Abstract
Preeclamptic women have enhanced blood pressure response to angiotensin II and extensive systemic vascular infiltration of neutrophils. Neutrophils release reactive oxygen species that might activate the RhoA kinase pathway to enhance vascular reactivity. We hypothesized that enhanced vascular reactivity in preeclampsia is due to neutrophil mediated reactive oxygen species activation of the RhoA kinase pathway. Omental arteries were obtained at cesarean section and studied using a myograph system. We found that arteries of preeclamptic women had extensive infiltration of neutrophils and enhanced reactivity to angiotensin II. Treatment of arteries of normal pregnant women with reactive oxygen species or activated neutrophils enhanced vessel reactivity to angiotensin II mimicking preeclamptic vessels. Pretreatment with superoxide dismutase/catalase to quench reactive oxygen species, or RhoA kinase inhibitor blocked enhanced responses in preeclamptic and normal vessels. Reactive oxygen species also enhanced vessel reactivity to norepinephrine, which was blocked by RhoA kinase inhibition. Treatment of arteries with reactive oxygen species increased RhoA kinase activity 3-fold, whereas culture of human vascular smooth muscle cells with angiotensin II and activated neutrophils or reactive oxygen species resulted in phosphorylation of key proteins in the RhoA kinase pathway. We conclude that enhanced vascular reactivity of omental arteries in preeclampsia is due to reactive oxygen species activation of the RhoA kinase pathway, and that enhanced vascular reactivity is likely due to the infiltration of neutrophils. We speculate that neutrophil infiltration into systemic vasculature of preeclamptic women is an important mechanism for hypertension.
Keywords: preeclampsia, neutrophils, reactive oxygen species, RhoA kinase, angiotensin II, hypertension
INTRODUCTION
Preeclampsia is a hypertensive disorder of pregnancy that complicates 5–7% of all pregnancies resulting in significant maternal and fetal morbidity and mortality 1. The cause of hypertension in preeclampsia has never been fully explained. In 1973, Gant et al described enhanced blood pressure response to angiotensin II (Ang II) in women who went on to develop preeclampsia 2. However, mechanisms underlying this increased vascular reactivity remained elusive. Increased blood pressure in preeclampsia is not due to elevated levels of Ang II because Ang II levels are not elevated 3–5, nor are the levels of other vasoconstrictive hormones, such as epinephrine or norepinephrine 6.
Recently, we showed significant infiltration of neutrophils into systemic vasculature of women with preeclampsia, which was associated with marked vascular inflammation 7, 8. Neutrophils release reactive oxygen species (ROS) that may enhance vascular reactivity via the RhoA kinase pathway. When RhoA kinase is activated, it phosphorylates myosin phosphatase target subunit 1 (MYPT1), which inhibits myosin light chain phosphatase (MLCP), so myosin light chains (MLC) remain phosphorylated, which enhances calcium sensitization 9, 10. ROS have been shown to activate this pathway in rat aorta and rat pulmonary arteries 11, 12 and ROS are mediators of Ang II signaling 13.
Neutrophils are usually thought of as part of the innate immune system and the first line of defense against infection at the site of a wound 14,15. A role for neutrophils in the control of blood pressure is not commonly considered, however, given the extensive infiltration of neutrophils into the systemic vasculature of women with preeclampsia, neutrophil release of ROS might activate the RhoA kinase pathway to enhance vessel reactivity.
In this study we used human omental arteries obtained from normal pregnant and preeclamptic women to test the hypothesis that enhanced vascular reactivity in preeclampsia is due to neutrophil mediated ROS activation of the RhoA kinase pathway.
MATERIALS and METHODS
Study Subjects
Omental fat biopsies (approximately 2 cm × 4 cm × 2 cm) were collected from 40 normal pregnant and 9 preeclamptic women undergoing C-section. Arteries were dissected, cleared of fat and used for the myograph experiments. The Office of Research Subjects Protection of Virginia Commonwealth University approved this study, all subjects gave informed consent, and the procedures followed were in accordance with institutional guidelines.
Please see the Online Supplement at http:/hyper.ajajournals.org for expanded materials and methods for clinical characteristics of the patient groups (Table S1), the myograph experiments, immunohistochemistry, Western blot, RhoA kinase activity assay, and data analysis.
RESULTS
Comparison of vascular reactivity between arteries of normal and preeclamptic pregnancy
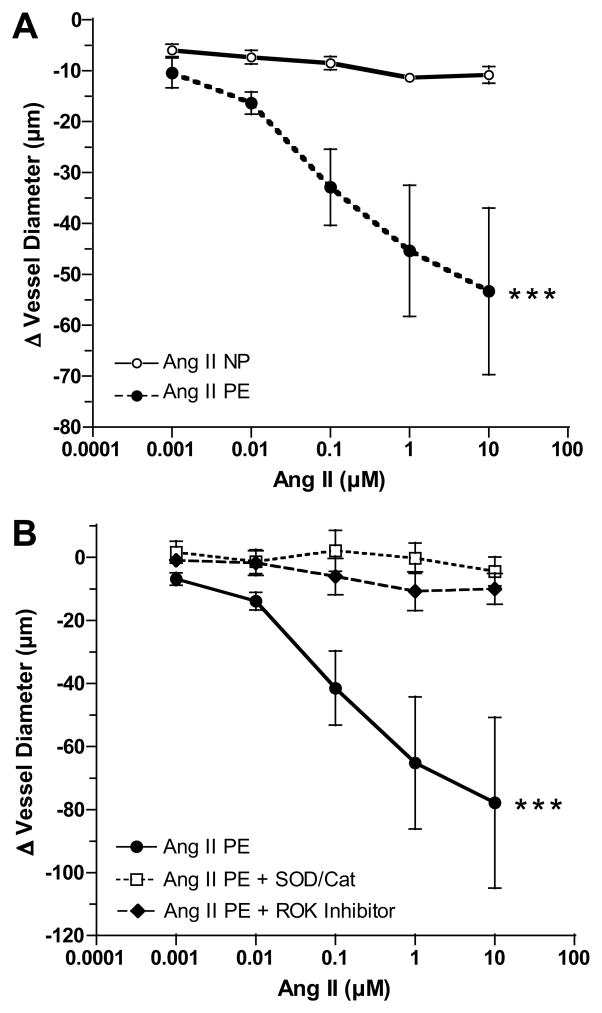
Figure 1A shows that vessel reactivity to Ang II was modest in omental arteries from normal pregnant women. In contrast, arteries from preeclamptic women showed significantly enhanced vessel reactivity as compared to arteries of normal pregnant women. Figure 1B shows that the enhanced reactivity of preeclamptic arteries was blocked by pretreatment with either superoxide dismutase (SOD)/catalase or RhoA kinase (ROK) inhibitor. Neither SOD/catalase nor ROK inhibitor alone significantly affected vessel diameter. Vascular reactivity of two nonpregnant subjects was also evaluated for comparison with pregnancy. Reactivity was between normal pregnant and preeclamptic patients (Online Supplement Figure S1).
Figure 1.
Comparison of vessel reactivity to angiotensin II (Ang II). A) Vessel reactivity to Ang II was significantly enhanced in endothelium intact omental arteries obtained from preeclamptic women (PE, n=9) as compared to normal pregnant women (NP, n=6). B) Enhanced vessel reactivity to Ang II was blocked by pretreatment with a combination of superoxide dismutase and catalase (SOD/Cat) or RhoA kinase (ROK) inhibitor (n=4). (*** p<0.001 for treatment effects)
Neutrophil infiltration into omental fat vessels
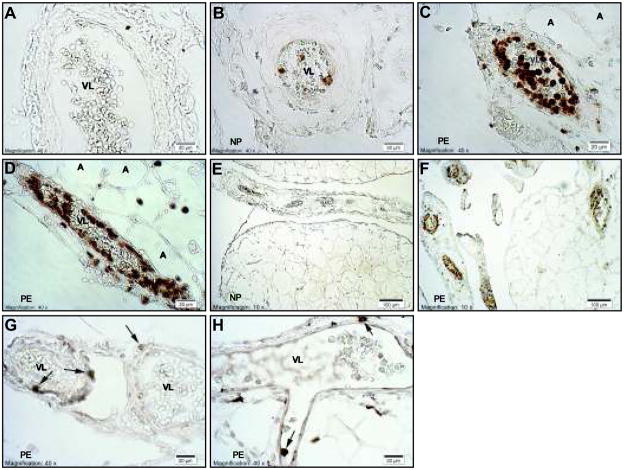
Figure 2 shows immunostaining for CD66b, a neutrophil antigen (Panels A–F), CD99, a lymphocyte antigen (Panel G) and CD14, a monocyte/macrophage antigen (Panel H) in omental fat vessels of normal pregnant (n=3) and preeclamptic patients (n=3). Visual staining scores and % vessels stained data are shown in Online Supplement Figure S2. The visual staining score (0–4) for CD66b was significantly greater for preeclamptic patients than for normal pregnant patients (2.8 ± 0.03 vs, 0.39 ± 0.26, P<0.01). Neutrophil staining was present in 82 ± 8 % of vessels of preeclamptic patients as compared to 25 ± 13 % of vessels in normal pregnant patients (P<0.05). Vessels of preeclamptic patients showed extensive staining of neutrophils in the lumen, adhered and flattened along the endothelium and infiltrated to the vascular smooth muscle (Panels C, D, F). Few vessels stained for CD99 or CD14 and there were no differences in staining between preeclamptic and normal pregnancy (CD99: visual score, 0.23 ± 0.06 vs. 0.25 ± 0.06 and % vessels stained, 16.6 ± 3.4 vs 17.3 ± 4.7%; CD14: visual score, 0.26 ± 0.03 vs 0.34 ± 0.05 and % vessels stained, 24.7 ± 1.0 vs 20.7 ± 2.2 %, respectively).
Figure 2.
Representative sections of omental fat vessels immunostained for CD66b, a neutrophil antigen, CD99, a lymphocyte antigen and CD14, a monocyte/macrophage antigen. A) Isotype negative control for CD66b (preeclamptic). B) normal pregnancy for CD66b. Vessels of normal pregnant patients had some brown staining for neutrophils primarily in the lumen. C and D) preeclamptic pregnancy for CD66b. Vessels of preeclamptic patients showed extensive brown staining of neutrophils in the lumen, adhered and flattened along the endothelium and infiltrated to the vascular smooth muscle. E and F) lower magnification of CD66b staining showing little to no staining of vessels in normal pregnancy (E) as compared to almost all vessels showing extensive staining in preeclampsia (F). G) CD99 staining and H) CD14 staining in preeclamptic vessels. Few vessels showed staining for CD99 or CD14 and when they did, there were only one or two lymphocytes or monocytes/macrophages per vessel (arrows). A, adipocyte; VL, vessel lumen; NP, normal pregnancy; PE, preeclamptic pregnancy. All images were taken with a 40X lens except panels E and F taken with a 10X lens.
Effect of neutrophil products, ROS and tumor necrosis factor-alpha (TNFα)
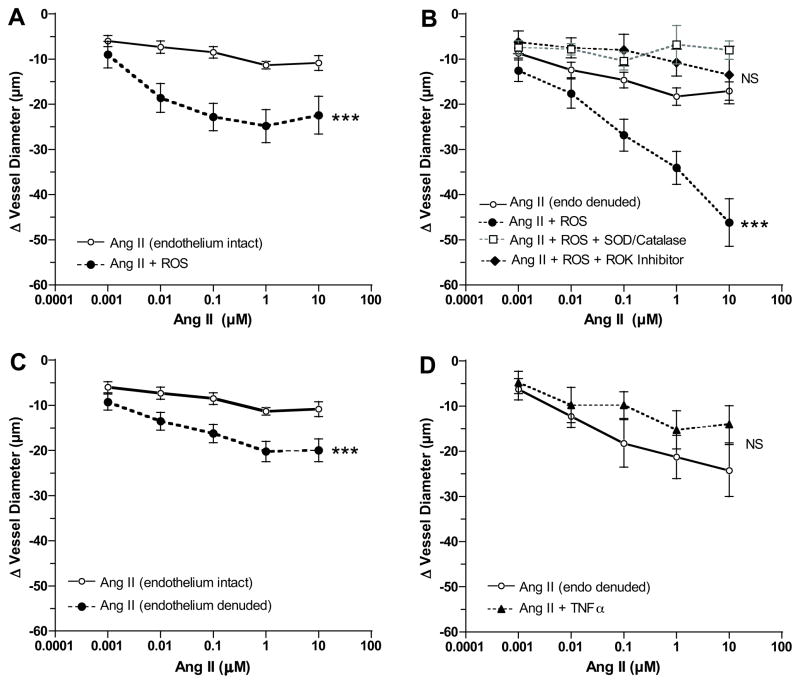
To determine if neutrophils or neutrophil products could enhance vessel reactivity, normal pregnant arteries were used. The effect of ROS was studied in both endothelium intact and endothelium denuded arteries to determine the role of the endothelium in enhanced vascular reactivity. ROS alone did not cause significant vessel contraction, however, ROS significantly enhanced vascular reactivity to Ang II in endothelium-intact vessels (Fig. 3A). When ROS was tested in endothelium-denuded vessels (Fig. 3B), reactivity to Ang II was much greater than in endothelium-intact vessels demonstrating that enhanced vascular reactivity was independent of the endothelium. Pretreatment with SOD/catalase to quench ROS or ROK inhibitor significantly inhibited ROS induced enhancement of vascular reactivity to Ang II (Fig. 3B). Removal of the endothelium resulted in a significant increase in vessel reactivity to Ang II (Fig. 3C), but less than that induced by ROS. In contrast to the enhancing effect of ROS, TNFα at a concentration higher than circulating levels in preeclampsia 16 did not alter vascular reactivity to Ang II (Fig. 3D).
Figure 3.
Vessel reactivity of omental arteries of normal pregnant women to angiotensin II (Ang II) in response to neutrophil products and presence or absence of endothelium. A) A reactive oxygen species generating solution (ROS) significantly enhanced vessel reactivity to Ang II in endothelium intact vessels (n=6). B) ROS enhanced vessel reactivity to Ang II to a greater extent when endothelium was removed (endo denuded) as compared to when endothelium was intact (n=19). Pretreatment with superoxide dismutase and catalase (SOD/Catalase) to quench ROS (n=5) or RhoA kinase (ROK) inhibitor (n=4) abolished enhanced vessel reactivity to Ang II in response to ROS. C) Removal of the endothelium enhanced vessel reactivity to Ang II (n=21) as compared to when endothelium was intact (n=6), but the enhancement was much less than that induced by ROS. D) Tumor necrosis factor-alpha (TNFα), another neutrophil product, did not enhance arterial reactivity to Ang II (n=4). (*** p<0.001 for treatment effects; NS, non-significant as compared to Ang II alone)
Effect of neutrophils
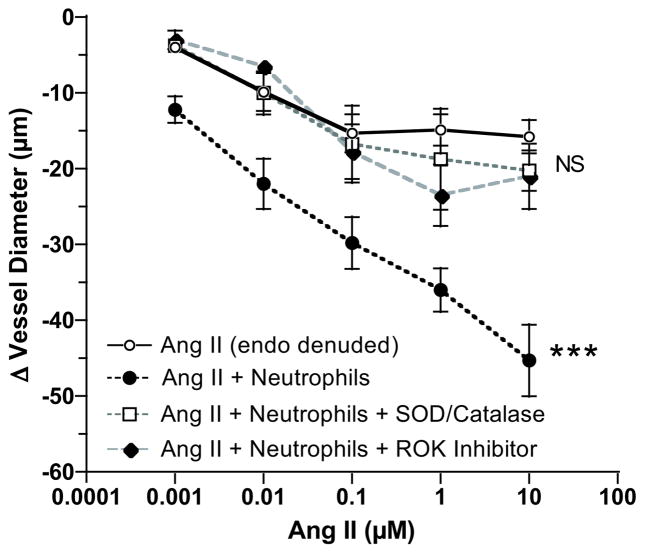
Perfusion of neutrophils activated with interleukin-8 through the vessel lumen did not significantly affect vessel contractility, however, when the Ang II dose response was repeated in the presence of activated neutrophils, vascular reactivity was significantly enhanced similar to ROS (Fig. 4). Pretreatment with SOD/catalase significantly blocked enhanced vascular reactivity to Ang II. ROK inhibitor also abolished enhanced vascular reactivity to Ang II in the presence of activated neutrophils. Perfusion through the vessel lumen of either un-activated neutrophils or interleukin-8 alone did not affect the Ang II dose response (Online Figure S3).
Figure 4.
Vessel reactivity of endothelium denuded (endo denuded) omental arteries of normal pregnant women to angiotensin II (Ang II) in response to activated neutrophils. Omental arterial reactivity to Ang II was significantly enhanced with perfusion of activated neutrophils through the vessel lumen (n=11) similar to that observed with ROS in Figure 3. Pretreatment with superoxide dismutase and catalase (SOD/Catalase) to quench ROS (n=4) or RhoA kinase (ROK) inhibitor (N=4) abolished neutrophil enhanced vessel reactivity to Ang II. (*** p<0.001 for treatment effect; NS, non-significant as compared to Ang II alone)
Change in resistance
Resistance is inversely proportional to the fourth power of the radius, so changes in vessel diameter induced by Ang II resulted in large increases in resistance in preeclamptic vessels and normal vessels treated with ROS or neutrophils. The highest dose of Ang II increased resistance 7.4-fold in preeclamptic vessels as compared to normal vessels. Similarly, treatment of normal vessels with ROS or neutrophils resulted in 3.6-fold and 4.1-fold increases, respectively.
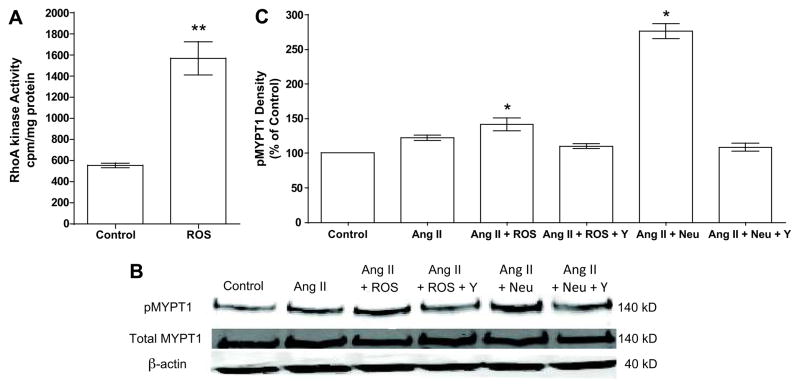
Effect of neutrophils and ROS on RhoA kinase activity and phosphorylation of MYPT1 and MLC
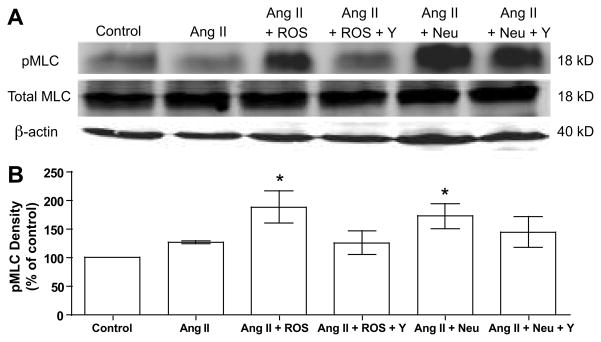
To study the direct effect of ROS on RhoA kinase activity in human vessels, omental arteries were treated with ROS. ROS resulted in a 3-fold increase in RhoA kinase activity as compared to untreated arteries (Fig. 5A). To study phosphorylation of key proteins in the RhoA kinase pathway, human vascular smooth muscle cells (VSMC) were used and exposed to the treatments used for vascular reactivity. Ang II alone slightly increased phosphorylation of MYPT1 and MLC, but not significantly (Fig. 5B & 5C, Fig. 6). However, when Ang II was combined with either ROS or neutrophils, phosphorylation of MYPT1 and MLC was significantly increased as compared to control. The increased phosphorylation of MLC is consistent with inhibition of myosin light chain phosphatase by phosphorylated MYPT1.
Figure 5.
RhoA kinase activity in human omental arteries and expression of phosphorylated myosin phosphatase target subunit 1 (pMYPT1) in human vascular smooth muscle cells. A) A reactive oxygen species generating solution (ROS) caused a 3-fold increase in RhoA kinase activity in omental arteries as compared to untreated control arteries (n=5, ** p<0.01). B) Representative Western blot of Thr696 pMYPT1, total MYPT1 and β-actin in cultured human vascular smooth muscle cells exposed to the treatments used in the myograph vascular reactivity experiments. C) Density of pMYPT1 plotted as percentage of control (n=3). Angiotensin II (Ang II) in the presence of ROS or activated neutrophils (Neu) significantly enhanced phosphorylation of MYPT1. (Y, RhoA kinase inhibitor Y-27632) (* P<0.05, ** P<0.01 as compared to control)
Figure 6.
Expression of phosphorylated myosin light chain (pMLC). A) Representative Western blot of Ser19-pMLC, total MLC and β-actin in cultured human vascular smooth muscle cells. B) Density of pMLC plotted as percentage of control (n=5). Vascular smooth muscle cells were treated as in Figure 5. Treatment with angiotensin II (Ang II) plus reactive oxygen species (ROS) or activated neutrophils (neu) significantly enhanced phosphorylation of MLC. (Y, RhoA kinase inhibitor Y-27632) (* P<0.05 as compared to control)
Norepinephrine (NE) dose response
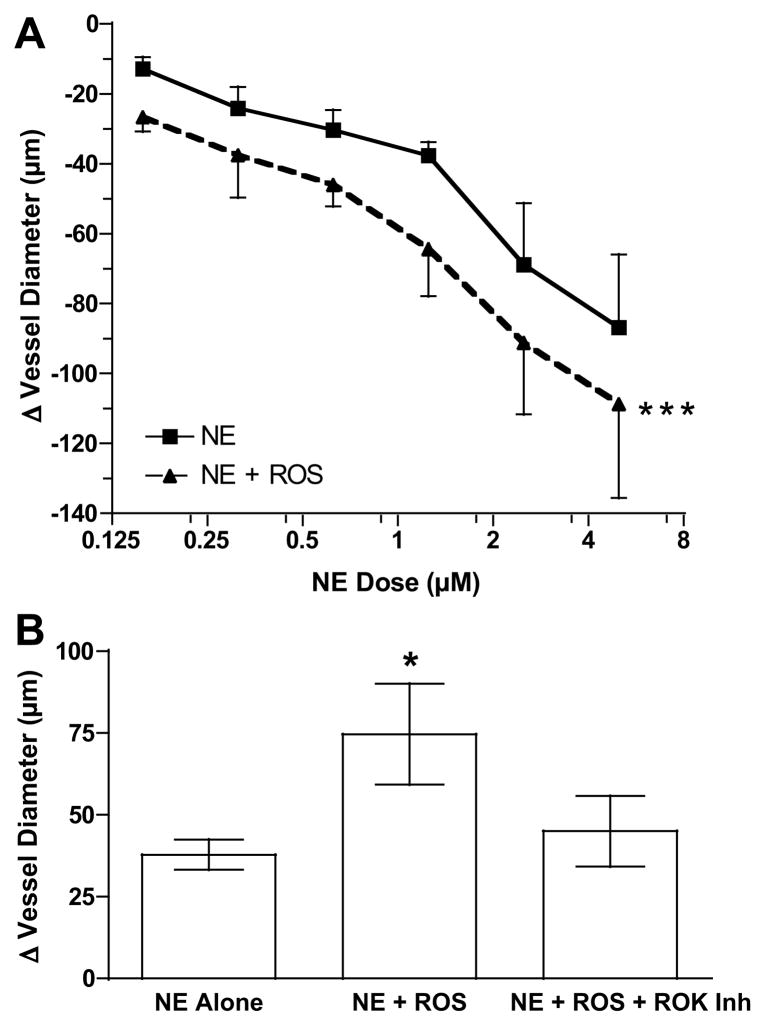
To determine if enhanced vascular reactivity was specific to Ang II, we examined another vasoconstrictor, NE. Since ROS was identified as the stimulator of the RhoA kinase pathway in the studies above, we evaluated vascular responsiveness to NE in the presence of ROS. As shown in Fig. 7A, ROS significantly enhanced the vasoconstrictive response to NE. To test the role of the RhoA kinase pathway, a midrange dose for NE of 1.25 μmol/L was chosen. The increase in vasoconstrictive response to NE by ROS was significantly inhibited by ROK inhibitor (Fig. 7B).
Figure 7.
Vessel reactivity to norepinephrine (NE). A) Omental arterial reactivity to NE was significantly enhanced in the presence of reactive oxygen species (ROS) (n=6, *** p<0.001 for treatment effect compared to NE alone). B) Addition of RhoA kinase (ROK) inhibitor abolished enhanced vessel reactivity to NE in response to ROS at 1.25 μmol/L dose of NE (n=4, * p<0.05).
DISCUSSION
In this study we demonstrate enhanced vascular reactivity to Ang II of omental arteries obtained from preeclamptic women as compared to omental arteries obtained from normal pregnant women. Enhanced vessel reactivity was due to ROS activation of the RhoA kinase pathway because it was abolished by pretreatment with either SOD/catalase or ROK inhibitor. Normal pregnant vessels were relatively resistant to the vasoconstrictor effect of angiotensin II, which is consistent with a previous report using a wire myograph system and omental vessel rings 17. If neutrophil infiltration begins before clinical symptoms, our study may explain the enhanced blood pressure response to angiotensin II observed by Gant et al in women who went on to develop preeclampsia2.
We also demonstrate extensive infiltration of neutrophils, but not monocytes or lymphocytes, into omental fat vessels in preeclamptic women which is consistent with our previous reports for subcutaneous fat vessels 7, 8, 18. We provide evidence that neutrophils are the likely source of ROS for enhanced vascular reactivity because enhanced reactivity of preeclamptic vessels could be mimicked in arteries of normal pregnant women by exposing them to ROS or perfusing them with activated neutrophils. In both cases, quenching ROS or inhibiting ROK blocked enhanced vessel reactivity. Neither ROS alone nor neutrophils alone caused a significant change in vessel diameter. Their effect was only manifest in the presence of a vasoconstrictor hormone. TNFα, another neutrophil product, was ineffective in acutely enhancing vessel reactivity, however we cannot rule out an effect of a longer exposure that would affect gene expression.
The number of neutrophils present in preeclamptic vessels is remarkable. Normal pregnancy is associated with a leukocytosis that is due to an increase in neutrophils. Their number increases 2.5-fold by 30 weeks of gestation 19 and increases significantly more in preeclampsia 20. Our data demonstrate that the increased number of neutrophils in preeclampsia represent activated neutrophils because CD66b is expressed on the cell surface of activated neutrophils 21. CD66b is thought to play a role in cell adhesion during extravasation. The fact that preeclampsia is most commonly diagnosed in the third trimester when neutrophil numbers are increased lends credence to the notion that neutrophils are causative of clinical symptoms.
To confirm the role of ROS activation of the RhoA kinase pathway, we measured RhoA kinase activity in omental arteries and phosphorylation of key proteins of the pathway in cultured human vascular smooth muscle cells. Treatment of omental arteries with ROS resulted in a 3-fold increase in the activity of RhoA kinase. Treatment of cultured cells with the treatments used for the vascular reactivity myograph studies demonstrated that Ang II in the presence of ROS or activated neutrophils significantly increased phosphorylation of MLC and MYPT1.
ROS activation of the RhoA kinase pathway may not be the only mechanism for enhanced vascular reactivity in preeclampsia. Mechanisms may also involve deficiencies in endothelial vasodilators, such as prostacyclin and nitric oxide. Decreased prostacyclin in preeclampsia is well documented 22, 23, and endothelial nitric oxide may also be decreased because peroxynitrite is increased in maternal endothelium in preeclampsia 24. Superoxide reacts with nitric oxide to form peroxynitrite; so increased peroxynitrite suggests an endothelial deficiency of nitric oxide. Both of these deficiencies could be due to the infiltration of neutrophils because ROS inhibit prostacyclin synthase 25, 26 and superoxide specifically reacts with nitric oxide to form peroxynitrite. To evaluate the role of endothelial factors, we compared vascular response in endothelium intact and endothelium denuded vessels. Vascular reactivity to Ang II was enhanced when the endothelium was removed, but the enhancement was only 21 – 31% of that induced by ROS, neutrophils or that present in preeclamptic vessels. This suggests enhanced vascular reactivity in preeclampsia is primarily due to ROS activation of the RhoA kinase pathway, although deficiency of endothelial vasodilators also contributes.
The ability of ROS to enhance vascular reactivity via the RhoA kinase pathway was not specific to Ang II as ROS also enhanced vascular reactivity to NE. This finding is pertinent to preeclampsia because NE is induced by stress and sympathetic activation, factors reported to be associated with preeclampsia 27–30, and previous work has shown that in vitro vascular reactivity to NE is increased in omental arteries of preeclamptic women as compared to normal pregnant women 31.
PERSPECTIVES
In this study we demonstrate that enhanced vascular reactivity of omental arteries of preeclamptic women is primarily due to ROS activation of the RhoA kinase pathway, and provide support for the idea that enhanced vascular reactivity is likely due to the infiltration of activated neutrophils. We previously proposed that neutrophils are activated as they circulate through the intervillous space and are exposed to increased levels of oxidized lipids secreted by the placenta 32–34. This contention is supported by the observation that neutrophils in the uterine vein express significantly higher levels of integrins than neutrophils in the antecubital vein in preeclamptic, but not normal pregnant, women 35. Other possible mechanisms for neutrophil activation include exposure to elevated plasma levels of matrix metalloproteinase-1 or activating autoantibodies against angiotensin II receptor 1 in preeclamptic women 36, 37. Matrix metalloproteinase-1 activates neutrophils 36 and activating autoantibodies against angiotensin II receptor 1 stimulate NADPH oxidase in vascular smooth muscle 38, and so, might also stimulate NADPH oxidase in neutrophils.
This study could provide novel avenues for treatment of hypertension based on regulation of neutrophils, their products or their cellular effects. Potential treatments that are becoming available for clinical studies are neutralizing antibodies against adhesion molecules necessary for neutrophil infiltration and selective RhoA kinase inhibitors. Antioxidant therapy with vitamins C and E has been tried, but was ineffective in preventing preeclampsia in clinical trials 39, 40. The high doses of vitamin C used in these trials may have been counter productive because vitamin C is a potent oxidant in the presence of free iron, which is elevated in blood of women with preeclampsia 41, 42. On the other hand, meta-analysis of low-dose aspirin trials demonstrates aspirin is effective in preventing preeclampsia 43, 44, the effectiveness of which is related to patient compliance 45. The ability of aspirin to reduce preeclampsia may be due to its ability to inhibit neutrophil superoxide production 46, which would be consistent with the findings of our study.
Supplementary Material
Acknowledgments
We would like to thank Dr. Srinivasan Karnam for his help and support with the RhoA kinase activity assay funded by NIH Grant RO1 DK15564, Department of Physiology & Biophysics, Virginia Commonwealth University Medical Center, Richmond, VA
SOURCES OF FUNDING
Supported by a grant to SWW from the NIH, National Heart, Lung and Blood Institute (R01 HL069851)
Footnotes
CONFLICT OF INTEREST/DISCLOSURE
None
References
- 1.Cunningham G. In: Williams Obstetrics. 22. Cunningham G, editor. McGraw Hill Inc. Companies; 2005. [Google Scholar]
- 2.Gant NF, Daley GL, Chand S, Whalley PJ, MacDonald PC. A study of angiotensin II pressor response throughout primigravid pregnancy. J Clin Invest. 1973;52:2682–2689. doi: 10.1172/JCI107462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown MA. The physiology of pre-eclampsia. Clin Exp Pharmacol Physiol. 1995;22:781–791. doi: 10.1111/j.1440-1681.1995.tb01937.x. [DOI] [PubMed] [Google Scholar]
- 4.Brown MA, Wang J, Whitworth JA. The renin-angiotensin-aldosterone system in pre-eclampsia. Clin Exp Hypertens. 1997;19:713–726. doi: 10.3109/10641969709083181. [DOI] [PubMed] [Google Scholar]
- 5.de Jong CL, Dekker GA, Sibai BM. The renin-angiotensin-aldosterone system in preeclampsia. A review. Clin Perinatol. 1991;18:683–711. [PubMed] [Google Scholar]
- 6.Pedersen EB, Christensen NJ, Christensen P, Johannesen P, Kornerup HJ, Kristensen S, Lauritsen JG, Leyssac PP, Rasmussen A, Wohlert M. Preeclampsia -- a state of prostaglandin deficiency? Urinary prostaglandin excretion, the renin-aldosterone system, and circulating catecholamines in preeclampsia. Hypertension. 1983;5:105–111. doi: 10.1161/01.hyp.5.1.105. [DOI] [PubMed] [Google Scholar]
- 7.Leik CE, Walsh SW. Neutrophils infiltrate resistance-sized vessels of subcutaneous fat in women with preeclampsia. Hypertension. 2004;44:72–77. doi: 10.1161/01.HYP.0000130483.83154.37. [DOI] [PubMed] [Google Scholar]
- 8.Shah TJ, Walsh SW. Activation of NF-kappaB and expression of COX-2 in association with neutrophil infiltration in systemic vascular tissue of women with preeclampsia. Am J Obstet Gynecol. 2007;196(48):e1–8. doi: 10.1016/j.ajog.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 9.Lee DL, Webb RC, Jin L. Hypertension and RhoA/Rho-kinase signaling in the vasculature: highlights from the recent literature. Hypertension. 2004;44:796–799. doi: 10.1161/01.HYP.0000148303.98066.ab. [DOI] [PubMed] [Google Scholar]
- 10.Somlyo AP, Somlyo AV. Signal transduction through the RhoA/Rho-kinase pathway in smooth muscle. J Muscle Res Cell Motil. 2004;25:613–615. doi: 10.1007/s10974-004-3146-1. [DOI] [PubMed] [Google Scholar]
- 11.Jin L, Ying Z, Webb RC. Activation of Rho/Rho kinase signaling pathway by reactive oxygen species in rat aorta. Am J Physiol Heart Circ Physiol. 2004;287:H1495–1500. doi: 10.1152/ajpheart.01006.2003. [DOI] [PubMed] [Google Scholar]
- 12.Jernigan NL, Walker BR, Resta TC. Reactive oxygen species mediate RhoA/Rho kinase-induced Ca2+ sensitization in pulmonary vascular smooth muscle following chronic hypoxia. Am J Physiol Lung Cell Mol Physiol. 2008;295:L515–529. doi: 10.1152/ajplung.00355.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griendling KK, Ushio-Fukai M. Reactive oxygen species as mediators of angiotensin II signaling. Regul Pept. 2000;91:21–27. doi: 10.1016/s0167-0115(00)00136-1. [DOI] [PubMed] [Google Scholar]
- 14.Edwards SW, editor. Biochemistry and Physiology of Neutrophil. Melbourne: Cambridge University Press; 1994. [Google Scholar]
- 15.Goldsby RA, Kindt TJ, Osborne BA. Kuby Immunology. 4. W. H. Freeman and Co; 2000. [Google Scholar]
- 16.Kupferminc MJ, Peaceman AM, Wigton TR, Rehnberg KA, Socol ML. Tumor necrosis factor-α is elevated in plasma and amniotic fluid of patients with severe preeclampsia. Am J Obstet Gynecol. 1994;170:1752–1759. [PubMed] [Google Scholar]
- 17.Aalkjaer C, Danielsen H, Johannesen P, Pedersen EB, Rasmussen A, Mulvany MJ. Abnormal vascular function and morphology in pre-eclampsia: a study of isolated resistance vessels. Clin Sci (Lond) 1985;69:477–482. doi: 10.1042/cs0690477. [DOI] [PubMed] [Google Scholar]
- 18.Cadden KA, Walsh SW. Neutrophils, but not lymphocytes or monocytes, infiltrate maternal systemic vasculature in women with preeclampsia. Hypertens Pregnancy. 2008;27:396–405. doi: 10.1080/10641950801958067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veenstra van Nieuwenhoven AL, Bouman A, Moes H, Heineman MJ, de Leij LF, Santema J, Faas MM. Cytokine production in natural killer cells and lymphocytes in pregnant women compared with women in the follicular phase of the ovarian cycle. Fertil Steril. 2002;77:1032–1037. doi: 10.1016/s0015-0282(02)02976-x. [DOI] [PubMed] [Google Scholar]
- 20.Lurie S, Frenkel E, Tuvbin Y. Comparison of the differential distribution of leukocytes in preeclampsia versus uncomplicated pregnancy. Gynecol Obstet Invest. 1998;45:229–231. doi: 10.1159/000009973. [DOI] [PubMed] [Google Scholar]
- 21.Ducker TP, Skubitz KM. Subcellular localization of CD66, CD67, and NCA in human neutrophils. J Leukoc Biol. 1992;52:11–16. doi: 10.1002/jlb.52.1.11. [DOI] [PubMed] [Google Scholar]
- 22.Mills JL, DerSimonian R, Raymond E, Morrow JD, Roberts LJ, 2nd, Clemens JD, Hauth JC, Catalano P, Sibai B, Curet LB, Levine RJ. Prostacyclin and thromboxane changes predating clinical onset of preeclampsia: a multicenter prospective study. JAMA. 1999;282:356–362. doi: 10.1001/jama.282.4.356. [DOI] [PubMed] [Google Scholar]
- 23.Wang YP, Walsh SW, Guo JD, Zhang JY. The imbalance between thromboxane and prostacyclin in preeclampsia is associated with an imbalance between lipid peroxides and vitamin E in maternal blood. Am J Obstet Gynecol. 1991;165:1695–1700. doi: 10.1016/0002-9378(91)90017-l. [DOI] [PubMed] [Google Scholar]
- 24.Roggensack AM, Zhang Y, Davidge ST. Evidence for peroxynitrite formation in the vasculature of women with preeclampsia. Hypertension. 1999;33:83–89. doi: 10.1161/01.hyp.33.1.83. [DOI] [PubMed] [Google Scholar]
- 25.Wang JA, Zhen EZ, Guo ZZ, Lu YC. Effect of hyperlipidemic serum on lipid peroxidation, synthesis of prostacyclin and thromboxane by cultured endothelial cells: protective effect of antioxidants. Free Radic Biol Med. 1989;7:243–249. doi: 10.1016/0891-5849(89)90131-7. [DOI] [PubMed] [Google Scholar]
- 26.Warso MA, Lands WE. Lipid peroxidation in relation to prostacyclin and thromboxane physiology and pathophysiology. Br Med Bull. 1983;39:277–280. doi: 10.1093/oxfordjournals.bmb.a071833. [DOI] [PubMed] [Google Scholar]
- 27.Leeners B, Neumaier-Wagner P, Kuse S, Stiller R, Rath W. Emotional stress and the risk to develop hypertensive diseases in pregnancy. Hypertens Pregnancy. 2007;26:211–226. doi: 10.1080/10641950701274870. [DOI] [PubMed] [Google Scholar]
- 28.Klonoff-Cohen HS, Cross JL, Pieper CF. Job stress and preeclampsia. Epidemiology. 1996;7:245–249. doi: 10.1097/00001648-199605000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Luecken LJ, Suarez EC, Kuhn CM, Barefoot JC, Blumenthal JA, Siegler IC, Williams RB. Stress in employed women: impact of marital status and children at home on neurohormone output and home strain. Psychosom Med. 1997;59:352–359. doi: 10.1097/00006842-199707000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Schobel HP, Fischer T, Heuszer K, Geiger H, Schmieder RE. Preeclampsia -- a state of sympathetic overactivity. N Engl J Med. 1996;335:1480–1485. doi: 10.1056/NEJM199611143352002. [DOI] [PubMed] [Google Scholar]
- 31.Belfort MA, Saade GR, Suresh M, Kramer W, Vedernikov YP. Effects of selected vasoconstrictor agonists on isolated omental artery from premenopausal nonpregnant women and from normal and preeclamptic pregnant women. Am J Obstet Gynecol. 1996;174:687–693. doi: 10.1016/s0002-9378(96)70451-9. [DOI] [PubMed] [Google Scholar]
- 32.Walsh SW. Lipid peroxidation in pregnancy. Hypertens Pregn. 1994;13:1–32. [Google Scholar]
- 33.Walsh SW. Maternal-placental interactions of oxidative stress and antioxidants in preeclampsia. Semin Reprod Endocrinol. 1998;16:93–104. doi: 10.1055/s-2007-1016256. [DOI] [PubMed] [Google Scholar]
- 34.Walsh SW, Vaughan JE, Wang Y, Roberts LJ., II Placental isoprostane is significantly increased in preeclampsia. FASEB J. 2000;14:1289–1296. doi: 10.1096/fj.14.10.1289. [DOI] [PubMed] [Google Scholar]
- 35.Mellembakken JR, Aukrust P, Olafsen MK, Ueland T, Hestdal K, Videm V. Activation of leukocytes during the uteroplacental passage in preeclampsia. Hypertension. 2002;39:155–160. doi: 10.1161/hy0102.100778. [DOI] [PubMed] [Google Scholar]
- 36.Estrada-Gutierrez G, Cappello RE, Mishra N, Romero R, Strauss JF, 3rd, Walsh SW. Increased expression of matrix metalloproteinase-1 in systemic vessels of preeclamptic women a critical mediator of vascular dysfunction. Am J Pathol. 2011;178:451–460. doi: 10.1016/j.ajpath.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siddiqui AH, Irani RA, Blackwell SC, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibody is highly prevalent in preeclampsia: correlation with disease severity. Hypertension. 2010;55:386–393. doi: 10.1161/HYPERTENSIONAHA.109.140061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dechend R, Viedt C, Muller DN, Ugele B, Brandes RP, Wallukat G, Park JK, Janke J, Barta P, Theuer J, Fiebeler A, Homuth V, Dietz R, Haller H, Kreuzer J, Luft FC. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation. 2003;107:1632–1639. doi: 10.1161/01.CIR.0000058200.90059.B1. [DOI] [PubMed] [Google Scholar]
- 39.Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet. 2006;367:1145–1154. doi: 10.1016/S0140-6736(06)68433-X. [DOI] [PubMed] [Google Scholar]
- 40.Roberts JM, Myatt L, Spong CY, Thom EA, Hauth JC, Leveno KJ, Pearson GD, Wapner RJ, Varner MW, Thorp JM, Jr, Mercer BM, Peaceman AM, Ramin SM, Carpenter MW, Samuels P, Sciscione A, Harper M, Smith WJ, Saade G, Sorokin Y, Anderson GB. Vitamins C and E to prevent complications of pregnancy-associated hypertension. N Engl J Med. 2010;362:1282–1291. doi: 10.1056/NEJMoa0908056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Entman SS, Kambam JR, Bradley CA, Cousar JB. Increased levels of carboxyhemoglobin and serum iron as an indicator of increased red cell turnover in preeclampsia. Am J Obstet Gynecol. 1987;156:1169–1173. doi: 10.1016/0002-9378(87)90134-7. [DOI] [PubMed] [Google Scholar]
- 42.Entman SS, Richardson LD, Killam AP. Elevated serum ferritin in the altered ferrokinetics of toxemia of pregnancy. Am J Obstet Gynecol. 1982;144:418–422. doi: 10.1016/0002-9378(82)90247-2. [DOI] [PubMed] [Google Scholar]
- 43.Askie LM, Duley L, Henderson-Smart DJ, Stewart LA. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369:1791–1798. doi: 10.1016/S0140-6736(07)60712-0. [DOI] [PubMed] [Google Scholar]
- 44.Bujold E, Roberge S, Lacasse Y, Bureau M, Audibert F, Marcoux S, Forest JC, Giguere Y. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116:402–414. doi: 10.1097/AOG.0b013e3181e9322a. [DOI] [PubMed] [Google Scholar]
- 45.Walsh SW. Eicosanoids in preeclampsia. Prostaglandins Leukot Essent Fatty Acids. 2004;70:223–232. doi: 10.1016/j.plefa.2003.04.010. [DOI] [PubMed] [Google Scholar]
- 46.Vaughan JE, Walsh SW, Ford GD. Thromboxane mediates neutrophil superoxide production in pregnancy. Am J Obstet Gynecol. 2006;195:1415–1420. doi: 10.1016/j.ajog.2006.02.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.