Abstract
Alpine soils undergo dramatic temporal changes in their microclimatic properties, suggesting that the bacteria there encounter uncommon shifting selection gradients. Pseudomonads constitute important members of the alpine soil community. In order to characterize the alpine Pseudomonas community and to assess the impact of shifting selection on this community, we examined the ability of cold-tolerant Pseudomonas isolates to grow on a variety of carbon sources, and we determined their phylogenetic relationships based on 16S ribosomal DNA sequencing. We found a high prevalence of Pseudomonas in our soil samples, and isolates from these soils exhibited extensive metabolic diversity. In addition, our data revealed that many of our isolates form a unique cold-adapted clade, representatives of which are also found in the Swedish tundra and Antarctica. Our data also show a lack of concordance between the metabolic properties and 16S phylogeny, indicating that the metabolic diversity of these organisms cannot be predicted by phylogeny.
High-alpine soil environments are characterized by dramatic seasonal shifts in physical and biochemical properties. Winter is characterized by intermittent snow cover and fluctuating subfreezing temperatures; summer has intense, desiccating sunshine punctuated by infrequent rains (8). Many organic compounds important to the microbial community fluctuate seasonally, including cellulose, hot-water-soluble organic pools (22), soil protein, and amino acids (23). Shifts in microbial community composition (21, 35) and microbial metabolic capability (36) appear to be correlated with periods of marked environmental change, suggesting that shifts in selection pressures occur over time. In addition, as soils change from wet to dry, the spatial distribution of sources of carbon for growth becomes more heterogeneous. Thus, high-alpine soil environments impose severe and shifting selection gradients on bacteria.
Bacteria are renowned for their rapid evolution in response to novel selection pressure, and any environment subject to varying selection, either spatially or temporally, may harbor suites of bacteria that are capable of rapid change. The emergence and spread of antibiotic resistance (1) are perhaps the best known examples. In addition, the bioremediation literature is full of references to bacteria that possess unique genes that metabolize toxic chemicals (e.g., 11, 38). Many more examples of rapid evolution of metabolic characters have been described for a diverse range of bacterial species, and most cases involve the emergence of novel genes and their spread in environments that are subject to marked human impact (10, 26). Although important information about the metabolic versatility of bacteria in human-impacted environments can be gleaned from the literature, whether such versatility is a general property of natural microbial communities is less well known.
Pseudomonas, an enormously diverse genus of the γ-Proteobacteria, is an important member of soil microbial communities (27). Members of the genus have been isolated from essentially all environments studied (28), including alpine soil, where it was identified as the most prevalent culturable genus in Kobresia alpine meadows (24). The genus exhibits remarkable metabolic variation (31), and a large number of different plasmids have been described for it, including enormous plasmids containing many genes (e.g., the IncP-9 TOL plasmid pWW0 in Pseudomonas putida is over 110 kb and contains 148 open reading frames [7]). The great metabolic flexibility of Pseudomonas species may allow them to inhabit variable environments. One strategy might be the evolution of strains that are capable of utilizing a large number of different carbon sources for growth. Alternatively, because the alpine environment is highly heterogeneous with pockets of specific carbon compounds, a large number of different strains that have recently gained or lost the ability to grow on particular sources of carbon may exist.
In this study we report the prevalence of fluorescent pseudomonads and describe the isolation and characterization of 17 cold-tolerant strains of Pseudomonas from high-alpine soil in Colorado. Strains were grown on 20 different carbon sources to assess their metabolic characteristics, and their 16S ribosomal DNA (rDNA) sequences were determined for phylogenetic analysis. Evaluation of the metabolic properties relative to the phylogeny revealed that Pseudomonas bacteria from high-alpine soil lack phylogenetic diversity but exhibit great metabolic versatility. The lack of concordance between the metabolic data and the inferred phylogeny may reflect rapid gains and losses of genes.
MATERIALS AND METHODS
Site and MPN counts.
Soils for the most-probable-number (MPN) counts were collected in the autumn and winter of 1997 to 1998 (5 October 1997, 16 November 1997, and 1 February 1998) from Niwot Ridge (40°03′N, 105°36′W), a large expanse of alpine tundra located 50 km west of Boulder, Colo. The soils on Niwot Ridge are Pergelic Cryumbrepts, partially aeolian in origin (32). We obtained the soil samples from xeric meadows within a kilometer of each other at an elevation of 3,500 m. The sedge Kobresia myosuroides (Vill.) Paol. and Fiori dominates the meadows. Previous work has shown that soil temperatures at this site range from 3.5°C in early October to −0.4°C in the winter (14, 23). The medium for the MPN count consisted of a mineral salt solution (1 g of MgSO4 · 7H2O, 0.14 g of K2HPO4, 0.02 g of KH2PO4, 0.10 g of NH4NO3, and 0.05 g of CaCl2 per liter of deionized H2O), 1 ml per liter of soil extract (10:1 [vol/wt], sterile filtered), and glutamate at a concentration of 1 mM, as described by Lipson et al. (23). MPN counts were done as described previously (21). Briefly, soil (10 g of dry mass equivalent) was blended (2 min on, 1 min off, 2 min on) in 100 ml of sterile MgSO4 · 7H2O solution (1 g/liter). The soil suspension (0.05 ml) was dispensed into 96-well plates (10-fold dilution steps, eight replicates per dilution, 0.15 ml of medium per well) and incubated for 6 weeks at 3°C. The wells were scored positive if they showed turbidity (total cells) or yellow fluorescence (yellow cells) visible to the naked eye.
Isolation.
To obtain pure Pseudomonas isolates that were significantly represented in the soils, we used two related isolation methods. The first method, a limiting dilution culture, isolated organisms that might otherwise be outcompeted in the laboratory (4, 20). In this method, the isolates came from the highest dilution showing growth. The second method, enrichment cultures, selected fast-growing organisms by using the 10−3 dilutions. The culture medium for the isolation, enrichment, dilution, and growth studies was the same mineral salt solution with sterile soil extract as that used for the MPN counts, but with a C source (0.2 g liter−1). Table 1 outlines the dates of soil collection and the methods, C sources, and temperatures of isolation.
TABLE 1.
Details of isolation conditions of study strains
| Isolate | Date of soil collection (mo/yr) | Degree of dilution at isolation | Isolation carbon sourcea | Temp of isolation (°C) |
|---|---|---|---|---|
| WE7°2b | 10/95 | 10−3 | Glutamate | 7 |
| BE3dil | 9/95 | 10−6 | Glutamate | 22 |
| BG2dil | 9/95 | 10−6 | Glycine | 22 |
| WR7°2 | 10/95 | 10−3 | Arginine | 7 |
| SE22°2 | 10/95 | 10−3 | Glutamate | 22 |
| BE1dil | 9/95 | 10−6 | Glutamate | 22 |
| 4/11SKenr22° | 4/98 | 10−3 | Salicylate | 22 |
| WE7°1b | 10/95 | 10−3 | Glutamate | 7 |
| SE7°1 | 10/95 | 10−3 | Glutamate | 7 |
| SE22°1a | 10/95 | 10−3 | Glutamate | 22 |
| R1enr | 9/95 | 10−3 | Arginine | 22 |
| 11/20CMCctl | 11/97 | 10−6 | Carboxymethylcellulose | 3 |
| WG7°1 | 10/95 | 10−3 | Glycine | 7 |
| WG22°2 | 10/95 | 10−3 | Glycine | 22 |
| BE4dil | 9/95 | 10−6 | Glutamate | 22 |
| 4/11GCS3°e | 4/98 | 10−6 | Glucose | 3 |
| 4/27CMCA2 | 4/97 | 10−6 | Carboxymethylcellulose | 3 |
Glutamate, arginine, glycine, and glucose were chosen because they have all been found in our soils; carboxymethylcellulose was chosen because these soils contain considerable plant organic matter.
Using the methods described above, we isolated 17 cold-tolerant, fluorescent Pseudomonas strains from the same Kobresia dry meadow soil as was used for the MPN counts. Specifically, 41 isolates showing visible siderophore fluorescence were originally cultured. We restricted our isolates to cold-tolerant strains by choosing only those 23 isolates showing growth at −2 and 22°C but not at 36°C. Subsequent difficulties in sequencing the entire small-subunit gene and the presence of redundant sequences narrowed our isolates to 17.
Metabolic studies using different carbon sources.
We determined whether each isolate grew on each of 20 different C sources. Table 2 lists the C sources that were tested. The growth experiments were done at 22°C. A standard 96-well plate with mineral salt-glutamate medium served as the inoculation master. Duplicates for each isolate grew in adjacent wells. To maintain the separation of the isolates, we grew our isolates only in every other column of the plates; the uninoculated columns served as control blanks and also as a method to guard against cross-contamination of the wells. Five replicate control plates contained only mineral salt medium and no C compound. Using a replicator (Boekel Scientific, Inc., Feasterville, Pa.), we inoculated cells from the master plate into each control and C source plate. A Spectra Max 340PC plate reader driven by Soft Max Pro 2.6.1 software (Molecular Devices Corporation, Milpitas, Calif.) measured growth at an optical density (OD) of 595 nm. To evenly suspend the cells in the medium, we manually agitated each plate and set the plate reader to agitate the plate just before each growth reading. Growth was measured daily for 7 days. Growth was defined as present if, over the duration of each growth study, any mean OD of the two replicates was recorded as being greater than 1 standard deviation above the mean control OD.
TABLE 2.
Growth on carbon sources for each isolate
| Isolate | Growth on indicated carbon sourcea
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycine | Casein | Citrate | Formate | Oxalate | Acetate | Benzoate | Phenol | Salicylate | Vanillate | Cellubiose | Carboxymethylcellulose | Levan | Starch | Maltose | Trehalose | Sucrose | Polyethylene glycol | Ethanol | Methanol | |
| WE7°2b | 0 | 0 | + | 0 | 0 | 0 | + | 0 | 0 | + | 0 | 0 | + | 0 | 0 | + | 0 | 0 | 0 | 0 |
| BE3dil | + | + | + | 0 | 0 | + | + | 0 | 0 | 0 | 0 | 0 | + | 0 | + | + | 0 | 0 | 0 | 0 |
| BG2dil | + | + | + | 0 | 0 | 0 | + | 0 | 0 | 0 | 0 | 0 | + | 0 | + | + | + | 0 | 0 | + |
| WR7°2 | + | 0 | + | 0 | 0 | + | + | 0 | 0 | + | 0 | 0 | + | + | 0 | + | + | 0 | 0 | 0 |
| SE22°2 | + | + | + | + | + | 0 | + | 0 | 0 | 0 | + | 0 | + | 0 | + | + | + | 0 | 0 | 0 |
| BE1dil | + | + | + | + | + | + | + | 0 | + | 0 | 0 | 0 | + | 0 | + | + | + | 0 | 0 | 0 |
| 4/11Skenr22° | + | + | + | 0 | + | 0 | + | + | + | 0 | 0 | + | + | 0 | 0 | + | + | 0 | + | + |
| WE7°1b | + | + | + | + | 0 | + | + | + | 0 | + | 0 | 0 | + | + | + | + | + | 0 | 0 | + |
| SE7°1 | + | + | + | + | + | 0 | + | + | 0 | 0 | + | + | + | 0 | + | + | + | + | 0 | 0 |
| SE22°1a | + | + | + | + | 0 | + | 0 | 0 | 0 | 0 | + | + | + | + | + | 0 | + | + | + | + |
| R1enr | + | + | + | + | + | + | + | 0 | + | 0 | 0 | + | + | 0 | + | + | + | + | 0 | 0 |
| 11/20CMCctl | + | + | + | 0 | + | + | + | + | + | 0 | 0 | + | + | 0 | + | + | + | 0 | + | + |
| WG7°1 | + | + | + | + | + | + | + | 0 | 0 | 0 | + | + | + | + | + | 0 | + | + | + | + |
| WG22°2 | + | + | + | + | + | + | + | 0 | + | 0 | + | + | + | 0 | + | + | + | + | + | + |
| BE4dil | + | + | + | + | + | + | + | 0 | 0 | 0 | + | + | + | + | + | + | + | + | + | + |
| 4/11GCS3°e | + | + | + | + | + | + | + | + | 0 | + | + | + | + | + | + | + | + | + | + | + |
| 4/27CMCA2 | + | + | + | + | + | + | + | + | 0 | + | + | + | + | + | + | + | + | + | + | + |
+, growth; 0, no growth.
Molecular studies and phylogenetic analysis.
We extracted DNA from fresh overnight cultures by using a QIAamp tissue kit (QIAGEN, Inc., Valencia, Calif.). We employed a PCR to obtain the 16S rDNA. PCR amplification was conducted with a total volume of 100 μl with the following final concentrations of reagents: 0.1 μM (each) primer, 0.2 mM (each) deoxynucleotide triphosphate, 10 mM Tris-HCl (pH 9.0), 50 mM KCl, 2.0 mM MgCl2, and 4 U of Taq DNA polymerase (Promega, Madison, Wis.). Thermocycling conditions consisted of an initial denaturation at 94°C for 1.5 min followed by 33 cycles of denaturation at 94°C for 1 min, annealing at 50°C for 1 min, and extension at 72°C for 2 min, as well as a final extension step of 72°C for 5 min. The detailed primer data for the PCRs and the sequencing reactions are shown in Table 3. We purified the PCR-amplified DNAs with QIAquick (QIAGEN Inc.) according to the manufacturer's PCR purification kit protocol, by using water to elute the DNA from the spin column. Six overlapping fragments (three forward and three reverse) of approximately 600 bp each were sequenced on a Perkin-Elmer (Foster City, Calif.) model 377 DNA sequencer with a PE Applied Biosystems BigDye terminator cycle sequencing ready reaction (Perkin-Elmer). For each isolate, we assembled the six fragments of 16S rDNA into one consensus sequence of at least 1,470 bp by using Sequencher (version 3.0; Gene Codes Corp., Ann Arbor, Mich.).
TABLE 3.
Primer dataa
| Primer | Sequenceb | E. coli positions (nt) | Designationc | Used ford: |
|---|---|---|---|---|
| F8-27 | AGAGTTTGATCMTGGCTCAG | 8-27 | 27f | P, S |
| F515 | GTGCCAGCMGCCGCGG | 515-530 | 530f | S |
| F1093 | AGTCCCGCAACGAGCGCAA | 1093-1114 | 1114f | S |
| R536 | GTATTACCGCGGCTGCTGG | 519-536 | 519r | S |
| R1110 | GGGTTGCGCTCGTTG | 1110-1124 | 1110r | S |
| R1492 | TACGGTTACCTTGTTACGACTT | 1492-1513 | 1492r | S |
| R1510 | CGGYTACCTTGTTACGACTT | 1494-1513 | none | P |
We aligned the sequences of the 17 cold-adapted isolates and representative sequences of Pseudomonas sensu stricto retrieved from GenBank (2) by using Clustal X (version 1.81) (16), with the alignment adjusted by eye with SeqPup 9 (Indiana University, Bloomington). Only the clearly unambiguous positions in the alignment were retained for the phylogenetic analysis.
Posterior probabilities were determined by Bayesian Markov chain Monte Carlo methods implemented by using MrBayes (13). A general time-reversible model with gamma rate heterogeneity was adopted; 500,000 generations were run, and the trees and model parameters were sampled every 100 generations. The posterior probability distribution stabilized after 36,000 generations, and so this number was adopted as the burn-in value (meaning that all parameter estimates prior to generation 36,100 were omitted). Branch lengths were estimated by using maximum likelihood and the modal parameter values estimated for the substitution model from the Bayesian analysis. The likelihood analysis was performed with PAUP (version 4.0b8a; Sinauer Associates, Inc., Sunderland, Mass.).
The concordance between the metabolic data and 16S rRNA evolution was examined in several ways. First, we constructed a tree based on the metabolic matrix by using parsimony with unordered characters, and we compared this tree with the Bayesian 16S rRNA tree by using the Shimodaira-Hasegawa likelihood-based test implemented in PAUP. Second, we explored the number of metabolic state changes by optimizing the data regarding the presence or absence of growth on the set of Bayesian 16S rDNA trees with the aid of MacClade (D. Maddison and W. Maddison, 1993). Before we did this, we rearranged the branch order on the tree for the nodes that were not resolved (i.e., nodes defined an ancestor for more than two lineages) such that the total number of metabolic character state changes was minimized. In this way, we avoided including polytomies when tracing character evolution. Significance was assessed by comparing the observed number of changes with the number of changes with assumption of no correlation between carbon source growth data and phylogeny. Significance was established by optimizing the growth data on 1,000 random trees and comparing the observed number of changes with the distribution of changes for the random trees. Only phylogenetically informative characters were subjected to this analysis. Finally, we plotted the branch lengths that were estimated for the DNA and metabolic data separately by using maximum likelihood and parsimony, respectively. Significance was assessed by linear regression.
Nucleotide sequence accession numbers. Table 4 lists the GenBank accession numbers of our newly identified isolates.
TABLE 4.
GenBank accession numbers of newly identified isolates
| Isolate | GenBank accession no. |
|---|---|
| BG2dil | AY263468 |
| WG7°1 | AY263469 |
| WG22°2 | AY263470 |
| BE1dil | AY263471 |
| BE3dil | AY263472 |
| BE4dil | AY263473 |
| WE7°1b | AY263474 |
| WE7°2b | AY263475 |
| SE7°1 | AY263476 |
| SE22°1a | AY263477 |
| SE22°2 | AY263478 |
| R1enr | AY263479 |
| WR7°2 | AY263480 |
| 4/11GCS3°e | AY263481 |
| 11/20CMC control | AY263482 |
| 4/11Skenr22° | AY263483 |
| 4/27CMC A2 | AY263484 |
RESULTS
Prevalence in alpine soil.
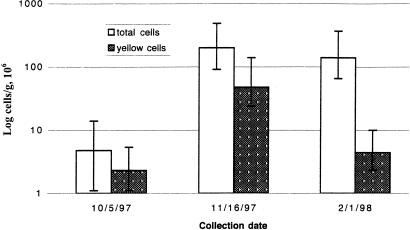
Fluorescent pseudomonads are prevalent in high numbers in the soil in the Colorado alpine area. Our MPN results demonstrate that such organisms (yellow cells) were present at 0.23 × 107 to 4.8 × 107 cells per gram (dry weight) of soil (Fig. 1). As a percentage of the total MPNs of cells, the fluorescent pseudomonads ranged from 3 to 48%. In addition, fluorescent pseudomonads showed their lowest relative prevalence in winter rather than autumn soils. Such cells made up only 3% of the total MPNs of cells on 1 February 1998 in contrast to 48 and 24% on 5 October 1997 and 16 November 1997, respectively.
FIG. 1.
MPNs of cells per gram (dry weight) of soil from a Kobresia alpine meadow on three dates (month/day/year) in autumn and winter. Yellow cells were those showing visible yellow fluorescence. Error bars represent 95% confidence intervals.
16S phylogeny.
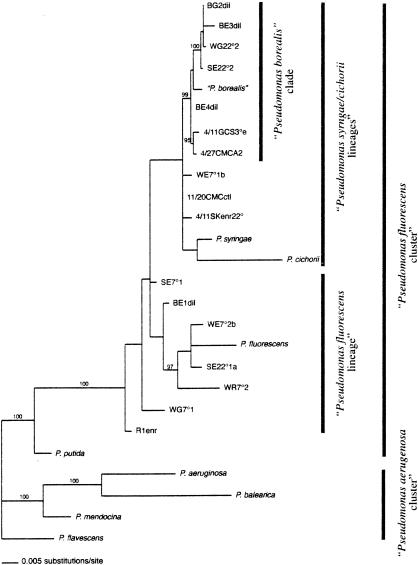
Bayesian analysis showed that the cold-tolerant, fluorescent pseudomonads that we isolated from the Colorado alpine site were all from the genus Pseudomonas sensu stricto (3, 25) but were not distributed throughout all of the accepted Pseudomonas lineages (Fig. 2). Moore et al. (25) used the 16S rRNA gene to define two major clusters of Pseudomonas, the P. aeruginosa cluster and the P. fluorescens cluster, with several lineages (or subclusters) in each. The organisms in our study all fell only within the P. fluorescens cluster, which is not surprising as the majority of described fluorescent Pseudomonas sensu stricto organisms fall into this cluster (12, 27, 28). However, all of our isolates grouped in only two of the five lineages described for the P. fluorescens cluster: the P. fluorescens lineage and the P. syringae lineage (Fig. 2). Interestingly, seven of our isolates in the P. syringae lineage are very closely related to “P. borealis,” an organism isolated in the Swedish tundra (M. Hokeberg, personal communication); they form a unique clade with “P. borealis” and also have this organism as their closest GenBank BLAST (2) match. This clade is phylogenetically distinct from the rest of the Pseudomonas phylotypes with a posterior probability of 99% (Fig. 2). The trees generated by distance and maximum parsimony methods showed relationships that were not materially different from those in Fig. 2 (trees not shown).
FIG. 2.
Bayesian phylogenetic tree of 16S rDNA sequences of our 17 isolates and selected Pseudomonas (sensu stricto) sequences obtained from GenBank. Posterior probability values of 95% or greater are noted. The tree was rooted with P. flavescens, which gave the same topology as Escherichia coli. GenBank accession numbers: P. aeruginosa, Z76651; P. balearica, U26418; “P. borealis,” AJ012712; P. cichorii, Z76658; P. flavescens, U01916; P. fluorescens, Z76662; P. mendocina, Z76664; P. putida, Z76667; and P. syringae, Z76669.
Diverse metabolic capabilities.
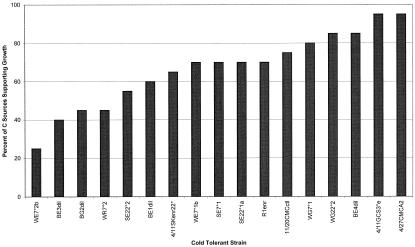
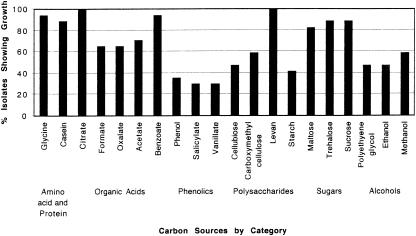
We measured the metabolic diversity of the cold-tolerant Pseudomonas isolates by testing their ability to grow on 20 different C compounds from several chemical groups (Table 2). Our Pseudomonas isolates showed much diversity in their range of metabolic capabilities. Two isolates, 4/11GCS3°e and 4/27CMCA2, were supported by the highest number (95%) of the C sources, and one isolate, WE7°2b, was supported by the least number (25%) (Fig. 3). Similarly, the frequency of isolates that were supported by a given C source ranged from a low of 30% for salicylate and vanillate to a high of 100% for citrate and levan (Fig. 4). The chemical groups supporting the most isolates were the amino acids and proteins, averaging 91% of the isolates, and the sugars, averaging 87%; the one supporting the least number of isolates was the phenolics at 32%. No isolate that grew on vanillate also grew on salicylate and vice versa (Table 2).
FIG. 3.
Frequency of growth on carbon sources. Seventeen cold-tolerant alpine isolates were tested for their ability to grow on 20 C sources. All isolates grew on at least one C source tested. Frequencies of growth on C sources ranged from 25 to 90%.
FIG. 4.
Frequency of utilization of carbon sources. Frequencies ranged from 30 to 100%. The phenolic category supported growth the least, and the amino acid and protein categories supported growth the most.
Lack of concordance between metabolic properties and phylogeny.
A comparison of the two trees, one generated from the 16S rDNA data and a parsimony tree of the metabolic characters (coded as presence or absence), performed with a Shimodaira-Hasegawa test based on DNA data, indicated that the two data sets supported significantly different sets of relationships (P < 0.001).
A comparison of the number of changes of carbon source preference on the inferred phylogeny with the number of changes required for random trees suggested that phylogeny provides a poor explanation for the evolution of carbon preference. For all of the carbon sources, the observed numbers of changes fell within the distribution that was expected if the character changes occurred randomly on the tree.
A bivariate plot of the branch lengths estimated for the DNA and carbon source data revealed a lack of correlation (r2 = 0; P = 0.97). Several lineages were noteworthy. Isolate SE22°1a was inferred to have undergone seven unique changes of carbon source preference since it last shared an ancestor with another strain about 0.003 substitution ago (0.3% change in sequence). At the other extreme, isolate WR7°2 underwent an approximately 1.2% change in sequence yet did not appear to change its preference for carbon from the inferred ancestral condition.
DISCUSSION
Metabolic diversity cannot be predicted by phylogeny.
The limited covariation between 16S rRNA phylogeny and metabolic properties suggests that the ability to use particular carbon sources cannot be predicted from a knowledge of phylogeny. This was evident in all tests of concordance between the two sets of data. Several implications emerge from this result. First, cold-adapted alpine Pseudomonas isolates manifest metabolic versatility. Second, these Pseudomonas isolates demonstrate great metabolic versatility independent of their evolution, indicating that these phenotypes did not evolve with the core 16S phylogeny. Third, this versatility emerges from an apparently dynamic process in which lineages gain and lose the ability to use different sources of carbon for growth.
Bacterial genomes are known to undergo high rates of evolution by a combination of point mutations, deletions, gene duplications, and acquisitions of foreign DNA. The fate of mutations is governed by selection. In the absence of a particular carbon source, the residence time for the necessary gene is probably short. Our evidence for a dynamic and versatile metabolic repertoire suggests that alpine soil environments may be tremendously heterogeneous with respect to the availability of different carbon sources, and such differential selection due to the various concentrations of different carbon pools may drive the gains and losses of metabolic genes.
Our findings of a lack of congruence between phenotypic and genotypic data are in contrast to many of the classic nutritional studies of Pseudomonas. These nutritional studies, combined with rDNA hybridization data, have been seen as giving good agreement in intrageneric clustering (27-29). However, many of the same and similar reports state that gene exchanges between clusters may also explain some of their results (9, 27, 29, 30), implying less than total congruence between genotypic (rDNA) and phenotypic (nutritional) data. Many studies of Pseudomonas have presented genotypic and phenotypic data and commented on their congruence (15, 18, 34, 39), but few have rigorously tested such congruence. Our results, rigorously showing no congruence, suggest that statements of congruence should be based on careful tests of this issue and that the famous metabolic versatility of the genus might well be founded in large measure on the easy ability to acquire opportunistic metabolic capabilities.
Unique cold-soil clade.
Our phylogenetic analysis reveals that 7 of our 17 isolates (41%) fall into a novel, well-supported clade (posterior probability, 99%) that is closely related to the P. syringae lineage (25) (Fig. 2). This clade includes “P. borealis,” an organism that was also isolated from cold soils. Although unpublished, the 16S rDNA sequence of “P. borealis” was deposited in GenBank after being isolated from Swedish tundra soil north of the arctic circle (M. Hokeberg, personal communication). An organism with 99.3% sequence identity has also been isolated from soil from Signy Island off Antarctica (B. Stallwood, personal communication). Since 41% of our isolates fall into this unique cold-soil clade, our data suggest that a significant proportion of the Pseudomonas isolates in our alpine soil are specifically adapted to cold soils. Also, the finding of very similar 16S isolates in widely separated, persistently cold climates suggests that this strain is not endemic but perhaps ubiquitous in extremely cold environments. This suggestion is in contrast to the results of a four-continent study of 38 mesophyllic soil strains of Pseudomonas sensu stricto which showed endemicity at distances of less than 197 km (5).
Significant role in the Kobresia ecosystem.
Our growth data alone strongly suggest that pseudomonads play a significant biogeochemical role in the Kobresia dry meadow community. All 17 of our isolates grow on levan (Fig. 4), a 2,6-linked polymer of fructose. This polysaccharide, thought to be important in the frost resistance of Kobresia, is specifically accumulated in significant quantities by Kobresia (T. Rosenstiel, personal communication). Since Kobresia dominates this community and is a significant source of litter and root-derived organic matter, we believe that levan is a major C source for the soil microbes here.
Several other interesting properties of alpine Pseudomonas emerged from our data. The isolates demonstrated a high use of maltose but a low use of starch (Fig. 4). Since alpine soil has significant amylase activity in both summer and winter (21), our results may indicate that Pseudomonas strains do not expend resources on excreting amylase but rather rely on the amylases of other organisms. More than 85% of the isolates grew on casein, a milk protein (Fig. 4), which supports other data suggesting that amino acids from the degradation of peptides account for most of the N cycled in the alpine (19). Also, in contrast to the low utilization of glycine by alpine microbes (20), our isolates have a high utilization of glycine (Fig. 4), suggesting that Pseudomonas strains may be important competitors with plants for amino acids, an important N source for plants in this ecosystem (33).
We found a high absolute and relative prevalence of fluorescent pseudomonads in our alpine soils, with the highest percentages present in autumn and the lowest percentage present in winter (Fig. 1). These data are in contrast to those of Mancinelli (24), who found Pseudomonas MPN counts to be 2 orders of magnitude lower and the lowest proportions to be present in autumn (24). These differences in results may represent year-to-year fluctuations, inadequate spatial sampling, or differences in technique. In any case, our results demonstrate large numbers of fluorescent pseudomonads in our study soils, suggesting that they play a prominent role in biogeochemical processes there, perhaps more actively in the autumn, when more substrate is available (6).
The interesting growth pattern of our isolates confirms earlier laboratory work on bacterial aromatic-compound degradation pathways. Many aromatics are converted to either protocatechuate or to catechol before ring cleavage (37). Of the phenolics we tested, earlier studies show that vanillate is converted to protocatechuate while salicylate is converted to catechol (37). Interestingly, the growth patterns of our isolates on vanillate and salicylate were mutually exclusive: those that grew on one did not grow on the other (Table 2), which is consistent with the laboratory work on vanillate and salicylate degradation.
Caveats.
Although we suspect that the observed variation in metabolic capacities among the strains reflects the characteristics of natural populations, the conditions used for the isolation of the strains may have enhanced the observed metabolic variation. Strains were isolated at five different times on seven different carbon sources and at three different temperatures. It is certainly true that different isolation conditions selected for different metabolic characteristics; however, it is also possible that specific metabolic pathways were lost due to mutation during isolation.
Acknowledgments
We thank K. Shiley, L. Courter, and K. Ouimet for laboratory and field assistance and M. Krest, E. Costello, and D. Nemergut for helpful comments on the manuscript.
This work was supported by NSF grants MCB-0084223 and IBN-9817164.
REFERENCES
- 1.Alberti, S., V. Ortali, and E. J. Salas. 1965. On the transduction of certain metabolic characters in staphylococci. Ann. Ist. Super. Sanita 1:61-66. [PubMed] [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, M. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anzai, Y., H. Kim, J. Y. Park, H. Wakabayashi, and H. Oyaizu. 2000. Phylogenetic affiliation of the pseudomonads based on 16S rRNA sequence. Int. J. Syst. E vol. Microbiol. 50:1563-1589. [DOI] [PubMed] [Google Scholar]
- 4.Button, D. K., F. Schut, P. Quang, R. Martin, and B. R. Robertson. 1993. Viability and isolation of marine bacteria by dilution culture: theory, procedures, and initial results. Appl. Environ. Microbiol. 59:881-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho, J.-C., and J. M. Tiedje. 2000. Biogeography and degree of endemicity of fluorescent Pseudomonas strains in soil. Appl. Environ. Microbiol. 66:5448-5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisk, M. C., and S. K. Schmidt. 1995. Nitrogen mineralization and microbial biomass nitrogen dynamics in three alpine tundra communities. Soil Sci. Soc. Am. J. 59:1036-1043. [Google Scholar]
- 7.Greated, A., L. Lambertsen, P. A. Williams, and C. M. Thomas. 2002. Complete sequence of the IncP-9 TOL plasmid pWW0 from Pseudomonas putida. Environ. Microbiol. 4:856-871. [DOI] [PubMed] [Google Scholar]
- 8.Greenland, D., and M. Losleben. 2001. Climate, p. 15-31. In W. D. Bowman and T. R. Seastedt (ed.), Structure and function of an alpine ecosystem Niwot Ridge, Colorado. Oxford University Press, New York, N.Y.
- 9.Grimont, P. A. D., M. Vancanneyt, M. Lefevre, K. Vandemeulebroecke, L. Vauterin, R. Brosch, K. Kersters, and F. Grimont. 1996. Ability of Biolog and Biotype-100 systems to reveal the taxonomic diversity of the pseudomonads. Syst. Appl. Microbiol. 19:510-527. [Google Scholar]
- 10.Hall, R. M., and C. M. Collis. 1998. Antibiotic resistance in gram-negative bacteria: the role of gene cassettes and integrons. Drug Resist. Updates 1:109-119. [DOI] [PubMed] [Google Scholar]
- 11.Hickey, W. J., G. Sabat, A. S. Yuroff, A. R. Arment, and J. Pérez-Lesher. 2001. Cloning, nucleotide sequencing, and functional analysis of a novel, mobile cluster of biodegradation genes from Pseudomonas aeruginosa strain JB2. Appl. Environ. Microbiol. 67:4603-4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holt, J. G., N. R. Krieg, P. Sneath, J. Staley, and S. Williams. 1994. Bergey's manual of determinative bacteriology, 9th ed. Williams & Wilkins, Baltimore, Md.
- 13.Huelsenbeck, J. P., and F. Ronquist. 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754-755. [DOI] [PubMed] [Google Scholar]
- 14.Jaeger, C. H., R. K. Monson, M. C. Fisk, and S. K. Schmidt. 1999. Seasonal partitioning of nitrogen by plants and soil microorganisms in an alpine ecosystem. Ecology 80:1883-1891. [Google Scholar]
- 15.Jaunet, T., G. Laguerre, P. Lemanceau, R. Frutos, and J. L. Notteghem. 1995. Diversity of Pseudomonas fuscovaginae and other fluorescent pseudomonads isolated from diseased rice. Phytopathology 85:1534-1541. [Google Scholar]
- 16.Jeanmougin, F., J. D. Thompson, M. Gouy, D. G. Higgins, and T. J. Gibson. 1998. Multiple sequence alignment with Clustal X. Trends Biochem. Sci. 23:403-405. [DOI] [PubMed] [Google Scholar]
- 17.Lane, D. J. 1991. 16s/23s rRNA sequencing, p. 115-173. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons Ltd., New York, N.Y.
- 18.Lemanceau, P., T. Corberand, L. Gardan, X. Latour, G. Laguerre, J.-M. Boeufgras, and C. Alabouvette. 1995. Effect of two plant species, flax (Linum usitatissinum L.) and tomato (Lycopersicon esculentum Mill.), on the diversity of soilborne populations of fluorescent pseudomonads. Appl. Environ. Microbiol. 61:1004-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipson, D. A., T. K. Raab, S. K. Schmidt, and R. K. Monson. 2001. An empirical model of amino acid transformations in an alpine soil. Soil Biol. Biochem. 33:189-198. [Google Scholar]
- 20.Lipson, D. A., T. K. Raab, S. K. Schmidt, and R. K. Monson. 1999. Variation in competitive abilities of plants and microbes for specific amino acids. Biol. Fertil. Soils 29:257-261. [Google Scholar]
- 21.Lipson, D. A., C. W. Schadt, and S. K. Schmidt. 2002. Changes in soil microbial community structure and function in an alpine dry meadow following spring snowmelt. Microb. Ecol. 43:307-314. [DOI] [PubMed] [Google Scholar]
- 22.Lipson, D. A., S. K. Schmidt, and R. K. Monson. 2000. Carbon availability and temperature control the post-snowmelt decline in alpine soil microbial biomass. Soil Biol. Biochem. 32:441-448. [Google Scholar]
- 23.Lipson, D. A., S. K. Schmidt, and R. K. Monson. 1999. Links between microbial population dynamics and nitrogen availability in an alpine ecosystem. Ecology 80:1623-1631. [Google Scholar]
- 24.Mancinelli, R. L. 1984. Population-dynamics of alpine tundra soil bacteria, Niwot Ridge, Colorado Front Range, USA. Arc. Alp. Res. 16:185-192. [Google Scholar]
- 25.Moore, E. R. B., M. Mau, A. Arnscheidt, E. C. Bottger, R. A. Hutson, M. D. Collins, Y. Van de Peer, R. DeWachter, and K. N. Timmis. 1996. The determination and comparison of the 16S rRNA gene sequences of species of the genus Pseudomonas (sensu stricto) and estimation of the natural intrageneric relationships. Syst. Appl. Microbiol. 19:478-492. [Google Scholar]
- 26.Nemergut, D. R., A. P. Martin, and S. K. Schmidt. Integron diversity in heavy metal contaminated mine tailings and inferences about integron origin and evolution. Appl. Environ. Microbiol., in press. [DOI] [PMC free article] [PubMed]
- 27.Palleroni, N. J. 1984. Genus I. Pseudomonas Migula 1894, p. 141-199. In N. R. Krieg and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 1. Williams & Wilkins, Baltimore, Md.
- 28.Palleroni, N. J. 1992. Introduction to the family Pseudomonadaceae, p. 3071-3079. In A. Balows, H. Truper, M. Dworkin, W. Harder, and K. H. Schleifer (ed.), The prokaryotes, 2nd ed., vol. 1. Springer-Verlag, New York, N.Y.
- 29.Palleroni, N. J. 2003. Prokaryote taxonomy of the 20th century and the impact of studies on the genus Pseudomonas: a personal view. Microbiology 149:1-7. [DOI] [PubMed] [Google Scholar]
- 30.Palleroni, N. J. 1993. Pseudomonas classification. A new case history in the taxonomy of gram-negative bacteria. Antonie Leeuwenhoek 64:231-251. [DOI] [PubMed] [Google Scholar]
- 31.Palleroni, N. J., and M. Doudoroff. 1972. Some properties and taxonomic subdivisions of genus Pseudomonas. Annu. Rev. Phytopathol. 10:73-100. [Google Scholar]
- 32.Pauker, S. J., and T. R. Seastedt. 1996. Effects of mobile tree islands on soil carbon storage in tundra ecosystems. Ecology 77:2563-2567. [Google Scholar]
- 33.Raab, T. K., D. A. Lipson, and R. K. Monson. 1996. Non-mycorrhizal uptake of amino acids by roots of the alpine sedge Kobesia myosuroides: implications for the alpine nitrogen cycle. Oecologia 108:488-494. [DOI] [PubMed] [Google Scholar]
- 34.Rainey, P. B., M. J. Bailey, and I. P. Thompson. 1994. Phenotypic and genotypic diversity of fluorescent pseudomonads isolated from field-grown sugar-beet. Microbiology 140:2315-2331. [DOI] [PubMed] [Google Scholar]
- 35.Schadt, C. W., A. P. Martin, D. A. Lipson, and S. K. Schmidt. 2003. Seasonal dynamics of novel fungal lineages in tundra soils. Science 301:1359-1361. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt, S. K., D. A. Lipson, R. E. Ley, M. C. Fisk, and A. E. West. Impacts of chronic nitrogen additions vary seasonally and by microbial functional group in tundra soils. Biogeochemistry, in press.
- 37.Stanier, R. Y., and L. N. Ornston. 1973. The β-ketodipate pathway. Adv. Microb. Physiol. 9:89-149. [PubMed] [Google Scholar]
- 38.Thakur, I. S., P. K. Verma, and K. C. Upadhaya. 2001. Involvement of plasmid in degradation of pentachlorophenol by Pseudomonas sp. from a chemostat. Biochem. Biophys. Res. Commun. 286:109-113. [DOI] [PubMed] [Google Scholar]
- 39.Wiedmann, M., D. Weilmeier, S. S. Dineen, R. Ralyea, and K. J. Boor. 2000. Molecular and phenotypic characterization of Pseudomonas spp. isolated from milk. Appl. Environ. Microbiol. 66:2085-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]