Abstract
Adipocytes have been studied with increasing intensity as a result of the emergence of obesity as a serious public health problem and the realization that adipose tissue serves as an integrator of various physiological pathways. In particular, their role in calorie storage makes adipocytes well suited to the regulation of energy balance. Adipose tissue also serves as a crucial integrator of glucose homeostasis. Knowledge of adipocyte biology is therefore crucial for understanding the pathophysiological basis of obesity and metabolic diseases such as type 2 diabetes. Furthermore, the rational manipulation of adipose physiology is a promising avenue for therapy of these conditions.
Adipose tissue has been ignored by anatomists and physicians for centuries, considered to be an energy storage depot with few interesting attributes. The well-documented rise in obesity during the past 30 years1 has contributed to the negative image of adipose tissue, particularly in the popular mind. The past two decades, however, have seen a wave of intense scientific interest in this cell type, fuelled in part by concerns about obesity and its attendant metabolic sequelae2, and also by the recognition that adipocytes integrate a wide array of homeostatic processes. In addition to regulating fat mass and nutrient homeostasis (discussed below), adipocytes are involved in the immune response, blood pressure control, haemostasis, bone mass, and thyroid and reproductive function3. These processes are coordinated mainly through the synthesis and release of peptide hormones by adipocytes.
Adipocytes also release fatty acids into the circulation, which are used by most organs for fuel when glucose is limiting. These fatty acids are generated by breaking down triacylglycerols, which contain more energy per unit mass than do carbohydrates and can essentially be stored anhydrously. By contrast, glycogen has only the half the energy content per unit of pure mass, and must be stored in association with water, further decreasing its efficiency.
Although most multicellular organisms have cells that store excess energy, adipocytes evolved to meet this need at the time of the vertebrate radiation. Mammals, birds, reptiles, amphibians and many (but not all) fish have cells that are readily identifiable as adipocytes, although the anatomical location of fat tissues varies considerably between species4. Most mammals have stereotypical adipose depots located throughout the body. Some of these depots are thought to be largely structural, providing mechanical support but contributing relatively little to energy homeostasis. Examples include the fat pads of the heels, fingers and toes, and the periorbital fat supporting the eyes. Other adipocytes exist in loose association with the skin, and have been termed subcutaneous fat. These cells are the cause of ‘cellulite’, and are the target of cosmetic procedures such as liposuction. Finally, there are several distinct depots within the body cavity, surrounding the heart and other organs, associated with the intestinal mesentery, and in the retroperitoneum. Some of these depots, known as visceral fat, drain directly into the portal circulation and have been linked to many of the morbidities associated with obesity, including type 2 diabetes and cardiovascular disease. Adipocytes and precursor cells from different depots have different replicative potential, different developmental attributes and different responses to hormonal signals, although the mechanistic basis for these distinctions is still unclear5.
In addition to depot-specific differences, a further distinction must be made between brown and white adipocytes. Brown adipocytes are found only in mammals, and differ from the more typical white adipocytes in that they express uncoupling protein-1 (UCP-1), which dissipates the proton gradient across the inner mitochondrial membrane that is produced by the action of the electron transport chain. This generates heat at the expense of ATP. Morphologically, brown adipocytes are multilocular and contain less overall lipid than their white counterparts, and are particularly rich in mitochondria. Rodents have a distinct brown fat pad, which lies in the interscapular region. In humans, brown adipose tissue surrounds the heart and great vessels in infancy but tends to disappear over time until only scattered cells can be found within white fat pads.
This review briefly examines the transcriptional basis of adipocyte development, and then discusses energy homeostasis in mammals and how adipocytes regulate components of that system. The second part of the review provides a similar look at the role of adipose tissue in glucose homeostasis. Adipocytes have a crucial role in regulating both of these physiological processes through a series of endocrine and non-endocrine mechanisms. These involve a widening array of adipose-derived secreted molecules (known as adipokines), neural connections and changes in whole-body physiology wrought by primary alterations in adipocyte cellular metabolism.
Transcriptional regulation of adipocyte differentiation
Adipocytes have been a popular model for the study of cell differentiation since the development of the murine adipose 3T3 cell culture system by Green and colleagues6. There have been several thorough reviews on this aspect of adipose biology recently7, 8, so we present only the core of this regulatory system. The central engine of adipose differentiation is peroxisome proliferator-activated receptor-γ (PPAR-γ)9–11. When this receptor is activated by an agonist ligand in fibroblastic cells, a full programme of differentiation is stimulated, including morphological changes, lipid accumulation and the expression of almost all genes characteristic of fat cells. Multiple CCAAT/enhancer-binding proteins (C/EBPs) also have a critical role in adipogenesis, with C/EBP-β and C/EBP-δ driving PPAR-γ expression in the early stages of differentiation and C/EBPα maintaining PPAR-γ expression later on in the process7. C/EBPs and PPAR-γ also directly activate many of the genes of terminally differentiated adipocytes. More recently, other factors have been implicated in the differentiation process, including several Krüppel-like factors12–14, early growth response 2 (Krox20)15 and early B-cell factors16.
This transcriptional cascade seems to function in both white and brown adipogenesis. However, ectopic expression of PPAR-γ or C/EBP proteins in fibroblasts results in the formation of adipocytes with the characteristics of white fat cells, with little or no expression of UCP-1 even when stimulated with β-adrenergic agents. Relatively little is known about brown fat differentiation, except for the important role of transcriptional cofactors, including pRb (ref. 17), p107 (ref. 18), SRC-1 and TIF2 (ref. 19), and PPAR-γ-coactivator-1α (PGC-1α)20. PGC-1α coactivates PPAR-γ and other transcription factors, is expressed at much higher levels in brown adipocytes than in white adipocytes, and is induced in brown cells upon cold exposure in vivo, or by β-adrenergic stimulation in isolated cells20,21. When introduced into white fat cells in culture or in vivo, PGC-1α ‘switches on’ many of the key features of brown cells, including mitochondrial biogenesis and UCP-1 activity20. Cells ectopically expressing PGC-1α also have an increased fraction of uncoupled respiration, a key characteristic of brown fat. Although PGC-1α is clearly a key effector of the thermogenic programme of brown fat, cells lacking PGC-1α still show a morphology that is brown-fat-like, and still express certain molecular markers of brown fat22. Thus, it seems likely that other factors function upstream of PGC-1α to control the determination of brown fat cells.
Principles of energy balance
Energy balance in animals is governed by the First Law of Thermo dynamics, and is often expressed as a simple equation:
| (1) |
Lipid storage in adipose tissue thus represents excess energy consumption relative to energy expenditure (Fig. 1). Although fundamentally true, this simple representation overlooks a few key features of energy homeostasis in vivo. First, although food intake is relatively easy to measure, it is not the precise parameter that determines the amount of energy brought into the system. The efficiency of calorie absorption in the gut, which is much more difficult to measure and is usually ignored in practice, must also be accounted for. A second consideration is that the body’s response to alterations in energy input or expenditure is not static. In general, energy homeostasis is regulated to defend the highest weight achieved23. Thus voluntary reductions in food intake are countered by involuntary reductions in energy expenditure, making weight loss more difficult than a simple interpretation of equation (1) would indicate. Overall, energy balance is responsive to various factors, including hormones and neural inputs, in addition to psychological and cultural factors24.
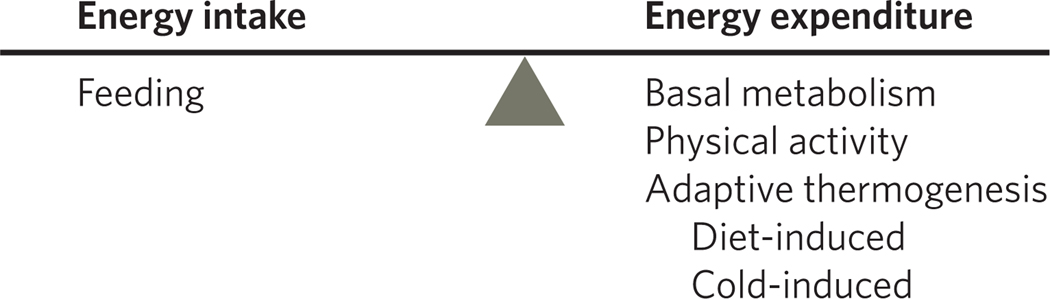
Figure 1. Energy homeostasis depends upon the balance between caloric intake and energy expenditure.
Although caloric intake is almost entirely due to the consumption of food (minus whatever fails to be absorbed), energy expenditure has more components, including basal metabolism, physical activity (voluntary and involuntary) and adaptive thermogenesis. The last category includes the small amount of energy spent in absorbing and processing the diet (known as diet-induced thermogenesis) as well as energy spent to maintain body temperature in the face of cold.
Adipocytes and energy balance
Adipose tissue contains most of the energy stores in normal healthy humans. An important but underappreciated fact is that having more fat cells does not make an animal fatter. In the absence of altered energy balance, an increase in adipogenesis will result in smaller fat cells with no change in total adiposity. Conversely, a reduction in adipocyte number without a change in energy balance would result in larger fat cells, but not less total adipose mass. For example, surgical removal of fat can have cosmetic effects but does not change the energy balance equation. Careful studies in animals have shown that total body fat usually recovers after surgical removal of fat pads25. If certain depots are removed entirely, the fat usually increases at other anatomical locations, though careful clinical studies have not yet been reported in humans. The fact that adipocyte differentiation does not in itself cause obesity does not mean that adipocytes have no role in energy balance. In fact, adipose tissues are critical integrators of organismal energy balance, through the regulation of both food intake and energy expenditure. These effects are mediated by both endocrine and non-endocrine means.
Leptin
Leptin was the first adipokine discovered to have a role in modulating adiposity, and it remains the best studied26–29. Leptin is secreted almost exclusively by fat, and serves as a major ‘adipostat’ by repressing food intake and promoting energy expenditure. Predictably, animals and humans with mutations in either leptin or the leptin receptor are obese. The leptin receptor is expressed at low levels in numerous tissues, but is found at high levels in the mediobasal hypothalamus, particularly the arcuate nucleus, the ventromedial nucleus and the dorsomedial nucleus30–32 (Fig. 2). Leptin receptor activation at these sites leads to repression of orexigenic pathways (for example, those involving neuropeptide Y, NPY, and agouti-related peptide, AgRP) and induction of anorexigenic pathways (such as those involving pro-opiomelanocortin, POMC, and cocaine and amphetamine-regulated transcript, CART). Leptin-dependent effects on food intake and energy expenditure ultimately diverge in the central nervous system (CNS), partly at the level of melanocortin MC4 receptor signalling33. Recently, increased attention has been paid to leptin action in non-hypothalamic sites such as the caudal brainstem34.
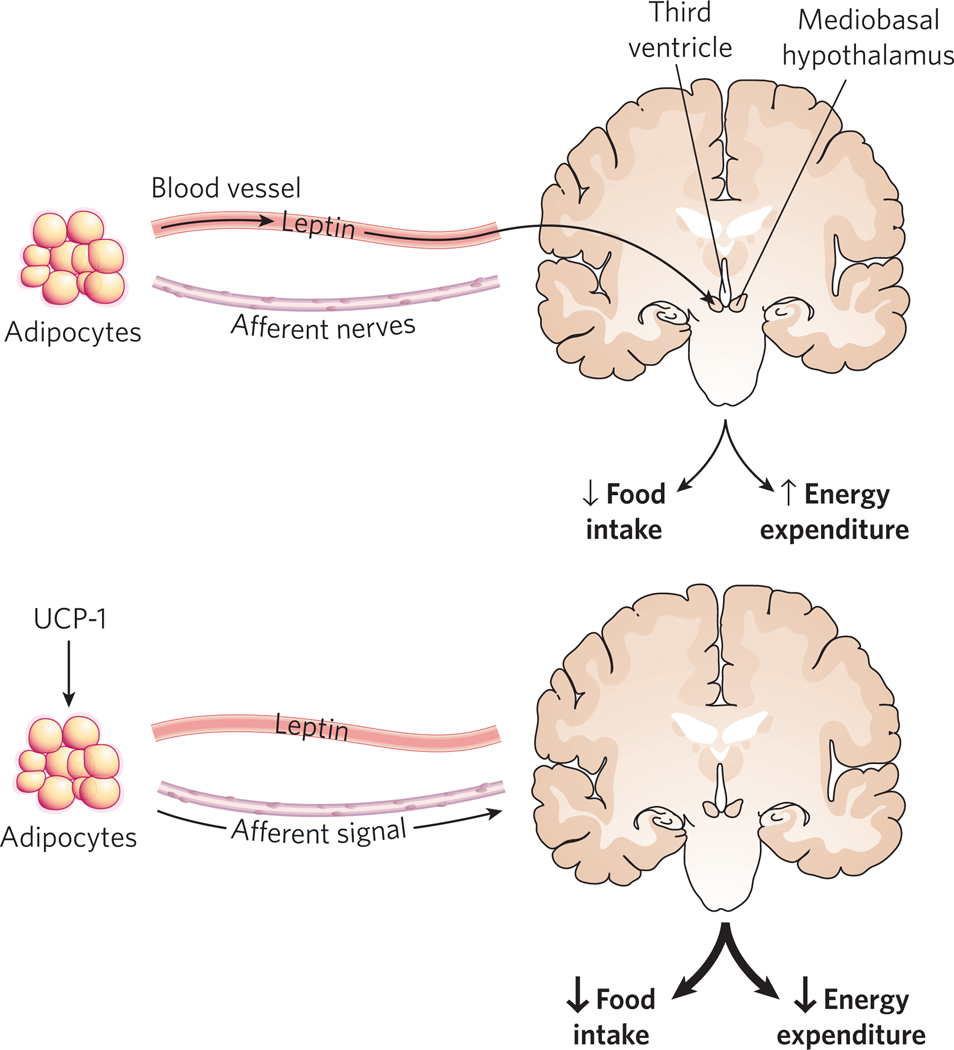
Figure 2. Adipocytes regulate energy balance by endocrine and non-endocrine mechanisms.
Adipocytes synthesize and secrete leptin, which circulates in the blood and acts on the CNS (primarily the hypothalamus) to reduce food intake and enhance energy expenditure. Adipose tissue also communicates with the CNS by means of a rich network of peripheral nerves, which can transmit afferent signals about energy status to the brain, such as when UCP-1 is expressed ectopically. This results in enhanced leptin sensitivity, which increases the effect of secreted leptin on energy balance.
Neural pathways
Fat pads are richly innervated by sympathetic fibres, and activation of these fibres is associated with enhanced lipolysis35,36. These nerves also regulate fat pad cellularity — denervation of specific depots results in a twofold elevation in adipocyte number in hamsters and rats35. Recent studies have also suggested that neural afferent signals from adipose tissue to the brain might also regulate adiposity. Direct introduction of low levels of UCP-1 into white adipose tissue causes a marked increase in leptin sensitivity in mice, with a concomitant reduction in food intake, and enhanced energy expenditure and weight loss37 (Fig. 2). This effect is abrogated entirely when the manipulated fat pad is denervated. The actual parameter being monitored by the adipocyte is unclear, but might include reactive oxygen species (ROS), ATP levels or even local heat production.
Alterations in adipocyte metabolism
Another way in which adipocytes can modulate whole-body energy balance is through alterations in their own metabolism. This is perhaps best understood for brown fat. Brown adipocytes are highly specialized to perform uncoupled respiration, which dissipates chemical energy in the form of heat. In classical mitochondrial dynamics, fuel oxidation is linked to electron transport, resulting in the development of an electrochemical gradient across the inner mitochondrial membrane. This gradient, which is formed by the extrusion of protons across the inner mitochondrial membrane by three protein complexes in the electron transport system, is normally dissipated at complex V, which couples proton inflow to ATP synthesis. Brown fat, however, expresses UCP-1, which allows protons to flow back across the inner mitochondrial membrane without concomitant ATP synthesis, resulting in heat generation. In rodents, brown adipose tissue makes a substantial contribution to whole-body energy metabolism. Mice lacking brown adipose tissue exhibit reduced energy expenditure and are prone to diet-induced obesity38,39. Interestingly, mice lacking UCP-1 are cold-sensitive but not obese40. Taken together, these genetic models suggest that brown adipose tissue might influence whole-body energy metabolism in ways not fully explained by the expression of UCP-1. It is clear, however, that overexpression of UCP-1 in white adipose tissue results in reduced adiposity. As mentioned earlier, at least part of this effect is neurally mediated37.
Enhancement of fatty acid oxidation in white adipocytes (without uncoupling) has also been proposed to reduce adiposity. Early examples of genetically altered mice with enhanced adipocyte fatty-acid oxidation and concomitant leanness were identified in global knockouts, making it difficult to pinpoint altered metabolism in adipose tissue as the primary cause of the leanness41. In one example, ablation of the transcriptional co-repressor receptor-interacting protein 140 (RIP140) results in lean mice with enhanced fatty-acid oxidation and oxygen consumption associated with increased UCP-1 expression in white adipocytes42. Another recent study showed that adipose-specific targeting of the pseudokinase TRB3 results in enhanced lipolysis and fatty-acid oxidation, with subsequent protection from diet-induced obesity43. This effect is caused by the TRB3-mediated ubiquitination and degradation of acetyl-coenzyme-A carboxylase (ACC), which catalyses the rate-limiting step in lipogenesis. Enhanced lipolysis alone is insufficient to promote weight loss; the liberated fatty acids must be oxidized. Furthermore, oxidation alone is also insufficient unless the ATP generated is consumed. Thus, the observed weight loss in the Trb3 transgenic mice leads us to infer that there is undetected uncoupling in the mitochondria or that an ATP-consuming process has been activated.
Principles of glucose homeostasis
Despite intermittent ingestion of dietary carbohydrates, serum glucose levels remain relatively steady throughout the day. This requires the concerted actions of several different tissues44,45 (Fig. 3). Pancreatic β-cells, for example, secrete insulin in response to the elevations in glucose that occur after eating. Insulin promotes glucose disposal in adipose tissue and muscle, and also prevents the liver from producing more glucose by suppressing glycogenolysis and gluconeogenesis. In the fasting state, low insulin levels combined with elevated counter-regulatory hormones such as glucagon, adrenaline and corticosteroids promote hepatic glucose production. Recently, evidence has emerged that the brain coordinates many of these effects as well, through direct and indirect glucose sensing and neural outputs to peripheral organs44.
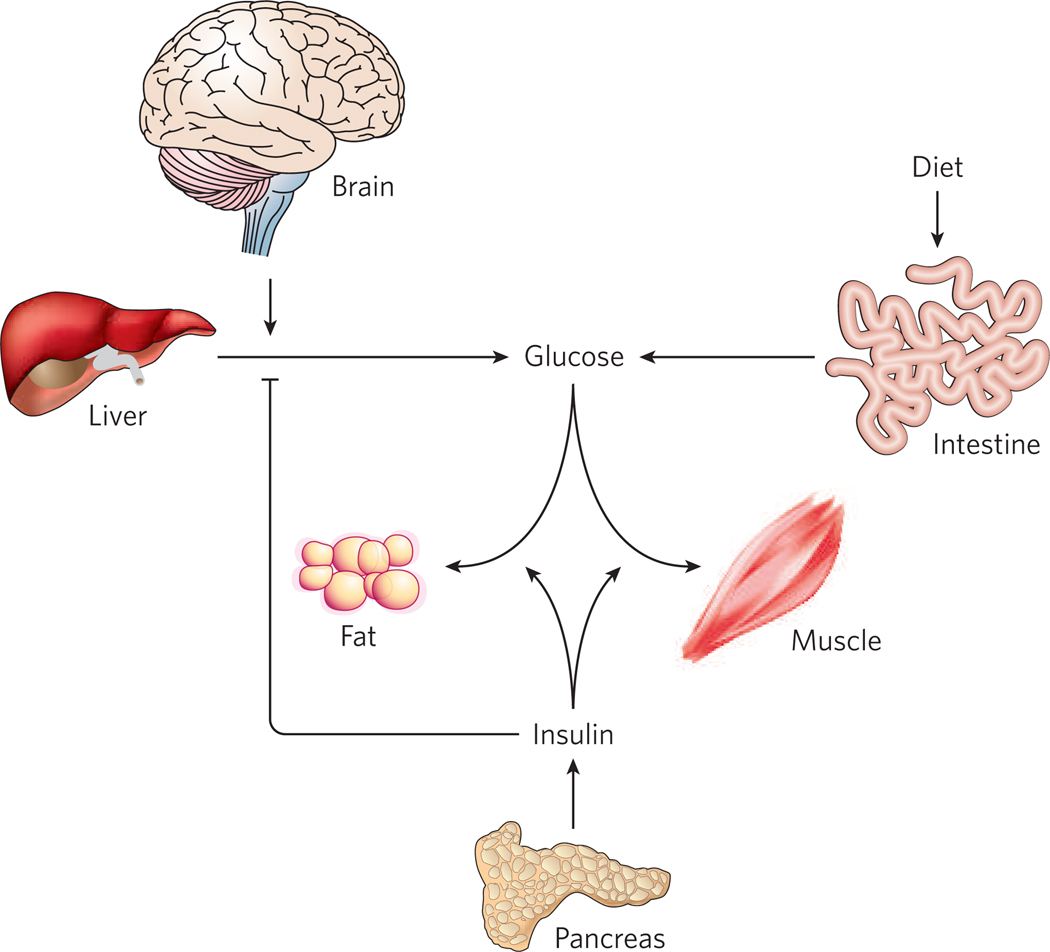
Figure 3. Glucose homeostasis requires the coordinated actions of various organs.
Inputs to serum glucose levels include absorption from the intestine and release from the liver. The latter occurs by breakdown of preformed glycogen as well as gluconeogenesis, and both processes are inhibited by insulin. Glucose is removed from the system by uptake into virtually all cell types, but most importantly into muscle and adipose tissue, which requires insulin. Recent evidence suggests that the CNS can also sense glucose and act to affect systemic glycaemia, at least in part by regulating gluconeogenesis.
Adipocytes as regulators of glucose homeostasis
At first, the idea that adipose tissue could have a considerable effect on global glycaemic control was not easy to accept. Early studies determined that adipose tissue accounts for only a fraction of glucose disposal after a meal (about 10–15%), with most of the rest taken up by muscle46. Nonetheless, it was equally clear that alterations in adiposity have profound implications for glucose homeostasis; too much fat (obesity) and too little fat (lipodystrophy) are both associated with insulin resistance and hyperglycaemia. In addition, PPAR-γ ligands such as thiazolidinedione (TZD) drugs have excellent anti-diabetic activity despite the fact that most PPAR-γ is found in adipose tissue and not muscle. We now understand that the profound effect of adipocytes on glucose balance is mediated by several different mechanisms, which can be loosely categorized (as before with energy balance) as endocrine (Fig. 4) and non-endocrine.
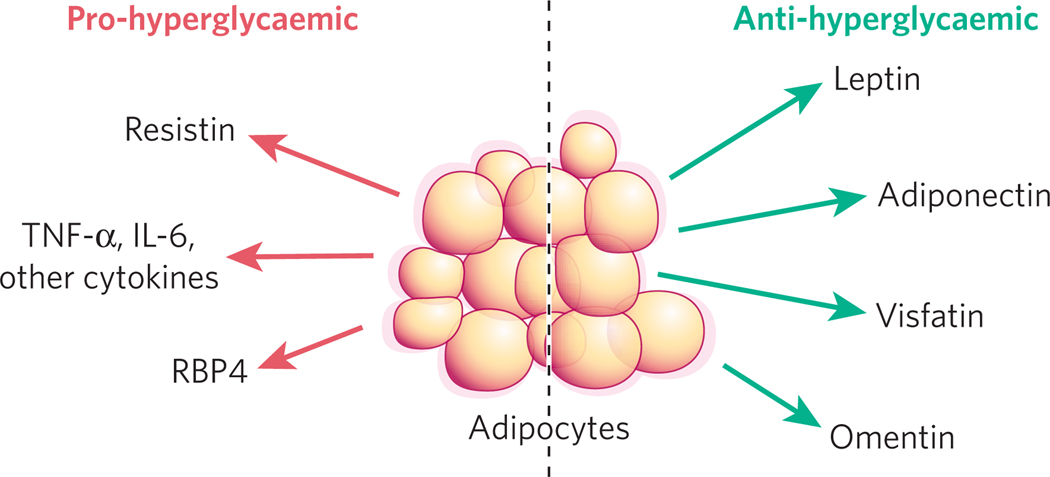
Figure 4. Adipocytes secrete proteins with varied effects on glucose homeostasis.
Adipocyte-derived proteins with anti-diabetic action (green arrows) include leptin, adiponectin, omentin and visfatin. Other factors tend to raise blood glucose (red arrows), including resistin, TNF-α and RBP4. TNF-α and human resistin are probably secreted by non-adipocytes within the fat pad. IL, interleukin.
Leptin
As described above, leptin is a multifunctional protein secreted primarily by adipocytes. In addition to its well-described role in energy balance, leptin has notable effects on glucose homeostasis, as it reverses hyperglycaemia in ob/ob mice before body weight is corrected29. Similarly, pair feeding ob/ob mice to match control animals does not restore glucose tolerance as well as exogenous leptin does47. Leptin also improves glucose homeostasis in lipodystrophic mice, and in humans with lipodystrophy or congenital leptin deficiency48–50. Results in humans with ‘typical’ obesity have been disappointing in this regard51. However, this is consistent with the high levels of leptin and apparent leptin resistance seen in these patients.
The anti-hyperglycaemic actions of leptin are mediated through several different organs. First, leptin improves insulin sensitivity in muscles. Leptin also reduces intra-myocellular lipid levels through a combination of direct activation of AMP-activated protein kinase (AMPK) and indirect actions mediated through central neural pathways52. The effect on lipid partitioning might help to explain the improved insulin sensitivity, according to the current idea that intracellular lipids contribute to insulin resistance. Leptin also improves insulin sensitivity in the liver, an effect seen with either peripheral or intracerebroventricular administration53. As in muscles, leptin functions in part to reduce hepatic intracellular triacylglycerol levels. There has also been discussion of an ‘adipo-insular axis’, with insulin promoting leptin secretion and leptin inhibiting insulin release54. Consistent with this idea, ablation of leptin receptors from β-cells results in enhanced basal insulin secretion and fasting hypoglycaemia55. The effect of leptin on insulin levels is due to inhibition of proinsulin synthesis as well as reduction of secretion54.
Adiponectin
The hormone adiponectin was identified by several different groups and given various names (for example, apM1, GBP28, AdipoQ and ACRP30)56. This 30-kDa protein has an amino-terminal collagen-like domain and a carboxy-terminal globular domain that mediates multimerization. Circulating adiponectin can exist as a trimer, hexamer or a higher-order multimer with 12–18 subunits57,58; there is some controversy about which is the active form of the hormone. Two types of receptor have been proposed. One comprises two similar transmembrane proteins with homology to G-protein-coupled receptors, known as adipoR1 and adipoR2 (ref. 59); a second molecule (T-cadherin) without a transmembrane domain has been proposed to act as a co-receptor for the high-molecular-weight form of adiponectin on endothelial and smooth muscle cells60. Interestingly, adiponectin circulates at extraordinarily high concentrations (5–10 µg ml−1), accounting for 0.01% of all plasma protein61. Unlike almost all other adipokines, however, adiponectin levels are inversely correlated with body mass62,63, for reasons that remain obscure.
Delivery of adiponectin to obese, diabetic mice stimulates AMP kinase activity in the liver and skeletal muscle, with profound effects on fatty acid oxidation and insulin sensitivity. Loss-of-function models of adiponectin have been more variable, with different groups reporting reduced insulin sensitivity on both chow and high-fat diets, high-fat diet only, or no effect on insulin action at all64–66. The reason for these differences is unknown. Two groups have shown that part of the anti-diabetic action of TZDs requires adiponectin67,68; TZDs significantly raise plasma adiponectin levels through effects on synthesis and secretion69. Adiponectin has no effect on insulin secretion in islets from healthy mice or humans, but does enhance glucose-stimulated insulin secretion in islets from mice with diet-induced obesity70.
Visfatin
The connection between visceral adiposity and insulin resistance has led several groups to try to identify secreted products derived specifically from this depot. The first such protein reported was visfatin, which had been identified in immune cells years earlier as pre-B-cell colony-enhancing factor (PBEF)71. There were several surprising aspects surrounding this discovery, including the fact that visfatin does not promote insulin resistance — on the contrary, it has a salutary effect on glucose uptake mediated by direct binding and activation of the insulin receptor. Visfatin circulates at concentrations well below those of insulin (<10%), however, and fasting and feeding do not regulate its expression, making it doubtful that visfatin alone is an important factor in insulin-receptor signalling. Other aspects of visfatin biology require further study. For example, serum levels of visfatin are variably correlated with type 2 diabetes and other insulin-resistant states72. Visfatin also has no signal sequence and has been shown to have enzymatic activity as a nicotinamide phosphoribosyltransferase with residence in the nucleus and cytosol73. It is not clear whether there is regulated secretion of visfatin or whether serum levels reflect leakage from dead or damaged cells.
Omentin
Omentin is another peptide secreted predominantly by visceral fat. Like visfatin, it has positive effects on glucose uptake, although omentin works as an insulin sensitizer and does not have insulin-mimetic properties74. Unlike visfatin, omentin seems to be made by stromal-vascular cells within the fat pad rather than adipocytes. Interestingly, omentin is produced in considerable quantities by adipose tissue in humans and macaques but not mice74. Omentin’s mechanism of action, including target tissues, a receptor or relevant signal transduction pathways, remains obscure.
Tumour necrosis factor-α and other cytokines
Tumour necrosis factor-α (TNF-α) was the first secreted adipose protein to be shown to have effects on glucose homeostasis75. TNF-α levels are elevated in obesity and in other insulin-resistant states (such as sepsis), addition of TNF-α to cells and mice reduces insulin action, and blockade of TNF-α action by biochemical or genetic means restores insulin sensitivity in vivo and in vitro. Interestingly, TNF-α seems to be derived from cell types other than adipocytes themselves. Macrophages in particular have been implicated in TNF-α production from murine fat pads76. Other cytokines, including interleukin-6, are produced by adipocytes, and there is conflicting evidence suggesting that they have both insulin-resistance-promoting and insulin-sensitizing effects77,78. Such ‘adipocytokines’ can promote insulin resistance through several mechanisms. These include c-Jun N-terminal kinase 1 (JNK1)-mediated serine phosphorylation of insulin receptor substrate-1 (IRS-1)79, IκB kinase (IKK)-mediated nuclear factor-κB (NF-κB) activation80, induction of suppressor of cytokine signalling 3 (SOCS3)81 and production of ROS82.
Resistin
Resistin is another small inflammatory molecule with hyperglycaemic action. Resistin (also known as FIZZ3) is one of a family of cysteine-rich resistin-like molecules (RELMs)83. Resistin was discovered as a secreted product of mouse adipocytes that was repressed by TZDs84. Levels of resistin are elevated in many murine models of obesity, and gain- and loss-of-function studies in rodents support a role for resistin in increasing hepatic glucose output85,86. Resistin might also be involved in reducing glucose uptake by muscles and fat, but this is not as robust an effect as that seen in the liver. As with adiponectin, there are several multimeric forms of resistin circulating in plasma, and action at the cellular level seems to depend on species with lower molecular weight87. There is considerable controversy surrounding the role of resistin in humans, in whom even the cellular source of resistin has been debated. The bulk of the data suggest that human resistin is the product of macrophages or other stromal cells within the fat pad88,89.
Retinol-binding protein 4
Specific ablation of glucose transporter 4 (Glut4) in mouse adipose tissue was shown to significantly reduce whole-body insulin sensitivity, whereas transgenic overexpression of Glut4 had the opposite effect. Because the magnitude of the effect was greater than that predicted by altered glucose flux into adipose tissue alone, an endocrine effect was postulated90. Transcriptional profiling of samples from these mice led to the discovery that a secreted member of the lipocalin superfamily, retinol-binding protein 4 (RBP4), was coordinately regulated by the changes in adipocyte GLUT4 levels. Subsequent studies showed that overexpression of RBP4 impairs hepatic and muscle insulin action, and Rbp4−/− mice show enhanced insulin sensitivity90. Fenretinide, a drug that enhances RBP4 clearance, also increases insulin sensitivity in mice fed a high-fat diet. Furthermore, high serum RBP4 levels are associated with insulin resistance in obese humans and those with type 2 diabetes as well as in lean, nondiabetic people with a family history of diabetes91. Much remains to be learned about how RBP4 impairs insulin action, and at present it is not clear whether the process involves the retinoid ligand or some other mechanism.
Non-esterified fatty acids
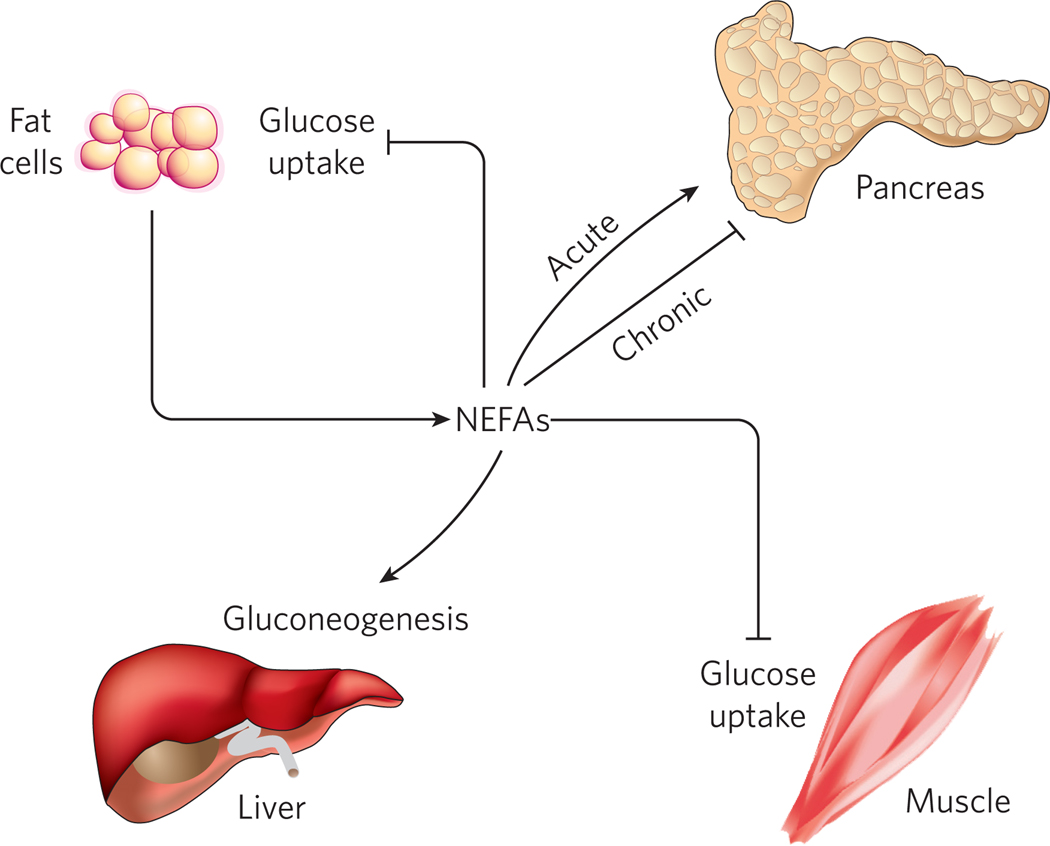
Although the concept of adipokines is relatively new, non-esterified fatty acids (NEFAs) have long been known to be an adipocyte-derived secreted product. NEFAs are primarily released during fasting as a nutrient source for the rest of the body, and have several actions on glucose homeostasis (Fig. 5). Circulating NEFAs reduce adipocyte and muscle glucose uptake, and also promote hepatic glucose output92,93. The net effect of these actions is to promote lipid burning as a fuel source in most tissues, while sparing carbohydrate for neurons (and red blood cells), which depend on glucose as an energy source. Several mechanisms have been proposed to account for the effects of NEFAs on muscle, liver and adipose tissue, including protein kinase C (PKC) activation94,95, oxidative stress96, ceramide formation97 and activation of the innate immune system98,99. These pathways are not mutually exclusive, and probably function interdependently.
Figure 5. Adipocyte-derived non-esterified fatty acids have several effects on glucose homeostasis.
Lipolysis in adipocytes is repressed by insulin, so it is normal in the fasted state when insulin levels are low. Insulin resistance, however, is also associated with lipolysis and NEFA release into the circulation. Elevated serum NEFAs inhibit insulin’s ability to promote peripheral glucose uptake into muscle and fat and to reduce hepatic glucose production. Transiently elevated NEFAs (such as occur after a meal) tend to enhance insulin secretion, whereas chronic elevations in NEFAs (such as occur in insulin resistance) tend to reduce insulin secretion.
Because lipolysis in adipocytes is repressed by insulin, insulin resistance from any cause can lead to NEFA elevation, which, in turn, induces additional insulin resistance as part of a vicious cycle. β-cells are also affected by NEFAs, depending in part on the duration of exposure; acutely, NEFAs induce insulin secretion (as after a meal), whereas chronic exposure to NEFAs causes a decrease in insulin secretion100. This effect is mediated by several mechanisms, including lipotoxicity-induced apoptosis of islet cells101. In β-cells that escape apoptosis, NEFAs decrease glucose sensing by inducing expression of uncoupling protein-2 (UCP-2), which decreases mitochondrial membrane potential, ATP synthesis and insulin secretion101.
Lipid sink
Another way, albeit indirect, in which adipose tissue can affect global glucose homeostasis is by serving as a lipid sink. This idea has emerged from the observation that animals and humans with lipodystrophy (in which adipose tissue fails to develop properly) have ectopic lipid deposition in the liver, muscles, β-cells and other organs, which can lead to insulin resistance and decreased insulin secretion102. The ability to store large amounts of esterified lipid in a manner that is not toxic to the cell or the organism as a whole might be one of the most critical functions of the adipocyte. However, the ability of exogenous leptin49,50, alone or in combination with adiponectin103, to improve whole-body glucose homeostasis in lipodystrophy suggests that the endocrine function of adipose tissue is also crucial to the regulation of glucose homeostasis.
Inflammation and adipose tissue in obesity
There is now overwhelming evidence that obesity and type 2 diabetes are inflammatory states, consistent with the production of TNF-α and other cytokines by adipose tissue, as described above. In obese animals and humans, bone-marrow-derived macrophages are recruited to the fat pad under the influence of proteins secreted by adipocytes, including macrophage chemoattractant protein-1 (MCP-1)76,104. Targeted ablation of either MCP-1 or its receptor reduces macrophage infiltration of fat depots and improves insulin sensitivity despite causing no change in body weight105, and overexpression of MCP-1 has the opposite effect106,107. These data, combined with studies (mentioned above) that indicate a role for pro-inflammatory cytokines in insulin resistance, strongly suggest that intra-adipose macrophages are dominant contributors to the insulin resistance that is associated with obesity. Thiazolidinediones, which are PPAR-γ agonists used clinically as insulin sensitizers, reduce MCP-1 levels and macrophage infiltration into adipose tissue108.
Alterations in adipocyte metabolism
Genetic manipulations of adipocyte metabolism can have consequences for global glucose homeostasis. Many of these manipulations affect fat mass, which accounts for the observed changes in insulin sensitivity. But the results of some experiments cannot be explained by an effect on adiposity. For example, transgenic overexpression of Glut4 in adipocytes improves insulin sensitivity (despite a slight increase in body weight)109, whereas adipose-specific Glut4-knockout mice are insulin resistant110. This effect is at least partly explained by changes in RBP4 (see above), although it is not clear whether RBP4 accounts for the full effect. Similarly, the direct injection of UCP-1 into limited areas of white adipose tissue is sufficient to improve whole-body glucose tolerance, an effect that is not neurally mediated and is not dependent on leptin signalling37. An endocrine mechanism is proposed here, but adiponectin levels seem unchanged in these mice, and, so far, a specific effector adipokine has not been discovered.
Future directions
The past decade has seen adipose tissue move from being a minor participant to having a central role in diverse homeostatic processes, with particular emphasis on energy balance and glucose homeostasis. The plethora of ways in which adipose tissue controls body weight and glycaemia has led to the idea that manipulation of adipocyte biology might be a useful therapeutic strategy in metabolic diseases such as obesity and type 2 diabetes. However, despite the clear connection between excess adiposity and a constellation of health problems, including insulin resistance, dyslipidaemia and cardiovascular disease, it is clear that simply reducing fat cell number is not a viable strategy to promote health. Surgical removal of adipose tissue does not improve metabolic parameters in obese animals or humans. Furthermore, subjects with lipodystrophy are affected by insulin resistance, hyperlipidaemia and steatohepatitis (often leading to cirrhosis) associated with ectopic lipid deposition. A much sounder strategy would be to manipulate adipocyte biology in ways that promote health: enhancement of leptin and adiponectin synthesis, and secretion and promotion of intra-adipocyte lipid oxidation are examples of approaches that might be expected to benefit patients.
The contribution of brown adipose tissue to the total energy balance of humans is largely unexplored. Although infants have recognizable brown adipose tissue depots in the interscapular and around the great vessels, these disappear as humans mature. Individuals with chronically elevated catecholamine secretion, such as those with phaeochromocytoma, redevelop distinct brown adipose tissue depots111, as do individuals chronically exposed to cold temperatures. Reactivation of brown adipose tissue in adult humans (or promotion of a brown adipose tissue-like phenotype in white adipocytes) remains a potentially viable therapeutic strategy in humans.
The pace of discovery indicates that this field is still growing rapidly, and new insights are unfolding with each passing month. It remains to be seen how well we will translate these discoveries to our patients, but hopes for breakthrough therapies for obesity and diabetes are as high as they have ever been.
Acknowledgements
We thank B. Lowell and M. Herman for a critical reading of the manuscript.
Footnotes
The authors declare no competing financial interests.
References
- 1.Mokdad AH, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. J. Am. Med. Assoc. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 2.Bray GA, Bellanger T. Epidemiology, trends, and morbidities of obesity and the metabolic syndrome. Endocrine. 2006;29:109–117. doi: 10.1385/ENDO:29:1:109. [DOI] [PubMed] [Google Scholar]
- 3.Trayhurn P. Endocrine and signalling role of adipose tissue: new perspectives on fat. Acta Physiol. Scand. 2005;184:285–293. doi: 10.1111/j.1365-201X.2005.01468.x. [DOI] [PubMed] [Google Scholar]
- 4.Pond CM. The Fats of Life. Cambridge: Cambridge Univ. Press; 1998. [Google Scholar]
- 5.Giorgino F, Laviola L, Eriksson JW. Regional differences of insulin action in adipose tissue: insights from in vivo and in vitro studies. Acta Physiol. Scand. 2005;183:13–30. doi: 10.1111/j.1365-201X.2004.01385.x. [DOI] [PubMed] [Google Scholar]
- 6.Green H, Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975;5:19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- 7.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen JB, Kristiansen K. Regulatory circuits controlling white versus brown adipocyte differentiation. Biochem. J. 2006;398:153–168. doi: 10.1042/BJ20060402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosen ED, et al. C/EBPα induces adipogenesis through PPARγ: a unified pathway. Genes Dev. 2002;16:22–26. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosen ED, et al. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol. Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 11.Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPARγ2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 12.Oishi Y, et al. Kruppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab. 2005;1:27–39. doi: 10.1016/j.cmet.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Mori T, et al. Role of Kruppel-like factor 15 (KLF15) in transcriptional regulation of adipogenesis. J. Biol. Chem. 2005;280:12867–12875. doi: 10.1074/jbc.M410515200. [DOI] [PubMed] [Google Scholar]
- 14.Banerjee SS, et al. The Kruppel-like factor KLF2 inhibits peroxisome proliferator-activated receptor-γ expression and adipogenesis. J. Biol. Chem. 2003;278:2581–2584. doi: 10.1074/jbc.M210859200. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z, Torrens JI, Anand A, Spiegelman BM, Friedman JM. Krox20 stimulates adipogenesis via C/EBPβ-dependent and -independent mechanisms. Cell Metab. 2005;1:93–106. doi: 10.1016/j.cmet.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Akerblad P, Lind U, Liberg D, Bamberg K, Sigvardsson M. Early B-cell factor (O/E-1) is a promoter of adipogenesis and involved in control of genes important for terminal adipocyte differentiation. Mol. Cell Biol. 2002;22:8015–8025. doi: 10.1128/MCB.22.22.8015-8025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen JB, et al. Retinoblastoma protein functions as a molecular switch determining white versus brown adipocyte differentiation. Proc. Natl Acad. Sci. USA. 2004;101:4112–4117. doi: 10.1073/pnas.0301964101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scime A, et al. Rb and p107 regulate preadipocyte differentiation into white versus brown fat through repression of PGC-1α. Cell Metab. 2005;2:283–295. doi: 10.1016/j.cmet.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Picard F, et al. SRC-1 and TIF2 control energy balance between white and brown adipose tissues. Cell. 2002;111:931–941. doi: 10.1016/s0092-8674(02)01169-8. [DOI] [PubMed] [Google Scholar]
- 20.Puigserver P, et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 21.Puigserver P. Tissue-specific regulation of metabolic pathways through the transcriptional coactivator PGC1-α. Int. J. Obes. (Lond.) 2005;29 Suppl. 1:S5–S9. doi: 10.1038/sj.ijo.0802905. [DOI] [PubMed] [Google Scholar]
- 22.Uldry M, et al. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 2006;3:333–341. doi: 10.1016/j.cmet.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Schwartz MW, et al. Is the energy homeostasis system inherently biased toward weight gain? Diabetes. 2003;52:232–238. doi: 10.2337/diabetes.52.2.232. [DOI] [PubMed] [Google Scholar]
- 24.Abizaid A, Gao Q, Horvath TL. Thoughts for food: brain mechanisms and peripheral energy balance. Neuron. 2006;51:691–702. doi: 10.1016/j.neuron.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 25.Mauer MM, Harris RB, Bartness TJ. The regulation of total body fat: lessons learned from lipectomy studies. Neurosci. Biobehav. Rev. 2001;25:15–28. doi: 10.1016/s0149-7634(00)00047-6. [DOI] [PubMed] [Google Scholar]
- 26.Friedman JM. Leptin and the regulation of body weight. Harvey Lect. 1999;95:107–136. [PubMed] [Google Scholar]
- 27.Friedman JM. The function of leptin in nutrition, weight, and physiology. Nutr. Rev. 2002;60:S1–S14. doi: 10.1301/002966402320634878. [DOI] [PubMed] [Google Scholar]
- 28.Halaas JL, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 29.Pelleymounter MA, et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269:540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J. Clin. Invest. 1996;98:1101–1106. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bjorbaek C, Kahn BB. Leptin signaling in the central nervous system and the periphery. Recent Prog. Horm. Res. 2004;59:305–331. doi: 10.1210/rp.59.1.305. [DOI] [PubMed] [Google Scholar]
- 32.Fei H, et al. Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc. Natl Acad. Sci. USA. 1997;94:7001–7005. doi: 10.1073/pnas.94.13.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balthasar N, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123:493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 34.Grill HJ. Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity (Silver Spring) 2006;14 Suppl. 5:216S–221S. doi: 10.1038/oby.2006.312. [DOI] [PubMed] [Google Scholar]
- 35.Bartness TJ, Bamshad M. Innervation of mammalian white adipose tissue: implications for the regulation of total body fat. Am. J. Physiol. 1998;275:R1399–R1411. doi: 10.1152/ajpregu.1998.275.5.R1399. [DOI] [PubMed] [Google Scholar]
- 36.Bartness TJ, Kay Song C, Shi H, Bowers RR, Foster MT. Brain-adipose tissue cross talk. Proc. Nutr. Soc. 2005;64:53–64. doi: 10.1079/pns2004409. [DOI] [PubMed] [Google Scholar]
- 37.Yamada T, et al. Signals from intra-abdominal fat modulate insulin and leptin sensitivity through different mechanisms: neuronal involvement in food-intake regulation. Cell Metab. 2006;3:223–229. doi: 10.1016/j.cmet.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Bachman ES, et al. βAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297:843–845. doi: 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- 39.Lowell BB, et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366:740–742. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- 40.Enerback S, et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–94. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- 41.Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–2616. doi: 10.1126/science.1056843. [DOI] [PubMed] [Google Scholar]
- 42.Parker MG, Christian M, White R. The nuclear receptor co-repressor RIP140 controls the expression of metabolic gene networks. Biochem. Soc. Trans. 2006;34:1103–1106. doi: 10.1042/BST0341103. [DOI] [PubMed] [Google Scholar]
- 43.Qi L, et al. TRB3 links the E3 ubiquitin ligase COP1 to lipid metabolism. Science. 2006;312:1763–1766. doi: 10.1126/science.1123374. [DOI] [PubMed] [Google Scholar]
- 44.Herman MA, Kahn BB. Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony. J. Clin. Invest. 2006;116:1767–1775. doi: 10.1172/JCI29027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tirone TA, Brunicardi FC. Overview of glucose regulation. World J. Surg. 2001;25:461–467. doi: 10.1007/s002680020338. [DOI] [PubMed] [Google Scholar]
- 46.Kahn BB. Lilly lecture 1995. Glucose transport: pivotal step in insulin action. Diabetes. 1996;45:1644–1654. doi: 10.2337/diab.45.11.1644. [DOI] [PubMed] [Google Scholar]
- 47.Schwartz MW, et al. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes. 1996;45:531–535. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- 48.Farooqi IS, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J. Clin. Invest. 2002;110:1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–76. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- 50.Oral EA, et al. Leptin-replacement therapy for lipodystrophy. N. Engl. J. Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- 51.Heymsfield SB, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. J. Am. Med. Assoc. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- 52.Minokoshi Y, et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 53.Kamohara S, Burcelin R, Halaas JL, Friedman JM, Charron MJ. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature. 1997;389:374–377. doi: 10.1038/38717. [DOI] [PubMed] [Google Scholar]
- 54.Kieffer TJ, Habener JF. The adipoinsular axis: effects of leptin on pancreatic β-cells. Am. J. Physiol. Endocrinol. Metab. 2000;278:E1–E14. doi: 10.1152/ajpendo.2000.278.1.E1. [DOI] [PubMed] [Google Scholar]
- 55.Covey SD, et al. The pancreatic β cell is a key site for mediating the effects of leptin on glucose homeostasis. Cell Metab. 2006;4:291–302. doi: 10.1016/j.cmet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 56.Kadowaki T, et al. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Invest. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waki H, et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J. Biol. Chem. 2003;278:40352–40363. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 58.Pajvani UB, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J. Biol. Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 59.Yamauchi T, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 60.Hug C, et al. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc. Natl Acad. Sci. USA. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arita Y, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 62.Yatagai T, et al. Hypoadiponectinemia is associated with visceral fat accumulation and insulin resistance in Japanese men with type 2 diabetes mellitus. Metabolism. 2003;52:1274–1278. doi: 10.1016/s0026-0495(03)00195-1. [DOI] [PubMed] [Google Scholar]
- 63.Hotta K, et al. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes. 2001;50:1126–1133. doi: 10.2337/diabetes.50.5.1126. [DOI] [PubMed] [Google Scholar]
- 64.Kubota N, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J. Biol. Chem. 2002;277:25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 65.Ma K, et al. Increased β-oxidation but no insulin resistance or glucose intolerance in mice lacking adiponectin. J. Biol. Chem. 2002;277:34658–34661. doi: 10.1074/jbc.C200362200. [DOI] [PubMed] [Google Scholar]
- 66.Maeda N, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nature Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 67.Nawrocki AR, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor γ agonists. J. Biol. Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- 68.Kubota N, et al. Pioglitazone ameliorates insulin resistance and diabetes by both adiponectin-dependent and -independent pathways. J. Biol. Chem. 2006;281:8748–8755. doi: 10.1074/jbc.M505649200. [DOI] [PubMed] [Google Scholar]
- 69.Maeda N, et al. PPARγ ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes. 2001;50:2094–2099. doi: 10.2337/diabetes.50.9.2094. [DOI] [PubMed] [Google Scholar]
- 70.Winzell MS, Nogueiras R, Dieguez C, Ahren B. Dual action of adiponectin on insulin secretion in insulin-resistant mice. Biochem. Biophys. Res. Commun. 2004;321:154–160. doi: 10.1016/j.bbrc.2004.06.130. [DOI] [PubMed] [Google Scholar]
- 71.Fukuhara A, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–430. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 72.Stephens JM, Vidal-Puig AJ. An update on visfatin/pre-B cell colony-enhancing factor, an ubiquitously expressed, illusive cytokine that is regulated in obesity. Curr. Opin. Lipidol. 2006;17:128–131. doi: 10.1097/01.mol.0000217893.77746.4b. [DOI] [PubMed] [Google Scholar]
- 73.Yang H, Lavu S, Sinclair DA. Nampt/PBEF/Visfatin: a regulator of mammalian health and longevity? Exp. Gerontol. 2006;41:718–726. doi: 10.1016/j.exger.2006.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang RZ, et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am. J. Physiol. Endocrinol. Metab. 2006;290:E1253–E1261. doi: 10.1152/ajpendo.00572.2004. [DOI] [PubMed] [Google Scholar]
- 75.Hotamisligil GS. The role of TNFα and TNF receptors in obesity and insulin resistance. J. Intern. Med. 1999;245:621–625. doi: 10.1046/j.1365-2796.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- 76.Xu H, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carey AL, et al. Interleukin-6 increases insulin-stimulated glucose disposal in humans and glucose uptake and fatty acid oxidation in vitro via AMP-activated protein kinase. Diabetes. 2006;55:2688–2697. doi: 10.2337/db05-1404. [DOI] [PubMed] [Google Scholar]
- 78.Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-α, overexpressed in human fat cells from insulin-resistant subjects. J. Biol. Chem. 2003;278:45777–45784. doi: 10.1074/jbc.M301977200. [DOI] [PubMed] [Google Scholar]
- 79.Hirosumi J, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 80.Shoelson SE, Lee J, Yuan M. Inflammation and the IKKβ/IκB/NF-κB axis in obesity-and diet-induced insulin resistance. Int. J. Obes. Relat. Metab. Disord. 2003;27 Suppl. 3:S49–S52. doi: 10.1038/sj.ijo.0802501. [DOI] [PubMed] [Google Scholar]
- 81.Howard JK, Flier JS. Attenuation of leptin and insulin signaling by SOCS proteins. Trends Endocrinol. Metab. 2006;17:365–371. doi: 10.1016/j.tem.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 82.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 83.Steppan CM, et al. A family of tissue-specific resistin-like molecules. Proc. Natl Acad. Sci. USA. 2001;98:502–506. doi: 10.1073/pnas.98.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Steppan CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 85.Banerjee RR, et al. Regulation of fasted blood glucose by resistin. Science. 2004;303:1195–1198. doi: 10.1126/science.1092341. [DOI] [PubMed] [Google Scholar]
- 86.Steppan CM, Lazar MA. The current biology of resistin. J. Intern. Med. 2004;255:439–447. doi: 10.1111/j.1365-2796.2004.01306.x. [DOI] [PubMed] [Google Scholar]
- 87.Patel SD, Rajala MW, Rossetti L, Scherer PE, Shapiro L. Disulfide-dependent multimeric assembly of resistin family hormones. Science. 2004;304:1154–1158. doi: 10.1126/science.1093466. [DOI] [PubMed] [Google Scholar]
- 88.Kaser S, et al. Resistin messenger-RNA expression is increased by proinflammatory cytokines in vitro. Biochem. Biophys. Res. Commun. 2003;309:286–290. doi: 10.1016/j.bbrc.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 89.Patel L, et al. Resistin is expressed in human macrophages and directly regulated by PPARγ activators. Biochem. Biophys. Res. Commun. 2003;300:472–476. doi: 10.1016/s0006-291x(02)02841-3. [DOI] [PubMed] [Google Scholar]
- 90.Yang Q, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 91.Graham TE, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N. Engl. J. Med. 2006;354:2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 92.Roden M, et al. Mechanism of free fatty acid-induced insulin resistance in humans. J. Clin. Invest. 1996;97:2859–2865. doi: 10.1172/JCI118742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roden M, et al. Effects of free fatty acid elevation on postabsorptive endogenous glucose production and gluconeogenesis in humans. Diabetes. 2000;49:701–707. doi: 10.2337/diabetes.49.5.701. [DOI] [PubMed] [Google Scholar]
- 94.Griffin ME, et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48:1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 95.Schmitz-Peiffer C, et al. Alterations in the expression and cellular localization of protein kinase C isozymes epsilon and theta are associated with insulin resistance in skeletal muscle of the high-fat-fed rat. Diabetes. 1997;46:169–178. doi: 10.2337/diab.46.2.169. [DOI] [PubMed] [Google Scholar]
- 96.Paolisso G, et al. Does free fatty acid infusion impair insulin action also through an increase in oxidative stress? J. Clin. Endocrinol. Metab. 1996;81:4244–4248. doi: 10.1210/jcem.81.12.8954022. [DOI] [PubMed] [Google Scholar]
- 97.Hajduch E, et al. Ceramide impairs the insulin-dependent membrane recruitment of protein kinase B leading to a loss in downstream signalling in L6 skeletal muscle cells. Diabetologia. 2001;44:173–183. doi: 10.1007/s001250051596. [DOI] [PubMed] [Google Scholar]
- 98.Shi H, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Song MJ, Kim KH, Yoon JM, Kim JB. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem. Biophys. Res. Commun. 2006;346:739–745. doi: 10.1016/j.bbrc.2006.05.170. [DOI] [PubMed] [Google Scholar]
- 100.Eldor R, Raz I. Lipotoxicity versus adipotoxicity — the deleterious effects of adipose tissue on beta cells in the pathogenesis of type 2 diabetes. Diabetes Res. Clin. Pract. 2006;74:S3–S8. [Google Scholar]
- 101.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 102.Simha V, Garg A. Lipodystrophy: lessons in lipid and energy metabolism. Curr. Opin. Lipidol. 2006;17:162–169. doi: 10.1097/01.mol.0000217898.52197.18. [DOI] [PubMed] [Google Scholar]
- 103.Yamauchi T, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nature Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 104.Weisberg SP, et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weisberg SP, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kanda H, et al. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J. Clin. Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kamei N, et al. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J. Biol. Chem. 2006;281:26602–26614. doi: 10.1074/jbc.M601284200. [DOI] [PubMed] [Google Scholar]
- 108.Di Gregorio GB, et al. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes. 2005;54:2305–2313. doi: 10.2337/diabetes.54.8.2305. [DOI] [PubMed] [Google Scholar]
- 109.Gnudi L, Tozzo E, Shepherd PR, Bliss JL, Kahn BB. High level overexpression of glucose transporter-4 driven by an adipose-specific promoter is maintained in transgenic mice on a high fat diet, but does not prevent impaired glucose tolerance. Endocrinology. 1995;136:995–1002. doi: 10.1210/endo.136.3.7867610. [DOI] [PubMed] [Google Scholar]
- 110.Abel ED, et al. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729–733. doi: 10.1038/35055575. [DOI] [PubMed] [Google Scholar]
- 111.Fukuchi K, et al. Radionuclide imaging metabolic activity of brown adipose tissue in a patient with pheochromocytoma. Exp. Clin. Endocrinol. Diabetes. 2004;112:601–603. doi: 10.1055/s-2004-830407. [DOI] [PubMed] [Google Scholar]