Abstract
Background
Chronic hepatitis C is more aggressive during HIV infection. Available data about risk factors of liver fibrosis in HIV/HCV co-infected patients derive from studies based on a single liver biopsy.
Objectives
To evaluate the risk factors of liver fibrosis progression (LFP) and to investigate the role of antiretroviral therapy (ARV) in HIV/HCV patients who underwent paired liver biopsy.
Patients and Methods
We retrospectively studied 58 patients followed at two Infectious Diseases Departments in Northern Italy during the period 1988-2005. All specimens were double-blinded and centrally examined by two pathologists. LFP was defined when an increase of at least one stage occurred in the second biopsy, according to the Ishak-Knodell classification.
Results
In a univariate analysis, serum levels of alanine aminotransferase (ALT) > 150 IU/L at the first biopsy (P = 0.02), and a > 20% decrease in CD4+ cell count between the two biopsies (P = 0.007), were significantly associated with LFP. In multivariate analysis, a > 20% decrease in CD4+ cell count remained independently associated to LFP (Odds Ratio, 3.99; 95% confidence interval, 1.25-12.76; P < 0.02). Analysis of life survival curves confirmed the correlation between CD4+ cell count and LFP.
Conclusions
Our findings highlight that in HIV/HCV coinfected patients, an effective antiretroviral therapy that assures a good immune-virological profile contributes to reducing the risk of LFP.
Keywords: HIV, HCV, Liver fibrosis, Antiretroviral therapy
1. Background
The use of highly active antiretroviral therapy (HAART) has significantly improved the life expectancy of HIV-infected patients mostly due to a drop in opportunistic infections, while on the other hand the mortality rate due to liver disease has dramatically increased [1][2][3][4][5]. Due to the similar routes of transmission of HCV and HIV, the prevalence of HCV infection in HIV-positive patients ranges from 30 to 50%, and can reach up to 90% among injecting drug users [6][7][8][9]. Several studies have shown that HIV/HCV co-infected patients show a more rapid progression to cirrhosis [10][11][12]. Different factors contribute to the accelerated evolution of liver disease; central among these is HIV-induced immunosuppression. The effect of HAART on liver fibrosis remains controversial. Recent data have demonstrated that HAART is associated with a reduction of liver-related mortality in HIV/HCV co-infected patients [13][14]. A few retrospective studies have reported a relationship between regimens of antiretroviral therapy containing protease inhibitors (PI), and slower fibrosis progression [15][16][17]. However, several studies revealed no association between HAART and liver fibrosis progression [18][19][20][21]. Available data on risk factors for liver fibrosis progression in HIV/HCV co-infected patients derive mostly from retrospective studies based on a single liver biopsy and an estimated duration of HCV infection, which make the assumptions that the reported date of infection was reliable and that liver fibrosis progressed at a linear rate. Considering these limitations, we carried out a study to analyze liver fibrosis progression in HIV/HCV co-infected patients who underwent paired liver biopsies.
2. Objectives
The first end point of this study was to evaluate liver fibrosis progression (LFP) and its associated risk factors. LFP was defined as an increase of at least one stage at the second biopsy. The fibrosis progression rate (FPR) was defined as the difference between scores at two consecutive biopsies divided by the time in years elapsed between these two biopsies. The second end point was to evaluate the effect of antiretroviral therapy on the progression of liver fibrosis.
3. Patients and Methods
We retrospectively studied HIV/HCV patients who underwent paired liver biopsies during the period 1988-2005. Patients enrolled were addressed at the II Department of Infectious Diseases of L. Sacco Hospital and at the Division of Infectious Diseases, S. Raffaele Scientific Institute, Milan, Italy. HCV infection was defined by a positive serology result after a second-generation enzyme-linked immunosorbent assay (ELISA), and positivity for plasma HCV-RNA through a branched-DNA PCR assay (Bayer). Only patients who underwent two sequential liver biopsies with an adequate sample for histological analysis and who had available clinical data were included in the study.
3.1. Patient evaluation
For each patient a case report was recorded, including epidemiological and clinical features [sex, age, risk factors for HIV infection, HIV stage (according to 1992 revised Centers for Disease Control and Prevention (CDC) classification), and history of high alcohol intake (defined as a consumption of > 50 g of alcohol per day)], biochemical data [levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT)], immune profile (CD4+ cell count), and HIV RNA level (available from 1996), recorded at the time of the first liver biopsy and subsequently every year until the second biopsy. The study was conducted with local Ethical Committee approval and all patients signed specific consent forms.
3.2. Histological evaluation
Percutaneous liver biopsies were performed using Menghini's needle to obtain specimens > 10 mm long, which were fixed in 10% formalin buffer and stained with hematoxylin-eosin. The threshold of adequacy for histological assessment was the presence of more than 10 portal tracts. All specimens were double-blinded and centrally examined by two experienced pathologists, who were not aware of the clinical and biological data of the patients. The Knodell score system, modified by Ishak (1995, revised in 2000) [22][23], was used to assess necroinflammatory activity and fibrosis. Patients at stage 5-6 during the baseline liver biopsy were excluded.
3.3. Statistical analysis
Software packages Graph Pad Prism (version 3.02) for Windows, Graph Pad Instat (version 3.05) for Windows 95 (Graph Pad Software, San Diego, CA, USA, www.graphpad.com), and Stata 7.0 (Stata Corporation - Lakeway Drive College Station, Texas USA, www.stata.com) were used. Descriptive statistics were expressed as medians and Interquartile Ranges (IQR) or mean and 95% Confidence Interval (95% CI), and as percentage, for continuous and categorical variables, respectively. Continuous variables were compared using the nonparametric Mann-Whitney test and the unpaired t test when appropriate. Differences in proportions were performed using Fisher's exact test. Investigated risk factors for LFP included: age at liver biopsy, gender, risk factors for HIV transmission, clinical stage of the disease, baseline and nadir CD4+ cell counts, HIV RNA at baseline, modifications of CD4+ cell counts and HIV RNA (when available) between two consecutive biopsies, ALT levels, HCV genotype, daily alcohol intake, antiretroviral therapy, and histological grading of activity (according to the Knodell-Ishak score). For each parameter, univariate logistic regression analysis was performed to calculate odds ratio (OR) and 95% CI for fibrosis progression versus non-progression. Only significant variables in univariate analysis were included in multivariate analysis by means of logistic regression. The Kaplan-Meir plot (log rank test) was used to identify differences in time for LFP in relation to the trends of CD4+ cell count between the two biopsies and to the use of HAART.
4. Results
4.1. Study population
During the study period, 65 HIV/HCV co-infected patients with paired liver biopsies were considered. Among them, 58 patients met the inclusion criteria. The main characteristics of the patients at the first biopsy are shown in Table 1. The mean time between the two biopsies was 42.64 months (95% CI 33.7-51.5). Most of the patients were male, with median age of 34 years. The median of the CD4+ cell count was high (> 400/mm(3)). 6 out of 58 patients (10.3%) had CD4+ cells count < 200/mm(3) at baseline and 33 (56.8%) had CD4+ cell count < 200 cells/mm(3) at nadir. The median ALT value was 114 IU/L; 17 out of 58 patients (29.3%) had ALT values > 150 IU/L. Most of the patients had a baseline stage of ≥ 2 (51.7%). At baseline, 20 patients (34.5%) received no antiretroviral therapy, 27 patients (46.5%) received monotherapy or a combination of two nucleoside reverse transcriptase inhibitors (NRTIs) and 11 patients (19%) received HAART. In the period between the two biopsies, six patients (10%) remained naive to antiretroviral therapy, 32 patients (55%) received monotherapy or a combination of two NRTIs, and 20 patients (35%) received HAART. Forty out of 58 (69%) patients were treated with interferon therapy after the first biopsy. All patients were non-responders or prematurely discontinued anti-HCV treatment.
Table 1. Characteristics of the patients at first liver biopsy.
| Characteristics | |
| Sex (Male), No. (%) | 43 (74) |
| Age, y (IQR a) | 34 (22-39.5) |
| Risk category, No. (%) | |
| IDU b | 49 (84) |
| Sexual contact | 9 (16) |
| CDC c stage, No. (%) | |
| A | 25 (43) |
| B | 17 (29) |
| C | 16 (28) |
| ART d received, No. (%) | |
| None | 20 (34.5) |
| Single or dual | 27 (46.5) |
| HAART e | 11 (19) |
| CD4+ T cells count at | |
| Liver biopsy, cells/mmc (IQR) | 449.5 (308-676) |
| Nadir, cells/mmc (IQR) | 194 (97-290) |
| HCV genotype, No. (%) | |
| 1 | 18 (31,1) |
| 2 | 1 (1,7) |
| 3 | 21 (36,2) |
| 4 | 4 (6,9) |
| Not available | 14 (24,1) |
| Staging, No. (%) | |
| 0-1 | 28(48.2) |
| 2 | 17(29.3) |
| 3 | 10(17.2) |
| 4 | 2 (3.3) |
| History of alcohol abuse, No. (%) | 21 (36) |
| ALT f at liver biopsy, IU/l (IQR) | 114 (72-163) |
| Grading mean 95%CI g | 4 (3.4-4.5) |
| Staging mean 95%CI | 1.67 (1.37-1.97) |
a IQR: interquartile range
b IDU: injecting drug use
c CDC: Centers for Disease Control and Prevention
d ART: antiretroviral therapy
e HAART: highly active antiretroviral therapy
f ALT: alanine aminotransferase
g CI: confidence interval
4.2. Liver fibrosis progression
LFP was observed in 27 patients (46.5%). 9 out of 58 patients (15.5%) showed an increase of more than two fibrosis stages in the second biopsy. Five out of 58 patients (8.6%) developed cirrhosis. The mean time between the two biopsies was comparable in the two groups: 38.69 months (95% CI 28.2-49.0) in patients who progressed versus 45.9 months (95% CI 31.6-60.2) in patients who did not (P = 0.93 Mann Whitney test). A comparison of patients who progressed and those who did not showed a significant difference in mean stage score at the first biopsy (1.3 ± 0.9 vs. 1.96 ± 1.22, P = 0.03; unpaired t test) and in mean ALT values at baseline (173.41 ± 26.52 IU/L vs. 111.55 ± 50.6 IU/L, P = 0.03; unpaired t test with Welch correction). Age, grading score, and CD4+ cell counts at baseline and at nadir were comparable between the two groups. In 25 patients out of 58 (43%) CD4+ cell count decreased by more than 20%, and in 22 patients (38%) increased by more than 20% between the two biopsies. In the group of patients who progressed, CD4+ cell count decreased by more than 20% in 17 out of 27 patients (62.9%). HIV RNA level data was available for the 20 patients who received HAART between the two biopsies. HIV RNA remained undetectable (less than the cutoff value) in most of the determinations recorded between the two biopsies in only three out of the 10 patients who progressed (30%), and in seven out 10 (70%) in the group of non-progressors.
4.3. Risk factors for fibrosis progression
We studied the correlation between the LFP and age, gender, route of HIV transmission, CDC stage of HIV infection, history of alcohol abuse, ALT > 150 IU/L at baseline (> grade 2, according to ACTG scale of liver toxicity), CD4+ cell count < 200/mm(3) at nadir and < 350/mm(3) at first biopsy, detectable HIV RNA during more than 50% of the time period considered (available in the patients treated with HAART between two biopsies), histological necroinflammatory index (grading), and antiretroviral therapy. The results of the univariate analysis are summarized in Table 2. A decrease of > 20% in CD4+ cell count between the two biopsies (OR 4.88, 95% CI 1.59-15, P = 0.007), and higher ALT values at first biopsy (OR 4.16, 95% CI 1.22-14.1, P = 0.02), were significantly associated with the progression of liver fibrosis. Moreover, detectable HIV RNA, high alcohol consumption, and a drop of CD4+ cell count to < 200/mm(3) between the two biopsies were also related to a high risk of progression even if they did not reach statistical significance.
Table 2. Univariate analysis of factors associated with liver fibrosis progression.
| Factors | OR a, 95% CI b | P value |
| Age (>35 y at liver biopsy) | 0.7 (0.24-2.04) | 0.59 |
| Sex (Male) | 1.43 (0.43-4.72) | 0.7 |
| History of alcohol abuse | 2.67 (0.88-8.04) | 0.067 |
| ALT c (>150, IU/L at first biopsy) | 4.16 (1.22-14.11) | 0.02 |
| No interferon treatment | 2.35 (0.75-7.36) | 0.16 |
| CD4 | ||
| Nadir, < 200/mmc | 2.1 (0.73-6.19) | 0.19 |
| At first biopsy, < 350/mmc | 0.69 (0.23-2.02) | 0.59 |
| Between two biopsies, < 200/mmc | 3.37 (0.9-12) | 0.06 |
| No ART d, Single or dual NRTIs evs. HAART f | ||
| At first biopsy | 0.67 (0.1-2.5) | 0.73 |
| Between two biopsies | 0.8 (0.2-2.39) | 0.7 |
| Decrease of CD4 (> 20% between two biopsies) | 4.88 (1.59-15) | 0.007 |
| Detectable HIVRNA | 5.44 (0.8-36.8) | 0.08 |
| Route of HIV transmission (IDU gvs. other) | 1.1 (0.2-4.6) | 1 |
| CDC h stage (C vs. A or B) | 1.7 (0.5-5.4) | 0.3 |
a OR: Odds ratio
b CI: Confidence interval
c ALT: Alanine aminotransferase
d ART: Antiretroviral therapy
e NRTIs: Nucleoside reverse transcriptase inhibitors
f HAART: Highly active antiretroviral therapy
g IDU: Injecting drug use
h CDC: Centres for Disease Control and Prevention
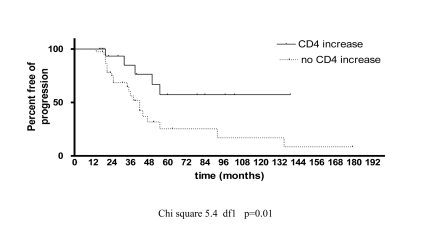
No significant correlation was found between LFP and the presence of antiretroviral therapy at the first biopsy and also between the two biopsies. In the multivariate analysis, a decrease in CD4+ cell count between the two biopsies remained independently associated with LFP (Table 3). Analysis of life survival curves confirmed that an increase in CD4+ cell count between the two biopsies was significantly correlated with slower LFP (Figure 1). We determined the liver fibrosis progression rate per years (FPR) in the 27 patients who progressed: the median FPR was 0.51 (IQR 0.31-0.64). At this rate of fibrosis progression, the median expected time to cirrhosis was 11.7 years (IQR 9.3-19.3).
Figure 1.
Analysis of life survival curve showing an increase of CD4 cells count between two biopsies and the correlation with slower LFP (Chi square 5.4 df1 P = 0.01)
Table 3. Multivariate analysis (logistic regression) of factors associated with liver fibrosis progression (staging increase of one).
| Factors | OR a | IL, 95% CI | SL, 95% CI b | P value |
| CD4+ decrease (> 20%) | 3.99 | 1.25 | 12.76 | 0.020 |
| ALT c (> 150 IU/L) | 3.10 | 0.85 | 11.31 | 0.087 |
a OR: Adjusted odds ratio
b CI: Confidence interval
c ALT: Alanine aminotransferase
5. Discussion
A more rapid progression of liver disease in HIV/HCV co-infected patients is well documented in a large number of studies [10][11][12][24]. However, the majority of available studies on liver fibrosis progression used a single liver biopsy with an estimated duration of HCV infection to calculate FPR [17][18][19][24][25][26]. The calculation of FPR has been extensively applied but assumes linear progression through all stages of infection. This assumption has effectively no clear evidence base. In the current study, we evaluated LFP in patients who underwent two consecutive biopsies. The rate of progression of liver fibrosis in our cohort was variable: in fact, we observed rapid progression even in patients with a lower stage score at baseline (median FPR 0.51/year) with an expected time to cirrhosis of less than 12 years, whereas the majority of patients (53.5%) did not show progression. Thus, this study provides strong evidence that progression of liver fibrosis in HIV/HCV co-infected patients is not linear.
In addition, we demonstrated that, even in patients with mild liver disease at first biopsy (stage 0-1), progression of fibrosis could occur in a significant proportion (27.5%) in < 43 months. This is consistent with the findings of Sulkowski et al. who demonstrated that significant fibrosis progression occurred in 24% of 174 non-cirrhotic HIV/HCV patients, although no or minimal fibrosis was detected in 77% of these patients in the first liver biopsy [27]. The rate of LFP in HIV/HCV co-infected patients is clearly higher than that observed in HCV-monoinfected patients, where available data showed rates from 8% to 12% [28][29]. Different parameters have been previously identified as risk factors for severe liver fibrosis in HCV-positive patient cohorts: increased age, duration of HCV infection, high alcohol intake, high ALT serum levels, and high necroinflammatory index [15][19][21][30][31][32][33][34][35][36]. In our study, only high ALT serum levels were significantly associated to LFP in a univariate analysis. In the study by Sulkowski et al, levels of serum AST also play an important role in predicting future liver disease, leading to support of the use of this prognostic marker in HCV treatment decision algorithms [27].
Other factors related to HIV infection may be also involved in LFP. Several studies have previously demonstrated that a low CD4+ cell count is strongly associated with rapid progression of fibrosis in HIV/HCV co-infected patients [15][21][36][37]. Immunosuppression due to HIV could induce modifications of cytokine patterns in the liver towards a Th2 response, which has been demonstrated to be associated with advanced fibrosis in animal models [37][38][39]. Regarding the role of antiretroviral therapy, in a recent study, Brau et al. [26] showed that HIV/HCV co-infected patients with HIV RNA < 400 cp/mL exhibited FPR similar to HCV monoinfected patients (0.122/year vs. 0.128), whereas patients with detectable HIV RNA had a higher FPR (0.15/year). In this study, HIV RNA, and not CD4+ cell count, was the best predictor of liver fibrosis. In another recent study, Verma et al. [25] compared 85 HIV/HCV co-infected and 296 HCV-monoinfected patients during a period of 10 years, and demonstrated that patients taking HAART as the first antiretroviral regimen had an FPR similar to HCV-monoinfected subjects; HAART, but not CD4+ cell count or HIV RNA level, was independently related to liver fibrosis. It is necessary to emphasize that in these studies, a single value of CD4+ cell count and HIV RNA, corresponding to the date of liver biopsy, were considered in the analysis, whereas in our study we evaluated not a single value, but the trend of the CD4+ cell count and, when available, HIV RNA levels in the period between the two biopsies. In fact, we observed that a decrease of more than 20% in CD4+ cell count between the two biopsies was independently correlated to LFP. Additionally, in 20 patients who were taking HAART, the presence of detectable HIV RNA in more than 50% of determinations was related to a faster LFP, although without statistical significance, probably due to the small number of patients with available HIV RNA. Furthermore, we observed that an increase of more than 20% in CD4+ cell count between the two biopsies, which can be related to efficient antiretroviral therapy, was also significantly related to slower LFP. However, we demonstrated no association between LFP and the absence or specific type of antiretroviral therapy (HAART, combination of two NRTIs, or monotherapy), underlining that only effective therapy, which results in an increase in CD4+ cell count and HIV RNA suppression, is really relevant to reduce LFP.
Our findings that ALT levels and a decrease in CD4+ cell count, but not other parameters, were significantly correlated to LFP, might be due to the small number of enrolled patients and to the retrospective nature of the analysis; therefore, an increase in sample size might provide further information. A recent study on paired liver biopsy in HIV/HCV co-infected patients has demonstrated the efficacy of PEG-IFN therapy in reducing or stabilizing liver fibrosis, in comparison to untreated patients, who presented faster progression even in the absence of a sustained virological response [40]. In our study, we did not find a correlation with anti-HCV therapy, probably due to the fact that all our patients had been treated in the past, mostly with suboptimal schedules based on standard IFN monotherapy, or had prematurely discontinued treatment.
In conclusion, our data suggest that in HIV/HCV co-infected patients the introduction of effective antiretroviral therapy is also relevant to reduce LFP. Therefore, significant efforts should be made to obtain patients' adherence to antiretroviral therapy, in order to reduce the occurrence of viral resistance and guarantee a good immune-virological profile. Thus, early introduction of antiretroviral therapy should be considered in HIV/HCV co-infected patients, especially in the presence of contraindications to anti-HCV therapy or in patients that were non-responsive to previous antiviral treatments. The evidence of rapid progression in subjects with mild liver disease suggests the need for adequate screening and aggressive treatment of HCV infection even in this subset of patients.
Footnotes
Implication for health policy/practice/research/medical education: Chronic hepatitis C is a common problem in HIV patients that leads to liver fibrosis. This study investigates ARV therapy in c0-infected HIV/HCV patients who are the case of paired liver biopsy. Therefore, study of this article is recommended to all researchers in the field of public health, diagnosis and treatment of HIV and/or HCV patients.
Please cite this paper as: Schiavini M, Angeli E, Mainini A, Uberti-Foppa C, Zerbi P, Sagnelli C, et al. Fibrosis progression in paired liver biopsies from HIV/HCV co-infected patients. Hepat Mon. 2011;11(7):525-31.
Financial support: None declared.
Conflict of interest: None declared.
References
- 1.Bica I, McGovern B, Dhar R, Stone D, McGowan K, Scheib R, Snydman DR. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32(3):492–7. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 2.Soriano V, Garcia-Samaniego J, Valencia E, Rodriguez-Rosado R, Munoz F, Gonzalez-Lahoz J. Impact of chronic liver disease due to hepatitis viruses as cause of hospital admission and death in HIV-infected drug users. Eur J Epidemiol. 1999;15(1):1–4. doi: 10.1023/a:1007506617734. [DOI] [PubMed] [Google Scholar]
- 3.Puoti M, Spinetti A, Ghezzi A, Donato F, Zaltron S, Putzolu V, Quiros-Roldan E, Zanini B, Casari S, Carosi G. Mortality for liver disease in patients with HIV infection: a cohort study. J Acquir Immune Defic Syndr. 2000;24(3):211–7. doi: 10.1097/00126334-200007010-00003. [DOI] [PubMed] [Google Scholar]
- 4.Weber R, Sabin CA, Friis-Møller N, Reiss P, El-Sadr WM, Kirk O, Dabis F, Law MG, Pradier C, De Wit S, Akerlund B, Calvo G, Monforte A, Rickenbach M, Ledergerber B, Phillips AN, Lundgren JD. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166(15):1632–41. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 5.Salmon-Ceron D, Lewden C, Morlat P, Bévilacqua S, Jougla E, Bonnet F, Héripret L, Costagliola D, May T, Chêne G. Liver disease as a major cause of death among HIV infected patients: role of hepatitis C and B viruses and alcohol. J Hepatol. 2005;42(6):799–805. doi: 10.1016/j.jhep.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Saillour F, Dabis F, Dupon M, Lacoste D, Trimoulet P, Rispal P, Monlun E, Ragnaud JM, Morlat P, Pellegrin JL, Fleury H, Couzigou P. Prevalence and determinants of antibodies to hepatitis C virus and markers for hepatitis B virus infection in patients with HIV infection in Aquitaine. Groupe d'Epidemiologie Clinique du SIDA en Aquitaine. BMJ. 1996;313(7055):461–4. doi: 10.1136/bmj.313.7055.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherman KE, Freeman S, Harrison S, Andron L. Prevalence of antibody to hepatitis C virus in patients infected with the human immunodeficiency virus. J Infect Dis. 1991;163(2):414–5. doi: 10.1093/infdis/163.2.414. [DOI] [PubMed] [Google Scholar]
- 8.Sherman K, Roustrer S, Chung R, Rajicic N. Hepatitis C prevalence in HIV-infected patients: a cross-sectional analysis of the US ACTG. Antivir Ther. 2000;5(suppl 1):64–5. [Google Scholar]
- 9.Soriano V, Kirk O, Antunes F, Johnson M, d'Arminio Monforte A, Teglbjorg LS, Goebel FD, Lundgren JD. The influence of Hepatitis C virus (HCV) on the prognosis of HIV-infected persons: The EuroSIDA Study. In: International AIDS Conference; 2000 Jul. 9-14. Madrid, Spain: NLM Gateway; 2000. p. abstract no. ThOrB655; [Google Scholar]
- 10.Lesens O, Deschenes M, Steben M, Belanger G, Tsoukas CM. Hepatitis C virus is related to progressive liver disease in human immunodeficiency virus-positive hemophiliacs and should be treated as an opportunistic infection. J Infect Dis. 1999;179(5):1254–8. doi: 10.1086/314720. [DOI] [PubMed] [Google Scholar]
- 11.Soto B, Sanchez-Quijano A, Rodrigo L, del Olmob JA, García-Bengoecheac M, Hernández-Querod J, Rey C, Abad MA, Rodrígueza M, Gilabertb MS, Gonzálezc F, Mirónd P., Caruz A, Relimpio F, Torronteras R, Leal M, Lissen E. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol. 1997;26(1):1–5. doi: 10.1016/s0168-8278(97)80001-3. [DOI] [PubMed] [Google Scholar]
- 12.Eyster ME, Diamondstone LS, Lien JM, Ehmann WC, Quan S, Goedert JJ. Natural history of hepatitis C virus infection in multitransfused hemophiliacs: effect of coinfection with human immunodeficiency virus. The Multicenter Hemophilia Cohort Study. J Acquir Immune Defic Syndr. 1993;6(6):602–10. [PubMed] [Google Scholar]
- 13.Qurishi N, Kreuzberg C, Luchters G, Effenberger W, Kupfer B, Sauerbruch T, Rockstroh J.K, Spengler U. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet. 2003;362(9397):1708–13. doi: 10.1016/S0140-6736(03)14844-1. [DOI] [PubMed] [Google Scholar]
- 14.Merchante N, Girón-González JA, González-Serrano M, Torre-Cisneros J, García-García JA, Arizcorreta A, Ruiz-Morales J, Cano-Lliteras P, Lozano F, Martínez-Sierra C, Macías J, Pineda JA. Survival and prognostic factors of HIV-infected patients with HCV-related end-stage liver disease. AIDS. 2006;20(1):49–57. doi: 10.1097/01.aids.0000198087.47454.e1. [DOI] [PubMed] [Google Scholar]
- 15.Benhamou Y, Di Martino V, Bochet M, Colombet G, Thibault V, Liou A, Katlama C, Poynard T. Factors affecting liver fibrosis in human immunodeficiency virus-and hepatitis C virus-coinfected patients: impact of protease inhibitor therapy. Hepatology. 2001;34(2):283–7. doi: 10.1053/jhep.2001.26517. [DOI] [PubMed] [Google Scholar]
- 16.Marine-Barjoan E, Saint-Paul MC, Pradier C, Chaillou S, Anty R, Michiels JF, Sattonnet C, Ouzan D, Dellamonica P, Tran A. Impact of antiretroviral treatment on progression of hepatic fibrosis in HIV/hepatitis C virus coinfected patients. AIDS. 2004;18(16):2163–70. doi: 10.1097/00002030-200411050-00008. [DOI] [PubMed] [Google Scholar]
- 17.Macias J, Mira JA, Lopez-Cortes LF, Santos I, Giron-Gonzalez JA, Gonzalez-Serrano M, Merino D, Hernandez-Quero J, Rivero A, Merchante N, Trastoy M, Carrillo-Gomez R, ARrizcorreta-Yarza A, Gómez-Mateos J, Pineda JA. Antiretroviral therapy based on protease inhibitors as a protective factor against liver fibrosis progression in patients with chronic hepatitis C. Antivir Ther. 2006;11(7):839–46. [PubMed] [Google Scholar]
- 18.Martinez-Sierra C, Arizcorreta A, Diaz F, Roldan R, Martin-Herrera L, Perez-Guzman E, Giron-Gonzalez JA. Progression of chronic hepatitis C to liver fibrosis and cirrhosis in patients coinfected with hepatitis C virus and human immunodeficiency virus. Clin Infect Dis. 2003;36(4):491–8. doi: 10.1086/367643. [DOI] [PubMed] [Google Scholar]
- 19.Mohsen AH, Easterbrook PJ, Taylor C, Portmann B, Kulasegaram R, Murad S, Wiselka M, Norris S. Impact of human immunodeficiency virus (HIV) infection on the progression of liver fibrosis in hepatitis C virus infected patients. Gut. 2003;52(7):1035–40. doi: 10.1136/gut.52.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta SH, Thomas DL, Torbenson M, Brinkley S, Mirel L, Chaisson RE, Moore RD, Sulkowski MS. The effect of antiretroviral therapy on liver disease among adults with HIV and hepatitis C coinfection. Hepatology. 2005;41(1):123–31. doi: 10.1002/hep.20541. [DOI] [PubMed] [Google Scholar]
- 21.Martin-Carbonero L, Benhamou Y, Puoti M, Berenguer J, Mallolas J, Quereda C, Arizcorreta A, Gonzalez A, Rockstroh J, Asensi V, Miralles P, Laguno M, Moreno L, Giron JA, Vogel M, Garcia-Samaniego J, Nunez M, Romero M, Moreno S, de la Cruz JJ, Soriano V. Incidence and predictors of severe liver fibrosis in human immunodeficiency virus-infected patients with chronic hepatitis C: a European collaborative study. Clin Infect Dis. 2004;38(1):128–33. doi: 10.1086/380130. [DOI] [PubMed] [Google Scholar]
- 22.Ishak K, Baptista A, Bianchi L, Callead F, De Groote J, Gudat F, Denkg H, Desmeth V, Korbi G, MacSweenj R.N.M, Phillipsk M.J, Portmannl B.G., Poulsenm H, Scheuer PJ., Schmidn M, Thaler H. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22(6):696–9. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 23.Ishak KG. Pathologic features of chronic hepatitis. A review and update. Am J Clin Pathol. 2000;113(1):40–55. doi: 10.1309/42D6-W7PL-FX0A-LBXF. [DOI] [PubMed] [Google Scholar]
- 24.Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, Coutellier A, Vidaud M, Bricaire F, Opolon P, Katlama C, Poynard T. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30(4):1054–8. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 25.Verma S, Wang CH, Govindarajan S, Kanel G, Squires K, Bonacini M. Do type and duration of antiretroviral therapy attenuate liver fibrosis in HIV-hepatitis C virus-coinfected patients? Clin Infect Dis. 2006;42(2):262–70. doi: 10.1086/499055. [DOI] [PubMed] [Google Scholar]
- 26.Brau N, Salvatore M, Rios-Bedoya CF, Fernandez-Carbia A, Paronetto F, Rodriguez-Orengo JF, Rodríguez-Torres M. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol. 2006;44(1):47–55. doi: 10.1016/j.jhep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Sulkowski MS, Mehta SH, Torbenson MS, Higgins Y, Brinkley SC, de Oca RM, Moore RD, Afdhal NH, Thomas DL. Rapid fibrosis progression among HIV/hepatitis C virus-co-infected adults. AIDS. 2007;21(16):2209–16. doi: 10.1097/QAD.0b013e3282f10de9. [DOI] [PubMed] [Google Scholar]
- 28.Ghany M.G., Kleiner D.A., Alter H., Doo E., Khokar F., Promrat K., Herion D., Park Y., Liang T.J., Hoofnagle J.H. Progression of fibrosis in chronic hepatitis C. Gastroenterology. 2003;124(1):97–104. doi: 10.1053/gast.2003.50018. [DOI] [PubMed] [Google Scholar]
- 29.Ryder SD, Irving WL, Jones DA, Neal KR, Underwood JC. Progression of hepatic fibrosis in patients with hepatitis C: a prospective repeat liver biopsy study. Gut. 2004;53(3):451–5. doi: 10.1136/gut.2003.021691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Graham CS, Baden LR, Yu E, Mrus JM, Carnie J, Heeren T, Koziel MJ. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33(4):562–9. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 31.Minuk GY. The influence of host factors on the natural history of chronic hepatitis C viral infections. J Viral Hepat. 1999;6(4):271–6. doi: 10.1046/j.1365-2893.1999.00158.x. [DOI] [PubMed] [Google Scholar]
- 32.Freeman AJ, Law MG, Kaldor JM, Dore GJ. Predicting progression to cirrhosis in chronic hepatitis C virus infection. J Viral Hepat. 2003;10(4):285–93. doi: 10.1046/j.1365-2893.2003.00436.x. [DOI] [PubMed] [Google Scholar]
- 33.Freeman AJ, Dore GJ, Law MG, Thorpe M, Von Overbeck J, Lloyd AR, Marinos G, Kaldor JM. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology. 2001;34(4 Pt 1):809–16. doi: 10.1053/jhep.2001.27831. [DOI] [PubMed] [Google Scholar]
- 34.Seeff LB. Natural history of hepatitis C. Hepatology. 1997;26(3 Suppl 1):21S–8S. doi: 10.1002/hep.510260704. [DOI] [PubMed] [Google Scholar]
- 35.Schiavini M, Angeli E, Mainini A, Zerbi P, Duca PG, Gubertini G, Vago L, Fociani P, Giorgi R, Cargnel A. Risk factors for fibrosis progression in HIV/HCV coinfected patients from a retrospective analysis of liver biopsies in 1985-2002. HIV Med. 2006;7(5):331–7. doi: 10.1111/j.1468-1293.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 36.Tural C, Fuster D, Tor J, Ojanguren I, Sirera G, Ballesteros A, Lasanta JA, Planas R, Rey-Joly C, Clotet B. Time on antiretroviral therapy is a protective factor for liver fibrosis in HIV and hepatitis C virus (HCV) co-infected patients. J Viral Hepat. 2003;10(2):118–25. doi: 10.1046/j.1365-2893.2003.00413.x. [DOI] [PubMed] [Google Scholar]
- 37.Puoti M, Bonacini M, Spinetti A, Putzolu V, Govindarajan S, Zaltron S, Favret M, Callea F, Gargiulo F, Donato F, Carosi G. Liver fibrosis progression is related to CD4 cell depletion in patients coinfected with hepatitis C virus and human immunodeficiency virus. J Infect Dis. 2001;183(1):134–7. doi: 10.1086/317644. [DOI] [PubMed] [Google Scholar]
- 38.Shi Z, Wakil AE, Rockey DC. Strain-specific differences in mouse hepatic wound healing are mediated by divergent T helper cytokine responses. Proc Natl Acad Sci U S A. 1997;94(20):10663–8. doi: 10.1073/pnas.94.20.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verma S. HAART attenuates liver fibrosis in patients with HIV/HCV co-infection: fact or fiction? J Antimicrob Chemother. 2006;58(3):496–501. doi: 10.1093/jac/dkl280. [DOI] [PubMed] [Google Scholar]
- 40.Rodriguez-Torres M, Rodriguez-Orengo JF, Rios-Bedoya CF, Fernández-Carbia A, Marxuach-Cuetara AM, Lopez-Torres A, Jimenez-Rivera J. Effect of hepatitis C virus treatment in fibrosis progression rate (FPR) and time to cirrhosis (TTC) in patients co-infected with human immunodeficiency virus: a paired liver biopsy study. J Hepatol. 2007;46(4):613–9. doi: 10.1016/j.jhep.2006.12.011. [DOI] [PubMed] [Google Scholar]