Abstract
Stress can have a lasting impact on the structure and function of brain circuitry that results in long-lasting changes in the behavior of an organism. Synaptic plasticity is the mechanism by which information is stored and maintained within individual synapses, neurons, and neuronal circuits to guide the behavior of an organism. Although these mechanisms allow the organism to adapt to its constantly evolving environment, not all of these adaptations are beneficial. Under prolonged bouts of physical or psychological stress, these mechanisms become dysregulated, and the connectivity between brain regions becomes unbalanced, resulting in pathological behaviors. In this review, we highlight the effects of stress on the structure and function of neurons within the mesocorticolimbic brain systems known to regulate mood and motivation. We then discuss the implications of these spine adaptations on neuronal activity and pathological behaviors implicated in mood disorders. Finally, we end by discussing recent brain imaging studies in human depression within the context of these basic findings to provide insight into the underlying mechanisms leading to neural dysfunction in depression.
Keywords: 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl) propanoic acid (AMPA); dendritic spines; depression; mesolimbic dopamine system; nuclear factor κB (NF-κB); synapse
Introduction
Neurons are integrative units that synthesize inputs from an array of connected neurons to generate outputs, in the form of action potentials that result in dynamic neuronal ensembles (Buzsáki, 2010). To allow for this coordinated activity among neurons, they contain a specialized structure known as the synapse, which is the focal point of information exchange between neurons. The brain contains billions of neurons that give rise to trillions of synapses, which are dynamic, regulated aspects of interneuronal communication capable of maintaining a history of neuronal activity (Hebb, 1949; Yasui et al., 2005).
The synapse is principally a unidirectional point of information transmission in the brain with a presynaptic axon terminal delivering a signal that is received and propagated by the post-synaptic aspect. Our ability to process and incorporate information is dependent on synaptic plasticity mechanisms (Holtmaat and Svoboda, 2009; Wilbrecht et al., 2010). A majority of the literature has focused on the synaptic plasticity at excitatory synapses that are present mainly on small protrusions of the dendrite called spines. Ramon y Cajal observed spines and speculated on their function over a century ago, but it is only in the past few decades that we have made fundamental progress in understanding how synaptic adaptations relate to brain functions such as learning and memory (Shepherd, 1996; Hayashi and Majewska, 2005; Bourne and Harris, 2007; Higley and Sabatini, 2008; Kasai et al., 2010; Penzes et al., 2011).
Dysregulation of synaptic plasticity has been implicated in a variety of psychiatric disorders (Russo et al., 2009; Cruz-Martín et al., 2010; Hayashi-Takagi et al., 2010; Penzes et al., 2010), neurological disorders (Akram et al., 2008; Bingol and Sheng, 2011), and in age-related cognitive impairment (Dumitriu et al., 2010). Genetic mutations have been identified that disrupt normal developmental plasticity mechanisms. Alternatively, life experience can cause changes in synaptic connectivity that result in pathological wiring of neural circuits. Uncovering the fundamental principles of synaptic adaptation is critical to both the understanding of the disease state as well as the discovery of novel therapeutics. In this review, we focus on stress-induced synaptic remodeling in animal models of depression and anxiety disorders. We also discuss the functional relevance of these synaptic adaptations, identify how these basic findings translate to the clinical populations, and finally how this information might aid in developing new therapeutics.
Animal models of stress
Stress is a well-established precipitating factor in several psychiatric diseases including major depressive disorder (MDD) and post-traumatic stress disorders (PTSDs) (Kessler, 1997). Therefore, animal models of these disorders often use stressors to understand the neural mechanisms underlying this form of experience-dependent behavioral plasticity (Figure 1). There are numerous stress paradigms available and each allows us to investigate unique aspects of stress and stress-induced behaviors providing the means to discover universal and stimulus-specific mechanisms of plasticity. Although none of these models recapitulate stress-related psychiatric disorders fully, they are useful tools to model specific symptoms of these disorders.
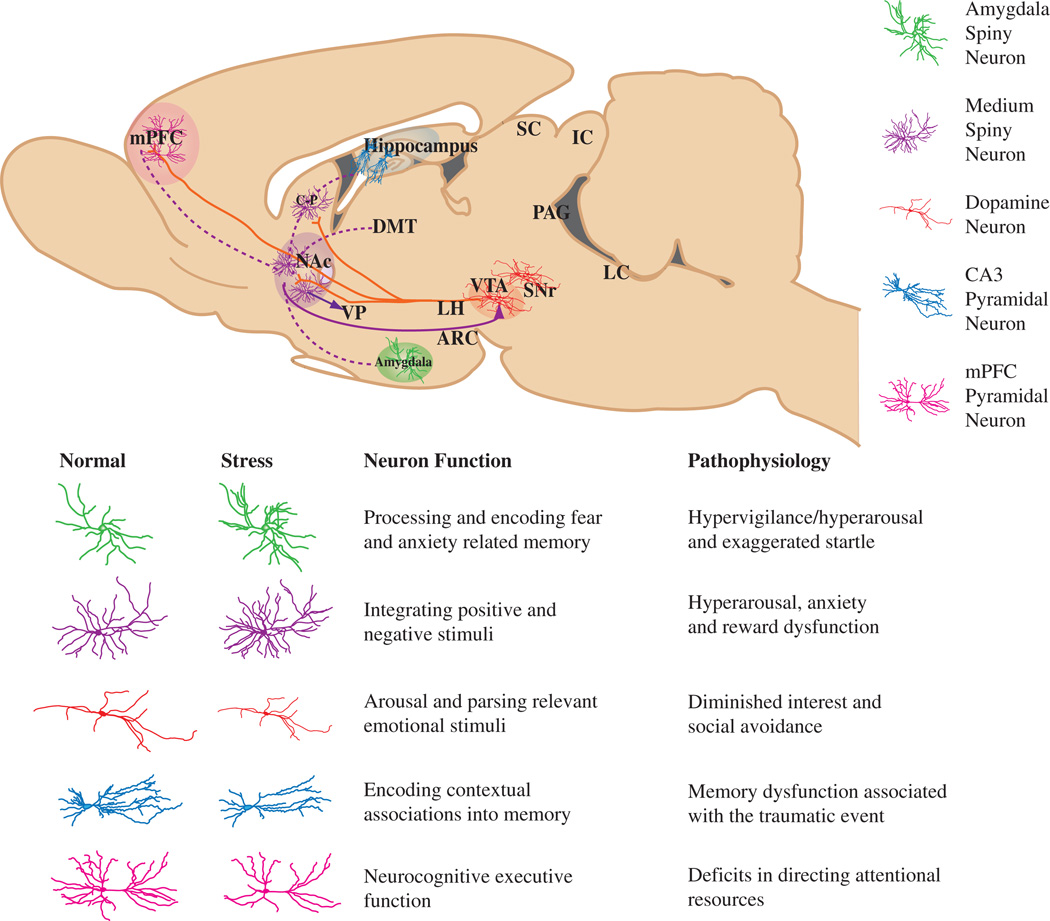
Figure 1. A sagittal brain slice showing the mesocorticolimbic reward circuitry of the brain, highlighting the major neuron type of each region.
Projections of VTA dopamine neurons (shown in solid red lines) impinge directly on NAc and mPFC neurons, as well as on amygdala and hippocampal neurons (the latter projections are not shown in the figure). The solid purple line represents GABAergic afferents (some direct, some indirect) from the NAc to the VTA, which provide feedback to VTA dopamine neurons. The dotted purple lines represent glutamatergic afferents to the NAc from mPFC, amygdala, and hippocampus. Each structure contains specialized neuronal cell types thought to play an integral role in the complex behavioral phenotypes associated with reward-related behavior. These cell types, color-coded in the key, include amygdala (green) and NAc (purple) spiny neurons, PFC (pink) and hippocampal CA3 (blue) pyramidal neurons, and VTA dopamine neurons (red). Below, cartoon renderings of the affect of stress on neuronal morphology, along with a description of the normal function and pathophysiology of plasticity within each mesocorticolimbic brain region.
The various stress paradigms employed rely either on acute or chronic stress. Although acute stress paradigms have been used as expedient means to test antidepressant efficacy and the pro- or antidepressant effect of gene knockouts and transgenics, they lack construct validity for most stress-related disorders and have limited face and predictive validity. Chronic stress paradigms may be more homologous to the human condition, and some of the behavioral phenotypes induced by these models uniquely respond only to chronic administration of antidepressants mimicking the therapeutic response in patients with MDD.
Acute models of stress
Tail suspension stress and forced swim stress are examples of acute stressors that were developed as tools to test the efficacy of antidepressant compounds (Porsolt et al., 1978; Steru et al., 1985). In both models, the critical response measured is immobility, which is believed to measure despair-like behavior. Both of these models have some success in predicting the efficacy of antidepressant compounds in humans; however, given that antidepressants have a rapid effect in these models, their utility in interpreting therapeutic effects is less clear. Moreover, given the acute nature of the stressors, these models do not mimic the pathophysiology of depressive disorders. Learned helplessness is the delivery of an uncontrollable stressor (i.e., footshock) over one or more sessions. In a subsequent testing condition, animals exposed to a stressor show reduced escape attempts to an escapable stressor (Weiss and Glazer, 1975). Although this model also has some predictive validity, it lacks face validity; the stressor produces a transient helplessness phenotype in these models that is typically gone 72 h after the stressor. The absence of long-lasting phenotypes impairs the utility of these models in determining long-lasting changes in neuronal morphology relevant to chronic relapsing stress disorders.
Chronic models of stress
Historically, various forms of chronic stress have been utilized to model depression-like behavior in mice, such as chronic social defeat stress (CSDS), chronic restraint stress (CRS), and chronic unpredictable stress (CUS) followed by behavioral measures of anhedonia (sucrose preference) or despair (forced swim test and tail suspension test) (Krishnan and Nestler, 2010; Nestler and Hyman, 2010). Importantly, behavioral phenotypes induced by all three of these chronic stressors have been identified that are reversible by chronic, but not acute, antidepressant treatments (Berton et al., 2006; Tsankova et al., 2006; Schmidt and Duman, 2010; Christiansena et al., 2011). This is an especially attractive feature compared with models that do respond to acute antidepressant treatments, as it allows for an efficacy comparison between novel fast-acting therapeutic interventions and the more classic slower-acting pharmacotherapies.
The CSDS model of depression results in a robust depression-like phenotype marked by anhedonia, anxiety, and social-avoidance behaviors (Kudryavtseva et al., 1991; Rygula et al., 2005, 2006a,b). In this model, c57BL/6J mice are subjected to 10 once-daily bouts of social defeat by a larger CD-1 mouse that has been screened for aggressive behavior (for detailed protocol see, Golden et al., 2011). Following completion of the social defeats, a majority of mice (65%) develop a constellation of depression-like symptoms and therefore are termed ‘susceptible’, whereas a minority (35%) fail to show these symptoms and are termed ‘resilient’. Taken together, social avoidance exhibited by susceptible mice is associated with a myriad of hedonic changes and weight gain, whereas both susceptible and resilient mice show increased anxiety and corticosterone reactivity (Krishnan et al., 2007). The behavioral syndrome induced by social defeat makes this model useful in studying individual differences in depression- and anxiety-associated behaviors and has the discriminative ability to distinguish animals on these behavioral domains. This feature is of great importance owing to the dynamic range of reactions an individual can exhibit in response to stressors, whether it is the development of a major depressive disorder, PTSD, or resiliency to such disease states (Yehuda et al., 2006). However, a major caveat of the CSDS model in c57BL/6J mice is that female mice are not easily defeated, and thus the model is limited at this point to studying male responses. Regardless, the ability to study individual responses to social stressors has become increasingly useful in modeling aspects of depression-like behavior (Kudryavtseva et al., 1991; Koolhaas et al., 1997; Butterweck et al., 2001; Krishnan and Nestler, 2008; Miczek et al., 2008; Rygula et al., 2008) with high construct, face, discriminative, and predictive validity (for an more in-depth review of these terms, see Nestler and Hyman, 2010).
CRS has been used extensively to determine the morphological, hormonal, and behavioral changes due to repeated stress. Typically, this model consists of restraining an animal for 1–6 h each day in a restraint device (bag or cage) for a period of 3 weeks or more (Watanabe et al., 1992; Radley et al., 2006). This model has been shown to produce changes in the morphology of neurons in the hippocampus, prefrontal cortex (PFC), and amygdala (Table 1). Behaviorally, this model induces depressive-like symptoms establishing a degree of face validity, and depending upon the behavioral measure examined following the stress (i.e., novelty suppressed feeding), it demonstrates predictive validity in responding only to chronic antidepressant treatment. A disadvantage of the CRS model is that during chronic stress, animals habituate over time and show no increase in hypothalamic-pituitary-adrenal (HPA) axis activation. This contrasts with the clinical situation, where it is well documented that depressed individuals show hyperactivity of the HPA axis (Carroll et al., 1976; Stetler and Miller, 2011).
Table 1.
Regions specific affects of multiple stressors on synaptic plasticity.
| Region | Stress | Structure/function adaptation (references) | Clinical findings (reference) |
|---|---|---|---|
| PL | Restraint | ↓Dendritic length (Cook and Wellman, 2004; Radley et al., 2006) | ↓Volume (Drevets et al., 1998; Videbech and Ravnkilde, 2004; Koolschijn et al., 2009) |
| ↓Spine density (Radley et al., 2006, 2008) | ↓CBF and metabolism (Drevets et al., 2008) | ||
| ↓Thin spines (Radley et al., 2008) | |||
| IL | Restraint | ↓Dendritic length (Goldwater et al., 2009; Shansky et al., 2009) | N/A |
| ↓Spine density (Goldwater et al., 2009) | |||
| BLA-projectinga (Shansky and Morrison, 2008) | |||
| oPFC | Restraint | ↑Dendritic length (Liston et al., 2009) | ↓Volume (Drevets et al., 2008) |
| Amy | Restraint† | ↑Dendritic length† (Vyas et al., 2003; Mitra et al., 2005) | ↑Volume (Bremner et al., 2000) |
| Inhibitory avoidance* | ↑Spine density† (Vyas et al., 2006) | ↓Volume (Caetano et al., 2004) | |
| ↓Spine density† (Bennur et al., 2007) | ↑CBF (Drevets et al., 2008) | ||
| ↑Firing rate* (Pelletier et al., 2005) | |||
| Hip | Restraint | ↓Dendritic length (Magarinos and McEwen, 1995; Sousa et al., 2000) | ↓Volume (Bremner et al., 2000; Videbech and Ravnkilde, 2004) |
| ↑Synaptic vesicle density (Magarinos et al., 1997) | ↓CBF and metabolism (Drevets et al., 2008) | ||
| NAc | CSDS‡ | ↑Stubby spines† (Christoffel et al., 2011) | ↓Volume (Pizzagalli et al., 2009; Wacker et al., 2009) |
| Forced swim** |
↑mEPSCs† (Christoffel et al., 2011) | ↓Reduced responsiveness to rewards (Pizzagalli et al., 2009; Wacker et al., 2009) | |
| ↑Inward rectification† (Vialou et al., 2010) | |||
| ↑AMPA/NMDA ratio* (Campioni et al., 2009) | |||
| VTA | CSDS | ↑Firing rate of DA neurons (Krishnan et al., 2007) | N/A |
Restraint stress.
Inhibtory avoidance.
CSDS.
Forced swim.
Amy, amygdala; BLA, basolateral amygdala; Hip, hippocampus; IL, infra-limbic cortex; NAc, nucleus accumbens; oPFC, orbital frontal cortex; PL, prelimbic cortex; VTA, ventral tegmental area; AMPA, α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate; CBF, cerebral blood flow; DA, dopamine; mEPSCs, miniature excitatory postsynaptic currents; NMDA, N-methyl-d-aspartate; N/A, not available.
No change.
CUS is a stress model developed to overcome stress habituation that occurs during CRS. It has also been recently adapted to study sex differences in stress responses and thus, unlike CSDS, is useful in studying mechanisms of depression in females (LaPlant et al., 2009). It involves subjecting animals to a variety of stressors (tail suspension, footshock, restraint, cage tilt, etc.) in a semi-random or unpredictable order over the course of several days to weeks (Papp et al., 1991). Owing to the gradual development of stress-induced behavioral deficits, this model demonstrates construct validity and has been particularly useful in studying anhedonic responses or loss of interest in pleasurable activities (i.e., sucrose consumption, sex, and social interaction). Some of these behavioral deficits are long lasting and are only reversed by chronic antidepressant treatment and thus are more relevant to human disease.
Structural and synaptic plasticity
Stress-induced structural plasticity of dendrites and spines was first identified in hippocampal pyramidal neurons (McEwen, 2000). Exposure to CUS was shown to induce dendritic atrophy in hippocampal regions (Magarinos and McEwen, 1995). Subsequently, other forms of stress, such as CRS in rats, which leads to a glucocorticoid-dependent atrophy of dendrites on CA3 (Magarinos et al., 1997) and CA1 pyramidal neurons (Sousa et al., 2000) demonstrated a more general effect of stress on hippocampal structural plasticity. The effect of stress on spine density in the hippocampus is less clear. One study shows an increase in spines on CA3 dendrites (Sunanda et al., 1995), whereas others observed no changes in spine density (Magarinos et al., 1996) or a decrease in spines that is reversible with recovery (Sandi et al., 2003; Stewart et al., 2005). Additionally, an ultrastructural study of CA1 synapses found an increase in the size of the postsynaptic density following CRS (Donohue et al., 2006). Collectively, these studies suggest that stress alters the morphology and, thus, the strength of hippocampal excitatory synapses, although it is not clear whether the discrepancy in overall spine number is due to methodological differences.
Paralleling changes in the hippocampus, the PFC shows a general atrophy of dendrites and spines in response to stress. For example, CRS has been shown to lead to dendritic atrophy and spine loss (Wellman, 2001; Cook and Wellman, 2004; Radley and Morrison, 2005; Goldwater et al., 2009); both of which are reversible following a period of recovery (Radley et al., 2006; Goldwater et al., 2009). These changes are shown to occur in both the prelimbic (PL) and infralimbic (IL) regions of the PFC. Interestingly a subpopulation of IL neurons that project to the basolateral amygdala (BLA) seem to be resistant to these stress-induced changes (Shansky and Morrison, 2009). Further demonstrating the circuit specific effects of stress on neuronal remodeling, one study found a 43% increase in the dendritic arborization of the orbital frontal cortex, an effect opposite to what is observed in other cortical neuron populations (Liston et al., 2009).
In the amygdala and the nucleus accumbens (NAc), subcortical limbic structures involved in mood regulation, stress generally results in an increase in spine density. CRS results in a hypertrophy of dendritic arborization and increased spine density in basolateral amygdala spiny neurons (Vyas et al., 2003, 2004, 2006; Mitra et al., 2005). Recovery following stress does not reverse these changes. Similarly, CSDS in mice increases spine density, mainly stubby spines, on NAc mediums spiny neurons (MSNs) (Figure 2A). These new spines are associated with generally smaller postsynaptic densities (PSDs) and an increase in the frequency of mini-excitatory postsynaptic currents (mEPSCs), indicating a greater number of functional glutamatergic synapses. To date, no studies have examined the effects of CSDS on the dendritic arborization in these neurons or other forms of synaptic plasticity. Interestingly, while neurons in the BLA and the NAc shell both undergo increases in spine density, these two neuron types differ greatly in their molecular composition. BLA neurons are generally thought to be glutamatergic and pyramidal-like, whereas NAc MSNs are GABAergic (Zahm, 2000; Sah et al., 2003). Perhaps surprisingly, spiny neurons in the medial amygdala, which are GABAergic and morphologically more similar to NAc MSNs, show a decrease in spine density after acute inhibitory avoidance stress (Bennur et al., 2007). What can account for these divergent density changes in varying neuronal types? It is possible that NAc and BLA neurons share some common downstream mechanisms or synaptic inputs that promote similar spine changes. Indeed the BLA projects to the NAc, and altered functioning of the BLA may induce similar plasticity in the NAc. Additionally, the shell of the NAc has been thought to be part of the extended amygdala (Zahm, 2000), and both the NAc and the amygdala are projection regions of the ventral tegmental area (VTA), which also undergoes stress-induced plasticity (Krishnan et al., 2007). Thus, future studies mapping the connections to and from these diverse neuronal populations undergoing synaptic plasticity will be critical to understand whether differences in synaptic morphology observed after chronic stress are due to their synaptic inputs.
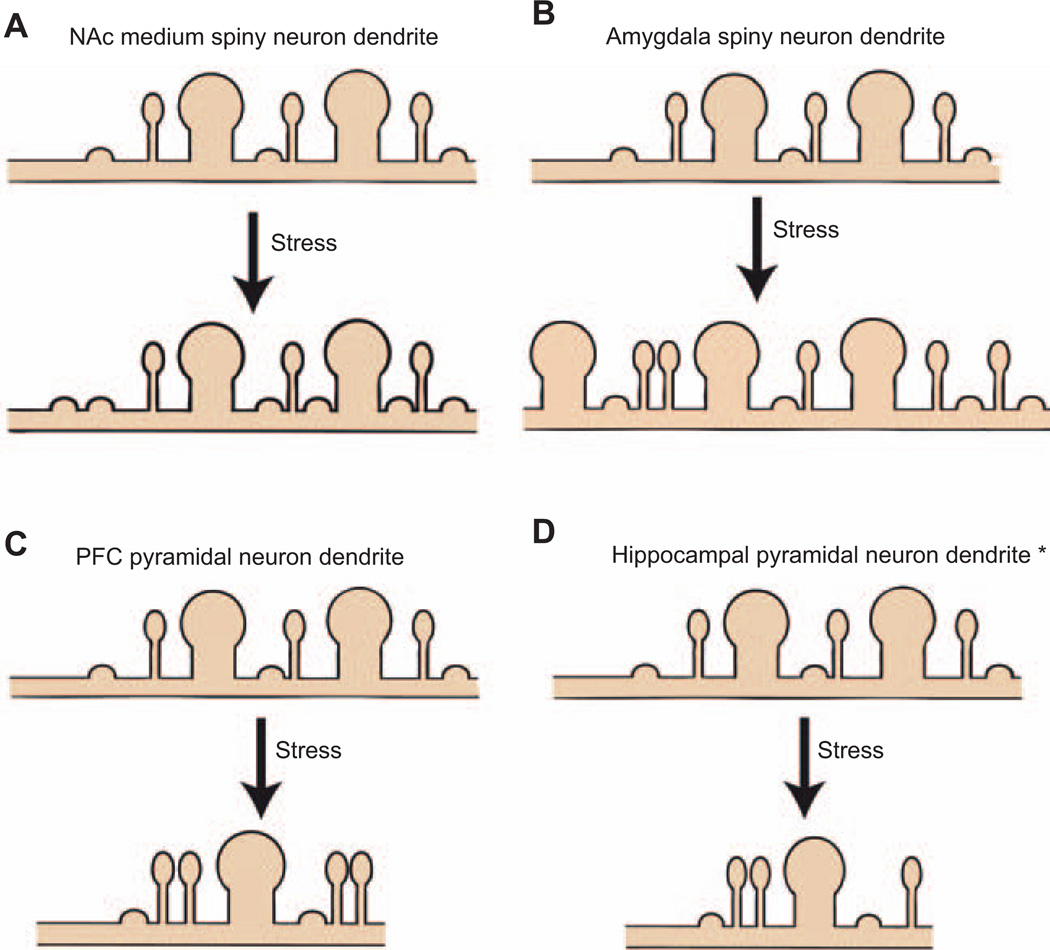
Figure 2. Region specific effects of stress on spine plasticity.
(A) In the NAc, one study has found a doubling of stubby spines after CSDS. To date, no other studies have examined spine reorganization following stress. (B) In the amygdala, several studies have found a stress-induced increase in dendritic length and spine density; however, there are no studies that have examined changes in spine morphologies. (C) In the PFC, the majority of studies found that stress causes a reduction in dendritic length and spine density. It seems that these changes in density are specific to spine types. Note the increase in thin spines but decrease in mushroom spines. (D) Although there are conflicting reports*, several studies would support the hypothesis that the dendritic atrophy of hippocampal pyramidal neurons is accompanied by spine loss.
Owing to recent advances in optical technologies over the past decade, it is now possible to perform high-throughput categorization of spines based on morphological characteristics. Although spines appear on a morphological continuum, the gross categorizations of thin, mushroom, and stubby spines has been useful. It has been argued that these different spine types serve different functions. Thin and stubby spines are prevalent during development and are considered to be immature plastic structures, whereas mushroom spines are more stable and have larger, stronger synapses (Harris and Kater, 1994; Petrak et al., 2005). It is hypothesized that the stubby spine, which has a nonrestrictive neck, is strongly coupled to the parent dendrite (Schmidt and Eilers, 2009). Changes in the percentage of these spines may then have a greater impact on neuronal excitability than others (Noguchi et al., 2005) (Figure 3). Interestingly, it has also been shown that stubby spines are mainly innervated by cortical afferents in the amygdala and undergo afferent specific plasticity (Humeau et al., 2005). In light of their differential functions, emphasis is now being placed more on the shifts in spine types and less on the overall density. For instance, although CRS has been shown to result in an overall spine loss in the PFC (Radley et al., 2006), a more in-depth investigation of spine type shows a decrease in large ‘mushroom’ spines but an increase in smaller ‘thin’ spines (Radley et al., 2008). Thus, although the net difference in spine number is the same, these morphological shifts may have profound effects on cell physiology (Figure 3).
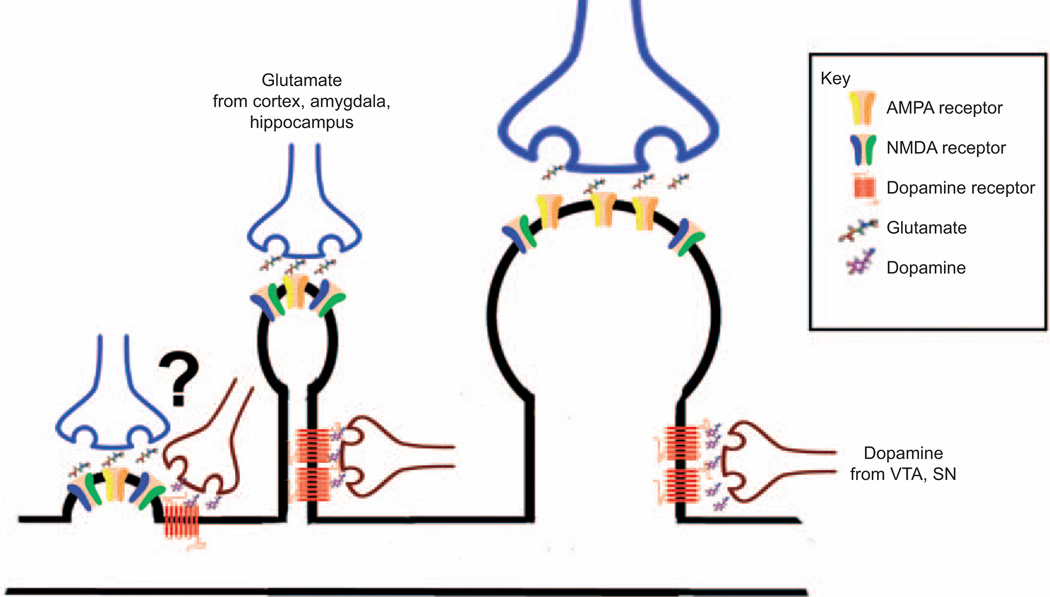
Figure 3. Model of interactions between spine type and dopamine and glutamate neurotransmitter systems in the NAc.
Spine morphology is an important determinant of synaptic strength. Larger mushroom spines have more AMPA receptors than smaller thin and stubby spines. However, morphology may also have an important impact on responsiveness to different neurotransmitters. Stubby spines, which lack a traditional spine neck, may either receive less or contain very different connections with dopamine terminals extending from the VTA and substantia nigra than thin or mushroom spines. Future studies aimed at characterizing the function of specific terminal populations will further elucidate neurotransmitter specific effects on postsynaptic signaling. (From left to right, stubby spine, thin spine, and mushroom spine.)
Although the focus on plasticity mechanisms has principally been on the postsynaptic aspect, the presynaptic axon terminal also undergoes reorganization in response to experience. In 1982, Kandel and Schwartz showed that serotonin-induced upregulation of cyclic adenosine mono-phosphate (cAMP) is responsible for the increased release of the neurotransmitter that results in sensitization in Aplysia (Kandel and Schwartz, 1982). Presynaptic vesicular release is a multistep process that involves docking of synaptic vesicles to the active zone of the presynaptic terminal or ‘bouton’, priming of the synaptic vesicles, and triggering of Ca2+ influx, that leads to the culmination of vesicular fusion and neurotransmitter release (for a complete review of this process see, Sudhof, 1995, 2004). The release probability for synaptic vesicles varies from synapse to synapse and depends on parameters such as the amount of Ca2+ influx per action potential, along with the sensitivity of vesicles to Ca2+. In primary hippocampal cultures, there are 5–10 docked vesicles ready to undergo immediate fusion, termed the ready releasable pool (Schikorski and Stevens, 1997). These terminals have been shown to be ‘unreliable’ because synaptic vesicle release occurs once for approximately 5–10 Ca2+ triggering signals (Dobrunz and Stevens, 1997). Long-term potentiation (LTP), a persistent increase in synaptic strength due to high-frequency stimulation of afferent fibers, at the mossy fiber-Ca3 synapse occurs via increased Ca2+ presynaptically, which increases the reliability of these terminals (Nicoll and Malenka, 1995). Induction of this form of LTP is impaired by acute stress (Chen et al., 2010). Interestingly, molecules known to be critical in vesicle docking and priming, such as Rab3-interacting molecules (RIMs), are required for this form of LTP (Castillo et al., 1997; Lonart et al., 2003). Although little is known regarding the role of these presynaptic proteins in psychiatric disorders, RIM α-knockout mice display schizophrenia-like symptoms, such as impaired social interaction and prepulse inhibition (Blundell et al., 2010). Further studies are needed to assess the relative contribution of the presynaptic machinery in stress-induced plasticity.
Molecular mechanisms of spine adaptations
Attempts to understand stress-induced alterations in dendritic spine morphology and their underlying mechanisms are disadvantaged by the diversity of morphological modifications seen across brain regions. This is prominently exemplified by the opposite changes in the dendritic structure observed in cortical and limbic structures following stressful stimuli. In the following section, we narrow our focus towards recent findings that have begun to elucidate the mechanisms of stress-induced dendritic spine remodeling in the NAc and hippocampus.
Although monoamine depletion has prevailed as the foremost hypothesis on the etiology of depression (Tissot, 1975; Heninger et al., 1996), in recent years, other systems, such as the inflammatory cytokines (Smith, 1991; Ur et al., 1992; de Beaurepaire, 2002), neurotrophic factors (Nibuya et al., 1995; Chen et al., 2001; Vidal et al., 2011), and the ubiquitous neurotransmitter glutamate (Palucha and Pilc, 2005; Li et al., 2010), have been shown to be crucial factors in the pathogenesis of depression. The actions of these systems are known to rapidly modulate dendritic spine morphology. A series of recent independently reported findings show that targets directly downstream of proinflammatory cytokines (Christoffel et al., 2011) and glutamate N-methyl-d-aspartate (NMDA) receptor (Autry et al., 2011; Li et al., 2011) directly affect stress-induced behavioral and synaptic plasticity. Interestingly, NMDA antagonists produce rapid antidepressant effects within 24 h following a single treatment. Considering the delayed onset of efficacy with traditional monoamine therapies, monoamines may simply be providing a framework that supports the direct actions of these other signaling systems (Kugaya and Sanacora, 2005). It is clear that investigating the mechanisms directly involved in synaptic remodeling offers new and exciting therapeutic avenues (Fuchs et al., 2006; Sandi and Bisaz, 2007; Andrade and Rao, 2010; Gorman and Docherty, 2010; Vidal et al., 2011).
Brain-derived neurotrophic factor (BDNF) has long been implicated in stress and depression-induced behavioral and synaptic plasticity in several brain regions (Tsankova et al., 2006; Krishnan et al., 2007; Monteggia et al., 2007; Taliaz et al., 2011). In the NAc, VTA-derived BDNF is required for the development of experience-dependent social avoidance behavior, an index of depression-like behavior in the CSDS model (Berton et al., 2006). Additionally, infusion of BDNF into this region is prodepressant, whereas blocking its action is antidepressant (Eisch et al., 2003). The hippocampus, by contrast, shows reductions in BDNF levels following multiple stress paradigms (Smith et al., 1995; Gersner et al., 2010; Zoladz et al., 2011). Supporting a brain region specific effect of BDNF levels on depressive behaviors, knockdown of BDNF in specific subregions of the hippocampus leads to the emergence of depressive behaviors (Taliaz et al., 2010). Moreover, chronic treatment with several different antidepressants following stress reverses this deficit in the hippocampus (Nibuya et al., 1995), and direct infusion of BDNF into the hippocampus also has antidepressant activity (Shirayama et al., 2002). Interestingly, both plasma and serum levels of BDNF are decreased in patients with MDD (Shimizu et al., 2003; Kima et al., 2007). Antidepressants normalize stress-induced changes in spine density, experimental evidence that strengthens the hypothesis that BDNF critically regulates spine plasticity in depression (Norrholm and Ouimet, 2001; Marchetti et al., 2010). These data support the common hypothesis that BDNF is involved in initiating plasticity mechanisms and not regulating mood states directly.
Recently, we found that a downstream target of BDNF and cytokines, inhibitor of kappa kinase (IKK), is significantly upregulated in the NAc following CSDS in mice (Christoffel et al., 2011). IKK is an upstream effector of nuclear factor κB (NFκB), which is a dimeric transcription factor composed of different combinations of the following subunits: p50, RelA/p65, c-Rel, RelB, and p52 (Häcker and Karin, 2006). In its inactive conformation, NFκB is sequestered in the cytoplasm by inhibitor of kappa B (IκB). After phosphorylation by IKK and subsequent polyubiquitylation, IκB is degraded by proteosomes. Now released and active, the NFκB subunits are free to translocate from the cytoplasm into the nucleus and initiate transcription of target genes (Gutierrez et al., 2005). Early studies found that NFκB is activated in a Ca(2+)/calmodulin-dependent kinase (CaMKII) dependent manner, that the p65:p50 heteromer is selectively localized to synapses, and that activated p65 is translocated into the nucleus following experience-dependant activation (Meffert et al., 2003). Furthermore, p65-deficient mice lacking synaptic NFκB expression were unable to learn spatial memory tasks, directly implicating NFκB-modulated gene transcription in behavioral and synaptic plasticity. Furthermore, evidence exists suggesting that NFκB signaling in neurons is far more complicated than initial reports indicate. Depending on the mechanism of NFκB p65 phosphorylation, NFκB is able to exert potent inhibitory or promotional effects on neurite growth in the same neurons (Gutierrez et al., 2008). In the NAc, viral-mediated gene transfer of a constitutively active IKK mutant results in increases in the number of dendritic spines on NAc neurons, whereas inhibition of NFκB by expression of a dominant negative IKK mutant decreases basal dendritic spine number (Russo et al., 2009). We also found that inhibition of IKK signaling following CSDS reverses the increase in spines as well as social avoidance behavior, which suggests that NFκB-dependent changes in neuronal morphology in the NAc are driving aspects of stress-induced behavioral plasticity.
These data all lead towards a singular question: what is the intracellular mechanism directly responsible for modifying the actin cytoskeleton following stressful stimuli? Dendritic spines are composed primarily of actin filaments modulated by cytoskeletal remodelers and capped by a postsynaptic density containing complexes of receptors along with signaling and scaffolding proteins (Arellano et al., 2007; Bourne and Harris, 2008). Although data examining stress-induced alterations at this level are sparse, there is considerable literature examining this question in other neuropsychiatric disorders (Bozdagi et al., 2010; Hayashi-Takagi et al., 2010; Peca et al., 2011) that clearly implicates actin reorganization as a critical step for synaptic and behavioral plasticity.
Reorganization of the actin cytoskeleton is modulated, to a large extent, by members of the small G protein RhoGTPase family, including Rac1, Cdc42, and RhoA, through activation by guanine nucleotide exchange factors (GEFs) or inactivation by GTPase-activating proteins (GAPs) (for a complete review, see Tolias et al., 2011). This family of proteins has been heavily implicated in spinogenesis in several neuronal systems (Nakayama and Luo, 2000; Luo, 2002; Meng et al., 2003; Negishi and Katoh, 2005; Newey et al., 2005; Penzes et al., 2008). Although 58 Rho-GEFs, 56 Rho-GAPs, and 20 Rho-GTPases have been identified in the mouse genome (Kiraly et al., 2010a), we currently have very little understanding of their roles in the NAc, basally, or in response to stressful stimuli. However, it is worth noting that administration of cocaine regulates the GEF Kalirin-7 in the NAc and results in alteration in dendritic spine morphology (Kiraly et al., 2010b). Based on the similarities observed in the NAc following either stress or administration of drugs of abuse (Miczek et al., 2008), it is interesting to speculate on the role of RhoGTPases in stress-induced dendritic spine morphology.
Live imaging of spines
Studies of spine density changes have become routine in animal models of disease, but the ultimate impact of these changes on neuronal activity is poorly understood. Changes in spine morphology have been shown to correlate with LTP and long-term depression (LTD) (Van Harreveld and Fifkova, 1975; Zhou et al., 2004). LTP is known to increase insertion of 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl) propanoic acid (AMPA) receptors into the synapse (Liao et al., 1995), and spine size and AMPA receptors levels are positively correlated. Interestingly, a near doubling of stubby spines occurs 10 min after LTP induction (Chang and Greenough, 1984; Arellano et al., 2007), yet ultrastructural studies have shown that the total synaptic area along a dendrite after LTP induction in the hippocampus remains the same (Bourne and Harris, 2010). More specifically, the authors found that the enlargement of synapses was accompanied by a loss of small synapses, verified in both excitatory and inhibitory synapses, suggesting that shifts in synapse size and number are accompanied by homeostatic regulation.
Early research on spines was limited to static snap shots, whereas recent live imaging techniques provide us with tools necessary to gain a more complete understanding of the temporal dynamics of dendritic spine structure and function. For example, using two-photon microscopy in combination with glutamate uncaging, Kasai and colleagues confirmed that single spine stimulation, via high frequency release of 4-methoxy-7-nitroindolinyl-caged-l-glutamate (MNI-glutamate), results in an increase in spine size and synaptic AMPA receptor currents, which was dependent on CaMKII in hippocampal pyramidal neurons (Matsuzaki et al., 2004). The same approach has been used to study single spine dynamics in other regions (Carter and Sabatini, 2004) and is currently the only method available to visually assess single spine plasticity mechanisms of subcortical regions.
In vivo two-photon studies have demonstrated that a subpopulation of spines are dynamic, emerging and retracting over the course of days, yet the majority of dendritic spines are stable across months and possibly even years (Grutzendler et al., 2002). Experience-dependent learning tends to induce a shift towards larger, more stable spines without changing the overall spine number, a finding that suggests that the balance between stable and immature structures is an important aspect of experience-dependent plasticity (Keck et al., 2008; Yang et al., 2009). Interestingly, Xu et al. demonstrated that spine formation correlates with the degree of learning in mice on the forelimb motor task and that the percentage of new spines that persisted predicted long-term performance (Xu et al., 2009). These findings beg the question: how do changes in spine density, type, or turnover translate into functional changes in neuronal activity? Although there is strong evidence that spines function as biochemical compartments (Denk et al., 1996; Svoboda et al., 1996), their direct role in controlling the cellular physiology of disease states is still not well understood (Tsay and Yuste, 2004; Spruston, 2008).
Stress and functional plasticity
Initial attempts to understand the functional implications of spine changes on stress-induced plasticity have used electro-physiological techniques to assess synaptic plasticity. Patch clamp recording of single neurons or field recordings from groups or areas in an ex vivo brain slice preparation have been the primary methods to assess the effects of stress on functional properties of neurons. For example, MSNs in the NAc shell show an increase in the AMPAR/NMDAR ratio following forced swim stress, suggesting an overall strengthening of synapses (Campioni et al., 2009). Similarly, CSDS causes a shift in the AMPAR profile with susceptible animals showing a decrease in GluA2 and an increase in GluA1, which is associated with increased excitability of these neurons (Vialou et al., 2010). As previously mentioned, CSDS also increases NAc MSN stubby spine formation and the frequency of mEPSCs, a functional correlate of increased synapse number (Christoffel et al., 2011). Interestingly, a mild stressor (performing the Morris water maze in 22°C water) enhances spatial learning and increases GluA2 at hippocampal synapses in rats. However, another study showed that in CD-1 mice undergoing an adolescent chronic social stress in an unstable hierarchy structure, there was a decrease in GLuA1 mRNA expression and an increase in GLuA2 mRNA expression in CA1 and the dentate gyrus, respectively (Schmidt et al., 2010). This effect highlights the importance of taking developmental, strain, and species differences into account when interpreting stress results.
Although less is known regarding other brain circuits, in the amygdala, inescapable footshock leads to a transient increase in the firing rate of spiny neurons (Pelletier et al., 2005). Similarly, VTA dopaminergic neurons display an increase in firing rate following CSDS; however, it is unknown whether structural alterations accompany either of these changes (Krishnan et al., 2007).
In many brain circuits, there seems to be fairly good overlap between structural and functional changes. For example, stress increases spine density and results in a hyperexcitability of amygdala and NAc neurons, whereas stress reduces spine density and impairs LTP in the PFC and hippocampus pyramidal neurons (Rosenkranz et al., 2010). Foy et al. showed that tail-shock and restraint stress impairs LTP and enhances LTD in the CA1 region of the hippocampus (Foy et al., 1987; Xu et al., 1997). Not surprisingly, plasticity impairments due to stress can result in dysfunction of signaling between brain regions. For example, CUS impairs LTP at PFC synapses receiving hippocampal innervations (Cerqueira et al., 2007), as well as at those receiving thalamic innervations (Quan et al., 2011). Collectively, these studies suggest that spines serve as biochemical compartments whose signaling pathways lead to alterations in the strength of a particular connection, which is dependent upon the presynaptic innervations they receive. Future studies to dissect circuit level changes in the structure and function of synapses undergoing plasticity will give us greater insight into how the brain functions during periods of stress to guide behavior.
Human studies
In clinical populations, experience of depression and stress is often correlated with changes in the total volume and activity of a region. Pizzagalli and colleagues demonstrated that anhedonia, a core symptom of depression, is correlated with a reduction in NAc volume and NAc responsiveness to rewarding stimuli (Pizzagalli et al., 2009; Wacker et al., 2009). Similarly, reduced volumes in other mesolimbic regions, such as the PFC, are observed in depressed patients (Drevets et al., 1998; Videbech and Ravnkilde, 2004; Koolschijn et al., 2009). Because longitudinal studies tracking individuals before the onset of depression are lacking, it is unclear whether these changes are due to stressful life events or preexisting vulnerabilities to developing depressive disorders. Further complicating the issue, clinical and basic science findings are only partly consistent. For example, in the hippocampus there is a stress-induced shrinkage of hippocampal neurons in rodents and reduced volume in depressed human patients. Additionally, elevated glucocorticoid levels, seen in depressive populations, can lead to hippocampal atrophy in rodents, a finding that suggests that HPA axis activation is partly responsible for reduced hippocampus volumes in humans (Woolley et al., 1990). However, the finding that NAc MSNs show increased spine density is not easily reconciled with a human decrease in NAc volume and responsiveness to rewarding stimuli. In fact, drug abuse models would suggest that increased branching of dendrites and spine density is correlated with behavioral sensitization to the rewarding effects of a drug (Russo et al., 2010). Moreover, there seems to be even greater inconsistency in the amygdala, where studies have shown an increase, decrease, or no change in volume (Bremner et al., 2000; Caetano et al., 2004; Lorenzetti et al., 2010). A meta-analysis of studies looking at amygdala volumes found no changes in depressed patients (Koolschijn et al., 2009); however, another study found an increase in cerebral blood flow to the amygdala, a correlate of neuronal activity (Drevets et al., 2008). Rodent models somewhat confirm these latter results and show that stress induces dendritic hypertrophy, increased spine density, and hyperexcitability within the amygdala. These apparently conflicting clinical and basic findings could be due to heterogeneous clinical populations, where depression is comorbid with other psychiatric illness, or medication effects, as some patients were undergoing treatment during the experiments. At the same time, the stress paradigms used in basic research are incomplete models not capable of fully recapitulating the human disease state. Furthermore, most of the basic science findings were gathered from adult rodent stress and do not take into account early life stress, which has been shown to play a pivotal role in the development of mood disorders.
As new technologies in basic and clinical research become available, we will be able to better determine the relevant functional and structural changes important in depression and other stress disorders. Recent progress in the development of new analytic techniques for functional magnetic resonance imaging (fMRI) data, such as independent component analysis or region-of-interest (ROI) analysis, is already providing a more comprehensive look at the interaction among brain regions in mood disorders (Fox et al., 2007). One study, using ROI analysis, uncovered a decreased connectivity of frontal-limbic circuits in depressed patients (Lui et al., 2011). Likewise, on the preclinical side, optogenetics now allows us to study the functional relevance of neuronal activity on circuit level fMRI responses and depression-like behavior. In a seminal study, Deisseroth and colleagues showed that stimulation of the thalamus with channel-rhodopsin (ChR) increased activity in the somatosensory cortex measured by fMRI (Lee et al., 2010). Furthermore, blue light stimulation of ChR in frontal cortex pyramidal neurons reduces the prodepressant effects of CSDS while stimulation of NAc alters reward-related behavior (Covington et al., 2010; Lobo et al., 2010). This approach is mirrored in clinical studies where deep brain stimulation (DBS) in the cortex and NAc is effective in alleviating symptoms in treatment-resistant depressed patients (Lozano et al., 2008; Bewernick et al., 2010). Optogenetics provides an excellent tool to parse circuits and cell types responsible for the therapeutics effect of DBS, and will assist in the refinement of this treatment modality. More broadly, we now have tools to uncover how system level signaling controls behavior. As more sophisticated tools are developed, our ability to take hypotheses from the basic sciences to the clinic will increase exponentially.
Conclusions
Correlative evidence about the function of spines has greatly informed neuroscience and aided in understanding the pathology of brain diseases. However, novel investigative tools are now uncovering causative evidence for synaptic plasticity in controlling depression-related behavior. Our ability to not only observe and characterize but also manipulate the biological function of spines is a revolutionary advance in the study of synaptic plasticity. Longitudinal studies of spine and neuronal dynamics, in combination with stress and genetic models of disease, will pave the way for clarification of the etiology of synaptic dysregulation in psychiatric disease. Yet, there are still many important questions that must be resolved (Box 1). Although we have moved beyond the monoamine hypothesis and recognize that psychiatric disease reflects a disruption in normal plasticity mechanisms, the influence of these complex adaptations on behavior is only beginning to be discovered. Furthermore, it is crucial to continue to improve clinical diagnostic and analytical tools. Doing so will aid both in the diagnosis of psychiatric disease and our ability to translate basic findings into effective therapies.
Box 1 Outstanding questions.
Are synaptic alterations the cause of maladaptive behavior or a correlative response of stressful experience?
Do different intracellular signaling pathways or different synaptic inputs account for the reversibility of spine changes on particular neuron types?
Do specific neurotransmitters cause distinct synaptic adaptations?
What is the functional relevance of shifts in dendritic spine size and/or number?
What mechanisms determine the directionality of changes in spine size and/or number?
Acknowledgments
This work was supported by a US National Institute of Mental Health grant 1R01MH090264-01A1 (S.J.R.).
Biographies

Daniel J. Christoffel BA, is a graduate student in the laboratory of Dr. Russo in the Neuroscience Department at the Mount Sinai School of Medicine. He obtained his BA in Psychology and Philosophy at New York University. His thesis studies focus on the role of inhibitor of kappaB kinase in stress-induced synaptic plasticity and depressive behaviors.

Scott J. Russo, PhD, is an Assistant Professor of Neuroscience at the Mount Sinai School of Medicine. He did his PhD in Biopsychology at The Graduate Center of CUNY and a postdoctoral fellowship in Psychiatry at The University of Texas Southwestern Medical Center. His work focuses on the molecular mechanisms that control synaptic plasticity of reward circuitry in stress- and addictive-disorders.

Sam A. Golden is a graduate student in the Russo laboratory at the Mount Sinai School of Medicine. Prior to his matriculation at the Mount Sinai School of Medicine, he was an Intramural Training Award Fellow at the National Institute of Drug Abuse. His work has focused on identifying the intracellular mechanisms underlying drug and stress-induced synaptic plasticity in the mesolimbic dopaminergic reward circuit, and evaluating their potential for therapeutic innovation.
References
- Akram A, Christoffel D, Rocher AB, Bouras C, Kövari E, Perl DP, Morrison JH, Herrmann FR, Haroutunian V, Giannakopoulos P, et al. Stereologic estimates of total spinophilin-immunoreactive spine number in area 9 and the CA1 field: relationship with the progression of Alzheimer’s disease. Neurobiol. Aging. 2008;29:1296–1307. doi: 10.1016/j.neurobiolaging.2007.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade C, Rao NS. How antidepressant drugs act: a primer on neuroplasticity as the eventual mediator of antidepressant efficacy. Indian J. Psychiatry. 2010;52:378–386. doi: 10.4103/0019-5545.74318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arellano JI, Benavides-Piccione R, DeFelipe J, Yuste R. Ultrastructure of dendritic spines: correlation between synaptic and spine morphologies. Front. Neurosci. 2007;1:131–143. doi: 10.3389/neuro.01.1.1.010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng P-f, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennur S, Shankaranarayana Rao BS, Pawlak R, Strickland S, McEwen BS, Chattarji S. Stress-induced spine loss in the medial amygdala is mediated by tissue-plasminogen activator. Neuroscience. 2007;144:8–16. doi: 10.1016/j.neuroscience.2006.08.075. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, DiLeone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Bewernick BH, Hurlemann R, Matusch A, Kayser S, Grubert C, Hadrysiewicz B, Axmacher N, Lemke M, Cooper-Mahkorn D, Cohen MX, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol. Psychiatry. 2010;67:110–116. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Bingol B, Sheng M. Deconstruction for reconstruction: the role of proteolysis in neural plasticity and disease. Neuron. 2011;69:22–32. doi: 10.1016/j.neuron.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Blundell J, Kaeser PS, Südhof TC, Powell CM. RIM1α and interacting proteins involved in presynaptic plasticity mediate prepulse inhibition and additional behaviors linked to schizophrenia. J. Neurosci. 2010;30:5326–5333. doi: 10.1523/JNEUROSCI.0328-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr. Opin. Neurobiol. 2007;17:381–386. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu. Rev. Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne JN, Harris KM. Coordination of size and number of excitatory and inhibitory synapses results in a balanced structural plasticity along mature hippocampal CA1 dendrites during LTP. Hippocampus. 2010;373:354–373. doi: 10.1002/hipo.20768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi O, Sakurai T, Papapetrou D, Wang X, Dickstein DL, Takahashi N, Kajiwara Y, Yang M, Katz AM, Scattoni ML, et al. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol. Autism. 2010;1:15. doi: 10.1186/2040-2392-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am. J. Psychiatry. 2000;157:115–118. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- Butterweck V, Winterhoff H, Herkenham M. St John’s wort, hypericin, and imipramine: a comparative analysis of mRNA levels in brain areas involved in HPA axis control following short-term and long-term administration in normal and stressed rats. Mol. Psychiatry. 2001;6:547–564. doi: 10.1038/sj.mp.4000937. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Neural syntax: cell assemblies, synapsembles, and readers. Neuron. 2010;68:362–385. doi: 10.1016/j.neuron.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano SC, Hatch JP, Brambilla P, Sassi RB, Nicoletti M, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. Anatomical MRI study of hippocampus and amygdala in patients with current and remitted major depression. Psychiatry Res. 2004;132:141–147. doi: 10.1016/j.pscychresns.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Campioni MR, Xu M, McGehee DS. Stress-induced changes in nucleus accumbens glutamate synaptic plasticity. J. Neurophysiol. 2009;101:3192–3198. doi: 10.1152/jn.91111.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll BJ, Curtis GC, Mendels J. Neuroendocrine regulation in depression. I. Limbic system-adrenocortical dysfunction. Arch. Gen. Psychiatry. 1976;33:1039–1044. doi: 10.1001/archpsyc.1976.01770090029002. [DOI] [PubMed] [Google Scholar]
- Carter AG, Sabatini BL. State-dependent calcium signaling in dendritic spines of striatal medium spiny neurons. Neuron. 2004;44:483–493. doi: 10.1016/j.neuron.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Castillo PE, Janz R, Sdhof TC, Tzounopoulos T, Malenka RC, Nicoll RA. Rab3A is essential for mossy fibre long-term potentiation in the hippocampus. Nature. 1997;388:590–593. doi: 10.1038/41574. [DOI] [PubMed] [Google Scholar]
- Cerqueira JJ, Mailliet F, Almeida OFX, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. J. Neurosci. 2007;27:2781–2787. doi: 10.1523/JNEUROSCI.4372-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang FL, Greenough WT. Transient and enduring morphological correlates of synaptic activity and efficacy change in the rat hippocampal slice. Brain Res. 1984;309:35–46. doi: 10.1016/0006-8993(84)91008-4. [DOI] [PubMed] [Google Scholar]
- Chen B, Dowlatshahi D, MacQueen GM, Wang J-F, Young LT. Increased hippocampal BDNF immunore-activity in subjects treated with antidepressant medication. Biol. Psychiatry. 2001;50:260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- Chen C-C, Yang C-H, Huang C-C, Hsu K-S. Acute stress impairs hippocampal mossy fiber-CA3 long-term potentiation by enhancing cAMP-specific phosphodiesterase 4 activity. Neuropsychopharmacology. 2010;35:1605–1617. doi: 10.1038/npp.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansena SH, Olesena MV, Wörtweina G, Woldbye DPD. Fluoxetine reverts chronic restraint stress-induced depression-like behaviour and increases neuropeptide Y and galanin expression in mice. Behav. Brain Res. 2011;216:585–591. doi: 10.1016/j.bbr.2010.08.044. [DOI] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, Krishnan V, Reyes CM, Han M-H, Ables JL, et al. IκB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J. Neurosci. 2011;31:314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J. Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Covington HE, III, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, LaPlant Q, Mouzon E, Ghose S, Tamminga CA, et al. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J. Neurosci. 2010;30:16082–16090. doi: 10.1523/JNEUROSCI.1731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Martín A, Crespo M, Portera-Cailliau C. Delayed stabilization of dendritic spines in fragile X mice. J. Neurosci. 2010;30:7793–7803. doi: 10.1523/JNEUROSCI.0577-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Beaurepaire R. Questions raised by the cytokine hypothesis of depression. Brain Behav. Immun. 2002;16:610–617. doi: 10.1016/s0889-1591(02)00005-3. [DOI] [PubMed] [Google Scholar]
- Denk W, Yusteb R, Svobodaa K, Tank DW. Imaging calcium dynamics in dendritic spines. Curr. Opin. Neurobiol. 1996;6:372–378. doi: 10.1016/s0959-4388(96)80122-x. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Donohue HS, Gabbott PLA, Davies HA, Rodríguez JJ, Cordero MI, Sandi C, Medvedev NI, Popov VI, Colyer FM, Peddie CJ, et al. Chronic restraint stress induces changes in synapse morphology in stratum lacunosum-moleculare CA1 rat hippocampus: a stereological and three-dimensional ultrastructural study. Neuroscience. 2006;140:597–606. doi: 10.1016/j.neuroscience.2006.02.072. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Öngür D, Price JL. Neuroimaging abnormalities in the subgenual prefrontal cortex: implications for the pathophysiology of familial mood disorders. Mol. Psychiatry. 1998;3:220–226. 190–191. doi: 10.1038/sj.mp.4000370. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct. Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WGM, Lou W, Rapp PR, Morrison JH. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J. Neurosci. 2010;30:7507–7515. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Bolaños CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, Verhaagen J, Nestler EJ. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol. Psychiatry. 2003;54:994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME. Intrinsic fluctuations within cortical systems account for inter-trial variability in human behavior. Neuron. 2007;56:171–184. doi: 10.1016/j.neuron.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Foy MR, Stanton ME, Levine S, Thompson RF. Behavioral stress impairs long-term potentiation in rodent hippocampus. Behav. Neural Biol. 1987;48:138–149. doi: 10.1016/s0163-1047(87)90664-9. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Flugge G, Czeh B. Remodeling of neuronal networks by stress. Front. Biosci. 2006;11:2746–2758. doi: 10.2741/2004. [DOI] [PubMed] [Google Scholar]
- Gersner R, Toth E, Isserles M, Zangen A. Site-specific antidepressant effects of repeated subconvulsive electrical stimulation: potential role of brain-derived neurotrophic factor. Biol. Psychiatry. 2010;67:125–132. doi: 10.1016/j.biopsych.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Golden SA, Covington HE, III, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat. Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, Morrison JH. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009;164:798–808. doi: 10.1016/j.neuroscience.2009.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman JM, Docherty JP. A hypothesized role for dendritic remodeling in the etiology of mood and anxiety disorders. J. Neuropsychiatry Clin. Neurosci. 2010;22:256–264. doi: 10.1176/jnp.2010.22.3.256. [DOI] [PubMed] [Google Scholar]
- Grutzendler J, Kasthuri N, Gan W-B. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420:812–816. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- Gutierrez H, Hale VA, Dolcet X, Davies A. NF-κB signalling regulates the growth of neural processes in the developing PNS and CNS. Development. 2005;132:1713–1726. doi: 10.1242/dev.01702. [DOI] [PubMed] [Google Scholar]
- Gutierrez H, O’Keeffe GW, Gavaldà N, Gallagher D, Davies AM. Nuclear factor κB signaling either stimulates or inhibits neurite growth depending on the phosphorylation status of p65/RelA. J. Neurosci. 2008;28:8246–8256. doi: 10.1523/JNEUROSCI.1941-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häcker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci. STKE. 2006:re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- Harris KM, Kater SB. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu. Rev. Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Majewska AK. Dendritic spine geometry: functional implication and regulation. Neuron. 2005;46:529–532. doi: 10.1016/j.neuron.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Hayashi-Takagi A, Takaki M, Graziane N, Seshadri S, Murdoch H, Dunlop AJ, Makino Y, Seshadri AJ, Ishizuka K, Srivastava DP. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat. Neurosci. 2010;13:327–332. doi: 10.1038/nn.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior; A Neuropsychological Theory. Mahwah, NJ: Wiley; 1949. [Google Scholar]
- Heninger GR, Delgado PL, Charney DS. The revised monoamine theory of depression: a modulatory role for monoamines, based on new findings from monoamine depletion experiments in humans. Pharmacopsychiatry. 1996;29:2–11. doi: 10.1055/s-2007-979535. [DOI] [PubMed] [Google Scholar]
- Higley MJ, Sabatini BL. Calcium signaling in dendrites and spines: practical and functional considerations. Neuron. 2008;59:902–913. doi: 10.1016/j.neuron.2008.08.020. [DOI] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat. Rev. Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Humeau Y, Herry C, Kemp N, Shaban H, Fourcaudot E, Bissière S, Lüthi A. Dendritic spine heterogeneity determines afferent-specific Hebbian plasticity in the amygdala. Neuron. 2005;45:119–131. doi: 10.1016/j.neuron.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH. Molecular biology of learning: modulation of transmitter release. Science. 1982;218:433–443. doi: 10.1126/science.6289442. [DOI] [PubMed] [Google Scholar]
- Kasai H, Hayama T, Ishikawa M, Watanabe S, Yagishita S, Noguchi J. Learning rules and persistence of dendritic spines. Eur. J. Neurosci. 2010;32:241–249. doi: 10.1111/j.1460-9568.2010.07344.x. [DOI] [PubMed] [Google Scholar]
- Keck T, Mrsic-Flogel TD, Vaz Afonso M, Eysel UT, Bonhoeffer T, Hübener M. Massive restructuring of neuronal circuits during functional reorganization of adult visual cortex. Nat. Neurosci. 2008;11:1162–1167. doi: 10.1038/nn.2181. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The effects of stressful life events on depression. Annu. Rev. Psychol. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- Kima T-S, Kima D-J, Leea H, Kimb Y-K. Increased plasma brain-derived neurotrophic factor levels in chronic smokers following unaided smoking cessation. Neurosci. Lett. 2007;423:53–57. doi: 10.1016/j.neulet.2007.05.064. [DOI] [PubMed] [Google Scholar]
- Kiraly DD, Eipper-Mains JE, Mains RE, Eipper BA. Synaptic plasticity, a symphony in GEF. ACS Chem. Neurosci. 2010a;1:348–365. doi: 10.1021/cn100012x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly DD, Ma XM, Mazzone CM, Xin X, Mains RE, Eipper BA. Behavioral and morphological responses to cocaine require kalirin7. Biol. Psychiatry. 2010b;68:249–255. doi: 10.1016/j.biopsych.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas JM, De Boer SF, De Rutter AJ, Meerlo P, Sgoifo A. Social stress in rats and mice. Acta Physiol. Scand. Suppl. 1997;640:69–72. [PubMed] [Google Scholar]
- Koolschijn PCMP, van Haren NEM, Lensvelt-Mulders GJ, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum. Brain Mapp. 2009;30:3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. Linking molecules to mood: new insight into the biology of depression. Am. J. Psychiatry. 2010;167:1305–1320. doi: 10.1176/appi.ajp.2009.10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han M-H, Graham DL, Berton O, Renthal W, Russo SJ, LaPlant Q, Graham A, Lutter M, Lagace DC, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Kudryavtseva NN, Bakshtanovskaya IV, Koryakinaa LA. Social model of depression in mice of C57BL/6J strain. Pharmacol. Biochem. Behav. 1991;38:315–320. doi: 10.1016/0091-3057(91)90284-9. [DOI] [PubMed] [Google Scholar]
- Kugaya A, Sanacora G. Beyond monoamines: glutamatergic function in mood disorders. CNS Spectr. 2005;10:808–819. doi: 10.1017/s1092852900010403. [DOI] [PubMed] [Google Scholar]
- LaPlant Q, Chakravarty S, Vialou V, Mukherjee S, Koo JW, Kalahasti G, Bradbury KR, Taylor SV, Maze I, Kumar A. Role of nuclear factor κB in ovarian hormone-mediated stress hypersensitivity in female mice. Biol. Psychiatry. 2009;65:874–880. doi: 10.1016/j.biopsych.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Durand R, Gradinaru V, Zhang F, Goshen I, Kim D-S, Fenno LE, Ramakrishnan C, Deisseroth K. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature. 2010;465:788–792. doi: 10.1038/nature09108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu R-J, Banasr M, Dwyer JM, Iwata M, Li X-Y, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, Li XY, Aghajanian G, Duman RS. Glutamate N-methyl-d-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol. Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Hessler NA, Malinow R. Activation of post-synaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc. Natl. Acad. Sci. USA. 2009;106:912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Covington HE, III, Chaudhury D, Friedman AK, Sun HS, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonart G, Schoch S, Kaeser PS, Larkin CJ, Südhof TC, Linden DJ. Phosphorylation of RIM1α by PKA triggers presynaptic long-term potentiation at cerebellar parallel fiber synapses. Cell. 2003;115:49–60. doi: 10.1016/s0092-8674(03)00727-x. [DOI] [PubMed] [Google Scholar]
- Lorenzetti V, Allen NB, Whittle S, Yücel M. Amygdala volumes in a sample of current depressed and remitted depressed patients and healthy controls. J. Affect. Disord. 2010;120:112–119. doi: 10.1016/j.jad.2009.04.021. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biol. Psychiatry. 2008;64:461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- Lui S, Wu Q, Qiu L, Yang X, Kuang W, Chan RCK, Huang X, Kemp GJ, Andrea Mechelli DM, Gong Q. Resting-state functional connectivity in treatment-resistant depression. Am. J. Psychiatry. 2011;168:642–648. doi: 10.1176/appi.ajp.2010.10101419. [DOI] [PubMed] [Google Scholar]
- Luo L. Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Annu. Rev. Cell Dev. Biol. 2002;18:601–635. doi: 10.1146/annurev.cellbio.18.031802.150501. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience. 1995;69:83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS, Flügge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J. Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos AM, Verdugo JM, McEwen BS. Chronic stress alters synaptic terminal structure in hippocampus. Proc. Natl. Acad. Sci. USA. 1997;94:14002–14008. doi: 10.1073/pnas.94.25.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti C, Tafi E, Middei S, Rubinacci MA, Restivo L, Ammassari-Teule M, Marie H. Synaptic adaptations of CA1 pyramidal neurons induced by a highly effective combinational antidepressant therapy. Biol. Psychiatry. 2010;67:146–154. doi: 10.1016/j.biopsych.2009.09.017. [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GCR, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D. NF-κB functions in synaptic signaling and behavior. Nat. Neurosci. 2003;6:1072–1078. doi: 10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- Meng Y, Zhang Y, Tregoubov V, Falls DL, Jia Z. Regulation of spine morphology and synaptic function by LIMK and the actin cytoskeleton. Rev. Neurosci. 2003;14:233–240. doi: 10.1515/revneuro.2003.14.3.233. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE., III Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol. Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc. Natl. Acad. Sci. USA. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteggia LM, Luikart B, Barrot M, Theobold D, Malkovska I, Nef S, Parade LF, Nestler EJ. Brain-derived neurotrophic factor conditional knockouts show gender differences in depression-related behaviors. Biol. Psychiatry. 2007;61:187–197. doi: 10.1016/j.biopsych.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Nakayama AY, Luo L. Intracellular signaling pathways that regulate dendritic spine morphogenesis. Hippocampus. 2000;10:582–586. doi: 10.1002/1098-1063(2000)10:5<582::AID-HIPO8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Negishi M, Katoh H. Rho family GTPases and dendrite plasticity. Neuroscientist. 2005;11:187–191. doi: 10.1177/1073858404268768. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newey SE, Velamoor V, Govek E-E, Aelst LV. Rho GTPases, dendritic structure, and mental retardation. J. Neurobiol. 2005;64:58–74. doi: 10.1002/neu.20153. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC. Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature. 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- Noguchi J, Matsuzaki M, Ellis-Davies GC, Kasai H. Spine-neck geometry determines NMDA receptor-dependent Ca2+ signaling in dendrites. Neuron. 2005;46:609–622. doi: 10.1016/j.neuron.2005.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Ouimet CC. Altered dendritic spine density in animal models of depression and in response to antidepressant treatment. Synapse. 2001;42:151–163. doi: 10.1002/syn.10006. [DOI] [PubMed] [Google Scholar]
- Palucha A, Pilc A. The involvement of glutamate in the pathophysiology of depression. Drug News Perspect. 2005;18:262–268. doi: 10.1358/dnp.2005.18.4.908661. [DOI] [PubMed] [Google Scholar]
- Papp M, Willner P, Muscat R. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl.) 1991;104:255–259. doi: 10.1007/BF02244188. [DOI] [PubMed] [Google Scholar]
- Peca J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, Lascola CD, Fu Z, Feng G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier JG, Likhtik E, Filali M, Pare D. Lasting increases in basolateral amygdala activity after emotional arousal: implications for facilitated consolidation of emotional memories. Learn. Mem. 2005;12:96–102. doi: 10.1101/lm.88605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Cahill ME, Jones KA, VanLeeuwen J-E, Woolfrey KM. Convergent CaMK and RacGEF signals control dendritic structure and function. Trends Cell Biol. 2008;18:405–413. doi: 10.1016/j.tcb.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Penzes P, Woolfrey KM, Srivastava DP. Epac2- mediated dendritic spine remodeling: implications for disease. Mol. Cell. Neurosci. 2010;46:368–380. doi: 10.1016/j.mcn.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat. Neurosci. 2011;14:285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrak LJ, Harris KM, Kirov SA. Synaptogenesis on mature hippocampal dendrites occurs via filopodia and immature spines during blocked synaptic transmission. J. Comp. Neurol. 2005;484:183–190. doi: 10.1002/cne.20468. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am. J. Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Quan M, Zheng C, Zhang N, Han D, Tian Y, Zhang T, Yang Z. Impairments of behavior, information flow between thalamus and cortex, and prefrontal cortical synaptic plasticity in an animal model of depression. Brain Res. Bull. 2011;85:109–116. doi: 10.1016/j.brainresbull.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Morrison JH. Repeated stress and structural plasticity in the brain. Ageing Res. Rev. 2005;4:271–287. doi: 10.1016/j.arr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WGM, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb. Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J. Comp. Neurol. 2008;1150:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Venheim ER, Padival M. Chronic stress causes amygdala hyperexcitability in rodents. Biol. Psychiatry. 2010;67:1128–1136. doi: 10.1016/j.biopsych.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Wilkinson MB, Mazei-Robison MS, Dietz DM, Maze I, Krishnan V, Renthal W, Graham A, Birnbaum SG, Green TA. Nuclear factor kappa B signaling regulates neuronal morphology and cocaine reward. J. Neurosci. 2009;29:3529–3537. doi: 10.1523/JNEUROSCI.6173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Dietz DM, Dumitriu D, Morrison JH, Malenka RC, Nestler EJ. The addicted synapse: mechanisms of synaptic and structural plasticity in nucleus accumbens. Trends Neurosci. 2010;2:1–10. doi: 10.1016/j.tins.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rygula R, Domenici AE, Hiemke C, Fuchs E. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav. Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flügge G, Fuchs E, Rüther E, Havemann-Reinecke U. Effects of fluoxetine on behavioral deficits evoked by chronic social stress in rats. Behav. Brain Res. 2006a;174:188–192. doi: 10.1016/j.bbr.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flügge G, Hiemke C, Fuchs E, Rüther E, Havemann-Reinecke U. Citalopram counteracts depressive-like symptoms evoked by chronic social stress in rats. Behav. Pharmacol. 2006b;17:19–29. doi: 10.1097/01.fbp.0000186631.53851.71. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Havemann-Reinecke U, Rüther E, Hiemke C, Zernig G, Fuchs E, Flügge G. Pharmacological validation of a chronic social stress model of depression in rats: effects of reboxetine, haloperidol and diazepam. Behav. Pharmacol. 2008;19:183–196. doi: 10.1097/FBP.0b013e3282fe8871. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ESL, Lopez de Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol. Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Sandi C, Bisaz R. A model for the involvement of neural cell adhesion molecules in stress-related mood disorders. Neuroendocrinology. 2007;85:158–176. doi: 10.1159/000101535. [DOI] [PubMed] [Google Scholar]
- Sandi C, Davies HA, Cordero MI, Rodriguez JJ, Popov VI, Stewart MG. Rapid reversal of stress induced loss of synapses in CA3 of rat hippocampus following water maze training. Eur. J. Neurosci. 2003;17:2447–2456. doi: 10.1046/j.1460-9568.2003.02675.x. [DOI] [PubMed] [Google Scholar]
- Schikorski T, Stevens CF. Quantitative ultrastructural analysis of hippocampal excitatory synapses. J. Neurosci. 1997;17:5858–5867. doi: 10.1523/JNEUROSCI.17-15-05858.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H, Eilers J. Spine neck geometry determines spino-dendritic cross-talk in the presence of mobile endogenous calcium binding proteins. J. Comput. Neurosci. 2009;27:229–243. doi: 10.1007/s10827-009-0139-5. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology. 2010;35:2378–2391. doi: 10.1038/npp.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MV, Trümbach D, Weber P, Wagner K, Scharf SH, Liebl C, Datson N, Namendorf C, Gerlach T, Kühne C. Individual stress vulnerability is predicted by short-term memory and AMPA receptor subunit ratio in the hippocampus. J. Neurosci. 2010;30:16949–16958. doi: 10.1523/JNEUROSCI.4668-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Morrison JH. Stress-induced dendritic remodeling in the medial prefrontal cortex: effects of circuit, hormones and rest. Brain Res. 2009;1293:108–113. doi: 10.1016/j.brainres.2009.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM. The dendritic spine: a multifunctional integrative unit. J. Neurophysiol. 1996;75:2197–2210. doi: 10.1152/jn.1996.75.6.2197. [DOI] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Watanabe H, Komatsu N, Okamura N, Koike K, Shinoda N, Nakazato M, Kumakiri C, Okada S. Serum brain-derived neurotrophic factor (BDNF) levels in schizophrenia are indistinguishable from controls. Neurosci. Lett. 2003;351:111–114. doi: 10.1016/j.neulet.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC-H, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J. Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS. The macrophage theory of depression. Med. Hypotheses. 1991;35:298–306. doi: 10.1016/0306-9877(91)90272-z. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J. Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat. Rev. Neurosci. 2008;9:206–221. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. A new method for screening antidepressants in mice. Behav. Neural Biol. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Stetler C, Miller GE. Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom. Med. 2011;73:114–126. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- Stewart MG, Davies HA, Sandi C, Kraev IV, Rogachevsky VV, Peddie CJ, Rodriguez JJ, Cordero MI, Donohue HS, Gabbott PL. Stress suppresses and learning induces plasticity in CA3 of rat hippocampus: a three-dimensional ultrastructural study of thorny excrescences and their postsynaptic densities. Neuroscience. 2005;131:43–54. doi: 10.1016/j.neuroscience.2004.10.031. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle. Annu. Rev. Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Sunanda, Rao MS, Raju TR. Effect of chronic restraint stress on dendritic spines and excrescences of hippocampal CA3 pyramidal neurons - a quantitative study. Brain Res. 1995;694:312–317. doi: 10.1016/0006-8993(95)00822-8. [DOI] [PubMed] [Google Scholar]
- Svoboda K, Tank DW, Denk W. Direct measurement of coupling between dendritic spines and shafts. Science. 1996;272:716–719. doi: 10.1126/science.272.5262.716. [DOI] [PubMed] [Google Scholar]
- Taliaz D, Stall N, Dar DE, Zangen A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol. Psychiatry. 2010;15:80–92. doi: 10.1038/mp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliaz D, Loya A, Gersner R, Haramati S, Chen A, Zangen A. Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. J. Neurosci. 2011;31:4475–4483. doi: 10.1523/JNEUROSCI.5725-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissot R. The common pathophysiology of monaminergic psychoses: a new hypothesis. Neuropsychobiology. 1975;1:243–260. doi: 10.1159/000117498. [DOI] [PubMed] [Google Scholar]
- Tolias KF, Duman JG, Um K. Control of synapse development and plasticity by Rho GTPase regulatory proteins. Prog. Neurobiol. 2011;94:133–148. doi: 10.1016/j.pneurobio.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat. Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Tsay D, Yuste R. On the electrical function of dendritic spines. Trends Neurosci. 2004;27:77–83. doi: 10.1016/j.tins.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Ur E, White PD, Grossman A. Hypothesis: cytokines may be activated to cause depressive illness and chronic fatigue syndrome. Eur. Arch. Psychiatry Clin. Neurosci. 1992;241:317–322. doi: 10.1007/BF02195983. [DOI] [PubMed] [Google Scholar]
- Van Harreveld A, Fifkova E. Swelling of dendritic spines in the fascia dentata after stimulation of the perforant fibers as a mechanism of post-tetanic potentiation. Exp. Neurol. 1975;49:736–749. doi: 10.1016/0014-4886(75)90055-2. [DOI] [PubMed] [Google Scholar]
- Vialou V, Robison AJ, LaPlant QC, Covington HE, III, Dietz DM, Ohnishi YN, Mouzon E, Rush AJ, III, Watts EL, Wallace DL. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat. Neurosci. 2010;13:745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal R, Pilar-Cuellar F, dos Anjos S, Linge R, Treceno B, Ines Vargas V, Rodriguez-Gaztelumendi A, Mostany R, Castro E, Diaz A. New strategies in the development of antidepressants: towards the modulation of neuroplasticity pathways. Curr. Pharm. Des. 2011;17:521–533. doi: 10.2174/138161211795164086. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am. J. Psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Vyas A, Bernalb S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Res. 2003;965:290–294. doi: 10.1016/s0006-8993(02)04162-8. [DOI] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143:387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Wacker J, Dillon DG, Pizzagalli DA. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. Neuroimage. 2009;46:327–337. doi: 10.1016/j.neuroimage.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Glazer HI. Effects of acute exposure to stressors on subsequent avoidance-escape behavior. Psychosom. Med. 1975;37:499–521. doi: 10.1097/00006842-197511000-00005. [DOI] [PubMed] [Google Scholar]
- Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J. Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- Wilbrecht L, Holtmaat A, Wright N, Fox K, Svoboda K. Structural plasticity underlies experience-dependent functional plasticity of cortical circuits. J. Neurosci. 2010;30:4927–4932. doi: 10.1523/JNEUROSCI.6403-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- Xu L, Anwyl R, Rowan MJ. Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature. 1997;387:497–500. doi: 10.1038/387497a0. [DOI] [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan W-B. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui T, Fujisawa S, Tsukamoto M, Matsuki N, Ikegaya Y. Dynamic synapses as archives of synaptic history: state-dependent redistribution of synaptic efficacy in the rat hippocampal CA1. J. Physiol. 2005;566:143–160. doi: 10.1113/jphysiol.2005.086595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Flory JD, Southwick S, Charney DS. Developing an agenda for translational studies of resilience and vulnerability following trauma exposure. Ann. NY Acad. Sci. 2006;1071:379–396. doi: 10.1196/annals.1364.028. [DOI] [PubMed] [Google Scholar]
- Zahm DS. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci. Biobehav. Rev. 2000;24:85–105. doi: 10.1016/s0149-7634(99)00065-2. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Homma KJ, Poo M-M. Shrinkage of dendritic spines associated with long-term depression of hippocampal synapses. Neuron. 2004;44:749–757. doi: 10.1016/j.neuron.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Zoladz PR, Park CR, Halonen JD, Salim S, Alzoubi KH, Srivareerat M, Fleshner M, Alkadhi KA, Diamond DM. Differential expression of molecular markers of synaptic plasticity in the hippocampus, prefrontal cortex, and amygdala in response to spatial learning, predator exposure, and stress-induced amnesia. Hippocampus. 2011 doi: 10.1002/hipo.20922. [DOI] [PubMed] [Google Scholar]