Abstract
We have previously shown that ExoU, a type III secreted cytotoxin of Pseudomonas aeruginosa, causes acute cytotoxicity towards corneal epithelial cells in vitro, and contributes to corneal disease pathology and ocular colonization in vivo. Subsequently, we reported that ExoU represses phagocyte infiltration of infected corneas in vivo. ExoU has patatin-like phospholipase activity that is required for cytotoxic activity in vitro (mammalian cell injury and death) and for disease in a murine model of pneumonia. We hypothesized that the phospholipase activity was required for ExoU-mediated corneal disease and ocular colonization. Using the murine scarification model, corneal disease pathology was examined after inoculation with ~106 cfu of a P. aeruginosa effector mutant (PA103ΔexoUexoT::Tc) complemented with either exoU (pUCPexoU), phospholipase-inactive exoU (pUCPexoUD344A) or a plasmid control (pUCP18). Eyes were photographed and disease severity scored at 24 and 48 h post-infection. Viable bacteria colonizing infected eyes were quantified at 6 and 48 h. Complementation with exoU caused significantly more pathology (increased disease severity scores) and enabled bacteria to better colonize (by ~1000-fold) at 48 h as compared to phospholipase-inactive exoU which did not differ from plasmid control. Surprisingly, exoU did not contribute to early (6 h) colonization. In-vitro assays confirmed that the phospholipase domain of exoU was required for cytotoxicity towards human corneal epithelial cells. Taken together these data show that the phospholipase activity of the P. aeruginosa cytotoxin, ExoU, plays a role in the pathogenesis of corneal infection via mechanism(s) occurring after initial colonization of a susceptible cornea.
Keywords: P. aeruginosa, keratitis, type III secretion, ExoU, phospholipase, virulence
1. Introduction
Pseudomonas aeruginosa keratitis is an acute sight-threatening infection that can occur as a complication of soft contact lens wear or corneal injury (Cheng et al., 1999; Alexandrakis et al., 2000; Lam et al., 2002; Watt and Swarbrick 2005; Verhelst et al., 2006; Bharathi et al., 2007; Pachigolla et al., 2007). Corneal isolates of P. aeruginosa can be divided into invasive or cytotoxic strain types based upon how they interact with epithelial cells (Fleiszig et al., 1996; Fleiszig et al., 1997; Cowell et al., 2003). We have shown previously that cytotoxic isolates are genetically different from invasive strains in that they encode the type III secreted toxin ExoU (Fleiszig et al., 1997). Possession of the exoU gene was identified as one of several virulence traits encoded by epidemic clones of P. aeruginosa isolated from patients with bacterial keratitis (Lomholt et al., 2001).
ExoU is delivered into mammalian cells upon contact with bacteria, and is required for acute (within 3 h) injury and death of several mammalian cell types in vitro, including epithelial cells and macrophages (Fleiszig et al., 1996; Fleiszig et al., 1997; Garrity-Ryan et al., 2000; Finck-Barbancon and Frank 2001). Cytotoxic isolates can also kill epithelial cells on the surface of intact corneas ex vivo (Fleiszig et al., 1998). Using a murine scarification model of keratitis, we previously demonstrated that ExoU also makes a significant contribution to ocular colonization and corneal disease, and that it represses phagocyte infiltration of the central region of infected corneas in vivo (Lee et al., 2003; Zolfaghar et al., 2006). Others have shown that complementation of exoU into an invasive strain of P. aeruginosa enhances its virulence in a murine model of acute pneumonia (Allewelt et al., 2000).
It has been shown that ExoU exhibits a patatin-like phospholipase activity (Sato et al., 2003; Sato and Frank 2004; Sato et al., 2005) in combination with host factors, e.g. superoxide dismutase (Sato et al., 2006). Multiple regions of ExoU have been found to be required for cytotoxic activity (Rabin and Hauser 2005). The N-terminal domain of the protein encodes the phospholipase activity. Mutations of the catalytic serine (S142) or aspartate (D344) residues abolishes both the phospholipase activity and cytotoxicity towards mammalian and yeast cells (Phillips et al., 2003; Sato et al., 2003; Rabin and Hauser 2005). Mutation of the N-terminal region of ExoU also reduces P. aeruginosa virulence of in a murine model of acute pneumonia (Pankhaniya et al., 2004).
Here, we hypothesized that ExoU-mediated colonization and disease-promoting activity in the cornea would require its phospholipase activity. This was tested by comparing corneal disease pathology and ocular colonization by a double effector mutant of the cytotoxic P. aeruginosa strain PA103 (PA103ΔexoUexoT::Tc) complemented with a functional exoU gene on the plasmid pUCP18 (pUCPexoU) with disease caused by the same strain when complemented with exoU containing a mutation in the catalytic aspartate region of the phospholipase domain (pUCPexoUD344A), or a control (pUCP18). Testing in an effector null background was done to remove any potential complicating factors due to ExoT, another type-III secreted effector encoded by strain PA103, that we have previously shown to act redundantly with ExoU in contributing to P. aeruginosa-induced pathology in the cornea (Lee et al., 2003).
2. Materials and Methods
2.1. Bacteria
The experiments described in this study were done using an effector null mutant of P. aeruginosa strain PA103 (PA103ΔexoUexoT::Tc) (PA103ΔUT). This mutant lacks both ExoU and ExoT, the two effectors of the type III secretion system known to be produced by this strain (Vallis et al., 1999). The PA103ΔUT mutant was complemented with plasmid pUCP18 containing either the exoU gene (pUCPexoU) which fully restores cytotoxic activity towards eukaryotic cells, or the exoU gene in which the aspartate catalytic site of the N-terminal phospholipase domain is mutated (pUCPexoUD344A), and thus it lacks phospholipase and in vitro cytotoxic activity, or an empty vector control (pUCP18) (Sato et al., 2003). Plasmid complemented mutants were grown on trypticase soy agar (TSA) supplemented with carbenicillin 300 μg/ml overnight (~18 h) at 37 °C. For use in experiments, bacteria were resuspended in tissue culture medium (equal parts DMEM/Hams F12) to an optical density (at 650 nm) of ~0.1 to 0.2 corresponding to a viable count of ~2–3 × 108 cfu/mL. Control experiments in this, and previous studies (Vallis et al., 1999; Lee et al., 2003) have confirmed that these plasmid-complemented strains grow equally well in vitro with or without exposure to mammalian cells.
2.2 Corneal epithelial cell culture and in vitro cytotoxicity assay
Telomerase-immortalized human corneal epithelial cells (HCEC) were cultured in 24-well tissue culture plates as previously described (Robertson et al., 2005). Cytotoxic activity of P. aeruginosa towards HCEC was examined by Trypan Blue staining (Fleiszig et al., 1996) after 5 h exposure to ~106 cfu of the plasmid-complemented PA103ΔUT mutants of P. aeruginosa strain PA103. For cytotoxicity assays bacteria were suspended in KBM cell culture medium (Robertson et al., 2005).
2.3. Murine corneal infection and P. aeruginosa colonization
The murine scarification model of infectious keratitis was used (Preston et al., 1995). C57/BL6 mice (5–12 weeks old) were anesthetized by intraperitoneal infection with an anesthetic cocktail (21 mg/mL ketamine, 2.4 mg/mL xylazine and 0.3 mg/mL acepromazine). Eyes were checked for corneal clarity using a stereomicroscope prior to the initiation of experiments. Three parallel scratches (~1mm in length) were made on the right cornea of each animal using a sterile 25 5/8-guage needle, and eyes topically inoculated with a bacterial suspension containing ~106 cfu in 5 μl of DMEM/Hams F12. Four mice were assigned to each treatment group. Animals were observed daily, and overall disease severity was graded in a masked fashion after 24 h and 48 h using the following scoring system (Beisel et al., 1983); Grade 0, eye macroscopically identical to the uninfected contralateral control eye; Grade 1, faint opacity partially covering the pupil; Grade 2, dense opacity covering the pupil; Grade 3, dense opacity covering the entire anterior segment; Grade 4, perforation of cornea and /or phthisis bulbi (shrinkage of the eyeball following inflammatory disease). Eyes were also graded using another 5-point grading system (grade 0 = no infection to grade 4 = severe infection) that assessed three different characteristics of the disease (Cowell et al., 1999). Thus, scores were assigned to the area and density of the opacity and the epithelial surface quality. The calculated sum of scores for these three characteristics ranged from a possible 0 (clear, normal) to a maximum of 12. Eyes were photodocumented using an Optronics 3-chip cooled camera (Goleta, CA) attached to a Zeiss Stemi 2000-C dissecting microscope (Jena, Germany).
To quantify bacterial colonization, mice that had been infected for 48 h were euthanatized, and infected eyes enucleated and homogenized in PBS (1 ml) containing Triton X-100 (0.25 % vol/vol). Viable counts were performed on homogenates using TSA containing carbenicillin 300ug/ml to select plasmid-bearing bacteria. We have previously observed that this plasmid is retained by P. aeruginosa in vivo over 48 h (Lee et al., 2003). In other experiments, mice were sacrificed at 6 h after infection to explore the role of ExoU and its phospholipase activity on early bacterial colonization at a point prior to significant immune cell infiltration. All procedures were conducted in accordance with the policies established by the Association for the Research in Vision and Ophthalmology, and were approved by the University of California, Berkeley Animal Care and Use Committee.
2.4 Statistical analysis
Data were expressed as a mean with standard deviation, and differences between groups compared for statistical significance using ANOVA with Fisher PLSD and Scheffe F-test post-hoc analysis. Differences in disease severity scores were compared using the Kruskal-Wallis and Mann-Whitney non-parametric tests. P values < 0.05 were considered significant. All experiments were repeated at least once.
3. Results
3.1 Phospholipase-inactive ExoU is not cytotoxic towards HCEC in vitro
Trypan blue staining confirmed that the ΔUT mutant of P. aeruginosa strain PA103 complemented with vector control (pUCP18) was not cytotoxic towards cultured human corneal epithelial cells (Fig. 1A). As expected, complementation with pUCPexoU, but not the phospholipase-inactive form of exoU, restored normal cytotoxic activity to the ΔUT double mutant (Fig. 1B and C respectively).
Figure 1.
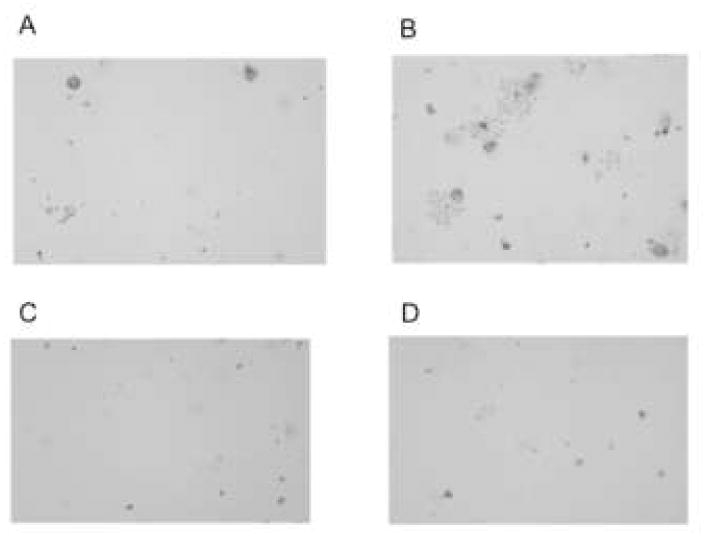
Trypan blue staining (dead or dying cells) of cultured HCEC after 5 h exposure to ~1 × 106 cfu strain PA103ΔexoUexoT::Tc (PA103ΔUT) complemented with empty vector pUCP18 (A), pUCPexoU (B), the phospholipase-inactive mutant pUCPexoUD344A (C) or media (KBM) only (D). ExoU-complemented bacteria caused a typical pattern of cell death. Bacteria complemented with phospholipase-inactive ExoU caused little or no cell death similar to vector and media only controls. Images were adjusted using Adobe Photoshop to equate brightness and contrast.
3.2 The Phospholipase domain of ExoU is required for effects on disease severity
At both 24 and 48 h, disease caused by bacteria complemented with exoU was of significantly increased severity compared to that involving the phospholipase-inactive form of exoU (pUCPexoUD344A) or the vector control (pUCP18) (p = 0.04 at 24 h, p = 0.004 at 48 h, Kruskal-Wallis Test) (Tables 1 and 2, Fig. 2). Indeed, disease involving the phospholipase-inactive form of exoU did not differ from that caused by bacteria complemented with the plasmid control at both time points (p > 0.05, Mann-Whitney test). These results were confirmed with both the 4-point overall grading system (Table 1) and the multiplex scoring system (Table 2). Representative photographs of infected eyes at 24 and 48 h (Fig. 2) illustrate differences in disease severity caused by bacteria expressing phospholipase-active or –inactive forms of exoU.
Table 1.
Overall disease severity scores (see methods) in the murine scarification model of P. aeruginosa keratitis after inoculation with 5 μl of cell culture medium containing ~1 × 106 cfu strain PA103ΔexoUexoT::Tc (PA103ΔUT) complemented with pUCP18 (control), pUCPexoU, or the phospholipase mutant pUCPexoUD344A.
| Time | PA103ΔUT + pUCP18 | PA103ΔUT + pUCPexoU | PA103ΔUT + pUCPexoUD344A |
|---|---|---|---|
| 24 h* | 1, 1, 1, 0 | 1, 2, 2, 2 | 0, 1, 1, 0 |
| 48 h* | 2, 2, 2, 2 | 3, 3, 3, 3 | 2, 2, 2, 2 |
Disease involving pUCPexoU was of significantly increased severity compared to both vector control and phospholipase catalytic mutant at each time point (p = 0.04 at 24 h; p = 0.004 at 48 h, Kruskal-Wallis Test).
Table 2.
Scoring individual characteristics of ocular disease severity (see methods) in the murine scarification model of P. aeruginosa keratitis after inoculation with 5 μl of cell culture medium containing ~1 × 106 cfu strain PA103ΔexoUexoT::Tc (PA103ΔUT) complemented with pUCP18, pUCPexoU, or pUCPexoUD344A.
| Total Disease Scores at 48 h [= Area + Density + Epithelial Quality] Each Scored From 0 to 4, Possible Maximum = 12 | |||
|---|---|---|---|
| PA103ΔUT + pUCP18 | PA103ΔUT + pUCPexoU* | PA103ΔUT + pUCPexoUD344A | |
| Mouse 1 | 8 [4+2+2] |
10 [4+3+3] |
9 [4+3+2] |
| Mouse 2 | 8 [4+2+2] |
10 [4+4+2] |
8 [4+3+1] |
| Mouse 3 | 9 [4+3+2] |
10 [4+4+2] |
7 [3+3+1] |
| Mouse 4 | 8 [4+2+2] |
11 [4+4+3] |
7 [3+3+1 ] |
Disease involving pUCPexoU was of increased overall severity compared to the phospholipase catalytic mutant (p = 0.017, Mann-Whitney Test) which was not different from control (p = 0.350, Mann-Whitney Test).
Figure 2.
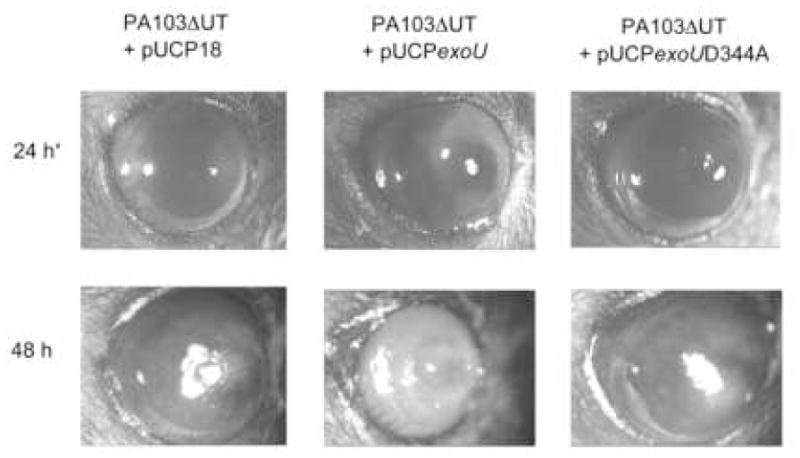
Overall disease pathology in the murine scarification model of P. aeruginosa keratitis at 24 h and 48 h after inoculation with 5 μl of cell culture medium containing ~1 × 106 cfu strain PA103ΔexoUexoT::Tc (PA103ΔUT) complemented with pUCP18, pUCPexoU, or the phospholipase mutant pUCPexoUD344A. Note the ring infiltrate of the eyes infected with bacteria expressing exoU (Zolfaghar et al., 2006). *24 h images were equally adjusted for brightness and contrast using Adobe Photoshop to match backgrounds of 48 h data.
A peripheral ring infiltrate, which we have previously reported to require ExoU, was observed only in those eyes infected with bacteria expressing intact exoU (Zolfaghar et al., 2006).
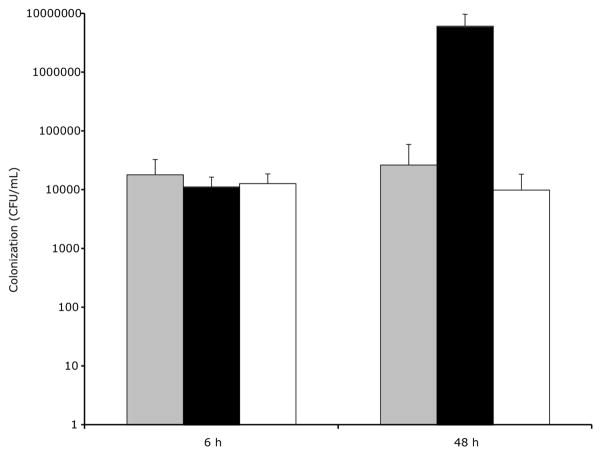
3.3 Loss of phospholipase activity abolishes ExoU-mediated corneal colonization at 48 h, but does not affect early (6 h) colonization
Mutation of the phospholipase active region of exoU was associated with a significant (~1000-fold) reduction in ocular colonization at 48 h (p < 0.05, ANOVA, Fig. 3). Indeed, there was no significant difference between the phospholipase-inactive form of exoU and the vector (plasmid) control at this time. In stark contrast, at 6 h post-inoculation, there was no significant difference in ocular colonization between bacteria complemented with vector control and either the phospholipase-active or the inactive forms of exoU (p > 0.05, ANOVA). In the absence of ExoU phospholipase activity, and its associated promotion of colonization noted at 48 h, there was little difference between colonization levels at 6 and 48 h post-infection (Fig. 3).
Figure 3.
Ocular colonization in the murine scarification model of P. aeruginosa keratitis at 6 h and 48 h (separate experiments) post-inoculation with 5 μl of medium containing ~1 × 106 cfu strain PA103ΔexoUexoT::Tc (PA103ΔUT) complemented with pUCP18 (gray), pUCPexoU (black), or pUCPexoUD344A (white). At 48 h, bacteria complemented with phospholipase-inactive exoU showed significantly reduced colonization compared to exoU-complemented bacteria (p < 0.05, ANOVA and Fisher PLSD and Scheffe F-test post-hoc analysis). At 6 h, there were no significant differences between the groups (p > 0.05, ANOVA and by Fisher PLSD and Scheffe F-test post-hoc analysis)
4. Discussion
We have previously shown that ExoU is involved in the ability of P. aeruginosa to colonize the cornea in vivo and to cause pathology (Lee et al., 2003) which involves inhibition of phagocyte infiltration of the central cornea (Zolfaghar et al., 2006). This followed our earlier work showing that ExoU was responsible for the acute cytotoxicity of cytotoxic strains of P. aeruginosa towards mammalian cells in vitro (Finck-Barbancon et al., 1997; Fleiszig et al., 1997). In this study we show that the mechanism for ExoU-mediated cytotoxicity towards corneal epithelial cells in vitro, and its promotion of ocular colonization and corneal disease pathology by 48 h in vivo, requires the phospholipase activity of this toxin.
ExoU has been shown to cause acute cell death of numerous types of mammalian cell including epithelial cells, fibroblasts, and even professional phagocytes in vitro (Finck-Barbancon et al., 1997; Fleiszig et al., 1997; Garrity-Ryan et al., 2000; Finck-Barbancon and Frank 2001; Evans et al., 2002). Thus, the in-vivo role of ExoU may involve its ability to kill host cells. For example, killing of corneal epithelial cells might increase bacterial binding to damaged epithelia, or it could increase the ability of bacteria to translocate the epithelium in vivo. Killing of phagocytes would reduce the capacity of the host to clear bacteria.
Considering that cytotoxic strains of P. aeruginosa can rapidly kill corneal epithelial cells in vitro (Fleiszig et al., 1996) and also when they are on the surface of intact corneas ex vivo (Fleiszig et al., 1998), it was interesting that we were not able to demonstrate a role for ExoU in early bacterial colonization (6 h). It is possible that epithelial cytotoxicity of P. aeruginosa is not required in this particular model of keratitis in which the epithelium is already damaged/breached by needle injury. It is also possible that in the absence of alterations to the ocular surface biochemistry, that likely occur in both contact lens- and dry eye-related infection, the cytotoxic effects of ExoU towards corneal epithelial cells are inhibited by natural in vivo factor(s). Indeed, we have demonstrated that human tear fluid can protect corneal epithelial cells from ExoU-mediated killing in vitro (Fleiszig et al., 2003), and protect both injured and healing murine corneas from cytotoxic P. aeruginosa infection in vivo (Kwong et al., 2007).
We previously described the inflammatory infiltrate induced by PA103ΔUT, wild-type PA103 and mutants in known TTSS effectors a murine model of infectious keratitis (Zolfaghar et al., 2006). In that study, ExoU was responsible for repression of phagocyte infiltration of infected corneas at 48 h in vivo, correlating with promotion of bacterial colonization, and with disease severity scores and appearance. Eyes infected with bacteria encoding exoU showed a distinctive “ring” infiltrate (also seen with some human corneal infections) that was consistent with the accumulation of phagocytes at the peripheral regions of the cornea and their complete absence from the central cornea. In contrast, eyes infected with exoU mutants showed dense phagocyte infiltration of the central cornea. In the present study, we found that the phospholipase activity of ExoU was required for enabling efficient colonization by 48 h. In addition, the “ring” infiltrate was only observed in eyes infected with bacteria complemented with phospholipase-active exoU. The loss of colonization, reduction in disease severity, and loss of ring infiltrate with phospholipase mutant ExoU to a level similar to background PA103ΔUT suggested that the use of more animals to do further histological experiments was not justifiable. Considering that ExoU’s phospholipase activity is required for cytotoxic activity, and for colonization at 48 h but not at 6 h time points, these data suggest that the in vivo contribution of ExoU towards corneal virulence involves cytotoxic effects on infiltrating neutrophils and monocytes.
It is also possible, however, that the effects of ExoU on later (48 h) colonization are also a consequence of earlier events. For example, cytotoxic effects of ExoU on epithelial cells at earlier time points, if they do occur, could hinder the production of proinflammatory cytokines that contribute to the chemotactic “driving force” for phagocyte infiltration and persistence in the infected murine cornea at later time points (Rudner et al., 2000; Thakur et al., 2002; Xue et al., 2003; Hazlett 2007; Willcox 2007).
In conclusion, our earlier work established a role for ExoU as an important contributor to corneal disease involving cytotoxic strains of P. aeruginosa. Here we show that the colonization and disease promoting activities of this toxin require the domain encoding its phospholipase activity. Inhibiting this activity could form the basis for a novel therapeutic intervention in P. aeruginosa keratitis albeit in conjunction with other therapeutic interventions which target the contributions of ExoU-independent virulence mechanisms, e.g. proteases, towards the pathogenesis of P. aeruginosa keratitis.
Acknowledgments
The authors wish to express their thanks to Dr. Dara Frank (Medical College of Wisconsin, WI, USA) for generously providing the complemented P. aeruginosa mutants used in this study. Thank you also to Dr. Danielle Robertson, Dr. Dwight Cavanagh (Southwestern Medical Center, TX, USA) and Dr. James Jester (University of California, Irvine, CA, USA) for generously providing the telomerase-immortalized HCEC. This work was supported by NIH Grant EY11221, and by an unrestricted gift fund from Allergan, Irvine, CA, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexandrakis G, Alfonso EC, Miller D. Shifting trends in bacterial keratitis in south Florida and emerging resistance to fluoroquinolones. Ophthalmology. 2000;107:1497–1502. doi: 10.1016/s0161-6420(00)00179-2. [DOI] [PubMed] [Google Scholar]
- Allewelt M, Coleman FT, Grout M, Priebe GP, Pier GB. Acquisition of expression of the Pseudomonas aeruginosa ExoU cytotoxin leads to increased bacterial virulence in a murine model of acute pneumonia and systemic spread. Infect Immun. 2000;68:3998–4004. doi: 10.1128/iai.68.7.3998-4004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisel KW, Hazlett LD, Berk RS. Dominant susceptibility effect on the murine corneal response to Pseudomonas aeruginosa. Proc Soc Exp Biol Med. 1983;172:488–491. doi: 10.3181/00379727-172-41592. [DOI] [PubMed] [Google Scholar]
- Bharathi MJ, Ramakrishnan R, Meenakshi R, Kumar CS, Padmavathy S, Mittal S. Ulcerative keratitis associated with contact lens wear. Indian J Ophthalmol. 2007;55:64–67. doi: 10.4103/0301-4738.29500. [DOI] [PubMed] [Google Scholar]
- Cheng KH, Leung SL, Hoekman HW, Beekhuis WH, Mulder PG, Geerards AJ, Kijlstra A. Incidence of contact-lens-associated microbial keratitis and its related morbidity. Lancet. 1999;354:181–185. doi: 10.1016/S0140-6736(98)09385-4. [DOI] [PubMed] [Google Scholar]
- Cowell BA, Weissman BA, Yeung KK, Johnson L, Ho S, Van R, Bruckner D, Mondino B, Fleiszig SM. Phenotype of Pseudomonas aeruginosa isolates causing corneal infection between 1997 and 2000. Cornea. 2003;22:131–134. doi: 10.1097/00003226-200303000-00010. [DOI] [PubMed] [Google Scholar]
- Cowell BA, Wu C, Fleiszig SM. Use of an Animal Model in Studies of Bacterial Corneal Infection. Ilar J. 1999;40:43–50. doi: 10.1093/ilar.40.2.43. [DOI] [PubMed] [Google Scholar]
- Evans DJ, Kuo TC, Kwong M, Van R, Fleiszig SM. Mutation of csk, encoding the C-terminal Src kinase, reduces Pseudomonas aeruginosa internalization by mammalian cells and enhances bacterial cytotoxicity. Microb Pathog. 2002;33:135–143. doi: 10.1006/mpat.2002.0521. [DOI] [PubMed] [Google Scholar]
- Finck-Barbancon V, Frank DW. Multiple domains are required for the toxic activity of Pseudomonas aeruginosa ExoU. J Bacteriol. 2001;183:4330–4344. doi: 10.1128/JB.183.14.4330-4344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck-Barbancon V, Goranson J, Zhu L, Sawa T, Wiener-Kronish JP, Fleiszig SM, Wu C, Mende-Mueller L, Frank DW. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol. 1997;25:547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- Fleiszig SM, Kwong MS, Evans DJ. Modification of Pseudomonas aeruginosa interactions with corneal epithelial cells by human tear fluid. Infect Immun. 2003;71:3866–3874. doi: 10.1128/IAI.71.7.3866-3874.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiszig SM, Lee EJ, Wu C, Andika RC, Vallas V, Portoles M, Frank DW. Cytotoxic strains of Pseudomonas aeruginosa can damage the intact corneal surface in vitro. Clao J. 1998;24:41–47. [PubMed] [Google Scholar]
- Fleiszig SM, Wiener-Kronish JP, Miyazaki H, Vallas V, Mostov KE, Kanada D, Sawa T, Yen TS, Frank DW. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect Immun. 1997;65:579–586. doi: 10.1128/iai.65.2.579-586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiszig SM, Zaidi TS, Preston MJ, Grout M, Evans DJ, Pier GB. Relationship between cytotoxicity and corneal epithelial cell invasion by clinical isolates of Pseudomonas aeruginosa. Infect Immun. 1996;64:2288–2294. doi: 10.1128/iai.64.6.2288-2294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity-Ryan L, Kazmierczak B, Kowal R, Comolli J, Hauser A, Engel JN. The arginine finger domain of ExoT contributes to actin cytoskeleton disruption and inhibition of internalization of Pseudomonas aeruginosa by epithelial cells and macrophages. Infect Immun. 2000;68:7100–7113. doi: 10.1128/iai.68.12.7100-7113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazlett LD. Bacterial infections of the cornea (Pseudomonas aeruginosa) Chem Immunol Allergy. 2007;92:185–194. doi: 10.1159/000099269. [DOI] [PubMed] [Google Scholar]
- Kwong MS, Evans DJ, Ni M, Cowell BA, Fleiszig SM. Human Tear Fluid Protects against Pseudomonas aeruginosa Keratitis in a Murine Experimental Model. Infect Immun. 2007;75:2325–2332. doi: 10.1128/IAI.01404-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam DS, Houang E, Fan DS, Lyon D, Seal D, Wong E. Incidence and risk factors for microbial keratitis in Hong Kong: comparison with Europe and North America. Eye. 2002;16:608–618. doi: 10.1038/sj.eye.6700151. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Cowell BA, Evans DJ, Fleiszig SM. Contribution of ExsA-regulated factors to corneal infection by cytotoxic and invasive Pseudomonas aeruginosa in a murine scarification model. Invest Ophthalmol Vis Sci. 2003;44:3892–3898. doi: 10.1167/iovs.02-1302. [DOI] [PubMed] [Google Scholar]
- Lomholt JA, Poulsen K, Kilian M. Epidemic population structure of Pseudomonas aeruginosa: evidence for a clone that is pathogenic to the eye and that has a distinct combination of virulence factors. Infect Immun. 2001;69:6284–6295. doi: 10.1128/IAI.69.10.6284-6295.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachigolla G, Blomquist P, Cavanagh HD. Microbial keratitis pathogens and antibiotic susceptibilities: a 5-year review of cases at an urban county hospital in north Texas. Eye Contact Lens. 2007;33:45–49. doi: 10.1097/01.icl.0000234002.88643.d0. [DOI] [PubMed] [Google Scholar]
- Pankhaniya RR, Tamura M, Allmond LR, Moriyama K, Ajayi T, Wiener-Kronish JP, Sawa T. Pseudomonas aeruginosa causes acute lung injury via the catalytic activity of the patatin-like phospholipase domain of ExoU. Crit Care Med. 2004;32:2293–2299. doi: 10.1097/01.ccm.0000145588.79063.07. [DOI] [PubMed] [Google Scholar]
- Phillips RM, Six DA, Dennis EA, Ghosh P. In vivo phospholipase activity of the Pseudomonas aeruginosa cytotoxin ExoU and protection of mammalian cells with phospholipase A2 inhibitors. J Biol Chem. 2003;278:41326–41332. doi: 10.1074/jbc.M302472200. [DOI] [PubMed] [Google Scholar]
- Preston MJ, Fleiszig SM, Zaidi TS, Goldberg JB, Shortridge VD, Vasil ML, Pier GB. Rapid and sensitive method for evaluating Pseudomonas aeruginosa virulence factors during corneal infections in mice. Infect Immun. 1995;63:3497–3501. doi: 10.1128/iai.63.9.3497-3501.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin SD, Hauser AR. Functional regions of the Pseudomonas aeruginosa cytotoxin ExoU. Infect Immun. 2005;73:573–582. doi: 10.1128/IAI.73.1.573-582.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson DM, Li L, Fisher S, Pearce VP, Shay JW, Wright WE, Cavanagh HD, Jester JV. Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Invest Ophthalmol Vis Sci. 2005;46:470–478. doi: 10.1167/iovs.04-0528. [DOI] [PubMed] [Google Scholar]
- Rudner XL, Kernacki KA, Barrett RP, Hazlett LD. Prolonged elevation of IL-1 in Pseudomonas aeruginosa ocular infection regulates macrophage-inflammatory protein-2 production, polymorphonuclear neutrophil persistence, and corneal perforation. J Immunol. 2000;164:6576–6582. doi: 10.4049/jimmunol.164.12.6576. [DOI] [PubMed] [Google Scholar]
- Sato H, Feix JB, Frank DW. Identification of superoxide dismutase as a cofactor for the pseudomonas type III toxin, ExoU. Biochemistry. 2006;45:10368–10375. doi: 10.1021/bi060788j. [DOI] [PubMed] [Google Scholar]
- Sato H, Feix JB, Hillard CJ, Frank DW. Characterization of phospholipase activity of the Pseudomonas aeruginosa type III cytotoxin, ExoU. J Bacteriol. 2005;187:1192–1195. doi: 10.1128/JB.187.3.1192-1195.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Frank DW. ExoU is a potent intracellular phospholipase. Mol Microbiol. 2004;53:1279–1290. doi: 10.1111/j.1365-2958.2004.04194.x. [DOI] [PubMed] [Google Scholar]
- Sato H, Frank DW, Hillard CJ, Feix JB, Pankhaniya RR, Moriyama K, Finck-Barbancon V, Buchaklian A, Lei M, Long RM, Wiener-Kronish J, Sawa T. The mechanism of action of the Pseudomonas aeruginosa-encoded type III cytotoxin, ExoU. Embo J. 2003;22:2959–2969. doi: 10.1093/emboj/cdg290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur A, Xue M, Stapleton F, Lloyd AR, Wakefield D, Willcox MD. Balance of pro- and anti-inflammatory cytokines correlates with outcome of acute experimental Pseudomonas aeruginosa keratitis. Infect Immun. 2002;70:2187–2197. doi: 10.1128/IAI.70.4.2187-2197.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallis AJ, Finck-Barbancon V, Yahr TL, Frank DW. Biological effects of Pseudomonas aeruginosa type III-secreted proteins on CHO cells. Infect Immun. 1999;67:2040–2044. doi: 10.1128/iai.67.4.2040-2044.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhelst D, Koppen C, Van Looveren J, Meheus A, Tassignon MJ. Contact lens-related corneal ulcers requiring hospitalization: a 7-year retrospective study in Belgium. Acta Ophthalmol Scand. 2006;84:522–526. doi: 10.1111/j.1600-0420.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- Watt K, Swarbrick HA. Microbial keratitis in overnight orthokeratology: review of the first 50 cases. Eye Contact Lens. 2005;31:201–208. doi: 10.1097/01.icl.0000179705.23313.7e. [DOI] [PubMed] [Google Scholar]
- Willcox MD. Pseudomonas aeruginosa infection and inflammation during contact lens wear: a review. Optom Vis Sci. 2007;84:273–278. doi: 10.1097/OPX.0b013e3180439c3e. [DOI] [PubMed] [Google Scholar]
- Xue ML, Thakur A, Willcox MD, Zhu H, Lloyd AR, Wakefield D. Role and regulation of CXC-chemokines in acute experimental keratitis. Exp Eye Res. 2003;76:221–231. doi: 10.1016/s0014-4835(02)00270-1. [DOI] [PubMed] [Google Scholar]
- Zolfaghar I, Evans DJ, Ronaghi R, Fleiszig SM. Type III secretion-dependent modulation of innate immunity as one of multiple factors regulated by Pseudomonas aeruginosa RetS. Infect Immun. 2006;74:3880–3889. doi: 10.1128/IAI.01891-05. [DOI] [PMC free article] [PubMed] [Google Scholar]