Abstract
Objective
The purpose of the present investigation was to determine if variation in the catechol-O-methyltransferase (COMT) and mu-opioid receptor (OPRM1) genes is associated with pain-related positive affective regulation in fibromyalgia (FM).
Design
Forty-six female FM patients completed an electronic diary that included daily assessments of positive affect and pain. Between- and within-person analyses were conducted with multilevel modeling.
Main Outcome Measure
Daily positive affect was the primary outcome measure.
Results
Analyses revealed a significant gene × experience interaction for COMT, such that individuals with met/met genotype experienced a greater decline in positive affect on days when pain was elevated than did either val/met or val/val individuals. This finding supports a role for catecholamines in positive affective reactivity to FM pain. A gene × experience interaction for OPRM1 also emerged, indicating that individuals with at least one asp40 allele maintained greater positive affect despite elevations in daily pain than those homozygous for the asn40 allele. This finding may be explained by the asp40 allele’s role in reward processing.
Conclusions
Together, the findings offer researchers ample reason to further investigate the contribution of the catecholamine and opioid systems, and their associated genomic variants, to the still poorly understood experience of FM.
Keywords: fibromyalgia, genetics, pain, affect
Introduction
Fibromyalgia (FM) is a chronic musculoskeletal disorder characterized by widespread pain, fatigue, and a variety of other comorbid physiological and psychological conditions (Thieme, Turk, & Flor, 2004; Wolfe, Smyth, Yunus et al., 1990). Recent research has identified a deficit in positive affect (PA) among FM patients, relative to other chronic pain groups, as a distinctive feature characteristic of the disorder (Finan, Zautra, & Davis, 2009; Zautra, Fasman, Reich et al., 2005). In the absence of PA, FM patients may be more vulnerable to the harmful effects of pain on well-being (Zautra, Johnson, & Davis, 2005). Despite these findings, there is still considerable variability in the symptom presentation of FM. In the current study, we sought to identify genetic factors that may contribute to different patterns of positive affective reactivity to pain within the FM population.
In the exploration of the etiopathology of FM, researchers have increasingly shifted focus to the role of genetics. Interindividual differences in pain sensitivity are often reported to be substantially greater than intraindividual differences, and so a genetic approach provides an opportunity to target biologically latent sources of that variation (Mogil, 1999). Further, evidence for a strong familial aggregation of FM has led researchers to similarly conclude that genetic variation may help explain part of its complex symptom presentation. As such, the focus of research on FM has shifted to a variety of genomic variants (Buskila & Sarzi-Puttini, 2006; Limer, Nicholl, Thomson, & McBeth, 2008). The majority of pain-related candidate gene studies have measured phenotypes of pain sensitivity, threshold, and tolerance. However, few have explored affect and affective reactivity to pain (e.g., Zubieta et al., 2003), and none have done so within the FM population. Two genes with functional single nucleotide polymorphisms (SNPs) have emerged as particularly attractive candidates for the study of the dynamic relations between pain and affect in FM: the catechol-O-methyltransferase gene (COMT/val158met) and the μ-opioid receptor gene (OPRM1/asn40asp). Both the catecholaminergic and opioidergic systems, in which COMT and OPRM1, respectively, have regulatory roles, are intricately involved in the dual processing of pain and affect (Price, 2000; Smolka, Schumann, Wrase et al., 2005; Zubieta et al., 2003).
The COMT gene codes for an enzyme that facilitates the degradation of the catecholamine neurotransmitters epinephrine, norepinephrine, and dopamine (Li, Warsh, & Godse, 1984). Individuals homozygous for the val158allele of the val158met polymorphism have a three to four-fold increase in enzymatic activity compared to those homozygous for met158 as a result of changes in thermostability of the enzyme (Spielman & Weinshilboum, 1981). The heterozygous genotype (val/met) produces intermediate enzymatic activity, indicating that the alleles are codominant. There is evidence to support a role for the met158 allele as a “risk” allele in the regulation of PA during chronic pain. The met158 allele has been associated with incidence of FM (e.g., Garcia-Fructoso et al., 2006; Vargas-Alarcon et al., 2005), and has also been associated with higher sensory and affective ratings of lab-induced pain (Zubieta et al., 2003). The met/met genotype has been associated with greater FM illness severity across domains of pain, fatigue, sleep disturbance, and psychological distress (Garcia-Fructoso et al., 2006).
The asp-allele of the asn40asp polymorphism in OPRM1 has been reported to result in a seven to ten-fold reduction in μ-opioid receptor protein in cultured cells (Beyer, Koch, Schroder, Shulz, & Hollt, 2004; Zhang, Wang, Johnson, Papp, & Sadee, 2005), and is considered an appropriate candidate for association with pain processing (Uhl et al., 1999). Despite the relevance of μ-opioid receptor activity to pain processing, the findings from candidate gene studies have been mixed. Two null findings have been reported in studies of pain sensitivity (Comptom, Geschwind, & Alarcon, 2003; Kim, Mittal, Iadarola, & Dionne, 2006). In contrast, Fillingim, Kaplan, Staud et al., (2005) reported significant OPRM1 genetic variability in self-reported pressure pain threshold. In that study, individuals with at least one copy of the minor asp40 allele had a higher pressure (mechanical) pain threshold than those homozygous for asn40, but the effects did not generalize to other forms of pain stimuli, including thermal and ischemic pain. There is some evidence in support of asn40asp-mediated reward processing among people with alcohol abuse problems (Oroszi & Goldman, 2004), but, to our knowledge, no studies have linked the variant to affective components of pain.
Given the converging evidence of genetically-regulated, dual-processing neurobiological pathways of pain and affect, we believe it is appropriate to test for genetic association with daily affective processes relevant to FM patients. As such, we have centered our focus on the process of positive affective reactivity to pain. Despite the recent identification of a PA deficit in FM (Zautra, Fasman, Reich et al., 2005), and replications of those findings (Finan et al., 2009; Zautra Johnson, & Davis, 2005), there is currently a gap in the knowledge of what genetic mechanisms may contribute to FM patients’ experience of positive affective dysregulation, and how that disturbance may perpetuate and prolong the experience of chronic pain.
The positive affective response to pain is a salient phenotype that describes a common affective profile found in FM. Largely missing from the literature of genetic associations in FM is attention to rich phenotypic assessment that captures the multifarious daily experiences with chronic pain and affective disturbance. A next step, then, is to examine the effects of COMT and OPRM1 in the context of PA reports as they occur throughout daily life in individuals with FM. The examination of naturalistic data extends the laboratory work that has been integral to our understanding of the influence of the catecholaminergic and opioidergic systems on these phenotypes, and provides a unique window into the role of COMT and OPRM1 in the dual processing of pain and affect in the FM condition.
In the current study, we hypothesized that the met158 allele in COMT would be associated with reduced PA, across 30 days of daily reporting, relative to the val158 allele. Further, we proposed that a gene × experience effect would be observed, such that individuals with the met158 allele would suffer a greater reduction in PA on days in which pain was elevated. It was expected that PA effects would be greater with higher levels of met158 loading. To test this, trichotomous genotype differences for COMT were examined. In contrast, we hypothesized that FM patients with the asp40 allele in OPRM1 would have greater PA across days, and would be better able to sustain PA on days when pain was elevated, relative to homozygous carriers of asn40.
Method
Participants
The data that were analyzed for the current study were collected as part of a larger project (R01 AR46034) designed to identify factors related to adaptation to pain and stress in FM. The larger study included female participants with FM, osteoarthritis, or a dual diagnosis of both FM and osteoarthritis. Of the 392 participants enrolled in the parent study, 46 qualified for the current study. 201 individuals were excluded from the current study because they had a diagnosis of osteoarthritis, 71 individuals were dropped due to an inability to confirm diagnosis, and 65 participants were dropped for the following reasons: too ill to participate; no time to participate; changed mind about participation; unable to reestablish contact; upset about insufficient payment for participation; unable to schedule a mutually convenient time to meet; and, unknown reasons. Genetic data was unavailable for an additional 7 participants, leaving the final total included in the current study 46. As stated above, only participants with a single diagnosis of FM were analyzed. This decision was made to minimize the potential for separate disease processes to confound the analysis of genetic influences in FM.
Participants were between the ages of 38 and 72 with a physician-confirmed diagnosis FM. Participants were recruited in the Phoenix, AZ metropolitan area from physician’s offices, advertisements, senior citizen groups, and mailings to members of the Arthritis Foundation. Included in the study were participants who had no diagnosed autoimmune or arthritic disorders, a past-month pain rating above 20 on a 0–100 scale, and/or who were not involved in litigation regarding their condition. All participants reported their diagnosis to research staff and subsequently signed a Health Insurance Portability and Accountability Act (HIPAA) release form. Research staff then contacted each participant’s physician, who sent a written confirmation of the participant’s FM diagnosis and disconfirmed diagnosis of other autoimmune and arthritic disorders.
Procedure
After being screened into the study, participants were visited by a clinician to reconfirm FM diagnosis. All participants underwent a tender point exam conducted by trained research personnel supervised by licensed rheumatologists in a method consistent with medical standards. Our clinician used a dolorimeter to palpate 18 musculoskeletal regions identified by the American College of Rheumatology as tender points that can aid in FM diagnosis (Wolfe et al., 1990). The results of the tender point exam were used primarily to identify outliers whose reported pain may have differed from expectations based on physician diagnosis. However, results discrepant with diagnostic expectations were not used as exclusionary criteria. A number of supplementary analyses, described fully in the Results section, were conducted to examine whether tender point endorsement was influential on the outcomes of interest in the current study.
After the clinician visit, participants were trained in our laboratory by a research assistant to use a laptop computer to complete daily diaries each night for 30 days. Participants were encouraged to call our laboratory staff immediately if a problem occurred with the laptop. A built-in date-checking software program prevented data entry on days other than the correct day. After completing the 30-day diary, participants were visited by a clinician, and buccal cells were collected via a cheek swab method that followed published procedures (Walker et al., 1999). Participants were compensated $90 for completion of the diary. The overall rate of diary completion was 92.5%.
Genotyping
Genomic DNA was purified from buccal cheek swab samples by the University of Connecticut Health Center GCRC Core Lab. DNA samples were placed in 96-well plates and genotyped using PCR based TaqMan 5’-nuclease allelic discrimination assay methods in the GCRC Core Lab. Ten percent of genotypes were randomly repeated to monitor reproducibility. The core lab repeated samples from each plate in a known fashion and included water blanks and DNA samples with known genotypes to monitor quality control. Assays for both markers were already in use in the GCRC Core Lab. The primer and probe combinations for these assays are: COMT val158met polymorphism (rs4680) primers (CCCAGCGGATGGTGGAT and AACGGGTCAGGCATGCA), and dual labeled probes (Vic-TCCTTCAcGCCAGCGA-MGB and Fam-TCCTTCAtGCCAGCGA-MGB); OPRM1 Asn40Asp polymorphism (rs1799971) primers (CCCAGCCCCGGTTCCT and TGATGGCCGTGATCATGGA), and probes (Vic-AGATGGCGACCTGTCC-MGB Fam-AGATGGCAACCTGTCC-MGB).
Measures
Positive and Negative Affect
PA and NA were measured in the daily diary using the Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988). Participants rated 10 mood adjectives each for PA and NA using a 5-point scale from 1 (very slightly or not at all) to 5 (extremely). Scores for the two scales were obtained by computing means, while between-person reliabilities were computed by aggregating each participant’s items across all days. Cronbach’s alpha was .95 for PA and .86 for NA.
Soft Tissue Pain
The daily diary included a body diagram that depicted the major quadrants of the body (Affleck et al., 1996), with instructions for participants to rate their soft tissue pain in 15 areas including parts of their neck, shoulders, chest, arms, legs, back, and buttocks. Ratings were made on a scale of zero-to-three where zero was “No pain” and a three meant “Severe pain.” Sum scores were computed from the 15 items to create an overall score of soft tissue pain. Cronbach’s alpha was .93.
Interpersonal Stress
Both positive and negative interpersonal life events were assessed by administering an abridged version of the Inventory of Small Life Events (ISLE; Zautra, Guarnaccia, & Dohrenwend, 1986). Events were grouped in four domains of the ISLE: a) friends or acquaintances, b) spouse or partner, c) family members, and d) employment and co-workers. Participants were first asked to report whether or not each event occurred during the day of reporting. After responding to each domain of items, participants were asked, “Overall, how stressful were your relations with your (domain) today?”. Responses were made on a four-point Likert scale where 1 = Not at all, 2 = A little, 3 = Moderately, and 4 = Extremely. Stressfulness ratings from each of the four domains were then aggregated to create a single daily index of perceived stress. This method has been reported elsewhere (Zautra, Hoffman, Potter et al., 1997).
Data Analysis
The frequencies of the val158met genotypes in COMT have been reported as follows in a Spanish-origin FM population: [12% (val/val), 51% (val/met), 37% (met/met); Vargas-Alarcon et al., 2007]. Due to the functionally intermediate action of the heterozygous val/met genotype, gene effects were expected to be largest for individuals with homozygous genotypes. No known genotype frequency estimates were available within the FM population for the asn40asp genotype in the OPRM1 gene. Given the rarity of the homozygous asp/asp genotype (2–3% in the general population; Ray & Hutchison, 2004), individuals were analyzed based on the presence or absence of at least one copy of the asp40 allele.
Repeated daily measurements resulted in a hierarchical nested data structure, with up to 30 observations nested within each person. Given such a data structure, multilevel modeling, executed using SAS PROC MIXED (Littell et al., 1996), was the appropriate data analytic tool. Predictor variables in the current study were centered within-person (Nezlek, 2001; Enders & Tofighi, 2007), yielding an index of daily change.
Both Level 1 (within-person) and Level 2 (between-person) variables, as well as cross-level interactions (Level 1 × Level 2), were modeled as predictors. As an example, we will highlight the basic equations used in the present study, involving daily PA as the criterion:
| (1) |
There are i observations of PA for j individuals. β0j yields an estimate of the average level of PA at the individual’s mean level of pain, when they are experiencing no NA. β1j is the coefficient for the daily influence of pain on PA and β2j is the coefficient for the relationship between NA and PA, serving in this model as a covariate. rij is the within-person error component. At Level 2, individual differences in the average level of PA are probed, along with cross-level interactions. The Level 2 intercept is specified as follows:
| (2) |
where the equation for β0j predicts each person’s Level 1 intercept from the grand mean, the mean level of pain, and the individual’s genotype. The Level 2 slopes are specified as follows:
| (3) |
The second Level 2 equation models a cross-level interaction, whereby between-person differences in genotype (Level 2) moderate the relationship of within-person changes in pain (Level 1) and the outcome, daily PA. In all models, a first-order autoregressive variance-covariance matrix was chosen to model the within-person variance on the dependent variables, as suggested by Singer (1998). Additionally, because PA and negative affect (NA) are correlated when pain is elevated (Zautra, Smith, Affleck, & Tennen, 2001), NA was used as a covariate in all analyses.
Here we are only concerned with fixed effects, and so the Level 2 equations lack a random error component. Thus, for the analyses presented in the current study, only the intercept was modeled as random. The decision to model only fixed effects was motivated by the expectation, supported by the literature, that the gene and gene × experience effect sizes (typically modeled as “gene × environment” effects in the literature) would be relatively small. In such cases, it may be considered statistically justifiable to model only fixed effects.
Baseline self-reported medication (anti-inflammatory, muscle-relaxant, opioid-analgesic, anti-depressant, and anti-anxiety) use was controlled for in all analyses. Ultimately, medication use was not significantly related to the outcomes and did not alter the results, and so was left out of the final analyses.
Population stratification is considered a threat to internal validity in association studies (Cardon & Bell, 2001) and was addressed in the current analyses. Population stratification occurs when a sample consists of individuals from several different subgroups in which mating over time has been non-random, potentially having caused intergroup differences in genotypic representation. To account for this potential threat, ethnic differences were probed on the primary outcome variable (e.g., PA), and all analyses were re-run excluding non-Caucasian participants to test the generalizability of the results for Caucasian-only participants. Due to missing data, when all primary COMT and OPRM1 analyses were re-run with a Caucasian-only sample, the N decreased from 46 to 35. The result was that significance estimates varied slightly, but the form and direction of all effects remained consistent. As a result, we have concluded that there is little evidence to suggest that population stratification was a threat to the hypotheses tested in the current study.
Results
Participant Characteristics
Participants mean age was 53 (SD=7.82); 93.0% reported Caucasian ethnicity, 67.5% had completed some college, with 25% holding post-graduate degrees, and 75.7% reported earning over $30,000 per year, with 41.5% earning over $70,000 per year (demographic data was missing on six participants). Thus, our sample was of a higher socioeconomic status than the general FM population (Penrod et al., 2004). All demographic variables were also tested as predictors of PA and pain among FM patients. However, no significant associations were found.
The observed genotype frequencies were in Hardy-Weinberg equilibrium, indicating that non-random selective forces did not bias the genotype distributions. The following genotype frequencies were observed for the val158met SNP: val/val=12 (26.1%), val/met=24 (52.2%), met/met=10 (21.7%). The following genotype frequencies were observed for asn40asp polymorphism: asn/asn=35 (76.1%), asp/asn=10 (21.7%), asp/asp=1 (2.2%). Genotype frequencies for both polymorphisms resembled population estimates (Ray & Hutchison, 2004; Tiihonen, Hallikainen, Lachman et al., 1999).
Gene and Gene × Experience Effects on PA
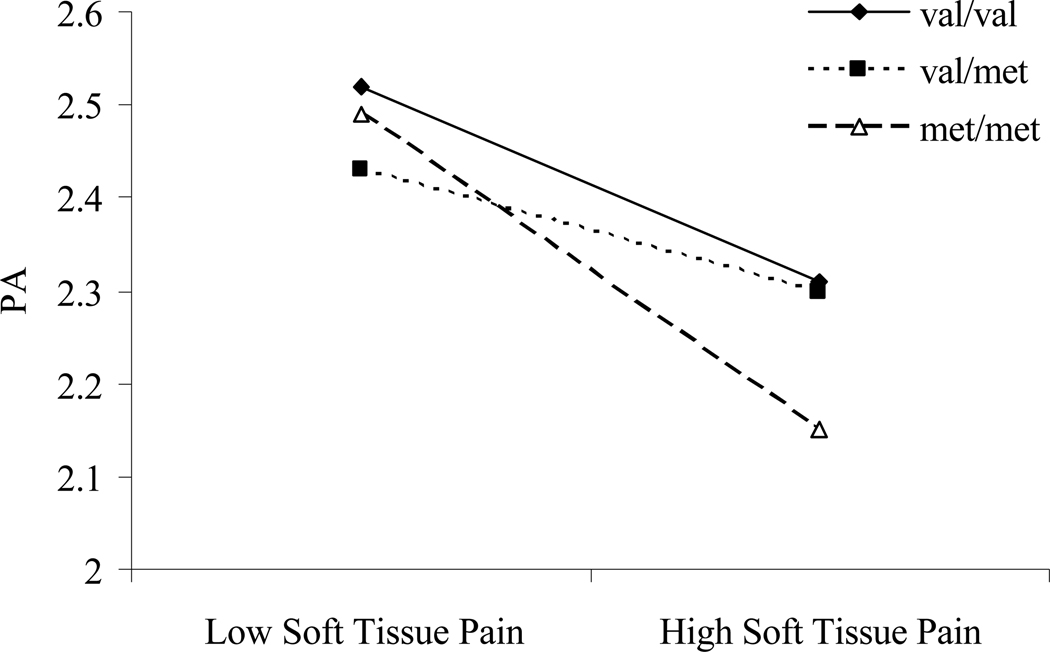
Our approach tested if there were any gene and/or gene × experience effects on PA, as reported across the span of diary days. COMT and OPRM1 genotypes were not correlated (r=−.035), so there was no concern that one genotype could confound the effect of the other genotype on the chosen phenotype. COMT genotype was not associated with PA across diary days, F(2, 42)=.05, p=.95 (for means and standard deviations, see Table 1). However, a significant gene × experience interaction was evident, F(2, 1217)=4.49, p<.05 (for complete multilevel statistics, see Table 2), such that individuals with met/met genotype experienced a greater decline in PA on days with elevated daily soft tissue pain than did either val/met or val/val individuals. Examination of the variance components (Singer, 1998) indicated that the model explained 6% of the within-person variance in PA, with COMT genotype contributing 1% of variance over and above the effect of pain on PA. The latter two genotypic groups were not significantly different from each other. Figure 1 depicts the COMT × daily pain interaction after dichotomizing pain into top and bottom thirds of responding within the sample. This dichotomization was done for graphical purposes; the significance of the interaction was still determined based on the coefficient for the continuous interaction.
Table 1.
Means and Standard Deviations of Positive Affect by Genotype
| Fibromyalgia Patients N=46 |
||
|---|---|---|
| Catechol-O-Methyltransferase | M | SD |
| val/val | 2.43a | .73 |
| val/met | 2.37a | .83 |
| met/met | 2.37a | .98 |
| μ-Opioid Receptor | ||
| asp-carrier | 2.78a | .80 |
| asn/asn | 2.26b | .74 |
Note. Means and standard deviations are provided for positive affect, aggregated across diary days. Comparisons were made between genotype, and values in each column (separately, for each gene) that do not share a common superscript are significantly different from each other, p<.05.
Table 2.
COMT Moderation of Daily Pain Effect on Daily Positive Affect
| Random Effects | |||||
|---|---|---|---|---|---|
| Covariance Parameter Estimates |
Subject | β | SE | Z | p |
| Intercept | ID | .06 | .13 | 4.40 | <.001 |
| Residual | ID | .32 | .02 | 20.23 | <.001 |
| Fixed Effects | |||||
| Predictor Variables | β | SE | df | t | p |
| Level 1 | |||||
| Centered Daily Pain | −.57 | .09 | 1217 | −6.07 | <.001 |
| Level 2 | |||||
| COMT (val/val vs. met/met) | .08 | .35 | 42 | .24 | .81 |
| COMT (val/met vs. met/met) | −.001 | .31 | 42 | −.01 | .99 |
| Covariate | |||||
| Negative Affect | −.29 | .04 | 1217 | −7.56 | <.001 |
| Level 1 × Level 2 | |||||
| Centered Daily Pain × COMT (val/val vs. met/met) | .37 | .16 | 1217 | 2.34 | <.05 |
| Centered Daily Pain × COMT (val/met vs. met/met) | .31 | .11 | 1217 | 2.78 | <.01 |
Note. The results of the multilevel analysis of the cross-level interaction of COMT genotype and daily pain (ΔPain) on daily positive affect among fibromyalgia patients are presented. The effects presented for each Level 2 and cross-level effect are separated according to contrast with the target group, met/met individuals.
met/met = individuals homozygous for the met allele; val/met = individuals with a heterozygous genotype; val/val = individuals homozygous for the val allele.
Figure 1. COMT × pain interaction.
Slopes reflect positive affective reactivity to high versus low pain days for patients with either val/val, val/met, or met/met genotypes. X-axis parameters were generated by dichotomizing centered pain scores into the top and bottom third of responses across participants.
To examine the specificity of the effects of COMT on the process of positive affective reactivity to pain, a regression of daily pain on COMT genotype was performed. No relation was evident, F(2, 42)=.71, p=.50. Additional analyses revealed useful information pertaining to the chosen phenotype. First, with regard to the outcome, COMT genotype was not related to NA across diary days, F(2, 42)=.52, p=.60, and did not interact with daily pain in the prediction of NA, F (2, 1217)=1.90, p=.15. Second, COMT did not interact with daily interpersonal stress in the prediction of PA, F(2, 1192)=1.01, p=.36, suggesting that daily pain was the appropriate predictor. Thus, the influence of COMT did not generalize to other common daily processes in FM.
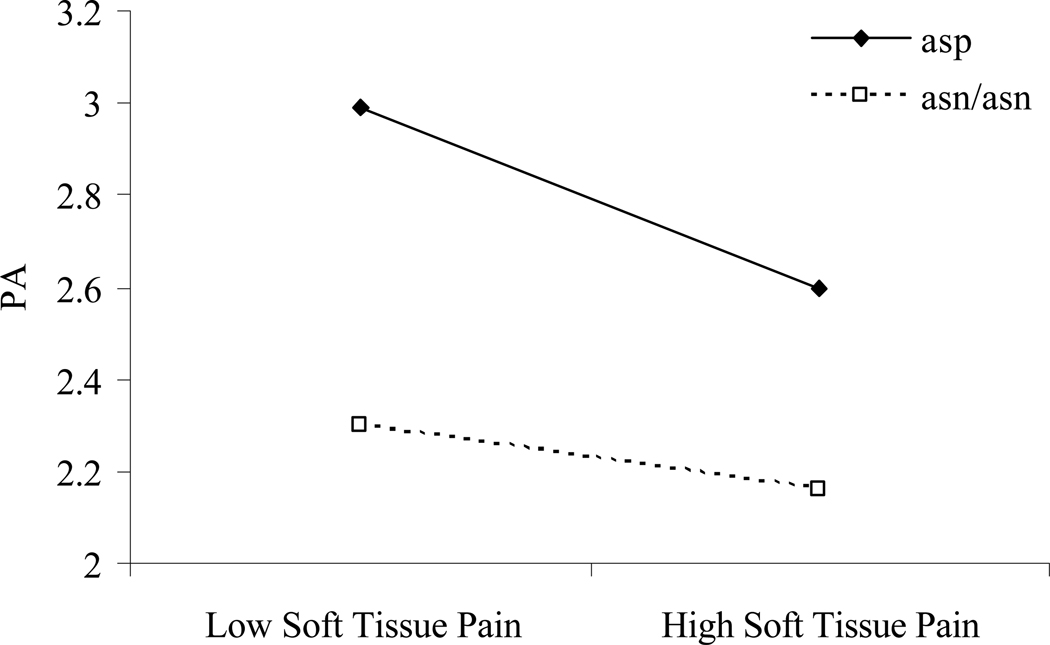
OPRM1 genotype evidenced a trend toward a significant prediction of PA across diary days, F(1, 43)=3.62, p=.06. Patients with an asp40 allele reported higher PA across diary days than those homozygous for asn40 (for means and standard deviations, see Table 1). The hypothesis that an OPRM1 × daily pain interaction would be observed was supported, F(1, 1218)=6.06, p<.05 (for complete multilevel statistics, see Table 3). This effect is graphically displayed in Figure 2. Patients with an asp40 allele experienced a greater decline in PA as daily soft tissue pain increased than did those carrying two asn40 alleles. Still, as Figure 2 indicates, asp40 carriers reported substantially greater PA than their counterparts on high pain days. Examination of the variance components indicated that the model explained 6% of the variance in PA, with OPRM1 genotype contributing 1% of variance over and above the effect of pain on PA.
Table 3.
OPRM1 Moderation of Daily Pain Effect on Daily Positive Affect
| Random Effects | |||||
|---|---|---|---|---|---|
| Covariance Parameter Estimates |
Subject | β | SE | Z | p |
| Intercept | ID | .53 | .12 | 4.43 | <.001 |
| Residual | ID | .32 | .02 | 20.27 | <.001 |
| Fixed Effects | |||||
| Predictor Variables | β | SE | df | t | p |
| Level 1 | |||||
| Centered Daily Pain | −.28 | .05 | 1218 | −5.18 | <.001 |
| Level 2 | |||||
| OPRM1 (asp vs. asn/asn) | .50 | .26 | 43 | 1.93 | .06 |
| Covariate | |||||
| Negative Affect | −.29 | .04 | 1218 | −7.39 | <.001 |
| Level 1 × Level 2 | |||||
| Centered Daily Pain × OPRM1 (asp vs. asn/asn) | −.32 | .13 | 1218 | −2.46 | <.05 |
Note. The results of the multilevel analysis of the cross-level interaction of OPRM1 genotype and daily pain on daily positive affect among fibromyalgia patients are presented.
asp = individuals either homozygous for the asp allele or heterozygous for the asp and asn alleles; asn/asn = individuals homozygous for the asp allele.
Figure 2. OPRM1 × pain interaction.
Slopes reflect positive affective reactivity to high and low pain days for patients who are either carriers or not carriers of the asp allele. X-axis parameters were generated by dichotomizing centered pain scores into the top and bottom third of responses across participants.
A series of analyses were also conducted to examine the specificity of the effect of OPRM1 on positive affective reactivity to pain. First, OPRM1 genotype was not related to daily pain, F(1, 43)=1.14, p=.29. However, further analyses revealed a significant OPRM1 × daily pain interaction on NA, F(1, 1218)=5.22, p<.05, such that FM-only asp40 carriers reported a greater increase in NA as pain increased than those without the asp40 allele. As with COMT, OPRM1 did not interact with daily interpersonal stress to predict PA, F(1, 1193)=.10, p=.76.
In separate analyses, each genotype was entered as a predictor of both soft tissue pain variance and PA variance. Met/met individuals evidenced significantly greater soft tissue pain variance than val/val individuals, F(2, 43)=3.40, p<.05, but did not differ from the heterozygous genotype. COMT genotype did not predict PA variance across diary days, and OPRM1 did not predict either pain or PA variance.
Influence of Tender Point Endorsement
When assessed by trained members of our research staff, some participants endorsed tender point pain discordant with their physician-confirmed diagnosis. For a diagnosis of FM, pain should be present in 11 of 18 tender points. Ten FM patients reported pain in fewer than 11 out of 18 tender points. One patient lacked tender point exam data. Ultimately, the physician-confirmed diagnosis is the gold-standard and was used to classify diagnosis for the analyses presented above. However, because the present study is concerned with FM-specific reactivity to daily pain, this finding motivated several tests for the possible confound of individual differences in tender point endorsement. First, correlations between tender point endorsement and genotype were examined. Tender point endorsement evidenced a non-significant trend for a relation to COMT, r=.13, p=.07, and was not significantly related to OPRM1, r=−.08, p=.26, genotype. Second, all analyses were run separately including only those FM patients who reported pain in 11 or more tender points. Results of these separate analyses did not significantly differ from results run with the full sample. Finally, the number of tender points endorsed was included as a covariate in analyses involving the full sample of FM patients. No effects were significantly altered through the inclusion of raw tender point endorsement as a covariate. Thus, it is unlikely that individual differences in tender point endorsement affected any of the present results.
Discussion
The purpose of the present study was to test the relation of candidate genes from the catecholaminergic and opioidergic systems to positive affective regulation during pain. As hypothesized, COMT moderated the relation of daily pain to daily PA such that elevations in pain were associated with lower PA to a greater degree among met/met individuals than their val/met and val/val counterparts. A gene-dose effect was not observed, as the heterozygous group closely resembled the homozygous val/val group. This finding suggests that the lower enzymatic activity on catecholamines conferred by the met/met genotype affects FM patients’ ability to maintain PA in the face of pain flares. Importantly, this finding stands in contrast to Zubieta et al.’s (2003) finding that COMT was not associated with the positive affective components of pain. The current study differs in sample constituency (FM patients vs. normative population), methodology (daily diary vs. laboratory pain manipulation), and measurement (PANAS vs. McGill Pain Questionnaire), and so comparing results must be qualified by those major differences. Still, the contrast offers a chance to highlight the potential role for COMT in the concomitant PA deficit with widespread pain in FM. Broadly speaking, the results suggest that the association of COMT and pain is variable and context-dependent.
Both gene and gene × experience effects on daily PA were found for OPRM1. FM patients with at least one asp40 allele reported more PA across diary days than those homozygous for asn40, although this was only a trend (p<.06). On days when pain was elevated, however, asp40 individuals report a greater decrease in PA than those homozygous for asn40. There is more than one possible explanation for this finding. One possibility is that asp40 individuals experience more PA, in general, than their counterparts, but face greater instability in their positive affective reactivity to pain. An alternate interpretation could be that the average PA level for asn40 individuals is so low that elevations in pain will not appreciably change that level. The likelihood of this possibility is bolstered by the comparison of PA levels in past studies using a similar sample. For example, Zautra et al. (2005) reported an average PA level of 2.78 (SD=.58) for the FM patients in that study. In comparison, the FM patients homozygous for asn40 in the current study reported a mean PA level across days of 2.26 (SD=.74). Thus, the average PA level experienced by homozygous asn40 carriers was low, even relative to those of other FM samples. This suggests that there may have been little room for those individuals to decline in PA on days of elevated pain. Either way, the findings suggest that the asp40 allele may be a source of resilience for FM patients. Even on high pain days, they are able to maintain higher PA levels than those of their counterparts.
For both the COMT and OPRM1 interaction effects, genotype explained just 1% of the variance over and above the effect of pain on PA. Although these are small effects, they are consistent with the size of effects generally reported in the gene × environment literature, which can be considered a useful comparator to the findings presented here. Further, effect sizes in daily diary studies should be interpreted in the context from which they are derived; small effects compounded daily over time may have greater long-term significance than can be measured by a traditional effect size estimate.
Genotypic differences emerged in the day-to-day variance of pain reports, such that met/met individuals evidenced significantly greater daily variance in their pain scores than those with the val/val genotype. How does this between-genotype difference relate to the finding that met/met individuals experienced less PA on days in which pain was elevated? It is possible, although speculative, that the greater daily variability in pain experiences for the met/met group led them to show more pronounced positive affective responses to pain elevations. The met/met group did not significantly differ from other COMT genotypes in daily PA variance, suggesting that their more highly variable pain scores could have influenced their relatively stable PA variance. Further evidence through direct testing will be needed to explicate these potential relations.
Our confidence in these findings is strengthened by the results of analyses that controlled for the influence of tender point pain on PA. Contrary to diagnostic criteria, about 23% of FM patients endorsed pain in fewer than the minimum 11 tender points needed to meet conditions for diagnosis. Still, despite controlling for the discrepant tender point endorsements in our analyses, the primary gene and gene × experience findings remained. That the effects of COMT and OPRM1 were observed among FM patients with and without the requisite number of tender points suggests that factors other than widespread pain may serve to cluster these individuals. This result supports recent assertions (Finan et al., 2009) that the diagnosis of FM may require a broader assessment than that put forth by the American College of Rheumatology (Wolfe et al., 1991).
A major strength of the current study is the attention to rich phenotypic measurement, a focus that is often absent from behavioral genetic research in chronic pain. An approach that emphasizes a case-control design fails to take into account the heterogeneity of symptoms within the FM population and the variable process of adaptation to pain and stress that occurs daily. A naturalistic phenotype approach, in which genetic association can be explored within the context of daily processes relevant to a disease, allows for greater flexibility in the choice of phenotype. Analyses in the present study revealed that, by and large, daily positive affective reactivity to pain was an appropriate phenotype with variation that can be explained by variation in both COMT and OPRM1. That neither COMT nor OPRM1 were related to positive affective reactivity to stress suggests that the phenotype of reactivity to pain appropriately approximated the neurobiological functions of the genes. Even more, COMT did not moderate the relation of pain to NA, providing further support for the specificity of the phenotype. The finding that OPRM1 genotype significantly moderated negative affective reactivity to pain suggests that more work must be done to refine this phenotype.
Relatively little empirical work has been directed toward elaborating phenotypes that reliably capture the wide variety of symptoms experienced by FM patients, including mood, fatigue, interpersonal instability, and stress reactivity. The current study capitalized on the methodological strength of the daily process design, which increases the power to detect small effects through reliable, repeated measurement. It is quite possible that a cross-sectional design with a similarly small sample size might not have detected the gene × experience effects observed in the current study. Thus, the current study highlights the utility in robust phenotypic measurement for clinical researchers interested in explicating the influence of genomic variants in complex chronic pain disorders with psychopathological comorbidities.
We prefer a cautious approach in discussing the implications of the gene × experience effects for diagnosis and treatment. No conclusions should be made in that regard until these findings are replicated in a different sample with more participants. If the findings are replicated, clinical trials might be warranted to determine if FM patients of certain genotypes respond more favorably to one treatment approach versus another.
Limitations
One major limitation of the current study was the single SNP approach inherent in its design. Enthusiasm over this approach has been recently tempered by the glut of small effect sizes and non-replications, as well as the potential utility of functional gene networks. Other currently available approaches could certainly expand the knowledge gained from the current study. For example, the haplotype approach, used in identifying COMT-moderated pain sensitivity by Diatchenko, Slade, Nackley et al. (2005), allows for the grouping of multiple SNPs to be analyzed based on their cumulative contribution to a behavioral phenotype. Diatchenko et al.’s work shows that it may not be the met158 allele, alone, that accounts for pain sensitivity, but rather a combined effect of that allele with alleles from SNPs in high linkage disequilibrium with val158met.
Second, the current study included only female participants. Although FM primarily affects women, gender differences are widely reported in candidate gene studies, and a male comparison group may have provided important information about genetic differences in dynamics of pain and affect. Future studies should include a male cohort to determine if the present findings are moderated by gender.
Third, due to the small sample, the varying reports of tender point pain, and the trend observed between tender point endorsement and COMT genotype, we cannot exclude the possibility that tender point pain may have been related to genotype, and may have influenced the primary findings. A larger sample will be needed to confidently dismiss this possible confounding relation.
Finally, it remains to be seen if the findings in the current study generalize beyond FM patients. The sample was recruited with some criteria (e.g. absence of other rheumatic diagnosis) that may limit the relevance of the current findings to other FM populations. The concept of dual processing of pain and emotion is relevant to other chronic pain groups. The analyses presented in this manuscript will need to be tested on other populations before conclusions can be made about whether the findings are specific to FM patients or common across chronic pain patients in general.
Summary and Conclusions
Evidence has been provided in support of the hypotheses that variants in COMT and OPRM1 would be associated with the dual processes of pain and affect in FM. The findings are unique in several respects. First, they make use of repeated measurements of pain and affect collected across 30 days of electronic diary entries. This methodology allowed for the examination of genetic associations with a stable PA phenotype, observed over time, as well as positive affective reactivity to naturally occurring perturbations in pain. Naturalistic data such as those presented in the current study are crucial to our understanding of FM because pain for this patient population is often closely related to life stress and interpersonal processes that are, at best, imperfectly simulated in the laboratory. By identifying two separate, but complimentary, neurobiological systems that have been shown to contribute to pain and affective processing, the current study sought to determine if candidate genes known to have regulatory roles within those systems were associated with a dual processing model of pain-related positive affective regulation in FM. To that end, hypotheses that the val158met polymorphism in COMT and the asn40asp polymorphism in OPRM1 would be associated with daily positive affective reactivity to pain, were supported. The findings offer researchers ample reason to further investigate the contribution of the catecholamine and opioid systems, and their associated genomic variants, to the still poorly understood experience of FM.
References
- Affleck G, Urrows S, Tennen H, Higgins P, et al. Sequential daily relations of sleep, pain intensity, and attention to pain among women with fibromyalgia. Pain. 1996;68(2–3):363–368. doi: 10.1016/s0304-3959(96)03226-5. [DOI] [PubMed] [Google Scholar]
- Beyer A, Koch T, Schroder H, Schulz S, Hollt V. Effect of the A118G polymorphism on binding affinity, potency and agonist-mediated endocytosis, desensitization, and resensitization of the human mu-opioid receptor. Journal of Neurochemistry. 2004;89:553–560. doi: 10.1111/j.1471-4159.2004.02340.x. [DOI] [PubMed] [Google Scholar]
- Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, et al. Single-nucleotide polymorphism in the human mu opioid receptor gene alters Beta-endorphin binding and activity: possible implications for opiate addiction. Neurobiology. 1998;95:9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buskila D, Sarzi-Puttini P. Genetic aspects of fibromyalgia syndrome. Arthritis Therapy and Research. 2006;8:218–222. doi: 10.1186/ar2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardon LR, Bell JI. Association study designs for complex diseases. Nature Reviews Genetics. 2001;2:91–99. doi: 10.1038/35052543. [DOI] [PubMed] [Google Scholar]
- Compton P, Geschwind DH, Alarcon M. Association between human mu-opioid receptor gene polymorphism, pain tolerance, and opioid addiction. American Journal of Medical Genetics. 2003;121B:76–82. doi: 10.1002/ajmg.b.20057. [DOI] [PubMed] [Google Scholar]
- Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, et al. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Human Molecular Genetics. 2005;14:135–143. doi: 10.1093/hmg/ddi013. [DOI] [PubMed] [Google Scholar]
- Enders CK, Tofighi D. Centering predictor variables in cross-sectional multilevel models: A new look at an old issue. Psychological Methods. 2007;12:121–138. doi: 10.1037/1082-989X.12.2.121. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, Kaplan L, Staud R, Ness TJ, Glover TL, Campbell CM, Mogil JS, Wallace MR. The A118G single nucleotide polymorphism of the mu-opioid receptor gene (OPRM1) is associated with pressure pain sensitivity in humans. The Journal of Pain. 2005;6:159–167. doi: 10.1016/j.jpain.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Finan PH, Zautra AJ, Davis MC. Daily affect relations in fibromyalgia patients reveal positive affective disturbance. Psychosomatic Medicine. 2009 doi: 10.1097/PSY.0b013e31819e0a8b. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Garcia-Fructoso FJ, Lao-Villadoniga JL, Beyer K, Santos C. Relationship between catechol-O-methyltransferase genotypes and fibromyalgia’s severity. Rheumatology Clinic. 2006;2:168–172. [Google Scholar]
- Hernandez-Avila CA, Wand G, Luo X, Gelernter J, Kranzler HR. Association between the cortisol response to opioid blockade and the Asn40Asp polymorphism at the mu-opioid receptor locus (OPRM1) American Journal of Medical Genetics. 2003;118B:60–65. doi: 10.1002/ajmg.b.10054. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Covault J, Wand G, Zhang H, Gelernter J, Kranzler HR. Population-specific effects of the Asn40Asp polymorphism at the mu-opioid receptor gene (OPRM1) on HPA-axis activation. Pharmacogenetics and Genomics. 2007;17:1031–1038. doi: 10.1097/FPC.0b013e3282f0b99c. [DOI] [PubMed] [Google Scholar]
- Kim H, Mittal DP, Iadorala Mj, Dionne RA. Genetic predictors for acute experimental cold and heat pain sensitivity in humans. Journal of Medical Genetics. 2006;43:e40. doi: 10.1136/jmg.2005.036079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limer KL, Nicholl BI, Thomson W, McBeth J. Exploring the genetic susceptibility of chronic widespread pain: the tender points in genetic association studies. Rheumatology. 2008;47:572–577. doi: 10.1093/rheumatology/ken027. [DOI] [PubMed] [Google Scholar]
- Li PP, Warsh JJ, Godse DD. Formation and clearance of norepinephrine glycol metabolites in mouse brain. Journal of Neurochemistry. 1984;43:1425–1433. doi: 10.1111/j.1471-4159.1984.tb05404.x. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS system for mixed models. Cary, NC: SAS Institute; 1996. [Google Scholar]
- Lotsch J, Skarke C, Grosch S, Darimont J, Schmidt H, Geisslinger G. The polymorphism A118G of the human mu-opioid receptor gene decreases the pupil constrictory effect of morphine-6-glucuronide but not that of morphine. Pharmacogenetics. 2002;12:3–9. doi: 10.1097/00008571-200201000-00002. [DOI] [PubMed] [Google Scholar]
- Mogil JS. The genetic mediation of individual differences in sensitivity to pain and its inhibition. Proceedings of the National Academy of the Sciences USA. 1999;96:7744–7751. doi: 10.1073/pnas.96.14.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezlek JB. Multilevel random coefficient analyses of event- and interval-contingent data in social and personality psychology research. Personality and Social Psychology Bulletin. 2001;27:771–785. [Google Scholar]
- Oroszi G, Goldman D. Alcoholism: Genes and mechanisms. Pharmacogenomics. 2004;5:1037–1048. doi: 10.1517/14622416.5.8.1037. [DOI] [PubMed] [Google Scholar]
- Penrod JR, Bernatsky S, Adam V, Baron M, Dayan N, Dobkin PL. Health services costs and their determinants in women with fibromyalgia. The Journal of Rheumatology. 2004;31:1391–1398. [PubMed] [Google Scholar]
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. doi: 10.1126/science.288.5472.1769. [DOI] [PubMed] [Google Scholar]
- Ray L, Hutchison KE. A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcoholism: Clinical & Experimental Research. 2004;28:1789–1795. doi: 10.1097/01.alc.0000148114.34000.b9. [DOI] [PubMed] [Google Scholar]
- Singer J. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. Journal of Educational and Behavioral Statistics. 1998;23:323–355. [Google Scholar]
- Smolka MN, Schumann G, Wrase J, Grusser SM, Flor H, Mann K, Braus DF, Goldman D, Buchel C, Heinz A. Catechol-O-Methyltransferase val158met genotypce affects processing of emotional stimuli in the amygdale and prefrontal cortex. Journal of Neuroscience. 2005;25:836–842. doi: 10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman RS, Weinshilboum RM. Genetics of red cell COMT activity: Analysis of thermal stability and family data. American Journal of Medical Genetics. 1981;10:279–290. doi: 10.1002/ajmg.1320100311. [DOI] [PubMed] [Google Scholar]
- Thieme K, Turk DC, Flor H. Comorbid depression and anxiety in fibromyalgia syndrome: relationship to somatic and psychosocial variables. Psychosomatic Medicine. 2004;66:837–844. doi: 10.1097/01.psy.0000146329.63158.40. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Hallikainen T, Lachman H, Saito T, Volavka J, Kauhanen J, et al. Association between the functional variant of the catechol-O-methyltransferase (COMT) gene and type 1 alcoholism. Molecular Psychiatry. 1999;4:286–289. doi: 10.1038/sj.mp.4000509. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Sora I, Wang Z. The mu-opiate receptor as a candidate gene for pain: polymorphisms, variations in expression, nociception, and opiate responses. Proceedings of the National Acadamy of the Sciences. 1999;96:7752–7755. doi: 10.1073/pnas.96.14.7752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Alarcon G, Fragoso JM, Cruz-Robles D, Vargas A, Vargas A, Lao-Villadoniga JI, Garcia-Fructoso F, Ramos-Kuri M, Hernandez F, Springall R. Catechol-O-methyltransferase gene haplotypes in Mexican and Spanish patients with fibromyalgia. Arthritis Research and Therapy. 2007;9:R10. doi: 10.1186/ar2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AH, Najarian D, White DL, Jaffe JF, Kanetsky PA, Rebbeck TR. Collection of genomic DNA by buccal swabs for polymerase chain reaction-based biomarker assays. Environmental Health Perspectives. 1999;107:517–520. doi: 10.1289/ehp.99107517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand GS, McCaul M, Yang X, Reynolds J, Gotjen D, Lee S, Ali A. The mu-opioid receptor gene polymorphism (A118G) alters HPA axis activation induced by opioid receptor blockade. Neuropsychopharmacology. 2002;26:106–114. doi: 10.1016/S0893-133X(01)00294-9. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology criteria for the classification of fibromyalgia: report of the multicenter criteria committee. Arthritis and Rheumatism. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- Zautra AJ, Fasman R, Reich JW, Harakas P, Johnson LM, Olmsted ME, Davis MC. Fibromyalgia: evidence for deficits in positive affect regulation. Psychosomatic Medicine. 2005;67:147–155. doi: 10.1097/01.psy.0000146328.52009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zautra AJ, Guarnaccia CA, Dohrenwend BP. Measuring small life events. American Journal of Community Psychology. 1986;56:608–617. doi: 10.1007/BF00931340. [DOI] [PubMed] [Google Scholar]
- Zautra AJ, Hoffman J, Potter P, Matt KS, Yocum D, Castro L. Examination of changes in interpersonal stress as a factor in disease exacerbations among women with rheumatoid arthritis. Annals of Behavioral Medicine. 1997;19:279–286. doi: 10.1007/BF02892292. [DOI] [PubMed] [Google Scholar]
- Zautra AJ, Johnson LM, Davis MC. Positive affect as a source of resilience for women in chronic pain. Journal of Consulting and Clinical Psychology. 2005;73:212–220. doi: 10.1037/0022-006X.73.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zautra AJ, Smith B, Affleck G, Tennen H. Examinations of chronic pain and affect relationships: applications of a dynamic model of affect. Journal of Consulting and Clinical Psychology. 2001;69:786–795. doi: 10.1037//0022-006x.69.5.786. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang D, Johnson AD, Papp AC, Sadee W. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. Journal of Biological Chemistry. 2005;280:32618–32624. doi: 10.1074/jbc.M504942200. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, et al. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 2003;299:1240–1241. doi: 10.1126/science.1078546. [DOI] [PubMed] [Google Scholar]