Abstract
Flavobacterium psychrophilum, a member of the Cytophaga-Flavobacterium-Bacteroides group, is an important pathogen of salmonid fish. Previous attempts to develop genetic techniques for this fastidious, psychrotrophic bacterium have met with failure. Here we describe the development of techniques for the genetic manipulation of F. psychrophilum and the identification of plasmids, selectable markers, a reporter system, and a transposon that function in several isolates of this fish pathogen. The antibiotic resistance genes ermF, cfxA, and tetQ function in F. psychrophilum. Cloning vectors based on the F. psychrophilum cryptic plasmid pCP1 which carried these selectable markers were introduced by conjugation from E. coli, resulting in antibiotic-resistant colonies of F. psychrophilum. Conjugative transfer of DNA into F. psychrophilum was strain dependent. Efficient transfer was observed for two of the seven strains tested (THC02-90 and THC04-90). E. coli lacZY functioned in F. psychrophilum when expressed from a pCP1 promoter, allowing its development as a reporter for studies of gene expression. Plasmids isolated from F. psychrophilum were efficiently introduced into F. psychrophilum by electroporation, but plasmids isolated from E. coli were not suitable for transfer by this route, suggesting the presence of a restriction barrier. DNA isolated from F. psychrophilum was resistant to digestion by Sau3AI and BamHI, indicating that a Sau3AI-like restriction modification system may constitute part of this barrier. Tn4351 was introduced into F. psychrophilum from E. coli and transposed with apparent randomness, resulting in erythromycin-resistant colonies. The techniques developed in this study allow for genetic manipulation and analysis of this important fish pathogen.
The gliding bacterium Flavobacterium psychrophilum is the causative agent of cold water disease (CWD) in salmonids. Outbreaks of this disease, which may result in up to 50% mortality, are most common in fingerlings and typically occur at water temperatures between 12 and 14°C. CWD has important economic consequences because of the large and growing salmonid aquaculture industry (for a review, see references 7 and 10). No vaccine to prevent CWD is available, and control of the outbreaks currently involves antimicrobial drug administration.
F. psychrophilum is somewhat fastidious, requiring additions such as serum for optimal growth and for efficient colony formation from single cells plated on solid media. The optimum temperature for growth is approximately 18°C, and most strains fail to grow above 20°C. Under optimal conditions, the population doubles in approximately 3 h. These growth properties have hampered studies of F. psychrophilum and the development of genetic techniques. Recent advances have been made in methods for cultivation (18) and detection (8, 29, 30, 31) of F. psychrophilum, and procedures allowing standardization of experimental infection have been developed (9).
The mechanisms of F. psychrophilum pathogenicity are poorly understood. Cells of F. psychrophilum produce a number of proteases that may play a role in the disease process (3). A correlation has been established between the presence of specific proteases and virulence (13, 20). The metalloprotease Fpp1, which may be involved in pathogenicity, has recently been purified and characterized (23). Lipopolysaccharide may also be associated with virulence and has been the subject of several recent studies (6, 12). The lack of genetic techniques for F. psychrophilum has hampered further progress toward defining the mechanisms of pathogenesis. The development of methods for genetic manipulation would allow for identification of virulence genes and would facilitate the rational design of vaccine strains.
F. psychrophilum is a member of the Cytophaga-Flavobacterium-Bacteroides (CFB) group and is very distantly related to organisms with well-developed genetic systems, such as members of the proteobacteria. In general, plasmids, selectable markers, and transposons that function in proteobacteria fail to function in members of the CFB group (16, 21). Methods for genetic manipulation of Bacteroides species (21) and of some Flavobacterium species (15, 16, 27) have been developed. In spite of the fact that the plasmid used to develop Flavobacterium cloning vectors was isolated from F. psychrophilum D12, repeated attempts by ourselves and others to demonstrate transfer of these plasmids or other genetic elements into F. psychrophilum have been unsuccessful (J. A. Guijarro, unpublished data; M. J. McBride, unpublished data; D. W. Hunnicutt, unpublished data).
In this study, we describe the development of methods for the introduction of DNA into F. psychrophilum by conjugation and electroporation. Selectable markers, plasmid cloning vectors, a lacZY reporter construct, and a transposon which functions in F. psychrophilum are also described, and evidence for a Sau3AI-like restriction modification system in F. psychrophilum THC02-90 is presented. The availability of these genetic techniques should allow analysis of the underlying mechanisms of F. psychrophilum virulence and facilitate the development of vaccine strains.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. EAO medium (18) was used, consisting of 5 g of tryptone, 0.5 g of yeast extract, 0.2 g of beef extract, and 0.2 g of sodium acetate per liter, with the pH adjusted to 7.2 to 7.4. EAOS medium was the same as EAO medium except that it was supplemented with 5% horse serum, 10 μM l-phenylalanine, 10 μM l-tyrosine, 10 μM l-tryptophan, 10 μM p-aminobenzoic acid, 10 μM 4-hydroxybenzoic acid, and 10 μM 2,3-dihydroxybenzoic acid. For growth on solid medium, 15 g of agar was added per liter of EAOS medium, and cultures were incubated at 20°C. Nutrient broth (NB; Promega) was used for growth of F. psychrophilum in liquid, and cultures were incubated at 18°C with shaking at 250 rpm. Escherichia coli strains were grown at 37°C in 2× TY medium (15 g of tryptone, 10 g of yeast extract, and 5 g of NaCl per liter), with 20 g of agar per liter added for solid medium. For selection of F. psychrophilum transconjugants or transformants, erythromycin, cefoxitin, or tetracycline was used at 10 μg/ml. For selective growth of E. coli strains, antibiotics were used when needed at the following concentrations: ampicillin, 100 μg/ml; chloramphenicol, 30 μg/ml; streptomycin, 50 μg/ml; and tetracycline, 15 μg/ml.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Source or reference |
|---|---|---|
| Bacterial strains | ||
| E. coli | ||
| S17-1 λ pir | λpir hsdR pro thi; chromosomal integrated RP4-2 Tc::Mu Km::Tn7 | 25 |
| BW19851 | RP4-2tet::Mu-1kan::Tn7 integrant; ΔuidA::pir+recA1 hsdR17 creB510 endA1 zbf-5 thi | 17 |
| F. psychrophilum | ||
| ATCC 49418 | Wild type | ATCCa |
| ATCC 49510 | Wild type | ATCC |
| ATCC 49511 | Wild type | ATCC |
| D12 | Wild type | 15 |
| OSU SH3-81 | Wild type | 4 |
| OSU THCO2-90 | Wild type | 4 |
| OSU THCO4-90 | Wild type | 4 |
| OSU Ch8-80 | Wild type | 4 |
| FPC 830 | Wild type | 4 |
| Plasmidsb | ||
| pCP29 | ColE1 ori; (pCP1 ori); Apr (Cfr Emr); E. coli-F. psychrophilum shuttle plasmid | 11 |
| pCP23 | ColE1 ori (pCP1 ori); Apr (Tcr); E. coli-F. psychrophilum shuttle plasmid | 1 |
| pIVET8 | pir-dependent oriR6K; Mob+ Apr | 14 |
| pCP23-β | 6-kb SphI fragment of pIVET8 containing lacZY genes cloned in pCP23 | This study |
| pEP4351 | pir-requiring R6K oriV; RP4 oriT; Cmr Tcr (Emr); vector for Tn4351 transfer | 5 |
ATCC, American Type Culture Collection, Rockville, Md.
Antibiotic resistance phenotypes: ampicillin, Apr; cefoxitin, Cfr; chloramphenicol, Cmr; erythromycin, Emr; tetracycline, Tcr. Antiobiotic resistance phenotypes and other features listed in parentheses are those expressed by F. psychrophilum but not by E. coli.
Manipulation of DNA and nucleic acid sequencing.
Standard procedures were used to isolate plasmid and genomic DNA and to clone and analyze DNA fragments (22). Nucleic acid sequencing was performed by the dideoxy nucleotide procedure, using an automated (Applied Biosystems) sequencing system. Sequences were analyzed with MacVector and AssemblyLign software (Accelrys, San Diego, Calif.), and comparisons to database sequences were made by use of the BLAST algorithm (2).
Conjugal transfer of DNA from E. coli to F. psychrophilum.
E. coli S17-1 λ pir was used for conjugative transfer of pCP29, pCP23, and pCP23-β into F. psychrophilum, and E. coli BW19851 was used to transfer pEP4351. Donor E. coli strains were grown to mid-log phase in 2× TY medium, and recipient F. psychrophilum strains were grown to mid-log phase in NB. F. psychrophilum cells (10 ml) were harvested by centrifugation, washed twice with TM buffer, consisting of 20 mM Tris-HCl and 20 mM MgSO4 with the pH adjusted to 7.2, and suspended in 50 μl of TM buffer. E. coli cells (10 ml) were harvested by centrifugation, washed twice with EAO medium, and suspended in 50 μl of EAO medium. Cells of F. psychrophilum and E. coli were mixed together (approximately 108 cells of each strain), spotted onto EAOS agar, and incubated at 20°C for 48 h to allow conjugative transfer of DNA. After conjugation, cells were scraped off the plates, diluted in 1 ml of EAO medium, and plated on EAOS agar containing the appropriate antibiotic (cefoxitin, erythromycin, or tetracycline) to select for plasmid or transposon transfer and to eliminate E. coli donor cells. Plates were incubated at 20°C for 4 to 7 days. A separate counterselection to eliminate E. coli was not needed, since cfxA, ermF, and tetQ are not expressed in E. coli. To calculate the frequencies of conjugative transfer per recipient, cells were plated on EAOS agar without antibiotics to determine the number of surviving F. psychrophilum cells.
Electroporation.
F. psychrophilum cells were harvested during exponential growth, washed three times with ice-cold double-distilled water, and resuspended to a density of approximately 1011 cells/ml in double-distilled water. Approximately 200 ng of pCP29 was added to 40 μl of cells. For the optimized procedure, each mixture was placed in a Bio-Rad 0.2-cm pulser cuvette and pulsed with a Bio-Rad gene pulser, with a field strength of 10 kV/cm, resistance of 400 Ω, and capacitance of 25 μF. DNA isolated from F. psychrophilum and E. coli strain S17-1 λ pir was used to electroporate F. psychrophilum cells. After electroporation, the cells were transferred to NB and incubated at 18°C, with shaking at 250 rpm, for 3 h to allow expression of antibiotic resistance. Afterwards, cells were diluted in NB and plated on EAOS agar with the appropriate antibiotic. Colonies were counted after 4 to 5 days of incubation at 20°C.
Southern blot analysis of Tn4351 insertions.
Tn4351 was introduced into F. psychrophilum THC02-90 by conjugation, as described above. Genomic DNA from erythromycin-resistant transconjugants was isolated, digested with XbaI, separated by gel electrophoresis, and transferred to nylon membranes essentially as described previously (22). The DIG DNA labeling and detection kit (Roche) was used to prepare probes and to perform hybridization. Two probes were used, a 6.2-kb SalI fragment from pEP4351 containing the transposon Tn4351 and the chloramphenicol acetyltransferase gene (cat), which is also present on pEP4351. The cat gene was amplified as a 633-bp PCR product from pIVET8 by use of the primers CAT-1 (CACTGGATATACCACCG) and CAT-2 (TGCCACTCATCGCAGTA).
In vitro β-galactosidase determination.
The SphI fragment of pIVET8 (14) containing the lacZY genes was inserted into the SphI site of pCP23 downstream of the ORF1 promoter to generate pCP23-β. pCP23-β was introduced by conjugation into F. psychrophilum THC02-90, and tetracycline-resistant transconjugants (THC02-90-β) were obtained. As a control, the THC02-90 strain containing the pCP23 plasmid (THC02-90-p) was used. For detection of β-galactosidase activity on the plates, 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) was added at 50 μg/ml. NB was used to detect the production of β-galactosidase. Cells from 1 ml of a culture which had been incubated for 48 h were collected by centrifugation at 12,000 × g for 5 min, suspended in buffer Z (19), and disrupted by sonication. β-Galactosidase activity was measured as previously described (19).
Nucleotide sequence accession number.
The sequence of pCP1 which is reported in this paper has been deposited in the GenBank database under accession number AY277637.
RESULTS
Conjugative transfer of plasmids into F. psychrophilum.
pCP29 was selected as a test plasmid to determine the optimal conditions for conjugative transfer of DNA into F. psychrophilum. Despite the fact that previous attempts to transfer pCP29 and other plasmids into this bacterium had all failed, we predicted that F. psychrophilum would support replication of pCP29 once barriers to transfer were overcome because this plasmid is derived from the cryptic F. psychrophilum D12 plasmid pCP1 (11, 15). pCP29 carries two antibiotic resistance genes (cfxA and ermF) that function in Flavobacterium johnsoniae, Bacteroides thetaiotaomicron, and many other members of the CFB group, so it seemed likely that pCP29 would confer erythromycin and cefoxitin resistance to F. psychrophilum. F. psychrophilum THC02-90, which does not carry pCP1, was chosen as the recipient strain to avoid problems of plasmid incompatibility. pCP29 was transferred by conjugation from E. coli to F. psychrophilum THC02-90, and cefoxitin- and erythromycin-resistant colonies arose after 4 to 5 days of incubation at 20°C. pCP29 plasmid DNA was isolated from the transconjugants, and phenotypic analysis and PCR analysis using specific F. psychrophilum primers (8) confirmed that the transconjugants were F. psychrophilum. A number of variables were tested to determine the optimal conditions for conjugative transfer. Recipient cells harvested during the early exponential phase of growth (optical density at 525 nm, 0.3 to 0.5) resulted in the highest frequencies of conjugation (1.3 × 10−5 to 1.7 × 10−5 antibiotic-resistant colonies per recipient cell). Cells harvested during stationary phase exhibited much lower rates of conjugative transfer (5.9 × 10−7 to 8.3 × 10−7 antibiotic-resistant colonies per recipient cell). The length of mating time (24, 48, or 72 h) was tested, and the maximum number of transconjugants per recipient cell was obtained with 48 h of incubation. We expected that conjugative transfer from E. coli would not be efficient at the low temperatures required for cultivation of F. psychrophilum and therefore used the highest temperature that F. psychrophilum would easily tolerate (20°C). Reduction of the temperature of incubation during mating to 18 and 15°C resulted in a reduction of 10- and 100-fold, respectively, in the number of antibiotic-resistant transconjugants. With control experiments, we found that conjugation of pCP29 from E. coli into F. johnsoniae, whose optimum growth temperature is 30°C, was also reduced 10-fold when conjugation was performed at 20°C instead of 30°C. cfxA and ermF functioned equally well as selectable markers in F. psychrophilum, since similar numbers of transconjugants were obtained with either cefoxitin or erythromycin as the selective agent. tetQ conferred tetracycline resistance on F. psychrophilum, but colonies did not appear until after 5 to 7 days of incubation when the tetQ-containing plasmid, pCP23, was transferred, and conjugative transfer frequencies were low (2.44 × 10−8 to 3.33 × 10−8 antibiotic-resistant colonies per recipient cell). Plasmids were also transferred by conjugation into several other strains of F. psychrophilum. pCP29 was transferred by conjugation into F. psychrophilum THC04-90 (6 × 10−6 colonies per recipient cell) and, less efficiently, into F. psychrophilum SH3-81. Despite repeated attempts, no transconjugants were obtained with F. psychrophilum strains ATCC 49418, ATCC 49510, ATCC 49511, Ch8-80, D12, and FPC 830.
Electroporation of F. psychrophilum.
A procedure was developed to allow for the direct introduction of DNA into F. psychrophilum cells by electroporation. pCP29 isolated from F. psychrophilum THC02-90 was used to optimize this procedure. Electrocompetent cells of F. psychrophilum were prepared by washing with either 10% glycerol or distilled water. Cells produced by either procedure were equally competent. The voltage (2.5 to 12.5 kV/cm) and resistance (200 to 1,000 Ω) were varied to optimize transfer. The optimal conditions for transfer were 7.5 to 12.5 kV/cm and 200 to 600 Ω. The highest number of transformants (2 × 106 to 2.6 × 106 transformants/μg of DNA) was obtained when the electrical parameters were 400 Ω, 10 kV/cm, and 25 μF and cefoxitin was used as the selective agent. The antibiotic used for selection had a marked effect on the efficiency of electrotransformation. When erythromycin was used instead of cefoxitin, only 4 × 104 to 5.3 × 104 colonies/μg of DNA were obtained.
Sequence analysis of pCP1.
The 3,407-bp nucleotide sequence of pCP1 was determined in order to enhance the usefulness of cloning vectors such as pCP29 and pCP23, which were derived from this plasmid. pCP1 contains four open reading frames: repA, ORF1, ORF2, and ORF3 (Fig. 1). RepA is similar to the Reimerella antatipestifer plasmid replication protein 1 from pCFC1 (49% identity over 317 amino acids; GenBank accession number AAC27555) and is probably involved in plasmid replication. The functions of the other open reading frames are not known. ORF1 and ORF2 are apparently not needed for replication in F. psychrophilum or F. johnsoniae, since ORF1 was disrupted during construction of pCP11 (15) and pCP29 (11) (Fig. 1) and ORF1 and ORF2 were disrupted during construction of pCP23 (1) (Fig. 1). Upstream of repA is a 21-bp sequence, AAACTTTCTTTTCGCTTATAA, that is tandemly repeated 4.7 times and may be involved in plasmid replication.
FIG. 1.
Maps of the plasmids pCP1, pCP23, and pCP29. Numbers in parentheses indicate map positions in nucleotides. The relative sizes of the maps do not correspond exactly to the sizes of the plasmids. Abbreviations: oriT, origin of transfer for conjugation; ori, ColE1 origin of replication, which functions in E. coli but not in F. psychrophilum; bla, β-lactamase, which confers resistance to ampicillin to E. coli but not to F. psychrophilum. The exact site of the pCP1 origin of replication, which functions in F. psychrophilum, has not been determined but is probably between ORF3 and repA.
Development of lacZY as a transcriptional reporter for F. psychrophilum.
The ORF1 promoter reads toward the multiple cloning site of pCP23 (Fig. 1). Promoterless lacZY was cloned into the multiple cloning site to test this promoter and to determine whether lacZ could be used as a reporter of gene expression for F. psychrophilum. Green tetracycline-resistant transconjugants appeared after 7 days of incubation (Fig. 2). Expression of lacZ from this promoter was measured under various conditions as a first test of its usefulness as a reporter of F. psychrophilum gene expression. The expression level was similar for cultures grown at 12 and 18°C and for cultures grown at pHs 6, 6.5, 7, and 7.5. In contrast, the addition of 1 to 5 mM CaCl2 resulted in decreased expression.
FIG. 2.
Expression of lacZ from the ORF1 promoter of pCP1. Transconjugants of F. psychrophilum containing the lacZY genes inserted into the SphI site of pCP23 downstream of the ORF1 promoter were grown on EAOS agar containing X-Gal. 1, F. psychrophilum THC02-90-p; 2, F. psychrophilum THC02-90-β.
Transposition of Tn4351 in F. psychrophilum.
Tn4351 was introduced into F. psychrophilum THC02-90 on pEP4351 by conjugation, and erythromycin-resistant transconjugants were obtained at a frequency of 0.8 × 10−8 to 1.8 × 10−8 per recipient cell. PCR and Southern blot analysis of transconjugants verified that Tn4351 had been transferred (Fig. 3). When analyzed by miniprep, none of the transconjugants carried pEP4351 as a free plasmid, indicating that as expected, pEP4351 did not replicate in F. psychrophilum. Some transconjugants carried only Tn4351 (Fig. 3B and C, lanes 3 to 6, 11, and 13), whereas others had the delivery vector integrated into the chromosome with Tn4351 (Fig. 3B and C, lanes 7 to 10 and 12). Insertions of plasmid DNA into the chromosome, which may be the result of homologous recombination after transposition of Tn4351, have been described previously for B. thetaiotaomicron, F. johnsoniae, and related bacteria (15, 24). The frequencies of such insertions are strain dependent. Most of the mutants that did not carry vector DNA had single insertions of Tn4351, but the presence of three bands in lane 6 of Fig. 3B suggests that multiple insertions may also occur. Tn4351 inserted at different locations in the chromosome (Fig. 3) and will probably be useful for mutagenesis of F. psychrophilum.
FIG. 3.
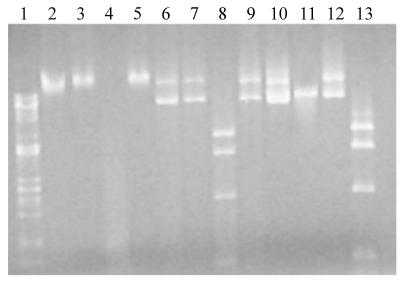
Southern blot hybridization of F. psychrophilum transconjugants carrying Tn4351. (A) Restriction map of pEP4351 showing the probes used for Southern blot hybridization. cat, a 633-bp fragment containing the structural part of the cat gene; Tn4351, the 6.2-kb SalI fragment containing the Tn4351 transposon. S, SalI; X, XbaI. (B and C) Chromosomal DNA from the transconjugants was digested with XbaI and Southern blot hybridization was carried out with the Tn4351 (B) and cat (C) probes. Lanes 3 to 13, different isolated transconjugants; lanes 2, DNA from the wild-type strain; lanes 14, pEP4351 DNA digested with SalI; lanes 1, λ DNA digested with PstI.
Evidence for a Sau3AI-like restriction modification system in F. psychrophilum.
Electroporation of pCP29 extracted from E. coli into F. psychrophilum resulted in no antibiotic-resistant transformants. We suspected that a restriction barrier was responsible for the lack of transformants. One indication of the presence of a restriction modification system is modification of DNA such that it resists digestion by the restriction enzyme produced by that strain. DNA propagated in F. psychrophilum was resistant to digestion by Sau3AI (Fig. 4, lanes 5 and 9) and BamHI (Fig. 4, lanes 3 and 7). Both enzymes cleave DNA that contains the core sequence GATC and are inhibited by methylation of cytosine in this sequence. MboI, which recognizes the same sequence as Sau3AI but is not inhibited by cytosine methylation, digested F. psychrophilum chromosomal DNA (Fig. 4, lane 4) and pCP29 DNA isolated from F. psychrophilum (Fig. 4, lane 8). In contrast, when pCP29 was extracted from E. coli S17-1 λ pir, it was susceptible to digestion by BamHI (Fig. 4, lane 11) and Sau3AI (Fig. 4, lane 13) but was resistant to MboI (Fig. 4, lane 12), an enzyme that fails to digest DNA that has been methylated on adenine residues by the dam system. F. psychrophilum DNA appears to be modified, and it is likely that this modification is part of a restriction modification system. This may account for some of the difficulties encountered when introducing DNA into F. psychrophilum by electroporation. Others have observed that conjugative transfer is generally less sensitive to the presence of restriction systems in the recipient strain (21), which may account for the success of conjugative transfer of DNA into F. psychrophilum.
FIG. 4.
Restriction analysis of genomic DNA and of pCP29 isolated from E. coli λ pir and F. psychrophilum THCO2-90. Genomic or plasmid DNA was obtained as described in the text and was digested with different restriction enzymes. Lanes 2 to 5, F. psychrophilum genomic DNA; lanes 6 to 9, pCP29 isolated from F. psychrophilum; lanes 10 to 13, pCP29 isolated from E. coli. DNA was digested with BamHI (lanes 3, 7, and 11), MboI (lanes 4, 8, and 12), or Sau3AI (lanes 5, 9, and 13). No restriction enzymes were added to the samples in lanes 2, 6, and 10. Lane 1 contained λ DNA digested with PstI as a reference.
DISCUSSION
F. psychrophilum, Flavobacterium columnare, and Flavobacterium branchiophilum are important fish pathogens. Techniques to genetically manipulate some members of the genus Flavobacterium have been developed (15, 16, 27), but despite the efforts of several laboratories, the fish pathogenic flavobacteria have resisted attempts of gene transfer. In this study, techniques that allow for the genetic manipulation of several strains of F. psychrophilum were developed, opening up the possibility of genetic analysis of pathogenesis by members of this genus.
DNA was introduced into F. psychrophilum by conjugation. Plasmids based on pCP1 replicated in F. psychrophilum, and the antibiotic resistance genes cfxA, ermF, and tetQ conferred cefoxitin resistance, erythromycin resistance, and tetracycline resistance, respectively. Conjugal transfer was strain dependent and was observed for three of the seven strains tested. F. psychrophilum THC02-90 and THC04-90, which were the best recipients for conjugative transfer, do not harbor any of the cryptic plasmids that are present in many strains (4). Since pCP1 was isolated from F. psychrophilum D12, it is possible that plasmid incompatibility accounts for some of the difficulties encountered when transferring pCP29 into some strains of F. psychrophilum. Another reason that F. psychrophilum may have resisted attempts of gene transfer until now is its inability to tolerate temperatures above 20°C. E. coli cells incubated at low temperatures exhibit decreased levels of conjugation (26). Low temperatures could alter the expression of genes involved in conjugation or the lipid composition of cell membranes (for a review, see reference 28), resulting in a decreased efficiency of conjugative transfer.
The conditions for the introduction of DNA into F. psychrophilum cells by electroporation were also determined. Antibiotic-resistant colonies arose when plasmid DNA isolated from F. psychrophilum was introduced into cells of F. psychrophilum, but DNA isolated from E. coli was not suitable for transfer by electroporation. A restriction barrier is likely to be responsible for our inability to introduce DNA isolated from E. coli into F. psychrophilum by electroporation. The resistance of F. psychrophilum DNA to digestion by Sau3AI and BamHI suggests that a Sau3AI-like restriction modification system may constitute at least part of the restriction barrier. The generation of a strain of F. psychrophilum with a restriction enzyme deficiency or the use of an E. coli strain containing the modification enzyme may remove the barrier to transfer of DNA by electroporation. Until then, conjugation will likely be a more reliable technique for the transfer of DNA from E. coli into F. psychrophilum.
Tn4351 was demonstrated to function in F. psychrophilum, inserting into the chromosome and conferring resistance to erythromycin. Tn4351 has been used for mutagenesis of a number of other members of the CFB group (15, 16, 21, 24) and is likely to be a useful genetic tool for mutagenesis and analysis of F. psychrophilum genes.
With the tools described above, a foreign gene (E. coli lacZ) was introduced into F. psychrophilum and expressed, apparently from the ORF1 promoter of pCP23. The results indicate that lacZ may be used in F. psychrophilum as a reporter of gene expression. They also suggest that the ORF1 promoter may be used to express genes for complementation analyses of F. psychrophilum mutants. The presence of calcium in the culture medium resulted in decreased levels of β-galactosidase activity from this construct. Calcium has previously been implicated in the control of expression of Fpp1 protease, a putative virulence factor of F. psychrophilum (23). Growth of F. psychrophilum is also sensitive to the level of calcium in the medium (P. Secades and J. A. Guijarro, unpublished data).
F. psychrophilum and the related bacteria F. columnare and F. branchiophilum are important pathogens of fish. The development of methods to genetically manipulate F. psychrophilum should allow for new approaches for the identification of virulence factors and determination of the mechanisms of pathogenesis and could result in the development of vaccine strains to prevent CWD. The results of this study may also aid the development of similar genetic systems for the other flavobacterial fish pathogens.
Acknowledgments
This work was supported by a grant (AGL2000-0869) from the Ministerio de Ciencia y Técnologia of Spain. B. Alvarez was the recipient of a grant from the FICYT. M. McBride was supported by grants from the National Science Foundation (MCB-0130967) and the Milwaukee Foundation.
We thank C. Rodicio for useful comments and assistance in the restriction modification experiments, J.-F. Bernardet for providing strains, and D. Saffarini and the members of the Saffarini and McBride laboratories for helpful suggestions.
REFERENCES
- 1.Agarwal, S., D. W. Hunnicutt, and M. J. McBride. 1997. Cloning and characterization of the Flavobacterium johnsoniae (Cytophaga johnsonae) gliding motility gene, gldA. Proc. Natl. Acad. Sci. USA 94:12139-12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Bertolini, J. M., H. Wakabayashi, V. G. Watral, M. J. Whipple, and J. S. Rohovec. 1994. Electrophoretic detection of proteases from selected strains of Flexibacter psychrophilus and assessment of their variability. J. Aquat. Anim. Health 6:224-233. [Google Scholar]
- 4.Chakroun, C., F. Grimont, M. C. Urdaci, and J.-F. Bernardet. 1998. Fingerprinting of Flavobacterium psychrophilum isolates by ribotyping and plasmid profiling. Dis. Aquat. Org. 33:167-177. [DOI] [PubMed] [Google Scholar]
- 5.Cooper, A. J., A. P. Kalinowski, N. B. Shoemaker, and A. A. Salyers. 1997. Construction and characterization of a Bacteroides thetaiotaomicron recA mutant: transfer of Bacteroides integrated conjugative elements is recA independent. J. Bacteriol. 179:6221-6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crump, E. M., M. B. Perry, S. C. Clouthier, and W. W. Kay. 2001. Antigenic characterization of the fish pathogen Flavobacterium psychrophilum. Appl. Environ. Microbiol. 67:750-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalsgaard, I. 1993. Virulence mechanisms in Cytophaga psychrophila and other Cytophaga-like bacteria pathogenic for fish. Annu. Rev. Fish Dis. 3:127-144. [Google Scholar]
- 8.Del Cerro, A., M. C. Mendoza, and J. A. Guijarro. 2002. Usefulness of a TaqMan-based polymerase chain reaction assay for the detection of the fish pathogen Flavobacterium psychrophilum. J. Appl. Microbiol. 93:149-156. [DOI] [PubMed] [Google Scholar]
- 9.Garcia, C., F. Pozet, and C. Michel. 2000. Standardization of experimental infection with Flavobacterium psychrophilum, the agent of rainbow trout Onchorhynchus mykiss fry syndrome. Dis. Aquat. Org. 42:191-197. [DOI] [PubMed] [Google Scholar]
- 10.Holt, R. A. 1987. Cytophaga psychrophila, the causative agent of bacterial coldwater disease in salmonid fish. Ph.D. thesis. Oregon State University, Corvallis.
- 11.Kempf, M. J., and M. J. McBride. 2000. Transposon insertions in the Flavobacterium johnsoniae ftsX gene disrupt gliding motility and cell division. J. Bacteriol. 182:1671-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacLean, L. L., E. Vinogradov, E. M. Crump, M. B. Perry, and W. W. Kay. 2001. The structure of the lipopolysaccharide O-antigen produced by Flavobacterium psychrophilum (259-93). Eur. J. Biochem. 268:2710-2716. [DOI] [PubMed] [Google Scholar]
- 13.Madsen, L., and I. Dalsgaard. 1998. Characterization of Flavobacterium psychrophilum; comparison of proteolytic activity and virulence of strains isolated from trout (Oncorhynchus mykiss), p. 45-52. In A. C. Barnes, G. A. Davidson, M. P. Hiney, and D. McIntosh (ed.), Methodology in fish disease research. Fisheries Research Service, Aberdeen, Scotland.
- 14.Mahan, M. J., J. W. Tobias, J. M. Slauch, P. C. Hanna, R. J. Collier, and J. J. Mekalanos. 1995. Antibiotic-based selection for bacterial genes that are specifically induced during infection of a host. Proc. Natl. Acad. Sci. USA 92:669-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McBride, M. J., and S. A. Baker. 1996. Development of techniques to genetically manipulate members of the genera Cytophaga, Flavobacterium, Flexibacter, and Sporocytophaga. Appl. Environ. Microbiol. 62:3017-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McBride, M. J., and M. J. Kempf. 1996. Development of techniques for the genetic manipulation of the gliding bacterium Cytophaga johnsonae. J. Bacteriol. 178:583-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metcalf, W. W., W. Jiang, and B. L. Wanner. 1994. Use of the rep technique for allele replacement to construct new Escherichia coli hosts for maintenance of R6Kγ origin plasmids at different copy numbers. Gene 138:1-7. [DOI] [PubMed] [Google Scholar]
- 18.Michel, C., D. Antonio, and R. P. Hedrick. 1999. Production of viable cultures of Flavobacterium psychrophilum approach and control. Res. Microbiol. 150:351-358. [DOI] [PubMed] [Google Scholar]
- 19.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 20.Ostland, V. E., P. J. Byrne, G. Hoover, and H. W. Ferguson. 2000. Necrotic myositis of rainbow trout, Oncorhynchus mykiss (Walbaum): proteolytic characteristics of a crude extracellular preparation from Flavobacterium psychrophilum. J. Fish Dis. 23:329-336. [Google Scholar]
- 21.Salyers, A. A., N. Shoemaker, A. Cooper, J. D'Elia, and J. A. Shipman. 1999. Genetic methods for Bacteroides species, p. 229-249. In M. C. M. Smith and R. E. Sockett (ed.), Genetic methods for diverse prokaryotes. Academic Press, London, United Kingdom.
- 22.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 23.Secades, P., B. Alvarez, and J. A. Guijarro. 2001. Purification and characterization of a psychrophilic calcium induced, growth-phase-dependent metalloprotease from the fish pathogen Flavobacterium psychrophilum. Appl. Environ. Microbiol. 67:2436-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shoemaker, N. B., C. Getty, J. F. Gardner, and A. A. Salyers. 1986. Tn4351 transposes in Bacteroides spp. and mediates the integration of plasmid R751 into the Bacteroides chromosome. J. Bacteriol. 165:929-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 26.Singleton, P., and A. E. Anson. 1983. Effect of pH on conjugal transfer at low temperatures. Appl. Environ. Microbiol. 46:291-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su, H., Z. Shao, L. Tkalec, F. Blain, and J. Zimmermann. 2001. Development of a genetic system for the transfer of DNA into Flavobacterium heparinum. Microbiology 147:581-589. [DOI] [PubMed] [Google Scholar]
- 28.Thieringer, H. A., P. G. Jones, and M. Inouye. 1998. Cold shock and adaptation. Bioessays 20:49-57. [DOI] [PubMed] [Google Scholar]
- 29.Toyama, T., K. Kita-Tsukamoto, and H. Wakabayashi. 1994. Identification of Cytophaga psychrophila by PCR targeted 16S ribosomal RNA. Fish Pathol. 29:271-275. [Google Scholar]
- 30.Urdaci, M. C., C. Chakroun, D. Faure, and J.-F. Bernardet. 1998. Development of a polymerase chain reaction assay for identification and detection of the fish pathogen Flavobacterium psychrophilum. Res. Microbiol. 149:519-530. [DOI] [PubMed] [Google Scholar]
- 31.Wiklund, T., L. Madsen, M. S. Bruun, and I. Dalsgaard. 2000. Detection of Flavobacterium psychrophilum from fish tissue and water samples by PCR amplification. J. Appl. Microbiol. 88:299-307. [DOI] [PubMed] [Google Scholar]