Synopsis
Reinnervation of a hand transplant ultimately dictates functional recovery but provides a significant regenerative challenge. The authors present a review highlighting interventions to enhance nerve regeneration through acceleration of axonal regeneration or augmentation of Schwann cell supportand discuss their relevance to composite tissue allotransplantation. Surgical techniques that may be performed at the time of transplantation to optimize intrinsic muscle recovery—including appropriate alignment of ulnar nerve motor and sensory components, transfer of the distal anterior interosseous nerve to the recurrent motor branch of the median nerve, and prophylactic release of potential nerve entrapment points—are also presented.
Keywords: peripheral nerve, nerve regeneration, composite tissue allotransplantation, hand transplantation, axon, Schwann cell
Introduction
Since the first successful case was reported in September 1998, at least 50 hand transplantation procedures have been performed worldwide[1]. Successful transplantation had initially been limited by availability of an immunosuppressant regimen able to prevent rejection of the composite tissue allograft (CTA). Now, realization of hand transplantation as a permanent tool in the reconstructive armamentarium and expansion of its clinical indications will no doubt depend on further breakthroughs in immunomodulation, such as development of new protocols that minimize maintenance immunosuppression or induce a state of graft tolerance. Just as important to the ultimate success of hand transplantation, however, will be improving its long-term functional results. With the ever-increasing cost of healthcare and with the growing pressure on health care providers to justify expenditures with published evidence of substantial clinical benefit, improving functionality must remain as high a priority as lessening risk and decreasing cost.
Peripheral nerve injury and regeneration
Axonal disruption in the peripheral nerve activates a highly regulated and sophisticated sequence of events[for a review [2]]. Wallerian degeneration occurs distal to the site of injury, with early changes revolving around the removal of cellular debris and degenerating distal axon segments by local macrophages and Schwann cells (SCs)[2–5]. Mitogenic cytokines released by the injured axons induce SCs to change from a myelinating phenotype to a proliferative phenotype[6–10]. These cytokine signals, as well as those secreted by infiltrating macrophages, induce SCs to line up into columns along the basement membrane of endoneurial tubes. These columns of SCs, termed bands of Büngner, secrete neurotrophic factors that stimulate axonal sprouting and guide axonal regeneration[11–14].
Proximal to the site of injury, axons are pruned back by one or two nodes of Ranvier[15]. Each axon then sprouts multiple growth cones, each growth cone giving rise to a daughter axon, with a ratio of approximately 5 daughter axons per parent axon[16–18]. The axon cell body changes the focus of its protein production from synthesis of neurotransmitters to structural proteins, which are used to lengthen axon projections. Each daughter axon searches to reconnect with a neuromuscular junction under the guidance of the endoneurial microstructure, regenerative SCs, and neurotrophic factors—all derived from the distal nerve stump. Regeneration proceeds slowly, at approximately 1 mm per day in humans[19]. Daughter axons that fail to reach the appropriate target organ undergo pruning. Time to reinnervation of the neuromuscular junctions may be on the order of months or, in cases of very proximal nerve injury, even years.
When nerve continuity cannot be restored after transection by a tension-free repair, a nerve graft must be interposed between the proximal and distal nerve stumps to act as a scaffold for axonal regeneration. Nerve autografts, harvested from the patient’s own noncritical sensory nerves, have served as the “gold standard” by providing both endoneurial microstructure and supportive SCs to aid regeneration. However, when the supply of available autografts is insufficient, such as in the repair of large, segmental, or complex nerve injuries, cadaveric or donor-related allografts provide a readily accessible alternative[20]. Experimental studies on rodents, large animals, and nonhuman primates, as well as the senior author’s clinical experience, have shown that nerve allografts in the presence of immunosuppression provide equal regeneration and functional recovery as autografts, if not slightly better due to the use of the regeneration-enhancing immunosuppressant tacrolimus[21–25]. Understanding the interaction between host tissues and nerve allografts and the unique regenerative challenges that are present in this clinical model is essential to predicting and optimizing nerve regeneration in the peripheral nerves of hand transplants. In the following section we will first discuss the clinical scenario of a nerve allograft alone, without the other CTA components. Subsequently, we will discuss the differences in nerve regeneration when the nerve allograft is part of a CTA.
What have we learned from nerve allotransplantation?
While its axonal content will degenerate following harvest, the fresh unprocessed nerve allograft contains both endoneurial architecture and supportive SCs to promote host axonal regeneration across the graft. Schwann cells are the most immunogenic component of human nerve[26–32]. Nerve allotransplantation, therefore, requires immunosuppression during the period of host axonal regeneration across the allograft to ensure donor SC support. The entire length of the graft must ultimately become populated with host SCs, however, so that immunosuppression can be safely withdrawn once host axons have regenerated through the graft[21]. Should large portions of the graft remain populated solely by donor SCs, removal of immunosuppression will result in massive donor SC loss and debilitating conduction block. Nerve conduction will only be supported long-term if regenerated host axons have been myelinated by host SCs that have migrated into the graft.
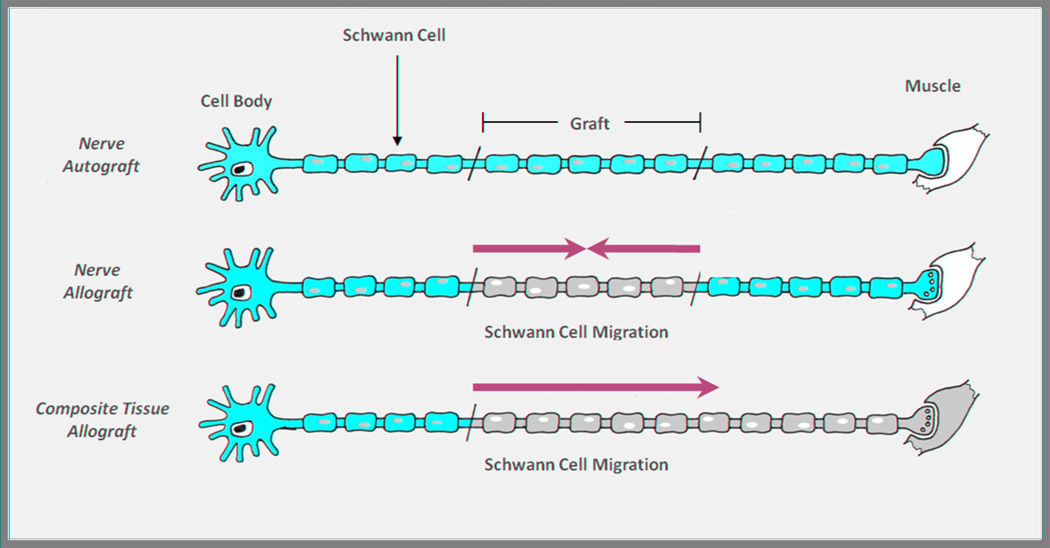
At the time of implantation, host and donor SCs exist in two distinct populations—donor SCs within the allograft and host SCs in the proximal and distal nerve stumps. In the presence of immunosuppression, both donor and host SCs proliferate and support axonal regeneration. Even with adequate traditional immunosuppression, however, the donor SC population will initially be depleted by short subclinical episodes of rejection, providing a stimulus for host SC migration from both the proximal and distal host nerve stumps[33] (Figure 1). Interestingly, in a nerve autograft situation, Aguayo has shown that SCs do not migrate, but rather stay in their original location, whether that be within the graft or within the proximal and distal nerve stumps[34,35].
Figure 1. Schwann cell migration.
In the nerve autograft (top), autologous Schwann cells (SCs) support axonal regeneration through the graft, and there is no migration of SCs from within the graft or from the proximal and distal stumps. In the nerve allograft (middle), donor SCs initially support axonal regeneration but may be replaced over time as the graft is repopulated by inward migration of host SCs from both the proximal and distal nerve stumps. In the composite tissue allograft (bottom), only unidirectional host SC migration is possible. It is unknown whether host SCs are capable of replicating and migrating enough to repopulate the distal-most extent of the nerve. (Adapted from Moore AM et al. Nerve allotransplantation as it pertains to composite tissue transplantation. Hand. 2009;4:239-44; with permission.)
Host SCs have been shown to migrate into the allograft from both the proximal and distal stumps of the recipient nerve[36–39].In vivo imaging of SC migration demonstrated a greater proportion of migration from the distal host nerve stump. In an accellularized nerve graft model (processed to remove immunogenic cells, including SCs), host SCs quickly repopulated the graft, within 10 days, while axonal regeneration lagged behind by an additional 10–15 days[37]. In a fresh, unprocessed nerve allograft model, a delay in host SC migration was observed, during which time donor SCs appeared to assist axonal regeneration across the graft. Host SCs later migrated into the graft. This was likely in response to episodes of subclinical acute rejection which diminished donor SC numbers, until what was observed was a coexisting population of host and donor SCs in the graft. Once axonal regeneration through the graft was complete and immunosuppression was withdrawn, host SCs immediately attempted to fill any gaps left by the loss of the remaining donor SCs[33].
What is different about Schwann cell migration in a CTA versus a nerve allograft?
The nerve components of a transplanted hand are similar in many ways to a nerve allograft—they provide the same intact nerve architecture and supportive, albeit immunogenic, donor SCs. Many of the same mechanisms for SC turnover described above likely apply in the CTA model. However, significant differences between the two models exist that could make nerve regeneration in a CTA model inferior to a “stand alone” nerve allograft. First, in a hand transplant, there is no host distal nerve stump and no distal source of host SCs. The entire distal neuromuscular unit is composed of donor tissue, and repopulation of the donor nerve with host SCs relies solely on proximal host SC proliferation and migration (Figure 1). It is unknown whether host SCs will be capable of meeting this demand and populating the most distal extents of the transplanted nerve, especially as the level of transplantation moves proximally up the arm[40].
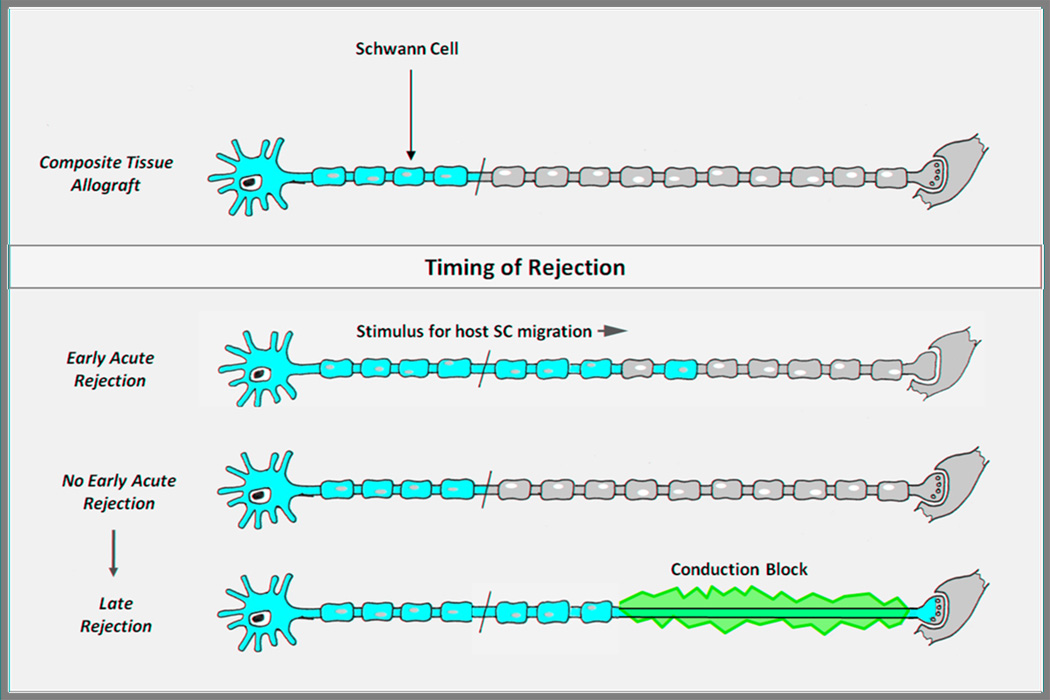
A second difference between allografts and CTA involves the impetus for SC migration. In nerve allografts, multiple subclinical rejection events in which the donor SC population is trimmed back but not eliminated likely provide the cue for host SC migration into the graft. In the hand transplant, other tissues such as skin and muscle have even greater antigenicity than SCs and require a more stringent suppression of the immune system[41]. Without the mini rejection episodes that are permissible and probably even desirable in nerve allografting alone, it is unknown to what extent host SCs will be triggered to proliferate and migrate into the graft. Should the graft remain exclusively populated by donor SCs, axonal regeneration will still occur. Donor SCs are intrinsically no different than host SCs in their support of regeneration. However, if a significant rejection episode occurs later that significantly impacts the donor SC population, the patient will be left with a long, unsupported segment of nerve and a devastating nerve conduction block that will render the hand transplant nonfunctional (Figure 2). Demyelination, which occurs with loss of SC support, is a major stimulus for SC migration. However, we know from our work with SC migration into acellularized nerve grafts that migration is limited to about 5 cm, even with SC migration occurring from both proximal and distal nerve stumps [unpublished data]. Until immunosuppressive strategies are developed that consistently induce tolerance of all donor tissue, the late loss of donor SCs due to unexpected episodes of rejection is a significant concern. Therefore, strategies to enhance nerve regeneration in CTA must take into account the delicate interplay between donor and host SCs, by promoting eventual complete repopulation of the donor nerve by host SCs, by keeping donor SCs alive long enough to support axonal regeneration until host SCs arrive, and by avoiding any damage to or rejection of the composite tissue transplant during accomplishment of the above objectives.
Figure 2. Schwann cell fate in relation to timing of rejection in composite tissue allotransplantation.
Minor early acute rejection episodes provide a stimulus for proximal host Schwann cell (SC) migration into the donor nerve. Without early rejection episodes, donor SCs will support axonal regeneration as well as host SCs. However, should late rejection occur without repopulation of the regenerated nerve by host SCs, the patient will be left with a debilitating conduction block. In this scenario, the host axon will not be supported by the rejected donor SCs, and host SCs cannot repopulate the entire length of the nerve from the proximal stump alone. (Adapted from Moore AM et al. Nerve allotransplantation as it pertains to composite tissue transplantation. Hand. 2009;4:239-44; with permission.)
Critical time window for regeneration
There is a critical window of time in which regeneration must occur for optimal recovery to be achieved. With regeneration proceeding at approximately 1 mm per day, many months may pass before target neuromuscular junctions are reinnervated[42–45]. As the months pass, with distal SCs lacking axonal contact and target muscle remaining denervated, irreversible changes begin that are detrimental to functional recovery. First, SCs that previously dedifferentiated from a myelinating phenotype to a regenerative phenotype settle back into a quiescent mode[46–48]. The SCs disarrange themselves from the bands of Büngner, decrease secretion of growth factors, and stop replication. Early reports suggest that SCs may even disappear altogether[49]. The result is inadequate support of regenerating axons. It is unknown exactly how long it takes in humans for denervated SCs to become quiescent. Clinical experience with proximal nerve injuries in humans seems to indicate that little additional motor functional recovery can be achieved by a patient past 1 year after injury[50]. Sensory recovery does not appear to be as time-dependent.
The second irreversible change that occurs with prolonged denervation is atrophy and fibrosis in the chronically denervated muscle[51–54]. In humans, these changes begin several months after denervation[19]. After this point, even if the muscle should become reinnervated, it can no longer achieve the same bulk or contractile force as pre-injury. In order to ensure optimal functional recovery, regeneration must occur during the critical time window. As the level of transplantation moves more and more proximally—from the hand to the forearm to the upper arm—we must be cognizant of these time constraints. Therefore, strategies to enhance nerve regeneration in CTA should include: (1) speeding the rate of axonal regeneration while ensuring quality of regeneration (2) maintaining perfect SC support during the entire time course of axonal regeneration, and (3) preserving the distal muscle until it can be reinnervated, all while preventing rejection of the composite tissue graft. In this review, we will highlight the experimental and clinical research that pertain to optimizing axonal regeneration and SC support in a CTA model.
Speeding axonal regeneration while ensuring quality of regeneration
A large proportion of the axon’s axoplasmic volume is amputated when it undergoes transection. This traumatic event will, at the least, lead to structural and functional changes in the neuronal cell body and may even lead to cell death[55,56]. At the start of regeneration, then, there may be fewer available axons than were originally present in the nerve, highlighting the need to ensure successful regeneration of all remaining axons. Within hours after injury, the neuronal cell body has altered its metabolic machinery to focus on structural protein synthesis and begins producing numerous growth cones, or terminal sprouts[57–59]. These represent the first wave of sprouts and are followed by a second wave within the first 2 days after injury[15,60]. The second wave of sprouts contains the definitive sprouts that proceed to regeneration, while the early sprouts degenerate. The time until appearance of the definitive sprouts is referred to as the “initial delay[61].” The time is variable and may be increased or decreased as a result of stress, activity, or drugs. The advancing sprouts must cross the critical “interstump zone[2],” the area of repair between the proximal and distal nerve stumps. They cross in a staggered fashion, and the final success of the nerve regeneration relies in great part on all regenerating axons successfully crossing this critical area. This highlights the importance of the growth-supportivemilieu and architectural cues provided by the SCs of the distal nerve stump. In the absence of appropriate neurotrophic and structural guidance, the regenerative axons may lose their way and form a neuroma, a disorganized collection of connective tissue and nerve fibers[61]. Strategies to promote perfect SC support will be discussed later in this review.
FK-506, also known as tacrolimus, is a potent calcineurin phosphatase inhibitor that is an essential part of immunosuppression regimens for a variety of organ transplants. Its immunosuppressive mechanism ultimately inhibits the activation of T-cell proliferation through binding and inhibition of calcineurin. In 1995, Gold et al first described the ability of FK-506 to enhance peripheral nerve regeneration in a rat model. Since that time, experimental studies have shown FK-506 to enhance regeneration after crush[62,63], transection[64], and chronic axotomy[65] injuries and in isograft[66] and allograft[21,67] models. Its ability to accelerate functional recovery has been shown in both small and large animal models. Although the exact molecular pathways leading to its enhanced regeneration are not completely clear, evidence of calcineurin-independent pathway of action suggests that mediators such as FKBP-52, growth associated protein 43 (GAP43), heat shock protein 90 (HSP-90), and cytoskeletal dynamics may be involved in its neuro-regenerative properties [68–73]. FK-506 has been shown to increase the number of regenerated myelinated and unmyelinated nerve fibers[63], stimulate even chronically axotomized motoneurons[65], and block neuronal apoptosis[74,75].
Correct timing of FK-506 administration is essential to its efficacy. Animal studies have shown that the neuroregenerative effect of FK-506 is decreased with delayed administration in both crush[76] injury and transection[77] models. Specifically, neuroregenerative benefit was diminished when administration was delayed by 3 days in a transection and immediate repair model, and was lost when administration was delayed by 5 days[77]. Therefore, as long as there is no individual contraindication to therapy, FK-506 should be provided as part of immunosuppression induction, as it was for all hand transplant patients between 1998 and 2006[20]. Ideally, the patient would receive FK-506 preloading for 3 days prior to nerve transection; this has been shown experimentally to further enhance FK-506’s neuroregenerative effect[78] and has been implemented clinically in elective cases of reconstructive nerve allotransplantation[79]. While this duration of preloading is not possible in hand transplantation due to the unpredictable nature of transplant availability, there is often a window of time on the order of 12 hours, in which the recipient is being notified of and prepared for surgery but the transplant is still being procured and prepared. Though it has not been definitively shown, we hypothesize that FK-506 preloading during this time window, given as a “loading dose” to reach therapeutic levels similar to those achieved with 3 days preloading, will similarly optimize the regenerative effects of FK-506.
Consideration should be given as well to including FK-506 in the patient’s immunosuppressive maintenance regimen. Unpublished data from our laboratory demonstrates that FK-506 continues to provide regenerative benefits for as long as it is administered. Specifically, for nerve transection injuries that posit a real regenerative challenge, FK-506 administration for a portion of the regenerative period shows better recovery than controls, but administration for the entire predicted regenerative period produces even more superior recovery. This corroborates with previous data that showed FK-506 to be advantageous even in the case of chronically axotomized axons[65]. The regenerating nerves in hand transplants, especially those of transplants at more proximal levels, are chronically axotomized for the many months they spend waiting to reach their distal targets. During this prolonged period, the patient will likely transition to a pared back immunosuppressive regimen. Incorporating FK-506 into this maintenance regimen will provide not only immunosuppressive graft protection, but also continued regenerative support.
Much research is being performed on how to minimize immunosuppression or, ideally, induce graft tolerance in CTA. As progress is made, we should remain aware that changing immunosuppressive regimens may differentially affect FK-506’s ability to stimulate regeneration. Animal studies have examined the regenerative impact of combining FK-506 administration with anti-CD40 ligand co-stimulatory blockade (CSB). Combining therapeutic doses of CSB with immunosuppressive doses of FK-506 significantly eliminated FK-506’s regenerative benefit. Using therapeutic CSB with a low dose of FK-506, however, was able to maintain the same regenerative benefit as therapeutic FK-506 alone[80]. Other studies have shown that FK-506 maintains neuroregenerative benefit at low doses[63,81–84], with a dose-benefit relationship that may not be strictly linear[85]. Although not specifically studied, it was hypothesized that the full immunosuppressive doses of each drug interfered with the mechanism of action of the other drug, each making the other less effective. Further understanding of the mechanisms by which these immunosuppressive agents potentiate and abrogate each other will progress our ability to create immunosuppressive or tolerance regimens that effectively protect the transplant while including the right dosing of FK-506 to promote regeneration.
It should also be noted that, in addition to impacting the rate of axonal regeneration, varying immunosuppressive regimens differentially impacted donor SC fate. In a study utilizing transgenic mice with SCs expressing green fluorescent protein under the S-100b (expressed by well-differentiated SCs) or nestin (expressed after a denervation stimulus) promoters, donor SC survival and phenotype in an allograft model were evaluated under different immunosuppressive regimens via serial imaging[86]. Mice immunosuppressed with FK-506 demonstrated mild acute rejection as well as somewhat hindered myelin formation and maturation of donor SCs (from a proliferative to myelinating phenotype) after axonal regeneration was complete. Mice treated with CSB, on the other hand, experienced virtually no graft rejection and showed optimal myelin formation and maturation of SCs after axonal regeneration. However, despite the improved restoration of mature SC-axonal relationships in CSB-treated mice, FK-506 treated mice demonstrated more robust regeneration of myelinated and unmyelinated axons, greater motor endplate reinnervation, and earlier functional recovery. While this study did not evaluate a combined FK-506 and CSB regimen, it would be useful to know whether an optimal combination could be found that blended the best of both regimens—the enhanced axonal regeneration of FK-506 treatment and the improved myelin formation of CSB treatment. The impact of this ideal combination regimen on host SC migration would then need to be studied, to determine the distribution and ratio of donor to host SCs that remain after axonal regeneration and what risk this might pose should late rejection occur.
Ensuring perfect Schwann cell support
As previously discussed, SCs are induced after nerve injury to change from a myelinating phenotype to a proliferative phenotype. Proliferative SCs are then intimately involved in the process of axonal regeneration, through provision of structural guidance via bands of Büngner and secretion of neurotrophic factors. Without axonal contact, however, SCs will not indefinitely maintain their new proliferative phenotype or their injury-induced increase in number[46–48]. An experimental study in a rat model demonstrated the detrimental effect of chronic denervation on SC ability to support axonal regeneration[87]. The common peroneal branch of the rat sciatic nerve was transected and the distal stump subjected to 0–24 weeks of chronic denervation. After the denervation period, the tibial branch of the sciatic nerve was transected and the proximal tibial stump repaired end-to-end to the chronically denervated peroneal distal stump. Retrograde labeling 12 months later demonstrated that denervation of SCs for up to 4 weeks did not negatively impact axonal regeneration, but axonal regeneration rapidly fell off when distal nerve SCs were denervated for 8 weeks or more. With SC chronic denervation for 24 weeks, the number of regenerating axons was diminished to less than 10%. Interestingly, the 10% of regenerated axons were well myelinated. Therefore, while chronically denervated SCs may not retain their ability to support regeneration, they retain their ability to myelinate, likely due to reversion back to a myelinating phenotype from a proliferative phenotype.
Perfect proliferative SC support is required, therefore, in order to optimize axonal regeneration. Perfect SC support is threatened, however, in a CTA model. As mentioned earlier, episodes of rejection threaten donor SCs, which are crucial to early nerve regeneration. Lack of short episodes of rejection, however, may remove the cue for migration of host SCs into the transplanted nerve, with the potential for a devastating conduction block if residual donor SCs are later rejected. Even if adequate cues for host SC migration are provided, lack of a distal host SC source may leave too large a proliferation and migration burden on proximal host SCs—proximal host SCs may not be able to populate the distal-most extent of the transplanted nerve.
Strategies to optimize SC support include re-activating SCs that have reverted from their proliferative phenotype to their original myelinating phenotype, or preventing this reversion. Alternatively, supplementation with cultured SCs or stem-cell derived SC-like cells after a period of chronic denervation might provide fresh support for regenerating axons, in essence “resetting the clock.”
Interaction of SCs with invading macrophages is important for subsequent SC support of regeneration. After nerve injury, transforming growth factor β (TGF-β) is secreted into the nerve by invading macrophages[88] as well as resident SCs[89]. Several in vitro studies have shown TGF-β to be essential in the transformation of SCs to a non-myelinating, proliferative phenotype[90–94]. Additionally, TGF-β is involved in mediating the neurotrophic effect of several other neurotrophic factors, such as glial cell line-derived neurotrophic factor (GDNF), a very potent neurotrophic factor for motoneurons[95,96]. An experimental study in a rat model of chronic SC denervation showed that TGF-β was able to attenuate the adverse effects of chronic denervation in vivo[97]. Schwann cells were isolated from rat distal nerve stumps that had been chronically denervated for 24 weeks. After incubation in culture medium with or without TGF-β, the SCs were placed into silastic tubes that were grafted into a freshly transected rat tibial nerve. Retrograde labeling 3 months later showed a four-fold increase in the number of distally regenerated motoneurons in the group that had received SC incubation with TGF-β[87,97]. It appears that TGF-β has the capability to reverse the detrimental effect of chronic denervation on SC ability to support axonal regeneration. Studies utilizing local delivery of TGF-β in a prolonged or chronic denervation model would be useful to assess its potential as a therapeutic in cases of human proximal nerve injury or hand transplantation, where prolonged denervation of distal SCs is anticipated.
If reawakening SCs into a proliferative mode is not possible or practical in vivo, supplementation with autologous cultured SCs or stem-cell derived SC-like cells may function to the same end. Supplementation also would be indicated in hand transplantation, for replacement of donor SCs that die off as a result of rejection episodes. Alternatively, prophylactic “replacement” of donor SCs with host SCs could be performed at the time of transplant to seed the entire donor nerve with preoperatively cultured cells, thereby creating a mixed population of host and donor SCs. In all cases except complete immune tolerance, this mixed population would be beneficial to better withstand rejection episodes.
Alternative sources for host Schwann cells
Isolation and culture of human SCs has been described in the peripheral nerve literature since 1991[98] but has been fraught with many difficulties in achieving good primary cell yields and in adequately excluding fibroblasts. Even though significant strides have been made, with a recently published protocol describing a relatively fast (21 day) and efficient (90–95% pure) culture of primary human SCs[99], cultured SCs have failed to achieve clinical translation over the past 20 years. Additionally, culture of human SCs requires invasive nerve biopsy with subsequent sensory loss and the risk of neuroma formation and neuropathic pain. More accessible and less morbid sources of SCs have, therefore, been sought.
Bone marrow, adipose tissue, and skin with its associated structures have arisen as sources of stem cells that may be induced to a SC-like character. Bone marrow stromal cells (BMSCs), harvested from the long bones, have been studied by a number of groups and shown to differentiate into an adherent SC-like phenotype when cultured in media with appropriate cytokines[100–102]. Though they have shown some promise in rodent and non-human primate experimental models of nerve conduits[101–104] and acellular grafts[100], interest has grown in less invasive sources of stem cells.
Adipose tissue has been identified as a source of multipotent stromal stem cells with the ability to differentiate into a SC phenotype under appropriate culture conditions. These adipose tissue-derived stem cells (ADSCs) have been shown to promote motor neurite outgrowth in vitro[105]. Two recent studies have evaluated cultured primary SCs, differentiated BMSCs, differentiated ADSCs, and undifferentiated ADSCs for supplementation of a 1 cm fibrin nerve conduit in a rat sciatic nerve model[106,107]. Evaluation of regenerative distance 2 weeks after conduit implantation showed that (1) supplementation with either cultured primary SCs, differentiated BMSCs, or differentiated ADSCs significantly increased the distance of axonal regeneration over that of conduits with media alone; (2) primary SCs were superior to both differentiated BMSCs and ADSCs; and (3) differentiated BMSCs and ADSCs were equivalent in their regeneration enhancement[106]. In a separate study, undifferentiated ADSCs seeded into the fibrin conduit were also able to increase regeneration distance over media alone. However, cell tracking 14 days after transplantation showed almost no remaining implanted cells[107]. This raises questions about the longevity of implanted cells that are undifferentiated versus differentiated, about the mechanisms by these cells work, and about the relationship between stem cell lifespan and regenerative benefit.
Finally, the skin dermis contains neural crest-related precursor cells, termed skin-derived precursor cells (SKPs), that have been shown to differentiate into SC-like cells in vitro when supplied with appropriate cues, both in rodents[108,109] and in humans[110,111]. Recent studies have tested the ability of SKPs to enhance regeneration in rodent acute injury and chronic denervation models. In a rat 12 mm sciatic nerve gap model, acellularized nerve allografts (ANAs) were seeded with cultured SCs or SKPs and compared to autografts (positive control) and ANAs with vehicle (negative control). Four and 8 week time points showed (1) continued survival of SKPs; (2) significantly better regeneration with SKP-seeded ANAs than with vehicle alone; (3) equivalent regeneration between SKP-seeded ANAs and autografts; and (4) equivalent or slightly better regeneration in SKP-seeded ANAs than SC-seeded ANAs[112]. Skin-derived precursor cells were then tested in a chronic denervation model and found to significantly enhance regeneration of motoneurons compared to vehicle control, in essence reawakening the regenerative capability of the chronically denervated distal stump[113]. The mechanism behind this phenomenon—whether this improved support is directly due to the influence of SKPs on regenerating axons or secondary to interactions between SKPs and host SCs—has yet to be elucidated.
There are a few practical considerations to discuss concerning the use of stem cells in peripheral nerve repair[114]. In order to optimize stem cell therapy, more research on the number, method, and timing of stem cell delivery is needed. Seeded stem cell numbers have varied widely across studies and injury models, and the optimal dosing regimen is unknown. Additionally, the degree of predifferentiation prior to seeding is being worked out. While it appears that survival is improved with predifferentiation[110], supplementation with naïve stem cells might better maintain the cells’ proliferative capacity, while allowing in vivo signals to prompt differentiation[115]. Survival should also be improved, as most studies to date have shown only between 0.5% and 38% survival of implanted stem cells[114]. Finally, the tumorigenic capabilities of these stem cells must be explored, especially before consideration in an immunosuppressed transplant population, as capacity for malignant transformation is a potential negative consequence of using multipotent precursors. While these parameters require further study prior to clinical translation, the use of stem cells for augmenting axonal regeneration is, nevertheless, an exciting area of research with the potential to significantly further peripheral nerve surgery outcomes.
Optimizing Surgical Technique
There are several surgical techniques that can be utilized to enhance motor and sensory recovery following hand transplantation. First, a thorough understanding of the motor and sensory topography of the ulnar and median nerves must guide their repair. This understanding will lead to proper alignment of ulnar nerve motor and sensory components, ultimately promoting appropriate reinnervation of motor and sensory targets. Secondly, with the realization that 95% of the median nerve at the wrist is sensory, nerve transfer of the distal anterior interosseous nerve to the median nerve deep motor branch optimizes motor reinnervation of the thenar musculature, enhancing specific functional recovery. Finally, as nerves that are regenerating will slow at areas of known nerve entrapment, decompression of nerve entrapment points distal to the nerve repair sites should be performed. Both the carpal tunnel and the ulnar nerve through Guyon’s canal can be decompressed through the same incision.
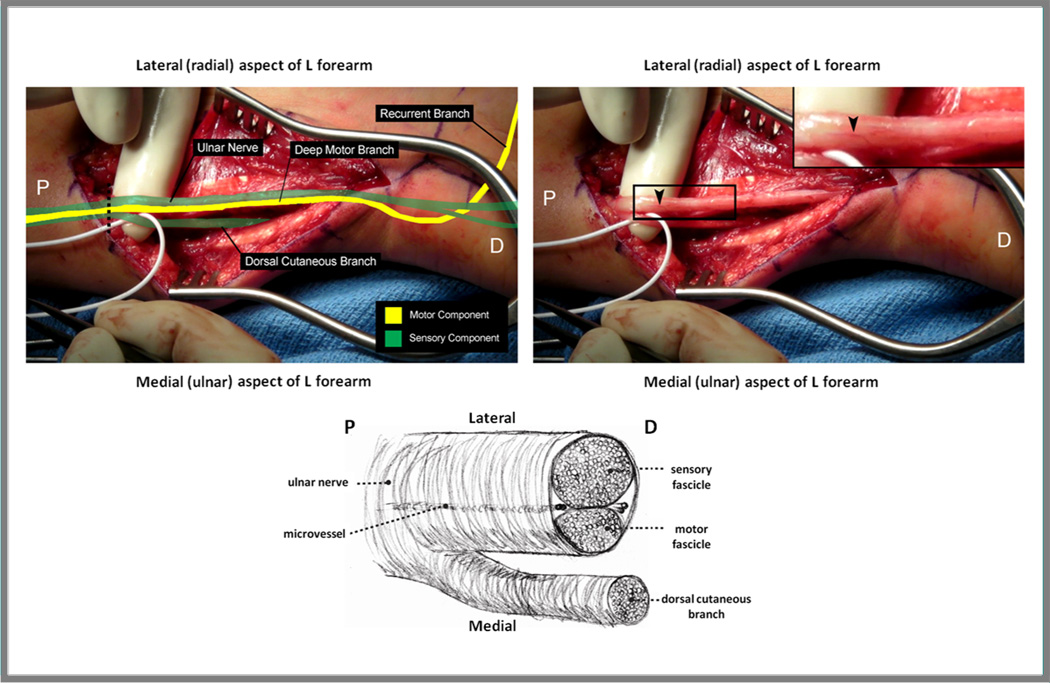
The intraneural topography of the ulnar nerve has been understood since Jabaley first described a preliminary report in 1980[116]. Specifically, distal to the takeoff of the dorsal cutaneous branch of the ulnar nerve (DCU), the sensory component that innervates the volar aspect of the hand makes up about 60% of the nerve cross-sectional area, and the motor component that continues as the deep motor branch makes up the remaining 40% (Figure 3). Proximal to the takeoff of the DCU, the motor fascicular group is “sandwiched” between the DCU medially and the main sensory component of the ulnar nerve laterally. The DCUbranches off from the main ulnar nerve about 10 cm above the wrist crease. As it travels distally, the motor component turns into the deep motor branch of the ulnar nerve (DMB) and moves from lying medial to the sensory portion of the ulnar nerve, to travelling under the sensory component as it turns around the hook of the hamate, where itfinally assumes a position lateral to the sensory component. There is an intraneural cleavage plane between the motor and sensory groups, as well as between the main ulnar nerve and the DCU, that is easily discernible using micro-pickups. Tapping across the surface of the nerve, micro-pickups will “fall” into the intraneural cleavage plane. Often, there is also a streak of fatty tissue or a micro-vessel that delineates this cleavage plane[117] (Figure 3). Certainly, in the donor hand the motor and sensory components can be clearly delineated by tracing the DMB proximally to identify the motor fascicular group. In the recipient forearm, at or proximal to the level of the wrist, the larger sensory component of the ulnar nerve will lie most laterally (i.e., most radially) compared to the motor component, and above 10 cm proximal to wrist level, there will also be a smaller fascicular group present on the most medial (ulnar) aspect that is the DCU. Appropriate alignment of motor and sensory fascicular components at the time of hand transplantation will optimize appropriate reinnervation of motor and sensory targets.
Figure 3. Ulnar nerve topography within the distal forearm.
Left: The motor (yellow) and sensory (green) fascicles should be identified at the time of transplantation to facilitate appropriate alignment during repair. Proximally, the ulnar nerve topography is sensory-motor-sensory, until the dorsal cutaneous branch separates from the remainder of the nerve approximately 10 cm proximal to the wrist crease. In order to confirm the identity of the motor fascicle, the deep motor branch should be identified within Guyon’s canal distally (not shown), then followed visually to where it joins the main sensory fascicular group proximally. Right: A prominent longitudinally oriented vessel (arrow) is frequently present, marking the natural cleavage plane between the sensory and motor fascicular groups of the ulnar nerve. Bottom: Ulnar nerve in cross section. Below the take-off of the dorsal cutaneous branch, the sensory fascicle comprises approximately 60% of the ulnar nerve, and the motor fascicle 40%. P = proximal; D = distal.Dashed line = cross section. (Adapted from Brown JM, Yee A, Mackinnon SE. Distal median to ulnar nerve transfers to restore ulnar motor and sensory function within the hand. Neurosurgery. 2009;65:966-78; with permission.)
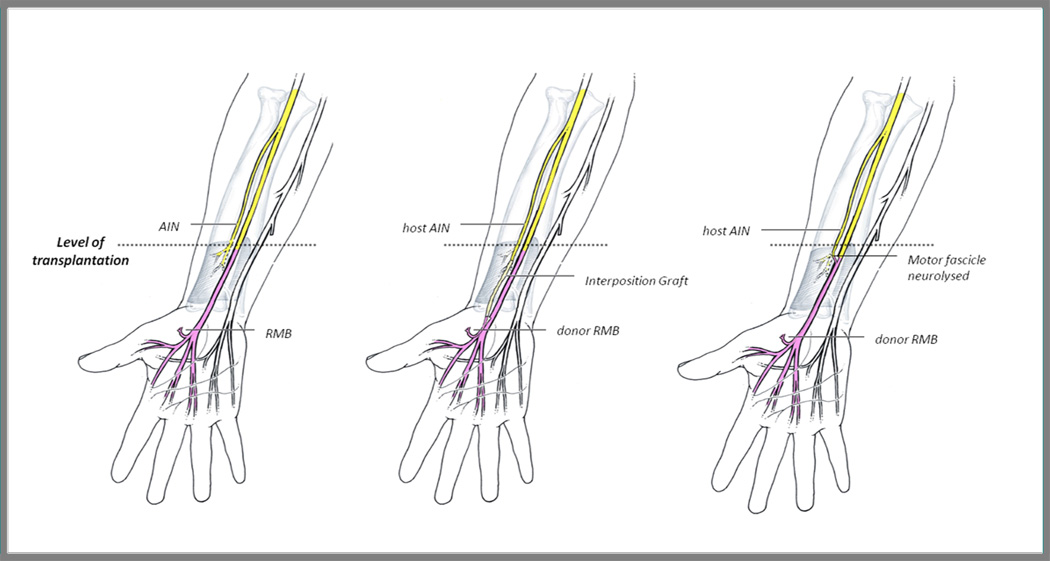
With respect to the median nerve, 95% of the nerve at wrist level is comprised of sensory axons. Topographical mapping of the recipient median nerveat this level during nerve repair is, therefore, not as feasible. While guidance of regenerating sensory axons to their sensory targets is practically assured, the ability to direct the remaining 5% of motor axons to the thenar muscles is tenuous. It is straight-forward, however, to identify the recurrent motor branch (RMB) of the median nerve in the donor hand and then to trace the motor fascicle proximally to the proposed repair site. Consideration can then be given to reinnervating the median nerve’s recurrentmotor branch (RMB) to the thenar muscles with a nerve transfer from the distal portion of the anterior interosseous nerve (AIN) (Figure 4).
Figure 4. Distal anterior interosseous nerve (AIN) to median recurrent motor branch (RMB) transfer.
For hand transplants at or below the level of the pronator quadratus muscle (left), the host AIN may serve as a reliable donor of motor axons to the thenar muscles, via nerve transfer to the RMB. The AIN may be transferred to the RMB either with the use of an interposing nerve graft (middle) or after more proximal motor fascicle dissection of the donor nerve (right). (Adapted from Colbert SH, Mackinnon SE. Nerve transfers in the hand and upper extremity. Tech Hand Up Extrem Surg 2008;12:20–33 and from Brown JM, Mackinnon SE. Nerve transfers in the forearm and hand. Hand Clinics. 2008;24:319-40; with permission.)
The successful transfer of the distal AIN to the RMB was first demonstrated by Huang in 1992 in rhesus monkeys[118], and then was reported in a cases series of 17 patients in 1997[119]. We also reported the use of this nerve transfer to restore thenar muscle function after resection of a median nerve neuroma-in-continuity[120].The distal AIN provides a suitable donor for transfer of motor axons to the RMB of the median nerve, because anatomically, the AIN and the RMB are comparable in size (AIN approximately 1.3–1.5 mm; RMB approximately 1.4–1.7 mm in diameter) and number of myelinated fibers (AIN approximately 866–912; RMB approximately 1020–1120 myelinated fibers)[121–124]. Also, while the AIN is a mixed nerve, it consists primarily of motor fibers[117]. The only limitation to this nerve transfer is the location of the AIN at 60 mm proximal to the distal wrist crease[123]. Either a nerve graft is necessary for successful tension-free nerve transfer or the motor branch of the donor median nerve would need to be neurolysed proximally enough in the donor forearm to facilitate a tension-free direct repair (Figure 4).
To perform the nerve transfer, the RMB can be identified in the carpal tunnel and then traced back proximally to the repair site. We use a technique that we call “neurolysing with our eyes;” we physically identify the recurrent motor branch as it enters the thenar muscles and the simply follow it proximally with micro-pickups until we can mark its position on the median nerve with blue ink. This allows us to determine the site at which we will transfer the AIN without the added operative time or unnecessary risks of physical neurolysis. The AIN may then be identified as it enters the pronator quadratus, transected just proximal to its branches at the midportion of the muscle, and then either transferred to the RMB with the use of an interposing nerve graft or transferred to the more proximal motor fascicle dissection on the donor nerve. Should a nerve graft be used, we would recommend use of an autologous nerve graft, as it provides immediate SC support and the potential to provide host SCs for migration and repopulation of donor nerves. We refer you to the following publications for a more detailed description of the AIN harvest[117,125] and transfer to the RMB[120].
Our final recommendation is to perform decompression of the carpal tunnel and Guyon’s canal with specific release of the DMB to prevent slowing of regeneration at these common areas of nerve entrapment. Both of these decompressions may be performed through a single longitudinal incision made approximately 6 mm ulnar to the thenar crease.
Summary
Reinnervation of a hand transplant ultimately dictates functional recovery but provides a significant regenerative challenge. In addition to high quality of regeneration, rapidity of regeneration is essential, as there is a critical time windowfor regeneration before detrimental changes occur in the distal nerve and target muscle. Interventions to enhance nerve regeneration include acceleration of axonal regeneration via FK-506 administration and augmentation of SC support, either by reactivation of chronically axotomized SCs or by SC supplementation . Optimizing surgical technique at the time of transplantation also better supports intrinsic muscle recovery. Appropriate alignment of ulnar nerve motor and sensory componentsand prophylactic release of potential entrapment points should be performed, and transfer of the distal anterior interosseous nerve to the recurrent motor branch of the median nerve considered. Improving long-term functional recovery through the above interventions will ultimately help determine the success and longevity of the field of hand transplantation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures/Funding:
The authors have nothing to disclose. No benefits or funding in any form have been received or will be received in relation to this article.
References
- 1.International Registry on Hand and Composite Tissue Transplantation, in. [Google Scholar]
- 2.Geuna S, Raimondo S, Ronchi G, et al. Chapter 3: Histology of the peripheral nerve and changes occurring during nerve regeneration. Int Rev Neurobiol. 2009;87:27–46. doi: 10.1016/S0074-7742(09)87003-7. [DOI] [PubMed] [Google Scholar]
- 3.Bigbee JW, Yoshino JE, DeVries GH. Morphological and proliferative responses of cultured Schwann cells following rapid phagocytosis of a myelin-enriched fraction. J Neurocytol. 1987;16:487–496. doi: 10.1007/BF01668503. [DOI] [PubMed] [Google Scholar]
- 4.Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- 5.Stoll G, Jander S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol. 1999;58:233–247. doi: 10.1016/s0301-0082(98)00083-5. [DOI] [PubMed] [Google Scholar]
- 6.Rogister B, Delree P, Leprince P, et al. Transforming growth factor beta as a neuronoglial signal during peripheral nervous system response to injury. J Neurosci Res. 1993;34:32–43. doi: 10.1002/jnr.490340105. [DOI] [PubMed] [Google Scholar]
- 7.Mews M, Meyer M. Modulation of Schwann cell phenotype by TGF-beta 1: inhibition of P0 mRNA expression and downregulation of the low affinity NGF receptor. Glia. 1993;8:208–217. doi: 10.1002/glia.440080308. [DOI] [PubMed] [Google Scholar]
- 8.Heumann R. Regulation of the synthesis of nerve growth factor. J Exp Biol. 1987;132:133–150. doi: 10.1242/jeb.132.1.133. [DOI] [PubMed] [Google Scholar]
- 9.Thoenen H, Bandtlow C, Heumann R, et al. Nerve growth factor: cellular localization and regulation of synthesis. Cell Mol Neurobiol. 1988;8:35–40. doi: 10.1007/BF00712909. [DOI] [PubMed] [Google Scholar]
- 10.Funakoshi H, Frisen J, Barbany G, et al. Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J Cell Biol. 1993;123:455–465. doi: 10.1083/jcb.123.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brushart TM. Preferential reinnervation of motor nerves by regenerating motor axons. J Neurosci. 1988;8:1026–1031. doi: 10.1523/JNEUROSCI.08-03-01026.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lundborg G, Dahlin LB, Danielsen N, et al. Tissue specificity in nerve regeneration. Scand J Plast Reconstr Surg. 1986;20:279–283. doi: 10.3109/02844318609004486. [DOI] [PubMed] [Google Scholar]
- 13.Lundborg Gr, Dahlin L, Danielsen N, et al. Trophism, Tropism and Specificity in Nerve Regeneration. J Reconstr Microsurg. 1994;10:345–354. doi: 10.1055/s-2007-1006604. [DOI] [PubMed] [Google Scholar]
- 14.Mackinnon SE, Dellon AL, Lundborg G, et al. A study of neurotrophism in a primate model. J Hand Surg Am. 1986;11:888–894. doi: 10.1016/s0363-5023(86)80244-1. [DOI] [PubMed] [Google Scholar]
- 15.Cajal SR. Degeneration and Regeneration of the Nervous System. New York: Oxford University Press; 1928. [Google Scholar]
- 16.Toft PB, Fugleholm K, Schmalbruch H. Axonal branching following crush lesions of peripheral nerves of rat. Muscle Nerve. 1988;11:880–889. doi: 10.1002/mus.880110813. [DOI] [PubMed] [Google Scholar]
- 17.Aitken JT, Sharman M, Young JZ. Maturation of regenerating nerve fibres with various peripheral connexions. J Anat. 1947;81:1–22. 22. [PubMed] [Google Scholar]
- 18.Mackinnon SE, Dellon AL, O'Brien JP. Changes in nerve fiber numbers distal to a nerve repair in the rat sciatic nerve model. Muscle Nerve. 1991;14:1116–1122. doi: 10.1002/mus.880141113. [DOI] [PubMed] [Google Scholar]
- 19.Mackinnon SE, Dellon AL. Surgery of the Peripheral Nerve. New York: Thieme; 1988. [Google Scholar]
- 20.Siemionow M, Sonmez E. Nerve allograft transplantation: a review. J Reconstr Microsurg. 2007;23:511–520. doi: 10.1055/s-2007-1022694. [DOI] [PubMed] [Google Scholar]
- 21.Mackinnon SE, Doolabh VB, Novak CB, et al. Clinical outcome following nerve allograft transplantation. Plast Reconstr Surg. 2001;107:1419–1429. doi: 10.1097/00006534-200105000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Bain JR, Mackinnon SE, Hudson AR, et al. Preliminary report of peripheral nerve allografting in primates immunosuppressed with cyclosporin A. Transplant Proc. 1989;21:3176–3177. [PubMed] [Google Scholar]
- 23.Midha R, Mackinnon SE, Evans PJ, et al. Comparison of regeneration across nerve allografts with temporary or continuous cyclosporin A immunosuppression. J Neurosurg. 1993;78:90–100. doi: 10.3171/jns.1993.78.1.0090. [DOI] [PubMed] [Google Scholar]
- 24.Nakao Y, Mackinnon SE, Mohanakumar T, et al. Monoclonal antibodies against ICAM-1 and LFA-1 (CD11A) induce specific tolerance to peripheral nerve allograft in rats. Transplant Proc. 1995;27:373–377. [PubMed] [Google Scholar]
- 25.Strasberg SR, Hertl MC, Mackinnon SE, et al. Peripheral nerve allograft preservation improves regeneration and decreases systemic cyclosporin A requirements. Exp Neurol. 1996;139:306–316. doi: 10.1006/exnr.1996.0104. [DOI] [PubMed] [Google Scholar]
- 26.Gulati AK. Immune response and neurotrophic factor interactions in peripheral nerve transplants. Acta Haematol. 1998;99:171–174. doi: 10.1159/000040832. [DOI] [PubMed] [Google Scholar]
- 27.Gulati AK, Cole GP. Nerve graft immunogenicity as a factor determining axonal regeneration in the rat. J Neurosurg. 1990;72:114–122. doi: 10.3171/jns.1990.72.1.0114. [DOI] [PubMed] [Google Scholar]
- 28.Pollard JD, Gye RS, McLeod JG. An assessment of immunosuppressive agents in experimental peripheral nerve transplantation. Surg Gynecol Obstet. 1971;132:839–845. [PubMed] [Google Scholar]
- 29.Trumble TE, Shon FG. The physiology of nerve transplantation. Hand Clin. 2000;16:105–122. [PubMed] [Google Scholar]
- 30.Lassner F, Schaller E, Steinhoff G, et al. Cellular mechanisms of rejection and regeneration in peripheral nerve allografts. Transplantation. 1989;48:386–392. doi: 10.1097/00007890-198909000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Mackinnon S, Hudson A, Falk R, et al. Nerve allograft response: a quantitative immunological study. Neurosurgery. 1982;10:61–69. doi: 10.1227/00006123-198201000-00011. [DOI] [PubMed] [Google Scholar]
- 32.Yu LT, Rostami A, Silvers WK, et al. Expression of major histocompatibility complex antigens on inflammatory peripheral nerve lesions. J Neuroimmunol. 1990;30:121–128. doi: 10.1016/0165-5728(90)90095-5. [DOI] [PubMed] [Google Scholar]
- 33.Whitlock EL, Myckatyn TM, Tong AY, et al. Dynamic quantification of host Schwann cell migration into peripheral nerve allografts. Exp Neurol. 2010;225:310–319. doi: 10.1016/j.expneurol.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aguayo AJ, Charron L, Bray GM. Potential of Schwann cells from unmyelinated nerves to produce myelin: a quantitative ultrastructural and radiographic study. J Neurocytol. 1976;5:565–573. doi: 10.1007/BF01175570. [DOI] [PubMed] [Google Scholar]
- 35.Aguayo AJ, Epps J, Charron L, et al. Multipotentiality of Schwann cells in cross-anastomosed and grafted myelinated and unmyelinated nerves: quantitative microscopy and radioautography. Brain Res. 1976;104:1–20. doi: 10.1016/0006-8993(76)90643-0. [DOI] [PubMed] [Google Scholar]
- 36.Fornaro M, Tos P, Geuna S, et al. Confocal imaging of Schwann-cell migration along muscle-vein combined grafts used to bridge nerve defects in the rat. Microsurgery. 2001;21:153–155. doi: 10.1002/micr.1029. [DOI] [PubMed] [Google Scholar]
- 37.Fukaya K, Hasegawa M, Mashitani T, et al. Oxidized galectin-1 stimulates the migration of Schwann cells from both proximal and distal stumps of transected nerves and promotes axonal regeneration after peripheral nerve injury. J Neuropathol Exp Neurol. 2003;62:162–172. doi: 10.1093/jnen/62.2.162. [DOI] [PubMed] [Google Scholar]
- 38.Tseng CY, Hu G, Ambron RT, et al. Histologic analysis of Schwann cell migration and peripheral nerve regeneration in the autogenous venous nerve conduit (AVNC) J Reconstr Microsurg. 2003;19:331–340. doi: 10.1055/s-2003-42502. [DOI] [PubMed] [Google Scholar]
- 39.Hayashi A, Koob JW, Liu DZ, et al. A double-transgenic mouse used to track migrating Schwann cells and regenerating axons following engraftment of injured nerves. Exp Neurol. 2007;207:128–138. doi: 10.1016/j.expneurol.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore AM, Ray WZ, Chenard KE, et al. Nerve allotransplantation as it pertains to composite tissue transplantation. Hand (N Y) 2009;4:239–244. doi: 10.1007/s11552-009-9183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hettiaratchy S, Melendy E, Randolph MA, et al. Tolerance to composite tissue allografts across a major histocompatibility barrier in miniature swine. Transplantation. 2004;77:514–521. doi: 10.1097/01.tp.0000113806.52063.42. [DOI] [PubMed] [Google Scholar]
- 42.Wang MS, Zeleny-Pooley M, Gold BG. Comparative dose-dependence study of FK506 and cyclosporin A on the rate of axonal regeneration in the rat sciatic nerve. J Pharmacol Exp Ther. 1997;282:1084–1093. [PubMed] [Google Scholar]
- 43.Pan YA, Misgeld T, Lichtman JW, et al. Effects of neurotoxic and neuroprotective agents on peripheral nerve regeneration assayed by time-lapse imaging in vivo. J Neurosci. 2003;23:11479–11488. doi: 10.1523/JNEUROSCI.23-36-11479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gutmann E, Guttmann L, Medawar PB, et al. The Rate of Regeneration of Nerve. J Exp Biol. 1942;19:14–44. [Google Scholar]
- 45.Mackinnon SE. Surgical management of the peripheral nerve gap. Clin Plast Surg. 1989;16:587–603. [PubMed] [Google Scholar]
- 46.Li H, Terenghi G, Hall SM. Effects of delayed re-innervation on the expression of c-erbB receptors by chronically denervated rat Schwann cells in vivo. Glia. 1997;20:333–347. doi: 10.1002/(sici)1098-1136(199708)20:4<333::aid-glia6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 47.Roytta M, Salonen V. Long-term endoneurial changes after nerve transection. Acta Neuropathol. 1988;76:35–45. doi: 10.1007/BF00687678. [DOI] [PubMed] [Google Scholar]
- 48.You S, Petrov T, Chung PH, et al. The expression of the low affinity nerve growth factor receptor in long-term denervated Schwann cells. Glia. 1997;20:87–100. doi: 10.1002/(sici)1098-1136(199706)20:2<87::aid-glia1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 49.Weinberg HJ, Spencer PS. The fate of Schwann cells isolated from axonal contact. J Neurocytol. 1978;7:555–569. doi: 10.1007/BF01260889. [DOI] [PubMed] [Google Scholar]
- 50.Sulaiman OA, Gordon T. Role of chronic Schwann cell denervation in poor functional recovery after nerve injuries and experimental strategies to combat it. Neurosurgery. 2009;65:A105–A114. doi: 10.1227/01.NEU.0000358537.30354.63. [DOI] [PubMed] [Google Scholar]
- 51.Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged denervation. J Neurosci. 1995;15:3886–3895. doi: 10.1523/JNEUROSCI.15-05-03886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: prolonged axotomy. J Neurosci. 1995;15:3876–3885. doi: 10.1523/JNEUROSCI.15-05-03876.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Finkelstein DI, Dooley PC, Luff AR. Recovery of muscle after different periods of denervation and treatments. Muscle Nerve. 1993;16:769–777. doi: 10.1002/mus.880160712. [DOI] [PubMed] [Google Scholar]
- 54.Kobayashi J, Mackinnon SE, Watanabe O, et al. The effect of duration of muscle denervation on functional recovery in the rat model. Muscle Nerve. 1997;20:858–866. doi: 10.1002/(sici)1097-4598(199707)20:7<858::aid-mus10>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 55.Horch K. Central responses of cutaneous sensory neurons to peripheral nerve crush in the cat. Brain Res. 1978;151:581–586. doi: 10.1016/0006-8993(78)91090-9. [DOI] [PubMed] [Google Scholar]
- 56.Kristensson K. Retrograde signalling of nerve cell body response to trauma. In: Gorio A, Millesi H, Mingrino S, editors. Posttraumatic Peripheral Nerve Regeneration. New York: Raven Press; 1981. [Google Scholar]
- 57.Ducker TB, Kempe LG, Hayes GJ. The metabolic background for peripheral nerve surgery. J Neurosurg. 1969;30:270–280. doi: 10.3171/jns.1969.30.3part1.0270. [DOI] [PubMed] [Google Scholar]
- 58.Lieberman AR. The axon reaction: a review of the principal features of perikaryal responses to axon injury. Int Rev Neurobiol. 1971;14:49–124. doi: 10.1016/s0074-7742(08)60183-x. [DOI] [PubMed] [Google Scholar]
- 59.Fu S, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Molecular Neurobiology. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- 60.Grafstein B, McQuarrie IG. Role of the nerve cell body in axonal regeneration. In: Cotman CW, editor. Neuronal Plasticity. New York: Raven Press; 1978. pp. 155–195. [Google Scholar]
- 61.Sunderland SS. Nerve and Nerve Injuries. ed Second. Edinburgh: Churchill Livingstone; 1978. [Google Scholar]
- 62.Lee M, Doolabh VB, Mackinnon SE, et al. FK506 promotes functional recovery in crushed rat sciatic nerve. Muscle Nerve. 2000;23:633–640. doi: 10.1002/(sici)1097-4598(200004)23:4<633::aid-mus24>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 63.Udina E, Ceballos D, Gold BG, et al. FK506 enhances reinnervation by regeneration and by collateral sprouting of peripheral nerve fibers. Exp Neurol. 2003;183:220–231. doi: 10.1016/s0014-4886(03)00173-0. [DOI] [PubMed] [Google Scholar]
- 64.Jost SC, Doolabh VB, Mackinnon SE, et al. Acceleration of peripheral nerve regeneration following FK506 administration. Restor Neurol Neurosci. 2000;17:39–44. [PubMed] [Google Scholar]
- 65.Sulaiman OA, Voda J, Gold BG, et al. FK506 increases peripheral nerve regeneration after chronic axotomy but not after chronic schwann cell denervation. Exp Neurol. 2002;175:127–137. doi: 10.1006/exnr.2002.7878. [DOI] [PubMed] [Google Scholar]
- 66.Doolabh VB, Mackinnon SE. FK506 accelerates functional recovery following nerve grafting in a rat model. Plast Reconstr Surg. 1999;103:1928–1936. doi: 10.1097/00006534-199906000-00018. [DOI] [PubMed] [Google Scholar]
- 67.Feng FY, Ogden MA, Myckatyn TM, et al. FK506 rescues peripheral nerve allografts in acute rejection. J Neurotrauma. 2001;18:217–229. doi: 10.1089/08977150150502631. [DOI] [PubMed] [Google Scholar]
- 68.Gold BG, Zeleny-Pooley M, Wang MS, et al. A nonimmunosuppressant FKBP-12 ligand increases nerve regeneration. Exp Neurol. 1997;147:269–278. doi: 10.1006/exnr.1997.6630. [DOI] [PubMed] [Google Scholar]
- 69.Klettner A, Baumgrass R, Zhang Y, et al. The neuroprotective actions of FK506 binding protein ligands: neuronal survival is triggered by de novo RNA synthesis, but is independent of inhibition of JNK and calcineurin. Brain Res Mol Brain Res. 2001;97:21–31. doi: 10.1016/s0169-328x(01)00286-8. [DOI] [PubMed] [Google Scholar]
- 70.Tanaka K, Fujita N, Higashi Y, et al. Neuroprotective and antioxidant properties of FKBP-binding immunophilin ligands are independent on the FKBP12 pathway in human cells. Neurosci Lett. 2002;330:147–150. doi: 10.1016/s0304-3940(02)00755-3. [DOI] [PubMed] [Google Scholar]
- 71.Gold BG, Villafranca JE. Neuroimmunophilin ligands: the development of novel neuroregenerative/ neuroprotective compounds. Curr Top Med Chem. 2003;3:1368–1375. doi: 10.2174/1568026033451880. [DOI] [PubMed] [Google Scholar]
- 72.Gold BG, Zhong YP. FK506 Requires Stimulation of the Extracellular Signal-Regulated Kinase 1/2 and the Steroid Receptor Chaperone Protein p23 for Neurite Elongation. Neurosignals. 2004;13:122–129. doi: 10.1159/000076565. [DOI] [PubMed] [Google Scholar]
- 73.Gold BG, Yew JY, Zeleny-Pooley M. The immunosuppressant FK506 increases GAP-43 mRNA levels in axotomized sensory neurons. Neurosci Lett. 1998;241:25–28. doi: 10.1016/s0304-3940(97)00960-9. [DOI] [PubMed] [Google Scholar]
- 74.Dawson TM, Steiner JP, Dawson VL, et al. Immunosuppressant FK506 enhances phosphorylation of nitric oxide synthase and protects against glutamate neurotoxicity. Proc Natl Acad Sci U S A. 1993;90:9808–9812. doi: 10.1073/pnas.90.21.9808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yardin C, Terro F, Lesort M, et al. FK506 antagonizes apoptosis and c-jun protein expression in neuronal cultures. Neuroreport. 1998;9:2077–2080. doi: 10.1097/00001756-199806220-00030. [DOI] [PubMed] [Google Scholar]
- 76.Gold BG, Gordon HS, Wang MS. Efficacy of delayed or discontinuous FK506 administrations on nerve regeneration in the rat sciatic nerve crush model: lack of evidence for a conditioning lesion-like effect. Neurosci Lett. 1999;267:33–36. doi: 10.1016/s0304-3940(99)00333-x. [DOI] [PubMed] [Google Scholar]
- 77.Sobol JB, Lowe IJ, Yang RK, et al. Effects of delaying FK506 administration on neuroregeneration in a rodent model. J Reconstr Microsurg. 2003;19:113–118. doi: 10.1055/s-2003-37817. [DOI] [PubMed] [Google Scholar]
- 78.Snyder AK, Fox IK, Nichols CM, et al. Neuroregenerative effects of preinjury FK-506 administration. Plast Reconstr Surg. 2006;118:360–367. doi: 10.1097/01.prs.0000227628.43867.5b. [DOI] [PubMed] [Google Scholar]
- 79.Fox IK, Mackinnon SE. Experience with nerve allograft transplantation. Semin Plast Surg. 2007;21:242–249. doi: 10.1055/s-2007-991194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brenner MJ, Mackinnon SE, Rickman SR, et al. FK506 and anti-CD40 ligand in peripheral nerve allotransplantation. Restor Neurol Neurosci. 2005;23:237–249. [PubMed] [Google Scholar]
- 81.Chunasuwankul R, Ayrout C, Dereli Z, et al. Low dose discontinued FK506 treatment enhances peripheral nerve regeneration. Int Surg. 2002;87:274–278. [PubMed] [Google Scholar]
- 82.Ellis RA, Brenner MJ, Mackinnon SE, et al. Use of mixed lymphocyte reaction to identify subimmunosuppressive FK-506 levels in mice. Microsurgery. 2003;23:276–282. doi: 10.1002/micr.10117. [DOI] [PubMed] [Google Scholar]
- 83.Udina E, Voda J, Gold BG, et al. Comparative dose-dependence study of FK506 on transected mouse sciatic nerve repaired by allograft or xenograft. J Peripher Nerv Syst. 2003;8:145–154. doi: 10.1046/j.1529-8027.2003.03020.x. [DOI] [PubMed] [Google Scholar]
- 84.Yang RK, Lowe JB, 3rd, Sobol JB, et al. Dose-dependent effects of FK506 on neuroregeneration in a rat model. Plast Reconstr Surg. 2003;112:1832–1840. doi: 10.1097/01.PRS.0000091167.27303.18. [DOI] [PubMed] [Google Scholar]
- 85.Udina E, Ceballos D, Verdu E, et al. Bimodal dose-dependence of FK506 on the rate of axonal regeneration in mouse peripheral nerve. Muscle Nerve. 2002;26:348–355. doi: 10.1002/mus.10195. [DOI] [PubMed] [Google Scholar]
- 86.Hayashi A, Moradzadeh A, Tong A, et al. Treatment modality affects allograft-derived Schwann cell phenotype and myelinating capacity. Exp Neurol. 2008;212:324–336. doi: 10.1016/j.expneurol.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sulaiman OA, Gordon T. Effects of short- and long-term Schwann cell denervation on peripheral nerve regeneration, myelination, and size. Glia. 2000;32:234–246. doi: 10.1002/1098-1136(200012)32:3<234::aid-glia40>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 88.Assoian RK, Fleurdelys BE, Stevenson HC, et al. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci U S A. 1987;84:6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ridley AJ, Davis JB, Stroobant P, et al. Transforming growth factors-beta 1 and beta 2 are mitogens for rat Schwann cells. J Cell Biol. 1989;109:3419–3424. doi: 10.1083/jcb.109.6.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chandross KJ, Chanson M, Spray DC, et al. Transforming growth factor-beta 1 and forskolin modulate gap junctional communication and cellular phenotype of cultured Schwann cells. J Neurosci. 1995;15:262–273. doi: 10.1523/JNEUROSCI.15-01-00262.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Einheber S, Hannocks MJ, Metz CN, et al. Transforming growth factor-beta 1 regulates axon/Schwann cell interactions. J Cell Biol. 1995;129:443–458. doi: 10.1083/jcb.129.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guenard V, Gwynn LA, Wood PM. Transforming growth factor-beta blocks myelination but not ensheathment of axons by Schwann cells in vitro. J Neurosci. 1995;15:419–428. doi: 10.1523/JNEUROSCI.15-01-00419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guenard V, Rosenbaum T, Gwynn LA, et al. Effect of transforming growth factor-beta 1 and - beta 2 on Schwann cell proliferation on neurites. Glia. 1995;13:309–318. doi: 10.1002/glia.440130407. [DOI] [PubMed] [Google Scholar]
- 94.Rosner BI, Hang T, Tranquillo RT. Schwann cell behavior in three-dimensional collagen gels: evidence for differential mechano-transduction and the influence of TGF-beta 1 in morphological polarization and differentiation. Exp Neurol. 2005;195:81–91. doi: 10.1016/j.expneurol.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 95.Krieglstein K, Henheik P, Farkas L, et al. Glial cell line-derived neurotrophic factor requires transforming growth factor-beta for exerting its full neurotrophic potential on peripheral and CNS neurons. J Neurosci. 1998;18:9822–9834. doi: 10.1523/JNEUROSCI.18-23-09822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schober A, Hertel R, Arumae U, et al. Glial cell line-derived neurotrophic factor rescues target-deprived sympathetic spinal cord neurons but requires transforming growth factor-beta as cofactor in vivo. J Neurosci. 1999;19:2008–2015. doi: 10.1523/JNEUROSCI.19-06-02008.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sulaiman OA, Gordon T. Transforming growth factor-beta and forskolin attenuate the adverse effects of long-term Schwann cell denervation on peripheral nerve regeneration in vivo. Glia. 2002;37:206–218. doi: 10.1002/glia.10022. [DOI] [PubMed] [Google Scholar]
- 98.Morrissey TK, Kleitman N, Bunge RP. Isolation and functional characterization of Schwann cells derived from adult peripheral nerve. J Neurosci. 1991;11:2433–2442. doi: 10.1523/JNEUROSCI.11-08-02433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Haastert K, Mauritz C, Chaturvedi S, et al. Human and rat adult Schwann cell cultures: fast and efficient enrichment and highly effective non-viral transfection protocol. Nat Protoc. 2007;2:99–104. doi: 10.1038/nprot.2006.486. [DOI] [PubMed] [Google Scholar]
- 100.Keilhoff G, Stang F, Goihl A, et al. Transdifferentiated mesenchymal stem cells as alternative therapy in supporting nerve regeneration and myelination. Cell Mol Neurobiol. 2006;26:1235–1252. doi: 10.1007/s10571-006-9029-9. [DOI] [PubMed] [Google Scholar]
- 101.Dezawa M, Takahashi I, Esaki M, et al. Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. Eur J Neurosci. 2001;14:1771–1776. doi: 10.1046/j.0953-816x.2001.01814.x. [DOI] [PubMed] [Google Scholar]
- 102.Tohill M, Mantovani C, Wiberg M, et al. Rat bone marrow mesenchymal stem cells express glial markers and stimulate nerve regeneration. Neurosci Lett. 2004;362:200–203. doi: 10.1016/j.neulet.2004.03.077. [DOI] [PubMed] [Google Scholar]
- 103.Chen CJ, Ou YC, Liao SL, et al. Transplantation of bone marrow stromal cells for peripheral nerve repair. Exp Neurol. 2007;204:443–453. doi: 10.1016/j.expneurol.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 104.Wakao S, Hayashi T, Kitada M, et al. Long-term observation of auto-cell transplantation in non-human primate reveals safety and efficiency of bone marrow stromal cell-derived Schwann cells in peripheral nerve regeneration. Exp Neurol. 2010;223:537–547. doi: 10.1016/j.expneurol.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 105.Kingham PJ, Kalbermatten DF, Mahay D, et al. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol. 2007;207:267–274. doi: 10.1016/j.expneurol.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 106.di Summa PG, Kingham PJ, Raffoul W, et al. Adipose-derived stem cells enhance peripheral nerve regeneration. J Plast Reconstr Aesthet Surg. 2010;63:1544–1552. doi: 10.1016/j.bjps.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 107.Erba P, Mantovani C, Kalbermatten DF, et al. Regeneration potential and survival of transplanted undifferentiated adipose tissue-derived stem cells in peripheral nerve conduits. J Plast Reconstr Aesthet Surg. 2010;63:e811–e817. doi: 10.1016/j.bjps.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 108.Fernandes KJ, McKenzie IA, Mill P, et al. A dermal niche for multipotent adult skin-derived precursor cells. Nat Cell Biol. 2004;6:1082–1093. doi: 10.1038/ncb1181. [DOI] [PubMed] [Google Scholar]
- 109.Toma JG, Akhavan M, Fernandes KJ, et al. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat Cell Biol. 2001;3:778–784. doi: 10.1038/ncb0901-778. [DOI] [PubMed] [Google Scholar]
- 110.McKenzie IA, Biernaskie J, Toma JG, et al. Skin-derived precursors generate myelinating Schwann cells for the injured and dysmyelinated nervous system. J Neurosci. 2006;26:6651–6660. doi: 10.1523/JNEUROSCI.1007-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Toma JG, McKenzie IA, Bagli D, et al. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells. 2005;23:727–737. doi: 10.1634/stemcells.2004-0134. [DOI] [PubMed] [Google Scholar]
- 112.Walsh S, Biernaskie J, Kemp SW, et al. Supplementation of acellular nerve grafts with skin derived precursor cells promotes peripheral nerve regeneration. Neuroscience. 2009;164:1097–1107. doi: 10.1016/j.neuroscience.2009.08.072. [DOI] [PubMed] [Google Scholar]
- 113.Walsh SK, Gordon T, Addas BM, et al. Skin-derived precursor cells enhance peripheral nerve regeneration following chronic denervation. Exp Neurol. 2010;223:221–228. doi: 10.1016/j.expneurol.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 114.Walsh S, Midha R. Practical considerations concerning the use of stem cells for peripheral nerve repair. Neurosurg Focus. 2009;26:E2. doi: 10.3171/FOC.2009.26.2.E2. [DOI] [PubMed] [Google Scholar]
- 115.Brannvall K, Corell M, Forsberg-Nilsson K, et al. Environmental cues from CNS, PNS, and ENS cells regulate CNS progenitor differentiation. Neuroreport. 2008;19:1283–1289. doi: 10.1097/WNR.0b013e32830bfba4. [DOI] [PubMed] [Google Scholar]
- 116.Jabaley ME, Wallace WH, Heckler FR. Internal topography of major nerves of the forearm and hand: a current view. J Hand Surg Am. 1980;5:1–18. doi: 10.1016/s0363-5023(80)80035-9. [DOI] [PubMed] [Google Scholar]
- 117.Brown JM, Yee A, Mackinnon SE. Distal median to ulnar nerve transfers to restore ulnar motor and sensory function within the hand: technical nuances. Neurosurgery. 2009;65:966–977. doi: 10.1227/01.NEU.0000358951.64043.73. discussion 977-968. [DOI] [PubMed] [Google Scholar]
- 118.Huang G. Experimental reconstruction on intrinsic hand muscle function by anterior interosseous nerve transference. Zhonghua Yi Xue Za Zhi. 1992;72:269–272. 318. [PubMed] [Google Scholar]
- 119.Wang Y, Zhu S. Transfer of a branch of the anterior interosseus nerve to the motor branch of the median nerve and ulnar nerve. Chin Med J (Engl) 1997;110:216–219. [PubMed] [Google Scholar]
- 120.Vernadakis AJ, Humphreys DB, Mackinnon SE. Distal anterior interosseous nerve in the recurrent motor branch graft for reconstruction of a median nerve neuroma-in-continuity. J Reconstr Microsurg. 2004;20:7–11. doi: 10.1055/s-2004-818043. [DOI] [PubMed] [Google Scholar]
- 121.Novak CB, Mackinnon SE. Distal anterior interosseous nerve transfer to the deep motor branch of the ulnar nerve for reconstruction of high ulnar nerve injuries. J Reconstr Microsurg. 2002;18:459–464. doi: 10.1055/s-2002-33326. [DOI] [PubMed] [Google Scholar]
- 122.Ustun ME, Ogun TC, Buyukmumcu M, et al. Selective restoration of motor function in the ulnar nerve by transfer of the anterior interosseous nerve. An anatomical feasibility study. J Bone Joint Surg Am. 2001;83-A:549–552. doi: 10.2106/00004623-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 123.Ustun ME, Ogun TC, Karabulut AK, et al. An alternative method for restoring opposition after median nerve injury: an anatomical feasibility study for the use of neurotisation. J Anat. 2001;198:635–638. doi: 10.1046/j.1469-7580.2001.19850635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang Y, Zhu S, Zhang B. Anatomical study and clinical application of transfer of pronator quadratus branch of anterior interosseous nerve in the repair of thenar branch of median nerve and deep branch of ulnar nerve. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 1997;11:335–337. [PubMed] [Google Scholar]
- 125.Brown JM, Mackinnon SE. Nerve transfers in the forearm and hand. Hand Clin. 2008;24:319–340. doi: 10.1016/j.hcl.2008.08.002. v. [DOI] [PubMed] [Google Scholar]