Abstract
BACKGROUND
There has been an ongoing debate in the reproductive field about whether mammalian spermatozoa rely on glycolysis, oxidative phosphorylation or both for their energy production. Recent studies have proposed that human spermatozoa depend mainly on glucose for motility and fertilization but the mechanism behind an efficient glycolysis in human spermatozoa is not well understood. Here, we demonstrate how human spermatozoa utilize exogenous pyruvate to enhance glycolytic ATP production, motility, hyperactivation and capacitation, events that are crucial for male fertility.
METHODS
Purified human spermatozoa from healthy donors were incubated under capacitating conditions (including albumin, bicarbonate and glucose) and tested for changes in ATP levels, motility, hyperactivation and tyrosine phosphorylation after treatment with pyruvate. The experiments were repeated in the presence of sodium cyanide in order to assess the contribution from mitochondrial respiration. The metabolism of 13C labeled glucose and pyruvate was traced by a combination of liquid chromatography and mass spectrometry.
RESULTS
The treatment of human spermatozoa with exogenous pyruvate increased intracellular ATP levels, progressive motility and hyperactivation by 56, 21 and 130%, respectively. In addition, added pyruvate induced a significant increase in tyrosine phosphorylation levels. Blocking of the electron transport chain did not markedly affect the results, indicating that the mechanism is independent of oxidative phosphorylation. However, the observed effects could be counteracted by oxamate, an inhibitor of lactate dehydrogenase (LDH). Metabolic tracing experiments revealed that the observed rise in ATP concentration resulted from an enhanced glycolytic flux, which was increased by more than 50% in the presence of exogenous pyruvate. Moreover, all consumed 13C labeled pyruvate added was converted to lactate rather than oxidized in the tricarboxylic acid cycle.
CONCLUSIONS
Human spermatozoa seem to rely mainly, if not entirely, on glycolysis as the source of ATP fueling the energy-demanding processes of motility and capacitation. The efficient glycolysis is dependent on exogenous pyruvate, which indirectly feeds the accelerated glycolysis with NAD+ through the LDH-mediated conversion of pyruvate to lactate. Pyruvate is present in the human female reproductive tract at concentrations in accordance with our results. As seen in other mammals, the motility and fertility of human spermatozoa seem to be dictated by the available energy substrates present in the conspecific female.
Keywords: capacitation, glycolysis, human spermatozoa, pyruvate, sperm metabolism
Introduction
Mammalian spermatozoa are dependent on an efficient generation of ATP to fuel the progressive and hyperactive motility crucial for capacitation and fertilization (Ho et al., 2002). While progressive motility is essential for spermatozoa to travel through the cervix to the oviduct, the vigorous and asymmetrical swimming pattern of hyperactivation aids the release of spermatozoa from the oviductal epithelium (Demott and Suarez, 1992) and subsequent penetration of the oocyte cell membrane (Stauss et al., 1995). Hyperactive motility and tyrosine phosphorylation represent two hallmarks of capacitation, a process required to prepare spermatozoa for fertilization.
The main question has been how spermatozoa provide the vast amount of ATP needed for these energy-demanding processes. It is known that mammalian spermatozoa solve their energy requirements by switching between different metabolic pathways depending on oxygen availability and the composition of metabolic substrates in their environment. Glucose, pyruvate and lactate are present at high concentrations in oviductal fluid (Ruiz-Pesini et al., 2007) and hence they are commonly utilized as energy substrates by mammalian spermatozoa (Hoshi et al., 1991; Williams and Ford, 2001; Mukai and Okuno, 2004). In addition, other carbon sources such as sorbitol (Cao et al., 2009), glycerol (Jones et al., 1992) and fructose (Murdoch and White, 1968; Rigau et al., 2001) can be utilized. The use of metabolic substrates for ATP production has been shown further to vary between species (Storey, 2008). Bull spermatozoa rely primarily on oxidative phosphorylation to support capacitation from oxidizable substrates (Hutson et al., 1977; Van et al., 1977), whereas human spermatozoa rely substantially on glucose-derived ATP under capacitation to perform hyperactivation (Hoshi et al., 1991; Williams and Ford, 2001), tyrosine phosphorylation (Travis et al., 2001) and fertilization in vitro (Mahadevan et al., 1997). Despite these findings, it has been unclear and debated in the field whether glycolysis or oxidative phosphorylation is the major contributor of the ATP needed for fertilization in humans (Ford, 2006; Ruiz-Pesini et al., 2007). In spermatozoa, these two metabolic pathways are sub-cellularly separated. The mitochondria are concentrated exclusively in the midpiece, whereas the glycolytic enzymes are tightly anchored to the fibrous sheath (FS) in the flagellum. As mitochondria are rather large organelles, this particular organization may have evolved to allow for the propeller motion of the tail. Because of the long distance between the mitochondria and the distal end of the speed-generating flagellum (35–256 µm) (Cummins and Woodall, 1985), it has been questioned if ATP diffusion- and carrier systems are able to supply the tail with mitochondrial ATP supporting motility and hyperactivation (Nevo and Rikmenspoel, 1970; Adam and Wei, 1975; Turner, 2003; Kim et al., 2007). In addition, the ability of glycolysis to provide sufficient ATP for sperm motility and capacitation is disputed, since one molecule of glucose yields only two molecules of ATP if incompletely metabolized by glycolysis and lactic acid fermentation. By contrast, complete aerobic combustion of one molecule of glucose theoretically generates more than 30 molecules of ATP.
In order to rely on glycolysis-derived ATP for motility and capacitation, spermatozoa are dependent on the sperm-specific variant of lactate dehydrogenase (LDH-C) (Blanco and Zinkham, 1963; Goldberg, 1963). LDH-C accounts for 80–100% of the LDH activity in human spermatozoa (Zinkham et al., 1964; Clausen and Ovlisen, 1965), catalyzing the reversible conversion of pyruvate to lactate with the concomitant conversion of nicotinamide adenine dinucleotide (NADH) to NAD+ during lactic acid fermentation. LDH-C has been shown to be essential for sperm motility (Rodriguez-Paez et al., 2002) and spermatozoa from mice carrying a targeted disruption of the LDH-C gene did not show tyrosine phosphorylation and hyperactive motility, resulting in impaired fertility (Odet et al., 2008).
In the present study, we investigated the effect of exogenous pyruvate on sperm ATP generation, motility and capacitation. The molecular mechanism was elucidated by metabolic tracing and liquid chromatography and mass spectrometry (LC-MS) experiments.
Materials and Methods
Media and reagents
Experiments were performed in sperm cell medium containing 138 mM NaCl, 5.3 mM KCl, 1.3 mM CaCl2, 0.5 mM MgCl2, 0.4 mM MgSO4, 0.4 mM KH2PO4, 0.3 mM Na2HPO4 and 4.2 mM NaHCO3, pH 7.4. For establishing capacitating conditions, spermatozoa were incubated in sperm cell medium supplemented with 1% human serum albumin (HSA) (Octapharma, Lachen, Switzerland) and 22 mM NaHCO3 (Sigma-Aldrich, St. Louis, USA) in a 5% CO2 atmosphere at 37°C (Burkman, 1991; Sukcharoen et al., 1995). Lysis buffer contained 1% sodium dodecyl sulfate (SDS) in phosphate-buffered saline (Sigma-Aldrich). Sodium cyanide (NaCN) was dissolved in distilled water (dH2O) to 1 M stocks, rotenone was dissolved in dimethylsulphoxide to 25 mM stocks, antimycin A was dissolved in ethanol to 1 mM stocks and aliquots were stored at −20°C (all from Sigma-Aldrich). Sodium oxamate (Sigma-Aldrich) was diluted in dH2O to 430 mM and stored at −20°C in aliquots. D-glucose, L-lactate and pyruvate (all obtained from Sigma-Aldrich) were stored as 1 M stocks at 4°C. 13C6 glucose and 13C1 pyruvate (both from Larodan, Malmø, Sweden) were diluted in dH2O to 1 M and stored at 4°C. Methylene blue solution was obtained from Sigma-Aldrich.
Sperm samples and preparation
Human spermatozoa were obtained from freshly ejaculated semen from healthy donors after 3 days of abstinence. Normal morphology and motility (WHO standards) was assessed in a computer-assisted sperm analyzer (CASA) (HTM-IVOS system, version 12, Hamilton-Thorne Research, Beverly, USA). Semen samples were liquefied at 37°C in a shaker for 30 min. Spermatozoa were purified by discontinuous percoll density centrifugation. One part 1.54 M NaCl and nine parts percoll (Sigma-Aldrich) were mixed to make a 90% percoll solution. This solution was diluted again in Hanks Balanced Salt Solution (HBSS) (catalogue/product number 24020 Invitrogen) to make 80 and 40% percoll working solutions. The semen samples were added on top of two cushions of 80 and 40% percoll and centrifuged at 1250g for 20 min. Motile cells were collected from the lower 80% percoll layer. Spermatozoa were then washed once in HBSS and kept in sperm cell medium supplemented with 5 mM glucose at room temperature. Immediately before the experiments, cells were washed twice in glucose-free sperm cell medium.
ATP measurements
Endogenous ATP concentrations were measured in a luciferase-based kit (ATPlite) from Perkin Elmer (Boston, USA). Human spermatozoa were diluted to 2 × 106/ml in sperm cell medium and incubated under capacitating conditions in a 96-well white microtiter plate (Nunc, Roskilde, Denmark). Luminescence was measured by a Gemini EM microplate spectrofluorometer (Molecular Devices, Sunnyvale, USA) and mol ATP was determined according to a standard curve. The effect of pyruvate and oxamate on endogenous ATP concentrations was studied by incubation of spermatozoa in the presence of 28 nM–5 mM and 3.6 µM–15 mM pyruvate and oxamate, respectively for 30 and/or 120 min. In the mitochondrial respiration inhibition experiments, purified spermatozoa were incubated for 120 min with increasing concentrations of NaCN (24 µM–25 mM), rotenone (128 pM–50 µM) and antimycin A (5 nM–2 µM) in the absence or presence of different metabolic substrates as described in the figure legends. The effect of methylene blue on ATP levels was investigated by incubation of spermatozoa with 10 mM NaCN, 10 µM rotenone or 1 µM antimycin A in the presence of 5 mM glucose and either 5 mM pyruvate or 50 µM methylene blue for 120 min. The concentration of methylene blue used was determined by a dose–response experiment.
Motility experiments
A HTM-IVOS system (Hamilton-Thorne Research) was used for motility analysis with the following settings: Slow spermatozoa were counted as static. Progressive cells were defined as average path velocity >25 µm/s and straightness >80%. Number of frames: 30, frame rate: 30 Hz. Parameters measured included curvilinear velocity (VCL, µm/s) which is defined as the time-average velocity of a sperm head along its actual curvilinear trajectory and amplitude of lateral head displacement (ALH, µm) which describes the magnitude of lateral displacement of a sperm head about its spatial average trajectory. Hyperactive spermatozoa were defined by Burkman (1991) and set to linearity (LIN, %) <65, ALH (µm) >7.5 and VCL (µm/s) >100. All motility experiments were performed under capacitating conditions. Cells were diluted to 2 × 107/ml and incubated in 48 wells cell culture plates (Corning, Schiphol-Rijk, The Netherlands) or eppendorf tubes. An aliquot of 5 µl of sperm solution was added to a 20 µm, two chambers slide (Leja, Nieuw-Vennep, The Netherlands) and a total of 20 fields were counted per sample. The effect of pyruvate and oxamate on sperm motility was studied by incubation of spermatozoa in the presence of 64 nM–5 mM and 39 µM–40 mM pyruvate and oxamate, respectively for 30 and/or 120 min. To study the effect of an inhibited mitochondrial respiration on sperm motility, spermatozoa were treated with 10 mM NaCN in the presence or absence of 5 mM glucose, pyruvate or a combination of the metabolites and incubated for 120 min. Regeneration of motility by methylene blue was investigated by incubation of spermatozoa with 10 mM NaCN, 10 µM rotenone or 1 µM antimycin A in the presence of 5 mM glucose and either 5 mM pyruvate or 50 µM methylene blue for 120 min. To confirm the viability of spermatozoa treated with NaCN, cells were incubated with 5 mM glucose and 10 mM NaCN under capacitating conditions for 120 min and motility was measured by CASA. Five mM of pyruvate were then added and reactivation was tested in CASA at time 180 min.
Immunoblotting
Purified spermatozoa were diluted in sperm cell medium to 5 × 106/ml and incubated in 48 well plates. The non-capacitated control sample was diluted in sperm cell medium and placed on ice directly after purification. The rest of the samples were treated with or without 10 mM NaCN, 5 mM glucose and 5 mM pyruvate under capacitating conditions for 30 and 120 min and put straight on ice after incubation. The samples were centrifuged at 4°C, the supernatant was removed and the pellet resuspended in lysis buffer with freshly added phosphatase inhibitor cocktails (Sigma-Aldrich) before being heavily vortexed for 3 × 20 s. The samples were loaded on a 10% tris–glycin SDS–PAGE gel (105 cells/lane) and the proteins were transferred onto a polyvinylidene difluoride membrane (Millipore, Billerica, USA). The membrane was blotted with anti-phosphotyrosine (pY) antibodies (catalogue/product number 9411, Cell Signaling Technologies, Danvers, USA) in tris-buffered saline (TBS) supplied with 0.1% tween 20 (Sigma-Aldrich) and 4% skimmed milk. As loading control, antibodies against α-tubulin (catalogue/product number T9026, Sigma-Aldrich) were used. All blots were treated with anti-mouse-horse-radish peroxidase as the secondary antibody (catalogue/product number 55563, MP Biomedicals, Aurora, USA) and developed by the Supersignal West Dura Extended Duration Substrate from Pierce (Rockford, USA).
Cellular respiration assay
Oxygen consumption of capacitating spermatozoa at 37°C was determined by high-resolution respirometry (Oroboros Oxygraph-2K, Innsbruck, Austria). The integrated software (datlab 4.2) presents respiration as oxygen flux; pmol O2/106 cells/s. The stirrer speed was set to 750 r.p.m. Spermatozoa (1.5 × 107/ml) were incubated under capacitating conditions for 120 min and transferred to the chambers maintained at 37°C. Basal respiration was measured for 15 min followed by stepwise mitochondrial uncoupling by carbonyl cyanide-P-trifluoromethoxy-phenylhydrazone (FCCP) at step titration of 0.5 μM until maximal respiration was obtained. This respiration rate corresponds to respiration capacity of the spermatozoa under the given condition. NaCN was subsequently added at 1 mM aliquots up to 10 mM. Respiration rates for the individual batches of spermatozoa were presented relative to respiration capacity of spermatozoa supplemented with glucose and pyruvate in combination.
Metabolic tracing
2 × 107/ml purified spermatozoa (100 µl) were incubated in eppendorf tubes for 0 and 120 min under capacitating conditions in the presence of 1 mM 13C6 glucose and a combination of 1 mM 13C6 glucose and 1 mM 13C1 pyruvate. The experiment was performed both in the absence and in the presence of 10 mM NaCN. The samples were deproteinized with 300 µl methanol immediately after incubation, centrifuged at 16 000g and supernatant was collected for LC-MS. The quantitative LC-MS measurements of metabolites in the metabolic tracing experiments were performed on a Perkin Elmer series 200 HPLC system interfaced with an API 2000™ triple quadrupole mass spectrometer (Applied Biosystems/MDS Sciex, Canada) equipped with a TurboIonSpray™ electrospray. Chromatographic separation of the analytes was achieved by isocratic elution on a 2.1 × 150 mm Sunfire™ 5 µm particles C18 column (Waters, Milford, USA) operated at ambient temperature. The mobile phase (50% methanol in water) was delivered at 150 µl/min and 30 µl samples were injected. All samples were run in duplicate. For calculation of metabolite concentrations, calibration curves based on the peak area of calibrators containing 0, 0.1, 0.25, 0.5, 0.75 and 1.0 mM of 13C1 labeled pyruvate were used, as well as 0, 0.1, 0.25, 0.5, 0.75, 1.0, 1.5 and 2.0 mM of unlabeled lactate. All calibrators were run in each batch and separate NaCN-containing calibrators were used for the inhibition experiments since NaCN influenced the MS signal intensity. The MS was tuned for maximum signal intensity for each analyte and the ion source parameters set as follows: nebulizer gas 60 psi, electrospray voltage −4500 V, curtain gas 20 psi, temperature 250°C, turbo gas 80 psi. The analytes were identified and quantified in single ion monitoring (SIM) mode as their deproteonized molecular ions (mw-1), using the following individually optimized settings [SIM mass (m/z), declustering potential (V), entrance potential (V)]: 13C6 glucose (185.1, 16, 9.5), 13C3 lactate (92.1, 16, 8), 13C1 lactate and 13C3 pyruvate (90, 14, 9.5) and 13C1 pyruvate (88, 11, 9.5).
Statistics and presentation
All experiments were repeated between three and five times (see figure legends) with new donor materials. Data sets are presented as mean ± SEM and P-values were calculated from a Student's paired t-test. Dose–response curves were created and half-maximal effective concentration (EC50) values calculated from the titration experiments by pyruvate and oxamate. Potencies were defined as percentage maximal increase/decrease compared with untreated controls.
Results
Exogenous pyruvate enhances motility and hyperactivation of human spermatozoa
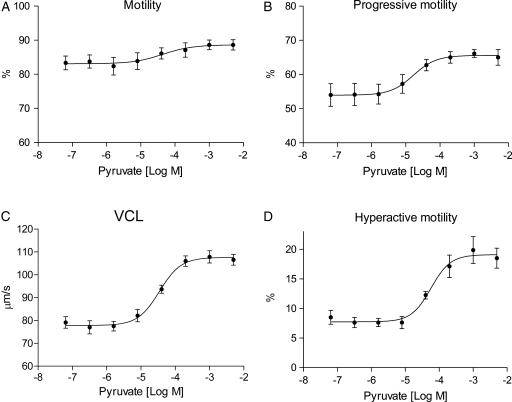
Previous studies on sperm pyruvate metabolism have focused on the role of pyruvate as an oxidizable substrate when present as sole metabolite. Our goal was to investigate the effect of pyruvate on motility and hyperactivation of human spermatozoa in combination with glucose. Purified spermatozoa were incubated under capacitating conditions (see Materials and Methods) in the presence of 5 mM glucose and a titration of pyruvate. Sperm motility was measured by CASA after 30 and 120 min incubation. In CASA, hyperactivation was defined according to criteria given by Burkman (1991). Addition of pyruvate enhanced all categories of sperm motility measured in a dose-dependent manner after 30 min (Fig. 1A–D). Maximal increase (%) and EC50 values (µM) induced by added pyruvate were calculated for motility, progressive motility, VCL, ALH and hyperactive motility and presented in Table I. Our findings demonstrate that exogenous pyruvate is a potent activator of sperm motility and hyperactivation.
Figure 1.
Pyruvate increases motility and hyperactivation of human spermatozoa in a dose-dependent manner. Purified human spermatozoa were incubated under capacitating conditions for 30 min in the presence of 5 mM glucose and incremental concentrations (64 nM–5 mM) of pyruvate (•). Effects of exogenous pyruvate on sperm motility were determined by CASA as (A) motility (%), (B) progressive motility (%), (C) VCL (µm/s) and (D) hyperactive motility (%). All data sets are presented as mean ± SEM from four separate experiments (n= 8).
Table I.
Effects of exogenous pyruvate on motility and intracellular ATP concentration in human spermatozoa incubated under capacitating conditions.
| Sperm parameter | EC50 (µM)a 30 min | Maximal increase (%)b 30 min | EC50 (µM)a 120 min | Maximal increase (%)b 120 min |
|---|---|---|---|---|
| Motility | 37 ± 3 | 9 ± 2 | ND | 0 |
| Progressive motility | 18 ± 5 | 21 ± 1 | 20 ± 6 | 15 ± 3 |
| VCL | 43 ± 14 | 36 ± 7 | 32 ± 5 | 26 ± 4 |
| ALH | 64 ± 13 | 19 ± 3 | 47 ± 4 | 13 ± 2 |
| Hyperactive motility | 58 ± 8 | 130 ± 26 | 41 ± 1 | 66 ± 22 |
| ATP | 34 ± 15 | 56 ± 14 | 20 ± 10 | 27 ± 3 |
Numbers are given as mean values ± SEM of four separate experiments. ND, not detectable.
aEC50 values (µM).
bMaximal increase (%) of sperm motility and ATP production by a titration of pyruvate after 30 and 120 min incubation.
Exogenous pyruvate increases intracellular ATP- and tyrosine phosphorylation levels in human spermatozoa
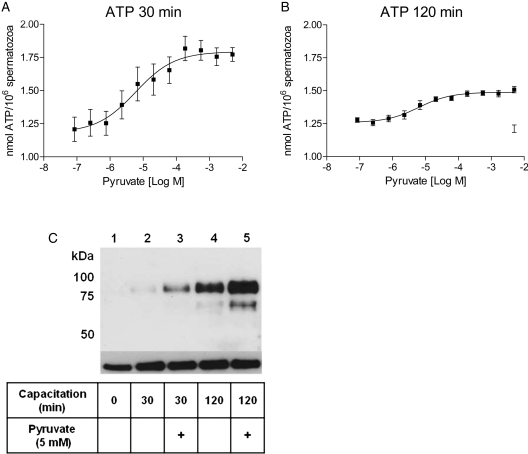
We next speculated that the observed increase in sperm motility and hyperactivation by exogenous pyruvate was correlated to endogenous ATP levels. A luminescence-based assay was used to measure endogenous ATP. Spermatozoa were incubated under capacitating conditions in the presence of increasing concentrations of pyruvate and 5 mM glucose. A dose-dependent rise in ATP concentrations by pyruvate was observed both after 30 and 120 min (Fig. 2A and B), with an average maximal increase of 56 ± 14 and 27 ± 3%, respectively (Table I). Successful capacitation was confirmed by immunoblotting using pY antibodies. Total tyrosine phosphorylation increased markedly under capacitating conditions (Fig. 2C, lanes 2 and 4) in comparison with spermatozoa incubated under non-capacitating conditions (lane 1). The presence of 5 mM pyruvate markedly increased tyrosine phosphorylation levels after both 30 and 120 min incubations (Fig. 2C, lanes 3 and 5). These results demonstrate that pyruvate-stimulated motility and hyperactivation correlate with increased tyrosine phosphorylation and endogenous ATP levels.
Figure 2.
Exogenous pyruvate enhances ATP production and tyrosine phosphorylation in human spermatozoa. Purified human spermatozoa were incubated under capacitating conditions in the presence of 5 mM glucose and increasing concentrations (28 nM–5 mM) of pyruvate for (A) 30 and (B) 120 min. The data sets are presented as mean ± SEM from four separate experiments (n= 12). (C) Sperm capacitation was confirmed by an increase in total tyrosine phosphorylation compared with spermatozoa incubated under non-capacitating conditions (0 min). The increase in tyrosine phosphorylation levels by 5 mM pyruvate (+) was measured after 30 and 120 min. Total tyrosine phosphorylation levels were detected by immunoblotting with pY antibodies. An antibody against α-tubulin was used as a loading control.
A combination of pyruvate and glucose maintains ATP production in human spermatozoa blocked for mitochondrial respiration
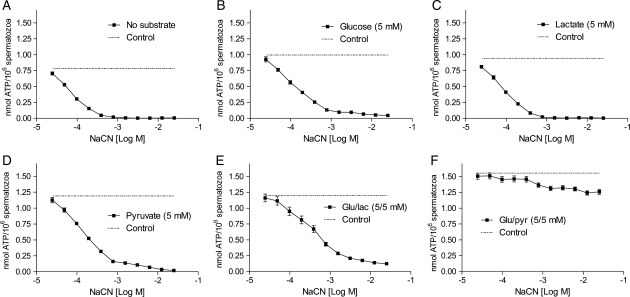
We next questioned whether the observed pyruvate-induced increase in ATP content was dependent on glycolysis alone or if oxidative phosphorylation was required. Human spermatozoa were treated with increasing concentrations of NaCN, an inhibitor of cytochrome c oxidase and the electron transport chain (ETC) in mitochondria (Way, 1984), in conditions promoting capacitation for 120 min. In the absence of metabolic substrates, the endogenous ATP concentrations were reduced by NaCN in a dose-dependent manner with ∼100% reduction at 1 mM NaCN (Fig. 3A). The decreased ATP levels by NaCN could not be restored when 5 mM of glucose, lactate or pyruvate was added separately (Fig. 3B–D). However, a combination of glucose and pyruvate restored intracellular ATP levels to a similar level to that seen in non-inhibited control cells (Fig. 3F). A combination of glucose and lactate could not reestablish the ATP levels under the same conditions (Fig. 3E). The experiment was repeated with two other inhibitors of mitochondrial respiration, rotenone and antimycin A, with similar results (Supplementary data, Fig. S1). These findings indicate that exogenous pyruvate enhances ATP production independently of mitochondrial respiration and that the mechanism is dependent on glucose.
Figure 3.
The effects of different metabolic substrates on ATP levels in human spermatozoa blocked for mitochondrial respiration. Human spermatozoa were incubated under capacitating conditions for 120 min in the presence of increasing concentrations (24 µM–25 mM) of NaCN without (A) or in the presence (B–F) of 5 mM of the metabolic substrates glucose, lactate, pyruvate or a combination of either glucose and lactate or glucose and pyruvate, respectively. Non-inhibited controls are presented as dotted lines (- -). All data sets are presented as mean ± SEM of five separate experiments (n = 10).
A combination of glucose and pyruvate generates ATP sufficient for sperm motility, hyperactivation and tyrosine phosphorylation in the presence of high doses of NaCN
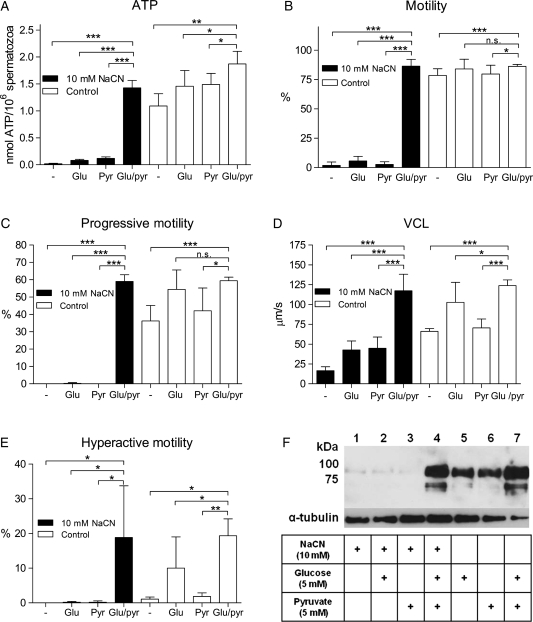
Sperm motility, hyperactivation and tyrosine phosphorylation are energy-demanding processes. Hence, we wanted to elucidate whether the ATP regenerated by glucose and pyruvate in the presence of NaCN could support these processes. Purified human spermatozoa were incubated under capacitating conditions in the presence of high doses of NaCN (10 mM, giving maximal reduction of ATP in Fig. 3A–D) together with 5 mM glucose and 5 mM pyruvate alone or in combination. Spermatozoa treated with NaCN without any metabolic substrate showed a complete depletion of endogenous ATP (Fig. 4A). Incubation with either glucose or pyruvate could not restore ATP to control levels. In contrast, spermatozoa treated a combination of glucose and pyruvate reestablished their ATP levels in the presence of high doses of NaCN blocking mitochondrial respiration.
Figure 4.
A combination of glucose and pyruvate generates ATP concentrations sufficient to support motility, hyperactivation and tyrosine phosphorylation when mitochondrial respiration is blocked by NaCN. Purified human spermatozoa were incubated under capacitating conditions for 120 min in the absence (white bars) or presence (black bars) of 10 mM NaCN and 5 mM of the metabolic substrates glucose, pyruvate or a combination of glucose and pyruvate. (A) ATP concentrations measured by luminescence. (B–E) Sperm motility evaluated by CASA. (B) Motility (%), (C) progressive motility (%), (D) VCL (µm/s) and (E) hyperactive motility (%). Experiment A is presented as mean ± SEM from three separate experiments (n= 9). Experiment B–E are presented as mean ± SEM from five separate experiments (n= 10). (F) Total tyrosine phosphorylation levels detected by immunoblotting with pY antibodies, anti-α-tubulin antibodies were used as loading control. *P < 0.05, **P < 0.01 and ***P < 0.001, n.s., not significant.
We further questioned whether these ATP levels were sufficient to support normal and hyperactive motility. Spermatozoa treated with 10 mM NaCN in the absence of metabolic substrates showed a decrease in all parameters of motility (Fig. 4B–E), with no progressive or hyperactive motility detected. The addition of either 5 mM glucose or 5 mM pyruvate to spermatozoa treated with NaCN did not recover the motility observed for the non-inhibited control cells (Fig. 4B–E). Importantly, these cells were not dead, as demonstrated by a reactivation experiment (Supplementary data, Fig. S3). NaCN-treated spermatozoa incubated in the presence of glucose and pyruvate in combination completely restored all categories of sperm movement measured and analyzed. Moreover, these spermatozoa were fully motile and performed the energetic swimming behavior of hyperactivation (Fig. 4E). (Videos and images with sperm tracks from CASA of human spermatozoa incubated under all the conditions presented in Fig. 4 are shown in the supplementary data, Fig. S2 and supplementary data, Video). The ability of glucose and pyruvate to stimulate tyrosine phosphorylation and thereby capacitation, in the presence of NaCN, was also investigated. With no mitochondrial inhibition, glucose and pyruvate supported tyrosine phosphorylation equally, whereas the addition of substrates in tandem led to improved phosphorylation levels (Fig. 4F). An inhibition of mitochondrial respiration by NaCN (10 mM) in the absence of metabolic substrates resulted in a lack of tyrosine phosphorylation. The presence of glucose or pyruvate alone could not restore the tyrosine phosphorylation levels. However, the addition of a combination of glucose and pyruvate resulted in tyrosine phosphorylation levels comparable to the non-inhibited control. Based on these results, we hypothesized the existence of an efficient mechanism of ATP generation in human spermatozoa, induced by glucose and pyruvate in concert, that fully supports motility and capacitation and that may well operate independently of oxidative phosphorylation.
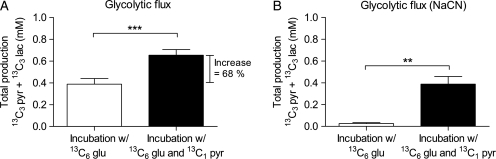
Exogenous pyruvate increases the glycolytic flux in capacitated human spermatozoa
Our initial observations demonstrated that pyruvate stimulates ATP production in human spermatozoa even in the presence of inhibitors of mitochondrial respiration. Glucose metabolism has been shown to be essential for sperm capacitation and we speculated that pyruvate indirectly affected glycolytic ATP production. The total production of 13C3 pyruvate and 13C3 lactate derived from 13C6 labeled glucose was used as a measure of glycolytic flux. This was based on the assumption that glycolysis in spermatozoa is highly effective, creating negligible levels of glycolytic intermediates. The metabolism of substrates was traced by a combination of chromatography, separating lactate and pyruvate in time (Supplementary data, Fig. S4), and MS, separating the different isotopes of the metabolites by mass. We found that when capacitated human spermatozoa were treated with 1 mM 13C1 pyruvate in addition to 13C6 glucose for 120 min, the glycolytic flux increased by more than 50% (Fig. 5A). Based on these findings and the fact that every pyruvate or lactate produced from glucose results in the synthesis of one ATP molecule, we speculated that the observed increase in ATP levels by exogenous pyruvate in our initial experiments was derived from glycolysis and not from oxidative phosphorylation. This hypothesis was supported by a second experiment where mitochondrial respiration was blocked by the treatment with 10 mM NaCN. Here, spermatozoa continued to produce glycolytic ATP in the presence of exogenous pyruvate (Fig. 5B). Interestingly, spermatozoa incubated with glucose as only metabolic substrate could not maintain glycolysis in the presence of NaCN. This finding indicates that mitochondria and oxidative phosphorylation have an important function in sperm energy production other than synthesis of ATP. Moreover, it seems that the mitochondrial function can be replaced by exogenous pyruvate. Spermatozoa are thus unique compared with healthy somatic cells in that they produce ATP mainly by glycolysis even in the presence of oxygen.
Figure 5.
Exogenous pyruvate increases glycolytic flux in human spermatozoa. Total endogenous levels of 13C3 pyruvate and 13C3 lactate derived from 13C6 glucose were quantified by LC-MS and used as a measure of glycolytic flux. Spermatozoa were incubated under capacitating conditions for 120 min in the presence of 1 mM 13C6 glucose (white bars) and a combination of 1 mM 13C6 glucose and 1 mM 13C1 pyruvate (black bars) under (A) normal oxygen levels and (B) in the presence of 10 mM NaCN to inhibit mitochondrial respiration. Data are presented as mean ± SEM from five separate experiments (n = 10). **P < 0.01 and ***P < 0.001.
Human spermatozoa metabolize exogenous pyruvate into lactate
The LC-MS experiments demonstrated that the observed increase in sperm ATP concentrations, motility, hyperactivation and tyrosine phosphorylation levels by exogenous pyruvate may have resulted from an accelerated glycolytic flux. To investigate the molecular mechanism behind this observation, we repeated the experiment and traced the metabolism of 13C1 labeled pyruvate. LDH is known to produce lactate from pyruvate under anaerobic conditions, but we hypothesized that this may be true for spermatozoa also under sufficient levels of oxygen. The conversion of exogenous 13C1 pyruvate to 13C1 lactate in capacitated spermatozoa after 120 min incubation was measured by LC-MS and is presented in Table II. This experiment showed that the added 13C1 pyruvate was rapidly consumed by the sperm cells (∼0.39 mM = 1.95 nmol/106 spermatozoa after 120 min). Strikingly, all 13C1 pyruvate consumed were converted to 13C1 lactate. This shows that pyruvate does not enter the tricarboxylic acid cycle even at excess concentrations and under normal oxygen conditions. Although the conversion of pyruvate to lactate by LDH does not generate ATP, the reaction depends on NADH as a cofactor that is concomitantly oxidized to NAD+ and necessary for a continued glycolysis. The concentration of NAD+ is rate-limiting in glycolysis and an accessible pool derived from exogenous pyruvate may allow for an accelerated glycolytic flux. The established method using labeled metabolites demonstrates that exogenous pyruvate enhances glycolytic ATP production and makes spermatozoa independent of mitochondria.
Table II.
Metabolic tracing of exogenous 13C1 labeled pyruvate in human spermatozoa by LC-MS.
| Measured metabolite | Start concentration (mM, t = 0) | End concentration (mM, t = 120 min) | Consumption (mM) | Production (mM) |
|---|---|---|---|---|
| 13C1 pyruvate | 0.91 ± 0.05 | 0.52 ± 0.01 | 0.39 ± 0.05 | |
| 13C1 lactate | 0 | 0.37 ± 0.04 | 0.37 ± 0.04 |
Concentration values (mM) represent total amount of metabolite in the sample (extra- and intracellular) and are presented as mean ± SEM from five different experiments (n = 10).
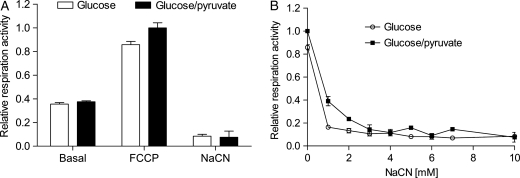
NaCN inactivates ETC-dependent oxygen consumption in human spermatozoa
Pyruvate can be oxidized in the process of mitochondrial respiration generating ATP. Thus, we next investigated whether the increase in endogenous ATP induced by the combination of glucose and pyruvate originated from insufficient inhibition of respiration by NaCN. The mitochondrial activities in capacitated spermatozoa were first assessed by monitoring the basal respiration rates during glucose and glucose/pyruvate administration. Then, the mitochondrial uncoupler FCCP was added to obtain total respiration capacity. Average total respiration capacity in the presence of glucose and pyruvate was 2.0 ± 0.3 pmol O2/106 cells/s (mean ± SEM) and with glucose alone it was 1.7 ± 0.2 pmol O2/106 cells/s. Finally, cyanide was titrated to unravel sensitivity to cyanide as well as to evaluate ETC-independent oxygen consumption. Under normal capacitation, spermatozoa respired at a rate that constituted less than 40 % of total respiration capacity (Fig. 6A). As seen in Fig. 6B, 1 mM NaCN was sufficient to inactivate mitochondrial respiration in spermatozoa metabolizing glucose, while 3 mM NaCN was required to completely block respiration during combined glucose/pyruvate administration. In comparison, 1 mM NaCN was sufficient to block succinate-supported respiration in freeze-thawed mouse heart mitochondria (data not shown). ETC-independent oxygen consumption (respiration activity during 10 mM NaCN) yielded ∼8% of respiration capacity in the spermatozoa.
Figure 6.
NaCN inactivates ETC-dependent oxygen consumption in human spermatozoa. Respiration rates for capacitated spermatozoa incubated in the presence of 5 mM glucose and a combination of 5 mM glucose and 5 mM pyruvate. The data are presented as relative to respiration capacity during glucose + pyruvate treatment (mean ± SEM from three separate experiments, n= 3). (A) Relative basal (basal), total (FCCP) and ETC-independent (NaCN) respiration capacity is shown. (B) NaCN blocks respiration during glucose (○) and glucose + pyruvate (▪) administration.
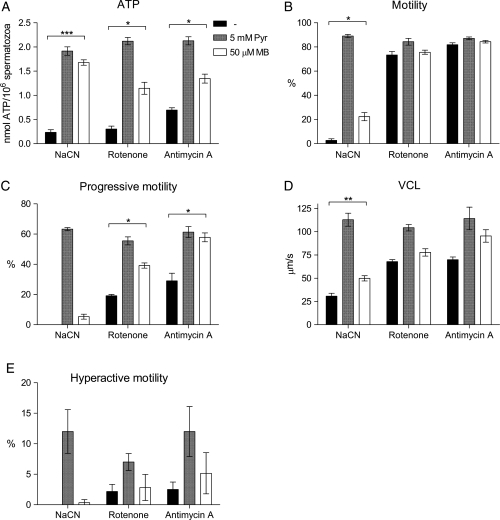
Methylene blue rescues ATP and motility levels in human spermatozoa blocked for mitochondrial respiration
Methylene blue is an oxidizing agent that can oxidize NADH to NAD+ (Sevcik and Dunford, 1991; Sevcik and Dunford, 1995). Hence, methylene blue should, in similar manner to pyruvate be able to rescue the ATP levels and motility observed for mitochondria-inhibited human spermatozoa. This hypothesis was investigated by the treatment of spermatozoa with 10 mM NaCN, 10 µM rotenone or 1 µM antimycin A under capacitating conditions (including 5 mM glucose) for 120 min. The presence of mitochondrial inhibitors reduced both ATP levels and all parameters of motility measured. Co-incubation with 50 µM methylene blue partly or totally regained the ATP (Fig. 7A) and motility levels (Fig. 7B–E) compared with the levels obtained with 5 mM pyruvate. These findings support the hypothesis that exogenous pyruvate promotes ATP and capacitation through an increase of intracellular NAD+ levels.
Figure 7.
The reduced levels of ATP and motility in human spermatozoa treated with mitochondrial inhibitors can be rescued by methylene blue. Spermatzoa were incubated under capacitating conditions supplemented with 5 mM glucose and 10 mM NaCN, 10 µM rotenone or 1 µM antimycin A for 120 min in the absence (black bars) and presence of 5 mM pyruvate (grey bars) or 50 µM methylene blue (white bars). (A) ATP concentrations measured by luminescence. (B–E) Sperm motility evaluated by CASA. The data are presented as mean ± SEM from three separate experiments (A; n=9, B–E; n= 6).
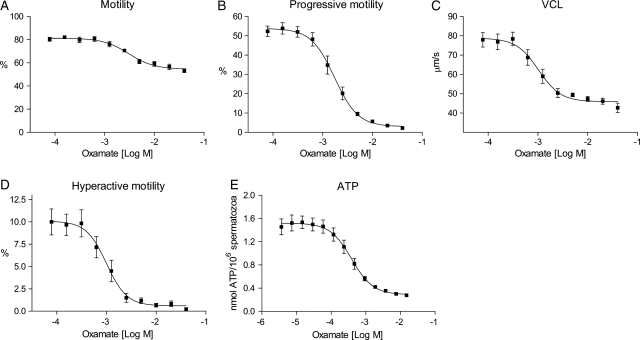
Oxamate inhibits motility, hyperactivation and ATP production in human spermatozoa
The metabolic tracing experiments demonstrated that human spermatozoa convert exogenous pyruvate exclusively to lactate, a reaction that is catalyzed by LDH in cells. An inhibition of LDH should therefore result in reduced motility and ATP levels. To test this, we treated human spermatozoa under capacitating conditions with increasing concentrations of oxamate, a known competitive substrate inhibitor of LDH (Adams et al., 1973; Wong et al., 1997), for 120 min. This resulted in a dose-dependent reduction in normal- and progressive motility, VCL, hyperactivation and ATP levels (Fig. 8). Maximum inhibition (%) and EC50 values (µM) of oxamate were calculated for motility and ATP concentrations and presented in Table III. Oxamate reduced progressive and hyperactive motility by 94 and 95%, respectively. Normal motility and ATP levels were on the other hand not completely depleted by oxamate, which may have resulted from a competition by glycolysis-derived pyruvate. Our findings strengthen the theory of LDH as an important enzyme responsible for the pyruvate-induced increase of sperm motility and capacitation.
Figure 8.
Oxamate reduces motility, hyperactivation and endogenous ATP in human spermatozoa in a dose-dependent manner. Purified human spermatozoa were incubated under conditions promoting capacitation for 120 min in the presence of increasing concentrations (A–D: 39 µM–40 mM, E: 3.6 µM–15 mM) of oxamate. Effects of oxamate on sperm cell motility were determined by CASA as (A) motility (%), (B) progressive motility (%), (C) VCL (µm/s) and (D) hyperactive motility (%). (E) ATP concentrations measured by luminescence. All data sets are presented as mean ± SEM from three (A–D; n= 6) and four (E; n= 8) separate experiments.
Table III.
Effects of oxamate on motility and endogenous ATP levels of capacitated human spermatozoa.
| Sperm parameter | EC50 (mM)a | Maximal inhibition (%)b |
|---|---|---|
| Motility | 2.9 ± 0.7 | 31 ± 1 |
| Progressive motility | 1.8 ± 0.4 | 94 ± 1 |
| VCL | 1.0 ± 0.3 | 42 ± 3 |
| Hyperactive motility | 1.0 ± 0.3 | 95 ± 2 |
| ATP | 0.5 ± 0.1 | 82 ± 3 |
Numbers are given as mean values ± SEM of three separate experiments.
aEC50 values (mM).
bMaximum inhibition (%) of sperm motility and ATP production by a titration of oxamate.
An increased and sustained ATP production by a combination of glucose and pyruvate is unaffected by the presence of high concentrations of exogenous lactate
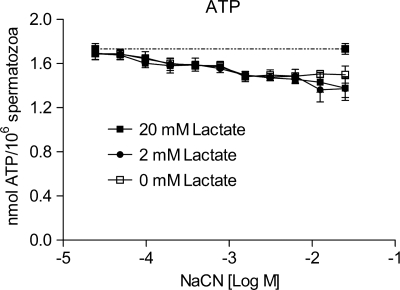
Spermatozoa are surrounded by high concentrations of lactate present in the female oviductal fluid (Dickens et al., 1995; Gardner et al., 1996; Tay et al., 1997). Since high lactate concentrations favor the reverse reaction of LDH and consequently an inhibition of lactic acid fermentation, we wanted to investigate whether an excess of lactate would block ATP production in our setup. We repeated the experiment where capacitated spermatozoa were treated with a titration of NaCN, this time in the presence of 5 mM glucose and 0.4 mM pyruvate. ATP production was sustained in the presence of up to 25 mM NaCN (Fig. 9). Addition of 5- and 50-fold molar excess of lactate compared to pyruvate did not affect ATP production induced by exogenous pyruvate and glucose in the presence of NaCN. These results suggest that sperm motility and hyperactivation stimulated solely by glycolytic ATP synthesis and lactic acid fermentation may operate in vivo.
Figure 9.
High concentrations of lactate do not affect ATP production induced by the combination of glucose and pyruvate when mitochondrial respiration is blocked by NaCN. Human spermatozoa were incubated under capacitating conditions for 120 min in the presence of 5 mM glucose, 0.4 mM pyruvate and increasing concentrations of NaCN (24 µM–25 mM). In addition, cells were exposed to 0 (□), 5 (•) or 50 (▪) fold excess concentrations of lactate compared with pyruvate. ATP levels of non-inhibited control cells are presented as a dotted line (- -). The data are presented as mean ± SEM from three separate experiments (n= 6).
Discussion
In this report, we demonstrate that sperm ATP levels, motility, hyperactivation and tyrosine phosphorylation increase in the presence of exogenous pyruvate in combination with glucose. Hence, pyruvate may promote male fertility. In the light of our findings, we suggest new criteria for sperm capacitation. Pyruvate, together with already known capacitating agents such as glucose, HSA and  should be included in standard IVF and laboratory protocols for capacitation of human spermatozoa. Furthermore, the effect of exogenous pyruvate could be developed into a clinical test for evaluation of glycolytic flux rates in human sperm samples of men having their fertility scrutinized.
should be included in standard IVF and laboratory protocols for capacitation of human spermatozoa. Furthermore, the effect of exogenous pyruvate could be developed into a clinical test for evaluation of glycolytic flux rates in human sperm samples of men having their fertility scrutinized.
ATP concentration and all parameters of motility measured increased at comparable EC50 values (∼0.04 mM) by the addition of pyruvate. These results suggest that endogenous ATP levels, motility and hyperactivation are coupled processes. After only 30 min incubation, we observed increased tyrosine phosphorylation and hyperactivation together with a peak in ATP levels in the presence of exogenous pyruvate. This indicates that the pyruvate-accelerated capacitation process is associated with an acute elevation in endogenous ATP levels. The hypothesis is in agreement with experiments performed by Ho et al. (2002) who concluded that the vigorous movement of hyperactivation requires increased ATP levels. It is possible that stimulation of spermatozoa with capacitating agents such as pyruvate and 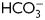 leads to an increase in endogenous ATP concentrations that further initiate hyperactivation and tyrosine phosphorylation. A connection between ATP content and capacitation is further supported by the observation that mouse spermatozoa lacking functional protein kinase A (PKA) showed reduced levels of tyrosine phosphorylation and endogenous ATP (Nolan et al., 2004). PKA is a serine/threonine kinase suggested to be a master regulator of sperm capacitation and tyrosine phosphorylation through Src (Baker et al., 2006).
leads to an increase in endogenous ATP concentrations that further initiate hyperactivation and tyrosine phosphorylation. A connection between ATP content and capacitation is further supported by the observation that mouse spermatozoa lacking functional protein kinase A (PKA) showed reduced levels of tyrosine phosphorylation and endogenous ATP (Nolan et al., 2004). PKA is a serine/threonine kinase suggested to be a master regulator of sperm capacitation and tyrosine phosphorylation through Src (Baker et al., 2006).
Spermatozoa incubated with glucose, pyruvate or lactate showed depleted ATP levels in the presence of mitochondrial inhibitors. However, when treated with a combination of glucose and pyruvate, ATP levels were restored to non-inhibited control levels. With this combination, even spermatozoa treated with high doses (10 mM) of NaCN produced sufficient ATP to support normal levels of progressive motility, hyperactivation and tyrosine phosphorylation. Our oxygen consumption experiments demonstrated a complete blockage of oxidative phosphorylation already at 3 mM NaCN, excluding any ATP contribution from mitochondria at 10 mM NaCN. Reports from the literature have been somewhat inconsistent regarding inhibitors of mitochondrial respiration and their effect on sperm motility (Ford and Harrison, 1981; Fraser and Quinn, 1981; Travis et al., 2001; Mukai and Okuno, 2004; Hung et al., 2008). To our knowledge, research on motility and capacitation of human spermatozoa has been performed in a variety of cell media, occasionally containing pyruvate (e.g. Biggers, Whitten and Whittingham and Ham's F10) (Rogers and Perreault, 1990; Williams and Ford, 2001; Nascimento et al., 2008; Barbonetti et al., 2010). This may have masked the important role of pyruvate in promoting sperm capacitation and reduced the potency of mitochondrial inhibitors on sperm motility.
The regeneration of ATP by exogenous pyruvate in spermatozoa blocked for mitochondrial respiration could be due to several mechanisms. Pyruvate might function as an antidote for the mitochondrial blockers (Way, 1984), an antioxidant against reactive oxygen species (ROS) (Andrae et al., 1985; de Lamirande and Gagnon, 1992; Brand and Hermfisse, 1997) or be utilized in alternative metabolic pathways. However, tracing of metabolites by LC-MS showed that spermatozoa treated with NaCN reestablished ATP levels via an enhanced glycolytic flux in the presence of pyruvate. In fact, all extracellular pyruvate consumed by spermatozoa was converted to lactate, even under aerobic conditions. This reaction is catalyzed by LDH in the final step of lactic acid fermentation with a concomitant production of NAD+ from NADH, which is necessary for a rapid glycolysis. This indicates that human spermatozoa display a slow glycolysis in the absence of exogenous pyruvate, possibly because of the low respiration rate observed in human spermatozoa (Storey, 1978) and thus a deficiency in the transfer of NAD+ to the cytosol.
Other authors have suggested that oxidative phosphorylation is an important source of ATP in spermatozoa based on experiments demonstrating that sperm motility and ATP levels are reduced when treated with mitochondrial blockers (and glucose is the sole metabolic substrate), (Ford and Harrison, 1981; Halangk et al., 1985; Halangk and Bohnensack, 1986; de Lamirande and Gagnon, 1992; Ruiz-Pesini et al., 2000). However, our findings show that blocking of mitochondrial respiration only inhibits glycolytic ATP production in the absence of pyruvate. Hence, human spermatozoa may operate independently of mitochondria in the presence of extracellular pyruvate. Since ATP levels, motility and hyperactivation are inhibited by NaCN in the absence of pyruvate, a possible role for mitochondria may be to deliver NAD+ to glycolysis under basal conditions in the seminal plasma where the demand for ATP is lower than under capacitation. Such a mechanism would also contribute to the prevention of premature capacitation. In addition, sperm mitochondria may also serve other functions such as supplying ATP to the spermatozoon head (Carey et al., 1981).
Spermatozoa from boar (Calvin and Tubbs, 1978) and mouse (Carey et al., 1981) utilize the glycerol-3-phosphate shuttle where cytoplasmic glycerol-3-phospate dehydrogenase (cGAP) reduces dihydroxyacetone phosphate (DHAP) to glycerol-3-phosphate (G3P) by oxidizing NADH to NAD+. The reaction is driven by a mitochondrial membrane bound glycerol-3-phospate dehydrogenase (mGAP) which catalyses the reverse reaction (G3P to DHAP) by reducing one molecule of mGAP-bound FAD to FADH2. FADH2 then reduces co-enzyme Q and the electrons finally enter oxidative phosphorylation. The G3P-shuttle both regenerates NAD+ in the cytoplasm that can fuel glycolysis and produces ATP in the mitochondria through the donated electrons. In addition, intramitochondrial LDH-C has been identified in spermatozoa from several mammalian species like rabbit (Storey and Kayne, 1977) and bull (Van et al., 1977). Located inside the mitochondria, LDH-C is able to directly oxidize lactate by a concomitant reduction of NAD+ to NADH and donating two electrons to the ETC. The pronounced effect on ATP levels and capacitation observed by exogenous pyruvate in this study indicates that human spermatozoa are undersupplied in both these pathways.
This hypothesis is further strengthened by the observation that the addition of methylene blue practically restored the same swimming pattern and ATP levels as exogenous pyruvate in the presence of three different mitochondrial inhibitors (Fig. 7). This is in contrast to a recent paper by Odet and co-workers where the addition of 5 µM methylene blue did not have any effect on sperm ATP levels or motility in wild type or LDH-C KO-mice (Odet et al., 2011). We chose to perform the experiment with 50 µM methylene blue based on dose–response curves (data not shown). In our experiments, 5 µM methylene blue was not sufficient to recover ATP levels. The discrepancy might also be due to species differences between human and mouse spermatozoa.
Spermatozoa possess several features that may favor glycolytic ATP production. Firstly, spermatozoa are reported to express several hexose transporter isoforms (Burant et al., 1992; Haber et al., 1993; Angulo et al., 1998) with different kinetic characteristics that support an efficient uptake of glucose and fructose. The tight organization of glycolytic enzymes along the FS (Krisfalusi et al., 2006; Kim et al., 2007) may further facilitate an efficient glycolytic flux (Mukai et al., 2009). Furthermore, spermatozoa are single cells that can easily access nutrients and excrete excess intracellular levels of lactate to the surroundings. In this way, a high rate of ATP production through glycolysis can be favored at the cost of a lower total yield. Another reason for cells to rely on aerobic glycolysis is that they avoid the production of ROS from oxidative phosphorylation. Hence, low mitochondrial activity might be of extra importance for spermatozoa whose main goal is to deliver intact DNA for fertilization. Finally, the biochemical properties separating LDH-C from the other LDH isoforms may contribute to the high glycolytic flux. LDH-C has a low Km for pyruvate (∼0.030 mM) and a high Km for lactate (∼2.0 mM) (Clausen and Ovlisen, 1965; Coronel et al., 1983; LeVan and Goldberg, 1991; Wong et al., 1997). This implies that LDH-C has an affinity for pyruvate that is >60-fold higher than for lactate, suggesting that pyruvate turnover to lactate may be high even at high concentrations of endogenous or extracellular lactate. This is supported by our experiments where addition of excess lactate (50-fold excess in relation to pyruvate) did not influence ATP production in capacitating spermatozoa. The relative composition of glucose, pyruvate and lactate in the female reproductive tract, although varying with the day of menstrual cycle and the exact place, are found to be in the range of 0.5–3.2 mM, 0.1–0.2 mM and 4.9–10.5 mM, respectively (Dickens et al., 1995; Gardner et al., 1996; Tay et al., 1997). Since the female reproductive tract contains glucose in the mM range and pyruvate at concentrations well above our calculated EC50 values (Table I), we argue that an increased glycolytic flux driven by glucose and pyruvate may operate in vivo.
We conclude that exogenous pyruvate and glucose together speed up the glycolytic machinery to produce sufficient ATP to support progressive motility and capacitation. This metabolic peculiarity may be vital for human fertilization. The fact that this process is independent of mitochondrial respiration indicates that human sperm cell motility and fertility are unaffected by low levels of oxygen as long as glucose and pyruvate are concurrently present at sufficient concentrations, as is the case in the female reproductive tract. Our findings emphasize how survival and function of spermatozoa require great flexibility as they are transcriptionally silent and exist in a constantly changing environment. Finally, the effect of exogenous pyruvate on human sperm ATP levels and capacitation is another example on how constituents of the female reproductive tract determine the metabolism of the conspecific spermatozoa.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors’ roles
T.H.H. designed the study, carried out and analyzed the experiments and wrote the manuscript. K.B.P.E. designed, performed and analyzed the LC-MS experiments. F.H.C. gave essential assistance in the LC-MS experiments and participated in critical discussions. L.E. performed the sperm respiration experiments. T.J. provided critical input on the interpretation of data and drafting of the manuscript. B.S.S. participated in critical discussions and drafting of the manuscript. K.R.R. gave essential assistance in the experiments, participated in the design of the study and critical discussions and wrote the manuscript. All authors approved the final manuscript.
Conflict of interest
T.H.H. is an employee and holds stock options in Spermatech AS. K.R.R. is an employee and holds stock options in Spermatech AS. T.J. is a board member and holds stock options in Spermatech AS. B.S.S. is a shareholder in Spermatech AS.
Funding
The work was funded by Spermatech AS and the Research Council of Norway. Funding to pay the Open Access publication charges for this article was provided by The University of Oslo (UiO).
Supplementary Material
Acknowledgements
The authors thank Alexander Rowe (Oslo University hospital Rikshospitalet) for critically reading the manuscript. We are grateful to Sissel Eikvar (UiO), Espen M. Gjems (Spermatech AS) and Joachim Krämer (Evotec AG) for excellent contributions to this work.
References
- Adam DE, Wei J. Mass transport of ATP within the motile sperm. J Theor Biol. 1975;49:125–145. doi: 10.1016/s0022-5193(75)80023-3. [DOI] [PubMed] [Google Scholar]
- Adams MJ, Buehner M, Chandrasekhar K, Ford GC, Hackert ML, Liljas A, Rossmann MG, Smiley IE, Allison WS, Everse J, et al. Structure-function relationships in lactate dehydrogenase. Proc Natl Acad Sci USA. 1973;70:1968–1972. doi: 10.1073/pnas.70.7.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrae U, Singh J, Ziegler-Skylakakis K. Pyruvate and related alpha-ketoacids protect mammalian cells in culture against hydrogen peroxide-induced cytotoxicity. Toxicol Lett. 1985;28:93–98. doi: 10.1016/0378-4274(85)90015-3. [DOI] [PubMed] [Google Scholar]
- Angulo C, Rauch MC, Droppelmann A, Reyes AM, Slebe JC, Delgado-Lopez F, Guaiquil VH, Vera JC, Concha II. Hexose transporter expression and function in mammalian spermatozoa: cellular localization and transport of hexoses and vitamin C. J Cell Biochem. 1998;71:189–203. [PubMed] [Google Scholar]
- Baker MA, Hetherington L, Aitken RJ. Identification of SRC as a key PKA-stimulated tyrosine kinase involved in the capacitation-associated hyperactivation of murine spermatozoa. J Cell Sci. 2006;119:3182–3192. doi: 10.1242/jcs.03055. [DOI] [PubMed] [Google Scholar]
- Barbonetti A, Vassallo MR, Fortunato D, Francavilla S, Maccarrone M, Francavilla F. Energetic metabolism and human sperm motility: impact of CB1 receptor activation. Endocrinology. 2010;151:5882–5892. doi: 10.1210/en.2010-0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco A, Zinkham WH. Lactate dehydrogenases in human testes. Science. 1963;139:601–602. doi: 10.1126/science.139.3555.601. [DOI] [PubMed] [Google Scholar]
- Brand KA, Hermfisse U. Aerobic glycolysis by proliferating cells: a protective strategy against reactive oxygen species. FASEB J. 1997;11:388–395. doi: 10.1096/fasebj.11.5.9141507. [DOI] [PubMed] [Google Scholar]
- Burant CF, Takeda J, Brot-Laroche E, Bell GI, Davidson NO. Fructose transporter in human spermatozoa and small intestine is GLUT5. J Biol Chem. 1992;267:14523–14526. [PubMed] [Google Scholar]
- Burkman LJ. Discrimination between nonhyperactivated and classical hyperactivated motility patterns in human spermatozoa using computerized analysis. Fertil Steril. 1991;55:363–371. [PubMed] [Google Scholar]
- Calvin J, Tubbs PK. Mitochondrial transport processes and oxidation of NADH by hypotonically-treated boar spermatozoa. Eur J Biochem. 1978;89:315–320. doi: 10.1111/j.1432-1033.1978.tb20929.x. [DOI] [PubMed] [Google Scholar]
- Cao W, Aghajanian HK, Haig-Ladewig LA, Gerton GL. Sorbitol can fuel mouse sperm motility and protein tyrosine phosphorylation via sorbitol dehydrogenase. Biol Reprod. 2009;80:124–133. doi: 10.1095/biolreprod.108.068882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JE, Olds-Clarke P, Storey BT. Oxidative metabolism of spermatozoa from inbred and random bred mice. J Exp Zool. 1981;216:285–292. doi: 10.1002/jez.1402160209. [DOI] [PubMed] [Google Scholar]
- Clausen J, Ovlisen B. Lactate dehydrogenase isoenzymes of human semen. Biochem J. 1965;97:513–517. doi: 10.1042/bj0970513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronel CE, Burgos C, Gerez de Burgos NM, Rovai LE, Blanco A. Catalytic properties of the sperm-specific lactate dehydrogenase (LDH X or C4) from different species. J Exp Zool. 1983;225:379–385. doi: 10.1002/jez.1402250305. [DOI] [PubMed] [Google Scholar]
- Cummins JM, Woodall PF. On mammalian sperm dimensions. J Reprod Fertil. 1985;75:153–175. doi: 10.1530/jrf.0.0750153. [DOI] [PubMed] [Google Scholar]
- de Lamirande E, Gagnon C. Reactive oxygen species and human spermatozoa. II. Depletion of adenosine triphosphate plays an important role in the inhibition of sperm motility. J Androl. 1992;13:379–386. [PubMed] [Google Scholar]
- Demott RP, Suarez SS. Hyperactivated sperm progress in the mouse oviduct. Biol Reprod. 1992;46:779–785. doi: 10.1095/biolreprod46.5.779. [DOI] [PubMed] [Google Scholar]
- Dickens CJ, Maguiness SD, Comer MT, Palmer A, Rutherford AJ, Leese HJ. Human tubal fluid: formation and composition during vascular perfusion of the fallopian tube. Hum Reprod. 1995;10:505–508. doi: 10.1093/oxfordjournals.humrep.a135978. [DOI] [PubMed] [Google Scholar]
- Ford WC. Glycolysis and sperm motility: does a spoonful of sugar help the flagellum go round? Hum Reprod Update. 2006;12:269–274. doi: 10.1093/humupd/dmi053. [DOI] [PubMed] [Google Scholar]
- Ford WC, Harrison A. The role of oxidative phosphorylation in the generation of ATP in human spermatozoa. J Reprod Fertil. 1981;63:271–278. doi: 10.1530/jrf.0.0630271. [DOI] [PubMed] [Google Scholar]
- Fraser LR, Quinn PJ. A glycolytic product is obligatory for initiation of the sperm acrosome reaction and whiplash motility required for fertilization in the mouse. J Reprod Fertil. 1981;61:25–35. doi: 10.1530/jrf.0.0610025. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Lane M, Calderon I, Leeton J. Environment of the preimplantation human embryo in vivo: metabolite analysis of oviduct and uterine fluids and metabolism of cumulus cells. Fertil Steril. 1996;65:349–353. doi: 10.1016/s0015-0282(16)58097-2. [DOI] [PubMed] [Google Scholar]
- Goldberg E. Lactic and malic dehydrogenases in human spermatozoa. Science. 1963;139:602–603. doi: 10.1126/science.139.3555.602. [DOI] [PubMed] [Google Scholar]
- Haber RS, Weinstein SP, O'Boyle E, Morgello S. Tissue distribution of the human GLUT3 glucose transporter. Endocrinology. 1993;132:2538–2543. doi: 10.1210/endo.132.6.8504756. [DOI] [PubMed] [Google Scholar]
- Halangk W, Bohnensack R. Quantification of sperm motility by a turbidimetric assay. Correlation to cellular respiration. Biomed Biochim Acta. 1986;45:331–341. [PubMed] [Google Scholar]
- Halangk W, Bohnensack R, Frank K, Kunz W. Effect of various substrates on mitochondrial and cellular energy state of intact spermatozoa. Biomed Biochim Acta. 1985;44:411–420. [PubMed] [Google Scholar]
- Ho HC, Granish KA, Suarez SS. Hyperactivated motility of bull sperm is triggered at the axoneme by Ca2+ and not cAMP. Dev Biol. 2002;250:208–217. doi: 10.1006/dbio.2002.0797. [DOI] [PubMed] [Google Scholar]
- Hoshi K, Tsukikawa S, Sato A. Importance of Ca2+, K+ and glucose in the medium for sperm penetration through the human zona pellucida. Tohoku J Exp Med. 1991;165:99–104. doi: 10.1620/tjem.165.99. [DOI] [PubMed] [Google Scholar]
- Hung PH, Miller MG, Meyers SA, Vandevoort CA. Sperm mitochondrial integrity is not required for hyperactivated motility, zona binding, or acrosome reaction in the rhesus macaque. Biol Reprod. 2008;79:367–375. doi: 10.1095/biolreprod.107.066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson SM, Van DC, Lardy HA. Mitochondrial metabolism of pyruvate in bovine spermatozoa. J Biol Chem. 1977;252:1309–1315. [PubMed] [Google Scholar]
- Jones AR, Chantrill LA, Cokinakis A. Metabolism of glycerol by mature boar spermatozoa. J Reprod Fertil. 1992;94:129–134. doi: 10.1530/jrf.0.0940129. [DOI] [PubMed] [Google Scholar]
- Kim YH, Haidl G, Schaefer M, Egner U, Mandal A, Herr JC. Compartmentalization of a unique ADP/ATP carrier protein SFEC (Sperm Flagellar Energy Carrier, AAC4) with glycolytic enzymes in the fibrous sheath of the human sperm flagellar principal piece. Dev Biol. 2007;302:463–476. doi: 10.1016/j.ydbio.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisfalusi M, Miki K, Magyar PL, O'Brien DA. Multiple glycolytic enzymes are tightly bound to the fibrous sheath of mouse spermatozoa. Biol Reprod. 2006;75:270–278. doi: 10.1095/biolreprod.105.049684. [DOI] [PubMed] [Google Scholar]
- LeVan KM, Goldberg E. Properties of human testis-specific lactate dehydrogenase expressed from Escherichia coli. Biochem J. 1991;273(Pt 3):587–592. doi: 10.1042/bj2730587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahadevan MM, Miller MM, Moutos DM. Absence of glucose decreases human fertilization and sperm movement characteristics in vitro. Hum Reprod. 1997;12:119–123. doi: 10.1093/humrep/12.1.119. [DOI] [PubMed] [Google Scholar]
- Mukai C, Okuno M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol Reprod. 2004;71:540–547. doi: 10.1095/biolreprod.103.026054. [DOI] [PubMed] [Google Scholar]
- Mukai C, Bergkvist M, Nelson JL, Travis AJ. Sequential reactions of surface-tethered glycolytic enzymes. Chem Biol. 2009;16:1013–1020. doi: 10.1016/j.chembiol.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch RN, White IG. Studies of the metabolism of human spermatozoa. J Reprod Fertil. 1968;16:351–361. doi: 10.1530/jrf.0.0160351. [DOI] [PubMed] [Google Scholar]
- Nascimento JM, Shi LZ, Tam J, Chandsawangbhuwana C, Durrant B, Botvinick EL, Berns MW. Comparison of glycolysis and oxidative phosphorylation as energy sources for mammalian sperm motility, using the combination of fluorescence imaging, laser tweezers, and real-time automated tracking and trapping. J Cell Physiol. 2008;217:745–751. doi: 10.1002/jcp.21549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevo AC, Rikmenspoel R. Diffusion of ATP in sperm flagella. J Theor Biol. 1970;26:11–18. doi: 10.1016/s0022-5193(70)80027-3. [DOI] [PubMed] [Google Scholar]
- Nolan MA, Babcock DF, Wennemuth G, Brown W, Burton KA, McKnight GS. Sperm-specific protein kinase A catalytic subunit Calpha2 orchestrates cAMP signaling for male fertility. Proc Natl Acad Sci USA. 2004;101:13483–13488. doi: 10.1073/pnas.0405580101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odet F, Duan C, Willis WD, Goulding EH, Kung A, Eddy EM, Goldberg E. Expression of the gene for mouse lactate dehydrogenase C (Ldhc) is required for male fertility. Biol Reprod. 2008;79:26–34. doi: 10.1095/biolreprod.108.068353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odet F, Gabel SA, Williams J, London RE, Goldberg E, Eddy EM. Lactate dehydrogenase C (LDHC) and energy metabolism in mouse sperm. Biol Reprod. 2011;85:556–564. doi: 10.1095/biolreprod.111.091546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigau T, Farre M, Ballester J, Mogas T, Pena A, Rodriguez-Gil JE. Effects of glucose and fructose on motility patterns of dog spermatozoa from fresh ejaculates. Theriogenology. 2001;56:801–815. doi: 10.1016/s0093-691x(01)00609-4. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Paez L, Guzman-Ibarra R, cuna-Gonzalez C, Santillan-Baez A, Moreno-Rodriguez R, Wong C. The study of N-isopropyl oxamate on sperm motility and fertility, in mice. Proc West Pharmacol Soc. 2002;45:171–173. [PubMed] [Google Scholar]
- Rogers BJ, Perreault SD. Importance of glycolysable substrates for in vitro capacitation of human spermatozoa. Biol Reprod. 1990;43:1064–1069. doi: 10.1095/biolreprod43.6.1064. [DOI] [PubMed] [Google Scholar]
- Ruiz-Pesini E, Lapena AC, Diez-Sanchez C, Perez-Martos A, Montoya J, Alvarez E, Diaz M, Urries A, Montoro L, Lopez-Perez MJ, et al. Human mtDNA haplogroups associated with high or reduced spermatozoa motility. Am J Hum Genet. 2000;67:682–696. doi: 10.1086/303040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Pesini E, ez-Sanchez C, Lopez-Perez MJ, Enriquez JA. The role of the mitochondrion in sperm function: is there a place for oxidative phosphorylation or is this a purely glycolytic process? Curr Top Dev Biol. 2007;77:3–19. doi: 10.1016/S0070-2153(06)77001-6. [DOI] [PubMed] [Google Scholar]
- Sevcik P, Dunford HB. Kinetics of the oxidation of NADH by methylene-blue in a closed system. J Phys Chem. 1991;95:2411–2415. [Google Scholar]
- Sevcik P, Dunford B. The rate-constant for aerial oxidation of NADH by methylene-blue. Int J Chem Kinet. 1995;27:925–928. [Google Scholar]
- Stauss CR, Votta TJ, Suarez SS. Sperm motility hyperactivation facilitates penetration of the hamster zona pellucida. Biol Reprod. 1995;53:1280–1285. doi: 10.1095/biolreprod53.6.1280. [DOI] [PubMed] [Google Scholar]
- Storey BT. Effect of ionophores and inhibitors and uncouplers of oxidative phosphorylation on sperm respiration. Arch Androl. 1978;1:169–177. doi: 10.3109/01485017808988334. [DOI] [PubMed] [Google Scholar]
- Storey BT. Mammalian sperm metabolism: oxygen and sugar, friend and foe. Int J Dev Biol. 2008;52:427–437. doi: 10.1387/ijdb.072522bs. [DOI] [PubMed] [Google Scholar]
- Storey BT, Kayne FJ. Energy metabolism of spermatozoa. VI. Direct intramitochondrial lactate oxidation by rabbit sperm mitochondria. Biol Reprod. 1977;16:549–556. [PubMed] [Google Scholar]
- Sukcharoen N, Keith J, Irvine DS, Aitken RJ. Definition of the optimal criteria for identifying hyperactivated human spermatozoa at 25 Hz using in-vitro fertilization as a functional end-point. Hum Reprod. 1995;10:2928–2937. doi: 10.1093/oxfordjournals.humrep.a135822. [DOI] [PubMed] [Google Scholar]
- Tay JI, Rutherford AJ, Killick SR, Maguiness SD, Partridge RJ, Leese HJ. Human tubal fluid: production, nutrient composition and response to adrenergic agents. Hum Reprod. 1997;12:2451–2456. doi: 10.1093/humrep/12.11.2451. [DOI] [PubMed] [Google Scholar]
- Travis AJ, Jorgez CJ, Merdiushev T, Jones BH, Dess DM, az-Cueto L, Storey BT, Kopf GS, Moss SB. Functional relationships between capacitation-dependent cell signaling and compartmentalized metabolic pathways in murine spermatozoa. J Biol Chem. 2001;276:7630–7636. doi: 10.1074/jbc.M006217200. [DOI] [PubMed] [Google Scholar]
- Turner RM. Tales from the tail: what do we really know about sperm motility? J Androl. 2003;24:790–803. doi: 10.1002/j.1939-4640.2003.tb03123.x. [DOI] [PubMed] [Google Scholar]
- Van DC, Hutson SM, Lardy HA. Pyruvate metabolism in bovine epididymal spermatozoa. J Biol Chem. 1977;252:1303–1308. [PubMed] [Google Scholar]
- Way JL. Cyanide intoxication and its mechanism of antagonism. Annu Rev Pharmacol Toxicol. 1984;24:451–481. doi: 10.1146/annurev.pa.24.040184.002315. [DOI] [PubMed] [Google Scholar]
- Williams AC, Ford WC. The role of glucose in supporting motility and capacitation in human spermatozoa. J Androl. 2001;22:680–695. [PubMed] [Google Scholar]
- Wong C, Rodriguez-Paez L, Nogueda B, Perez A, Baeza I. Selective inhibition of the sperm-specific lactate dehydrogenase isozyme-C4 by N-isopropyl oxamate. Biochim Biophys Acta. 1997;1343:16–22. doi: 10.1016/s0167-4838(97)00090-3. [DOI] [PubMed] [Google Scholar]
- Zinkham WH, Blanco A, Clowry LJ., Jr An unusual isozyme of lactate dehydrogenase in mature testis: localization, ontogeny, and kinetic properties. Ann NY Acad Sci. 1964;121:571–588. doi: 10.1111/j.1749-6632.1964.tb14227.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.