Abstract
This work studied the viabilities of five types of cells (two yeast cells, Saccharomyces cerevisiae CBS 1171 and Candida utilis; two bacterial strains, Escherichia coli and Lactobacillus plantarum; and one human leukemia K562 cell) as a function of cooling rate during freezing. The range of investigated cooling rates extended from 5 to 30,000°C/min. Cell viability was classified into three ranges: (i) high viability for low cooling rates (5 to 180°C/min), which allow cell water outflow to occur completely and do not allow any intracellular crystallization; (ii) low viability for rapid cooling rates (180 to 5,000°C/min), which allow the heat flow to prevail over water outflow (in this case, cell water crystallization would occur as water was flowing out of the cell); (iii) high viability for very high cooling rates (>5,000°C/min), which allow the heat flow to be very rapid and induce intracellular crystallization and/or vitrification before any water outflow from the cell. Finally, an assumption relating cell death to the cell water crystallization as water is flowing out of the cell is made. In addition, this general cell behavior is different for each type of cell and seems to be moderated by the cell size, the water permeability properties, and the presence of a cell wall.
The freeze-thawing process remains the principal method of cell preservation to date, and the high survival rates achieved by this method are of interest from both the biophysical and practical points of view. This is to ensure that the recovery of entire cell populations is free from the risk of possible subsequent alteration of its genetic composition.
Cell cryopreservation, which is commonly used in the food and pharmaceutical industries, requires optimization for each type of microorganism. Moreover, each type of cell has its own protocol for freezing. Numerous researchers have attempted to develop methods that permit 100% preservation of freeze-thawing of diverse cellular specimens (3, 6), but some microorganisms cannot yet be preserved by freezing.
For a better cell preservation some cryoprotectants such as glycerol or dimethyl sulfoxide can be used (8). These molecules improve the cell preservation by minimizing the cell water content (6) and/or supporting the vitrification occurrence (1) and finally by protecting the cell's constitutive macromolecules (2, 5).
The freeze-thawing process constitutes a double stress for the cell, i.e., thermal and hyperosmotic stresses, which act simultaneously during cooling (15, 16). The scenario of cell evolution during slow freezing is well known (13). The water surrounding the cell freezes before the cell contents, because the cytoplasm is more concentrated than the growth medium, and because thermodynamically, the component with the largest volume will nucleate first (6, 16). This freezing increases the osmotic pressure of the medium, and the extracellular solutes become concentrated in the remaining liquid extracellular water. Consecutive osmosis will then dehydrate cells as water diffuses from the cytoplasm into the more concentrated external solution. If the water outflow is faster than the heat outflow, then progressively, as the temperature decreases, the water contained in the medium will freeze and the osmotic pressure of the medium will continuously increase. The lower the temperature of the medium, the greater the concentration of the unfrozen solution and the greater the loss of intracellular water. This mass transfer is restricted by the hydraulic permeability (Lp) of the cell membrane.
In a previous study (6), we have shown that the cooling rate plays a major role in yeast cell preservation. Furthermore, it was shown that contrary to the generally proposed concept, low cooling rates are not the only ones that can be used for cell preservation; in fact, by using the yeast Saccharomyces cerevisiae, we have shown that very rapid cooling rates make it possible to obtain a cell viability equivalent to that obtained with low cooling rates.
The aim of this work was to study the viabilities of different types of cells (e.g., yeast cells, bacterial cells, and human cells) as a function of applied cooling rate. A large range of cooling rates, between 5 and 30,000°C/min, were investigated. Then, the properties of the cells were used to explain the cell viability results.
MATERIALS AND METHODS
(i) Biological materials.
Five types of cells were used in this study: two yeast cells (S. cerevisiae and Candida utilis), two bacterial strains (Escherichia coli and Lactobacillus plantarum), and one human cell (human myelogenous leukemia cells).
Cultures of the yeast S. cerevisiae CBS 1171 were grown in 250-ml conical flasks containing 100 ml of aerated, modified Wickerham medium and stirred at 250 rpm, while being maintained at 25°C. The medium was synthesized from 10 g of glucose, 3 g of pancreatic peptone, 3 g of yeast extract, and 1.5 g of Na2HPO4 in 1 liter of distilled water. The pH of the medium was adjusted to 5.35 by adding orthophosphoric acid. Inoculation was carried out by using 0.1 ml of a yeast suspension from a 48-h subculture grown under analogous conditions. Cells were harvested after a 65-h growth period (stationary phase) and used immediately in the experiments.
Starter cultures of C. utilis (ATCC 9950) were grown in yeast extract-malt extract broth (YM broth) (Difco Laboratories, Elancourt, France) at 30°C on a rotary shaker operating at 250 rpm for 24 h. The cultures were then used to inoculate 100 ml of YM broth at a final concentration of 1% (vol/vol) and were incubated at 30°C in a shaker operating at 250 rpm for 24 h (stationary phase).
L. plantarum 103151 T (Institut Pasteur, Paris, France) was maintained on petri dishes with a de Man, Rogosa, Sharpe (MRS) medium, supplemented with 20 g of agar (VWR International, Fontenay sous Bois, France) per liter. L. plantarum was grown in 250-ml conical flasks containing 100 ml of the MRS medium and maintained at 30°C for 18 h without shaking. One milliliter of culture was transferred into a conical flask containing the same medium and allowed to grow to the early stationary phase. The water activity of this medium was 0.992.
Escherichia coli K12 strain TG 1 [supE hsd Δ5thi Δ (lac-proAB) F′ (traD 36proA+ proB+ lacIq lacZ ΔM15)] was maintained on Luria-Bertani (LB) broth on agar plates (Sigma Chemical Co., St, Louis, Mo.) with a water activity value equal to 0.992. For the experiments, liquid cultures were prepared by inoculation with colonies into test tubes containing 9 ml of LB broth maintained at 37°C for 12 h. A subculture was then prepared by injecting 0.1 ml of bacterial suspension into 9 ml of LB broth, and this was grown at the same temperatures as those described above. Cells were harvested after a 24-h growth period (stationary phase) and used immediately in the experiments.
Human myelogenous leukemia K562 cells (etoposide-resistant cell line) were maintained at 37°C (in 5% CO2) and cultured in an RPMI 1640 medium serum (VWR International) supplemented with 1% (vol/vol) glutamine and 10% (vol/vol) fetal bovine serum. The cells were cultured for 48 h before use, which corresponds to the early stationary phase.
(ii) Freeze-thawing conditions.
Freezing was always carried out with liquid nitrogen at a temperature (T) of −196°C, and the nucleation temperature was not controlled. Cells were stored for 5 min at −196°C before thawing. For each experiment, cold stress was applied in a water-glycerol solution at an osmotic pressure (Π) of 1.38 MPa (5.1 g of glycerol was added to 100 g of water), which corresponds to the osmotic pressure of a classical growth medium. The different methods that were used to obtain the cooling rates under investigation are described in Table 1.
TABLE 1.
Observed cooling rates for supports and sample volumes
| Cooling rate (°C/min) | Support | Vol of sample (ml) |
|---|---|---|
| 5 | Sarstedt cryotube in plastic tube | 1 |
| 180 | Sarstedt cryotube | 1 |
| 250 | Nunc cryotube | 0.5 |
| 650 | Sarstedt cryotube | 0.5 |
| 5,000 | Cylinder in glass | 0.01 |
| 15,000 | Thermocouple in metal | 0.0014 |
| 30,000a | Thermocouple in metal | 0.0014 |
Value calculated from Fourier's second law.
For low cooling rates (5°C/min), samples in Sarstedt cryotubes were placed in 50-ml plastic tubes (PolyLabo, Paris, France) for centrifugation to protect them from the cold gradient by using a gas layer. For the cooling rate of 180°C/min, a Sarstedt cryotube containing 1 ml of the cell suspension was used. For cooling rates of 250 and 650°C/min, a Nunc cryotube and a Sarstedt cryotube containing 0.5 ml of the cell solution were used. For the cooling rate of 5,000°C/min, 10 μl of the sample was contained within a glass cylinder. For the fastest cooling rate (30,000°C/min), cells were fixed on a 0.5-mm-diameter type T thermocouple (TCSA, Dardilly, France) acting as the support. This thermocouple was used because cells can be fixed onto its surface. It provided effective measurement of the cooling rate at 15,000°C/min. The cooling rate in the first 20-μm layer, corresponding to the thickness of the cell suspension layer around the thermocouple, was calculated by using classical heat transfer equations as being 30,000°C/min.
The influence of the thawing period was not studied during the experiments, and thawing was always carried out by dropping the frozen sample into a temperature-controlled water bath maintained at 37°C. Indeed, Mazur (12) has shown that it is necessary to thaw samples as rapidly as possible to avoid conditions that could lead to recrystallization of the medium. In the case of freezing performed by use of the thermocouple, a water bath could not be used. So, cells were taken from the thermocouple by washing it three times with a controlled volume of 1 ml at 37°C of the water-glycerol solution previously described.
(iii) Viability measurements.
Viability measurements for the microorganisms were performed by using the CFU method, in which plating cells in the corresponding medium were supplemented with 15 g of agar per liter. The petri dishes were then incubated at the temperature corresponding to the growth conditions of the microorganisms. The viability was determined by comparing the experimental and control microorganisms.
For K562 cells, the viability measurements were followed by trypan blue staining. There were at least three repetitions for each experiment, and average values were calculated as well as confidence intervals (CI) at the 90% means level. The CI values were found to be inferior by 7% to the mean viability.
RESULTS
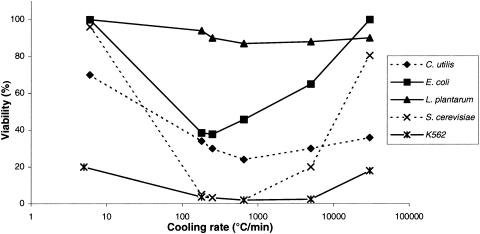
The viabilities of the five types of cells with regard to the five cooling rates used (5, 180, 250, 5,000, and 30,000°C/min) are shown in Fig. 1.
FIG. 1.
Relation of cell viability to cooling rate. Symbols: ♦, C. utilis; *, K562; ×, S. cerevisiae; ▴, L. plantarum; ▪, E. coli. The CI values at 90% were always less than 7% of the corresponding viability.
The cells of the yeast S. cerevisiae remained viable at a cooling rate of 5°C/min (i.e., >80% of the control). The results were different for cooling rates higher than 180°C/min and lower than 5,000°C/min. Indeed, the S. cerevisiae viability fell to a low point of 2% for a cooling rate of 180°C/min and then remained stable at about 3%. For higher cooling rates, the yeast viability increased from 20% at a cooling rate of 5,000°C/min to 80% at a cooling rate of 30,000°C/min.
The other yeast, C. utilis, showed its highest viability of 70% for a cooling rate of 5°C/min. Then, the viability fell gradually to 24% at a cooling rate of 250°C/min. For faster cooling rates, the viability was higher, with viabilities of 30% at a cooling rate of 5,000°C/min and 35% at a cooling rate of 30,000°C/min.
The bacterium L. plantarum showed no significant change in its viability between cooling rates of 5 and 30,000°C/min. The viability of this bacterium remained quite stable considering the limits of its viability, which were 100% for a cooling rate of 5°C/min and 87% for a cooling rate of 250°C/min.
The results were quite different for the other bacterium, E. coli, which showed changes in its viability that were comparable to the U pattern obtained for S. cerevisiae. Indeed, the viability of E. coli was 100% for a cooling rate of 5°C/min. Then, the viability fell progressively to 38% for a cooling rate of 250°C/min. For higher cooling rates, the viability increased until reaching 100% for a cooling rate of 30,000°C/min.
Such levels of high cell viability were not obtained with the human leukemia K562 cells. Indeed, the maximum viability value was observed for a cooling rate of 5°C/min and only reached 20% of the initial cell viability. For cooling rates of 180, 250, and 5,000°C/min, the viability fell and remained stable at about 2%. For a cooling rate of 30,000°C/min, the cell viability returned to nearly the maximum cell viability value (about 18%).
A cell's cryopreservation depends on numerous factors that influence the entire cell's viability. These results clearly show that the viabilities of the five microorganisms studied are influenced by the cooling rate. From an overall perspective, with the observed results it is possible to extract three types of cell behavior with regard to the freezing rate conditions.
The first type of behavior is the U-pattern curve, as obtained for S. cerevisiae, as a function of increasing cooling rate. In this case, a high viability is observed for low cooling rates (5°C/min) but also for ultrarapid cooling rates (30,000°C/min). The yeast S. cerevisiae and the bacterium E. coli both show this type of behavior.
The second type of behavior, as shown by C. utilis and K562 cells, shows a low resistance to freezing. Cell mortality was observed even with very rapid cooling rates.
The third type of behavior, as shown by L. plantarum cells, shows the cells maintaining a high viability irrespective of cooling rate.
These results illustrate that the cooling rate strongly influences cell viability. From a general point of view, in each case the minimal cell viability value was obtained for intermediate cooling rates (i.e., between 180 and 5,000°C/min). Lower cooling rates and very rapid cooling rates are less detrimental for the cells. To explain these different behaviors, it is necessary to focus on the factors that can influence the cell viability during the freezing process.
DISCUSSION
From an overall perspective, the results showed that, for most of the cells used and in the range of cooling rates studied, a U-pattern curve generally represented the evolution of the cell viability. Previous works have already shown that the cooling rate is one of the main factors (6, 12) that influence cell viability during the freeze-thawing process.
A major link between cooling rate, velocity of water outflow from the cell across the plasma membrane, and cell mortality is proposed in this work, based on previous work (6) on the correlation of viability of S. cerevisiae with cooling rate. In that study, at lower cooling rates (less than 100°C/min) cell water outflow occurred slowly, and this did not damage the yeast cells. In contrast, at intermediate cooling rates (between 100 and 1,000°C/min), cell viability was dramatically reduced. Then, for very rapid cooling rates (from 1,000 to 30,000°C/min), the recovery in cell viability was observed after thawing.
The understanding of these results is based on the hypothesis that if the water flow is faster than the thermal flow, then no intracellular crystallization will occur, and the cell survival rate will be high. In contrast, as soon as the thermal flow is equal to the water flow, then the freezing rate will induce intracellular water crystallization during the osmotic exit of water, a process which seems to provoke cell death.
Cell death can be prevented in two cases: the first case corresponds to a slow cooling rate during which intracellular water can flow out of the cell, and the second case corresponds to very rapid cooling rates, which involve a complete freezing of the intracellular water before any water transport occurs. In regards to the three kinds of cell viability behavior, it is interesting to study the factors that can induce differences between all the studied cells. Some determinant factors for cryopreservation utilizing heat and mass transfer are shown in Table 2. The first factor is cell size, which influences the surface-to-volume (S/V) ratio and directly defines the transfer surface for intracellular heat and mass. The second factor corresponds to the cell permeability properties, which determine the resistance of the cell membrane to water transfer. The presence of a cell wall is the third factor, which is of interest in terms of its mechanical protective properties.
TABLE 2.
Characteristics of the cells used
| Organism | Type of cell | Shape | Mean vola (μm3) | S/V ratio (μm−1) | Description of cell wall | Lp (m · s−1 · Pa−1) | Time to reach 50% of final cell vol variation |
|---|---|---|---|---|---|---|---|
| E. coli | Bacterium; gram negative | Straight rods | 6 | 3.3 | Very thin, high in lipid content | 3.4 × 10−11b | 0.075d |
| L. plantarum | Bacterium; gram positive | Rods with rounded ends | 6 | 3.7 | High in peptidoglycan content | ||
| S. cerevisiae | Yeast | Ellipsoidal | 250 | 0.7 | Predominantly glucan, mannan, and chitin polymers | 6.00 × 10−11c | 0.2 |
| C. utilis | Yeast | Spherical | 270 | 0.75 | Primarily cellulose, mannan, and glucan polymers | ||
| K562 | Leukemia cell | Spherical | 523 | 0.6 | No cell wall | 1.7 × 10−13c | 5 |
The S/V ratio can be linked to both thermal and water flow. The larger the cell radius is, then the smaller the S/V ratio is, i.e., larger cells offer a smaller transfer surface compared to their volume than smaller cells do, for both heat and mass transfer. Therefore, water and heat will flow out faster from cells with a high S/V ratio (i.e., smaller cells). Moreover, the cylindrical shape of bacteria gives them a significant excess surface area compared to their volume (the S/V ratio is about five times larger for bacteria than for yeast cells [Table 2]). The minimum viability values obtained during freezing could be related to this factor, irrespective of the cooling rate. Indeed, as shown in Fig. 1, the bacterial cells, which have the smallest size, were less destroyed by the freeze-thawing process (i.e., minimal viabilities of 87 and 37.8% for L. plantarum and E. coli, respectively) than were the yeast cells (i.e., minimal viabilities of 2 and 24% for S. cerevisiae and C. utilis, respectively). The large human cell K562 was found to be the most fragile of the cells, with a minimum viability of 2% obtained at cooling rates in the range of 250 to 5,000°C/min.
In the hypothesis discussed above, it was explained that cell mortality could result from the competition between water and heat efflux. Indeed, in the heat exchange occurring during freezing, a large part of the heat flow is taken up by the latent heat of crystallization, which is directly proportional to the mass of water to be frozen. This parameter would strongly influence the freezing time lag of high-volume cells, irrespective of cooling rate. Then, for this reason, in high-volume cells, the water would have more time to exit the cell before crystallization. Therefore, it could be proposed that for the same cooling rate of 30,000°C/min, the intracellular water of the K562 cells had begun to flow out of the cells, leading to cellular death, in contrast to what occurred with S. cerevisiae, which crystallized instantaneously owing to its smaller size.
The existence of the latent heat of water crystallization allows us to propose that the water mass transfer is more influenced by the S/V ratio than the heat transfer properties. Given that it is lethal for a cell to freeze when it loses some of its intracellular water and that the larger the cell, the more time it will take to freeze, then it is easier to freeze smaller cells without involving any loss of water and thus obtain a higher survival rate.
Therefore, the smaller size of the bacterial cells determines their better preservation properties after freezing than those of larger cells, such as yeast cells and animal cells. If heat efflux is significantly faster than the water outflow for high freezing rates, then cells can be frozen without any water loss and cell viability can be preserved.
Concurrent with the important role played by the cellular size, the cell water permeability can also be considered a factor that effectively influences the mass transfer, because the time lags under consideration are very short. Since water outflow will be the main phenomenon observed during the first stage of cell volume decrease (7), only the hydraulic permeability values of the cells need to be considered. The water permeability value is represented by the water permeability coefficient, Lp (in units of m · s−1 · Pa−1), and this varies with each type of cell.
Considering that cells with the same water permeability value are able to lose intracellular water for a given osmotic stress at the same rate whatever their sizes, then a comparison of water permeability values will allow for a prediction of a cell's cryopreservation. Therefore, looking at the data related to E. coli and S. cerevisiae (Table 2), one can observe that the Lp values of these two cells are of the same magnitude. Consequently, water can flow out from both cells at the same rate. Moreover, the difference in their cell viability results (100 and 80% viability at a cooling rate of 30,000°C/min for E. coli and S. cerevisiae, respectively) can be attributed to their different cell volumes alone, as S. cerevisiae is 40 times larger than E. coli. The major role of the S/V ratio is still to be highlighted in this case.
However, the considerations are different in regard to K562 cells and S. cerevisiae cells. Indeed, for a mean volume that is twice that of S. cerevisiae, the data in Table 2 show that K562 has an Lp value that is 400 times smaller than that of the S. cerevisiae yeast.
With regard to the viability obtained with low cooling rates (i.e., between 20 and 100°C/min, as shown in Fig. 1), the difference in the Lp values can explain why the viability of the yeast cells is higher than that of the human cells (about 20% viability for K562 versus 70% for S. cerevisiae at a cooling rate of 5°C/min). Indeed, at this cooling rate, the more quickly water can flow out of a cell for a given osmotic perturbation, then the more the cell is protected from internal crystallization. Consequently, S. cerevisiae is less affected by the cooling rate than are the K562 cells.
Concerning the rapid cooling rates (i.e., between 100 and 5,000°C/min), the time lag permitting the cellular osmotic response before crystallization commences is shorter than for slow cooling rates. In this case, the K562 cell damage due to crystallization increases and the viability decreases from 10 to 2%. For S. cerevisiae, this range of cooling rate sees the onset of intracellular crystallization, and the yeast viability dramatically decreases from 80 to 2%.
At the very rapid cooling rates (i.e., from 5,000 to 30,000°C/min), the K562 cells should be able to freeze without any change in cell volume because of their low Lp value. In contrast, the high Lp value of S. cerevisiae should aid the outflow of cell water, even at very rapid cooling rates, and therefore, cell viability should rapidly increase for K562 cells as the cooling rate increases. However, as shown in Fig. 1, the results do not verify this hypothesis. Indeed, even though a better preservation of the K562 cell is possible for very rapid cooling rates, their viability did not exceed 10%. In comparison, S. cerevisiae, in spite of its higher water permeability value, showed a better viability (80%).
Therefore, water permeability and size values alone cannot explain all the observed results. With regard to the proposed death scenario, i.e., the combination of water outflow and water crystallization, such a combination provokes irreversible membrane damage and then cell death. In the case of S. cerevisiae, the existence of a cell wall could certainly provide additional support to explain previous results. Indeed, a cell wall could prevent or repair membrane damages, such as membrane vesiculations, induced by simultaneous crystallization and water outflow (10, 14, 19).
Conclusions. This work has shown that a U-pattern behavior of cell viability versus cooling rate occurs for cooling rates between 5 and 30,000°C/min. For all types of cells investigated, the viability was found to be higher at low and very high cooling rates. This evolution of viability during the freeze-thawing process can be explained by a competition between the heat flow and the water outflow from the cell.
The S/V ratio plays a major role in the determination of the viability results, as it determines the time constant of the heat and mass transfer into and out of the cells. For slower cooling rates, the latent heat of cell water crystallization allows time for water to exit the cell and so prevents internal crystallization. For faster cooling rates, the latent heat delays intracellular crystallization and so becomes a negative factor for cell viability.
Cell death is proposed to occur from crystallization during water outflow from the cell, which involves lethal membrane damage. Thus, the S/V ratio, the latent heat of water crystallization, and the Lp value play a role in a cell's survival after freezing. High S/V values (i.e., small cell sizes), high Lp values, and high water content seem to influence positively the survival rate for low cooling rates. In contrast, low Lp values should be positive for high cooling rates.
REFERENCES
- 1.Ablett, S., J. M. Izzard, and P. J. Lillford. 1992. Differential scanning calorimetric study of frozen sucrose and glycerol solutions. J. Chem. Soc. Faraday Trans. 88:789-794. [Google Scholar]
- 2.Adam, M. M., K. J. Rana, and B. J. McAndrew. 1994. Effect of cryoprotectants on activity of selected enzymes in fish embryos. Cryobiology 32:92-104. [Google Scholar]
- 3.Albrecht, R. M., G. R. Orndorff, and A. P. MacKenzie. 1973. Survival of certain microorganisms subjected to rapid and very rapid freezing on membrane filters. Cryobiology 10:233-239. [DOI] [PubMed] [Google Scholar]
- 4.Alemohammad, M. M., and C. J. Knowles. 1974. Osmotically induced volume and turbidity changes of Escherichia coli due to salt sucrose and glycerol, with particular reference to the rapid permeation of glycerol into the cell. J. Gen. Microbiol. 82:125-142. [DOI] [PubMed] [Google Scholar]
- 5.Anchordoguy, T. J., A. S. Rudolph, J. F. Carpenter, and J. H. Crowe. 1987. Modes of interaction of cryoprotectants with membrane phospholipids during freezing. Cryobiology 24:324-331. [DOI] [PubMed] [Google Scholar]
- 6.Dumont, F., P. A. Marechal, and P. Gervais. 2003. Influence of cooling rate on Saccharomyces cerevisiae destruction during freezing: unexpected viability at ultra-rapid cooling rates. Cryobiology 46:33-42. [DOI] [PubMed] [Google Scholar]
- 7.Gervais, P., and L. Beney. 2001. Osmotic mass transfer in the yeast Saccharomyces cerevisiae. Cell. Mol. Biol. 47:831-839. [PubMed] [Google Scholar]
- 8.Hubalek, Z. 2003. Protectants used in the cryopreservation of microorganisms. Cryobiology 46:205-229. [DOI] [PubMed] [Google Scholar]
- 9.Kandler, O., and N. Weis. 1986. Regular, nonsporing gram-positive rods, p. 1208-1234. In N. R. Krieg and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 2. Williams & Wilkins, Baltimore, Md.
- 10.Klis, F. M., P. Mol, K. Hellingwerf, and S. Brul. 2002. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 26:239-256. [DOI] [PubMed] [Google Scholar]
- 11.Martinez De Marañon, I., P. Gervais, and P. Molin. 1997. Determination of cells' water membrane permeability: unexpected high osmotic permeability of Saccharomyces cerevisiae. Biotechnol. Bioeng. 56:63-70. [DOI] [PubMed] [Google Scholar]
- 12.Mazur, P. 1966. Theoretical and experimental effects of cooling and warming velocity on the survival of frozen and thawed cells. Cryobiology 2:181-192. [DOI] [PubMed] [Google Scholar]
- 13.Mazur, P. 1970. Cryobiology: the freezing of biological systems. Science 168:939-949. [DOI] [PubMed] [Google Scholar]
- 14.Mille, Y., L. Beney, and P. Gervais. 2002. Viability of Escherichia coli after combined osmotic and thermal treatment: a plasma membrane implication. Biochim. Biophys. Acta 1567:41-48. [DOI] [PubMed] [Google Scholar]
- 15.Morris, G. J., G. E. Coulson, and K.-J. Clarke. 1988. Freezing injury in Saccharomyces cerevisiae: the effect of growth conditions. Cryobiology 25:471-482. [Google Scholar]
- 16.Muldrew, K., and L. E. McGann. 1990. Mechanisms of intracellular ice formation. Biophys. J. 57:525-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orskov, F. 1984. Facultatively anaerobic gram-negative rods, p. 420-422. In N. R. Krieg and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 1. Williams & Wilkins, Baltimore, Md.
- 18.Rose, A. H., and J. S. Harrison. 1987. The yeasts, 2nd ed., vol. 1. Biology of yeasts. Academic Press, London, United Kingdom.
- 19.Steponkus, P. 1987. Membrane destabilisation resulting from freeze-induced dehydration. Cryobiology 24:555. [Google Scholar]