Abstract
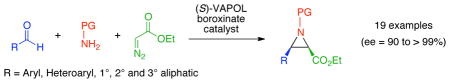
The first multi-component catalytic asymmetric aziridination reaction is developed to give aziridine-2-carboxylic esters with very high diastereo- and enantioselectivity from aromatic and aliphatic aldehydes. This new method pushes the boundary of the aziridination reaction to substrates that failed with pre-formed imines.
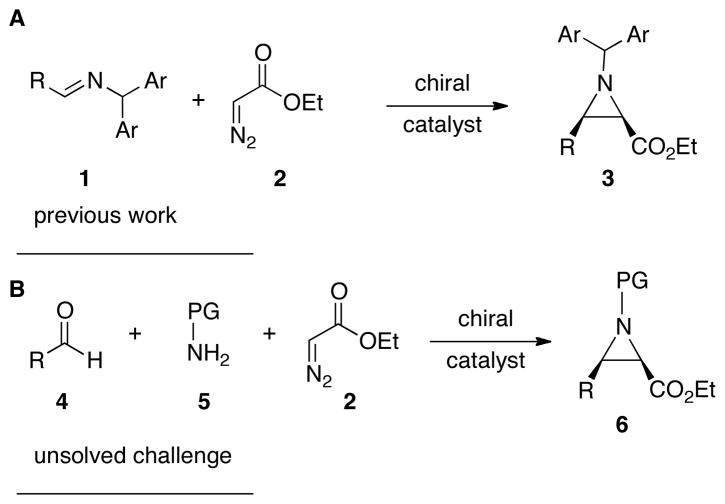
In recent times, multicomponent reactions have been quite extensively studied and applied in the field of asymmetric catalysis.1 Over the past few years, considerable advances have been made in the field of catalytic asymmetric aziridination.2 However, to the best of our knowlegde, no example of a multi-component catalytic asymmetric aziridination has been reported.1,3–5 A true multi-component reaction involves the reaction of three or more reagents added simultaneously.1 Multicomponent reactions that involve reaction between two reagents and then interception of the resulting intermediate by the addition of a third reagent are sequential component reactions.1,4 Herein, we report the first multi-component catalytic asymmetric aziridination (MCAZ) which incorporates the most simplified protocol yet developed for our catalytic asymmetric aziridination reaction (Figure 1).
Figure 1.
A: catalytic asymmetric aziridination of imines. B: three-component catalytic asymmetric aziridination.
We have previously developed chiral catalysts for the asymmetric synthesis of aziridines from the reaction of diazo compounds and imines (AZ reaction) (Figure 1A).6
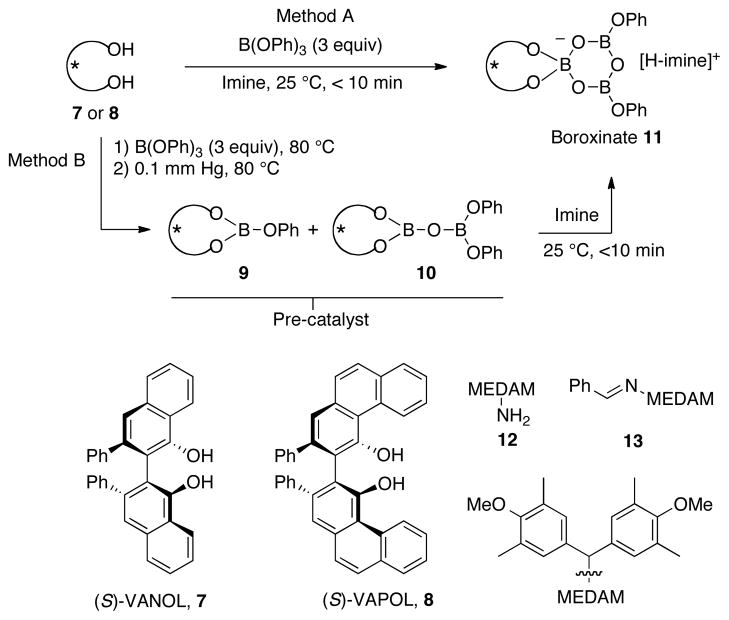
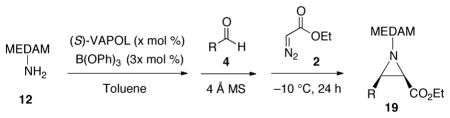
With chiral Brønsted acid catalysts generated from the VANOL and VAPOL ligands (Scheme 1), it is possible to obtain aziridine-2-carboxylates of the type 3 with very high enantioselectivities and diastereo-selectivities. The highest stereoselectivities are typically delivered by imines derived from the amine 12 which bears a bis-(dimethylanisyl)methyl group (MEDAM).6e The catalyst for the AZ reaction has been identified as the novel boroxinate species 11 which is an ion-pair consisting of a boroxinate anion and a protonated iminium.6d,g,i The boroxinate catalyst is typically generated with either of two methods (Scheme 1). Heating VANOL or VAPOL with B(OPh)3 and then removing volatiles under vacuum with heating generates a mixture of 9 and 10 as a pre-catalyst, both of which are converted to the boroxinate upon treatment with the imine at ambient temperature (Method B). Although B(OPh)3 and VAPOL are inert to each other at room temperature, the boroxinate is formed immediately upon addition of imine (Method A).6i
Scheme 1.
Methods for Catalyst Formation.
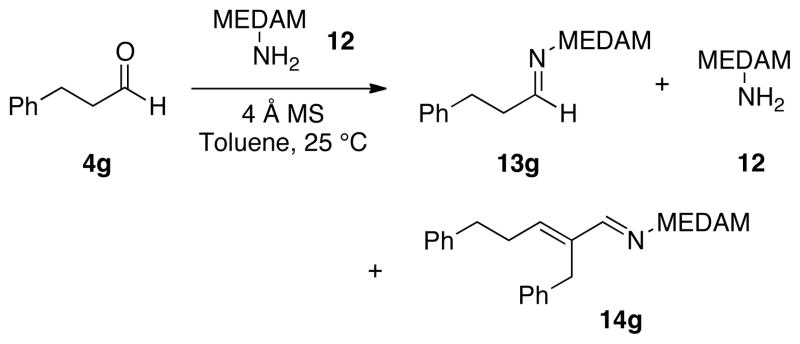
Up to this point, all of the aziridination reactions we have published have involved pre-formed imines. A drawback to the use of imines of course is the requirement of an additional step. The difficulty in the purification of imines can vary from drawback to limitation. The method of choice for purification is crystallization since most imines tend to decompose on silica gel or upon distillation. For non-crystalline imines this usually necessitates the use of non-purified imines. This can be an serious problem, especially for unbranched aliphatic aldehydes where in many cases no aziridine product is observed at all.7 We found in these cases, the imine can’t be generated in a clean fashion. For example, when aldehyde 4g is treated with amine 12, long before complete formation of the imine 13g can be realized, self-condensation of the imine begins to occur which gives rise to the conjugated imine 14g (Scheme 2).8 This mixture of species appears to kill the catalyst and this may very well be due to the amine 12 which is released in the formation of 14g. This imposes a serious limitation on the implimentation of the AZ reaction and the molecules in Figure 2 are illustrative of the point.9
Scheme 2.
Self-condensation of Imine 13g.
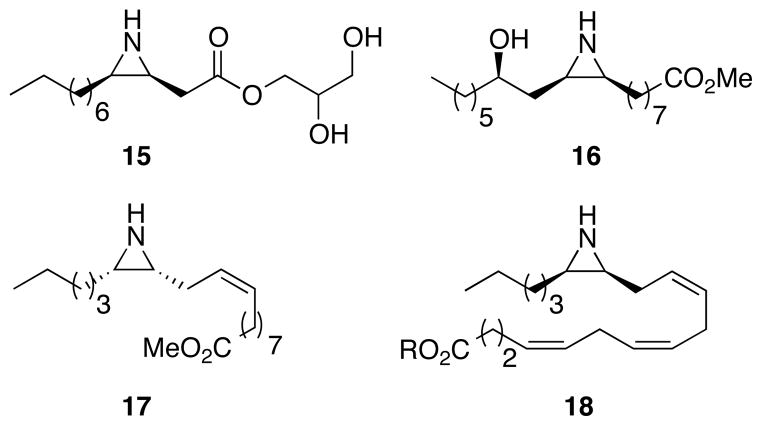
Figure 2.
Biologically Important Alkyl Aziridines. 15: antimicrobial activity against Gram-positive bacteria.9b 16 and 17: cytotoxic and antimicrobial activity.9c 18: inhibitor of arachidonate epoxygenase.9d
Given the greater basicity of an amine versus an imine, it was anticipated that the MEDAM amine 12 could also generate the boroxinate catalyst from VAPOL and B(OPh)3 in much the same way as does imine 13a (Scheme 1) and indeed this was confirmed by NMR analysis (see supporting information). What was not clear was whether the amine would be subsequently converted completely to the imine such that the more basic amine won’t tie up the catalytst and stop the reaction.
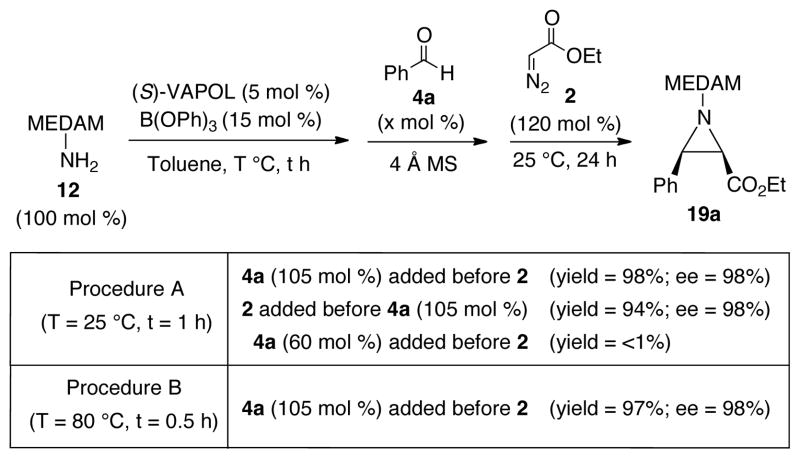
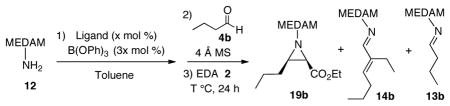
Given the problems with aliphatic aldehydes described above, the multi-component aziridination was first examined with benzaldehyde (Scheme 3). The VAPOL ligand was mixed with 3 equiv of B(OPh)3 and 20 equiv of amine 12 and stirred in toluene at 80 °C for 0.5 h to ensure complete formation of the boroxinate catalyst. This was followed by the addition of 4 Å MS and benzaldehyde. After subsequent addition of EDA 2, the resulting mixture was stirred at 25 °C for 24 h to give the aziridine 19a in 97% yield and 98% ee (Procedure B, Scheme 3). It was also found that generating the catalyst at 25 °C for 1 h gave the same results. (Procedure A, Scheme 3). Very similar results were obtained if the diazo compound 2 was added before the aldehyde 4a and this reveals that this is a true multi-component reaction since imine formation can occur in the presence of all other components.10 Interestingly, the reaction stops if 0.6 equiv of 4a is added (Scheme 3) which suggests that the amine can kill the catalyst which is consistent with the failure of pre-formed imine 13g to give any aziridine (Scheme 2).
Scheme 3.
Multicomponent AZ reaction of Benzaldehyde 4a.
The problem of imine self-condensation was encountered in the multi-component aziridination (MCAZ) of n-butanal 4b (Table 1). The three-component aziridination of n-butanal with MEDAM amine 12 at room temperature with 5 mol % VANOL catalyst gave only a 25% yield of aziridine 19b along with a 20% yield of the condensation product 14b. However, the formation of the side-product 14b was found to be disfavored at lower temperatures (entry 2). The yield was increased to 74% at 0 °C with 10 mol % catalyst (entry 4). With 5 mol% catalyst, increased yields up to 94% were observed with increased amounts of ethyl diazoacetate 2 (entries 8 and 9).11 Finally, it was found that reaction with 3 mol % catalyst and 2 equiv EDA provides 2.4 g (5.5 mmol) of aziridine 19b in 92% yield and 96% ee (entry 12).
Table 1.
Optimization of the Multicomponent AZ reaction for n-butanal 4b.a
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| entry | ligand | proc | catalyst (x mol %) | T (°C) | equiv 2 | yield % 19bb | ee % 19bc | yield % 14bd | yield % 13bd |
| 1 | (R)-VANOL | A | 5 | 25 | 1.2 | 25d | nd | 20 | 38 |
| 2 | (R)-VANOL | A | 5 | 0 | 1.2 | 50d | nd | 6 | 21 |
| 3 | (R)-VANOL | B | 5 | 0 | 1.2 | 53d | nd | 6 | 17 |
| 4 | (R)-VANOL | B | 10 | 0 | 1.2 | 74 | −95 | 2 | < 1 |
| 5 | (R)-VANOL | B | 10 | −10 | 1.2 | 80 | −96 | < 1 | < 1 |
| 6 | (R)-VANOL | B | 10 | −30 | 1.2 | 45e | −96 | 11 | 30 |
| 7 | (S)-VAPOL | B | 10 | −10 | 1.2 | 82 | 98 | < 1 | < 1 |
| 8 | (S)-VAPOL | B | 5 | −10 | 8 | 91 | 96 | < 1 | < 1 |
| 9 | (S)-VAPOL | A | 5 | −10 | 8 | 94 | 96 | < 1 | < 1 |
| 10 | (S)-VAPOL | A | 5 | −10 | 3 | 85f | 96 | < 1 | < 1 |
| 11 | (S)-VAPOL | A | 4 | −10 | 6 | 97g | 95 | < 1 | < 1 |
| 12 | (S)-VAPOL | A | 3 | −10 | 2 | 92 | 96 | < 2 | < 2 |
Unless otherwise specified, all reactions were performed with 0.5 mmol amine 12 (0.5 M) and 1.05 equiv of n-butanal 4b and 1.2 equiv EDA 2 with Procedure A or B (see Scheme 3) and went to 100% completion. nd = not determined.
Isolated yield.
Determined by chiral HPLC.
Yield determined by 1H NMR with Ph3CH as internal standard.
Reaction went to 61% completion.
Reaction on 2.5 mmol scale.
Reaction on 6 mmol scale.
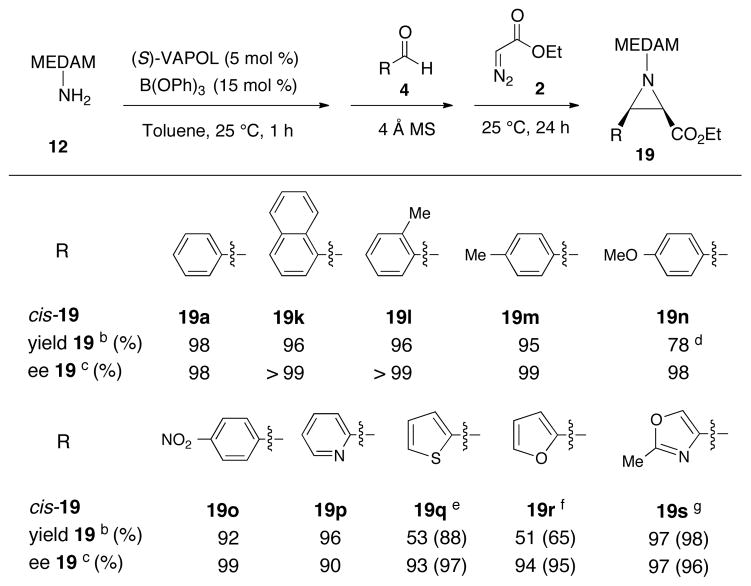
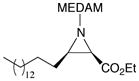
A series of eight additional aliphatic aldehydes were screened in the multi-component aziridination reaction (Table 2). Excellent asymmetric inductions were observed for the aziridination of a number of functionalized unbranched, α-branched and α,α-branched aliphatic aldehydes. These include a number of “problem” aldehydes that have failed to give aziridines via in-situ generated imines such as dihydrocinnamyl aldehyde 4g, phenyl acetaldehyde 4h and ethyl-5-oxopentanoate 4e due to the problem pertaining to the condensation product (Scheme 2). 7,8 Even with the three-component protocol, the aziridination of the “problem” aldehyde 4g proceeds to give only a 24% yield of the aziridine 19g (entry 8). The yield can be increased to 96% if excess (8 equiv) of EDA is used and this is interpreted to mean that the imine undergoes aziridination before it can self condense (entry 9).11 The MCAZ is also readily scalable as aziridine 19c can be obtained with essentially the same induction and in slightly higher yield when the scale is increased ten-fold (Table 2, entries 1 and 2). It was observed that excellent results could obtained for 2° and 3° aliphatic aldehydes at room temperature. (Table 2).
Table 2.
Multicomponent AZ reaction of Alkyl Aldehydes. a
 | ||||||
|---|---|---|---|---|---|---|
| entry | proc | catalyst (x mol %) | equiv 2 | yield % 19 b | ee % 19 c | aziridine 19 |
| 1 | B | 10 | 1.2 | 80 | 96 |
 19c |
| 2 | B | 10 | 3 | 85 | 95d | |
| 3 | B | 10 | 1.2 | 80 | 98 |
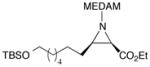 19d |
| 4 | B | 10 | 1.2 | 70 | 94 |
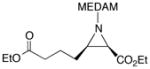 19e |
| 5 | A | 5 | 8 | 82 | 97 | |
| 6 | B | 10 | 1.2 | 55 | 93 |
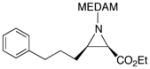 19f |
| 7 | A | 5 | 8 | 50 | 90 | |
| 8 | B | 10 | 1.2 | 24e | nd |
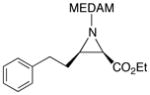 19g |
| 9 | B | 10 | 8 | 96 | 97 | |
| 10 | A | 5 | 8 | 91 | 96 | |
| 11 | B | 10 | 1.2 | 19e | nd |
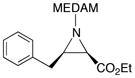 19h |
| 12 | B | 10 | 8 | 94 | 98 | |
| 13 | A | 5 | 8 | 86 | 98 | |
| 14f | A | 5 | 1.2 | 95 | 90 |
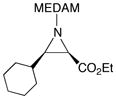 19i |
| 15f | B | 10 | 1.2 | 89 | 94 |
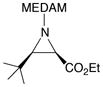 19j |
| 16f | A | 10 | 2 | 95 | 92 | |
| 17f | A | 10 | 8 | 97 | 90 | |
Unless otherwise specified, all reactions were performed with 0.5 mmol of amine 12 (0.5 M) with 1.05 equiv aldehyde 4 and 1.2 equiv EDA 2 with Procedure A or B (see Scheme 3) and went to 100% completion with a cis/trans ratio of >50:1.
Isolated yield.
Determined by chiral HPLC.
Reaction on 5.0 mmol scale.
Yield determined by 1H NMR with Ph3CH as standard; nd = not determined.
Reaction at 25 °C.
A survey of the scope of the multi-component protocol for aromatic aldehydes is given in Scheme 4. Excellent yields and asymmetric inductions were observed for a number of substituted benzaldehydes including those with both electron-withdrawing and electron-donating groups. The reaction with benzaldehyde 4a was complete in 1 h with 5 mol % catalyst by 1H NMR.12 Excellent inductions could also be obtained with heteroaryl aldehydes giving aziridines 19p – 19s in 90–97% ee.11,13
Scheme 4.
Multicomponent AZ reaction of Aryl Aldehydes.a
a Unless otherwise specified, all reactions were performed as described in Table 2 with 1.2 equiv of 2 with Procedure A and went to 100% completion and gave aziridine 19 with a cis/trans ratio of >50:1. Data in parentheses is with 8 equiv EDA 2. b Isolated yield. c Determined by chiral HPLC. d cis/trans = 20:1. e cis/trans = 25:1 with 8 equiv EDA. f cis/trans = 8.3:1 with 8 equiv EDA. g Reaction with 2 equiv EDA 2.
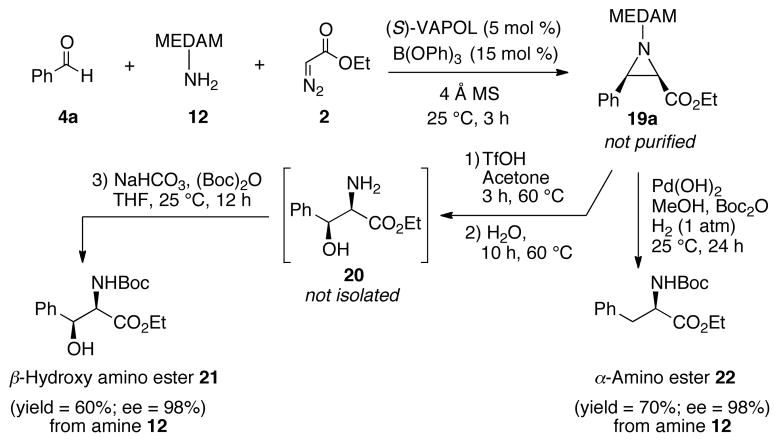
The synthetic utility of MCAZ reaction is illustrated by the direct transformation of aldehyde 4a to α-amino esters 21 and 22 in a one-pot fashion. The crude aziridine 19a obtained from MCAZ reaction is subjected to deprotection followed by water assisted ring opening and boc protection of free amine in 20 to afford β-hydroxy-α- amino ester 21 in very high optical purity in 60% overall yield from amine 12. Alternatively, the crude aziridine 19a can be treated under reductive deprotection and ringopening conditions to afford α-amino ester 22 in high optical purity and in 70% overall yield from amine 12.
In summary, a highly robust multicomponent catalytic asymmetric aziridination reaction has been realized.14 This method incorporates a very simplified protocol and it provides an effective solution to the long-standing problem with imines from unbranched aliphatic aldehydes in two-component methods. The fact that the imines are generated in-situ permits introduction of functionality in the aziridine not possible with two-step methods.
Supplementary Material
Scheme 5.
One-pot Synthesis of amino esters 21 and 22.
Acknowledgments
This work was supported by NSF Grant CHE-0750319 and the National Institute of General Medical Sciences (GM094478).
Footnotes
Supporting Information Available Synthetic procedures and spectral data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Guillena G, Ramon DJ, Yus M. Tetrahedron-Asymmetry. 2007;18:693–700. [Google Scholar]; (b) Ramon DJ, Yus M. Angew Chem Int Ed. 2005;44:1602–1634. doi: 10.1002/anie.200460548. [DOI] [PubMed] [Google Scholar]; (c) Zhu J, Bienayme H, editors. Multicomponent Reactions. Wiley-VCH; 2005. [Google Scholar]
- 2.Pellissier H. Tetrahedron. 2010;66:1509. [Google Scholar]
- 3.For multi-component aziridinations with non-chiral catalysts, see: Nagayama S, Kobayashi S. Chem Lett. 1998:685–686.Kubo T, Sakaguchi S, Ishii Y. Chem Commun. 2000:625–626.Yadav JS, Reddy BVS, Rao MS, Reddy PN. Tetrahedron Lett. 2003;44:5275–5278.Yadav JS, Reddy BVS, Reddy PN, Rao MS. Syn. 2003:1387–1390.Ishii Y, Sakaguchi S. Bull Chem Soc Jpn. 2004;77:909–920.
- 4.For a sequential component aziridination with a chiral catalyst, see: Akiyama T, Suzuki T, Mori K. Org Lett. 2009;11:2445–2447. doi: 10.1021/ol900674t.
- 5.For a stoichiometric asymmetric multi-component aziridination, see: Minakata S, Ando T, Nishimura M, Ryu I, Komatsu M. Angew Chem Int Ed. 1998;37:3392–3394. doi: 10.1002/(SICI)1521-3773(19981231)37:24<3392::AID-ANIE3392>3.0.CO;2-G.Nishimura M, Minakata S, Takahashi T, Oderaotoshi Y, Komatsu M. J Org Chem. 2002;67:2101–2110. doi: 10.1021/jo016146d.
- 6.(a) Zhang Y, Desai A, Lu Z, Hu G, Ding Z, Wulff WD. Chem–Eur J. 2008;14:3785–3803. doi: 10.1002/chem.200701558. [DOI] [PubMed] [Google Scholar]; (b) Zhang Y, Lu Z, Desai A, Wulff WD. Org Lett. 2008;8:5429–5432. doi: 10.1021/ol802431v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zhang Y, Lu Z, Wulff WD. SynLett. 2009:2715–2739. [Google Scholar]; (d) Hu G, Huang L, Huang RH, Wulff WD. J Am Chem Soc. 2009;131:15615–15617. doi: 10.1021/ja904589k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Mukherjee M, Gupta AK, Lu Z, Zhang Y, Wulff WD. J Org Chem. 2010;75:5643–5660. doi: 10.1021/jo101160c. [DOI] [PubMed] [Google Scholar]; (f) Desai AA, Wulff WD. J Am Chem Soc. 2010;132:13100–13103. doi: 10.1021/ja1038648. [DOI] [PubMed] [Google Scholar]; (g) Vetticatt MJ, Desai AA, Wulff WD. J Am Chem Soc. 2010;132:13104–13107. doi: 10.1021/ja103863j. [DOI] [PubMed] [Google Scholar]; (h) Ren H, Wulff WD. Org Lett. 2010;12:4908–4911. doi: 10.1021/ol102064b. [DOI] [PubMed] [Google Scholar]; (i) Hu G, Gupta AK, Huang RH, Mukherjee M, Wulff WD. J Am Chem Soc. 2010;132:14669–14675. doi: 10.1021/ja1070224. [DOI] [PubMed] [Google Scholar]
- 7.Wulff WD, Antilla JA, Pulgam VR, Zhang Y, Gilson-Osminksi W. unpublished results. [Google Scholar]
- 8.For a related self-condensation of a DAM imine, see reference 6e.
- 9.Ismail FMD, Levitsky DO, Dembitsky VM. Eur J Med Chem. 2009;44:3373. doi: 10.1016/j.ejmech.2009.05.013.and references therein Kabara JJ, Vrable R, Lie Ken Jie MSF. Lipids. 1977;12:753–759. doi: 10.1007/BF02570908.Metzger JO, Fürmeier S. Eur J Org Chem. 1999:661–664.Falck JR, Manna S, Viala J, Siddhanta AK, Moustakis CA, Capdevila J. Tetrahedron Lett. 1985;26:2287–2290.
- 10.In this experiement, EDA 2 was added with the VAPOL, B(OPh)3 and amine 12.
- 11.No attempt was made to optimize the amount of EDA.
- 12.Minimum reaction times were not determined for all substrates.
- 13.(a) We have compared the preparation of aziridine 19r by the MCAZ method described here with that of a two-step method involving the isolation of the imine from aldehyde 4r and amine 12. Substantial material loss is encountered in the two-step method as a result of purification of the imine (see supporting information). (b) pyridine-3-carboxaldehyde gave no aziridine (not shown)
- 14.Part of this work was presented at the 240th American Chemical Society National Meeting, Boston (2010), ORGN 636.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.