Abstract
Semiconductor quantum dot-conjugated antibodies were successfully developed to label Cryptosporidium parvum and Giardia lamblia. This novel fluorescence system exhibited superior photostability, gave 1.5- to 9-fold-higher signal-to-noise ratios than traditional organic dyes in detecting C. parvum, and allowed dual-color detection for C. parvum and G. lamblia.
The presence of Cryptosporidium and Giardia in various water sources is commonly determined by using immunofluorescent antibody (IFA) techniques (3, 4, 7). However, different water samples can contain inert particles or algal cells with strong autofluorescence and light scatter characteristics similar to those of immunofluorescently labeled protozoan cells. This can significantly impede the detection specificity of IFA for Cryptosporidium and Giardia but can be minimized by carefully selecting fluorescent dyes with minimal interference from fluorescent waterborne particles. It has been suggested (8) that fluorescein thiocyanate excited at 488 nm is the best fluorochrome for labeling oocysts in untreated water samples, while other fluorophores (i.e., cyanine dye 3, phycoerythrin, and tetramethylrhodamine isothiocyanate) excited at 542 nm are the best for use in drinking water samples. Nevertheless, these fluorescent dyes all are susceptible to photobleaching and have broad excitation and emission spectra, which often limit their uses in multiplexing detection.
Semiconductor quantum dots (QDs) or nanocrystals (∼5 to 50 nm in size) have recently emerged as a novel and promising class of fluorophores for cellular imaging (1, 2, 6, 9). Unlike conventional organic dyes, QDs can be excited by a wide spectrum of wavelengths to give great photostability, and their emission spectra, which differ according to size and material composition, are narrow, symmetrical, and tunable (1, 2). With these characteristics, QDs have minimal interference from natural autofluorescent particles and can be a superior fluorophore in the multiplexing detection of different molecular targets in various biological specimens (5, 6, 9). In this study, QDs were successfully demonstrated to be an excellent fluorophore in the IFA detection of microbial cells, such as those of Cryptosporidium parvum and Giardia lamblia.
Labeling strategies.
Two strategies were used to label C. parvum and G. lamblia cells with QD antibody bioconjugates. In strategy 1, the target cells were first bound with biotinylated antibodies before conjugation of QDs to the cell-attached antibodies. In detail, 5 μl of C. parvum or G. lamblia preparation (Waterborne Inc., New Orleans, La.) (concentration, 107 cells/ml) was spotted on a poly-l-lysine-coated glass slide and was air dried. The fixed cells were combined with 20 μl of blocking buffer (Waterborne Inc.) and were incubated for 20 min in a humidified chamber. After being washed with phosphate-buffered saline (PBS) (pH 7.4) three times for 5 min, the cells were combined with 20 μl of 1× biotinylated antibodies (anti-C. parvum or anti-G. lamblia from Waterborne Inc.) and were incubated for 30 min at 37°C. The cells were further washed with PBS three times for 5 min and were incubated with 20 μl of diluted QD solution for 30 min at 37°C. Two streptavidin-coated QDs (2 mM) with maximum emission wavelengths near 565 and 605 nm were purchased from Quantum Dot Corporation (Hayward, Calif.). After a final wash with PBS (three times for 5 min), the slide was mounted with mounting solutions and was observed under an Olympus BX51 epifluorescence microscope equipped with a cooled CCD camera SPOT-RT Slider (Diagnostic Instruments), a 100-W mercury short arc lamp bulb, Olympus fluorescence filter sets (U-MWB2 and U-MF2), and Chroma QD filter sets (32003 and 32005) (Chroma Inc.). Image exposure time varied from 50 to 100 ms for QD and organic dyes, respectively. Image analysis of the fluorescent signal-to-noise (S/N) ratio was performed with at least 10 cells by using Metamorph (Universal Imagine Corp.). In strategy 2, QDs were linked with antibodies followed by reaction with target cells. Biotinylated antibody (1× dilution) was first incubated with QDs for 30 min according to the manufacturer's protocol (Quantum Dot Corporation). Twenty microliters of the preincubated solution was added to a slide containing fixed target cells. The slide was incubated for 30 min at 37°C, washed with PBS three times for 5 min, and observed under a microscope.
Labeling efficiency.
In theory, one QD coated with multiple streptavidin molecules can accept multiple biotinylated antibodies. Thus, to maximize the illumination efficiency (one antibody per QD), strategy 1 or 2 could provide excessive QDs to those cell-bound antibodies after the removal of excessive free antibodies in the reaction system or could reduce the numbers of antibodies binding to one QD before labeling target cells with antibody-QD conjugates, respectively. Our results indicated that, under the manufacturer's suggested QD working concentration (20 nM), both strategies achieved a similar maximal S/N ratio of 17 for the labeling of C. parvum. Strategy 1 further showed a slight but gradual decrease in the S/N ratio to 16.5, 15.1, 10.8, and 7.5 under reduced QD concentration at 10×, 100×, 1,000×, and 10,000× dilution of the working concentration, respectively. In contrast, strategy 2 exhibited a rapid decrease in the S/N ratio to 15.0, 6.3, 6.0, and 3.8 under reduced QD concentrations at 3×, 9×, 27×, and 160× dilution of the suggested concentration, respectively. Further 3- to 10-fold increases in the QD working concentration with both strategies also caused an increase in background noise and a decrease in the S/N ratio to 9.2 to 12.1. Thus, a 3×- to 10-diluted QD working concentration was optimal for both strategies to maximize the S/N ratio.
Photostability.
In this study, QD labeling exhibited better photostability and higher brightness than the two most commonly used commercial staining kits, A100DF AquaGlo Dual Fluorochrome Kit (Waterborne Inc.) and KR1 Crypto-Cel IF test kit (Cellabs Pty. Ltd., Brookvale, New South Wales, Australia). It was observed that the QD-labeled Cryptosporidium cells remained photostable (fluorescent S/N ratio of ∼16 to 17) under continuous UV excitation for 5 min. In contrast, those two organic dyes bleached rapidly under continuous excitation, with a significant reduction in the S/N ratios from 5 to 11 to 2 to 3.5 and 1 to 2 after 1.5 and 5 min, respectively. The types of mounting solutions used were further found to affect the photostability of QDs significantly. Among all the antifade mounting solutions tested under 3-min continuous UV excitation, to our surprise immersion oil (Immersol 518F; Carl Zeiss, Göttingen, Germany) was the best one, with almost no reduction in the S/N ratio. Other solutions, which included 10% bovine serum albumin in 1× PBS used by Wu et al. (9), 90% glycerol suggested by Quantum Dot Corporation, antifade solutions provided in KR1 and A100 DF kits, water, and pure glycerol, showed 10 to 70% reduction in the S/N ratio.
Multiplexing detection.
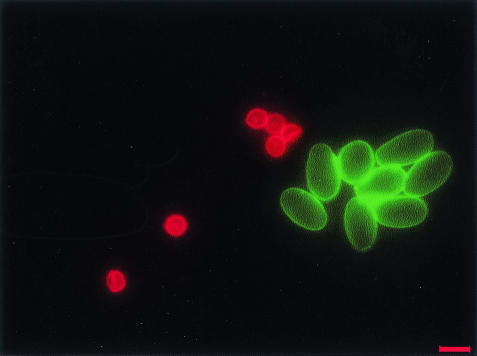
All QDs, irrespective of size and composition, were observed to be easily conjugated with biomolecules by using a universal approach, in contrast to traditional organic dyes, which required customized chemistry for conjugation of biomolecules to each fluorophore. The emission properties of QDs mentioned earlier further facilitated the multicolor imaging of one cell labeled with different QDs or different target cells labeled with different QDs, as illustrated in Fig. 1 with a dual-color labeling of C. parvum and G. lamblia.
FIG. 1.
A dual-color image of QD 605-labeled C. parvum (red) and QD 565-labeled G. lamblia (green) using strategy 2. Scale bar, 10 μm.
In summary, the general principle of QD labeling with C. parvum and G. lamblia could be applied to any other environmental microorganism if a specific antibody to that particular microorganism is available. This novel detection system could provide quantitative measurement with great sensitivity and photostability and potentially could revolutionize microbial detection in environmental microbiology studies.
Acknowledgments
This work was supported by grant R-265-000-125-305 from A*STAR to W.-T.L. and S.A.
REFERENCES
- 1.Bruchez, M., Jr., M. Moronne, P. Gin, S. Weiss, and A. P. Alivisatos. 1998. Semiconductor nanocrystals as fluorescent biological labels. Science 281:2013-2016. [DOI] [PubMed] [Google Scholar]
- 2.Chan, W. C., and S. Nie. 1998. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 281:2016-2018. [DOI] [PubMed] [Google Scholar]
- 3.Egyed, Z., T. Sreter, Z. Szell, and I. Varga. 2003. Characterization of Cryptosporidium spp.—recent developments and future needs. Vet. Parasitol. 111:103-114. [DOI] [PubMed] [Google Scholar]
- 4.Fayer, R., U. Morgan, and S. J. Upton. 2000. Epidemiology of Cryptosporidium: transmission, detection and identification. Int. J. Parasitol. 30:1305-1322. [DOI] [PubMed] [Google Scholar]
- 5.Goldman, E. R., E. D. Balighian, H. Mattoussi, M. K. Kuno, J. M. Mauro, P. T. Tran, and G. P. Anderson. 2002. Avidin: a natural bridge for quantum dot-antibody conjugates. J. Am. Chem. Soc. 124:6378-6382. [DOI] [PubMed] [Google Scholar]
- 6.Jaiswal, J. K., H. Mattoussi, J. M. Mauro, and S. M. Simon. 2003. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat. Biotechnol. 21:47-51. [DOI] [PubMed] [Google Scholar]
- 7.Quintero-Betancourt, W., E. R. Peele, and J. B. Rose. 2002. Cryptosporidium parvum and Cyclospora cayetanensis: a review of laboratory methods for detection of these waterborne parasites. J. Microbiol. Methods 49:209-224. [DOI] [PubMed] [Google Scholar]
- 8.Vesey, G., D. Deere, M. R. Gauci, K. R. Griffiths, K. L. Williams, and D. A. Veal. 1997. Evaluation of fluorochromes and excitation sources for immunofluorescence in water samples. Cytometry 29:147-154. [DOI] [PubMed] [Google Scholar]
- 9.Wu, X., H. Liu, J. Liu, N. Haley Kari, J. A. Treadway, J. P. Larson, N. Ge, F. Peale, and M. P. Bruchez. 2003. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat. Biotechnol. 21:41-46. [DOI] [PubMed] [Google Scholar]