Abstract
We previously showed that ADP-ribosylation (ADP-r) activity of ExoS, a type III secreted toxin of Pseudomonas aeruginosa, enables bacterial replication in corneal and respiratory epithelial cells and correlates with bacterial trafficking to plasma membrane blebs (bleb-niche formation). Here, we explored another type III secreted toxin, ExoY, for its impact on intracellular trafficking and survival, and for virulence in vivo using a murine corneal infection model. Chromosomal or plasmid-mediated expression of exoY in invasive P. aeruginosa (strain PAO1) enabled bacteria to form and traffic to epithelial membrane blebs in the absence of other known effectors. In contrast, plasmid expression of any of four adenylate cyclase mutant forms of exoY did not enable bleb-niche formation, and bacteria localized to perinuclear vacuoles as for effector null mutant controls. None of the plasmid-complemented bacteria used in this study showed ADP-r activity in the absence of ExoS and ExoT. In contrast to ADP-r activity of ExoS, bleb-niche formation induced by ExoY’s adenylate cyclase activity was not accompanied by enhanced intracellular replication. In vivo results showed that ExoY adenylate cyclase activity promoted P. aeruginosa corneal virulence in susceptible mice. Together the data show that adenylate cyclase activity of P. aeruginosa ExoY, similarly to the ADP-r activity of ExoS, can mediate bleb-niche formation in epithelial cells. While this activity did not promote intracellular replication in vitro, ExoY conferred increased virulence in vivo in susceptible mice. Mechanisms for bleb-niche formation and relationships to intracellular replication and virulence in vivo require further investigation for both ExoS and ExoY.
Keywords: Pseudomonas aeruginosa, ExoY, adenylate cyclase, epithelial cells, membrane blebs, intracellular survival, cornea, virulence
1. Introduction
Pseudomonas aeruginosa is a versatile opportunistic pathogen ubiquitously present in water and soil and is recognized as a leading cause of nosocomial pneumonia, respiratory infection in cystic fibrosis, burn wound and urinary catheter infections, and ocular infections (e.g. microbial keratitis) [1-4]. While the pathogenesis of P. aeruginosa infection is complex, involving multiple virulence mechanisms, many recent studies have shown the importance of Type III Secretion System (T3S) in virulence through the manipulation of mammalian cell function [5, 6].
For the P. aeruginosa T3S system, four known effector proteins are delivered into host cells to modify cell function and/or viability; ExoS, ExoT, ExoU, and ExoY. Both ExoS and ExoT have dual enzymatic activity: N-terminal Rho-GTPase activating protein activity and C-terminal ADP ribosyl-transferase activity which exert cytopathic effects on host cells [7], and demonstrated contributions to virulence [8, 9]. The only known activity of ExoY is adenylate cyclase activity [10]. The virulence contributions are unclear even though ExoY causes the retraction and rounding of host cells [11, 12], and inhibits epithelial cell invasion by P. aeruginosa [13]. ExoU is a powerful phospholipase which kills epithelial cells [14, 15], and also contributes to virulence [9, 16, 17], but is not encoded by invasive P. aeruginosa that survive intracellularly such as strain PAO1 [15, 18].
Recently we reported that strains of P. aeruginosa expressing ExoS, ExoT and ExoY induce the formation of membrane bleb-niches in epithelial cells correlating with bacterial intracellular survival and replication [19]. Without these effectors, intracellular bacteria traffic to perinuclear vacuoles which label with the late endosomal marker LAMP-3, and do not thrive [19], suggesting a “default” trafficking pathway exists that inhibits P. aeruginosa replication, from which bacteria must escape to survive and then presumably traffic to bleb-niches. Subsequently, we showed that the ADP-ribosylation domain of ExoS was sufficient to enable both bleb-niche formation and intracellular survival [20]. However, we also noted in that study that mutants in both exoS and exoT (i.e. expressing only ExoY) conferred a low level capacity to form bleb-niches, particularly if the incubation time was extended [20].
Here, we tested the hypothesis that the adenylate cyclase activity of ExoY could mediate bleb-niche formation and intracellular survival/replication in epithelial cells, and could contribute to virulence in vivo in the absence of other known T3S system effectors. The results showed roles for the adenylate cyclase activity in bleb-niche formation in epithelial cells in vitro and in virulence in vivo, but not in intracellular survival in cultured epithelial cells, suggesting that these capacities are not always related.
2. Results
2.1 P. aeruginosa ExoY mediates bleb-niche formation in corneal epithelial cells
Real time phase-contrast microscopy was used to compare bleb-niche formation in cultured human corneal epithelial cells (hTCEpi) after 8 h of infection with wild-type P. aeruginosa strain PAO1, an isogenic effector null mutant (PAO1ΔexoSΔexoTΔexoY), and a double effector mutant that expresses ExoY, but not ExoS or ExoT (PAO1ΔexoSΔexoTΔ) (Fig. 1). Uninfected epithelial cells appeared healthy with small perinuclear vacuoles present in their cytoplasm (Fig. 1A). As expected from previous studies [19], cells infected with wild-type P. aeruginosa, displayed numerous membrane bleb-niches containing motile bacteria swimming at a speed observable in real-time (Fig. 1B). Cells infected with the triple (known effector-null) mutant or PAO1ΔexoSΔexoY (expresses only ExoT) showed no bleb-niche formation with bacteria localized to perinuclear vacuoles (Fig. 1C, data not shown, respectively). In contrast, cells infected with P. aeruginosa mutants expressing only ExoY (mutants in both exoS and exoT) showed a combination of the above phenotypes; with some bacteria localized within perinuclear vacuoles (Fig. 1D), and others demonstrating real time observable swimming motility in plasma membrane blebs (Fig. 1E). Thus ExoY, in the absence of ExoS or ExoT, can enable P. aeruginosa to form and traffic to bleb-niches.
Fig. 1.
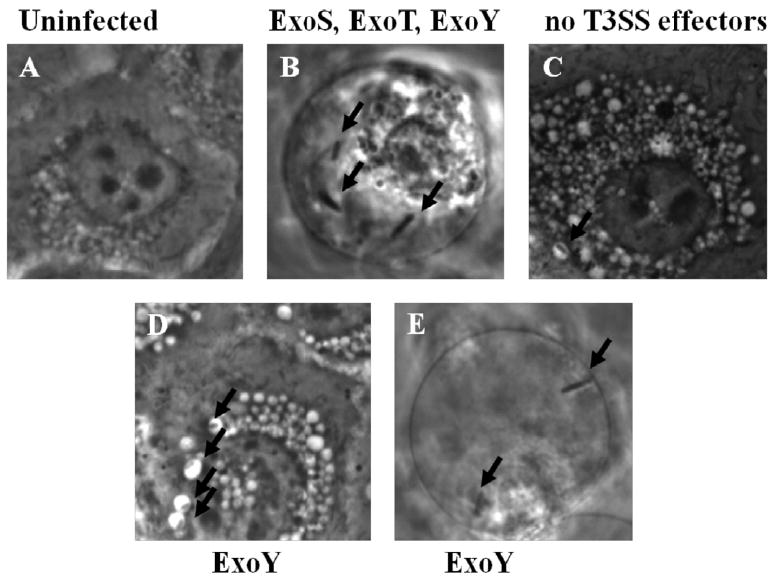
Phase-contrast microscopy of human corneal epithelial cells showing the intracellular localization of P. aeruginosa PAO1 versus various T3SS mutants at 8 h after inoculation with ~2 × 107 cfu bacteria. (A) Uninfected cells. (B) Wild-type PAO1 (positive control expressing ExoS, ExoT and ExoY) localized to bleb-niches as expected (arrows). (C) A negative control mutant lacking all known effectors (PAO1ΔexoSΔexoTΔexoY) localized to perinuclear vacuoles. (D and E) Bacteria expressing only ExoY (PAO1ΔexoSΔexoT) displayed both phenotypes, i.e. bacteria were found in both perinuclear vacuoles (D) and bleb-niches (E). Magnification 1000X. Images representative of three independent experiments.
2.2 ExoY mediation of bleb-niche formation requires adenylate cyclase activity
The only known enzymatic action of ExoY is adenylate cyclase (AC) activity. To determine if AC activity is required for ExoY to induce membrane bleb-niche formation, triple effector mutants of P. aeruginosa (PAO1ΔexoSΔexoTΔexoY) each complemented with one of four different mutants forms of exoY lacking adenylate cyclase activity (pUCPexoY K81M, pUCPexoY K88I, pUCPexoY D212N and pUCPExoY D214N) were compared to mutants complemented with native adenylate cyclase active exoY (pUCPexoY) (Fig. 2). As expected numerous bleb-niches were observed in cells infected with bacteria complemented with the native form of exoY (Fig. 2C, and Fig. 2H, middle panel). In contrast, cells infected with bacteria expressing adenylate cyclase inactive forms of ExoY did not display bleb niches containing bacteria (Fig. 2H, middle panel), and intracellular bacteria were found to be in perinuclear vacuoles, similar to the cells infected with the vector control (Figs. 2D-G, Fig. 2H, right panel). Quantitative differences between exoY-complemented bacteria and exoY-adenylate cyclase domain mutant-complemented bacteria were significant with regard to bleb-niche formation (with and without bacteria), and with regard to bacteria-filled perinuclear vacuole formation (see Fig. 2H). Interestingly, there were also significant differences between the exoY-domain mutant-complemented bacteria and the triple effector mutant with regard to empty bleb formation (Fig. 2H, left panel), and perinuclear vacuole localization (Fig. 2H, right panel). These results show that ExoY-mediated bleb-niche formation, in the absence of other known T3S effectors, requires adenylate cyclase activity, without which bacteria traffic to perinuclear vacuoles.
Fig. 2.
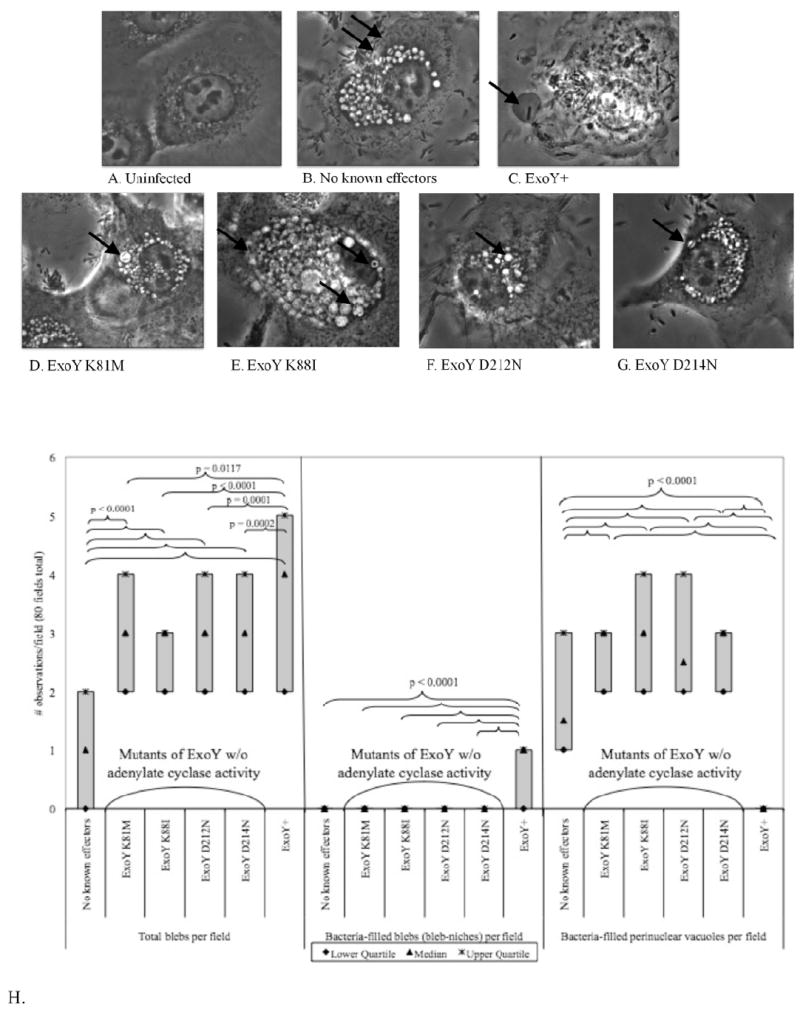
Phase-contrast microscopy of human corneal epithelial cells showing the intracellular localization of a triple effector mutant of P. aeruginosa PAO1 (PAO1ΔexoSΔexoTΔexoY) complemented with ExoY (pUCPexoY) or with four different ExoY adenylate cyclase domain mutants (pUCPexoYK81M, pUCPexoYK88I, pUCPexoYD212N, or pUCPexoYD214N) at 8 h after inoculation with ~2 × 107 cfu bacteria. (A) Uninfected cells. (B) Complementation with pUCP18 (control) caused bacteria to traffic to perinuclear vacuoles (arrows). (C) Complementation with plasmid-encoded ExoY enabled P. aeruginosa to traffic to membrane bleb-niches. (D, E, F, G) Complementation with the adenylate cyclase domain mutants resulted in the loss of bleb-niche formation, and bacteria trafficking to perinuclear vacuoles (arrows). Magnification 1000X. (H) Quantification of empty blebs, bacteria-filled blebs, and bacteria-filled perinuclear vacuoles from experiments represented in panels C-G. ExoY+ bacteria caused significantly more empty- and bacteria-filled blebs per field than adenylate cyclase mutant forms of ExoY or the plasmid control, and did not travel to perinuclear vacuoles.
2.3 ExoY, in the absence of other known effectors, does not confer capacity for intracellular replication
Previous studies with ExoS showed that ADP-r activity enabled bacteria to replicate intracellularly not only to form and occupy membrane bleb-niches, suggesting a relationship between these phenotypes. Having found ExoY can enable bleb-niche formation, the impact on intracellular survival/replication was next tested. Thus, native exoY was compared to an empty vector control and an adenylate cyclase mutant form of exoY (exoYK81M) in the triple effector (exoS/exoT/exoY-) mutant background for intracellular replication rates using gentamicin survival assays. The results showed similar intracellular survival rates for vector controls and bacteria expressing ExoY. Thus, ExoY’s adenylate cyclase activity does not enable intracellular survival despite involvement in bleb-niche formation. Surprisingly, there was a small but statistically significant intracellular survival advantage for bacteria expressing the adenylate cyclase mutant form of ExoY compared to the vector control (Fig. 3).
Fig. 3.
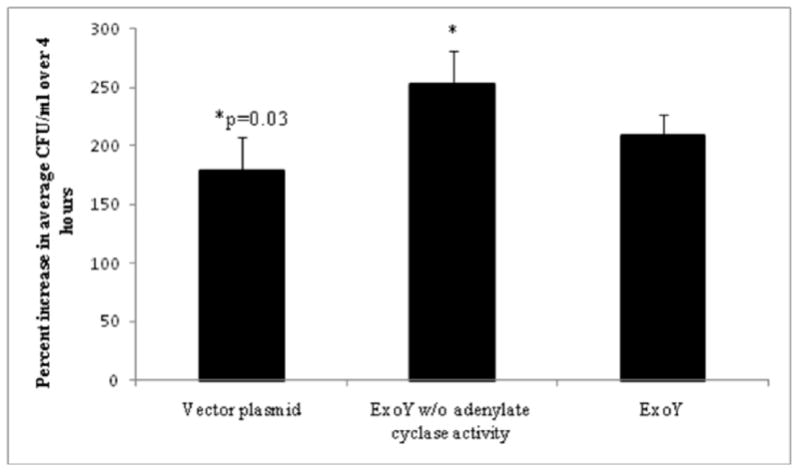
Intracellular replication of the effector-null mutant PAO1ΔexoSΔexoTΔexoY complemented with pUCPexoY (ExoY), or pUCPexoYK81M (adenylate cyclase mutant) or pUCP18 (vector plasmid) within human corneal epithelial cells. Cells were incubated with ~106 CFU bacteria for 3 h, followed by 1 h or 4 h of gentamicin treatment to kill extracellular bacteria. Plasmid-expressed ExoY did not significantly increase intracellular replication as compared to the plasmid control (p = 0.197) or adenylate cyclase mutant (p = 0.087). Interestingly, the increased replication of bacteria expressing the adenylate cyclase mutant form of ExoY compared to plasmid control was significant (p = 0.03). Data are expressed as the mean ± SD % increase in intracellular bacteria over 3 h (i.e. 1 h versus 4 h gentamicin treatments) for each complemented mutant over 3 independent experiments.
2.4 Plasmid encoded ExoY does not activate bacterial ADP-r activity
It is curious that two effectors (ExoS and ExoY) that do not have any known overlapping enzymatic activities could each independently enable bacterial bleb-niche formation. A potential explanation would be if the adenylate cyclase activity of ExoY activated ExoS independent ADP-r activity, which would then provide a common mechanism. To test that hypothesis, the P. aeruginosa triple effector mutant (PAO1ΔexoSΔexoTΔexoY) complemented with pUCPexoY or pUCPexoYK81M was tested for ADP-r activity using an in vitro assay with a Rab5-derived substrate and ExoS positive controls (see methods). No ADP-r activity was detected associated with ExoY in the absence of ExoS and ExoT (Fig. 4), suggesting that ADP-r activity was not involved in ExoY-mediated bleb-niche formation.
Fig. 4.
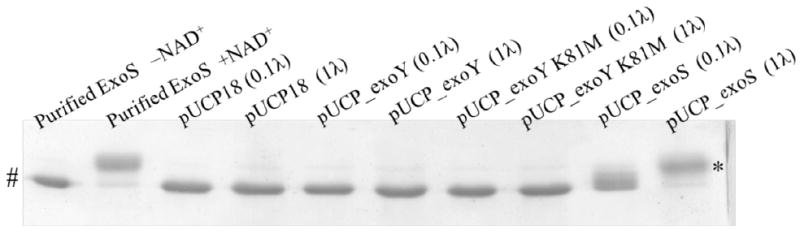
ADP-ribosylation of purified Rab5ΔCAAX (see methods) after exposure to purified ExoS with NAD (positive controls) or without NAD (negative controls), or culture supernatants of PAO1ΔexoSΔexoTΔexoY complemented with pUCPexoY, pUCPexoYK81M or pUCP18. Data are representative of two experiments. Shift in apparent molecular weight (*) indicates ADP-ribosylated Rab5ΔCAAX, # indicates unmodified Rab5 CAAX. None of the plasmid-complemented bacteria used in this study showed ADP-r activity towards this substrate.
2.5 The impact of ExoY adenylate cyclase activity in vivo
Having shown that the adenylate cyclase activity of ExoY could enable bleb-niche formation in corneal epithelial cells without providing an intracellular survival advantage, at least in cultured cells, experiments tested if adenylate cyclase activity provides an advantage in pathogenesis in vivo, using an in vivo model involving the same epithelial cell type (corneal). ExoY was studied plasmid expressed and in the absence of other known effectors. Thus, virulence of the triple effector mutant of P. aeruginosa PAO1ΔexoSΔexoTΔexoY complemented with pUCPexoY was compared to the mutant complemented with pUCPexoYK81M in a previously established murine model of bacterial keratitis that allows bacteria to interact with a healing (susceptible) epithelium [21]. Black Swiss Surfactant Protein-D (SP-D) knock-out mice were used since the corneal epithelium of these mice have increased susceptibility to penetration by wild-type P. aeruginosa in vivo [22]. Corneal disease severity (scores) after 24 h (Fig. 5 A) and 48 h (Fig. 5 B) post-inoculation showed significantly reduced disease scores for eyes that had been infected with the adenylate cyclase mutant ExoY as compared to eyes infected with the active form of ExoY (Figs 5 A and B). This corresponded with significantly reduced bacterial colonization at 48 h for mutant ExoY compared to adenylate cyclase active ExoY (Fig. 5 C). Thus, the adenylate cyclase activity of ExoY can contribute to virulence of P. aeruginosa in a susceptible mouse cornea.
Fig. 5.
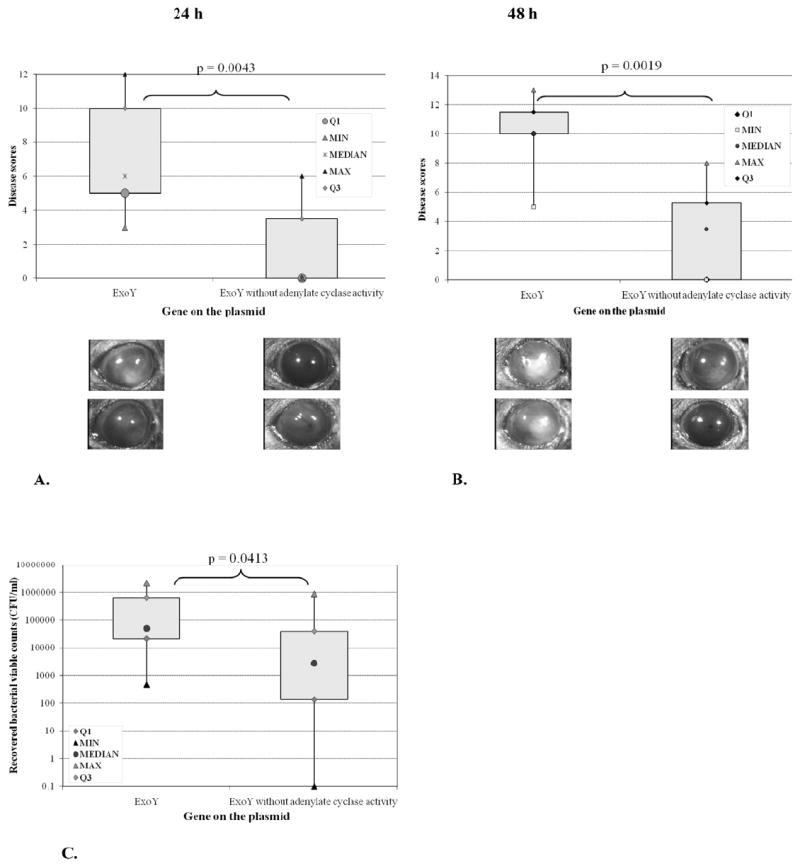
Corneal disease severity scores and images at 24 h (A) and 48 h (B) after inoculation with ~106 cfu of PAO1ΔexoSΔexoTΔexoY complemented with pUCPexoY or adenylate cyclase mutant pUCPexoYK81M. Corneas of SP-D (-/-) mice were scarified and allowed to heal for 6 h before inoculation (see methods). Complementation with exoY was associated with increased disease severity at both time points (A, B) and ocular colonization at 48 h (C) compared to the adenylate cyclase mutant.
3. Discussion
Our previously published work with P. aeruginosa demonstrated that membrane bleb-niche formation in epithelial cells and intracellular replication each required the bacterial T3S system, and that both events were enabled by ExoS in the absence of other known T3S effectors, which was attributable to ADP-r activity [19, 20]. That result suggested that bleb-niche formation and the capacity for intracellular survival by P. aeruginosa were mechanistically related. Here we show that ExoY, a different T3S effector of P. aeruginosa also enables P. aeruginosa to form membrane bleb-niches in epithelial cells, this time dependent upon adenylate cyclase activity. ExoY mediated bleb niche formation was not accompanied by increased replicative capacity. In vivo, the adenylate cyclase activity of ExoY, in the absence of other effectors, conferred a virulence advantage as demonstrated by enhanced corneal colonization rates and increased disease severity scores.
There are several potential mechanisms by which ExoY might mediate membrane bleb formation in epithelial cells. This effector has previously been correlated with actin cytoskeleton disruption in host cells with associated cell rounding [12, 13]. Thus, ExoY may disrupt cytoskeleton “anchoring” of the plasma membrane providing membrane “flexibility” needed for bleb-niche formation. ExoY could also mediate re-organization of host cell microtubules to cause cell retraction [11], providing an additional potential mechanism for blebbing. Involvement of the adenylate cyclase domain in ExoY-mediated blebbing suggests a role for elevated cytosolic cAMP [10]. It is known that ExoY-induced cAMP localizes to a cytosolic domain distinct from the endogenous cAMP pool located near the plasma membrane, and that these cAMP pools have opposing effects on endothelial cell junctions and barrier permeability [23]. Indeed, ExoY-mediated endothelial cell retraction and hyperpermeability was subsequently linked to cAMP-mediated microtubule dysfunction a potential mechanism alluded to above [11]. Further studies will be required to elucidate the details of both ExoY- and ExoS-mediated bleb-niche formation.
It is puzzling that ExoS and ExoY each enabled membrane bleb-niche formation because they do not have common enzymatic activities. Experiments were done to rule out the possibility that the adenylate cyclase activity of ExoY activates ExoS-independent ADP-r activity. However, the opposite may be true; i.e. when all the effectors are present, the ADP-r domain of ExoS (or ExoT) activates the adenylate cyclase activity of ExoY to cause blebbing. These separate activities may also converge downstream at a common induction pathway in the host cell. Finally, these activities may actually have different effects on cells, and the blebs that they induce are not the same, despite their similar appearance. That, in turn, could explain their different effects on bacterial intracellular survival.
Different types of blebbing could be one potential explanation for why ExoS, but not ExoY, enables intracellular survival. However, membrane bleb-niche formation may not confer intracellular survival, and ExoS has other actions that enable intracellular growth by the bacteria. Supporting that possibility, we previously showed that the popB mutant, which lacks the T3S translocon but can still secrete T3S effectors, can survive and replicate intracellularly without forming bleb-niches [19]. Another possibility is that ExoY is less efficient at trafficking bacteria to bleb-niches than ExoS. Indeed, ExoY-expressing bacteria were found in both perinuclear vacuoles and bleb-niches, while bacteria expressing ExoS localized more consistently to membrane blebs [20]. However, ExoY may promote intracellular survival, but adenylate cyclase activity could modulate host cell plasma membrane permeability to gentamicin (present in the extracellular fluid of our intracellular survival assays), which would inhibit intracellular replication and mask the effect. Further research will be needed to better understand the relationship between T3S-mediated intracellular survival and bleb-niche formation, and the different effects of ExoY and ExoS.
There is a paucity of data regarding ExoY contributions to virulence, and some previous studies have shown ExoY alone has little or no impact on virulence in vivo [24, 25]. To enable ExoY a fair opportunity to demonstrate virulence in vivo when expressed without other effectors, an SP-D gene knockout mouse model was used that has increased epithelial susceptibility to P. aeruginosa traversal [22]. The infection model used (6 hour heal model) requires bacteria to interact with the epithelium prior to accessing the vulnerable underlying stroma where pathological effects leading to visible disease are initiated. The results showed that the adenylate cyclase domain of ExoY contributed to virulence, manifesting as increased tissue colonization by bacteria and more severe visible pathological effects as compared to eyes infected with a mutant form of ExoY lacking adenylate cyclase activity. Thus, the capacity to form bleb niches in vitro correlated with enhanced virulence in vivo for this set of mutants. Proving a direct cause and effect relationship between ExoY-mediated bleb niche formation and ExoY-mediated virulence is more difficult. While we have observed that bleb-niche formation does occur during P. aeruginosa corneal infection in vivo (unpublished data), the actual contribution of this phenomenon to virulence still needs to be established. It is, however, interesting to consider that adenylate cyclase activity of ExoY increased bacterial colonization and disease in vivo during infection, but did not confer a survival advantage in infected cells in vitro. This raises the possibility that ExoY-mediated effects on bacterial trafficking within cells contributes to virulence independently of an impact on intracellular survival.
The adenylate cyclase mutant form of exoY differed from the vector control lacking exoY in multiple ways, having induced low levels of empty membrane blebs, enhanced the number of bacterial filled perinuclear vacuoles present in cells, and also impacting intracellular survival. In a previous study, we found that an adenylate cyclase mutant form of exoY impacted cell invasion by P. aeruginosa [13]. Together, these data suggest that ExoY could have additional activity domains with the potential to impact pathogenesis.
In conclusion, this study showed that the adenylate cyclase domain of ExoY could mediate bleb-niche formation in epithelial cells resembling bleb-niche formation by ExoS. In contrast to ExoS mediated bleb niches, ExoY-mediated bleb niches did not promote intracellular growth of bacteria and required adenylate cyclase rather than ADP-r activity. Importantly, the adenylate cyclase activity of ExoY was found to contribute to virulence in vivo. Mechanistic relationships between bleb-niche formation, intracellular bacterial localization and survival inside epithelial cells, and virulence in vivo will require further investigation in order to understand how ExoY and other T3S effectors of P. aeruginosa initiate disease at epithelial-lined tissue surfaces.
4. Materials and methods
4.1 Bacterial strains and culture conditions
P. aeruginosa strain PAO1 was used. The T3S of this strain expresses three known effectors (ExoS, ExoT and ExoY). To study ExoY-mediated effects without other effectors, a triple effector mutant of strain PAO1 (PAO1ΔexoSΔexoTΔexoY) was complemented with plasmid pUCP18 encoding the exoY gene (pUCPexoY), or the exoY gene with different mutations in the adenylate cyclase active domain (pUCPexoYK81M, pUCPexoYK88I, pUCPexoYD212N, or pUCPexoYD214N). These exoY domain mutants were generated using site-directed mutagenesis as previously described [10], and shown to have no adenylate cyclase activity. Plasmids and the triple effector mutant were generously provided by Dr. Dare Frank (Medical College of Wisconsin). The exoYK81M construct was already expressed in pUCP18 and used in our previous work [13]. However, for this study the remaining mutant forms of ExoY (K88I, D212N, D214N) were sub cloned from the E. coli plasmid vector pET16b into P. aeruginosa plasmid pUCP18 using restriction enzymes RsrII and SacII. Some initial experiments also included a chromosomal effector mutant lacking expression of ExoS and ExoT, but retaining ExoY (PAO1ΔexoSΔexoT). Bacteria were grown overnight at 37°C on Tryptic Soy Agar (TSA) plates supplemented with carbenicillin 400 μg/mL when needed for selection of plasmid-bearing strains. Inocula were prepared in KGM-2 cell culture media without antibiotics (OD of 0.1 at λ=650 nm, ~1 × 108 cfu/ml) and diluted to 1 × 107 cfu/ml for use in experiments.
4.2 Cell culture
Human telomerase-immortalized corneal epithelial cells (hTCEpi) were used [26], and cultured using serum-free keratinocyte grown medium (KGM-2) (Lonza, MD). Cells were subcultured onto 75mm tissue culture flasks with vented caps, grown in 5% CO2 at 37°C until confluent. Every two days, cells were washed with sterile PBS (Sigma, MO) and media replaced. One week before each experiment, cells were subcultured onto 22 mm glass cover-slips placed in non-tissue-culture-treated 6-well plates and grown to ~80% confluence. For intracellular replication assays, cells were cultured on 12-well tissue culture treated plates. Cells were incubated in 5% CO2 at 37°C during all experiments.
4.3 Quantification of bleb-niches and perinuclear vacuoles by phase-contrast microscopy
Epithelial cells grown on coverslips were washed twice with 2 ml of sterile PBS and infected with P. aeruginosa or its T3S mutants for 3 h at 37 °C. After that time, cells were washed twice with sterile PBS and fresh medium containing 200 μg/ml of gentamicin was added. Coverslips were then placed into an Attofluor Cell Chamber (Molecular Probes) maintained at 37°C in the presence of the gentamicin-containing media. Cells were viewed by phase-contrast microscopy (~1000X magnification) for up to 2 h while bacterial interactions with epithelial cells were documented using real-time movies and still images. For each experimental variable, images were collected from 20 random fields (minimum 8 cells per field). For each field the following parameters were manually quantified: total blebs per field, blebs containing bacteria per field, bacteria-filled perinuclear vacuoles per field. Each experiment was repeated at least three times.
4.4 Intracellular replication
Intracellular replication assays were performed as described previously [20], but with a small modification of the time allowed for intracellular replication. After incubation with bacteria (3 h), cells containing intracellular bacteria were incubated in antibiotic-containing cell culture medium for 4 h (instead of 5 h reported previously). Intracellular replication was then expressed as a percentage increase over baseline for each strain, and shown as a mean ± SD from three independent experiments.
4.5 ADP-r activity
A Rab5ΔCAAXconstruct was expressed in E.coli BL21 (DE3), purified through affinity chromatography, and used to assess in vitro ADP-ribosylation activity of bacterial culture supernatants prepared from P. aeruginosa PAO1ΔexoSΔexoTΔexoY complemented with pUCPexoY, or pUCPexoYK81M, or pUCP18. Bacterial culture supernatants prepared from P. aeruginosa pUCPexoS and purified ExoS were used as positive controls. Assays were performed as previously described [27, 28]. Briefly, the mixture (20 μL) for each reaction contained: 10 mM Tris, 20 mM (T10N20) NaCl, 2 μM His6-RabΔCAAX, either 20 ng purified ExoS or PAO1 cell lysate (1μL lysate or 1μL of a 1:10 dilution in T10N20 buffer), 150 nM FAS, and 40 μM NAD+. Reactions were incubated at room temperature for 1 h, boiled for 5 min to inactivate enzymatic activity and analyzed by SDS-PAGE and Coomassie staining.
4.6 In vivo infection model
Female Black Swiss Surfactant Protein-D (SD-D) knock-out mice (age: 15 weeks) were used. We have recently shown that these mice show increased susceptibility to P. aeruginosa epithelial traversal in the cornea in vivo [22], and as such provide a model which could allow the more subtle virulence effects of exoY-complemented T3S mutant bacteria to be determined. Corneas were inoculated with ~106 cfu of P. aeruginosa suspension after corneal scarification and 6 h epithelial healing [21]. Disease severity was scored using 16 point system at 24 and 48 h post-inoculation and eyes photographed. Bacterial colonization was assessed by viable counts from ocular homogenates at 48 h. All procedures were approved by Animal Care and Use Committee, the University of California, Berkeley.
4.7 Statistical analysis
The Mann-Whitney U-test was used for statistical analysis of bleb-niche quantification and in vivo studies. ANOVA was used for statistical analysis of intracellular replication with Fisher PLSD for post-hoc analysis. P values < 0.05 were considered significant.
Highlights.
ExoY, a P. aeruginosa type three-secreted toxin, causes epithelial cell bleb-niches
ExoY also contributes to P. aeruginosa corneal infection in SP-D deficient mice
Both of these ExoY-mediated effects required the toxin’s adenylate cyclase domain
ExoY-induced blebs did not coincide with increased bacterial intracellular survival
ExoY and ExoS likely target different host cell pathways to induce bleb-niches
Acknowledgments
This work was supported by research grants from the National Institutes of Health; R01 AI079192 (SMJF) and AI030162 (JTB). Dr. Hritonenko was supported by T32 EY07043-30 and EY020111. We also wish to express our gratitude to Drs. Dara Frank, Maria Plotkowski, and Arne Riestch for generously providing parent and mutant strains used in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agrafiotis M, Siempos II, Falagas ME. Infections related to airway stenting: a systematic review. Respiration. 2009;78:69–74. doi: 10.1159/000213244. [DOI] [PubMed] [Google Scholar]
- 2.Willcox MD. Pseudomonas aeruginosa infection and inflammation during contact lens wear: a review. Optom Vis Sci. 2007;84:273–278. doi: 10.1097/OPX.0b013e3180439c3e. [DOI] [PubMed] [Google Scholar]
- 3.Wagner VE, Iglewski BH. P. aeruginosa Biofilms in CF Infection. Clin Rev Allergy Immunol. 2008;35:124–134. doi: 10.1007/s12016-008-8079-9. [DOI] [PubMed] [Google Scholar]
- 4.Kerr KG, Snelling AM. Pseudomonas aeruginosa: a formidable and ever-present adversary. J Hosp Infect. 2009;73:338–344. doi: 10.1016/j.jhin.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 5.Engel J, Balachandran P. Role of Pseudomonas aeruginosa type III effectors in disease. Curr Opin Microbiol. 2009;12:61–66. doi: 10.1016/j.mib.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Hauser AR. The type III secretion system of Pseudomonas aeruginosa: infection by injection. Nat Rev Microbiol. 2009;7:654–665. doi: 10.1038/nrmicro2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbieri JT, Sun J. Pseudomonas aeruginosa ExoS and ExoT. Rev Physiol Biochem Pharmacol. 2004;152:79–92. doi: 10.1007/s10254-004-0031-7. [DOI] [PubMed] [Google Scholar]
- 8.Lee EJ, Cowell BA, Evans DJ, Fleiszig SM. Contribution of ExsA-regulated factors to corneal infection by cytotoxic and invasive Pseudomonas aeruginosa in a murine scarification model. Invest Ophthalmol Vis Sci. 2003;44:3892–3898. doi: 10.1167/iovs.02-1302. [DOI] [PubMed] [Google Scholar]
- 9.Shaver CM, Hauser AR. Relative contributions of Pseudomonas aeruginosa ExoU, ExoS, and ExoT to virulence in the lung. Infect Immun. 2004;72:6969–6977. doi: 10.1128/IAI.72.12.6969-6977.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yahr TL, Vallis AJ, Hancock MK, Barbieri JT, Frank DW. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc Natl Acad Sci U S A. 1998;95:13899–13904. doi: 10.1073/pnas.95.23.13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prasain N, Alexeyev M, Balczon R, Stevens T. Soluble adenylyl cyclase-dependent microtubule disassembly reveals a novel mechanism of endothelial cell retraction. Am J Physiol Lung Cell Mol Physiol. 2009;297:L73–83. doi: 10.1152/ajplung.90577.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vallis AJ, Finck-Barbancon V, Yahr TL, Frank DW. Biological effects of Pseudomonas aeruginosa type III-secreted proteins on CHO cells. Infect Immun. 1999;67:2040–2044. doi: 10.1128/iai.67.4.2040-2044.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cowell BA, Evans DJ, Fleiszig SM. Actin cytoskeleton disruption by ExoY and its effects on Pseudomonas aeruginosa invasion. FEMS Microbiol Lett. 2005;250:71–76. doi: 10.1016/j.femsle.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 14.Sato H, Frank DW. ExoU is a potent intracellular phospholipase. Mol Microbiol. 2004;53:1279–1290. doi: 10.1111/j.1365-2958.2004.04194.x. [DOI] [PubMed] [Google Scholar]
- 15.Finck-Barbancon V, Goranson J, Zhu L, Sawa T, Wiener-Kronish JP, Fleiszig SM, Wu C, Mende-Mueller L, Frank DW. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol. 1997;25:547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- 16.Zolfaghar I, Evans DJ, Ronaghi R, Fleiszig SM. Type III secretion-dependent modulation of innate immunity as one of multiple factors regulated by Pseudomonas aeruginosa RetS. Infect Immun. 2006;74:3880–3889. doi: 10.1128/IAI.01891-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tam C, Lewis SE, Li WY, Lee E, Evans DJ, Fleiszig SM. Mutation of the phospholipase catalytic domain of the Pseudomonas aeruginosa cytotoxin ExoU abolishes colonization promoting activity and reduces corneal disease severity. Exp Eye Res. 2007;85:799–805. doi: 10.1016/j.exer.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleiszig SM, Wiener-Kronish JP, Miyazaki H, Vallas V, Mostov KE, Kanada D, Sawa T, Yen TS, Frank DW. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect Immun. 1997;65:579–586. doi: 10.1128/iai.65.2.579-586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Angus AA, Lee AA, Augustin DK, Lee EJ, Evans DJ, Fleiszig SM. Pseudomonas aeruginosa induces membrane blebs in epithelial cells, which are utilized as a niche for intracellular replication and motility. Infect Immun. 2008;76:1992–2001. doi: 10.1128/IAI.01221-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angus AA, Evans DJ, Barbieri JT, Fleiszig SM. The ADP-ribosylation domain of Pseudomonas aeruginosa ExoS is required for membrane bleb niche formation and bacterial survival within epithelial cells. Infect Immun. 2010;78:4500–4510. doi: 10.1128/IAI.00417-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee EJ, Evans DJ, Fleiszig SM. Role of Pseudomonas aeruginosa ExsA in penetration through corneal epithelium in a novel in vivo model. Invest Ophthalmol Vis Sci. 2003;44:5220–5227. doi: 10.1167/iovs.03-0229. [DOI] [PubMed] [Google Scholar]
- 22.Alarcon I, Tam C, Mun JJ, Ledue J, Evans DJ, Fleiszig SM. Factors Impacting Corneal Epithelial Barrier Function Against Pseudomonas aeruginosa Traversal. Invest Ophthalmol Vis Sci. 2011 doi: 10.1167/iovs.10-6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sayner SL, Frank DW, King J, Chen H, VandeWaa J, Stevens T. Paradoxical cAMP-induced lung endothelial hyperpermeability revealed by Pseudomonas aeruginosa ExoY. Circ Res. 2004;95:196–203. doi: 10.1161/01.RES.0000134922.25721.d9. [DOI] [PubMed] [Google Scholar]
- 24.Vance RE, Rietsch A, Mekalanos JJ. Role of the type III secreted exoenzymes S, T, and Y in systemic spread of Pseudomonas aeruginosa PAO1 in vivo. Infect Immun. 2005;73:1706–1713. doi: 10.1128/IAI.73.3.1706-1713.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee VT, Smith RS, Tummler B, Lory S. Activities of Pseudomonas aeruginosa effectors secreted by the Type III secretion system in vitro and during infection. Infect Immun. 2005;73:1695–1705. doi: 10.1128/IAI.73.3.1695-1705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robertson DM, Li L, Fisher S, Pearce VP, Shay JW, Wright WE, Cavanagh HD, Jester JV. Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Invest Ophthalmol Vis Sci. 2005;46:470–478. doi: 10.1167/iovs.04-0528. [DOI] [PubMed] [Google Scholar]
- 27.Knight DA, Barbieri JT. Ecto-ADP-ribosyltransferase activity of Pseudomonas aeruginosa exoenzyme S. Infect Immun. 1997;65:3304–3309. doi: 10.1128/iai.65.8.3304-3309.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu S, Kulich SM, Barbieri JT. Identification of glutamic acid 381 as a candidate active site residue of Pseudomonas aeruginosa exoenzyme S. Biochemistry. 1996;35:2754–2758. doi: 10.1021/bi952340g. [DOI] [PubMed] [Google Scholar]


