The cardiovascular community understands that the QT interval is the surface electrocardiographic representation of ventricular repolarization. However, despite tremendous advances in our understanding of the molecular and cellular basis for cardiac repolarization and its surface ECG representation, clinicians, regulators, and drug developers are increasingly faced with uncertainty over the best way to interpret this parameter. The evidence described below demonstrates that
Prolongation of the QT interval is a reliable predictor of ventricular arrhythmias in some settings, and may be less predictable in others
The arrhythmogenic potential of a given degree of QT prolongation varies among individuals and drugs. Further, the relationship between QT prolongation and arrhythmia susceptibility is non-linear. Multiple mechanisms may underlie QT prolongation, and these may carry different arrhythmogenic potential.
There is little doubt that further understanding of the cellular and molecular mechanisms underlying repolarization hold the promise of developing better indices of the arrhythmogenic potential of abnormalities of repolarization, but that science has not yet matured
A caveat: The QT interval is hard to measure
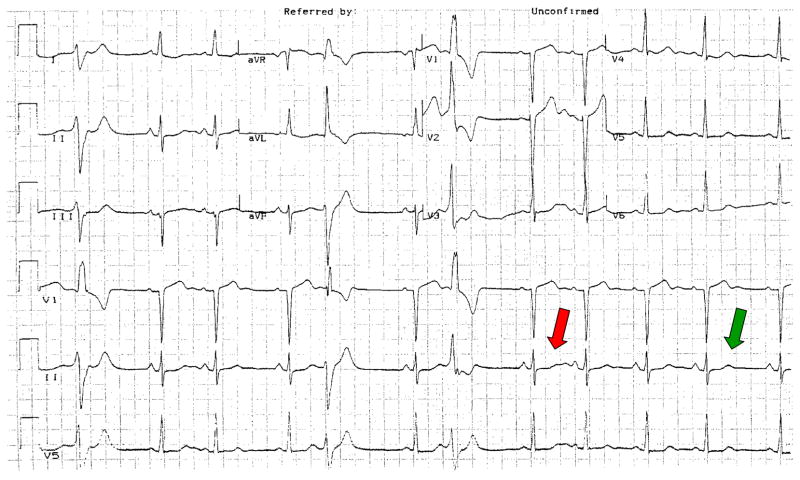
Measuring the QT is hard enough when it is normal, and it is almost impossible to come up with a workable number in the face of common disease-associated ECG changes such as diffuse ST segment changes, inverted T-waves, small or large U-waves, or pauses (see Figure 1). A second problem is rate: while there is absolutely no doubt that action potential durations in ventricular tissue, and the QT interval, are exquisitely dependent on previous cycle length or cycle lengths, the “best” method to correct for rate, and thus allow QT intervals recorded at different rates to be compared, is not known. Indeed, comparing two “rate-corrected” QT values presupposes that whatever the intervention between the two recordings – often a drug – itself has no effect on rate. Further, even if a drug has no direct effect on rate, common conditions for which drugs are prescribed may be associated with increases in heart rate (e.g. due to fever or to autonomic activation); any effective drug may thus affect heart rate indirectly.
Figure 1.
Which QT predicts arrhythmias? The complex indicated by the green arrow is at steady state, and normal. However, the complex indicated in red, which follows a pause, displays grotesque deformity. This example reinforces the concept that the answer to the “controversy” of whether the QT should be retained or jettisoned as an indicator of arrhythmias depends on what we now know – and what we need to learn – about its physiology and pathophysiology: discarding the QT as a measure of arrhythmia susceptibility in his case because the steady state QT is normal would be a mistake.
QT interval prolongation is associated with arrhythmias
The initial associations between striking QT prolongation in a range of settings and an increased susceptibility to arrhythmias and sudden death were first established in the 1950s, 60s, and 70s. However, these examples – congenital syndromes, AV block, drug-induced torsades – remained electrophysiologic curiosities until the late 1980s, when the problem of terfenadine-associated QT prolongation and torsades de pointes first caught the eye of regulatory agencies including the Food and Drug Administration (FDA). At that time, almost 100 million prescriptions of terfenadine had been written, and the drug was being considered for over-the-counter status. The initial case report and subsequent review of the FDA database established that the drug could produce dramatic QT prolongation and torsades de pointes and/or sudden death, and that the vast majority of cases had demonstrable co-morbidities, including drug interactions, advanced liver disease, or overdose.1 In fact, small clinical trials in the early 1990s mounted to characterize the effect of terfenadine in normal individuals and in patients with heart disease were punctuated by QT prolongation (exceeding 600 msec) and deformity, and at least one sudden death.2,3 The mechanism underlying terfenadine-induced QT prolongation and torsades is now reasonably well understood: the drug is a potent IKr blocker (the common mechanism for all drug-induced torsades) that undergoes near-complete presystemic bio-inactivation, so conditions that inhibit or avoid that metabolism, such as drug interactions, liver disease, overdose, lead to systemic accumulation of high drug concentrations.
Another setting in which QT prolongation has been incontrovertibly associated with arrhythmia risk is the congenital long QT syndromes. In a large series reported by Priori et al,4 mutation carriers with conventionally rate-corrected QT intervals in the lowest quartile (<446 ms) had a forty-year event rate under 20%, compared to an event rate of almost 80% in the highest quartile (>499 ms).
Finally, an extensive dataset from many clinical settings has implicated QT prolongation as a predictor of subsequent mortality; these include convalescence from acute myocardial infarction,5 diabetes, advancing age, chronic heart failure, autonomic dysfunction, hypertrophic cardiomyopathy, patients awaiting heart transplantation, antihypertensive therapy, and muscular dystrophy.
Variable arrhythmogenic potential of QT prolongation
Antiarrhythmic drugs that block IKr and prolong the QT interval (sotalol, dofetilide, ibutilide, and others) carry a risk of torsades de pointes. For these drugs, available evidence indicates that both torsades risk and QT interval prolongation by the drugs are dose-related.6–8 While these relationships speak to links among drug dose, IKr block, QT prolongation, and torsades risk, they should not be over-interpreted. Specifically, it is clear that the extent of arrhythmia risk with a given drug dose or degree of QT prolongation varies among individuals, reflecting both known factors such as comedications, pauses, or underlying rhythm9 as well as undetermined factors, including possible genetic contributors. Indeed, establishing a tight relationship between steady-state QT and torsades risk borders on the impossible, for two reasons. First, the arrhythmia frequently occurs after a pause, and post-pause beats are those most likely to display variability in duration and in morphology (e.g. Figure 1); a patient may have only modest QT prolongation during sinus rhythm but display obvious post-pause changes, and it is the pause and the electrophysiologic events that accompany it that generate the arrhythmia. Second, it seems clear that the relationship between QT prolongation and arrhythmia risk – if one could even be crudely quantified – is highly non-linear. That is, if a 200 msec prolongation confers a high risk, say 50%, with a given drug, it cannot be assumed that a 20 msec prolongation confers a 5% risk, or a 2 msec prolongation a 0.5% risk. Risks at such lower degrees of QT prolongation are difficult to quantify, but seem extremely small based on experience with drugs such as moxifloxacin or ziprasidone. These drugs do prolong QT by 10–20 msec, but cause torsades extremely rarely (perhaps <1/100,000).
While there is a rough relationship between drug dose and risk of torsades de pointes with pure IKr blockers, the situation is murkier for IKr blocking drugs with other important electrophysiologic effects: amiodarone, verapamil, and ranolazine fall into this category. Every clinician who uses amiodarone understands that “not all QT interval prolongation is created equally”: amiodarone very rarely causes torsades despite prolonging the QT. A “chain of evidence” linking IKr block to torsades de pointes includes not only drug interactions with the channel itself, but also the extent to which other electrophysiologic mechanisms modulate QT interval or the relative importance of IKr in overall repolarization, and presumably other factors such as cell–cell coupling, inter-individual variability in anatomy (that might determine susceptibility to reentry), underlying rhythm,9 the details of time- and voltage-dependence of IKr block,10 and drug access to its target site on the channel via passive or active transport mechanisms. It is especially important to note that interventions that prolong action potentials, such as block of IKr or IKs, or augmentation of plateau INa, do not, themselves, generate arrhythmias. Rather, they enable the action potential trajectory to enter into a region of voltage–time space in which arrhythmogenic inward currents (L-type calcium currents, or sodium–calcium exchangers seem likely candidates) are engaged, thereby further prolonging action potentials and contributing to triggered upstrokes. To the extent that any of these responses are heterogeneous among cells in the myocardium, this positive feedback loop serves to reinforce heterogeneities of repolarization and thus, reentry potential. It is conceivable that a drug that blocked the channel potently at depolarized potentials, but rapidly came off the channel during repolarization could prolong action potentials without allowing them to spend sufficient time in the region of voltage–time space to enable arrhythmogenic inward currents. This is speculative.
Moving forward: questions that need answering
Available data from studies of drug action in human beings as well as in the congenital long QT syndrome support the contention that QT prolongation beyond some boundary predicts ventricular arrhythmias. Tracings such as those shown in Figure 1 argue against risk stratification based on a number, and ignoring underlying pathophysiology. There is an increasing body of science that is defining subtleties in the relationship between QT interval and arrhythmias, and I have no difficulty accepting the idea that these that may lead to better predictors of arrhythmias than the duration of the QT interval itself. These subtleties include:
How is the QT interval best measured, especially in disease?
Does it matter how the QT interval became prolonged? The area that has received the greatest focus is IKr block, reflecting the problem of drug-induced torsades and the burden this puts on drug development. However, the recognized association between QT interval prolongation and increased mortality in other settings may or may not reflect the same mechanisms and thus may or may not carry the same implications. Indeed, it is entirely possible that QT interval prolongation in settings such as convalescence from acute myocardial infarction is a marker of electrophysiologic instability, but itself has little to do with the direct mechanism contributing to increased mortality in that setting.
Can the same mechanism(s) that cause arrhythmias when the QT interval is prolonged also cause arrhythmias in the absence of QT interval prolongation?
Can we describe the relationship between the extent of QT interval prolongation (by a drug or by a disease) and arrhythmia risk? Is there a “zero-risk” level?
The discussion above makes it clear that the QT interval is a predictor of ventricular arrhythmias. Is it perfect? By no means. But it would be a terrible mistake for clinical medicine and for drug development to entirely jettison this marker until new science has evolved and been validated to provide comfort and superiority in the many settings in which QT interval measurements are used.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Woosley RL, Chen Y, Freiman JP, et al. Mechanism of the cardiotoxic actions of terfenadine. JAMA. 1993;269:1532–1536. [PubMed] [Google Scholar]
- 2.Honig PK, Wortham DC, Zamani K, et al. Terfenadine-ketoconazole interaction. Pharmacokinetic and electrocardiographic consequences. JAMA. 1993;269:1513–1518. [PubMed] [Google Scholar]
- 3.Pratt CM, Ruberg S, Morganroth J, et al. Dose-response relation between terfenadine (Seldane) and the QTc interval on the scalar electrocardiogram: distinguishing a drug effect from spontaneous variability. Am Heart J. 1996;131:472–480. doi: 10.1016/s0002-8703(96)90525-6. [DOI] [PubMed] [Google Scholar]
- 4.Priori SG, Schwartz PJ, Napolitano C, et al. Risk Stratification in the Long-QT Syndrome. N Engl J Med. 2003;348:1866. doi: 10.1056/NEJMoa022147. [DOI] [PubMed] [Google Scholar]
- 5.Straus SM, Kors JA, De Bruin ML, et al. Prolonged QTc interval and risk of sudden cardiac death in a population of older adults. J Am Coll Cardiol. 2006;47:362–367. doi: 10.1016/j.jacc.2005.08.067. [DOI] [PubMed] [Google Scholar]
- 6.Singh S, Zoble RG, Yellen L, et al. Efficacy and safety of oral dofetilide in converting to and maintaining sinus rhythm in patients with chronic atrial fibrillation or atrial flutter: the symptomatic atrial fibrillation investigative research on dofetilide (SAFIRE-D) study. Circulation. 2000;102:2385–2390. doi: 10.1161/01.cir.102.19.2385. [DOI] [PubMed] [Google Scholar]
- 7.Soyka LF, Wirtz C, Spangenberg RB. Clinical safety profile of sotalol in patients with arrhythmias. Am J Cardiol. 1990;65:74A–81A. doi: 10.1016/0002-9149(90)90207-h. [DOI] [PubMed] [Google Scholar]
- 8.Ellenbogen KA, Stambler BS, Wood MA, et al. Efficacy of intravenous ibutilide for rapid termination of atrial fibrillation and atrial flutter: A dose-response study. J Am Coll Cardiol. 1996;28:130–136. doi: 10.1016/0735-1097(96)00121-0. [DOI] [PubMed] [Google Scholar]
- 9.Darbar D, Kimbrough J, Jawaid A, et al. Persistent atrial fibrillation is associated with reduced risk of torsades de pointes in patients with drug-induced long QT syndrome. J Am Coll Cardiol. 2008;51:836–842. doi: 10.1016/j.jacc.2007.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kamiya K, Niwa R, Mitcheson JS, et al. Molecular Determinants of hERG Channel Block. Mol Pharm. 2006;69:1709–1716. doi: 10.1124/mol.105.020990. [DOI] [PubMed] [Google Scholar]