SUMMARY
Homeostatic control of oxygen availability allows cells to survive oxygen deprivation. Although the transcription factor hypoxia-inducible factor 1α (HIF-1α) is the main regulator of the hypoxic response, the upstream mechanisms required for its stabilization remain elusive. Here, we show that p75 neurotrophin receptor (p75NTR) undergoes hypoxia-induced γ-secretase-dependent cleavage to provide a positive feed-forward mechanism required for oxygen-dependent HIF-1α stabilization. The intracellular domain of p75NTR directly interacts with the evolutionary conserved zinc finger domains of the E3 RING ubiquitin ligase seven in absentia homologue 2 (Siah2), which regulates HIF-1α degradation. p75NTR stabilizes Siah2 by decreasing its auto-ubiquitination. Genetic loss of p75NTR dramatically decreases Siah2 abundance, HIF-1α stabilization and induction of HIF-1α target genes in hypoxia.p75NTR−/− mice show reduced HIF-1α stabilization, vascular endothelial growth factor (VEGF) expression and neoangiogenesis after retinal hypoxia. Thus, hypoxia-induced intramembrane proteolysis of p75NTR constitutes an apical oxygen-dependent mechanism to control the magnitude of the hypoxic response.
INTRODUCTION
Oxygen regulates fundamental cellular functions, such as energy production, synthesis of cellular constituents and enzymatic activities (Semenza, 2007). Aerobic organisms have evolved sophisticated cellular processes to sense oxygen gradients and maintain adequate oxygen supply for metabolic functions. The heterodimeric transcription factor hypoxia-inducible factor 1 α (HIF-1α) is a master regulator of cellular adaptation to hypoxia in physiology and a wide range of diseases, such as ischemic disorders and cancer (Semenza, 2000). HIF-1α orchestrates the genetic response to hypoxia by activating the transcription of a wide set of genes that regulate multiple biological processes, including cellular proliferation, survival, angiogenesis, energy, and metabolism (Majmundar et al., 2010). In response to high oxygen levels, prolyl hydroxylases (PHDs) degrade HIF-1α, while in low-oxygen conditions PHDs cease their enzymatic activity to allow instantaneous stabilization of HIF-1α (Semenza, 2001a). Attenuation of PHD activity in low oxygen levels is facilitated by the upregulation of seven in absentia homologue 2 (Siah2), a RING finger E3 ubiquitin ligase that targets substrate proteins for proteasomal degradation (Nakayama et al., 2004). Although the downstream role of Siah2 as a regulator of PHD abundance and HIF-1α degradation is well defined, the upstream adaptor proteins required for Siah2-mediated, oxygen-dependent stabilization of HIF-1α during hypoxia are unknown.
In the present study, we examine the crosstalk between p75NTR signaling and the HIF-1α-mediated hypoxic response. p75NTR, a member of the tumor necrosis factor receptor superfamily, is upregulated during development and in various pathologic conditions, including CNS injury and disease (Chao, 2003). p75NTR is widely expressed in non-neuronal tissues and mediates diverse cellular functions, including apoptosis (Lee et al., 2001), differentiation (Deponti et al., 2009; Passino et al., 2007), myelination (Cosgaya et al., 2002), and extracellular matrix remodeling (Sachs et al., 2007). Like amyloid precursor protein (APP) and Notch, p75NTR undergoes γ-secretase-dependent regulated intramembrane proteolysis (Jung et al., 2003). Ectodomain shedding of p75NTR by α-secretases, such as ADAM metalloproteases, results in the formation of a membrane-bound carboxy-terminal fragment (CTF), a prerequisite for a second proteolytic cleavage by a γ-secretase complex that targets the CTF to liberate the soluble intracellular domain (p75ICD) (Bronfman, 2007). The p75ICD has signaling functions in apoptosis (Kenchappa et al., 2006; Majdan et al., 1997), neurite outgrowth (Domeniconi et al., 2005), and transcriptional activation of cell-cycle genes (Parkhurst et al., 2010). Combinations of ligand binding, co-receptor interactions, intracellular compartmentalization, and regulated intramembrane proteolysis determine which cytoplasmic partners p75NTR recruits to mediate its pleiotropic cellular functions (Barker, 2004).
Here we show that hypoxia-induced γ-secretase cleavage of p75NTR triggers oxygen-dependent stabilization of HIF-1α. We demonstrate an unanticipated function for p75NTR as an adaptor protein that stabilizes the ubiquitin ligase Siah2 by decreasing its auto-ubiquitination to provide a positive feed-forward mechanism that enhances HIF-1α stability in hypoxic conditions. In vitro and in vivo, hypoxia induces γ-secretase-mediated cleavage of p75NTR to stabilize HIF-1α. In response to hypoxia, p75NTR−/− mice show a decrease in stabilization of HIF-1α and VEGF expression, leading to decreased retinal angiogenesis. Overall, p75NTR intramembrane cleavage is an apical mechanism in the regulation of hypoxic signaling.
RESULTS
p75NTR Regulates HIF-1α Stabilization in Hypoxia
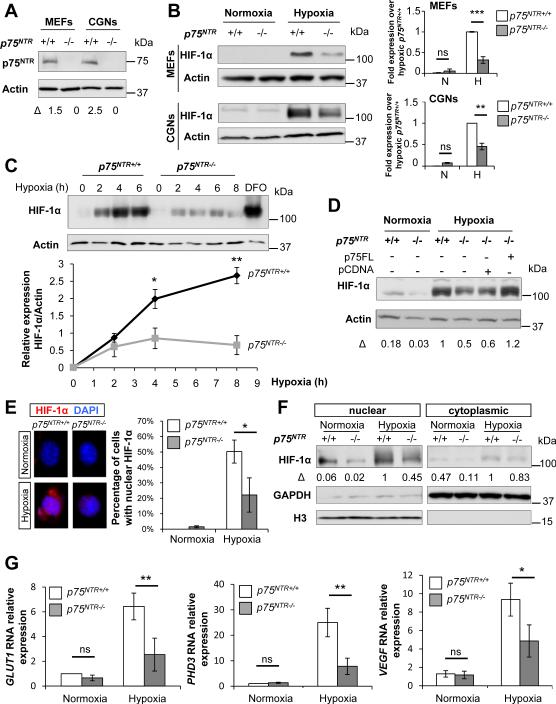
p75NTR plays a crucial role liver (Kendall et al., 2009; Passino et al., 2007), lung fibrosis (Sachs et al., 2007), and cancer (Johnston et al., 2007). Since the HIF-1α mediated-hypoxic response is a hallmark of fibrotic diseases and tumorigenesis (Higgins et al., 2008; Semenza, 2000), we investigated p75NTR as a potential regulator of the hypoxic response. We first compared the protein levels of HIF-1α in p75NTR+/+ (WT) and p75NTR−/− mouse embryonic fibroblasts (MEFs) and cerebellar granule neurons (CGNs) under normoxic (21% O2) or hypoxic (1% O2) conditions. p75NTR is expressed in MEFs as shown by western blot (Figure 1A and S1A–C), real-time PCR (Figure S1D) and immunocytochemistry (Figure S1E) at similar levels as in CGNs (Figure 1A). During hypoxia, HIF-1α stabilization was 68% lower in p75NTR−/− MEFs compared to WT (Figure 1B). Similarly, loss of endogenous p75NTR in primary CGNs decreased HIF-1α stabilization by 55% (Figure 1B). As expected, in normoxic conditions, HIF-1α was constitutively degraded in WT and p75NTR−/− cells (Figure 1B). Conversely, overexpression of p75NTR in stably transfected NIH3T3 fibroblasts increased HIF-1α stabilization (Figure S1F). Non-transfected NIH3T3 fibroblasts do not express endogenous p75NTR [(Sachs et al., 2007) and Figure S3D]. In contrast to HIF-1α, loss of p75NTR did not affect accumulation of the HIF-1α homolog HIF-2α in hypoxia (Figure S1G).
Figure 1. Stabilization of HIF-1α Is Impaired in p75NTR−/− Cells during Hypoxia.
(A) Western blot for p75NTR in MEFs and CGNs. β-Actin loading controls were performed on the same membrane. Representative western blot from n=2 independent experiments is shown.
(B) Western blot for HIF-1α during normoxia and after 5 hours of hypoxia (1% O2) in WT and p75NTR−/− MEFs and CGNs. β-Actin loading controls were performed on the same membrane. Values are mean ± SEM of the HIF1-α/β-actin ratio normalized to hypoxic WT cells as determined by densitometry (MEFs, n=4; CGNs, n=3 independent experiments, ***P< 0.001 and **P<0.01 by one-way ANOVA). N, normoxia; H, hypoxia.
(C) Kinetics of HIF-1α stabilization in hypoxia (1% O2) in WT and p75NTR−/− MEFs by western blot analysis. β-Actin loading controls were performed on the same membrane. The pharmacologic inhibitor DFO that stabilizes HIF-1α was used as a positive control. HIF1-α/β-actin ratio determined by densitometry is shown. Values are mean ± SEM of the HIF1-α/β-actin ratio (n=3 independent experiments, **P< 0.01 and *P<0.05 by unpaired Student's t test).
(D) Western blot for HIF-1α after 5 hours of hypoxia (1% O2) in p75NTR−/− MEFs infected with lentivirus expressing p75FL. β-Actin loading controls were performed on the same membrane. The ratio HIF1-α/β-actin is normalized to hypoxic WT cells.
(E) Immunocytochemical analysis of HIF-1α nuclear accumulation (WT normoxia, n=226; WT hypoxia, n=163; p75NTR−/− normoxia, n=195; p75NTR−/− hypoxia, n=240) after 5 hours of normoxia and hypoxia (1% O2) in WT and p75NTR−/− MEFs. Values are mean ± SD of the HIF1-α/β-actin ratio (n=2 independent experiments, *P<0.05 by unpaired Student's t test).
(F) Western blot for HIF-1α accumulation in nuclear and cytoplasmic fractions after 5 hours of normoxia or hypoxia (1% O2) in WT and p75NTR−/− MEFs. GAPDH and histone H3 (H3) were used as a loading control for cytoplasmic and nuclear fractions respectively. Representative western blot from n=2 independent experiments is shown. N, nuclear; NC, nucleo-cytoplasmic; C, cytoplasm.
(G) Real-time PCR analysis of GLUT1, PHD3 and VEGF expression in WT and p75NTR−/− cells after 5 hours of normoxia and hypoxia (1% O2). Values are mean ± SEM (GLUT1 and PHD3; MEFs, n=4; VEGF; CGNs, n=3 independent experiments, **P<0.01; *P<0.05 and ns, not significant, by one-way ANOVA). See also Figure S1.
In a kinetic experiment, HIF-1α accumulated continuously in WT cells but failed to stabilize in p75NTR−/− cells, even over 8 hours of hypoxia (Figure 1C), suggesting a sustained defect in HIF-1α stabilization. Gene expression analysis showed no significant differences in HIF-1α RNA between WT and p75NTR−/− cells (Figure S1H), indicating that HIF-1α expression is regulated at the protein level in p75NTR−/− cells in response to hypoxia. Lentiviral delivery of full-length p75NTR (p75FL) in p75NTR−/− cells rescued HIF-1α stabilization (Figure 1D). During hypoxia, stabilized HIF-1α translocates to the nucleus to activate its target genes (e.g., GLUT1, PHD3 and VEGF) (Marxsen et al., 2004; Semenza, 2001b). In p75NTR−/− cells, nuclear accumulation of HIF-1α was decreased, as shown by immunocytochemistry (Figure 1E) and subcellular fractionation (Figure 1F). HIF-1α decrease in the nuclear fraction of p75NTR−/− cells of 55% (Figure 1F) was similar to its decrease in whole-cell extracts of 68% (Figure 1B). In accordance, GLUT1, PHD3 and VEGF RNA expression were 61%, 69% and 48% lower respectively in hypoxia-treated p75NTR−/− cells (Figure 1G). Overall, these results suggest that p75NTR regulates HIF-1α stabilization and expression of HIF-1α target genes.
p75NTR Regulates PHD-dependent Proteasomal Degradation of HIF-1α
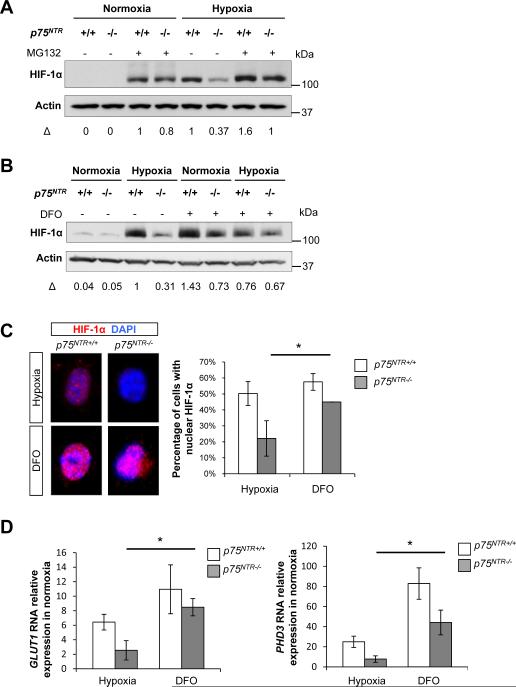
Oxygen-dependent hydroxylation of HIF-1α by PHDs triggers HIF-1α ubiquitination and its subsequent proteasomal degradation (Semenza, 2001a). We thus used two complementary approaches to determine whether p75NTR regulates proteasomal degradation of HIF-1α. The proteasomal inhibitor MG132 increased HIF-1α to similar levels in WT and p75NTR−/− cells (Figure 2A), suggesting that the defect in HIF-1α stabilization in the p75NTR−/− cells results from excessive proteasomal degradation. Moreover, desferroxamine (DFO), an iron chelator that inhibits the PHD activity restored protein abundance (Figure 2B) and nuclear accumulation (Figure 2C) of HIF-1α to comparable levels in WT and p75NTR−/− cells. Consistent with the nuclear accumulation of HIF-1α, DFO increased expression of GLUT1 and PHD3 by 70% and 82% respectively in p75NTR−/− cells, indicating that DFO restores HIF-1α target gene transcription in p75NTR−/− cells (Figure 2D). Thus, increased PHD activity appears to be responsible for the impaired HIF-1α stabilization in p75NTR−/− cells under hypoxia.
Figure 2. Inhibition of Proteasomal Degradation and PHD Activity Restores the HIF-1α-Mediated Hypoxic Response in p75NTR−/− Cells.
(A) Western blot for HIF-1α stabilization in WT and p75NTR−/− MEFs treated with MG132 for 1 hour before hypoxia (1% O2). β-Actin loading controls were performed on the same membrane. The HIF1-α/β-actin ratio is normalized to the hypoxic WT cells. Representative image from n=3 independent experiments is shown.
(B) Western blot for HIF-1α stabilization in WT and p75NTR−/− MEFs treated with DFO for 1 hour before hypoxia (1% O2). β-Actin loading controls were performed on the same membrane. The HIF1-α/β-actin ratio is normalized to hypoxic WT cells. Representative image from n=3 independent experiments is shown.
(C) Immunocytochemistry for HIF-1α nuclear accumulation in WT and p75NTR−/− MEFs treated with DFO for 5 hours (1% O2) (WT hypoxia, n=163; p75NTR−/− hypoxia, n=240; WT hypoxia, n=114; p75NTR−/− hypoxia, n=189). Values are mean ± SD of the HIF1-α/β-actin ratio (n=2 independent experiments, *P<0.05 by unpaired Student's t test).
(D) Real-time PCR analysis of GLUT1 and PHD3 gene expression in WT and p75NTR−/− MEFs treated with DFO for 5 hours (200 μM). Values are mean ± SEM (n=3 independent experiments, *P<0.05; by one-way ANOVA).
Siah2 Zinc Fingers Directly Associate with the Juxtamembrane Domain of p75NTR to Regulate HIF-1α Stabilization
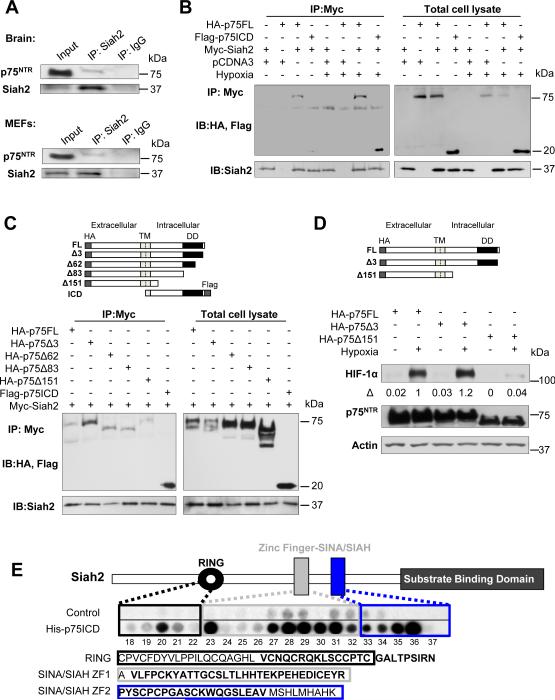
PHD activity is regulated by Siah2-dependent proteasomal degradation (Nakayama et al., 2004). Siah and the tumor necrosis factor receptor-associated factor (TRAF) family of proteins are components of the E3 ubiquitin ligase complex that share similar domain architecture (a RING finger domain, followed by a series of zinc fingers and the substrate binding domain), three-dimensional structure and functional activity (Polekhina et al., 2002). p75NTR directly interacts with TRAF family members, such as TRAF 6 (Khursigara et al., 1999). Given the structural similarity of Siah proteins and the TRAF binding partners of p75NTR, we hypothesized that p75NTR interacts with Siah2. Endogenous co-immunoprecipitation showed that p75NTR interacts with Siah2 in whole brain extracts and MEFs (Figure 3A), and N2a neuroblastoma cells (Figure S2A) in hypoxic conditions. Deletion mapping mutagenesis showed that a p75NTR deletion mutant lacking the entire ligand-binding extracellular domain (p75ICD) co-immunoprecipitated with Siah2 (Figures 3B, C). p75FL formed a complex with Siah2 under normoxic conditions and complex formation increased during hypoxia (Figure 3B). The interaction of Siah2 with p75ICD was also enhanced after hypoxia (Figure 3B). Control experiments using empty pCDNA3 vector showed that p75FL and p75ICD were not immunoprecipitated in Siah2 pull-downs (Figure S2B). Thus, the interaction of p75NTR with Siah2 appears to be oxygen-dependent but independent of ligand binding.
Figure 3. Siah2 Zinc Fingers Directly Associate with the Juxtamembrane Domain of the p75ICD.
(A) Endogenous co-immunoprecipitation of p75NTR with Siah2 in whole brain extracts from P9 mice exposed to hypoxia (9% O2 for 6 hours) and in MEFs after hypoxia (1% O2 for 6 hours in presence of MG132). Lysates were immunoprecipitated with anti-Siah2 or IgG control antibodies, and western blots were developed with anti-Siah2 or anti-p75NTR antibodies.
(B) Immunoprecipitation of Myc-Siah2 with HA-p75FL and Flag-p75ICD transfected in HEK293T cells subjected to normoxia or hypoxia (1% O2) for 5 hours in presence of MG132. Lysates were immunoprecipitated with anti-Myc antibody, and western blots were developed with anti-HA and anti-Siah2 antibodies.
(C) Mapping of the p75NTR sites required for interaction with Siah2. Immunoprecipitation of Myc-Siah2 with truncated forms of HA-p75 and Flag-p75 transfected in HEK293T cells subjected to hypoxia (1% O2) for 5 hours in the presence of MG132. Lysates were immunoprecipitated with anti-Myc antibody, and western blots were developed with anti-HA, anti-flag and anti-Siah2 antibodies. Schematic diagram of amino-terminal and carboxy-terminal truncated forms of HA-p75. TM, transmembrane domain; DD, death domain; ICD, intracellular domain.
(D) Western blot for HIF-1α and p75NTR in p75NTR−/− MEFs transfected with the HA-p75Δ151 mutant that lacks the p75NTR juxtamembrane domain, HA-p75FL, or the HA-p75Δ3 control mutant that lacks 3 distal aminoacids of the p75ICD. Cells were subjected to 5 hours of hypoxia (1% O2). β-Actin loading controls were performed on the same membrane. Representative images from n=3 independent experiments is shown. TM, transmembrane domain; DD, death domain; ICD, intracellular domain.
(E) Peptide array mapping of the Siah2 sites required for the interaction with p75ICD. Schematic diagram of the Siah2 protein shows the domain organization. Peptide library screened with recombinant His-p75ICD revealed two distinct domains of Siah2 that interact with the ICD of p75NTR: the RING domain (peptide 20) and the two SIAH/SINA zinc finger domains (peptides 27–36). ZF1: Zinc finger domain 1, ZF2: zinc finger domain 2. Sequences of RING and ZF domains are outlined and the Siah2 peptides interacting with p75NTR are shown in bold. See also Figure S2.
To characterize the p75NTR domains involved in Siah2 interaction, mapping studies were conducted with deletion mutants after hypoxia. Siah2 interacted with p75FL and with mutants lacking the distal 3, 62, and 83 amino acids (aa), but not with a mutant lacking the distal 151 aa (Figure 3C). Thus, Siah2 evidently interacts with the juxtamembrane region of p75NTR between residues 275 and 343. Since Siah2 binds the juxtamembrane domain of p75NTR (Figure 3C), we examined the contribution of the p75NTR juxtamembrane domain to HIF-1α stabilization. A mutant lacking the juxtamembrane domain (p75Δ151) failed to stabilize HIF-1α upon hypoxia (Figure 3D). p75FL or a control mutant containing the juxtamembrane domain, but lacking the 3 distal aminoacids of the p75ICD (p75Δ3) stabilized HIF-1α upon hypoxia (Figure 3D).
To further characterize the interaction, we used peptide array technology, which has defined sites of direct interaction for other p75NTR partners (Sachs et al., 2007) and many other proteins (Bolger et al., 2006). Using His-p75ICD, we screened a peptide array library of overlapping 25-mer peptides that spanned the sequence of Siah2. The strongest interactions were within the RING finger (peptide 20, aa 101–125) and SINA/SIAH zinc finger domains (peptides 27–36, aa 135–185) (Figure 3E). Binding was undetectable in the N-terminal domain of Siah2, while p75NTR also bound to peptide 53 (aa 266–290) within the Siah2 substrate binding domain (Figure S2C), which is required for substrate ubiquitination. The p75NTR-interacting sequences in the RING and zinc finger domains are highly conserved in the Siah2/SINA family members from plants to mammals. These results suggest a direct interaction between p75NTR and Siah2 that primarily requires sequences in the juxtamembrane region of p75NTR and the evolutionary conserved RING and zinc finger domains of Siah2.
p75NTR Regulates Siah2 Auto-ubiquitination
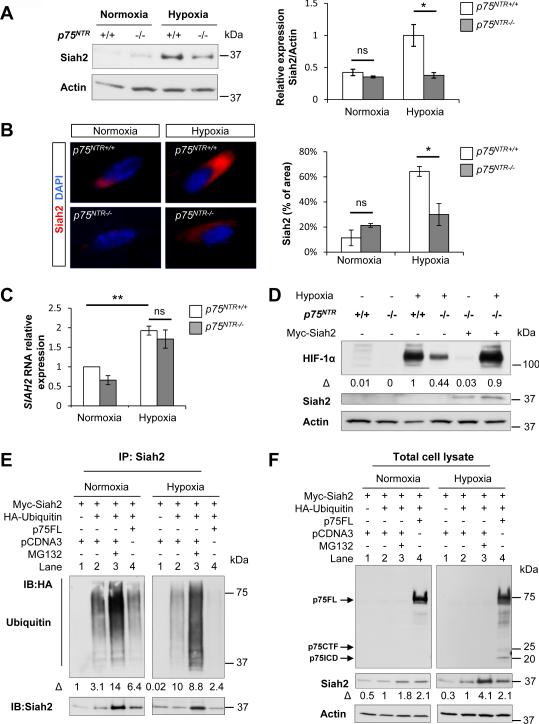
Siah2 is upregulated in hypoxia and induces a positive feed-forward mechanism to enhance HIF-1α stability under hypoxic conditions (Nakayama et al., 2004). Steady-state levels of Siah2 are regulated by its RING domain, which controls Siah2 auto-ubiquitination resulting in its self-degradation (Hu and Fearon, 1999). Since p75NTR binds the Siah2 RING domain (Figure 3E), we hypothesized that p75NTR increases Siah2 abundance in hypoxia by regulating its auto-ubiquitination. Indeed, in p75NTR−/− cells, Siah2 was reduced by 63% as shown by western blot (Figure 4A) and by 55% by immunocytochemistry (Figure 4B). No differences were observed in transcriptional regulation of SIAH2 between WT and p75NTR−/− cells during hypoxia (Figure 4C). Transfection with a Siah2 expression vector rescued the defect of HIF-1α accumulation in p75NTR−/− cells (Figure 4D), suggesting that p75NTR regulates HIF-1α stabilization through Siah2. In hypoxia, p75FL reduced the in vivo auto-ubiquitination of Siah2 by ~70% compared to pCDNA control vector (Figure 4E; hypoxia, lane 2 compared to lane 4). Consequently, total levels of Siah2 were increased by 50% in the presence of p75FL compared to pCDNA control (Figure 4F; hypoxia, lane 2 compared to lane 4). In contrast, in normoxia p75FL did not decrease Siah2 auto-ubiquitination (Figure 4E; normoxia lane 2 compared to lane 4). As expected, inhibition of proteasomal activity with MG132 increased the accumulation of ubiquitinated forms of Siah2 (Figure 4E, F; lane 3). Overall, these results suggest that in low oxygen levels p75NTR regulates Siah2 turnover by decreasing Siah2 self-degradation.
Figure 4. p75NTR Regulates HIF-1α Stabilization through Siah2.
(A) Western blot for Siah2 in WT and p75NTR−/− MEFs after 5 hours of normoxia or hypoxia (1% O2). β-Actin loading controls were performed on the same membrane. Values are mean ± SEM of the Siah2/β-actin ratio (n = 3 independent experiments, *P<0.05; ns, not significant, by oneway ANOVA), normalized to hypoxic WT cells.
(B) Immunocytochemistry of Siah2 accumulation in WT and p75NTR−/− MEFs after 5 hours of normoxia and hypoxia (1% O2). Values are mean ± SEM of the percentage of Siah2+ area, normalized to the total cell number (WT normoxia, n=244; WT hypoxia, n=455; p75NTR−/− normoxia, n=130; p75NTR−/− hypoxia, n=319; *P<0.05; ns, not significant, by one-way ANOVA).
(C) Real-time PCR analysis of SIAH2 expression in WT and p75NTR−/− MEFs after 5 hours of normoxia or hypoxia (1% O2). Values are mean ± SEM (n = 3 independent experiments, **P< 0.01; ns, not significant, by one-way ANOVA).
(D) Western blot for HIF-1α and Siah2 in WT and p75NTR−/− MEFs electroporated with Myc-Siah2 after 5 hours of normoxia or hypoxia (1% O2). β-Actin loading controls were performed on the same membrane. The HIF1-α/β-actin ratio is normalized to hypoxic WT cells. Representative image from n=2 independent experiments is shown.
(E) Co-immunoprecipitation of Myc-Siah2 with HA-ubiquitin (HA-Ub) transfected in HEK293T cells with p75FL or pCDNA3 control vector. Cells were subjected to normoxia or hypoxia (1% O2) for 5 hours in the presence of MG132 as indicated. Lysates were immunoprecipitated with anti-Myc antibody, and western blots were developed with anti-HA and anti-Siah2. The Ubiquitin/Siah2 ratio determined by densitometry is shown. Representative image from n=3 independent experiments is shown.
(F) Western blot for p75NTR and Siah2 in total lysates of HEK293T cells transfected with Myc-Siah2, HA-ubiquitin (HA-Ub), p75FL or pCDNA3 control vector. Cells were subjected to normoxia or hypoxia (1% O2) for 5 hours in the presence of MG132 as indicated. Western blots were developed with anti-p75NTR, anti-Siah2 and anti-β-Actin antibodies. The Siah2/β-actin ratio determined by densitometry is shown. Representative image from n=3 independent experiments is shown.
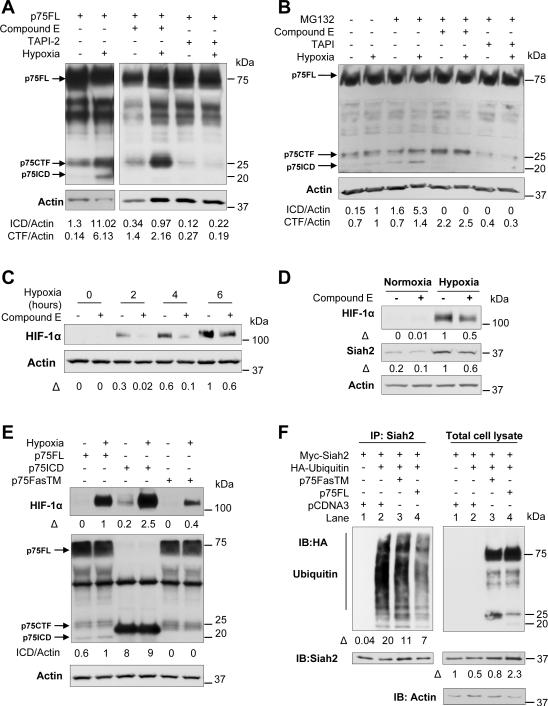
Hypoxia Stimulates γ-Secretase-mediated Proteolytic Cleavage of p75NTR to Control Siah2 Abundance and HIF-1α Stabilization
To determine whether oxygen levels regulate p75NTR, we analyzed the effects of hypoxia on p75NTR expression and intramembrane cleavage. Hypoxia did not increase p75NTR RNA (Figure S3A) or p75NTR protein levels (Figure S3B), but it stimulated the intramembrane proteolysis of p75NTR (Figure 5A, B and Figure 4F, total cell lysate, hypoxia). Within 5 hours, hypoxia increased the accumulation of the p75CTF and induced the formation of p75ICD in MEFs transfected with p75FL (Figure 5A). Hypoxia-induced intramembrane cleavage was also confirmed for endogenous p75NTR in WT MEFs (Figure 5B) and in N2a neuroblastoma cells (Figure S3C). To determine if the proteolytic cleavage of p75NTR in hypoxia is mediated by metalloprotease and γ-secretase, we used pharmacological inhibitors of α-secretase (TAPI-2) and γ-secretase (compound E). TAPI abolished the formation of both p75NTR proteolytic fragments, suggesting that metalloprotease-mediated shedding precedes ICD release during hypoxia (Figure 5A, B and S3C, D). In presence of the γ-secretase inhibitor, the CTF of p75NTR accumulated without subsequent ICD formation, demonstrating that γ-secretase mediates the second cleavage reaction to release the ICD of p75NTR in hypoxia (Figure 5A, B and S3C, D). Hypoxia induced γ-secretase cleavage of p75NTR in neurons (Figure S3C) and fibroblasts (Figure 5A, B and S3D) irrespective of whether p75NTR was endogenously expressed (Figure 5B and S3C) or transfected (Figure 5A and S3D). Differences in the ratio of the ICD and CTF in fibroblasts and neurons are in accordance with previous studies showing that p75NTR processing is dependent on cell type, primarily due to differences in α-secretase levels (Kanning et al., 2003). Inhibition of γ-secretase activity decreased both HIF-1α stabilization (Figures 5C and 5D) and Siah2 abundance (Figure 5D), suggesting that γ-secretase dependent intramembrane cleavage is necessary for HIF-1α stabilization.
Figure 5. Hypoxia Induces Proteolytic Cleavage of p75NTR by γ-secretase to Control Siah2 Abundance and HIF-1α Stabilization.
(A) Western blot for p75NTR in p75NTR−/− MEFs electroporated with p75FL and subjected to 5 hours of normoxia or hypoxia (1% O2). Cells were treated with 1 μM of compound E and 20 μM of TAPI for 14 and 2 hours before hypoxia. Based on the predicted molecular weight, the arrows indicate the full length receptor (~75 kDa, FL), the carboxy-terminal fragment (~25 kDa, CTF) or the intracellular domain (~20 kDa, ICD). β-Actin loading controls were performed on the same membrane. Representative image from n=3 independent experiments is shown.
(B) Western blot for p75NTR in WT MEFs subjected to 5 hours of normoxia or hypoxia (1% O2). Cells were treated with 1 μM of compound E and 20 μM of TAPI for 14 and 2 hours before hypoxia. MG132 was added 4 hours before hypoxia. Representative image from n=3 independent experiments is shown.
(C) Western blot for HIF-1α expression in WT MEFs in normoxia and during 2, 4 and 6 hours of hypoxia (1% O2). Cells were pretreated with 1 μM of compound E 14 and 2 hours before hypoxia. β-actin loading controls were performed on the same membrane. The ratio HIF1-α/β-actin is normalized to the non-treated WT cells after 6 hours of hypoxia.
(D) Western blot for HIF-1α and Siah2 in WT MEFs treated with 1 μM of compound E for 14 hours before 5 hours of hypoxia (1% O2). β-Actin loading controls were performed on the same membrane. Representative images from n=3 independent experiments are shown.
(E) Western blot for HIF-1α and p75NTR in p75NTR−/− MEFs transfected with p75FL, p75ICD and p75-FasTM and subjected to 5 hours of normoxia of hypoxia (1% O2). β-Actin loading controls were performed on the same membrane. Representative image from n=3 independent experiments is shown.
(F) Co-immunoprecipitation of Myc-Siah2 with HA-ubiquitin (HA-Ub) in HEK293T cells transfected with p75FL, p75FasTM or pCDNA3 subjected to normoxia or hypoxia (1% O2) for 5 hours. Lysates were immunoprecipitated with anti-Myc antibody, and western blots were developed with anti-HA, and anti-Siah2 antibodies. Western blot of total cell lysates developed with anti-p75NTR and anti-Siah2 antibodies is shown. The Ubiquitin/Siah2 ratio and the Siah2/β-actin ratio determined by densitometry are shown for the IP fraction and total cell lysate respectively. Representative images from n=2 independent experiments are shown. See also Figure S3.
To rule out the possibility that γ-secretase inhibitors were blocking the proteolysis of proteins other than p75NTR, we analyzed the effects of p75-FasTM, a p75NTR mutant that is resistant to γ-secretase cleavage (Domeniconi et al., 2005; Zampieri et al., 2005) or p75ICD on HIF-1α accumulation during hypoxia. p75-FasTM failed to increase HIF-1α stabilization upon hypoxia, in contrast to p75FL or p75ICD (Figure 5E). Moreover, p75FasTM increased the in vivo auto-ubiquitination of Siah2 in hypoxia by ~40% compared to p75FL (Figure 5F; IP: Siah2, lane 3 compared to lane 4), suggesting that release of p75ICD is necessary for Siah2 stabilization. Consequently, total levels of Siah2 were decreased in the presence of p75-FasTM by ~60% compared to p75FL (Figure 5F; Total cell lysate, lane 3 compared to lane 4). In addition, p75ICD increased HIF-1α stabilization at higher levels compared to p75FL in hypoxia and in normoxia (Figure 5E), indicating that p75ICD is sufficient to trigger HIF-1α accumulation. p75-FasTM and p75FL were expressed at similar levels and p75ICD cleavage product was not detected for the p75-FasTM construct (Figure 5E, F). p75NTR−/− MEFs were used for transfections of p75ICD and p75-FasTM to avoid potential interference of the endogenous p75NTR expression. These results suggest that hypoxia-induced intramembrane cleavage of p75NTR is necessary for HIF-1α stabilization and Siah2 accumulation.
p75NTR Regulates VEGF Expression and Neoangionesis in Retinal Hypoxia in vivo
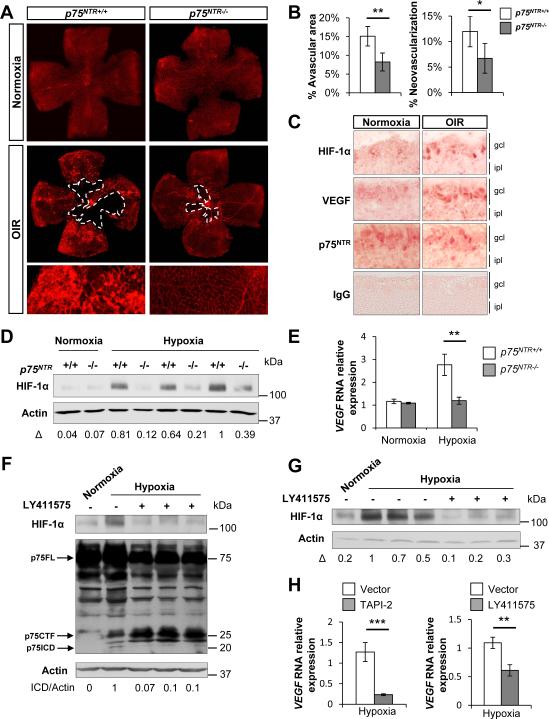
To determine the biological significance of p75NTR-mediated stabilization of HIF-1α, we examined the role of p75NTR in regulating the hypoxic response in vivo. Since p75NTR is expressed in the retina during development (Frade and Barde, 1999; Lim et al., 2008) and in response to injury (Harada et al., 2000) and HIF-1α is essential for retinal angiogenesis after hypoxia (Arjamaa and Nikinmaa, 2006), we investigated the role of p75NTR in oxygen-induced retinopathy (OIR), a model of retinal hypoxia (Connor et al., 2009). OIR has been used in vivo to examine the biological role of HIF-1α (Xia et al., 2008; Yoshida et al., 2010) and is an established model to validate new regulators of hypoxia-induced pathological neoangiogenesis, such as JNK (Guma et al., 2009), Notch (Hellstrom et al., 2007) and Robo4 (Jones et al., 2008). In OIR, hyperoxia produces regression of the vascular network (vaso-obliteration) in the center of the retina, which becomes hypoxic upon return to normoxia (Connor et al., 2009). After vaso-obliteration, compensatory neovascularization occurs to meet metabolic requirements. During this process, chaotically orientated and inefficient blood vessels can form, leading to pathological angiogenesis characterized by neovascular tufts. WT and p75NTR−/− mice were subjected to hyperoxia (75% oxygen) from postnatal day 7 (P7) to postnatal day 12 (P12), followed by a return to ambient air (normoxia) until postnatal day 17 (P17). In the p75NTR−/− mice, the vaso-obliterated area was 45% smaller than in WT mice, and pathological neovascularization was reduced by 43% (Figure 6A, OIR and 6B). High magnification images show robust neovascular tuft formation in WT, compared to p75NTR−/− mice (Figure 6A, OIR). During normoxia, no major differences were detected between WT and p75NTR−/− mice (Figure 6A, normoxia).
Figure 6. Loss of p75NTR Reduces HIF-1α Stabilization and VEGF Expression and Regulates Neovascularization in Retinal Hypoxia.
(A) Whole-mount retinas from WT and p75NTR−/− P17 mice exposed to normoxia or OIR were stained with the vascular marker Alexa-Fluor 594–conjugated isolectin B4 from Griffonia simplicifolia. Dashed lines outline the vaso-obliterated area.
(B) Quantification of the vaso-obliterated area and neovascular tuft formation in normoxia in WT and p75NTR−/− mice (n=3 per genotype) or in hypoxia WT (n=8) and p75NTR−/− mice (n=9). Results are presented as mean ± SEM. (*P<0.05 and **P<0.01, by unpaired Student's t test). Analysis was performed on animals from 3 independent litters.
(C) Immunohistochemical staining for HIF-1α, VEGF and p75NTR in normoxia and hypoxia in the central retina of P12 mice 12 hours after OIR. gcl, ganglion cell layer; ipl, inner plexiform layer.
(D) Western blot for HIF-1α in normoxic and hypoxic retinas of WT and p75NTR−/− P12 mice 6 hours after return to room air. β-Actin loading controls were performed on the same membrane. Three mice from n=3 independent litters are shown.
(E) Real-time PCR analysis of VEGF gene expression in WT and p75NTR−/− P12 mice 6 hours after return to room air. WT normoxia, n=3; p75NTR−/− normoxia, n=5; WT hypoxia, n=9; p75NTR−/− hypoxia, n=9; **P<0.01 by one-way ANOVA. Values are mean ± SEM. Analysis was performed on animals from three independent litters.
(F) Western blot for p75NTR and HIF-1α in normoxic and hypoxic retinas of WT P12 mice treated with LY411575 or vehicle. β-actin loading controls were performed on the same membrane. p75ICD is detected in lane 2. Representative western blot is shown from an average of n=6 animals in each experimental condition.
(G) Western blot for HIF-1α in normoxic and hypoxic retinas of WT P12 mice treated with LY411575 or vehicle. β-actin loading controls were performed on the same membrane. Representative western blot is shown from an average of n=6 animals in each experimental condition.
(H) Real-time PCR analysis of VEGF gene expression in WT P12 mice treated with TAPI-2, LY411575 or vehicle. WT vehicle, n=5; WT TAPI-2, n=6; WT LY411575, n=6; **P<0.01, ***P<0.001 by unpaired Student's t test. Values are mean ± SEM. See also Figure S4.
Since the p75NTR−/− mice showed similar deficits in vaso-obliteration and angiogenesis as mice with HIF-1α depletion (Xia et al., 2008; Yoshida et al., 2010), we examined whether p75NTR regulates HIF-1α stabilization in vivo. After hypoxia, HIF-1α is upregulated in the inner retina and is prominent in retinal ganglion cells (RGCs) (Mowat et al., 2010). Indeed, we observed increase of HIF-1α in the ganglion cell layer (GCL) after hypoxia (Figure 6C). The HIF-1α-target gene VEGF, which is implicated in ischemic retinopathies by promoting pathological neovascularization (Guma et al., 2009), was also increased after hypoxia in the GCL (Figure 6C). While in the adult p75NTR is expressed in Muller glial cells (Hu et al., 1998; Lebrun-Julien et al., 2010), during embryonic and early postnatal life, p75NTR is mostly expressed in RGCs at the GCL (Frade and Barde, 1999; Lim et al., 2008; Nakamura et al., 2007). Indeed, p75NTR expression during hypoxia was observed in the GCL, similar to that of HIF-1α and VEGF (Figure 6C). To confirm that intramembrane proteolysis of p75NTR occurs after hypoxia in vivo, we examined p75NTR cleavage in the retina. p75NTR proteolytic fragments corresponding to the p75CTF and p75ICD formed as early as 6 hours after retinal hypoxia and their formation was sustained 5 days after hypoxia (Figure S4A). In accordance with our in vitro studies (Figures S3A, B), hypoxia did not alter p75NTR protein levels in vivo (Figure S4B), suggesting that hypoxia primarily regulates p75NTR by inducing its proteolytic cleavage. Consistent with our in vitro findings, p75NTR−/− mice showed a dramatic reduction of HIF-1α stabilization in the retina after 6 hours of hypoxia compared to WT controls (Figure 6D) that showed the highest HIF-1α accumulation at the 6 hour timepoint (Figure S4C). The functional consequence of reduced HIF-1α stabilization in p75NTR−/− mice was assessed by measuring changes in the expression of VEGF. While VEGF was upregulated in the retina of WT mice, its expression did not increase in p75NTR−/− mice after retinal hypoxia (Figure 6E).
We then examined whether in vivo inhibition of p75NTR cleavage with α-secretase or γ-secretase inhibitors affects the HIF-1α mediated-hypoxic response. Formation of the p75CTF by α-secretase cleavage is a prerequisite for a second proteolytic cleavage by γ-secretase to release p75ICD (Bronfman, 2007). Administration of the γ-secretase inhibitor LY411575 or the α-secretase inhibitor TAPI-2 decreased HIF-1α stabilization (Figures 6G, S4D) and VEGF induction (Figure 6H) in the retina. In addition to its effects on HIF-1α and VEGF, TAPI-2 reduced the formation of p75CTF and p75ICD in the retina (Figure S4D). In vivo inhibition of γ-secretase activity by LY411575 decreased p75ICD formation in the retina (Figure 6F). As described (Zampieri et al., 2005) and in accordance with our in vitro data (Figure 5A, B and S3C, D), inhibition of γ-secretase activity in vivo increased CTF formation in the retina (Figure 6F). Inhibition of p75ICD formation by blocking either α-secretase or γ-secretase suggests that in vivo intramembrane proteolysis is necessary for the release of the p75ICD fragment detected in the retinal extracts after hypoxia (Figure 6F, S4A, D). These results demonstrate a crucial role for the oxygen-dependent intramembrane cleavage of p75NTR in the regulation of HIF-1α-mediated angiogenesis in vivo.
DISCUSSION
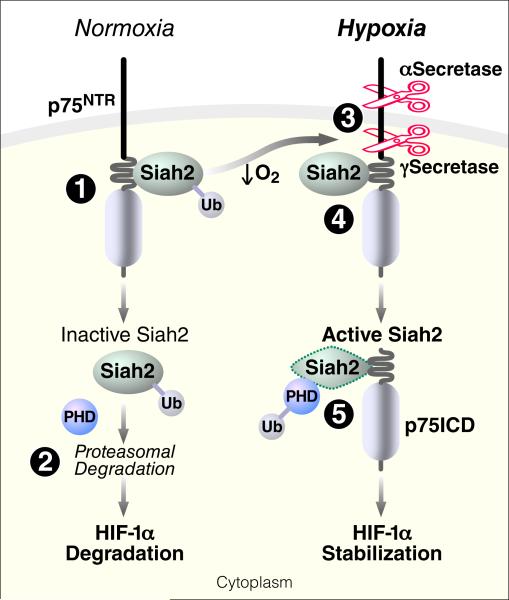
In this study, we identified p75NTR intramembrane proteolysis as an apical mechanism that regulates HIF-1α activity through Siah2 in response to hypoxia. Our study identifies p75NTR as an oxygen-dependent substrate of γ-secretase and demonstrates a new biological function for the p75NTR intracellular domain in HIF-1α stabilization in hypoxic conditions. Moreover, we identify a neurotrophin-independent, oxygen-regulated molecular pathway downstream p75NTR through its interaction with the ubiquitin ligase Siah2 that regulates the hypoxic response. Our study demonstrates a major in vivo biological role for p75NTR in retinal angiogenesis through the regulation of HIF-1α stabilization and VEGF expression. Our findings suggest the following model for the crosstalk between p75NTR signaling and the HIF-1α-mediated hypoxic response (Figure 7). (1) In normoxia, the juxtamembrane domain of p75NTR constitutively interacts with the zinc finger domains of Siah2, which is present at low, basal levels. (2) In this context, Siah2 undergoes ubiquitination and proteasomal degradation, allowing the PHDs to catalyze HIF-1α hydroxylation and subsequent HIF-1α degradation. (3) Conversely, reduction in oxygen levels stimulates the activity of metalloprotease and γ-secretase, leading to cleavage of p75NTR and release of its soluble ICD. (4) Intracellular accumulation of p75ICD results in increased interaction with Siah2 that decreases its auto-ubiquitination, thereby increasing its accumulation. (5) Siah2 targets PHDs for proteasomal degradation, which results in HIF-1α stabilization. Consequently, under hypoxic conditions, genetic loss of p75NTR decreases Siah2 abundance and HIF-1α activation, resulting in a failure to induce the hypoxic response. Regulation of hypoxia responses by p75NTR cleavage demonstrates an oxygen-dependent signaling mechanism upstream of prolyl hydroxylases and ubiquitin ligases that triggers HIF-1α stabilization.
Figure 7.
Working Model for the Role of p75NTR in the Regulation of HIF-1α-Mediated Hypoxic Response through the Ubiquitin Ligase Siah2
Our study demonstrates that p75NTR interacts with Siah2 and regulates HIF-1α in both fibroblasts and neurons as shown in both endogenous and overexpressing systems. Importantly, our in vitro findings derived from primary fibroblasts and neurons are corroborated in vivo using the retinal hypoxia model. p75NTR is expressed in fibroblasts in the liver (Suzuki et al., 2008), skin (Adly et al., 2009), eye (Micera et al., 2007), dental pulp (Sarram et al., 1997), bone marrow (Cattoretti et al., 1993), and myofibroblasts in the liver (Passino et al., 2007; Trim et al., 2000). Moreover, p75NTR is expressed in neurons including CGNs (Domeniconi et al., 2005). MEFs and CGNs have the intramembrane proteolytic machinery to cleave p75NTR (Domeniconi et al., 2005; Kanning et al., 2003), indicating that they are physiologically relevant primary cell systems to study p75NTR signaling. Indeed, regulation of HIF-1α stabilization and target gene expression by p75NTR, interaction of p75NTR with Siah2 and hypoxia-induced γ-secretase cleavage of p75NTR occurred in both neurons and fibroblasts. Since p75NTR is expressed in vivo not only in neurons, but also by other cell types, such as fibroblasts, outside of the nervous system, our study suggests that regulation of HIF-1α stabilization could be a physiologic and general mechanism for p75NTR function.
We identified regulation of the E3 ubiquitin ligase Siah2 as a novel signaling mechanism downstream of p75NTR using endogenous co-immunoprecipitation, deletion mapping mutagenesis, peptide array mapping and in vivo auto-ubiquitination assay. Our results support regulation of Siah2 by p75NTR primarily at a protein level, since loss of p75NTR decreases Siah2 during hypoxia without affecting its transcription. Depending on the bioavailability of other extracellular stimuli in addition to oxygen deprivation, alternate signaling pathways regulated by p75NTR might contribute to the Siah2-dependent p75NTR – mediated stabilization of HIF-1α. Two structurally similar components of E3 ubiquitin ligase complexes, Siah2 and TRAF6, interact with the juxtamembrane region of p75NTR (Khursigara et al., 1999) and our study). The RING domains of Siah2 and TRAF6 are essential for the transfer of polyubiquitin chains on their substrates (Joazeiro and Weissman, 2000). Since p75NTR binds the RING domain of Siah2 and prevents Siah2 auto-ubiquitination in hypoxia, p75NTR could also potentially act as an adaptor to regulate the ubiquitination of Siah2 substrates depending on oxygen levels. Conversely, since TRAF6 ubiquitinates p75NTR (Powell et al., 2009) and p75NTR directly interacts with the substrate binding domain of Siah2 (our study), Siah2 could also target p75NTR for degradation. Ubiquitination plays crucial roles in gene transcription, signal transduction, and the cell cycle by regulating protein stability. Thus, defects in protein degradation may lead to severe pathogenic disorders like neurodegeneration and cancer (Hoeller et al., 2006; Tai and Schuman, 2008). Indeed, members of the Siah family have been associated with Parkinson's disease (Liani et al., 2004), tumorigenesis, and metastasis (Qi et al., 2008). Therefore, since p75NTR regulates Siah2, we speculate that p75NTR might contribute to the pathological outcome of diseases characterized by dysregulation of Siah2.
Our study establishes hypoxia as a novel mechanism that induces regulated intramembrane proteolysis of p75NTR. Hypoxia-induced cleavage of p75NTR transduces the decrease in oxygen levels by initiating the release of p75ICD, which interacts with Siah2 to regulate HIF-1α expression. Previous findings showed that hypoxia induces expression of γ-secretase (Wang et al., 2006). Consistent with this notion, within 6 hours after retinal hypoxia, p75NTR underwent γ-secretase-mediated proteolytic cleavage to regulate HIF-1α-mediated angiogenesis. Hypoxia also increases α-secretase activity of ADAM17 (Charbonneau et al., 2007), which is the major metalloprotease mediating intramembrane cleavage of p75NTR (Kenchappa et al., 2010). Since ADAM17-dependent p75NTR cleavage is a prerequisite for the γ-secretase cleavage and thus a limiting step for the generation of p75ICD (Kenchappa et al., 2010), it is possible that ADAM17 might be a limiting factor for HIF-1α stabilization. Hypoxia also stimulates proteolytic cleavage of other cell-surface receptors, such as Notch and APP (Chen et al., 2007; Gustafsson et al., 2005; Sun et al., 2006), suggesting a convergent mechanism between low oxygen conditions and intramembrane proteolysis. Hypoxia-induced γ-secretase cleavage of Notch liberates Notch ICD (Gustaffson et al, 2005). While Notch ICD interacts with HIF-1α to regulate transcriptional activation of the hypoxic response (Gustaffson et al, 2005), oxygen-induced γ-secretase cleavage of p75NTR regulates HIF-1α at a protein level.
Several studies have delineated the important role of γ-secretase in vasculogenesis and angiogenesis (Boulton et al., 2008). Similar to the effects of genetic loss of p75NTR, γ-secretase inhibitors decrease the vaso-obliterated area in the OIR model (Hellstrom et al., 2007). While the effects of γ-secretase inhibitors have been attributed to inhibition of Notch cleavage, our findings identify p75NTR as a physiologically relevant substrate of γ-secretase in retinal hypoxia. Cleavage of p75NTR mediated by γ-secretase in response to hypoxia is an upstream mechanism to control HIF-1α stabilization. Therefore, release of p75ICD may be a component of the hypoxic signaling cascade that modulates the angiogenic response in retinal hypoxia.
HIF-1α is a master regulator of vascular homeostasis, driving transcriptional activation of several angiogenic genes in hypoxic and ischemic conditions (Semenza, 2001b). HIF-1α regulates the pathogenic feature of neovascularization in oxygen-dependent diseases of the retina by regulating angiogenic factors such as VEGF (Arjamaa and Nikinmaa, 2006). p75NTR−/− mice have reduced HIF-1α stabilization in the retina and decreased VEGF, which is transcriptionally regulated by HIF-1α. The vascular pattern in p75NTR−/− mice after OIR is similar to that after pharmacologic or genetic depletion of HIF-1α (Xia et al., 2008; Yoshida et al., 2010). Since the HIF pathway has been proposed as a potential target for therapeutic intervention in ischemic retinopathies, p75NTR might be a therapeutic target upstream of HIF-1α. Our study demonstrates that HIF-1α is the major target for p75NTR, while HIF-2α levels remained unaffected. Since HIF-2α is also involved in retinal angiogenesis (Weidemann et al., 2010), future studies will examine the potential involvement of HIF-2α in p75NTR-regulated retinal angiogenesis. The role of p75NTR in the retina has been primarily associated with axon guidance (Lim et al., 2008) and neurotrophin-mediated neuronal apoptosis (Frade and Barde, 1999; Lebrun-Julien et al., 2010). Our study reveals an unanticipated function of p75NTR as a regulator of HIF-1α angiogenic target genes and pathogenic angiogenesis and provides a molecular mechanism governing aberrant retinal angiogenesis after hypoxia. Since p75NTR expression is not restricted to the CNS, it is possible that p75NTR might contribute to the hypoxic response in other tissues.
In conclusion, our findings reveal a previously unrecognized mechanism for the regulation of the hypoxic response initiated by oxygen-dependent cleavage of p75NTR, which triggers stabilization of HIF-1α through the ubiquitin ligase Siah2. Although hypoxia induces the expression of the γ-secretase complex resulting in the cleavage of Notch and APP, our study reveals p75NTR as a new oxygen-regulated substrate of γ-secretase. Identifying Siah2 as an interacting partner of p75ICD provides a molecular link between regulated intramembrane proteolysis and proteasomal degradation. Our study also revealed that p75NTR cleavage regulates hypoxia-induced angiogenesis in the retina, a hitherto unrecognized function for p75NTR. Since p75NTR and HIF-1α are expressed in several tissues affected by hypoxia, regulation of HIF-1α stabilization by p75NTR might be a general mechanism for regulating oxygen-dependent angiogenesis and tissue remodeling. Regulated intramembrane cleavage of p75NTR and subsequent interaction with Siah2 might be targets for therapeutic intervention to modulate the hypoxic response in ischemia and cancer characterized by dysregulation of HIF-1α.
EXPERIMENTAL PROCEDURES
Mice
p75NTR−/− mice (Lee et al., 1992) (Jackson Laboratory) were backcrossed for 11–12 generations on a C57BL/6 background. Mice were fed standard chow and had access to food and water ad libitum. All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco, and are in accordance with those set by the National Institutes of Health.
In vitro Cell Hypoxia
Isolated MEFs and CGNs, HEK293T, N2a and NIH3T3 and NIH3T3-p75NTR cells were cultured at 37°C in a 5% CO2/95% air incubator. For hypoxic exposure, cells were incubated at 37°C for 5 hours in a modular incubator chamber (C-Chamber, Biospherix) flushed with 1% O2 and 5% CO2 and balanced with N2. For chemical hypoxia, MEFs were treated with 100 μM desferroxamine (DFO) (Sigma) for 5 hours. For secretase inhibition, 1 μM of compound E (Calbiochem) or 20 μM of TAPI-2 (Calbiochem) was added at 14 hours and 2 hours before hypoxia. The proteasome inhibitor MG132 (10 μM) was added alone or with PMA (100 ng/ml, Sigma) 1 hour before hypoxia.
Mouse Model of OIR
OIR was induced as described (Connor et al., 2009; Smith et al., 1994). Briefly, 7-day-old (P7) WT and p75NTR−/− mouse pups with nursing mothers were exposed to 75% O2 for 5 days in an O2 chamber controlled by the ProOx P110 and ProCO2 P120 systems (BioSpherix). At P12, the animals were returned to room air for 2–12 hours for biochemical analysis or for 5 days until P17 for retinal neovascular response. Control mice were kept in the same room under ambient O2 and were exposed to the same level of light.
Statistics
Differences between experimental conditions were analyzed with GraphPad Prism software (GraphPad). The unpaired t test was used for isolated pairs. Analysis of variance for multiple comparisons was performed with one-way ANOVA and Bonferroni post-test. Data are shown as mean ± SEM. P < 0.05 was considered significant.
Supplementary Material
ACKNOWLEDGMENTS
We thank Moses V. Chao, Rebecca Aron, Paul Muchowski and Jing Yang for reagents, Di Wu and Ari Green for the retinal whole mount preparations, Matthew Helmrick, Eirini Vagena, Dimitri Smirnoff and Alex Santillan for technical assistance, Gary Howard and Stephen Ordway for editorial support, John Carroll and Teresa Roberts for Graphics. The J. David Gladstone Institutes received support from a National Center for Research Resources Grant RR18928. Supported by the National Multiple Sclerosis Society postdoctoral fellowship to N.L.M., and NIH/NINDS Grants R01NS051470 and R01NS052189 to K.A. N.L.M. performed all in vivo and in vitro hypoxia experiments, N.L.M. and D.H. performed western blot experiments and data analysis, F.C. and M.D.H. performed the peptide array analysis and analyzed data, K.A. and N.L.M. designed the study, analyzed data and wrote the manuscript. The authors have no conflicting financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION Supplemental information includes four figures, Supplemental Experimental Procedures and Supplemental References.
REFERENCES
- Adly MA, Assaf HA, Hussein MR. Expression pattern of p75 neurotrophin receptor protein in human scalp skin and hair follicles: Hair cycle-dependent expression. J Am Acad Dermatol. 2009;60:99–109. doi: 10.1016/j.jaad.2008.09.060. [DOI] [PubMed] [Google Scholar]
- Arjamaa O, Nikinmaa M. Oxygen-dependent diseases in the retina: role of hypoxia-inducible factors. Exp Eye Res. 2006;83:473–483. doi: 10.1016/j.exer.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Barker PA. p75NTR is positively promiscuous: novel partners and new insights. Neuron. 2004;42:529–533. doi: 10.1016/j.neuron.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Bolger GB, Baillie GS, Li X, Lynch MJ, Herzyk P, Mohamed A, Mitchell LH, McCahill A, Hundsrucker C, Klussmann E, et al. Scanning peptide array analyses identify overlapping binding sites for the signalling scaffold proteins, beta-arrestin and RACK1, in cAMP-specific phosphodiesterase PDE4D5. Biochem J. 2006;398:23–36. doi: 10.1042/BJ20060423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton ME, Cai J, Grant MB. gamma-Secretase: a multifaceted regulator of angiogenesis. J Cell Mol Med. 2008;12:781–795. doi: 10.1111/j.1582-4934.2008.00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfman FC. Metalloproteases and gamma-secretase: new membrane partners regulating p75 neurotrophin receptor signaling? J Neurochem. 2007;103(Suppl 1):91–100. doi: 10.1111/j.1471-4159.2007.04781.x. [DOI] [PubMed] [Google Scholar]
- Cattoretti G, Schiro R, Orazi A, Soligo D, Colombo MP. Bone marrow stroma in humans: anti-nerve growth factor receptor antibodies selectively stain reticular cells in vivo and in vitro. Blood. 1993;81:1726–1738. [PubMed] [Google Scholar]
- Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- Charbonneau M, Harper K, Grondin F, Pelmus M, McDonald PP, Dubois CM. Hypoxia-inducible factor mediates hypoxic and tumor necrosis factor alpha-induced increases in tumor necrosis factor-alpha converting enzyme/ADAM17 expression by synovial cells. J Biol Chem. 2007;282:33714–33724. doi: 10.1074/jbc.M704041200. [DOI] [PubMed] [Google Scholar]
- Chen Y, De Marco MA, Graziani I, Gazdar AF, Strack PR, Miele L, Bocchetta M. Oxygen concentration determines the biological effects of NOTCH-1 signaling in adenocarcinoma of the lung. Cancer Res. 2007;67:7954–7959. doi: 10.1158/0008-5472.CAN-07-1229. [DOI] [PubMed] [Google Scholar]
- Connor KM, Krah NM, Dennison RJ, Aderman CM, Chen J, Guerin KI, Sapieha P, Stahl A, Willett KL, Smith LE. Quantification of oxygen-induced retinopathy in the mouse: a model of vessel loss, vessel regrowth and pathological angiogenesis. Nat Protoc. 2009;4:1565–1573. doi: 10.1038/nprot.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgaya JM, Chan JR, Shooter EM. The neurotrophin receptor p75NTR as a positive modulator of myelination. Science. 2002;298:1245–1248. doi: 10.1126/science.1076595. [DOI] [PubMed] [Google Scholar]
- Deponti D, Buono R, Catanzaro G, De Palma C, Longhi R, Meneveri R, Bresolin N, Bassi MT, Cossu G, Clementi E, Brunelli S. The low-affinity receptor for neurotrophins p75NTR plays a key role for satellite cell function in muscle repair acting via RhoA. Mol Biol Cell. 2009;20:3620–3627. doi: 10.1091/mbc.E09-01-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domeniconi M, Zampieri N, Spencer T, Hilaire M, Mellado W, Chao MV, Filbin MT. MAG induces regulated intramembrane proteolysis of the p75 neurotrophin receptor to inhibit neurite outgrowth. Neuron. 2005;46:849–855. doi: 10.1016/j.neuron.2005.05.029. [DOI] [PubMed] [Google Scholar]
- Frade JM, Barde YA. Genetic evidence for cell death mediated by nerve growth factor and the neurotrophin receptor p75 in the developing mouse retina and spinal cord. Development. 1999;126:683–690. doi: 10.1242/dev.126.4.683. [DOI] [PubMed] [Google Scholar]
- Guma M, Rius J, Duong-Polk KX, Haddad GG, Lindsey JD, Karin M. Genetic and pharmacological inhibition of JNK ameliorates hypoxia-induced retinopathy through interference with VEGF expression. Proc Natl Acad Sci U S A. 2009;106:8760–8765. doi: 10.1073/pnas.0902659106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Harada T, Harada C, Nakayama N, Okuyama S, Yoshida K, Kohsaka S, Matsuda H, Wada K. Modification of glial-neuronal cell interactions prevents photoreceptor apoptosis during light-induced retinal degeneration. Neuron. 2000;26:533–541. doi: 10.1016/s0896-6273(00)81185-x. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- Higgins DF, Kimura K, Iwano M, Haase VH. Hypoxia-inducible factor signaling in the development of tissue fibrosis. Cell Cycle. 2008;7:1128–1132. doi: 10.4161/cc.7.9.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeller D, Hecker CM, Dikic I. Ubiquitin and ubiquitin-like proteins in cancer pathogenesis. Nat Rev Cancer. 2006;6:776–788. doi: 10.1038/nrc1994. [DOI] [PubMed] [Google Scholar]
- Hu B, Yip HK, So KF. Localization of p75 neurotrophin receptor in the retina of the adult SD rat: an immunocytochemical study at light and electron microscopic levels. Glia. 1998;24:187–197. [PubMed] [Google Scholar]
- Hu G, Fearon ER. Siah-1 N-terminal RING domain is required for proteolysis function, and C-terminal sequences regulate oligomerization and binding to target proteins. Mol Cell Biol. 1999;19:724–732. doi: 10.1128/mcb.19.1.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joazeiro CA, Weissman AM. RING finger proteins: mediators of ubiquitin ligase activity. Cell. 2000;102:549–552. doi: 10.1016/s0092-8674(00)00077-5. [DOI] [PubMed] [Google Scholar]
- Johnston AL, Lun X, Rahn JJ, Liacini A, Wang L, Hamilton MG, Parney IF, Hempstead BL, Robbins SM, Forsyth PA, Senger DL. The p75 neurotrophin receptor is a central regulator of glioma invasion. PLoS Biol. 2007;5:e212. doi: 10.1371/journal.pbio.0050212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CA, London NR, Chen H, Park KW, Sauvaget D, Stockton RA, Wythe JD, Suh W, Larrieu-Lahargue F, Mukouyama YS, et al. Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nat Med. 2008;14:448–453. doi: 10.1038/nm1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KM, Tan S, Landman N, Petrova K, Murray S, Lewis R, Kim PK, Kim DS, Ryu SH, Chao MV, Kim TW. Regulated intramembrane proteolysis of the p75 neurotrophin receptor modulates its association with the TrkA receptor. J Biol Chem. 2003;278:42161–42169. doi: 10.1074/jbc.M306028200. [DOI] [PubMed] [Google Scholar]
- Kanning KC, Hudson M, Amieux PS, Wiley JC, Bothwell M, Schecterson LC. Proteolytic processing of the p75 neurotrophin receptor and two homologs generates C-terminal fragments with signaling capability. J Neurosci. 2003;23:5425–5436. doi: 10.1523/JNEUROSCI.23-13-05425.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenchappa RS, Tep C, Korade Z, Urra S, Bronfman FC, Yoon SO, Carter BD. p75 neurotrophin receptor-mediated apoptosis in sympathetic neurons involves a biphasic activation of JNK and up-regulation of tumor necrosis factor-alpha-converting enzyme/ADAM17. J Biol Chem. 2010;285:20358–20368. doi: 10.1074/jbc.M109.082834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenchappa RS, Zampieri N, Chao MV, Barker PA, Teng HK, Hempstead BL, Carter BD. Ligand-dependent cleavage of the P75 neurotrophin receptor is necessary for NRIF nuclear translocation and apoptosis in sympathetic neurons. Neuron. 2006;50:219–232. doi: 10.1016/j.neuron.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Kendall TJ, Hennedige S, Aucott RL, Hartland SN, Vernon MA, Benyon RC, Iredale JP. p75 Neurotrophin receptor signaling regulates hepatic myofibroblast proliferation and apoptosis in recovery from rodent liver fibrosis. Hepatology. 2009;49:901–910. doi: 10.1002/hep.22701. [DOI] [PubMed] [Google Scholar]
- Khursigara G, Orlinick JR, Chao MV. Association of the p75 neurotrophin receptor with TRAF6. J Biol Chem. 1999;274:2597–2600. doi: 10.1074/jbc.274.5.2597. [DOI] [PubMed] [Google Scholar]
- Lebrun-Julien F, Bertrand MJ, De Backer O, Stellwagen D, Morales CR, Di Polo A, Barker PA. ProNGF induces TNFalpha-dependent death of retinal ganglion cells through a p75NTR non-cell-autonomous signaling pathway. Proc Natl Acad Sci U S A. 2010;107:3817–3822. doi: 10.1073/pnas.0909276107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KF, Li E, Huber LJ, Landis SC, Sharpe AH, Chao MV, Jaenisch R. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell. 1992;69:737–749. doi: 10.1016/0092-8674(92)90286-l. [DOI] [PubMed] [Google Scholar]
- Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- Liani E, Eyal A, Avraham E, Shemer R, Szargel R, Berg D, Bornemann A, Riess O, Ross CA, Rott R, Engelender S. Ubiquitylation of synphilin-1 and alpha-synuclein by SIAH and its presence in cellular inclusions and Lewy bodies imply a role in Parkinson's disease. Proc Natl Acad Sci U S A. 2004;101:5500–5505. doi: 10.1073/pnas.0401081101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YS, McLaughlin T, Sung TC, Santiago A, Lee KF, O'Leary DD. p75(NTR) mediates ephrin-A reverse signaling required for axon repulsion and mapping. Neuron. 2008;59:746–758. doi: 10.1016/j.neuron.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdan M, Lachance C, Gloster A, Aloyz R, Zeindler C, Bamji S, Bhakar A, Belliveau D, Fawcett J, Miller FD, Barker PA. Transgenic mice expressing the intracellular domain of the p75 neurotrophin receptor undergo neuronal apoptosis. J Neurosci. 1997;17:6988–6998. doi: 10.1523/JNEUROSCI.17-18-06988.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marxsen JH, Stengel P, Doege K, Heikkinen P, Jokilehto T, Wagner T, Jelkmann W, Jaakkola P, Metzen E. Hypoxia-inducible factor-1 (HIF-1) promotes its degradation by induction of HIF-alpha-prolyl-4-hydroxylases. Biochem J. 2004;381:761–767. doi: 10.1042/BJ20040620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micera A, Lambiase A, Stampachiacchiere B, Sgrulletta R, Normando EM, Bonini S. Nerve growth factor has a modulatory role on human primary fibroblast cultures derived from vernal keratoconjunctivitis-affected conjunctiva. Mol Vis. 2007;13:981–987. [PMC free article] [PubMed] [Google Scholar]
- Mowat FM, Luhmann UF, Smith AJ, Lange C, Duran Y, Harten S, Shukla D, Maxwell PH, Ali RR, Bainbridge JW. HIF-1alpha and HIF-2alpha are differentially activated in distinct cell populations in retinal ischaemia. PLoS One. 2010;5:e11103. doi: 10.1371/journal.pone.0011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Namekata K, Harada C, Harada T. Intracellular sortilin expression pattern regulates proNGF-induced naturally occurring cell death during development. Cell Death Differ. 2007;14:1552–1554. doi: 10.1038/sj.cdd.4402173. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Frew IJ, Hagensen M, Skals M, Habelhah H, Bhoumik A, Kadoya T, Erdjument-Bromage H, Tempst P, Frappell PB, et al. Siah2 regulates stability of prolyl-hydroxylases, controls HIF1alpha abundance, and modulates physiological responses to hypoxia. Cell. 2004;117:941–952. doi: 10.1016/j.cell.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Parkhurst CN, Zampieri N, Chao MV. Nuclear localization of the p75 neurotrophin receptor intracellular domain. J Biol Chem. 2010;285:5361–5368. doi: 10.1074/jbc.M109.045054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passino MA, Adams RA, Sikorski SL, Akassoglou K. Regulation of hepatic stellate cell differentiation by the neurotrophin receptor p75NTR. Science. 2007;315:1853–1856. doi: 10.1126/science.1137603. [DOI] [PubMed] [Google Scholar]
- Polekhina G, House CM, Traficante N, Mackay JP, Relaix F, Sassoon DA, Parker MW, Bowtell DD. Siah ubiquitin ligase is structurally related to TRAF and modulates TNF-alpha signaling. Nat Struct Biol. 2002;9:68–75. doi: 10.1038/nsb743. [DOI] [PubMed] [Google Scholar]
- Powell JC, Twomey C, Jain R, McCarthy JV. Association between Presenilin-1 and TRAF6 modulates regulated intramembrane proteolysis of the p75NTR neurotrophin receptor. J Neurochem. 2009;108:216–230. doi: 10.1111/j.1471-4159.2008.05763.x. [DOI] [PubMed] [Google Scholar]
- Qi J, Nakayama K, Gaitonde S, Goydos JS, Krajewski S, Eroshkin A, Bar-Sagi D, Bowtell D, Ronai Z. The ubiquitin ligase Siah2 regulates tumorigenesis and metastasis by HIF-dependent and -independent pathways. Proc Natl Acad Sci U S A. 2008;105:16713–16718. doi: 10.1073/pnas.0804063105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs BD, Baillie GS, McCall JR, Passino MA, Schachtrup C, Wallace DA, Dunlop AJ, MacKenzie KF, Klussmann E, Lynch MJ, et al. p75 neurotrophin receptor regulates tissue fibrosis through inhibition of plasminogen activation via a PDE4/cAMP/PKA pathway. J Cell Biol. 2007;177:1119–1132. doi: 10.1083/jcb.200701040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarram S, Lee KF, Byers MR. Dental innervation and CGRP in adult p75-deficient mice. J Comp Neurol. 1997;385:297–308. doi: 10.1002/(sici)1096-9861(19970825)385:2<297::aid-cne8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1 and human disease: one highly involved factor. Genes Dev. 2000;14:1983–1991. [PubMed] [Google Scholar]
- Semenza GL. HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell. 2001a;107:1–3. doi: 10.1016/s0092-8674(01)00518-9. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med. 2001b;7:345–350. doi: 10.1016/s1471-4914(01)02090-1. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Life with oxygen. Science. 2007;318:62–64. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]
- Smith LE, Wesolowski E, McLellan A, Kostyk SK, D'Amato R, Sullivan R, D'Amore PA. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- Sun X, He G, Qing H, Zhou W, Dobie F, Cai F, Staufenbiel M, Huang LE, Song W. Hypoxia facilitates Alzheimer's disease pathogenesis by up-regulating BACE1 gene expression. Proc Natl Acad Sci U S A. 2006;103:18727–18732. doi: 10.1073/pnas.0606298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Tanaka M, Watanabe N, Saito S, Nonaka H, Miyajima A. p75 Neurotrophin receptor is a marker for precursors of stellate cells and portal fibroblasts in mouse fetal liver. Gastroenterology. 2008;135:270–281. e273. doi: 10.1053/j.gastro.2008.03.075. [DOI] [PubMed] [Google Scholar]
- Tai HC, Schuman EM. Ubiquitin, the proteasome and protein degradation in neuronal function and dysfunction. Nat Rev Neurosci. 2008;9:826–838. doi: 10.1038/nrn2499. [DOI] [PubMed] [Google Scholar]
- Trim N, Morgan S, Evans M, Issa R, Fine D, Afford S, Wilkins B, Iredale J. Hepatic stellate cells express the low affinity nerve growth factor receptor p75 and undergo apoptosis in response to nerve growth factor stimulation. Am J Pathol. 2000;156:1235–1243. doi: 10.1016/S0002-9440(10)64994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Zhang YW, Zhang X, Liu R, Hong S, Xia K, Xia J, Zhang Z, Xu H. Transcriptional regulation of APH-1A and increased gamma-secretase cleavage of APP and Notch by HIF-1 and hypoxia. FASEB J. 2006;20:1275–1277. doi: 10.1096/fj.06-5839fje. [DOI] [PubMed] [Google Scholar]
- Weidemann A, Krohne TU, Aguilar E, Kurihara T, Takeda N, Dorrell MI, Simon MC, Haase VH, Friedlander M, Johnson RS. Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. Glia. 2010;58:1177–1185. doi: 10.1002/glia.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XB, Xiong SQ, Xu HZ, Jiang J, Li Y. Suppression of retinal neovascularization by shRNA targeting HIF-1alpha. Curr Eye Res. 2008;33:892–902. doi: 10.1080/02713680802416670. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Zhang H, Iwase T, Shen J, Semenza GL, Campochiaro PA. Digoxin inhibits retinal ischemia-induced HIF-1alpha expression and ocular neovascularization. FASEB J. 2010;24:1759–1767. doi: 10.1096/fj.09-145664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampieri N, Xu CF, Neubert TA, Chao MV. Cleavage of p75 neurotrophin receptor by alpha-secretase and gamma-secretase requires specific receptor domains. J Biol Chem. 2005;280:14563–14571. doi: 10.1074/jbc.M412957200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.