Abstract
Background and Purpose
Plasmin is a direct-acting thrombolytic (DAT) agent with a striking hemostatic safety advantage over plasminogen activators (PA) in animal models of thrombolysis and bleeding. In contradistinction to PAs, which risk bleeding at any effective thrombolytic dose, plasmin is tolerated without bleeding at several-fold higher amounts than needed for thrombolysis. Plasmin has been safe in a current trial in patients with peripheral arterial or graft occlusion, and efforts are now directed towards therapy of stroke caused by cerebral artery occlusion.
Methods
A rabbit (4 kg body weight) model of 2-hour, thrombin-induced middle cerebral artery (MCA) occlusion using angiographic documentation of vascular patency and recanalization was utilized to perform a dose-ranging study of plasmin, delivered by catheter over a median duration of 10 minutes.
Results
Plasmin induced early recanalization in all animals (3 per group) within 10 minutes after discontinuation of 3, 2, or 1 mg of agent infusion. Control saline infusion failed to induce recanalization in 3 of 3 subjects.
Conclusions
Plasmin rapidly induces MCA recanalization, as determined in an angiogram-based animal model of arterial occlusion. Based on these data and other information, a phase 1/2a clinical trial of plasmin in human MCA ischemic stroke has been initiated.
Keywords: Thrombolysis, plasmin
Introduction
There is currently only one FDA-approved treatment for ischemic stroke, namely, recombinant tissue plasminogen activator (rt-PA) administered by intravenous (IV) infusion within 3 hours of symptom onset1. As IV therapy may not provide sufficient thrombolytic agent to the thrombus that totally occludes a cerebral artery, IA delivery of thrombolytic agents has been assessed with recombinant pro-urokinase (pro-UK)2 and as combined IV plus IA rt-PA3.
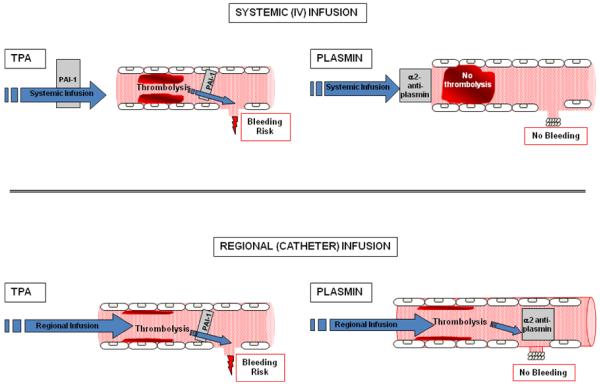
Direct-acting thrombolytics such as plasmin possess a distinctly different mode of action than plasminogen activators (PA)4, and preclinical studies suggest that catheter-delivered plasmin achieves thrombolytic efficacy without causing bleeding, as first formulated in 2001 by Marder and colleagues5. According to this scheme (Figure 1), plasmin administered by catheter binds to thrombus, where it is shielded from α2-antiplasmin and induces thrombolysis. Upon release from (or after bypassing) the thrombus into the circulation, plasmin is rapidly neutralized by α2-antiplasmin, thus preventing bleeding at remote sites of vascular injury. Plasmin administered intravenously is rapidly (within seconds) neutralized by α2-antiplasmin6 and neither dissolves thrombus nor induces bleeding. This explains prior observations7 that plasmin (“fibrinolysin”) administered intravenously is safe but not effective, and underscores the requirement for plasmin to be delivered locally by catheter to a thrombus in order to achieve effective thrombolysis.
Figure 1.
Schematic representation of mode of action of systemic (intravenous) and local (intra-arterial) administration of plasminogen activators (e.g., tPA) and plasmin. Modified from Marder and Novokhatny4. The top panel shows systemic (intravenous) administration, and the bottom panel shows local (catheter) administration of tissue-type plasminogen activator (t-PA) (left) and plasmin (right), as typical representatives of direct and indirect fibrinolytic agents. PAI-1, plasminogen activator inhibitor type 1.
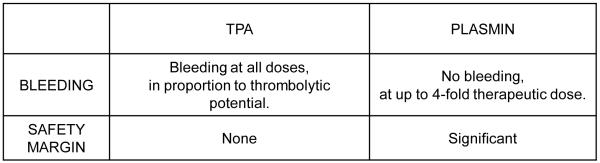
Whereas rt-PA has potential for hemorrhage at any dose that has thrombolytic effect, plasmin possesses a significant safety margin5,8, at approximately 4-fold greater dose than is needed to dissolve experimental thrombi (Figure 2). There is considerable confidence9 in this safety margin for plasmin use in peripheral arterial or graft occlusion10, where plasmin is quickly and efficiently neutralized by α2-antiplasmin after perfusing the lower extremity. However, judgment must necessarily be reserved for intra-arterial treatment of ischemic stroke4, as plasmin could enter a vulnerable distal cerebral artery after dissolution of a thrombo-embolus, and real-time blunting of its enzymatic effect needs to be accomplished by ambient α2-antiplasmin.
Figure 2.
Margin of safety against bleeding for rt-PA and plasmin. Conclusions based on data provided by experimental models of intra-arterial thrombolysis and systemic circulation of agent5,8.
Three direct-acting thrombolytic agents have been assessed for treatment of ischemic stroke in experimental models, only one of which has been used in human disease trials4.
Micro-plasmin consists of the serine protease domain of plasmin alone11, and after systemic (intravenous) administration, showed less intracranial hemorrhage and decreased cerebral injury equal to or better than with rt-PA in models of cerebral ischemia4. Micro-plasmin used intravenously (not by the intra-arterial route) in middle cerebral artery ischemic stroke showed a trend toward better recanalization compared to placebo, but further trial awaits a partner12, and Phase IIa results of intra-arterial micro-plasmin in basilar artery stroke are not published13.
Alfimeprase is a derivative of the Southern copperhead snake venom fibrolase, made by recombinant technology14 to slightly modify the N-terminus. Pre-clinical studies showed superior thrombolysis compared with UK in the rat, pig and dog model of thrombosed carotid artery4, but planned studies of alfimeprase ischemic stroke were never initiated15.
Mini-plasmin consists of the serine protease domain plus kringle 1 of plasmin16, and reduced ischemic injury after intravenous administration in middle cerebral artery ligation models17, but the agent has not been tested clinically.
Plasmin is currently under pre-clinical study as foundation for clinical trial. To assess its potential, we have established a model for angiographically-documented recanalization of the rabbit middle cerebral artery (MCA)18, and now utilize this selective MCA occlusion model to evaluate the recanalization efficacy of three dosages of plasmin.
Methods
Animal studies were approved by the David Geffen School of Medicine at UCLA Animal Research Committee and conducted on 3.5-4.3 kg New Zealand White rabbits. A 4F sheath (Terumo Pinnacle, Boston Scientific, Natick, MA) was placed into the right femoral artery and a 4F glidecatheter (Terumo Glidecath, Boston Scientific, Natick, MA) was inserted to reach the right common carotid artery (CCA). A 1.2 French Magic Balt flow-directed microcatheter and Sorcerer guidewire (BALT extrusion, Montmorency, France) were introduced into the right common carotid artery co-axially through the Terumo glidecatheter, and utilizing roadmap technique. Coagulant mixture of bovine thrombin (10 NIH units/100 μL) (Enzyme Research, South Bend, IN) with 1:10 saline-diluted rabbit brain thromboplastin (Neoplastine® CI PLUS, Diagnostica Stago, Asnieres, France) was administered over 10 minutes into the MCA, and occlusion documented by angiography immediately and at 2 hours to rule out spontaneous MCA recanalization. Plasmin (Human, TAL-05-00018, Talecris Biotherapeutics, Research Triangle Park, NC) was administered over 10-30 minutes by infusion pump just proximal to the MCA occlusion, after which angiography was performed at 15, 30, 45 and 60 minutes to determine if arterial recanalization had occurred. Angiographic perfusion was classified according to Thrombolysis in Myocardial Infarction (TIMI) grades19.
Results
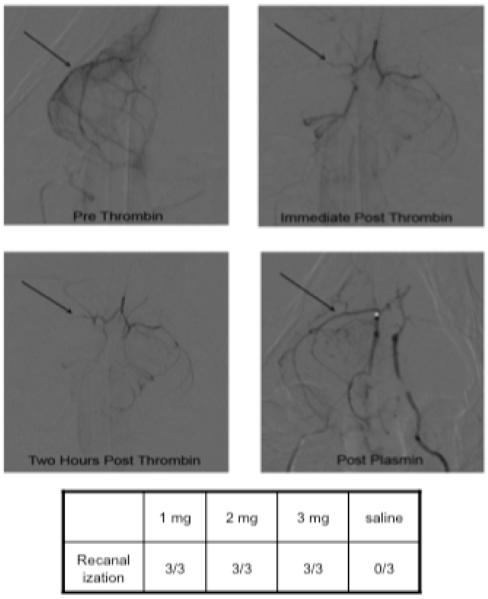
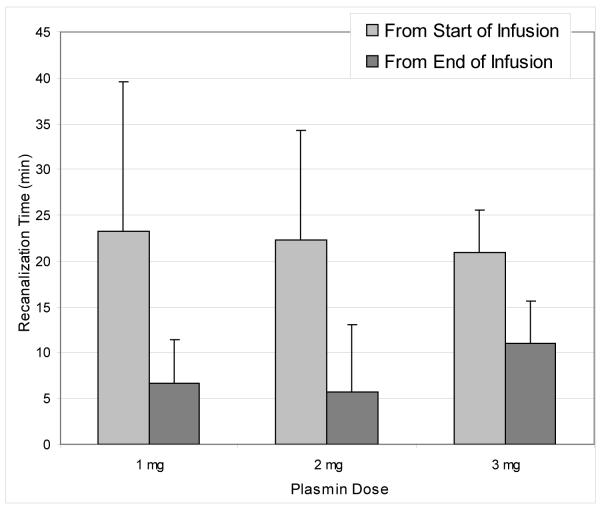
MCA occlusion (TIMI 0) was successfully accomplished in all 12 animals receiving IA thrombin, and the 2-hour follow-up angiograms showed no instance of spontaneous recanalization (Table 1). TIMI IIb or III MCA recanalization was noted on the first post-treatment angiogram in all 9 animals that received plasmin (see Figure 3 for representative result). In contrast, all of the saline-treated animals showed persistent TIMI 0 MCA occlusion. The mean elapsed times from the start of plasmin infusion until MCA recanalization varied between 13 and 42 minutes, depending upon technical delays before performing the angiogram, but were the same for the 1, 2, and 3 mg dosages (23.3, 22.3 and 21.0 minutes, respectively) (Figure 4). Recanalization after termination of plasmin infusion was always documented with the first follow-up angiogram, at 7.7, 5.7 and 11.0 minutes for the 1, 2, and 3 mg dosages, respectively (Figure 4).
Table 1.
Plasmin infusions into occluded middle cerebral artery of the rabbit.
| # | Duration of MCA Occlusion (min) |
Agent | Infusion Details | Time until Recanalization (min) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| mg/ mL |
mL/ min |
min | mL | From Start of Infusion |
From End of Infusion |
||||||
| Mean | Mean | Mean | |||||||||
| 1 | 128 | 131 | Plasmin (1 mg) |
0.2 | 0.17 | 30 | 5 | 42 | 23.3 | 12 | 7.7 |
| 2 | 132 | 0.2 | 0.5 | 10 | 5 | 15 | 5 | ||||
| 3 | 132 | 0.2 | 0.5 | 10 | 5 | 13 | 3 | ||||
| 4 | 130 | 129 | Plasmin (2 mg) |
1 | 0.2 | 10 | 2 | 10 | 22.3 | 0 | 5.7 |
| 5 | 136 | 0.2 | 0.5 | 20 | 10 | 34 | 14 | ||||
| 6 | 120 | 0.2 | 0.5 | 20 | 10 | 23 | 3 | ||||
| 7 | 101 | 109 | Plasmin (3 mg) |
0.6 | 0.5 | 10 | 5 | 20 | 21.0 | 10 | 11.0 |
| 8 | 113 | 0.6 | 0.5 | 10 | 5 | 26 | 16 | ||||
| 9 | 113 | 0.6 | 0.5 | 10 | 5 | 17 | 7 | ||||
| 10 | 97 | 120 | Saline | NA | 0.5 | 10 | 5 | >94 | >69.0 | >84 | >59.0 |
| 11 | 130 | 0.5 | 10 | 5 | >58 | >48 | |||||
| 12 | 132 | 0.5 | 10 | 5 | >55 | >45 | |||||
Figure 3.
Representative results of local (intra-arterial) infusion of plasmin into the thrombo-occluded rabbit MCA. Pre-thrombin image (upper left panel) shows the patent right MCA (arrow), which on the immediate post thrombin image (upper right panel) is occluded (arrow) and remains occluded on the 2-hour post thrombin image (lower left panel). After intra-arterial plasmin infusion, flow through the MCA is re-established (arrow, lower right panel).
Figure 4.
Mean delays until MCA recanalization after the start and from the end of plasmin infusions (1, 2, or 3 mg doses).
Discussion
Our focus on MCA recanalization is clinically relevant, as analysis of human ischemic stroke trial data indicates that arterial recanalization is the key to successful clinical outcome20. Specifically, a clinical (functional) advantage at 3 months occurs in patients in whom the occluded vessel had recanalized (58.1% vs 24.8%, odds ratio 4.43), and more importantly, the overall mortality was reduced in patients whose occluded artery had recanalized (14.4% vs 41.6%, odds ratio 0.24). Our model is ideal for demonstrating recanalization efficacy, as it is based upon routine angiographic documentation of MCA occlusion and patency.
The safety of these dosages of plasmin relative to complicating intracranial hemorrhage must still be assessed in comparative pre-clinical studies. Nevertheless, our results provide guidance for selecting appropriate initial dosages for clinical trials of ischemic stroke. Considering that a 1 mg dose of plasmin administered into the occluded MCA of the rabbit induced rapid (within 10 minutes) recanalization, one could calculate a starting dose for human MCA occlusion based on relative body weight, thrombus histology, and delay until recanalization as indicated in Table 2. On the basis of body weight and relative size of the MCA thrombus, the human dose would be approximately 10-20-fold greater than in the rabbit (20 mg instead of 1 mg). However, the induced MCA occlusion in the rabbit was caused by thrombin-clotted whole blood, while human ischemic stroke is caused by vascular occlusion of more mature and organized thrombo-embolic material21. The latter are therefore likely to be more resistant to and require relatively larger amounts of any thrombolytic agent than is effective in our model. As to the duration of plasmin infusion into or near the human thrombo-embolus, it is difficult to predict an appropriate regimen, but based on rt-PA infusions of about one hour, it is reasonable to utilize 30 minute infusions, ascertain vessel patency status at 15 minutes, and continue to monitor by angiogram for 30 minutes after the end of plasmin infusion.
Table 2.
Dose considerations for plasmin in human ischemic stroke.
| RABBIT | HUMAN | IMPLICATION | |
|---|---|---|---|
| BODY WEIGHT | 4 kg | 70-80 kg | 20-fold higher dose needed in humans (20 mg vs 1 mg) |
| THROMBUS HISTOLOGY |
Simple whole blood clot |
Mature, complex thrombo-embolus |
More plasmin needed in humans than on weight basis alone. |
| DELAY UNTIL RECANALIZATION |
20-25 min from start of infusion |
t-PA infusion time 60 min |
Plasmin infusion time of 30 min (early look at 15 min). |
Based on these and other considerations, a Phase 1 safety and dose finding study of intra-arterial plasmin has been initiated for patients with ischemic MCA stroke at up to 8.5 hours after onset, with focal deficit and a rather broad NIHSS score of 4-2522. MCA occlusion of the M1, M2 or M1-2 branches must be documented by artiography, and escalating dosages of 20, 40, and 60 mg are planned, administered as 50% over 15 minutes, followed by the second infusion, with angiograms at 15, 30, and 60 minutes after the start of infusion. Endpoints for safety are intracranial hemorrhage and functional neurologic changes, and for efficacy, partial or complete recanalization (TICI scores of 2a, 2b or 3). As of today, 7 patients have been entered into the study and have received the planned 20 mg plasmin dose.
Conclusions
Plasmin is the prototypical direct-acting thrombolytic agent, and has shown impressive hemostatic safety in comparison with rt-PA in an experimental model of fibrinolytic hemorrhage5. Efficacy of plasmin is documented in the rabbit MCA recanalization model18 (Figures 3 and 4), and a Phase 1/2a clinical trial of plasmin has been initiated in acute ischemic stroke22.
Acknowledgements
Funding Support for this work provided by grants, NINDS P50 NS044378 and NHLBI RO1 HL074051, from the National Institutes of Health, Bethesda, Maryland, and from Talecris Biotherapeutics, Inc., Research Triangle Park, North Carolina.
Footnotes
Conflicts of Interest VM and RJ are consultants for and receive research support from, and VA is an employee of, Talecris Biotherapeutics, Inc., Research Triangle Park, North Carolina.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, Pessin M, Ahuja A, Callahan F, Clark WM, Silver F, Rivera F. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: A randomized controlled trial. Prolyse in acute cerebral thromboembolism. JAMA. 1999;282:2003–11. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 3.IMS Study Investigators Combined intravenous and intra-arterial recanalization for acute ischemic stroke: The interventional management of stroke study. Stroke. 2004;35:904–11. doi: 10.1161/01.STR.0000121641.77121.98. [DOI] [PubMed] [Google Scholar]
- 4.Marder VJ, Novokhatny V. Direct fibrinolytic agents: biochemical attributes, preclinical foundation and clinical potential. J Thromb Haemost. 2009;8:433–444. doi: 10.1111/j.1538-7836.2009.03701.x. [DOI] [PubMed] [Google Scholar]
- 5.Marder VJ, Landskroner K, Novokhatny V, Zimmerman TP, Kong M, Kanouse JJ, Jesmok G. Plasmin induces local thrombolysis without causing hemorrhage: A comparison with tissue plasminogen activator in the rabbit. Thromb Haemost. 2001;86:739–45. [PubMed] [Google Scholar]
- 6.Collen D. On the regulation and control of fibrinolysis. Edward Kowalski memorial lecture. Thromb Haemost. 1980;43:77–89. [PubMed] [Google Scholar]
- 7.Boyles PW, Meyer WH, Graff J, Ashley CC, Ripic RC. Comparative effectiveness of intravenous and intra-arterial fibrinolysis therapy. In: Cliffton EC, editor. Am J Cardiol; Symposium on Fibrinolysis; 1960. pp. 539–46. [DOI] [PubMed] [Google Scholar]
- 8.Stewart D, Kong M, Novokhatny V, Jesmok G, Marder VJ. Distinct dose-dependent effects of plasmin and t-PA on coagulation and hemorrhage. Blood. 2003;101:3002–07. doi: 10.1182/blood-2002-08-2546. [DOI] [PubMed] [Google Scholar]
- 9.Marder VJ. Preclinical studies of plasmin: superior benefit-to-risk ratio compared to tissue plasminogen activator (tPA) Thromb Res. 2008;122:S9–S15. doi: 10.1016/j.thromres.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US National Institutes of Health service; 2010. A dose escalation and safety study of plasmin (Human) in acute lower extremity native artery or bypass graft occlusion (PRIORITY) NCT 00418483. ClinicalTrials.gov. [Google Scholar]
- 11.Wu HL, Shi GY, Wohl RC, Bender ML. Structure and formation of microplasmin. Proc Natl Acad Sci USA. 1987;84:8793–8795. doi: 10.1073/pnas.84.24.8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thijs VNS, Peeters A, Vosko MR, Aichner F, Schellinger PS, Schneider D, Neumann-Haefelin T, Roether J, Davalos A, Walgren N, Verhamme P. Randomized, placebo-controlled, dose-ranging clinical trial of intravenous microplasmin in patients with acute ischemic stroke. Stroke. 2009;40:3789–3795. doi: 10.1161/STROKEAHA.109.560201. [DOI] [PubMed] [Google Scholar]
- 13.U.S. National Institutes of Health. A service of the U.S. National Institutes of Health; 2009. Intra-Arterial Microplasmin Administration in Patients With Acute Intracranial Vertebrobasilar Artery Occlusion (MITI-IA) NCT 00123266. ClinicalTrials.gov. [Google Scholar]
- 14.Randolph A, Chamberlain SH, Chu HL, Retzios AD, Markland FS, Jr, Masiarz FR. Amino acid sequence of fibrolase, a direct-acting fibrinolytic enzyme from Agkistrodon contortrix contortrix venom. Protein Sci. 1992;1:590–600. doi: 10.1002/pro.5560010505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah AR, Scher L. Drug evaluation: Alfimeprase, a plasminogen-independent thrombolytic. IDrugs. 2007;10:329–35. [PubMed] [Google Scholar]
- 16.Sottrup-Jensen L, Claeys H, Zajdel M, Petersen TE, Magnusson S. The primary structure of human plasminogen: isolation of two lysine-binding fragments and one “mini” plasminogen (MW 38000) by elastase-catalyzed specific limited proteolysis. Progr Chem Fibrinolysis Thrombolysis. 1978;3:191–209. [Google Scholar]
- 17.Nagai N, De Mol M, Van Hoef B, Verstreken M, Collen D. Depletion of circulating alpha(2)-antiplasmin by intravenous plasmin or immunoneutralization reduces focal cerebral ischemic injury in the absence of arterial recanalization. Blood. 2001;97:3086–92. doi: 10.1182/blood.v97.10.3086. [DOI] [PubMed] [Google Scholar]
- 18.Jahan R, Stewart D, Vinters HV, Yong W, Vinuela F, Vandeberg P, Marder VJ. Middle cerebral artery occlusion in the rabbit using selective angiography: Application for assessment of thrombolysis. Stroke. 2008;39:1613–1615. doi: 10.1161/STROKEAHA.107.507376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Special report: The thrombolysis in myocardial infarction (TIMI) trial. N Engl J Med. 1985;312:932–936. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- 20.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: A meta-analysis. Stroke. 2007;38:967–973. doi: 10.1161/01.STR.0000258112.14918.24. [DOI] [PubMed] [Google Scholar]
- 21.Marder VJ, Chute DJ, Starkman S, Abolian AM, Kidwell C, Liebeskind D, Ovbiagele B, Vinuela F, Duckwiler G, Jahan R, Vespa PM, Selco S, Rajajee V, Kim D, Sanossian N, Saver JL. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke. 2006;37:2086–2093. doi: 10.1161/01.STR.0000230307.03438.94. [DOI] [PubMed] [Google Scholar]
- 22.US National Institutes of Health service; 2010. A safety and dose finding study of plasmin (human) administered into the middle cerebral artery of stroke patients. NCT 01014975. ClinicalTrials.gov. [Google Scholar]