Abstract
Pulmonary hypertension (PH) in sickle cell disease (SCD) is an emerging and important clinical problem. In a single-institution adult cohort of 365 patients, we investigated lipid and lipoprotein levels and their relationship to markers of intravascular hemolysis, vascular dysfunction and PH. In agreement with prior studies, we confirm significantly decreased plasma levels of total cholesterol, high-density lipoprotein-cholesterol (HDL-C), and low-density lipoprotein-cholesterol (LDL-C) in SCD vs. ethnically-matched healthy controls. Several cholesterol parameters correlate significantly with markers of anemia, but not endothelial activation or PH. More importantly, serum triglyceride levels are significantly elevated in SCD compared to controls. Elevated triglyceride levels correlate significantly with markers of hemolysis (lactate dehydrogenase and arginase; both p<0.0005), endothelial activation (soluble E-selectin, p<0.0001; soluble P-selectin, p=0.02; soluble vascular cell adhesion molecule-1, p=0.01), inflammation (leukocyte count, p=0.0004; erythrocyte sedimentation rate, p=0.02) and PH (amino-terminal brain natriuretic peptide, p=0.002; prevalence of elevated tricuspid regurgitant velocity (TRV), p<0.001). In a multivariate analysis, triglyceride levels correlate independently with elevated TRV (p=0.002). Finally, forearm blood flow studies in adult patients with SCD demonstrate a significant association between increased triglyceride/HDL-C ratio and endothelial dysfunction (p<0.05). These results characterize elevated plasma triglyceride levels as a potential risk factor for PH in SCD.
Introduction
Sickle cell disease (SCD) is a hemoglobinopathy characterized by red cell rigidity, compromised perfusion and tissue infarction. Chronic hemolysis, another pathological feature of SCD drawing increased attention, gives rise to diminished bioavailability of nitric oxide (NO), oxidant stress and endothelial activation(Aslan, et al 2001, Kato, et al 2006, Kaul, et al 2000, Nath, et al 2000, Reiter, et al 2002). It is now appreciated that certain complications of SCD may derive from progressive hemolysis-associated vasculopathy, including pulmonary hypertension (PH), cutaneous leg ulceration, priapism, and possibly stroke(Kato, et al 2007).
In recent years, PH, a proliferative vascular disease of the lung, has been recognized as a major complication and independent correlate with death among adults with SCD. Pulmonary artery (PA) systolic pressure (PASP) can be estimated by Doppler echocardiography, utilizing the tricuspid regurgitant velocity (TRV). A TRV of 2.5–2.9 m/s is at least two standard deviations above the mean and is considered representative of borderline or mildly elevated PASP, whereas a TRV 3.0 m/s of higher, approximately three standard deviations above the mean, represents significantly elevated PASP, often meeting criteria for pulmonary arterial hypertension. Increased TRV is estimated to be present in approximately one-third of adults with SCD and is associated with early mortality(Ataga, et al 2006, Gladwin, et al 2004). In the more severe cases, increased TRV is associated with histopathologic changes such as plexogenic changes and hyperplasia of the pulmonary arterial intima and media(Adedeji, et al 2001, Graham, et al 2007, Haque, et al 2002, Manci, et al 2003). These histopathological changes are very similar to those seen in the arterial wall thickening of atherosclerosis. Indeed, PH and atherosclerosis share several overlapping pathophysiologic features, including vascular smooth muscle proliferation, decreased NO bioavailability, oxidant stress, endothelial dysfunction, endothelial activation, increased levels of endogenous NOS inhibitors, platelet activation, in situ thrombosis, and accelerated renal insufficiency(Kato and Gladwin 2008).
Atherosclerosis is characterized by increased accumulation of cholesterol in arterial wall macrophages and is exacerbated by oxidant stress. PH in SCD is also characterized by oxidant stress caused by intravascular hemolysis, but atheromas are not typically present in SCD patients. This might be due to low levels of plasma total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) in SCD patients(Buchowski, et al 2007, el-Hazmi, et al 1987, el-Hazmi, et al 1995, Marzouki and Khoja 2003, Sasaki, et al 1983, Shores, et al 2003, Stone, et al 1990, Westerman 1975). Thus, the vasculopathy of PH in SCD does not seem attributable to increased TC or LDL-C levels. Intriguingly, however, there have been scattered positive reports of low HDL-C(Sasaki, et al 1983, Stone, et al 1990) and increased triglyceride(Buchowski, et al 2007, Kato, et al 2005, Morris, et al 2005)in SCD patients – features widely recognized in the general population as important contributory factors in cardiovascular disease. Investigation of potential roles for low HDL and high triglyceride levels in the development of PH in SCD therefore is of interest.
Our group has recently shown that besides decreased NO bioavailability, factors associated with PH in SCD include altered apolipoprotein levels and other features shared with atherosclerosis(Hebbel, et al 2004, Kato and Gladwin 2008, Morris, et al 2005, Yuditskaya, et al 2009). Proteomics analysis in a small cohort of 56 patients with and without pulmonary hypertension identified lower apoA-I and suggested higher apoA-II and serum amyloid A levels in SCD patients with PH(Yuditskaya, et al 2009). Furthermore, in a physiological test of endothelial function, patients with lower apoA-I had a blunted vasodilatory response to infusion of the endothelium-dependent vasodilator acetylcholine(Yuditskaya, et al 2009). Patients with SCD have 3-fold higher levels than healthy controls of the endogenous NO synthase inhibitor asymmetric dimethylarginine, particularly in those patients with elevated TRV(Kato, et al 2009, Landburg, et al 2008). Additionally, triglyceride levels have also been suggested to be elevated in patients with increased endothelial activation(Kato, et al 2005) and increased plasma arginase levels(Morris, et al 2005), which in turn were linked to increased pulmonary pressures.
These findings and the therapeutic potential to modulate serum lipids with several commonly used drugs prompted us to investigate in greater detail the serum lipid profile in patients with SCD and possible relationship to vasculopathic complications such as PH. In this study, we present our findings on the status of lipid and lipoprotein levels in a large adult sickle cell cohort at the National Institutes of Health. We confirm decreased serum levels of total cholesterol, LDL-C and HDL-C and increased serum levels of triglycerides in SCD patients, compared to ethnically-matched healthy controls. Decreased total cholesterol, LDL-C and HDL-C were significantly associated with severity of anemia, whereas increased triglyceride levels were associated with hemolysis, vascular dysfunction, and increased prevalence of pulmonary hypertension.
Materials and Methods
Patient/Cohort Characteristics
The National Heart Lung and Blood Institute’s Institutional Review Board approved this protocol (ClinicalTrials.gov identifier NCT00011648). All subjects provided written informed consent. Patients at least 18 years of age with all genotypes of SCD were eligible. Patients with SCD were recruited to participate in the study while they were in steady state, defined as a normal baseline status without acute pain requiring hospitalization. Our patient cohort consisted of 365 patients with SCD (Supplemental Table 1). Nucleotide sequence for the β-globin gene was available for 328 patients, of whom 76% harbor the homozygous SS or the Sβ0-thalassemia mutations, and 24% with either hemoglobin SC or Sβ+-thalassemia double heterozygosity. The characteristics of the cohort were as follows: 56.7% are female and 43.3% are male, ranging in age from 27–45 years with a median age of 33. Thirty-nine percent of the patients were on hydroxycarbamide. 39 African-American control subjects with age and sex distributions similar to those of the patients were also enrolled. All participants were screened prospectively for pulmonary hypertension by echocardiography, measuring the tricuspid regurgitant velocity (TRV) as previously described(Gladwin, et al 2004). Laboratory evaluations were performed in the Clinical Center Department of Laboratory Medicine at the National Institutes of Health by standard clinical laboratory assays, including standard complete blood counts, amino terminal brain natriuretic peptide (NT-proBNP), serum chemistry and lipid panels, including triglycerides, total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol. Glomerular filtration rate was estimated by the abbreviated Modification of Diet in Renal Disease Study (MDRD) equation. Lipid levels were similarly measured at the time patients were enrolled into a forearm blood flow plethysmography study. The patients and the study have been previously described (ClinicalTrials.gov identifier NCT00009581)(Gladwin, et al 2003).
Statistical Methods
Characteristics of study participants are presented as median and interquartile range (IQR) or percentage of participants with a given characteristic (Supplemental Table 1). Comparisons of mean lipid panels (triglycerides, cholesterol, high density lipoprotein, and low density lipoprotein levels) were made using analysis of variance (ANOVA) between healthy African-American controls and subjects with sickle cell disease grouped by phenotype of similar clinical severity (SC, Sβ+-thalassemia or SS, Sβ0-thalassemia). Mean levels of lipid panel variables were also compared by TRV grouped into 3 levels (<2.5, 2.5–2.9, and ≥ 3.0 m/sec) among all sickle cell phenotypes combined. The Cohran-Armitage Chi-Square test for trend was used to examine trends in the prevalence of moderately and highly elevated TRV by quartile of triglyceride level. Bivariate correlations were assessed using the Spearman rank correlation coefficient.
Logistic regression models were used to investigate the associations between a variety of factors, both lipid variables and other characteristics, with TRV. Two separate models were developed to generate odds ratio estimates of risk for subjects with moderate and high TRV (≥2.5 m/s) and subjects with only high TRV (≥ 3 m/s). For continuous variables, odds ratios are given for the 75th relative to the 25th percentile. Assessment of significance was by likelihood ratio test for the overall model and by ratio of coefficient to estimated standard error for the individual predictors. Cox proportional hazards regression was used to examine associations of lipid variables with mortality. All analyses were performed using SAS (version 9.1) or Stata (version 9).
Results
Serum lipid and lipoprotein levels in SCD compared to healthy controls
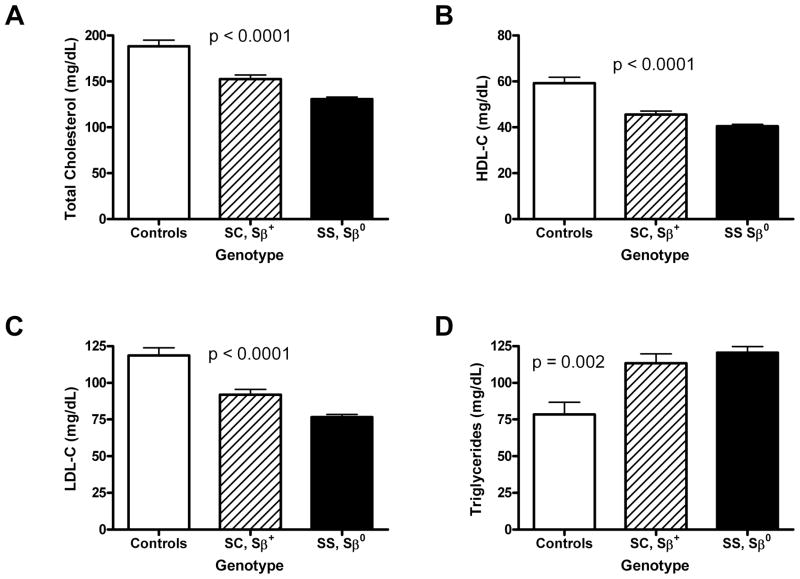
To characterize serum lipid levels in patients with SCD and to explore potential relationships between anemia, hemolysis, vascular disease and dysregulated lipids, we measured serum TC, HDL-C, LDL-C and triglyceride levels in the following three groups with varying degrees of sickle-cell anemia: a) 39 healthy African-American adult control subjects with absence of sickle-cell anemia; b) 78 adults with mild SCD (SC, Sβ+); and c) 250 adults with severe SCD (SS or Sβ0) (Fig. 1). All SCD patients were at steady state. TC, HDL-C and LDL-C levels varied inversely with the severity of the phenotype (Fig. 1A–C): non-anemic controls had highest levels, mildly anemic SC and Sβ+ patients had intermediate levels, and severely anemic SS and Sβ0 patients had the lowest levels of total cholesterol, HDL-C and LDL-C (Fig. 1A–C). In contrast, triglyceride levels were highly elevated in SCD compared to healthy controls, and correlated with SCD phenotype (Fig. 1D). These data establish a relationship between serum lipid levels and SCD severity.
Fig. 1. Serum Lipid Levels in Sickle Cell Patients Compared with Healthy Controls.
Serum lipid levels were determined in either healthy control subjects, patients with mild forms of SCD (SC or Sβ+-thalassemia) or patients with more severe forms of SCD (SS or Sβ0-thalassemia). (A) Serum total cholesterol levels were determined for 39 control subjects 78 patients with SC or Sβ+-thalassemia, or 249 patients with SS or Sβ0-thalassemia. (B) Serum HDL-cholesterol levels were determined for 39 control subjects, 78 patients with SC or Sβ+-thalassemia, or 249 patients with SS or Sβ0-thalassemia. (C) Serum LDL-cholesterol levels were determined for 39 control subjects, 78 patients with SC or Sβ+-thalassemia, or 250 patients with SS or Sβ0-thalassemia. (D) Serum triglyceride levels were determined for 32 control subjects, 77 patients with SC or Sβ+-thalassemia, or 245 patients with SS or Sβ0-thalassemia. Only those patients with genotypes determined by nucleotide sequencing (n=328, total) were included in this analysis. p values were determined from ANOVA F test of difference in means between sickle cell groups and controls.
Patients with the most severe forms of SCD (SS or Sβ0) phenotypes have the most severe hemolytic anemia, with highest prevalence of vascular disease and pulmonary hypertension(Taylor, et al 2008). Because they also have the most extreme disturbance in serum lipid levels, we explored the relationship between lipid parameters, anemia, hemolysis and pulmonary hypertension in greater detail.
Decreased serum cholesterol subclasses correlate with severity of anemia
We first investigated the relationship between cholesterol and anemia. The results in table 1 demonstrate that serum cholesterol (total cholesterol, HDL-C and LDL-C) inversely correlates with hematocrit and hemoglobin in patients with SCD of all phenotypes, indicating that increased cholesterol is associated with less severe anemia and decreased cholesterol is associated with more severe anemia. Consistently, TC, HDL-C and LDL-C all inversely correlated with absolute reticulocyte count and total bilirubin in patients with SCD of all phenotypes (Table 1). Consistent with the results in Fig. 1A–C, Table 1 thus indicates that serum cholesterol (TC, HDL-C and LDL-C) correlates inversely with severity of anemia.
Table 1.
Correlations of serum lipid levels to selected variables in patients with sickle cell disease.
| Total Cholesterol | HDL-C | LDL-C | Triglycerides | |||||
|---|---|---|---|---|---|---|---|---|
| N | r (p) | N | r (p) | N | r (p) | N | r (p) | |
| Hematocrit | 362 | 0.15 (0.004) | 362 | 0.19 (0.0003) | 363 | 0.15 (0.005) | 357 | −0.17 (0.001) |
| Hemoglobin | 362 | 0.15 (0.004) | 362 | 0.18 (0.0006) | 363 | 0.15 (0.003) | 357 | −0.17 (0.0009) |
| Absolute reticulocyte count | 355 | −0.28 (<0.0001) | 355 | −0.30 (<0.0001) | 356 | −0.18 (0.0006) | 350 | n.s. |
| Total bilirubin | 360 | −0.31 (<0.0001) | 360 | −0.28 (<0.0001) | 361 | −0.23 (<0.0001) | 356 | n.s. |
| LDH | 333 | n.s. | 332 | −0.26 (<0.0001) | 333 | n.s. | 329 | 0.20 (0.0004) |
| Arginase | 119 | n.s. | 119 | n.s. | 120 | n.s. | 114 | 0.36 (0.0001) |
| sE-selectin | 152 | n.s. | 153 | n.s. | 154 | n.s. | 147 | 0.33 (<0.0001) |
| sP-selectin | 156 | n.s. | 156 | n.s. | 157 | n.s. | 151 | 0.19 (0.02) |
| sVCAM-1 | 161 | n.s. | 161 | n.s. | 162 | n.s. | 156 | 0.20 (0.01) |
| sICAM-1 | 156 | n.s. | 157 | n.s. | 158 | n.s. | 151 | n.s. |
| WBC count | 360 | −0.02 (0.7) | 360 | −0.19 (0.003) | 361 | 0.04 (0.5) | 355 | 0.19 (0.0004) |
| ESR | 330 | 0.14 (0.01) | 330 | 0.06 (0.3) | 330 | 0.20 (0.002) | 325 | 0.13 (0.02) |
| NT-proBNP | 213 | n.s. | 213 | n.s. | 214 | n.s. | 208 | 0.22 (0.002) |
| Age | 363 | 0.27 (<0.0001) | 363 | 0.23 (<0.0001) | 364 | 0.16 (0.003) | 358 | 0.17 (0.001) |
| ALT | 362 | 0.04 (0.5) | 362 | 0.004 (0.9) | 363 | 0.03 (0.6) | 357 | 0.01 (0.8) |
The level of hemolysis in SCD and other diseases is linked to the development of increased oxidative stress and vascular dysfunction. To determine whether decreased cholesterol levels are associated with increased hemolysis as well as increased severity of anemia, two biomarkers for hemolysis, LDH and arginase, were measured in patients with SCD of all phenotypes. HDL-C levels did show a significant negative association with LDH, but not with arginase. TC and LDL-C did not have significant associations with LDH or arginase. In general, TC and LDL-C appear to correlate more closely to red cell mass than to severity of hemolysis.
Consistent with the hemolysis results, serum cholesterol parameters also did not closely correlate with markers of vascular dysfunction. None of the cholesterol parameters (total cholesterol, HDL-C or LDL-C) showed any significant association to any of the markers for endothelial activation (sE-selectin, sP-selectin, s-ICAM-1 or sVCAM-1) (Table 1). Cholesterol levels were inconsistently associated with markers of inflammation (ESR but not WBC count for TC and LDL; WBC count but not ESR for HDL) (Table 1). Cholesterol levels correlated positively with age but did not correlate with the liver disease marker, serum ALT levels (Table 1).
Pulmonary hypertension is known to correlate with hemolysis and vascular dysfunction. The absence of correlations between serum cholesterol parameters and hemolysis and vascular dysfunction would predict absence of correlations between serum cholesterol parameters and markers for pulmonary hypertension. Indeed, TC, HDL-C and LDL-C levels did not correlate with levels of the PH marker NT-pro BNP (Table 1). These cholesterol parameters were also not significantly associated with TRV, although TC showed an interesting trend (147 mg/dl +/− 45.8 for patients with highly elevated pulmonary pressure (n=57), vs. 134 +/− 33.0 mg/dl for patients with mildly elevated pulmonary pressure (n=94) and 136 +/− 34.8 for patients with normal pulmonary pressure (n=194)) that did not reach significance (p=0.07, ANOVA F test of difference in means between TRV groups). The results were qualitatively similar after adjusting for gender or hydroxycarbamide (not shown).
The combined results firmly establish that TC and LDL-C levels are associated with severity of anemia but not quite so significantly with hemolysis or vascular dysfunction. HDL-C levels are also associated with severity of anemia and, while negatively associated with LDH levels, does not show significant associations with arginase or markers of vascular dysfunction. Consistent with this lack of evidence for vascular dysfunction, serum TC, HDL-C and LDL-C were not associated with NT-proBNP or TRV. Thus, decreased serum cholesterol parameters, while associated with severity of anemia, are not significantly associated with PH.
Serum triglyceride levels correlate with hemolysis and endothelial activation
Serum triglyceride levels correlate significantly with markers of hemolytic severity, including decreased total hemoglobin and hematocrit, and increased LDH and arginase (Table 1). Serum triglyceride levels also correlate positively with other known markers of endothelial activation, including sE-selectin, sP-selectin, and sVCAM-1, as well as inflammatory markers such as WBC count and ESR (Table 1). Proline, a downstream product of arginine metabolism by arginase release with a potential role in fibrosis (for a recent review, see reference (Morris 2007)) is also significantly correlated with triglyceride levels (R=0.41; p<0.0001, n=213). Triglyceride levels correlated positively with age but did not correlate with levels of ALT (Table 1). In sickle cell patients, body mass index (BMI) was a weak but statistically significant predictor of triglyceride levels, with a Spearman r value of 0.15 (p = 0.002). In addition, SCD patients with blood glucose levels above the normal range (i.e., 116 mg/dL or higher) have significantly higher triglyceride levels compared to SCD patients with normal glucose levels (median triglyceride levels 145 vs. 104.5 mg/dL, p = 0.004, Mann-Whitney). Triglyceride levels did not differ significantly between patients that did or did not receive hydroxycarbamide; consistently, adjusting for hydroxycarbamide use did not alter serum triglyceride effects in statistical analyses. The combined data indicate a positive association between serum triglyceride levels and markers of hemolysis and vascular dysfunction.
Serum triglyceride levels independently correlate with markers of pulmonary hypertension
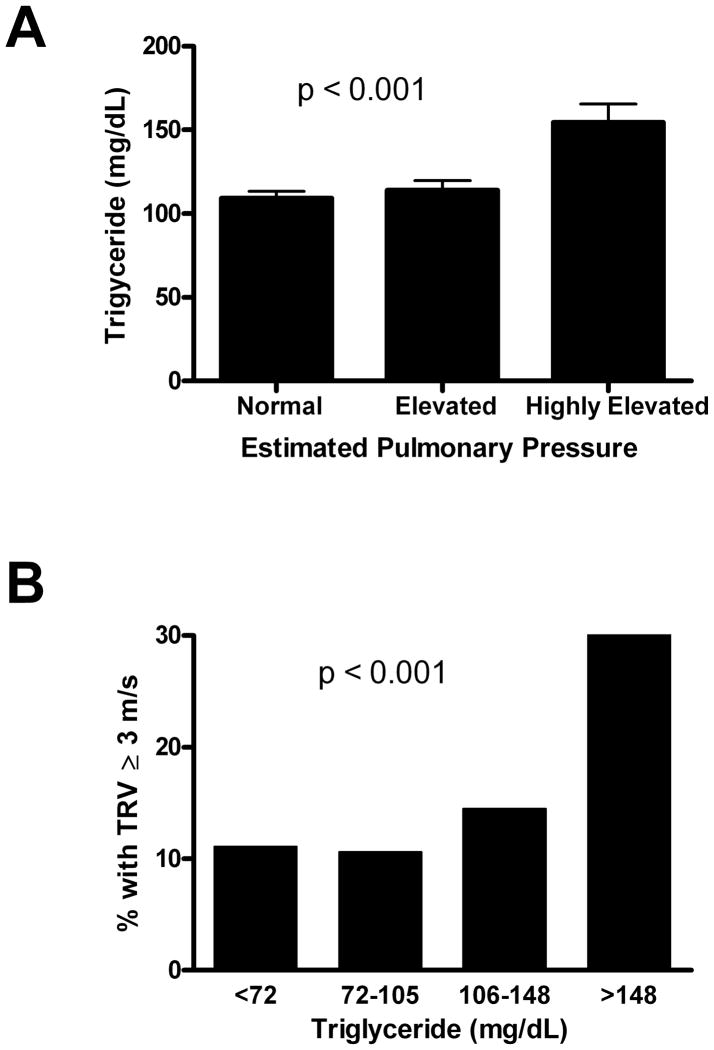
Serum levels of NT-proBNP, a biomarker for pulmonary hypertension, were measured in sickle-cell patients of all phenotypes (Table 1). Serum triglyceride levels and NT-proBNP showed a significant positive association (Table 1). To directly test for correlations between pulmonary hypertension and serum triglyceride levels, TRV levels for SCD patients of all phenotypes were measured and stratified into three groups according to estimated pulmonary pressures: normal (TRV < 2.5 m/sec), mildly elevated (TRV 2.5 – 2.9 m/sec), or highly elevated (TRV ≥ 3.0 m/sec). Serum triglyceride levels were determined for each group. Serum triglyceride levels and TRV showed a significant positive association, as shown in Fig. 2A (p<0.001).
Fig. 2. Serum triglycerides and pulmonary hypertension.
(A) Mean serum triglyceride levels were determined for sickle cell patients (all phenotypes) with pulmonary pressures that were normal (TRV<2.5 m/sec), mildly elevated (2.5 ≤ TRV ≤ 2.9 m/sec) or highly elevated (TRV ≥ 3 m/sec). Statistical significance was determined from ANOVA F test of difference in means between TRV groups. (B) Prevalence of highly elevated pulmonary pressures by triglyceride level among sickle cell patients. Patients were divided into quartiles according to their serum triglyceride levels and the percentage of patients in each quartile with highly elevated pulmonary pressure (TRV ≥ 3 m/sec) was determined. p-values were determined by Cohran-Armitage χ2 test for trend.
In addition, the prevalence of high TRV was increased in quartiles of patients with the highest triglyceride levels. In Fig. 2B, triglyceride levels of SCD patients of all phenotypes were stratified into quartiles, and the percentage of patients with highly elevated TRV (3.0 m/s or higher) in each quartile was calculated. Consistent with Fig. 2A, Fig. 2B demonstrates a higher prevalence of PH in patients with higher serum triglyceride levels in this analysis (P = .0006, χ2 test for trend). The combined data demonstrate a significant correlation between triglycerides and markers of pulmonary hypertension.
Increased systolic blood pressure, decreased transferrin levels, a marker for iron overload, and increased lactate dehydrogenase levels, a marker for hemolysis, in serum have previously been shown to correlate with increased TRV among 195 SCD patients at the National Institutes of Health(Gladwin, et al 2004). The results in Table 2 confirm that these findings remain significant in the current expansion of the previously studied cohort(Gladwin, et al 2004). To determine whether increased serum triglyceride levels are also independently associated with elevated TRV, logistic regression analysis of moderate TRV (≥ 2.5 m/sec) and high TRV (≥ 3.0 m/sec) levels among SCD patients of all phenotypes was performed (Table 2). Triglyceride levels did in fact independently correlate with TRV levels. The odds of a moderate elevation in TRV (≥ 2.5 m/sec) in the 75th vs. the 25th percentile for triglycerides increases by 1.47-fold (p=0.04) whereas the odds of highly elevated TRV (>3.0 m/sec) increase by 2.05 fold (p=0.002) (Table 2). Thus, triglyceride levels are an additional significant correlate of TRV, independent of systolic blood pressure, low transferrin or increased lactate dehydrogenase.
Table 2.
Logistic Regression Analyses of TRV≥ 2.5 m/sec or TRV> 3 m/sec among Sickle Cell Patients.
| TRV ≥ 2.5 m/sec | TRV > 3 m/sec | |||
|---|---|---|---|---|
| Independent Variable | OR (95% CI)1 | P Value | OR (95% CI)1 | P Value |
| Systolic blood pressure | 1.87 (1.3–2.7) | 0.001 | 1.53 (1.0–2.4) | 0.07 |
| Transferrin | 0.37 (0.2–0.6) | <0.0001 | 0.29 (0.2–0.5) | <0.0001 |
| Lactate dehydrogenase | 2.04 (1.4–3.0) | <0.0001 | 3.44 (2.0–6.1) | <0.0001 |
| Triglycerides | 1.47 (1.0–2.1) | 0.04 | 2.05 (1.3–3.2) | 0.002 |
For continuous variables, odds ratio is given for the 75th relative to 25th percentile.
Endothelial dysfunction and triglyceride/HDL-C ratio
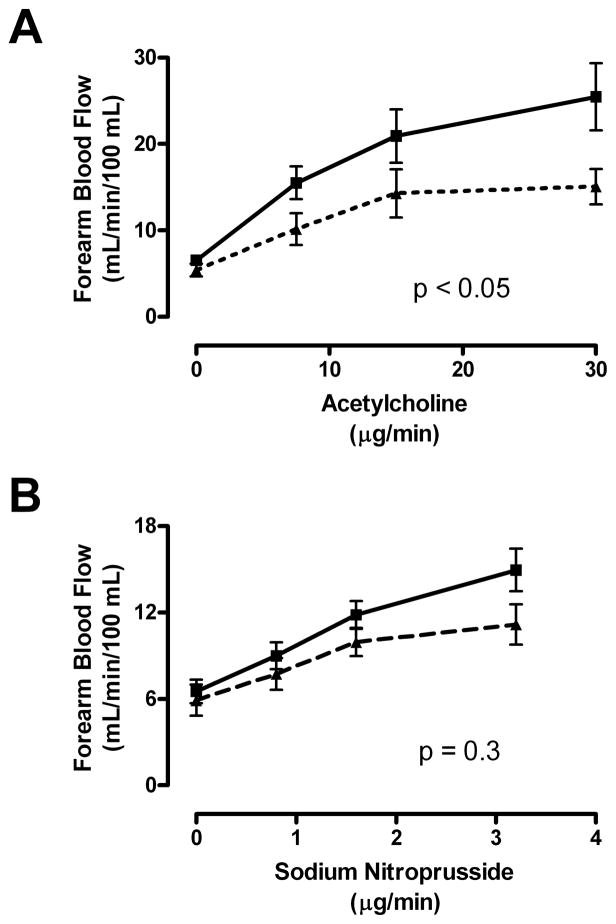
Further biologic support of a link between elevated TG and endothelial dysfunction was sought in 20 adult patients with HbSS who underwent forearm blood flow physiological studies(Gladwin, et al 2003). Patients with HbSS with higher than median levels of TG did not have significantly blunted mean responses to the endothelium-dependent vasodilator acetylcholine (ACh) compared to patients with HbSS with lower than median levels of TG (not shown).
The triglyceride/HDL-cholesterol (TG/HDL-C) ratio, which has been implicated in endothelial dysfunction associated with insulin resistance(Reaven 2002), did, however, associate with endothelial function (Fig. 3). Patients with HbSS with higher than median TG/HDL-C ratios (TG/HDL-C > 2, n = 10) demonstrated remarkably blunted mean responses to the endothelium-dependent vasodilator acetylcholine, but those with lower TG/HDL-C values (TG/HDL-C <2, n = 10) had significantly better vasodilatory responses (P < .001, two-way ANOVA; Fig. 3A). In contrast, the two TG/HDL-C groups showed no significant difference in the rate of blood flow increase in response to the endothelium-independent vasodilator sodium nitroprusside (SNP; Figure 3B). This distinctive pattern of diminished endothelial-dependent response with unaltered endothelial-independent response in HbSS patients with higher TG/HDL-C values is once again parallel to that seen in other disorders of endothelial dysfunction, including atherosclerosis.
Fig. 3. High TG/HDL-C ratio is a marker for endothelial dysfunction.
Forearm blood flow was measured in 20 patients with SCD with venous occlusion plethysmography following test doses of (A) acetylcholine (ACh) and (B) sodium nitroprusside (SNP) infused into the brachial artery. (A) Patients with lower than median TG/HDL-C values (median = 2.0) demonstrated dose-dependent vasodilation to ACh close to previously published normal values (solid line). In sharp contrast, those with higher than median TG/HDL-C values (dashed line) had markedly blunted responses, measured as absolute blood flow (P < .05, 2-way ANOVA). (B) The absolute blood flow at baseline and all doses of SNP did not differ by TG/HDL-C status.
These findings prompted us to ask whether the serum TG/HDL-C ratio was a better predictor of endothelial dysfunction and pulmonary hypertension than serum TG levels alone. While the TG/HDL-C ratio is indeed a good marker for endothelial dysfunction (Supplemental Table 2), it does not correlate better than TG alone with genotype, estimated pulmonary pressure or prevalence of pulmonary hypertension (Supplementary Figures 1A–C). Moreover, logistic regression analysis of moderate TRV (≥ 2.5 m/sec) and high TRV (≥ 3.0 m/sec) levels among SCD patients of all phenotypes indicated that the TG/HDL-C ratio did not predict TRV levels independent of systolic blood pressure, transferrin, and lactate dehydrogenase. Thus, the serum TG/HDL-C ratio was a better predictor of endothelial dysfunction but was not a better predictor of pulmonary hypertension than serum TG levels alone. The combined data indicate that whereas HDL levels do factor into forearm blood flow response to acetylcholine and other markers of endothelial dysfunction, TG levels appear to have greater predictive value in estimating increased risk of pulmonary hypertension.
Discussion
Hypocholesterolemia and, to a lesser extent, hypertriglyceridemia have been documented in SCD cohorts worldwide for over 40 years, yet the mechanistic basis and physiological ramifications of these altered lipid levels have yet to be fully elucidated. Here we examine serum levels of TC, HDL-C, LDL-C and triglyceride in a large cohort of SCD patients from NIH and their relationships to severity of anemia, markers of hemolysis and vascular dysfunction, and pulmonary hypertension. Cholesterol (TC, HDL-C and LDL-C) levels decreased and triglyceride levels increased in relation to severity of anemia. While not true for cholesterol levels, triglyceride levels show a strong correlation with markers of severity of hemolysis, endothelial activation, and pulmonary hypertension. Logistic regression analysis indicated that triglyceride levels are a significant correlate of TRV, independent of systolic blood pressure, low transferrin or increased lactate dehydrogenase, all previously reported correlates of PH in SCD.
Decreased TC and LDL-C in SCD has been documented in virtually every study that examined lipids in SCD adults(el-Hazmi, et al 1987, el-Hazmi, et al 1995, Marzouki and Khoja 2003, Sasaki, et al 1983, Shores, et al 2003, Stone, et al 1990, Westerman 1975), with slightly more variable results in SCD children. Several studies have described increased cholesterol content per RBC associated with decreased plasma or serum cholesterol in SCD(Akinyanju and Akinyanju 1976, Marzouki and Khoja 2003, Muskiet and Muskiet 1984, Sasaki, et al 1983, Westerman, et al 1979). Although it might be hypothesized that SCD hypocholesterolemia results from increased cholesterol utilization during the increased erythropoiesis of SCD, cholesterol is largely conserved through the enterohepatic circulation, at least in healthy individuals, and biogenesis of new RBC membranes would likely use recycled cholesterol from the hemolyzed RBCs. Westerman demonstrated that hypocholesterolemia was not due merely to increased RBC synthesis by showing that it is present in both hemolytic and non-hemolytic anemia(Westerman 1975). Serum cholesterol is proportional to the hematocrit, suggesting serum cholesterol may be in equilibrium with the cholesterol reservoir of the total red cell mass(Westerman 1975). Consistent with such equilibration, tritiated cholesterol incorporated into sickled erythrocytes is rapidly exchanged with plasma lipoproteins(Ngogang, et al 1989). Thus, low plasma cholesterol appears to be a consequence of anemia itself rather than increased RBC production(Westerman 1975).
Total cholesterol, in particular LDL-C, has a well-established role in atherosclerosis. The low levels of LDL-C in SCD are consistent with the low levels of total cholesterol and the virtual absence of atherosclerosis among SCD patients. Our results also clearly show a decrease in HDL-C in SCD vs. controls. Decreased HDL-C in SCD has been documented in some but not all previous studies(Sasaki, et al 1983, Stone, et al 1990). As in lipid studies for other disorders in which HDL-C is variably low, potential reasons for inconsistencies between studies include differences in age, diet, weight, smoking, gender, small sample sizes, different ranges of disease severity, and other diseases and treatments(Choy and Sattar 2009, Gotto A 2003). Decreased HDL-C and apoA-I is a known risk factor for endothelial dysfunction in the general population and in SCD, a potential contributor in SCD to PH, although the latter effect size might be small(Yuditskaya, et al 2009).
Our studies also convincingly show increased triglyceride levels in serum of SCD vs. control subjects. Triglycerides have not been as widely studied as cholesterol in SCD, and previous studies have given mixed results. Increased triglyceride have been reported in several previous studies of SCD adults(Buchowski, et al 2007, Kato, et al 2005, Morris, et al 2005); in addition, triglyceride levels were found to increase during crisis. However, two studies did not find increased triglyceride levels in adult SCD patients(Shores, et al 2003). In children, one study found increased triglyceride whereas another reported normal triglyceride in SCD. Potential reasons for inconsistencies between studies are similar to reasons for inconsistencies in HDL-C noted above. In addition, of all dietary lipids, plasma triglyceride levels are the most dependent on fasting vs. nonfasting status of the subject at the time blood is drawn, and in nonfasting subjects, the amount and type of fat or fatty acids in the diet and time elapsed since the fats were consumed can strongly affect the blood triglyceride levels.
Why is increased triglyceride but not cholesterol in serum associated with vascular dysfunction and pulmonary hypertension? Studies in atherosclerosis have firmly established that lipolysis of oxidized LDL in particular results in vascular dysfunction. Lipolysis of triglycerides present in triglyceride-rich lipoproteins releases neutral and oxidized free fatty acids that induce endothelial cell inflammation(Wang, et al 2009). Many oxidized fatty acids are more damaging to the endothelium than their non-oxidized precursors; for example, 13-hydroxy octadecadienoic acid (13-HODE) is a more potent inducer of ROS activity in HAECs than linoleate, the nonoxidized precursor of 13-HODE(Wang, et al 2009). Lipolytic generation of arachadonic acid, eicosanoids, and inflammatory molecules leading to vascular dysfunction is a well-established phenomenon(Boyanovsky and Webb 2009). Although LDL-C levels are decreased in SCD patients, LDL from SCD patients is more susceptible to oxidation and cytotoxicity to endothelium(Belcher, et al 1999)and an unfavorable plasma fatty acid composition has been associated with clinical severity of SCD(Ren, et al 2006). Lipolysis of phospholipids in lipoproteins or cell membranes by secretory phospholipase A2 (sPLA2) family members releases similarly harmful fatty acids, particularly in an oxidative environment(Boyanovsky and Webb 2009)and in fact selective PLA2 inhibitors are currently under development as potential therapeutic agents for atherosclerotic cardiovascular disease(Rosenson 2009). Finally, sPLA2 activity has been linked to lung disease in SCD. sPLA2 is elevated in acute chest syndrome of SCD and in conjunction with fever preliminarily appears to be a good biomarker for diagnosis, prediction and prevention of acute chest syndrome(Styles, et al 2000). The deleterious effects of phospholipid hydrolysis on lung vasculature predicts similar deleterious effects of triglyceride hydrolysis, particularly in the oxidatively stressed environment of SCD.
Elevated triglycerides have been documented in autoimmune inflammatory diseases with increased risk of vascular dysfunction and pulmonary hypertension, including systemic lupus erythematosus, scleroderma, rheumatoid arthritis, and mixed connective tissue diseases(Choy and Sattar 2009, Galie, et al 2005). In fact, triglyceride concentration is a stronger predictor of stroke than LDL-C or TC(Amarenco and Labreuche 2009). Even in healthy control subjects, a high-fat meal induces oxidative stress and inflammation, resulting in endothelial dysfunction and vasoconstriction(O’Keefe, et al 2008). Perhaps having high levels of plasma triglycerides promotes vascular dysfunction, with the clinical outcome of vasculopathy mainly in the coronary and cerebral arteries in the general population, and with more targeting to the pulmonary vascular bed in SCD and autoimmune diseases. Using forearm blood flow physiological studies, we demonstrated a link between endothelial dysfunction and increased TG/HDL-C ratio, previously proposed as an index of vascular dysfunction(Reaven 2002). The common factor in the above examples, regardless of mechanism, is an increase in plasma triglyceride levels. The combined data indicate that increased serum triglycerides and vascular dysfunction are linked not only to each other but to pulmonary hypertension as well.
The mechanisms leading to hypocholesterolemia and hypertriglyceridemia in plasma or serum of SCD patients are not completely understood. In normal individuals, triglyceride levels are determined to a significant degree by body weight, diet and physical exercise, as well as concurrent diabetes. While data on diet and physical exercise were not available for our study population, these factors very likely impact body weight and triglyceride levels in SCD patients. These findings indicate that standard risk factors for high triglycerides are also relevant to SCD patients. Mechanisms of SCD-specific risk factors for elevated plasma triglycerides are not as clear. RBCs do not have de novo lipid synthesis (Kuypers 2008). In SCD the rate of triglyceride synthesis from glycerol is elevated up to 4-fold in sickled reticulocytes (Lane, et al 1976), but SCD patients have defects in postabsorptive plasma homeostasis of fatty acids (Buchowski, et al 2007). Lipoproteins and albumin in plasma can contribute fatty acids to red blood cells for incorporation into membrane phospholipids (Kuypers 2008), but RBC membranes are not triglyceride-rich and contributions of RBCs to plasma triglyceride levels have not been described, to our knowledge. Interestingly, chronic intermittent or stable hypoxia just by exposure to high altitudes, with no underlying disease, is sufficient to increase triglyceride levels in healthy subjects (Siques, et al 2007). Thus, it is possible that hypoxia in SCD may contribute at least partially to the observed increase in serum triglyceride. Finally, there is a known link of low cholesterol and increased triglycerides that occurs in any primate acute phase response, such as infection and inflammation (Khovidhunkit, et al 2004). Perhaps because of their chronic hemolysis, SCD patients have a low level of acute phase response, which is also consistent with the other inflammatory markers. Further studies are required to elucidate the mechanisms leading to hypocholesterolemia and hypertriglyceridemia in SCD.
Our past and current studies indicate that pulmonary hypertension is a disease of the vasculature that shows many similarities with the vascular dysfunction that occurs in coronary atherosclerosis (Kato and Gladwin 2008). The similarities and differences are: They both have proliferative vascular smooth muscle cells – just in different vascular beds. They both have an impaired nitric oxide axis, increased oxidant stress, and vascular dysfunction. Most importantly, serum triglyceride levels, previously linked to vascular dysfunction, are definitely shown to correlate with NT-proBNP and TRV and thus, with pulmonary hypertension. Moreover, triglyceride levels are predictive of TRV independent of systolic blood pressure, low transferrin or increased lactate dehydrogenase.
Conclusions
To our knowledge, this constitutes the first evidence that hypertriglyceridemia in SCD is correlated with markers of intravascular hemolysis, vascular dysfunction, and pulmonary hypertension. Further study is needed to explore the mechanistic basis for these correlations; however, one possibility is that the fatty acids carried by triglycerides may become oxidized in SCD patients with hemolytic oxidative stress and serve as signaling molecules, or alternatively, hemolytic oxidative stress may coordinately regulate these pathways. Our findings provide a link between plasma lipids, particularly triglyceride levels, and vascular dysfunction in SCD. They support a model in which known risk factors in the general population for atherosclerosis may often also be risk factors for pulmonary hypertension in patients with SCD and suggest that lifestyle changes and lipid-lowering drug treatments used for the prevention of cardiovascular disease should be investigated for their ability to ameliorate the long-term vascular consequences of SCD.
Acknowledgments
The authors gratefully acknowledge the contributions of James Nichols, Amy Chi, Catherine Seamon, Marlene Peters-Lawrence, Oswaldo Castro and Jane Little, and protocol management by Mary K. Hall.
Footnotes
Authorship Contributions:
Suzana Zorca: Analyzed data, wrote manuscript
Lita Freeman: Analyzed data, wrote manuscript
Mariana Hildesheim: Performed statistical analysis
Darlene Allen: Enrolled subjects, collected clinical research data
Alan Remaley: Supervised lipid panel assays, edited manuscript
James Taylor: Enrolled subjects, performed gene sequencing analysis, edited manuscript
Gregory Kato: Generated hypothesis, analyzed data, wrote manuscript, enrolled subjects and supervised enrollment.
Disclosure of Conflicts of Interest: None.
References
- Adedeji MO, Cespedes J, Allen K, Subramony C, Hughson MD. Pulmonary thrombotic arteriopathy in patients with sickle cell disease. Arch Pathol Lab Med. 2001;125:1436–1441. doi: 10.5858/2001-125-1436-PTAIPW. [DOI] [PubMed] [Google Scholar]
- Akinyanju PA, Akinyanju CO. Plasma and red cell lipids in sickle cell disease. Ann Clin Lab Sci. 1976;6:521–524. [PubMed] [Google Scholar]
- Amarenco P, Labreuche J. Lipid management in the prevention of stroke: review and updated meta-analysis of statins for stroke prevention. Lancet Neurol. 2009;8:453–463. doi: 10.1016/S1474-4422(09)70058-4. [DOI] [PubMed] [Google Scholar]
- Aslan M, Ryan TM, Adler B, Townes TM, Parks DA, Thompson JA, Tousson A, Gladwin MT, Patel RP, Tarpey MM, Batinic-Haberle I, White CR, Freeman BA. Oxygen radical inhibition of nitric oxide-dependent vascular function in sickle cell disease. Proc Natl Acad Sci U S A. 2001;98:15215–15220. doi: 10.1073/pnas.221292098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataga KI, Moore CG, Jones S, Olajide O, Strayhorn D, Hinderliter A, Orringer EP. Pulmonary hypertension in patients with sickle cell disease: a longitudinal study. Br J Haematol. 2006;134:109–115. doi: 10.1111/j.1365-2141.2006.06110.x. [DOI] [PubMed] [Google Scholar]
- Belcher JD, Marker PH, Geiger P, Girotti AW, Steinberg MH, Hebbel RP, Vercellotti GM. Low-density lipoprotein susceptibility to oxidation and cytotoxicity to endothelium in sickle cell anemia. J Lab Clin Med. 1999;133:605–612. doi: 10.1016/s0022-2143(99)90191-9. [DOI] [PubMed] [Google Scholar]
- Boyanovsky BB, Webb NR. Biology of secretory phospholipase A2. Cardiovasc Drugs Ther. 2009;23:61–72. doi: 10.1007/s10557-008-6134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchowski MS, Swift LL, Akohoue SA, Shankar SM, Flakoll PJ, Abumrad N. Defects in postabsorptive plasma homeostasis of fatty acids in sickle cell disease. JPEN J Parenter Enteral Nutr. 2007;31:263–268. doi: 10.1177/0148607107031004263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy E, Sattar N. Interpreting lipid levels in the context of high-grade inflammatory states with a focus on rheumatoid arthritis: a challenge to conventional cardiovascular risk actions. Ann Rheum Dis. 2009;68:460–469. doi: 10.1136/ard.2008.101964. [DOI] [PubMed] [Google Scholar]
- el-Hazmi MA, Jabbar FA, Warsy AS. Cholesterol and triglyceride level in patients with sickle cell anaemia. Scand J Clin Lab Invest. 1987;47:351–354. [PubMed] [Google Scholar]
- el-Hazmi MA, Warsy AS, al-Swailem A, al-Swailem A, Bahakim H. Red cell genetic disorders and plasma lipids. J Trop Pediatr. 1995;41:202–205. doi: 10.1093/tropej/41.4.202. [DOI] [PubMed] [Google Scholar]
- Galie N, Manes A, Farahani KV, Pelino F, Palazzini M, Negro L, Romanazzi S, Branzi A. Pulmonary arterial hypertension associated to connective tissue diseases. Lupus. 2005;14:713–717. doi: 10.1191/0961203305lu2206oa. [DOI] [PubMed] [Google Scholar]
- Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, Brown B, Coles WA, Nichols JS, Ernst I, Hunter LA, Blackwelder WC, Schechter AN, Rodgers GP, Castro O, Ognibene FP. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med. 2004;350:886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- Gladwin MT, Schechter AN, Ognibene FP, Coles WA, Reiter CD, Schenke WH, Csako G, Waclawiw MA, Panza JA, Cannon RO., 3rd Divergent nitric oxide bioavailability in men and women with sickle cell disease. Circulation. 2003;107:271–278. doi: 10.1161/01.cir.0000044943.12533.a8. [DOI] [PubMed] [Google Scholar]
- Gotto APH. Manual of Lipid Disorders: Reducing the Risk for Coronary Heart Disease. Lippincott, Williams and Wilkins; Philadelphia, PA: 2003. [Google Scholar]
- Graham JK, Mosunjac M, Hanzlick RL, Mosunjac M. Sickle cell lung disease and sudden death: a retrospective/prospective study of 21 autopsy cases and literature review. Am J Forensic Med Pathol. 2007;28:168–172. doi: 10.1097/01.paf.0000257397.92466.50. [DOI] [PubMed] [Google Scholar]
- Haque AK, Gokhale S, Rampy BA, Adegboyega P, Duarte A, Saldana MJ. Pulmonary hypertension in sickle cell hemoglobinopathy: a clinicopathologic study of 20 cases. Hum Pathol. 2002;33:1037–1043. doi: 10.1053/hupa.2002.128059. [DOI] [PubMed] [Google Scholar]
- Hebbel RP, Osarogiagbon R, Kaul D. The endothelial biology of sickle cell disease: inflammation and a chronic vasculopathy. Microcirculation. 2004;11:129–151. [PubMed] [Google Scholar]
- Kato GJ, Gladwin MT. Evolution of novel small-molecule therapeutics targeting sickle cell vasculopathy. Jama. 2008;300:2638–2646. doi: 10.1001/jama.2008.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2007;21:37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato GJ, Martyr S, Blackwelder WC, Nichols JS, Coles WA, Hunter LA, Brennan ML, Hazen SL, Gladwin MT. Levels of soluble endothelium-derived adhesion molecules in patients with sickle cell disease are associated with pulmonary hypertension, organ dysfunction, and mortality. Br J Haematol. 2005;130:943–953. doi: 10.1111/j.1365-2141.2005.05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato GJ, McGowan V, Machado RF, Little JA, Taylor Jt, Morris CR, Nichols JS, Wang X, Poljakovic M, Morris SM, Jr, Gladwin MT. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension, and death in patients with sickle cell disease. Blood. 2006;107:2279–2285. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato GJ, Wang Z, Machado RF, Blackwelder WC, Taylor JGt, Hazen SL. Endogenous nitric oxide synthase inhibitors in sickle cell disease: abnormal levels and correlations with pulmonary hypertension, desaturation, haemolysis, organ dysfunction and death. Br J Haematol. 2009;145:506–513. doi: 10.1111/j.1365-2141.2009.07658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul DK, Liu XD, Fabry ME, Nagel RL. Impaired nitric oxide-mediated vasodilation in transgenic sickle mouse. Am J Physiol Heart Circ Physiol. 2000;278:H1799–1806. doi: 10.1152/ajpheart.2000.278.6.H1799. [DOI] [PubMed] [Google Scholar]
- Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45:1169–1196. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- Kuypers FA. Red cell membrane lipids in hemoglobinopathies. Curr Mol Med. 2008;8:633–638. doi: 10.2174/156652408786241429. [DOI] [PubMed] [Google Scholar]
- Landburg PP, Teerlink T, Muskiet FA, Duits AJ, Schnog JJ. Plasma concentrations of asymmetric dimethylarginine, an endogenous nitric oxide synthase inhibitor, are elevated in sickle cell patients but do not increase further during painful crisis. Am J Hematol. 2008;83:577–579. doi: 10.1002/ajh.21184. [DOI] [PubMed] [Google Scholar]
- Lane TA, Ballas SK, Burka ER. Lipid synthesis in human erythroid cells: the effect of sickling. Blood. 1976;47:189–195. [PubMed] [Google Scholar]
- Manci EA, Culberson DE, Yang YM, Gardner TM, Powell R, Haynes J, Jr, Shah AK, Mankad VN. Causes of death in sickle cell disease: an autopsy study. Br J Haematol. 2003;123:359–365. doi: 10.1046/j.1365-2141.2003.04594.x. [DOI] [PubMed] [Google Scholar]
- Marzouki ZM, Khoja SM. Plasma and red blood cells membrane lipid concentration of sickle cell disease patients. Saudi Med J. 2003;24:376–379. [PubMed] [Google Scholar]
- Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, Hazen SL, Vichinsky EP, Morris SM, Jr, Gladwin MT. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. Jama. 2005;294:81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SM., Jr Arginine metabolism: boundaries of our knowledge. J Nutr. 2007;137:1602S–1609S. doi: 10.1093/jn/137.6.1602S. [DOI] [PubMed] [Google Scholar]
- Muskiet FD, Muskiet FA. Lipids, fatty acids and trace elements in plasma and erythrocytes of pediatric patients with homozygous sickle cell disease. Clin Chim Acta. 1984;142:1–10. doi: 10.1016/0009-8981(84)90095-0. [DOI] [PubMed] [Google Scholar]
- Nath KA, Shah V, Haggard JJ, Croatt AJ, Smith LA, Hebbel RP, Katusic ZS. Mechanisms of vascular instability in a transgenic mouse model of sickle cell disease. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1949–1955. doi: 10.1152/ajpregu.2000.279.6.R1949. [DOI] [PubMed] [Google Scholar]
- Ngogang J, Mouray H, Lebreton de Vonne T, Raisonnier A. Erythrocyte and plasma cholesterol exchange in sickle cell anemia. Clin Chim Acta. 1989;179:295–304. doi: 10.1016/0009-8981(89)90092-2. [DOI] [PubMed] [Google Scholar]
- O’Keefe JH, Gheewala NM, O’Keefe JO. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J Am Coll Cardiol. 2008;51:249–255. doi: 10.1016/j.jacc.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Reaven G. Metabolic syndrome: pathophysiology and implications for management of cardiovascular disease. Circulation. 2002;106:286–288. doi: 10.1161/01.cir.0000019884.36724.d9. [DOI] [PubMed] [Google Scholar]
- Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, 3rd, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- Ren H, Ghebremeskel K, Okpala I, Ugochukwu CC, Crawford M, Ibegbulam O. Abnormality of erythrocyte membrane n-3 long chain polyunsaturated fatty acids in sickle cell haemoglobin C (HbSC) disease is not as remarkable as in sickle cell anaemia (HbSS) Prostaglandins Leukot Essent Fatty Acids. 2006;74:1–6. doi: 10.1016/j.plefa.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Rosenson RS. Future role for selective phospholipase A2 inhibitors in the prevention of atherosclerotic cardiovascular disease. Cardiovasc Drugs Ther. 2009;23:93–101. doi: 10.1007/s10557-008-6148-1. [DOI] [PubMed] [Google Scholar]
- Sasaki J, Waterman MR, Buchanan GR, Cottam GL. Plasma and erythrocyte lipids in sickle cell anaemia. Clin Lab Haematol. 1983;5:35–44. doi: 10.1111/j.1365-2257.1983.tb00494.x. [DOI] [PubMed] [Google Scholar]
- Shores J, Peterson J, VanderJagt D, Glew RH. Reduced cholesterol levels in African-American adults with sickle cell disease. J Natl Med Assoc. 2003;95:813–817. [PMC free article] [PubMed] [Google Scholar]
- Siques P, Brito J, Leon-Velarde F, Barrios L, De La Cruz JJ, Lopez V, Herruzo R. Hematological and lipid profile changes in sea-level natives after exposure to 3550-m altitude for 8 months. High Alt Med Biol. 2007;8:286–295. doi: 10.1089/ham.2007.8405. [DOI] [PubMed] [Google Scholar]
- Stone WL, Payne PH, Adebonojo FO. Plasma-vitamin E and low plasma lipoprotein levels in sickle cell anemia patients. J Assoc Acad Minor Phys. 1990;1:12–16. [PubMed] [Google Scholar]
- Styles LA, Aarsman AJ, Vichinsky EP, Kuypers FA. Secretory phospholipase A(2) predicts impending acute chest syndrome in sickle cell disease. Blood. 2000;96:3276–3278. [PubMed] [Google Scholar]
- Taylor JGt, Ackah D, Cobb C, Orr N, Percy MJ, Sachdev V, Machado R, Castro O, Kato GJ, Chanock SJ, Gladwin MT. Mutations and polymorphisms in hemoglobin genes and the risk of pulmonary hypertension and death in sickle cell disease. Am J Hematol. 2008;83:6–14. doi: 10.1002/ajh.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Gill R, Pedersen TL, Higgins LJ, Newman JW, Rutledge JC. Triglyceride-rich lipoprotein lipolysis releases neutral and oxidized FFAs that induce endothelial cell inflammation. J Lipid Res. 2009;50:204–213. doi: 10.1194/jlr.M700505-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerman MP. Hypocholesterolaemia and anaemia. Br J Haematol. 1975;31:87–94. doi: 10.1111/j.1365-2141.1975.tb00835.x. [DOI] [PubMed] [Google Scholar]
- Westerman MP, Diloy-Puray M, Streczyn M. Membrane components in the red cells of patients with sickle cell anemia. Relationship to cell aging and to irreversibility of sickling. Biochim Biophys Acta. 1979;557:149–155. doi: 10.1016/0005-2736(79)90097-x. [DOI] [PubMed] [Google Scholar]
- Yuditskaya S, Tumblin A, Hoehn GT, Wang G, Drake SK, Xu X, Ying S, Chi AH, Remaley AT, Shen RF, Munson PJ, Suffredini AF, Kato GJ. Proteomic identification of altered apolipoprotein patterns in pulmonary hypertension and vasculopathy of sickle cell disease. Blood. 2009;113:1122–1128. doi: 10.1182/blood-2008-03-142604. [DOI] [PMC free article] [PubMed] [Google Scholar]