.87. [DOI] [PubMed] [Google Scholar]
- Saczynski JS, Jonsdottir MK, Sigurdsson S, Eiriksdottir G, Jonsson PV, Garcia ME, et al. White matter lesions and cognitive performance: the role of cognitively complex leisure activity. J Gerontol A Biol Sci Med Sci. 2008;63:848–54. doi: 10.1093/gerona/63.8.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saczynski JS, Sigurdsson S, Jonsdottir MK, Eiriksdottir G, Jonsson PV, Garcia ME, et al. Cerebral infarcts and cognitive performance: importance of location and number of infarcts. Stroke. 2009;40:677–82. doi: 10.1161/STROKEAHA.108.530212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse T, Babcock R. Decomposing adult age differences in executive function. Developmental Psychology. 1991;27:763–76. [Google Scholar]
- Schaie KW, Maitland SB, Willis SL, Intrieri RC. Longitudinal invariance of adult psychometric ability factor structures across 7 years. Psychol Aging. 1998;13:8–20. doi: 10.1037/0882-7974.13.1.8. [DOI] [PubMed] [Google Scholar]
- Scher AI, Gudmundsson LS, Sigurdsson S, Ghambaryan A, Aspelund T, Eiriksdottir G, et al. Migraine headache in middle age and late-life brain infarcts. JAMA. 2009;301:2563–70. doi: 10.1001/jama.2009.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuteri A, Tesauro M, Appolloni S, Preziosi F, Brancati AM, Volpe M. Arterial stiffness as an independent predictor of longitudinal changes in cognitive function in the older individual. J Hypertens. 2007;25:1035–40. doi: 10.1097/HJH.0b013e3280895b55. [DOI] [PubMed] [Google Scholar]
- Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clinical Gerontology: a guide to assessment and intervention. New York: The Haworth Press; 1986. pp. 165–73. [Google Scholar]
- Stroop J. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–62. [Google Scholar]
- Sutton-Tyrrell K, Najjar SS, Boudreau RM, Venkitachalam L, Kupelian V, Simonsick EM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–90. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- van Elderen SG, Brandts A, Westenberg JJ, van der GJ, Tamsma JT, van Buchem MA, et al. Aortic stiffness is associated with cardiac function and cerebral small vessel disease in patients with type 1 diabetes mellitus: assessment by magnetic resonance imaging. Eur Radiol. 2010;20:1132–8. doi: 10.1007/s00330-009-1655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal JS, Sigurdsson S, Jonsdottir MK, Eiriksdottir G, Thorgeirsson G, Kjartansson O, et al. Coronary artery calcium, brain function and structure: the AGES-Reykjavik Study. Stroke. 2010;41:891–7. doi: 10.1161/STROKEAHA.110.579581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51:99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. Manual. New York: The Psychological Corporation; 1955. [Google Scholar]
- Willum-Hansen T, Staessen JA, Torp-Pedersen C, Rasmussen S, Thijs L, Ibsen H, et al. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–70. doi: 10.1161/CIRCULATIONAHA.105.579342. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Bennett DA, Beckett LA, Morris MC, Gilley DW, Bienias JL, et al. Cognitive activity in older persons from a geographically defined population. J Gerontol B Psychol Sci Soc Sci. 1999;54:155–60. doi: 10.1093/geronb/54b.3.p155. [DOI] [PubMed] [Google Scholar]
- Zijdenbos AP, Forghani R, Evans AC. Automatic "pipeline" analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging. 2002;21:1280–91. doi: 10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.