Abstract
Saccharomyces boulardii is gaining in popularity as a treatment for a variety of diarrheal diseases as well as inflammatory bowel disease. This study was designed to examine the effect of this yeast on infection by Shigella flexneri, a highly infectious and human host-adapted enteric pathogen. We investigated key interactions between the bacteria and host cells in the presence of the yeast in addition to a number of host responses including proinflammatory events and markers. Although the presence of the yeast during infection did not alter the number of bacteria that was able to attach or invade human colon cancer-derived T-84 cells, it did positively impact the tight junction protein zonula occluden-2 and significantly increase the barrier integrity of model epithelia. The yeast also decreased ERK, JNK, and NF-κB activation in response to S. flexneri, events likely responsible for the observed reductions in IL-8 secretion and the transepithelial migration of polymorphonuclear leukocytes across T-84 monolayers. These results, suggesting that the yeast allowed for a dampened inflammatory response, were confirmed in vivo utilizing a highly relevant model of human fetal colonic tissue transplanted into scid mice. Furthermore, a cell-free S. boulardii culture supernatant was also capable of reducing IL-8 secretion by infected T-84 cells. These data suggest that although the use of S. boulardii during infection with S. flexneri may alleviate symptoms associated with the inflammatory response of the host, it would not prevent infection.
Keywords: probiotic, inflammation, neutrophil, barrier function
Shigella species are gram-negative enteric bacilli belonging to the family of Enterobacteriaceae. Infection of the human colonic mucosa by Shigella results in bacillary dysentery (shigellosis), an acute inflammatory disease characterized by abdominal cramps, fever, and severe diarrhea often containing blood and mucus. This highly infectious, host- and tissue-specific pathogen has a distinct mode of pathogenesis that involves entry into colonic epithelial cells from the basolateral surface (31), thereby requiring its relocation from the lumenal to the underlying surface of the epithelium. This translocation event has historically been attributed to the uptake and transport by M cells (44). However, it has since been established that Shigellae are also capable of altering components of the tight junctional complex, allowing the bacteria to traverse the paracellular space to reach the basolateral surface, an event that also decreases barrier function (41). Once at the basolateral surface, Shigellae rapidly invade and disseminate through the epithelium, causing a further decrease in barrier function (4, 18, 41) through the action of a type-three secretion system and additional proteins encoded on a large virulence plasmid (4, 42, 43, 46). The 220-kb virulence plasmid also encodes a number of other effector proteins that are not required for invasion but have been shown to play significant roles in instigating and balancing inflammatory processes (2, 23, 25, 40, 53).
During infection, Shigella initiates a variety of proinflammatory events that involve the activation of NF-κB (19, 36) as well as mitogen-activated protein kinases (MAPKs), including ERK and JNK (19, 24). The activation of these molecules leads to the production of various cytokines, including IL-8 (36, 48), and other signaling molecules that guide polymorphonuclear leukocytes (PMNs) (24, 27, 28) from the bloodstream to the subepithelium and, in an independent step, from the subepithelium to the lumen (27–29). The massive infiltration of PMNs into the lumen is a hallmark of shigellosis and, along with damage caused by bacterial dissemination, results in significant tissue damage and an additional loss in barrier function (3).
Saccharomyces boulardii is a thermophilic, nonpathogenic yeast shown to be effective against a variety of diarrheal diseases, including inflammatory bowel disease (15, 20) and infection by enteric pathogens. S. boulardii displays antagonistic activities against several bacterial pathogens, including Clostridium difficile (10, 17), Vibrio cholerae (16), enteropathogenic and enterohemorrhagic Esherichia coli (EPEC and EHEC, respectively) (12, 14), and Salmonella typhimurium (39). The mechanisms by which S. boulardii exerts its protective effects are numerous (see Ref. 13 for review) and have thus far been demonstrated on multiple levels of pathogenesis. Examples of such protective events include proteolytic cleavage of C. difficile toxin A and B (7, 37), inhibition of cholera toxin-stimulated cAMP during infection with V. cholerae (11), binding and elimination of cholera toxin (6), interference of bacterial-induced signaling pathways (9, 12, 14), and stabilization of tight junctional proteins and barrier integrity (12). Although these activities have been established with regard to a variety of enteric pathogens, there has been only one study to date that examined the lone effects of S. boulardii on Shigella infection (39). This study by Rodrigues et al. (39), performed using gnotobiotic mice, showed that S. boulardii provided a protective effect against Shigella flexneri, as evidenced by decreased tissue damage without a reduction in the pathogen load within the intestines (39). Although this study demonstrated a general protective effect, no specific cellular or inflammatory events were monitored.
In this study, we examined the effect(s) of S. boulardii on infection by S. flexneri utilizing highly relevant models of infection both in vitro (i.e., model intestinal epithelia comprised of polarized monolayers of human colon cells) and in vivo (human fetal intestinal xenograft model). Based on previous findings, we hypothesized that S. boulardii would attenuate Shigella-induced inflammation while not necessarily having a direct effect on the pathogen or its ability to invade host cells. Our study reveals that although S. boulardii did not reduce the number of bacteria that successfully invaded or attached to epithelial cells, it was able to ameliorate a number of host cell processes directly leading to alterations in inflammatory states and barrier integrity.
MATERIALS AND METHODS
Cell culture and T-84 monolayer preparation
The human colon cancer-derived cell line T-84 (passages 57–77) was maintained in Dulbecco’s modified Eagle’s medium (DMEM)/F-12 (Invitrogen, Carlsbad, CA) supplemented with 15 mM HEPES, 14 mM NaHCO3, 40 μg/ml penicillin, 80 μg/ml ampicillin, 90 μg/ml streptomycin, and 6% fetal calf serum. Monolayers were grown on 0.33- and 4.7-cm2 suspended collagen-coated permeable polycarbonated filters with pore sizes of 5 and 3 μm, respectively (Costar, Cambridge, MA). Inverted monolayers used for the accessibility to the basolateral surface and PMN transmigrations were constructed as previously described with cells maintained on the underside of the filters (26, 33–35). Monolayers were utilized 14 days later once having reached a confluent, polarized, and differentiated state. A steady-state transepithelial cell resistance of at least 500 Ω × cm2 was reached in all monolayers used. Before treatment or infection, monolayers were drained of media, washed extensively, and allowed to equilibrate in Hanks’ balanced salt solution (HBSS) containing Mg2+, Ca2+, and 10 mM HEPES (HBSS+; pH 7.4; Sigma, St. Louis, MO) for 30 min at 37°C.
Growth conditions and preparation of S. flexneri and S. boulardii
S. flexneri 2457T, a wild-type strain of S. flexneri serotype 2a, is invasive in both HeLa and T-84 cells (30). S. flexneri cultures were grown aerobically overnight at 37°C in Bacto tryptic soy broth (TSB; Becton Dickinson, Sparks, MD). The following morning, bacteria were diluted 1:100 into fresh TSB and grown aerobically for 2 to 2.5 h at 37°C to a midexponential phase of growth (final concentration of 4.5 × 107 bacteria/ml). Cultures of S. boulardii were inoculated from lyophilized yeast (Biocodex, Montrouge, France) and grown in Difco sabouraud dextrose broth (SDB; Becton Dickinson) in a 37°C shaking incubator. After 20 h of growth, the yeast was diluted 1:200 in fresh SDB and grown aerobically at 37°C for 40 h (final concentration of 6 × 107 yeast/ml). After incubation, bacteria and yeast were pelleted by centrifugation, washed, and suspended to their appropriate concentrations in HBSS+. S. flexneri were added to either the basolateral or apical surface of polarized T-84 monolayers at the indicated multiplicity of infection (MOI) and length of time, as noted per specific experimental procedure. For all experiments, S. boulardii was added to the apical surface during infection with S. flexneri in a ratio of 10 yeast to each epithelial cell, a ratio previously shown to have anti-inflammatory effects when used in conjunction with other bacterial pathogens (12, 14). Volumes of bacteria and/or yeast suspensions were normalized so that 25 μl and 1 ml of the appropriate suspension were added to the monolayers grown on 0.33- and 4.7-cm2 filters, respectively.
Preparation of S. boulardii supernatant
Lyophilized S. boulardii was cultured in RPMI 1640 cell culture medium for 24 h in 37°C. The suspension was centrifuged at 7,400 rpm for 15 min, and the supernatant was passed through a 0.22-mm filter (Fisher Scientific, Agawam, MA) and then fractionated through a 10-kDa filter (Millipore, Bedford, MA).
Bacterial invasion and attachment assay of T-84 intestinal epithelial monolayers
The infection of T-84 monolayers was performed as previously described (27) with a slight modification (30). Normal and inverted T-84 cell monolayers were drained of medium and gently washed with HBSS+. S. flexneri were administered to the apical or basolateral surface of T-84 cell monolayers at a MOI of 50 bacteria/epithelial cell in the presence or absence of 3.3 × 106 yeast on the apical surface and incubated for 90 min at 37°C. After being rigorously washed, the monolayers were either stored at 4°C (for associated bacteria) or treated with 480 μg of gentamicin/ml for 90 min at 37°C (for internalized bacteria). The monolayers were next washed extensively and lysed with Triton X-100 (Sigma), and the cell lysates were diluted and plated on MacConkey agar plates. As a control for direct effect(s) on S. flexneri by the yeast, S. flexneri were coincubated with S. boulardii in an Eppendorf tube at the same ratio as occurred in the experimental invasion assays (5 bacteria/yeast; 1.65 × 107 bacteria per 3.3 × 106 yeast per 3.3 × 105 epithelial cells) for 90 min. After incubation, the mixtures were diluted and plated to determine whether the yeast had any toxic effects on the bacteria. Data are expressed as colony forming units (CFU) of associated (adhered and internalized) or internalized bacteria per monolayer.
Transepithelial electrical resistance measurements
Transepithelial electrical resistance (TER) measurements were used to monitor the integrity of epithelial monolayers using a Millicell ERS Volt-ohm meter (World Precision Instruments, New Haven, CT). Only monolayers exhibiting initial TER measurements of ≥500 Ω × cm2 were used for experimentation. T-84 monolayers on 0.33-cm2 filters were infected on the apical surface with S. flexneri at a MOI of 200 in the presence or absence of S. boulardii at 37°C. TER was measured in 30-min intervals of infection/treatment up to 3 h.
Barrier integrity assay
To determine whether the yeast prevented the loss of barrier integrity during S. flexneri infection, the flux of horseradish peroxidase (HRP) across infected monolayers exposed to yeast was compared with those that were infected in the absence of yeast. T-84 polarized monolayers seeded on 0.33-cm2 filters were apically infected with S. flexneri at a MOI of 200 in the presence or absence of S. boulardii and incubated at 37°C for 10, 30, 90, and 120 min. Uninfected monolayers were treated with trypsin-EDTA as a positive control. After incubation, the monolayers were washed and transferred to fresh 24-well plates with 600 μl of HBSS+ in each well. HRP (5 μg/ml) in HBSS+ was added to the apical chamber. Monolayers were incubated for 2 h at 37°C, after which the amount of HRP that had passed into the basolateral chamber (bottom) was quantified by an HRP activity assay (22).
PMN transepithelial migration assay
The PMN transepithelial migration assay has been previously described in detail (21, 34, 35) and modified for Shigella as outlined in McCormick et al. (30). Briefly, human PMNs were purified from whole blood (anticoagulated with 13.2 g of citrate and 11.2 g of dextrose in 500 ml of water; pH 6.5) collected by venipuncture from healthy human volunteers of both sexes, in accordance with and approval of the Massachusetts General Hospital Institutional Review Board (IRB) (protocol number P-007782/7). The buffy coat was obtained by centrifugation at 400 g at room temperature. Plasma and mononuclear cells were removed by aspiration, and the majority of erythrocytes were removed by a 2% gelatin sedimentation technique (34). Residual erythrocytes were removed by lysis in cold NH4Cl lysis buffer. After isolation, PMNs were suspended at a concentration of 5 × 107 ml in modified HBSS (without Ca2+ and Mg2+) and kept at 4°C (up to 1.5 h) until used. PMNs prepared in this manner were 95% pure with 98% viability (29).
Inverted T-84 polarized monolayers seeded on 0.33-cm2 filters were basolaterally infected with 25 μl S. flexneri suspension at 37°C for 90 min (see Ref. 30 for a description of the experimental design) in the presence or absence of yeast on the apical surface. After infection, bacteria and yeast were removed by washing, and the monolayers were transferred into fresh 24-well culture trays containing 1 ml HBSS+ in the bottom chamber (apical side). HBSS+ (100 μl) was placed over the basolateral surface of the monolayers, and 20 μl of prepared PMNs (1 × 106 PMN) were added to the basolateral bath. The monolayers were incubated at 37°C for 3 h, after which the monolayers and nonmigrating PMNs were gently removed, having left only those PMNs that had traversed the monolayer into the HBSS+ contained within the apical chamber. Positive-control transmigration assays were performed by the addition of 1 μM of the potent PMN chemoattractant N-formylmethionyl-leucyl-phenylalanine (fMLP; Sigma, St. Louis, MO) to the apical chamber. Transmigration was quantified by assaying for the PMN azurophilic granule marker myeloperoxidase (MPO), as previously described (26, 34, 35).
IL-8 assay
Inverted T-84 monolayers seeded on 0.33-cm2 filters were basolaterally infected with 25 μl S. flexneri suspension for 90 min at 37°C. During infection, the monolayers were coincubated with S. boulardii or S. boulardii supernatant (SbS) on the apical surface. As a positive control, the monolayers were incubated with 1 μg/ml phorbol 12-myristate 13-acetate for 90 min. After infection, monolayers were washed and transferred to fresh 24-well trays with 1 ml HBSS+ in the well and 100 μl HBSS+ in the upper chamber (basolateral surface). The monolayers were incubated at 37°C for 5 h to allow the basolateral secretion of IL-8. After incubation, 100 μl were collected from the basolateral chamber, and IL-8 was measured per manufacturer’s instructions using the human IL-8 ELISA kit (Pierce Endogen, Rockford, IL).
Cell lysates, Western blot analysis, and antibodies
T-84 monolayers seeded on 4.7-cm2 filters were apically infected with S. flexneri in the presence or absence of S. boulardii at 37°C for 10, 30, 45, and 90 min. To examine changes in IκB, a separate set of monolayers was treated with cycloheximide (20 μg/ml) for 1 h before and during infection/treatment. After infection, monolayers were gently washed with warm HBSS+ to remove bacteria and/or yeast. Cell lysates were collected, and the proteins were electrophoresed and transferred as previously described (24). Antibodies against claudin-1 and zonula occluden (ZO)-2 were used at dilutions of 1:1,500 (Zymed, San Francisco, CA). Phosphorylated (p)ERK, pJNK1, pIκBα, ERK1, and JNK1 primary antibodies and all HRP-labeled secondary antibodies were used at dilutions of 1:1,500 (Santa Cruz Biotechnology, Santa Cruz, CA). Bands were visualized by enhanced chemiluminescence (ECL) with an ECL system (Pierce Endogen) and analyzed using ImageJ software (38).
Preparation and infection of xenografts
Human fetal colon was obtained from Brigham and Women’s Hospital (Boston, MA; mean age, 14.2 ± 1.6 wk) after therapeutic abortion. Procurement and procedures involving the xenografting of human fetal tissues into CB17 scid/scid mice were performed in accordance with the local ethics committee, with IRB approval from Massachusetts General Hospital and Brigham and Women’s Hospital (protocol number P-003833). Fetal colonic tissues were washed in DMEM and xeno-transplanted subcutaneously into the subscapular region of scid mice as described previously (47). Grafts were allowed to develop for at least 10 wk before use. Human colonic xenografts (3/treatment) were infected by direct intralumenal inoculation with 100 μl of prepared suspension containing sterile HBSS+, 1 × 107 S. flexneri alone, 5 × 104 S. boulardii alone, or a combination of 1 × 107 S. flexneri and 5 × 104 S. boulardii. At 24 h following infection, animals were euthanized, and the grafts were removed for histopathological analysis. The amount of S. flexneri was established by previous xenograft studies (51, 52), and the quantity of S. boulardii was based on the relative amount of Shigella to yeast that was used in the in vitro studies. This was the most appropriate approximation in the absence of being able to estimate the number of epithelial cells within the graft to maintain the 10 yeast per epithelial cell ratio held in the in vitro experiments.
Histological procedures
After experimentation, fetal intestinal xenograft tissues were removed from the mice, placed in optimal cutting temperature compound (Sakura, Torrance, CA), and snap frozen in liquid nitrogen. Cryosections (5 μm) were mounted on glass slides before either hematoxylin and eosin (HE) or fluorescent staining. HE staining was carried out according to the method of Chen et al. (8). Fluorescent staining was carried out as follows: slides were fixed in acetone on ice for 10 min before being submerged in a 1% bovine serum albumin (BSA) solution. The slides were then blocked by first adding an avidin solution in 1% BSA for 15 min, followed by washing and the addition of biotin in 1% BSA for an additional 15 min (Vector, Burlingame, CA). Sections were stained using a fluorescein isothiocyanate-labeled anti-mouse Ly-6G and Ly-6C (Gr-1) antibody for PMNs (BD Biosciences Pharmingen, Franklin Lakes, NJ) for 90 min. After being washed, DNA was stained and coverslips were mounted using the 4′,6′-diamidino-2-phenylindol-containing solution Vectashield (Vector).
Presentation of data and statistical analysis
PMN transmigration results are represented as mean PMN cell equivalents and SD of PMNs that had completely traversed the monolayer, as derived from a daily standard PMN dilution curve. PMN isolation was limited to repetitive donations by 10 different donors over the course of the experiments. Because of variations in both PMN and TER between monolayers (baseline resistance of between 500 and 1,000 Ω × cm2), data were analyzed within an individual experiment and not between experiments. However, the overall trends associated within an experiment are reproducible between experiments. For all other assays, values are expressed as means ± SD of the individual experiments done in triplicate at least three times. Statistical analysis was performed by Student’s t-test.
RESULTS
S. boulardii has no effect on the ability of S. flexneri to invade and attach to T-84 monolayers
It has been previously reported that S. boulardii is able to reduce the number of “intracellular” EPEC by 50% (12); therefore, we investigated the ability of the yeast to alter S. flexneri attachment and invasion into model intestinal epithelial cells. To accomplish this, 10 yeast per epithelial cell were applied to the apical surface of T-84 monolayers, a ratio previously shown to have anti-inflammatory effects when used in conjunction with other bacterial pathogens (12, 14). S. flexneri is only capable of entering host cells from the basolateral surface (31); however, when applied apically, the bacteria have been shown to transition through the paracellular space to gain access to the basolateral surface (41). Thus we examined the ability of S. boulardii to interfere with the pathogen’s route to and direct entry from the basolateral surface by applying S. flexneri apically and basolaterally in separate experiments.
There were no significant differences between the numbers of host cell-associated S. flexneri(attached and invaded bacteria) when the yeast was present during infection compared with bacteria alone. When S. flexneri alone was applied to the apical surface of the T-84 cells, 24.7 ± 1.9 × 103 CFU were able to associate with the monolayer compared with 32.1 ± 2.4 × 103 CFU/monolayer when S. boulardii was present during the infection. Similarly, when S. flexneri was applied basolaterally in the absence of the yeast, 106.0 ± 21.9 × 103 CFU attached and/or invaded each monolayer, whereas 88.0 ± 11.3 × 103 CFU associated in the presence of the yeast.
Correspondingly, no significant reductions were observed when extracellular bacteria were killed with gentamicin, thereby providing an estimate of intracellular S. flexneri only. When S. flexneri alone was applied apically, 16.0 ± 2.8 × 103 CFU invaded the monolayer; however, when S. boulardii was present during the infection, 26.5 ± 3.5 × 103 CFU invaded the monolayer (P < 0.05), indicating that more S. flexneri invaded the T-84 monolayers in the presence of S. boulardii than in the absence of S. boulardii. This effect was not observed when S. flexneri was applied basolaterally with 18.7 ± 1.3 × 103 and 15.1 ± 1.4 × 103 CFU invasion in the absence and presence of the yeast, respectively. In addition, the coincubation of S. flexneri directly with S. boulardii did not result in bacterial death, indicating that the yeast was neither toxic to the bacteria nor caused an increase or decrease in bacterial numbers under these experimental conditions.
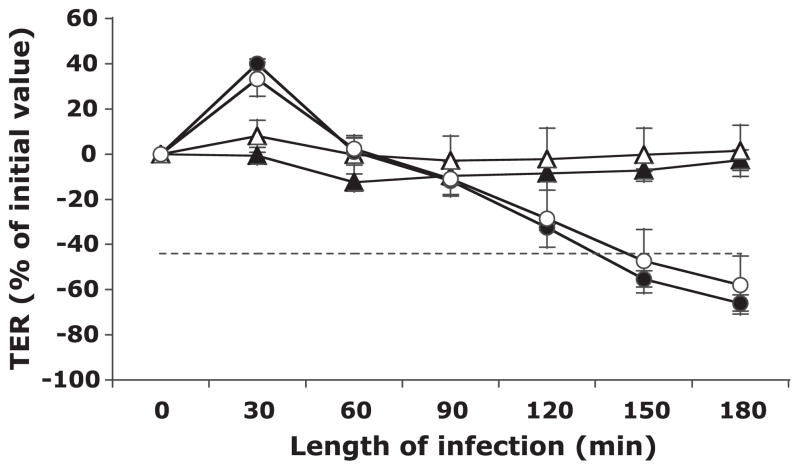
S. boulardii fails to inhibit the S. flexneri-induced decrease in T-84 monolayer TER
To establish the effect of S. boulardii on the loss of TER, which is known to occur with infection by S. flexneri, T-84 monolayers were apically infected with S. flexneri serotype 2a alone or in the presence of S. boulardii, and the TER was measured every 30 min for 3 h. As shown in Fig. 1, S. boulardii alone had no effect on TER over the course of 3 h, with the TER remaining at similar levels to those of uninfected controls. Conversely, S. flexneri infection resulted in a dramatic decrease beginning within 2 h of the start of the infection (P < 0.05, with respect to buffer controls) and reaching a level at which the barrier would likely be permeable to large molecules (indicated by dashed line) at 2.5 h (P < 0.01, with respect to buffer controls). The presence of S. boulardii during S. flexneri infection did not prevent this loss in TER and resulted in monolayer resistance measurements that were not significantly different from S. flexneri alone. This result was also observed when the monolayers were basolaterally infected and apically exposed to S. boulardii, with the loss of TER occurring slightly earlier during the infection (90-min poststart of infection) and no protection from the overall loss (data not shown).
Fig. 1.
Transepithelial electrical resistance (TER) of human colon cancer-derived cell line T-84 monolayers after apical exposure to Shigella flexneri in the absence and presence of Saccharomyces boulardii over 3 h. The TER values are displayed as percentages of initial values. The data are expressed as means ± SD of triplicate samples for all conditions tested and represent 1 of at least 3 independent experiments performed with similar results. Buffer control (▲), S. boulardii only (△), S. flexneri only (●), and S. flexneri and S. boulardii together (○) are shown. The dashed line represents the point at which the barrier would likely be permeable to large molecules. No significant differences were observed in loss of TER between monolayers infected with S. flexneri only and those infected with S. flexneri in the presence of S. boulardii.
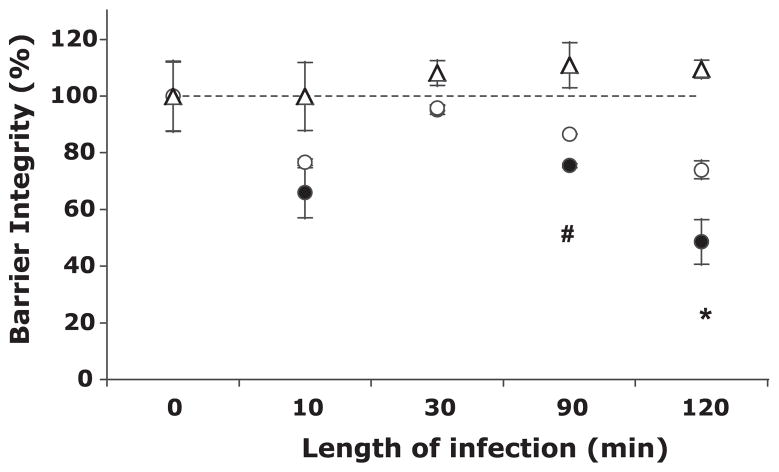
S. boulardii protects barrier function as measured through HRP flux
Because S. boulardii was unable to prevent the loss of TER, we utilized a more functional approach to examining the potential effects of S. boulardii on barrier function, specifically as measured through HRP (40 kDa) flux across the monolayer. Barrier integrity rapidly decreased after 10 min of infection to 65.9 ± 8.8% and 76.6 ± 1.1% in monolayers apically infected with S. flexneri alone and in the presence of S. boulardii, respectively (Fig. 2). After this initial decrease, barrier function was temporarily restored to near that of uninfected controls (indicated by dashed line) before substantially decreasing again after 90 min and a further decrease after 120 min. Although barrier function was significantly reduced in monolayers exposed to S. flexneri at later time points, both with and without S. boulardii (compared with uninfected controls; P < 0.05), there were significant increases in barrier integrity when the yeast was present following 90 and 120 min of infection. After 2 h of infection, monolayers infected with the pathogen exhibited only ~50% of the barrier function of uninfected controls, whereas monolayers exposed to both bacteria and yeast displayed ~75% of the barrier function compared with uninfected controls. This indicates that although barrier integrity was hampered with infection, the yeast was able to partially and significantly restore the integrity.
Fig. 2.
The effect of S. boulardii on S. flexneri-induced barrier permeability, as shown by horseradish peroxidase (HRP) flux across T-84 monolayers. Values are expressed as percentages of the amount of HRP that crossed buffer-treated monolayers (i.e., untreated, unexposed monolayers set to 100% barrier integrity). The data are expressed as means ± SD of triplicate samples for all conditions tested and represent 1 of at least 3 independent experiments performed with similar results. △, S. boulardii only; ●, S. flexneri only; ○, S. flexneri and S. boulardii together. The dashed line represents 100% barrier integrity, as set by the amount of HRP that crossed uninfected, unexposed monolayers. *P < 0.05; #P < 0.01 with respect to the absence or presence of S. boulardii.
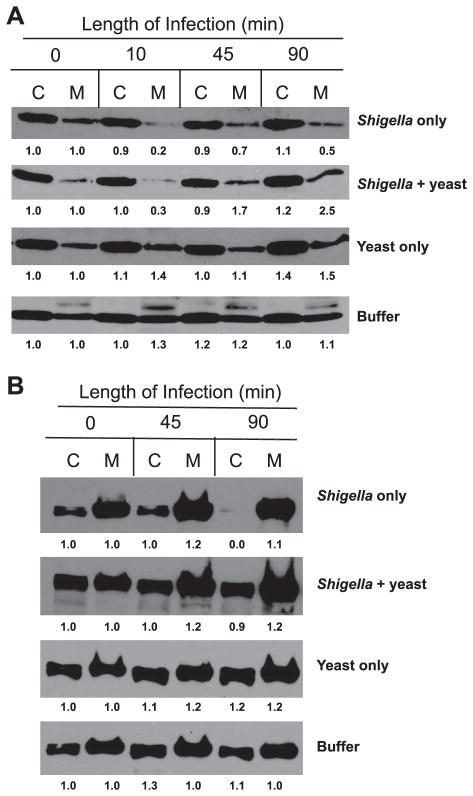
S. boulardii has varying effects on the ability of S. flexneri to modulate components of the tight junction
In light of the disparity between TER and functional barrier integrity, we evaluated a number of individual tight junction proteins to determine the ability of the yeast to interfere with S. flexneri regulation of tight junction components. As was previously established by Sakaguchi et al. (41) and is shown in Fig. 3A, S. flexneri causes a rapid reduction in the amount of claudin-1 found in the membrane fraction of cellular lysates from apically infected monolayers. This event, occurring as early as 10 min into infection, is thought to be a critical step in the pathogenesis of Shigella by providing the initial tight junction disruption allowing for paracellular passage. As seen in Fig. 3A, there is a dramatic loss of claudin-1 in the membrane fraction (70% to 80% loss as determined by densitometry) in S. flexneri-infected monolayers within 10 min, after which levels began to increase to between 50% and 70% of that of uninfected monolayers. Interestingly, although the presence of S. boulardii during infection was unable to prevent this initial loss in claudin-1, monolayers exposed to the yeast exhibited considerably larger amounts of claudin-1 in the membrane fraction at later times during infection. This suggests that the yeast provides the host cell with an enhanced ability to restore claudin-1 levels.
Fig. 3.
Representative Western blots for 2 individual tight junction proteins following a time course of apical infection with S. flexneri in the absence and presence of S. boulardii. C, cytosolic (Triton X-100 soluble) fraction; M, membrane (Triton X-100 insoluble) fraction. Thirty micrograms of protein were loaded per well. Densitometry was performed using ImageJ software (38). The fold change is displayed below each band and was calculated with respect to the change from uninfected, unexposed monolayers (buffer control) at time 0 within each individual Western blot. A: claudin-1 (22 kDa). B: zonula occluden (ZO)-2 (160 kDa). Western blots shown represent 1 of at least 3 independent experiments performed with similar results.
ZO-2 localization is also affected by apical infection with S. flexneri. Infection with the bacteria alone resulted in minor increases in ZO-2 in the membrane fraction within 45 min (compared with uninfected controls) and a complete loss from the cytosolic pool at 90 min after the start of infection (Fig. 3B). The presence of S. boulardii during infection resulted in an identical profile to bacteria alone after 45 min and a similar increase in ZO-2 in the membrane fraction thereafter; however, depletion of the ZO-2 cytosolic pool after 90 min did not occur (only ~10% loss compared with uninfected controls).
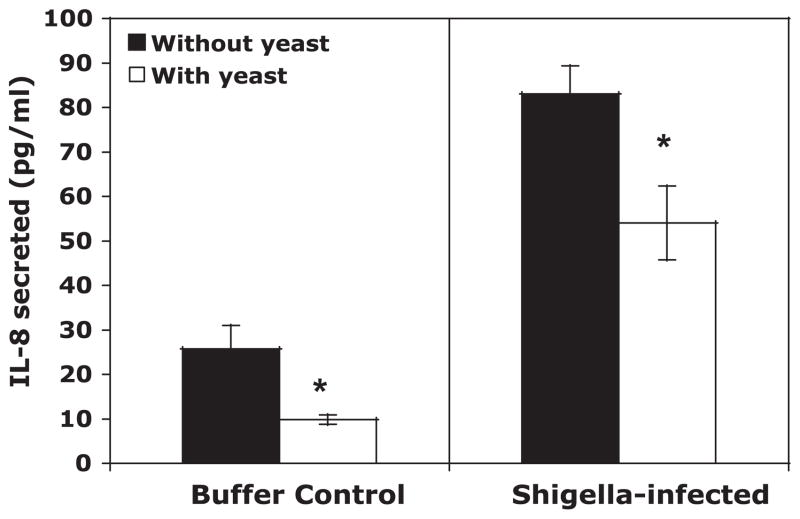
S. flexneri-induced IL-8 production and PMN transepithelial migration is reduced in the presence of S. boulardii
We next examined the ability of S. boulardii to reduce or prevent key inflammatory responses associated with S. flexneri infection. T-84 monolayers were basolaterally infected with S. flexneri in the absence and presence of S. boulardii on the apical surface. It has been well documented that intestinal epithelial cells produce and basolaterally release the proinflammatory cytokine IL-8 in response to S. flexneri (36, 45) and that this cytokine performs a key step in guiding PMNs from the bloodstream to the subepithelium (29). As shown in Fig. 4, model intestinal epithelia exposed to the yeast under both uninfected and infected conditions secreted significantly less IL-8 than those not exposed. Most notably, the level of IL-8 released from S. flexneri-infected monolayers was reduced by 35% when S. boulardii was present (Fig. 4). It is also worth noting that yeast applied 30 min after starting the bacterial infection was unable to reduce levels of secreted IL-8 despite being present for the remainder of the infection (data not shown).
Fig. 4.
IL-8 secretion from T-84 monolayers in response to infection by S. flexneri in the absence and presence of S. boulardii. Buffer was collected from the basolateral compartment of transwells, and the amount of IL-8 that was released in the fraction was quantified by ELISA. The data are expressed as means ± SD of triplicate samples for all conditions tested and represent 1 of at least 3 independent experiments performed with similar results. *P < 0.05 with respect to the absence or presence of S. boulardii.
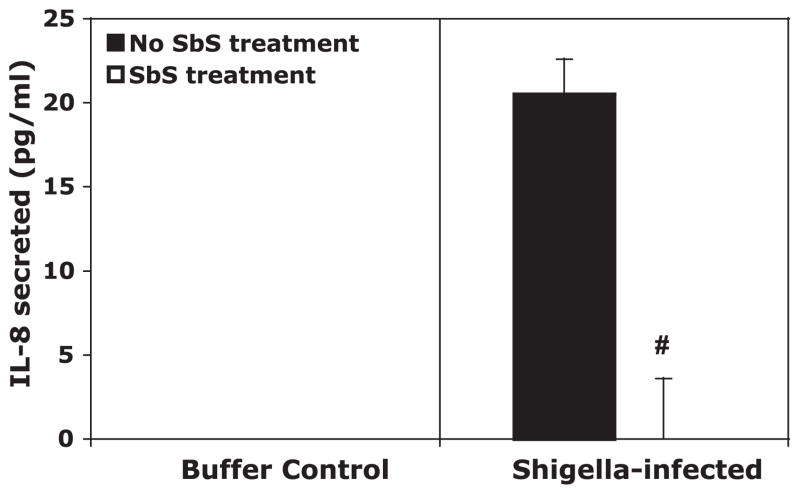
We next examined whether a cell-free, filtered supernatant of S. boulardii, SbS, could also alter IL-8 production by T84 monolayers during infection with S. flexneri. SbS was apically applied to T-84 monolayers during infection with S. flexneri in place of the yeast suspension in buffer. As shown in Fig. 5, the prepared fraction containing no viable yeast also prevented IL-8 production from S. flexneri-infected T-84 monolayers to an even greater extent than the whole yeast.
Fig. 5.
IL-8 secretion from T-84 monolayers in response to infection by S. flexneri with and without S. boulardii supernatant (SbS) treatment. Buffer was collected from the basolateral compartment of transwells, and the amount of IL-8 that was released in the fraction was quantified by ELISA. The data are expressed as means ± SD of triplicate samples for all conditions tested and represent 1 of at least 3 independent experiments performed with similar results. #P < 0.01 with respect to the absence or presence of S. boulardii.
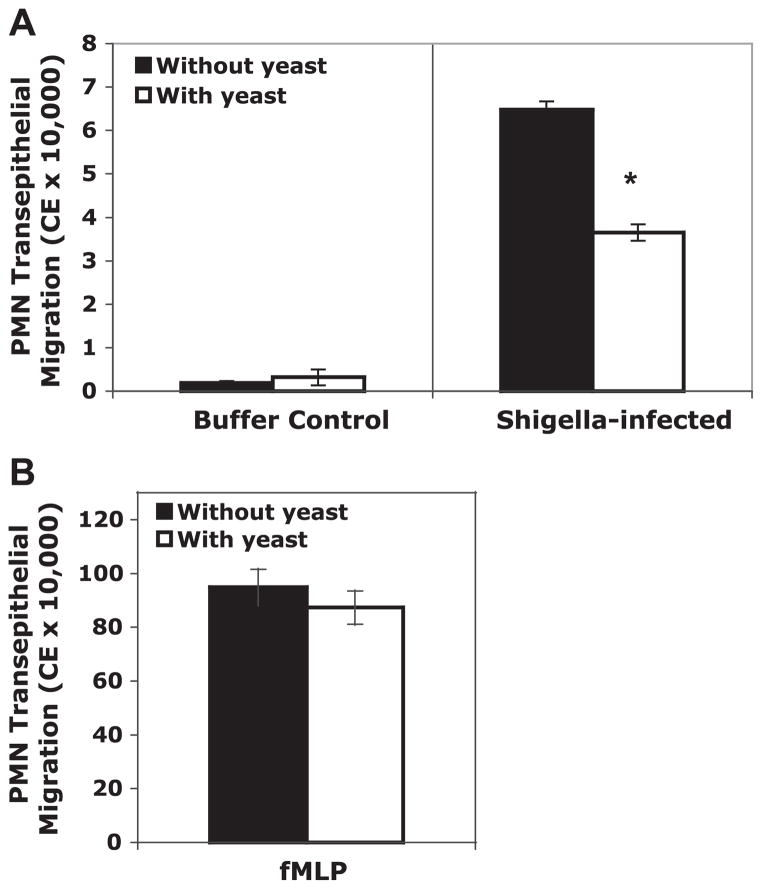
To gauge the effect of the yeast on a separate and distinct step in PMN transmigration, we utilized an in vitro assay that models PMN migration across a monolayer in the physiologically relevant basolateral to apical direction (26, 29, 33–35). As previously demonstrated, S. flexneri induced an increase in PMN transmigration across T-84 monolayers compared with uninfected monolayers (30) (Fig. 6A). We found that the apical presence of S. boulardii during infection caused a significant decrease (~40%) in the number of PMNs lured from the basolateral to the apical compartment (Fig. 6A). In contrast to the IL-8 release, there was no change in PMN migration with uninfected or fMLP-induced controls with exposure to the yeast (Fig. 6B).
Fig. 6.
The effect of S. boulardii on polymorphonuclear leukocyte (PMN) transepithelial migration across T-84 monolayers. A: S. flexneri-induced PMN migration in the presence and absence of S. boulardii. B: positive control, N-formylmethionin-leucyl-phenylalanine (fMLP)-induced PMN migration in the presence and absence of S. boulardii. The data are expressed as means cell equivalents (CE) ± SD of triplicate samples for all conditions tested and represent 1 of at least 3 independent experiments performed with similar results. *P < 0.05 with respect to the absence or presence of S. boulardii during S. flexneri infection.
Effect of S. boulardii on S. flexneri-induced activation of MAPK and NF-κB signaling pathways
To determine at what level S. boulardii may be interacting with the host cell to impede proinflammatory events, we next evaluated different signaling pathways that are involved in IL-8 production and PMN movement across the monolayer. It has been previously shown that S. boulardii is able to reduce activation of MAPK-(9, 12, 14) and NF-κB-related pathways (14), both of which lead to IL-8 production in Shigella-infected epithelial cells (24, 36). In addition, the activation of one MAPK, ERK1/2, is involved in S. flexneri-induced PMN migration, as shown by the use of PD-98059 (a specific MEK inhibitor) having significantly reduced PMN transepithelial migration in response to Shigella (24).
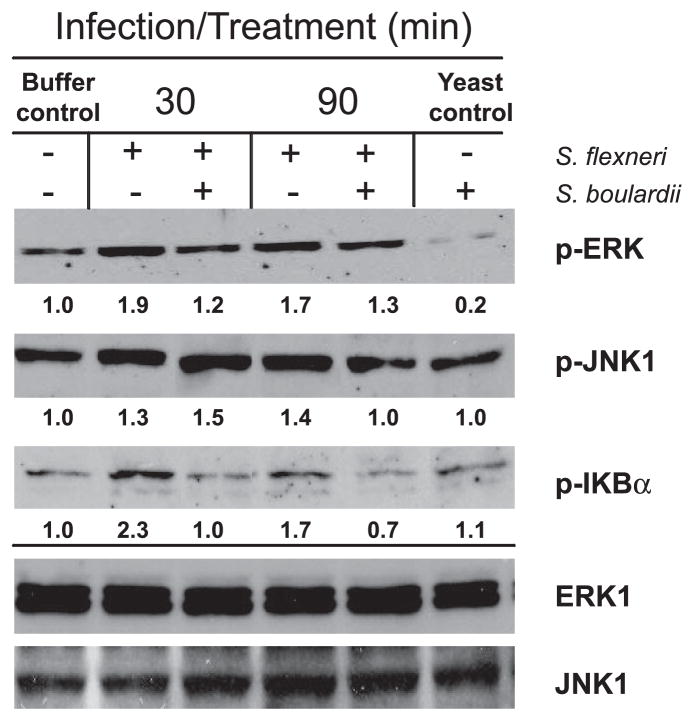
There is a notable increase in ERK1/2 activation in response to infection by S. flexneri within 30 min of infection, and the level remains elevated over the course of 90 min (Fig. 7). Even at this early time point, there is a dramatic decrease in the phosphorylation of ERK1/2 when S. boulardii is simultaneously applied with the bacteria. Densitometry revealed that S. flexneri infection resulted in nearly twice the ERK1/2 activation (compared with the uninfected control) and that the presence of S. boulardii during the infection reduced the levels of pERK1/2 by 35% and 23% after 30 and 90 min of infection, respectively, while not altering the amount of total ERK1 (Fig. 7). Additionally, uninfected monolayers exposed to the yeast alone exhibited considerably lower levels of pERK1/2 than the uninfected, unexposed monolayers. To establish the preventative capability and extent of the anti-inflammatory effects of S. boulardii, we pretreated monolayers with S. boulardii for 30 min before infection and found that this led to further decreases in pERK1/2 and IL-8 secretion (data not shown). Interestingly, however, removal of the yeast for >2 h resulted in a reversal of this effect, and treatment with 5 yeast/epithelial cell, as opposed to 10 yeast/epithelial cell used throughout this study, was unable to alter Shigella-induced ERK or JNK activation at any stage (data not shown).
Fig. 7.
Representative Western blots for phosphorylated (p)-ERK (44/42 kDa), p-JNK1 (45 kDa), and p-IκBα (36 kDa) following 30 and 90 min of infection with S. flexneri in the presence and absence of S. boulardii. Twenty micrograms of protein were loaded per well. Total ERK1 and JNK1 are shown as loading controls and did not change with each condition over time. Densitometry was performed using ImageJ software (38). Fold change, compared with the uninfected, unexposed monolayers (buffer control), is shown below each blot. Western blots shown represent 1 of at least 3 independent experiments performed with similar results.
Similarly, JNK1 activation in response to S. flexneri also occurred within 30 min of infection and resulted in a 30% increase in pJNK1 compared with uninfected monolayers, as measured by densitometry. This increase remained constant through the remainder of the infection, with no effect by the yeast observed until 90 min at which point there was a 25% reduction compared with the monolayers exposed to S. flexneri alone (Fig. 7). There was no difference in JNK activation between yeast- and buffer-exposed monolayers or in the amount of total JNK1 under any of the conditions and times.
Activation of the NF-κB signaling pathway during infection by S. flexneri has been previously documented (19, 36). To examine the effect of S. boulardii on NF-κB activation, we evaluated phosphorylation of IκB, which directly results in the release of NF-κB from its inhibitory complex. As shown in Fig. 7, S. flexneri caused over a twofold increase in pIκBα within the first 30 min of infection. During this time, the presence of S. boulardii prevented this event, thereby allowing the level of pIκBα to remain at that of uninfected control monolayers. After 90 min, the level of pIκBα in infected monolayers remained elevated, whereas those exposed to the yeast during infection showed nearly a 60% decrease with respect to its unexposed counterpart (Fig. 7). Monolayers exposed to the yeast alone also displayed a minor increase in the amount of pIκBα, although not to the levels of those infected with S. flexneri alone. To confirm this effect, we monitored the level of total IκB during infection in conjunction with cycloheximide treatment to prevent its resynthesis following degradation. After 90 min of infection by S. flexneri, T-84 monolayers were depleted of nearly half the intracellular IκB pool, a reflection of phosphorylated IκB being degraded. This degradation was prevented in monolayers exposed to S. boulardii during infection, resulting in total IκB levels similar to that of uninfected monolayers (data not shown). These data confirm that S. boulardii inhibits IκB phosphorylation and degradation during S. flexneri infection, thereby preventing NF-κB activation.
Anti-inflammatory effects of S. boulardii on S. flexneri infection were also observed in vivo using a human fetal intestinal xenograft model
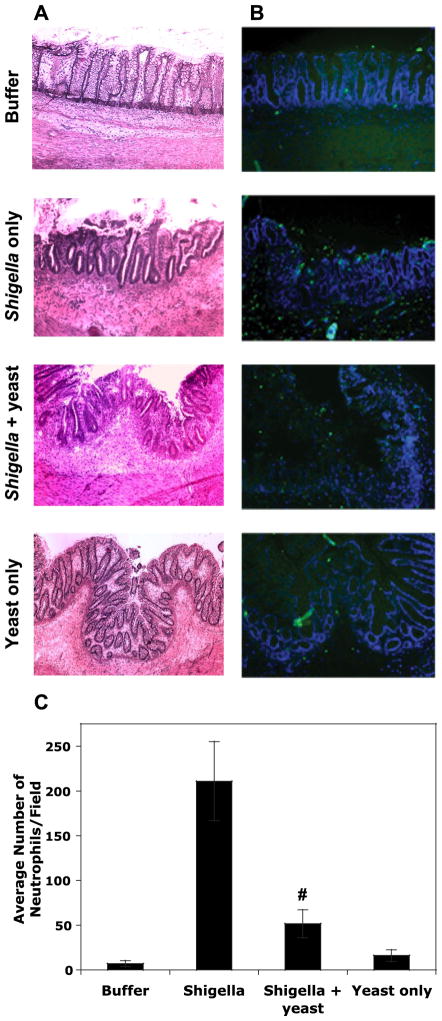
Given that S. flexneri is highly host adapted to human intestinal tissue and does not infect mice under appropriate conditions, we employed a highly relevant in vivo model of human fetal colonic tissue transplanted into scid mice to verify the observed anti-inflammatory effects of S. boulardii in vivo. Xenografts (3 mice/treatment) were injected with S. flexneri alone, S. flexneri with S. boulardii, S. boulardii alone, or buffer for 24 h after which the grafts were removed, sectioned, and stained for an examination by microscopy. HE staining of the human fetal colonic tissue sections revealed a severe pathology in the xenografts exposed to S. flexneri. Such markers of inflammation include thickening/edema of the wall of the colon, crypt destruction, and occasional breakage due to the extensive mucosal damage (Fig. 8A). The infiltration of inflammatory cells was also noticed in the lamina propria of the colonic tissue. Although these effects were still observed in S. flexneri/S. boulardii-exposed xenografts, there is a lesser degree of damage and inflammation.
Fig. 8.
Histopathology following 24 h of infection of human fetal colonic xenografts with S. flexneri in the absence and presence of S. boulardii, sterile HBSS+, or yeast alone. A: hematoxylin and eosin-stained (see MATERIALS AND METHODS) xenotransplanted colon sections shown at ×100 magnification. B: fluorescent-stained xenotransplanted colon sections at ×100 magnification. Sections were stained with 4′,6′-diamidino-2-phenylindol, and PMNs were stained using a FITC-labeled antibody specific for PMN surface markers (Ly-6G and Ly-6C). Photographs are representative tissues for each condition (n = 3 mice for each condition). C: the average number (means ± SD) of PMNs counted in 15 fields for each of the conditions. #P < 0.01 with respect to S. flexneri with and without S. boulardii.
More convincing differences in inflammation between S. boulardii-treated and -untreated xenografts were provided with regard to PMN infiltration in response to infection/treatment. Unfortunately, we were unable to examine PMN influx by the MPO assay due to a difficulty in homogenizing the xenograft tissue. Therefore, we utilized fluorescent staining using an antibody specific for a PMN surface marker (reacts with a common epitope on Ly-6G and Ly-6C) to provide an indication of PMN infiltration induced by S. flexneri in the presence and absence of S. boulardii (Fig. 8B). PMNs present in each of 15 fields per condition were counted and averaged (Fig. 8C). There is a dramatic decrease in the number of PMNs drawn into the xenograft during S. flexneri infection when S. boulardii was present (P < 0.01). This decrease is noticeably higher than was observed in vitro and likely reflects the culmination of both the reduction in IL-8 release and additional chemoattractants responsible for directing PMNs directly across the epithelium, events that were evaluated separately in vitro. It is important to note that although there is a large reduction in PMN infiltration in the presence of S. boulardii (~75% reduction), xenografts injected with both the pathogen and the yeast still resulted in significantly greater PMN influx than in xenografts exposed to buffer or S. boulardii alone (P < 0.01). These in vivo data substantiate the effects found in vitro in that although there is a large anti-inflammatory effect, S. boulardii does not completely prevent inflammatory processes.
DISCUSSION
In the age of increased antibiotic resistance, public awareness of common and emerging pathogens, and interest in more natural remedies, the use of probiotics for the treatment and prevention of gastrointestinal disorders has become an attractive option. Although it has been used for decades as a treatment for diarrheal diseases, S. boulardii is increasing in popularity and use, thereby promoting the need to understand its actions, capabilities, and limitations. Only in recent years have the mechanisms for the protective and therapeutic effects of S. boulardii begun to be deciphered, and studies have found that this yeast has a number of pathogen-specific strategies for conferring its positive effects, including proteases with specific bacterial substrates (7, 37), as well as interference with host signaling pathways (9, 12, 14). In addition, the yeast has been shown to stabilize tight junctional proteins and barrier integrity (12), an effect that could have potential use beyond the scope of bacterial-induced diarrhea. In light of such broad-reaching effects, we hypothesized that S. boulardii would attenuate inflammation induced by a unique enteric pathogen that is highly infectious and host adapted: S. flexneri. In this study, we have identified three important aspects when considering the utility of S. boulardii for the treatment of shigellosis. First, S. boulardii did not affect the adherence or invasion of S. flexneri into host cells. Second, the yeast was able to modestly increase barrier integrity of infected monolayers. Finally, S. boulardii exhibited an anti-inflammatory effect on S. flexneri-infected monolayers and tissues.
Through the use of a relevant in vitro model of polarized monolayers comprised of human colonic epithelial cells (T-84), we showed that S. boulardii did not reduce the number of S. flexneri that attached or invaded monolayers. In fact, we observed an increase in host cell invasion by Shigella with apical application in the presence of the yeast compared with Shigella alone. This increase is most likely not directly due to S. boulardii but is more a reflection of the effect of additional cells (yeast and bacteria together) within the single suspension above the apical surface of a monolayer. This is in contrast with basolateral infection, where Shigella and yeast were located on opposite surfaces of the monolayer. Thus the weight of the yeast within this suspension may have “forced” the bacteria into closer association with the monolayer, thereby encouraging bacterial translocation through the tight junctions to the basolateral surface for entry (41). This is supported by the small, although not significant, increase in association when Shigella and yeast were both applied apically compared with Shigella alone. Despite this, the more noteworthy aspect of this interaction is the fact that the yeast did not reduce invasion, thereby suggesting that any effects of the yeast on host cell responses to Shigella were not due to a reduction in infected cells. These results corroborate a previous study where mice were orally challenged with S. flexneri in the absence and presence of S. boulardii and where there was found to be no reduction in bacterial load within the intestines (39). Similar observations have been made for Salmonella typhimurium in mice (39) and in vitro for EHEC (14) and EPEC, with the exception of the coapplication of S. boulardii with EPEC resulting in a marked decrease in intracellular bacteria (12). Interestingly, these studies also showed that monolayers exposed to EHEC or EPEC and S. boulardii were protected from a loss of TER, an effect that was not observed with S. flexneri (Fig. 1). This difference may be due, in part, to the unique and complex interactions between Shigella and the host cell compared with other enteric pathogens (1, 32, 50).
Despite the persistent loss of TER, there was a modest increase in barrier integrity between S. flexneri-infected monolayers alone and those also exposed to S. boulardii (Fig. 2). Although the yeast offered some protection from barrier permeability, differences were not detected until the later stages of infection, and monolayers exposed to both bacteria and yeast still exhibited 25% more passage of HRP than that of buffer-treated monolayers. A similar effect has also been observed with EPEC using inulin, where S. boulardii was able to partially counteract the loss in barrier function in T-84 monolayer-associated infection by EPEC for 6 h, although EPEC/S. boulardii-exposed monolayers still exhibited more than twice the inulin flux compared with uninfected monolayers (12). Data shown here would indicate that although the amount of damage was reduced, the model epithelia were not intact or free from impairment, lending confirmation for the persistent loss in TER observed in Fig. 1. Figures 1 and 2 taken together indicate that although it takes 2 to 2.5 h of exposure and infection with Shigella to disrupt the monolayer to a point where most tight junctions are irreversibly disrupted and the monolayer is significantly damaged, it takes only 10 min for there to be membrane permeability. However, following 10 min, the barrier function is temporarily restored until 90 min postinfection, where function is again lost with the yeast being able to partially offset this effect. This moderate protective effect on the barrier may have been partly due to the yeast altering the ability of Shigella to modify specific tight-junction proteins (i.e., claudin-1, ZO-1, ZO-2, occludin, and E-cadherin). A protective activity by the yeast has previously been demonstrated in regard to ZO-1 distribution with EPEC (12) and such activity by the yeast and could be significant for the ability of Shigella to transition from the apical to basolateral surface for entry (41). We examined the effect of S. flexneri and S. boulardii on two of these proteins and determined the effects to be protein specific. The earliest of these events, and the one that is most attributed to the invasive phenotype, is the removal of claudin-1, which was not altered in the presence of S. boulardii (Fig. 3A). This removal also corresponds to a temporary increase in barrier permeability as evidenced by increased HRP passage with Shigella infection at 10 min in the presence and absence of S. boulardii (Fig. 2). The fact that claudin-1 loss and increased permeability at 10 min are not altered in monolayers exposed to both Shigella and S. boulardii is not surprising, since a lack of claudin-1 modification would likely have resulted in an invasion defect, which did not occur. On the contrary, ZO-2 profiles were altered later during infection (Fig. 3B) and correspond to the loss in barrier function with Shigella and also the modest increase in barrier function observed in the presence of S. boulardii. Again, despite ZO-2 being positively affected, the yeast was unable to counteract the dramatic loss of TER. Further investigation into whether other tight-junction proteins modified by S. flexneri (i.e., ZO-1, E-cadherin, and occludin) are affected by the yeast is needed.
In light of the fact that S. flexneri was able to attach to, invade, and spread within the T-84 monolayer, we wished to examine the ability of the yeast to alter inflammatory processes that would occur thereafter. One hallmark of shigellosis is the massive infiltration of PMNs into the intestinal lumen. Thus we chose to examine IL-8 production, which serves to guide PMNs from the bloodstream to the subepithelium and has previously been demonstrated to be decreased in the presence of S. boulardii following EHEC infection (14). We determined that T-84 monolayers exposed to S. boulardii during S. flexneri infection released significantly less IL-8, an effect that also occurred in buffer-treated monolayers (Fig. 4). This decrease is likely the result of an interference with signaling pathways induced by Shigella that play a role in IL-8 production, namely ERK and NF-κB activations (24, 36) that were inhibited in the presence of S. boulardii (Fig. 7), and corroborates previous publications (9, 12, 14). Consistent with these results, SbS also exhibited an anti-inflammatory effect on IL-8 production induced by S. flexneri, although to a greater extent than that of the whole, live yeast (Fig. 5). The more dramatic reduction may be the result of the responsible factor(s) produced by the yeast having accumulated in the supernatant over a longer duration, thereby allowing the factor(s) to be present at higher concentrations. This fraction of the supernatant has since been further purified and its effects on NF-κB described elsewhere (9, 49), although only in regard to its ability to alleviate IL-1B, TNF-α, or LPS-induced NF-κB activation.
Because IL-8 acts to direct PMNs to the subepithelium, we utilized our well-established in vitro model system to evaluate the transepithelial migration of PMNs. It has been previously established that S. flexneri induces PMN migration across a monolayer (30) and that migration across infected monolayers exposed to S. boulardii was reduced by >40% (Fig. 6A) without S. boulardii having a direct effect on PMNs, as evidenced by positive control fMLP-induced PMN migration being unaltered in the presence of the yeast (Fig. 6B). Interestingly, it has been demonstrated that PMN migration in response to Shigella is dependent on ERK phosphorylation (24), which is reduced in the presence of S. boulardii (Fig. 7), suggesting that changes in ERK activation are likely the mechanisms behind the ability of yeast to reduce PMN migration in response to S. flexneri. Importantly, the effects of S. boulardii on the activation of ERK, JNK, and NF-κB are not unique to S. flexneri infection, and similar observations have also been made with EPEC, EHEC, and C. difficile toxin A-induced enteritis (9, 12, 14).
These anti-inflammatory properties of the yeast were also observed in vivo employing a human tissue model, where human fetal colonic tissue was xenotransplanted into mice. This model allowed bacterial interaction with human tissue and the generation of signaling molecules. Through the use of this relevant model, we were able to recapitulate our in vitro findings and demonstrate that although the S. boulardii- and S. flexneri-exposed xenografts still suffered epithelial damage and PMN infiltration (Fig. 8, A–C), there was considerably more inflammation and damage associated with xenografts infected with S. flexneri alone.
The results presented here clearly demonstrate the anti-inflammatory effects of S. boulardii on infection with Shigella. However, in as much as S. boulardii was unable to affect the ability of S. flexneri to invade and, therefore, still instigate infection or result in complete inhibition of inflammatory processes, it is important to question the likely therapeutic efficacy of the yeast. Case in point, anti-inflammatory effects were observed when S. boulardii was present throughout infection and were more pronounced when monolayers were exposed to the yeast before infection. However, S. boulardii was applied to T-84 monolayers following the onset of infection, and it was unable to cause a reduction in IL-8 secretion (data not shown). In addition, lower doses of the yeast (5 yeast/epithelial cell vs. 10) were unable to reduce signaling or inflammatory processes, and the majority of the effects shown herein are reversible following the removal of the yeast for more than 2 h (data not shown). The aspect of a required minimum population and the duration of anti-inflammatory effect(s) is relevant from the therapeutic standpoint of administration in that the beneficial effects of the yeast may likely be nonexistent if gut populations fall below 5 yeast/epithelial cell. As such, the stringent timing of S. boulardii exposure is critical in that the yeast does not permanently colonize the human gut (5) and would, therefore, require continuous administration to maintain a minimum yeast population in preparation for illness. Such details are significant to consider and could bring to question the consequence of using S. boulardii therapeutically with S. flexneri or similar pathogens. Furthermore, it is unclear at this stage whether concomitant use with S. flexneri could result in reduced severity of symptoms, at the cost of delayed pathogen clearance since the inflammatory diarrhea may aid in limiting the duration of infection. Nonetheless, the potential therapeutic use of S. boulardii for nonbacterial gastrointestinal disorders is also of current interest, and the results thus far are promising for its use with inflammatory bowel disease (15, 20).
The increased interest in S. boulardii and other probiotic treatments necessitates a better understanding of their capability to prevent or eliminate bacterial-induced diarrhea. A comprehension of its capabilities, mechanisms of action, and outcomes for use with different bacterial pathogens is essential for the successful use of S. boulardii in a clinical setting.
Acknowledgments
We thank Jeffrey D. Bien, Jennifer Pasko, Michael A. Pazos, and Deke P. Huntley for technical assistance and Drs. Bryan P. Hurley, Hai Ning Shi, and Daniel M. Wall for advice and technical expertise. We also thank the Xenograft Core Facility for preparation of xenografts.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-33506 (to B. A. McCormick) and a T32 Training Grant sponsored by Harvard Medical School and the Department of Surgery at Massachusetts General Hospital (to K. L. Mumy).
References
- 1.Adam T, Giry M, Boquet P, Sansonetti P. Rho-dependent membrane folding causes Shigella entry into epithelial cells. EMBO J. 1996;15:3315–3321. [PMC free article] [PubMed] [Google Scholar]
- 2.Arbibe L, Kim DW, Batsche E, Pedron Mateescu BT, Muchardt C, Parsot C, Sansonetti PJ. An infected bacterial effector targets chromatin access for transcription factor NF-κB to alter transcription of host genes involved in immune responses. Nat Immun. 2007;8:47–56. doi: 10.1038/ni1423. [DOI] [PubMed] [Google Scholar]
- 3.Bennish ML. Potentially lethal complications of shigellosis. Rev Infect Dis. 1991;13:319–324. doi: 10.1093/clinids/13.supplement_4.s319. [DOI] [PubMed] [Google Scholar]
- 4.Bernardini ML, Mounier J, d’Hauteville H, Coquis-Rondon M, Sansonetti PJ. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci USA. 1989;86:3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bléhaut H, Massot J, Elmer GW, Levy RH. Disposition kinetics of Saccharomyces boulardii in man and rat. Biopharm Drug Dispos. 1989;10:353–364. doi: 10.1002/bdd.2510100403. [DOI] [PubMed] [Google Scholar]
- 6.Brandao R, Castro IM, Bambirra EA, Amaral SC, Fietto LG, Tropia MJM, Neves MJ, Dos Santos RG, Gomes NCM, Nicoli J. Intracellular signal triggered by cholera toxin in Saccharomyces boulardiiand Saccharomyces cerevisiae. Appl Environ Microbiol. 1998;64:564–568. doi: 10.1128/aem.64.2.564-568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castagliuolo I, Riegler MF, Valenick L, Lamont JT, Pothoulakis C. Saccharomyces boulardii protease inhibits the effects of Clostridium difficile toxin A and B in human colonic mucosa. Infect Immun. 1999;67:302–307. doi: 10.1128/iai.67.1.302-307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CC, Louie S, Shi HN, Walker WA. Preinoculation with the probiotic Lactobacillus acidophilus early in life effectively inhibits murine Citrobacter rodentium colitis. Pediatr Res. 2005;58:1185–1191. doi: 10.1203/01.pdr.0000183660.39116.83. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Kokkotou EG, Mustafa N, Bhaskar KR, Sougioultzis S, O’Brien M, Pothoulakis C, Kelly CP. Saccharomyces boulardii inhibits ERK1/2 mitogen-activated protein kinase activation both in vitro and in vivo, and protects against Clostridium difficile toxin A-induced enteritis. J Biol Chem. 2006;281:24449–24454. doi: 10.1074/jbc.M605200200. [DOI] [PubMed] [Google Scholar]
- 10.Corthier G, Dubos F, Ducluzeau R. Prevention of Clostridium difficile induced mortality in gnotobiotic mice by Saccharomyces boulardii. Can J Microbiol. 1986;32:894–896. doi: 10.1139/m86-164. [DOI] [PubMed] [Google Scholar]
- 11.Czerucka D, Roux I, Rampal P. Saccharomyces boulardii inhibits secretagogue-mediated adenosine 3′,5′-cyclic monophosphate induction in intestinal cells. Gastroenterology. 1994;106:65–72. doi: 10.1016/s0016-5085(94)94403-2. [DOI] [PubMed] [Google Scholar]
- 12.Czerucka D, Dahan S, Mograbi B, Rossi B, Rampal P. Saccharomyces boulardii preserves the barrier function and modulates the signal transduction pathway induced in enteropathogeneic Escherichia coli-infected T-84 cells. Infect Immun. 2000;68:5998–6004. doi: 10.1128/iai.68.10.5998-6004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czerucka D, Rampal P. Experimental effects of Saccharomyces boulardii on diarrheal pathogens. Microbes Infect. 2002;4:733–739. doi: 10.1016/s1286-4579(02)01592-7. [DOI] [PubMed] [Google Scholar]
- 14.Dahan S, Dalmasso G, Imbert V, Peyron JF, Rampal P, Czerucka D. Saccharomyces boulardii interferes with enterohemorrhagic Escherichia coli-induced signaling pathways in T-84 cells. Infect Immun. 2003;71:766–776. doi: 10.1128/IAI.71.2.766-773.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dalmasso G, Cottrez F, Imbert V, Lagadec P, Peyron JF, Rampal P, Czerucka D, Groux H. Saccharomyces boulardii inhibits inflammatory bowel disease by trapping T cells in mesenteric lymph nodes. Gastroenterology. 2006;131:1812–1825. doi: 10.1053/j.gastro.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Dias RS, Bambirra EA, Silva ME, Nicoli RJ. Protective effect of Saccharomyces boulardii against the cholera toxin in rats. Braz J Med Biol Res. 1995;28:323–325. [PubMed] [Google Scholar]
- 17.Elmer GW, McFarland LV. Biotherapeutic agents. A neglected modality for the treatment and prevention of selected intestinal and vaginal infections. JAMA. 1996;275:870–876. doi: 10.1001/jama.275.11.870. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez IM, Silva M, Schuch R, Walker WA, Siber AM, Maurelli AT, McCormick BA. Cadaverine prevents the escape of Shigella flexneri from the phagolysosome: a connection between bacterial dissemination and neutrophil transepithelial signaling. J Infect Dis. 2001;184:743–753. doi: 10.1086/323035. [DOI] [PubMed] [Google Scholar]
- 19.Girardin SE, Tournebize R, Mavris M, Page AL, Li X, Stark GR, Bertin J, DiStefano PS, Yaniv M, Sansonetti PJ, Philpott DJ. CARD4/Nod1 mediates NF-κB and JNK activation by invasive Shigella flexneri. EMBO J. 2001;21:736–742. doi: 10.1093/embo-reports/kve155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guslandi M, Mezzi G, Sorhi M, Testoni PA. Saccharaomyces boulardii in maintenance treatment of Crohn’s disease. Dig Dis Sci. 2000;45:1462–1464. doi: 10.1023/a:1005588911207. [DOI] [PubMed] [Google Scholar]
- 21.Henson P, Oades ZG. Stimulation of human neutrophils by soluble and insoluble immunoglobulin aggregates. J Clin Invest. 1975;56:1053–1061. doi: 10.1172/JCI108152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurley BP, Siccardi D, Mrsny RJ, McCormick BA. Polymorphonuclear cell transmigration induced by Pseudomonas aeruginosa requires the eicosanoid hepoxilin A3. J Immunol. 2004;173:5712–5720. doi: 10.4049/jimmunol.173.9.5712. [DOI] [PubMed] [Google Scholar]
- 23.Kim DW, Lenzen G, Page AL, Legrain P, Sansonetti PJ, Parsot C. The Shigella flexneri effector OspG interferes with innate immune responses by targeting ubiquitin-conjugating enzymes. Proc Natl Acad Sci USA. 2005;102:14046–14051. doi: 10.1073/pnas.0504466102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Köhler H, Rodrigues SP, McCormick BA. Shigella flexneri interactions with the basolateral membrane domain of polarized model intestinal epithelium: role of lipopolysaccharide in cell invasion and in activation of the mitogen-activated protein kinase ERK. Infect Immun. 2002;70:1150–1158. doi: 10.1128/IAI.70.3.1150-1158.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H, Xu H, Zhou Y, Zhang J, Long C, Li S, Chen S, Zhou JM, Shao F. The phosphothreonine lyase activity of a bacterial type III effector family. Science. 2007;315:1000–1003. doi: 10.1126/science.1138960. [DOI] [PubMed] [Google Scholar]
- 26.Madara JL, Parkos C, Colgan S, MacLeod RJ, Nash S, Matthews J, Delp C, Lencer W. Cl-secretion in a model intestinal epithelium induced by a neutrophil-derived secretagogue. J Clin Invest. 1992;89:1938–1944. doi: 10.1172/JCI115800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCormick BA, Colgan SP, Delp-Archer C, Miller SI, Madara JL. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signaling to subepithelial neutrophils. J Cell Biol. 1993;123:895–907. doi: 10.1083/jcb.123.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCormick BA, Hofman PM, Kim J, Carnes DK, Miller SI, Madara JL. Surface attachment of Salmonella typhimurium to intestinal epithelia imprints the subepithelial matrix with gradients chemotactic for neutrophils. J Cell Biol. 1995;131:1599–1608. doi: 10.1083/jcb.131.6.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCormick BA, Parkos CA, Colgan SP, Carnes DK, Madara JL. Apical secretion of a pathogen-elicited epithelial chemoattractant activity in response to surface colonization of intestinal epithelia by Salmonella typhimurium. J Immunol. 1998;160:455–466. [PubMed] [Google Scholar]
- 30.McCormick BA, Siber AM, Maurelli AT. Requirement of the Shigella flexneri virulence plasmid in the ability to induce trafficking of neutrophils across polarized monolayers of the intestinal epithelium. Infect Immun. 1998;66:4237–4243. doi: 10.1128/iai.66.9.4237-4243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mounier J, Vasselon T, Hellio R, Lesourd M, Sansonetti PJ. Shigella flexneri enters human colonic Caco-2 epithelial cells through the basolateral pole. Infect Immun. 1992;60:237–248. doi: 10.1128/iai.60.1.237-248.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mounier J, Laurent V, Hall A, Fort P, Carlier MF, Sansonetti PJ, Egile C. Rho family GTPases control entry of Shigella flexneri into epithelial cells but not intracellular motility. J Cell Sci. 1999;112:2069–2080. doi: 10.1242/jcs.112.13.2069. [DOI] [PubMed] [Google Scholar]
- 33.Nash S, Stafford J, Madara JL. Effects of polymorphonuclear leukocyte transmigration on the barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1987;80:1104–1113. doi: 10.1172/JCI113167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parkos CA, Delp C, Arnaout MA, Madara JL. Neutrophil migration across a cultured intestinal epithelium. Dependence on a CD11b/CD18-mediated event and enhanced efficiency in physiological direction. J Clin Invest. 1991;88:1605–1612. doi: 10.1172/JCI115473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parkos CA, Colgan SP, Delp C, Arnaout MA, Madara JL. Neutrophil migration across a cultured epithelial monolayer elicits a biphasic resistance response representing sequential effects on transcellular and paracellular pathways. J Cell Biol. 1992;117:757–764. doi: 10.1083/jcb.117.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Philpott DJ, Yamaokao S, Israel A, Sansonetti PJ. Invasive Shigella flexneri activates NF-κB through a lipopolysaccharide-dependent innate intracellular response and leads to IL-8 expression in epithelial cells. J Immunol. 2000;165:903–914. doi: 10.4049/jimmunol.165.2.903. [DOI] [PubMed] [Google Scholar]
- 37.Pothoulakis C, Kelly CP, Joshi MA, Gao N, O’Keane CJ, Castagliuolo I, Lamont JT. Saccharomyces boulardii inhibits Clostridium difficile-toxin A binding and enterotoxicity in rat ileum. Gastroenterology. 1993;104:1108–1115. doi: 10.1016/0016-5085(93)90280-p. [DOI] [PubMed] [Google Scholar]
- 38.Rasband WS. ImageJ. U.S. National Institutes of Health; Bethesda, Maryland: 1997–2007. http://rsb.info.nih.gov/ij/ [Google Scholar]
- 39.Rodrigues AC, Nardi RM, Bambirra EA, Vieira EC, Nicoli JR. Effect of Saccharomyces boulardii against experimental oral infection with Salmonella typhimurium and Shigella flexneri in conventional and gnotobiotic mice. J Appl Bacteriol. 1996;81:251–256. doi: 10.1111/j.1365-2672.1996.tb04325.x. [DOI] [PubMed] [Google Scholar]
- 40.Rohde JR, Breitkreutz A, Chenal A, Sansonetti PJ, Parsot C. Type III secretion effectors of the IpaH family are E3 ubiquitin ligases. Cell Host & Microbe. 2007;1:77–83. doi: 10.1016/j.chom.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Sakaguchi T, Köhler H, Gu X, McCormick BA, Reinecker HC. Shigella flexneri regulates tight junction-associated proteins in human intestinal epithelial cells. Cell Microbiol. 2002;4:367–381. doi: 10.1046/j.1462-5822.2002.00197.x. [DOI] [PubMed] [Google Scholar]
- 42.Sansonetti PJ, Kopecko DJ, Formal SB. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982;35:852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sansonetti PJ, d’Hauteville H, Ecobichon C, Pourcel C. Molecular comparison of virulence plasmids in Shigella and enteroinvasive Escherichia coli. Ann Microbiol (Paris) 1983;134A:295–318. [PubMed] [Google Scholar]
- 44.Sansonetti P, Phalipon A. Shigellosis: from molecular pathogenesis of infection to protective immunity and vaccine development. Res Immunol. 1996;147:595–602. doi: 10.1016/s0923-2494(97)85227-3. [DOI] [PubMed] [Google Scholar]
- 45.Sansonetti PJ, Arondel J, Huerre M, Harada A, Matsushima K. Interleukin-8 controls bacterial transepithelial translocation at the cost of epithelial destruction in experimental shigellosis. Infect Immun. 1999;67:1471–1480. doi: 10.1128/iai.67.3.1471-1480.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sasakawa C, Komatsu K, Tobe T, Suzuki T, Yoshikawa M. Eight genes in region 5 that form an operon are essential for invasion of epithelial cells by Shigella flexneri 2a. J Bacteriol. 1993;175:2334–2346. doi: 10.1128/jb.175.8.2334-2346.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Savidge TC, Morey AL, Ferguson DJP, Fleming KA, Shmakov AN, Phillips AD. Human intestinal development in a severe-combined immunodeficient xenograft model. Differentiation. 1995;58:361–371. doi: 10.1046/j.1432-0436.1995.5850361.x. [DOI] [PubMed] [Google Scholar]
- 48.Singer M, Sansonetti PJ. IL-8 is a key chemokine regulating neutrophil recruitment in a new mouse model of Shigella-induced colitis. J Immunol. 2004;173:4197–4206. doi: 10.4049/jimmunol.173.6.4197. [DOI] [PubMed] [Google Scholar]
- 49.Sougioultzis S, Simeonidis S, Bhaskar KR, Chen X, Anton PM, Keates S, Pothoulakis C, Kelly CP. Saccharomyces boulardii produces a soluble anti-inflammatory factor that inhibits NF-κB-mediated IL-8 gene expression. Biochem Biophys Res Commun. 2006;343:69–76. doi: 10.1016/j.bbrc.2006.02.080. [DOI] [PubMed] [Google Scholar]
- 50.Watarai M, Funato S, Sasakawa C. Interaction of Ipa proteins of Shigella flexneri with alpha5beta1 integrin promotes entry of the bacteria into mammalian cells. J Exp Med. 1996;183:991–999. doi: 10.1084/jem.183.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Z, Jin L, Champion G, Seydel KB, Stanley SL., Jr Shigella infection in a SCID mouse-human intestinal xenograft model: role for PMNs in containing bacterial dissemination in human intestine. Infect Immun. 2001;69:3240–3247. doi: 10.1128/IAI.69.5.3240-3247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Z, Stanley SL., Jr Stereotypic and specific elements of the human colonic response to Entamoeba histolyticaand Shigella flexneri. Cell Microbiol. 2004;6:535–554. doi: 10.1111/j.1462-5822.2004.00381.x. [DOI] [PubMed] [Google Scholar]
- 53.Zurawski DV, Chieko M, Mumy KL, McCormick BA, Maurelli AT. OspF and OspC1 are Shigella flexneri type III secretion system effectors that are required for postinvasion aspects of virulence. Infect Immun. 2006;74:5964–5976. doi: 10.1128/IAI.00594-06. [DOI] [PMC free article] [PubMed] [Google Scholar]