Abstract
ATP is an extracellular signal for the immune system, particularly during an inflammatory response. It is sensed by the P2X7 receptor, the expression of which is upregulated by pro-inflammatory cytokines. Activation of the P2X7 receptor opens a cation-specific channel that alters the ionic environment of the cell, activating several pathways, including (i) the inflammasome, leading to production of IL-1β and IL-18; (ii) the stress-activated protein kinase pathway, resulting in apoptosis; (iii) the mitogen-activated protein kinase pathway, leading to generation of reactive oxygen and nitrogen intermediates; and (iv) phospholipase D, stimulating phagosome-lysosome fusion. The P2X7 receptor can initiate host mechanisms to remove pathogens, most particularly those that parasitise macrophages. At the same time, the P2X7 receptor may be subverted by pathogens to modulate host responses. Moreover, recent genetic studies have demonstrated significant associations between susceptibility or resistance to parasites and bacteria, and loss-of-function or gain-of-function polymorphisms in the P2X7 receptor, underscoring its importance in infectious disease.
Introduction
In addition to its role in cellular metabolism, the purine nucleotide ATP acts as an important extracellular messenger in a range of physiological processes, including synaptic transmissions, taste, bone formation/resorption, male fertility, blood pressure regulation, and inflammation [1]–[3]. Its effects are mediated through activation of purinergic receptors such as the P1 adenosine and the P2 nucleotide receptors [4]. Purinergic receptors are found on all types of cells in mammalian tissues, with many cells expressing multiple P1 and P2 subtypes [5].
P2 receptors are classified into two subfamilies—the P2X ligand-gated ion channels and the P2Y G protein–coupled receptors [4]. To date, seven P2X subunits (P2X1–P2X7) and eight P2Y subunits (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, P2Y14) have been identified [5]. P2X receptors are fast acting and open a cation-selective channel within milliseconds of ATP binding, whereas P2Y receptors are slower acting because their activation proceeds through second-messenger pathways, in the form of G proteins. Most P2X receptors have a low affinity for ATP, usually within the µM–mM range, while P2Y receptors are responsive to nM concentrations of ATP [6].
The P2X Family
There are seven mammalian P2X subunits that assemble as homo- or heterotrimers to form functional receptors [7]. Each subunit consists of intracellular N and C termini and two membrane-spanning segments, separated by an extracellular loop containing ten conserved cysteine residues, thought to form disulfide bonds, and lysine and phenylalanine residues involved in activation by ATP [7]. The C termini of the various P2X subunits vary in length from 25 amino acids in the P2X6 receptor to 240 amino acids in the P2X7 receptor and are associated with the functional properties specific to each receptor [2], [8]. Each subunit is able to bind a molecule of ATP although the sensitivity to ATP binding varies widely within the family, with the P2X1 receptor requiring nM levels for activation, whereas the P2X7 receptor requires mM concentrations [3]. Brief exposure of all P2X receptors to ATP opens up a channel that renders the cell permeable to Na+, K+, and Ca2+, causing an increase in intracellular Ca2+ and Na+ concentrations and a decrease in intracellular K+. This leads to depolarisation of the cell membrane and initiation of downstream Ca2+ signalling pathways [3]. Prolonged exposure of the P2X1 and P2X3 receptors to ATP results in desensitisation and closure of the pore; however, prolonged exposure of the P2X2, P2X4, P2X5, and P2X7 receptors to ATP results in sustained membrane depolarisation and the opening of large transmembrane pores [2] that are permeable to hydrophilic molecules between 314 Da and 900 Da, depending on the cell type studied [9], [10].
The P2X7 Receptor
The P2X7 receptor is highly expressed by cells of the haemopoietic lineage and can mediate cell death, killing of infectious organisms, and regulation of the inflammatory response [7], [11], [12]. The receptor is constitutively expressed. Under normal physiological conditions, activity of the receptor is kept at a low level by the extracellular concentration of divalent cations such as Ca2+ and Mg2+, which appear to alter the affinity of ATP binding in an allosteric manner, and this is believed to prevent unnecessary cell permeability and pore formation [7], [13]. Under pathophysiological conditions, for example at sites of inflammation or infection, expression is up-regulated by inflammatory cytokines [14]. Extracellular concentrations of Ca2+ and Mg2+ decrease as a consequence of dead and damaged cells releasing their cytosolic contents. This “dilution”, combined with an increase in ATP (also released from lysing cells), enhances P2X7 receptor activation [7], [13].
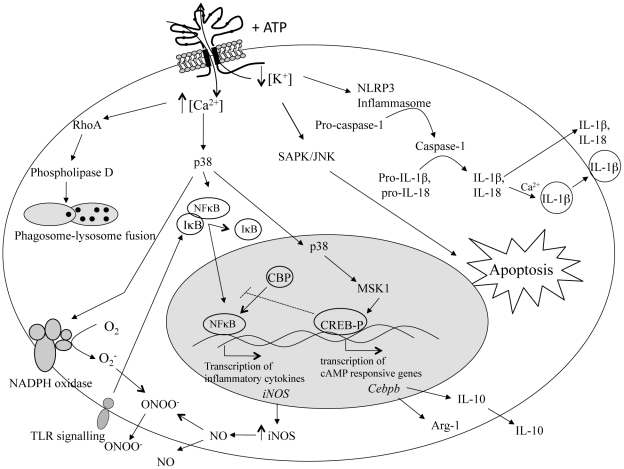
The alteration in the ionic environment of the cell, resulting from P2X7 receptor activation, triggers a number of cellular pathways, depending on cell type (Figure 1), including: (i) the inflammasome, leading to production of IL-1β and IL-18 [15]–[17]; (ii) the stress-activated protein kinase pathway, resulting in apoptosis [18]; (iii) the mitogen-activated protein kinase pathway, leading to generation of reactive oxygen and nitrogen intermediates [11], [19]–[21]; and (iv) phospholipase D, stimulating phagosome–lysosome fusion [22]. The involvement of the P2X7 receptor in these pathways suggests that it functions as a major regulator of inflammation. Indeed, absence of the P2X7 receptor alters immune cell function with the effect seen dependent on context. For example, in a murine model of arthritis, P2X7 receptor–deficient animals showed a reduction in the incidence and severity of the disease compared with wild-type animals [23]; however, in a study of autoimmune encephalomyelitis, P2X7 receptor–deficient animals had exacerbated neuroinflammation compared with wild-type animals [24]. Moreover, a number of change-of-function polymorphisms, most of which render the receptor inactive or with reduced function, have also been noted in the human population (Table 1) [25]–[32], and genetic association studies have uncovered links between some of these polymorphisms and resistance/susceptibility to mood disorders, bone diseases, and, most particularly, infectious disease [26]. It should be noted, however, that immune cells also express P2Y receptors, such as P2Y2, which are also activated by inflammatory cytokines and, so, may also contribute to the regulation of inflammation [5].
Figure 1. Intracellular pathways in immune cells stimulated by P2X7 receptor activation.
Activation of the P2X7 receptor with extracellular ATP opens a cation-specific ion channel that results in the influx of Ca2+ and Na+ and the efflux of K+. Prolonged exposure to ATP creates a pore in the cell membrane that further increases the intracellular Ca2+ concentration as well as allowing passage of larger molecules. This alteration in the ionic environment of the cell triggers a number of cellular pathways. Efflux of K+ stimulates the formation of the inflammasome, resulting in the activation of caspase-1. Caspase-1 then cleaves pro-IL-1β and pro-IL-18 to produce IL-1β and IL-18, which are then secreted from the cell as part of the inflammatory response. The efflux of K+ and influx of Na+ also activates the stress-activated protein kinase (SAPK)/c-Jun N-terminal kinases (JNK) pathway, resulting in the induction of apoptosis. The influx of Ca2+ activates phospholipase D via RhoA, leading to phagosome/lysosome fusion and the killing of intracellular pathogens. Influx of Ca2+ can also activate the mitogen-activated protein kinase p38, stimulating a number of downstream effects. Phosphorylation of p38 leads to the assembly of NADPH oxidase at the plasma membrane, and the subsequent production of superoxide (O2 −), enhances nuclear factor kappa B (NFκB) activation via toll-like receptor (TLR) signalling and the subsequent transcription of inducible nitric oxide synthase (iNOS) and production of nitric oxide (NO), as well as production of tumour necrosis factor (TNF) and IL-6. It can also lead to the phosphorylation of cAMP response elements binding protein (CREB) via mitogen- and stress-activated kinase 1 (MSK1). Phosphorylated CREB (CREB-P) sequesters CREB binding protein (CBP), a co-transcription factor required for NFκB-mediated gene transcription, and inhibits transcription of NFκB-controlled genes. Phosphorylated CREB/CBP also stimulates the production of cAMP-responsive genes such as Cebpb that act to modulate the inflammatory response through the production of arginase-1 (Arg-1) and IL-10.
Table 1. Single nucleotide polymorphisms identified in the human P2X7 receptor.
| P2X7R Gene Polymorphism (Nucleotide Position and Base Change) | Amino Acid Change | Location in Receptor | Effect on Receptor Function | Disease Association | dbSNP ID |
| 151+1 g>t | Produces null allele | Exon1/intron1 boundary | Loss of function – nonsense-mediated mRNA decay [25], [26] | None known | rs35933842 |
| 253 T>C | Val-76>Ala | Extracellular loop | Loss of function – partial reduction in pore formation [27] | None known | rs1752809 |
| 474 G>A | Gly-150>Arg | Extracellular loop | Loss of function – disrupted protein folding, no pore formation [27], [28] | None known | rs28360447 |
| 489 C>T | His-155>Tyr | Extracellular loop | Gain of function – enhanced pore formation and Ca2+ influx [29] | None known | rs208294 |
| 835 G>A | His-270>Arg | Extracellular loop | Gain of function – enhanced pore formation [27] | None known | rs7958311 |
| 853 G>A | Arg-276>His | Extracellular loop | Loss of function – no pore formation [27] | None known | rs7958316 |
| 946 G>A | Arg-307>Gln | ATP binding site | Loss of function – no channel or pore formation, loss of phospholipase D activity [30] | None known | rs28360457 |
| 1068 G>A | Ala-348>Thr | Cytoplasmic tail | Gain of function –enhanced pore formation and IL-1β secretion [27] | None known | rs1718119 |
| 1096 C>G | Thr-357>Ser | Cytoplasmic tail | Loss of function – partial reduction in channel and pore formation [25] | Impaired mycobacterial killing | rs2230911 |
| 1405 A>G | Glu-460>Arg | Cytoplasmic tail | Loss of function – partial reduction in pore formation [26], [27] | Bipolar disorder, major depressive disorder | rs2230912 |
| 1513 A>C | Glu-496>Ala | Cytoplasmic tail | Loss of function – suboptimal receptor assembly affecting pore formation [28], [31] | Susceptibility to reactivating tuberculosis; chronic lymphocytic leukaemia | rs3751143 |
| 1729 T>A | Ile-568>Asn | Cytoplasmic tail | Loss of function – prevention of receptor trafficking and surface expression [32] | None known | rs1653624 |
The P2X7 Receptor and Intracellular Bacteria
Mycobacteria, such as Mycobacterium tuberculosis, the causative agent of human tuberculosis, are able to survive and replicate in phagosomes within macrophages by inhibiting phagosomal fusion with lysosomes [33]. Treatment of infected macrophages with ATP, however, can overcome the phagosome–lysosome fusion block, leading to the killing of intracellular bacilli [34]. The process appears to be mediated by P2X7 receptors; bactericidal activity is markedly reduced in P2X7 receptor–deficient macrophages [34]. It involves activation of phospholipase D; thus, phospholipase D blockers inhibit killing of intracellular mycobacteria following ATP treatment [34]. Blocking macrophage phospholipase D activity, however, does not inhibit macrophage apoptotic death, demonstrating that, while ATP stimulation leads to macrophage apoptosis and mycobacterial death, these processes can be uncoupled [34]. More recently, autophagy has been shown to have a role in the control of mycobacterial infections. ATP treatment rapidly induces autophagy and mycobacterial killing in a process dependent on P2X7 receptor activation and Ca2+ influx [35].
Evidence for P2X7 receptor involvement in mycobacterial killing comes from studies showing that loss-of-function polymorphisms in the human P2X7 receptor gene lead to increased susceptibility to M. tuberculosis. Fernando et al. [36] investigated the prevalence of the 1513 A>C polymorphism in two independent Southeast Asian cohorts, and found a strong association with the 1513 A>C polymorphism and extrapulmonary tuberculosis. Subsequent studies have also found that the 1513C allele is a risk factor in the development of extrapulmonary and pulmonary tuberculosis in numerous ethnic populations, including Mexican [37], Russian Slavic [38], and a North Indian Punjabi population [39]. Recently, an association with the 1513C allele and the development of extrapulmonary tuberculosis in Turkish children was also identified [40]. Additional studies have shown associations between tuberculosis and alleles other than 1513C. Li et al. [41] studied various P2X7 receptor polymorphisms in a Gambian population. They found that a protective effect against tuberculosis was associated with a P2X7 receptor promoter polymorphism at position −762, but the 1513 A>C polymorphism discussed above did not show any significant association. Interestingly, Sambasivan et al. [42] found an association between development of clinical tuberculosis and the presence of the −762C or 1729T allele but not the 1513C allele in an Indian cohort, while Xiao et al. [43] did not find an association between either the 1513C allele or the −762C allele and the development of pulmonary tuberculosis in a Chinese Han cohort. Overall, though, a recent meta-analysis of P2X7 receptor gene polymorphism association studies revealed a strong association between the 1513 A>C polymorphism and susceptibility to tuberculosis [44].
Data from in vitro studies provide evidence for potential mechanistic explanations for the association of polymorphisms in the human P2X7 receptor gene with susceptibility to tuberculosis. Saunders et al. [45] activated P2X7 receptors on BCG-infected macrophages from wild-type and homozygous 1513C donors and showed that P2X7 receptor–activated wild-type macrophages undergo apoptosis and kill intracellular bacteria; however, macrophages homozygous for the 1513 A>C loss-of-function polymorphism fail to undergo apoptosis upon exposure to ATP, resulting in mycobacterial survival. The effect of other P2X7 receptor polymorphisms was further assessed by Fernando et al. [46] and Shemon et al. [25], who showed that several P2X7 receptor polymorphisms (946 G>A, 1729 T>A, and 155+1 g>t) result in reduced macrophage apoptosis and mycobacterial killing. This effect was augmented in compound heterozygous donors (donors with a heterozygous loss of function polymorphism at more than one position on the P2X7 receptor gene).
The P2X7 receptor has also been implicated in the innate response to obligate intracellular bacteria of the Chlamydia genus. The macrophage is an important reservoir and source of dissemination of Chlamydia [47], [48]. Like mycobacteria, Chlamydia within macrophages are susceptible to ATP treatment, which results in the death of ∼70%–90% of all intracellular bacteria [49]. This appears to be the result of P2X7 receptor–dependent stimulation of phospholipase D activity and subsequent phagolysosome fusion. Macrophages from P2X7 receptor gene knockout mice show no phospholipase D activation after treatment with ATP and are completely unable to induce ATP-dependent chlamydial death [50]. Furthermore, inhibiting phospholipase D activation in normal murine macrophages, by the addition of butan-1-ol, restores the levels of infection to ∼50%, indicating that phospholipase D is at least partly responsible for chlamydial death [50]. Moreover, treatment of epithelial cells (the preferred target cell for chlamydiae) with P2X7 receptor agonists reduces the infectiveness of the bacteria, again associated with activation of phospholipase D [51].
Although Chlamydia is clearly vulnerable to P2X7 receptor–dependent killing, it has developed some resistance to this killing. Chlamydia-infected J774 murine macrophages are resistant to apoptosis following treatment with ATP, whereas uninfected cells undergo apoptosis via P2X7 receptor–dependent pathways [49]. Infection results in markedly reduced activation of the P2X7 receptor; the mechanism by which this is achieved remains to be elucidated, but it is known that live, growing organisms are required [49].
The P2X7 Receptor and Intracellular Parasites
The P2X7 receptor may also play a role in clearance of intracellular parasites of the Leishmania genus. Murine macrophages and cells cultured from cutaneous lesions of mice infected with Leishmania amazonensis are more sensitive to P2X7 receptor–mediated pore formation, inhibiting growth of the intracellular parasite [52]. Killing of L. amazonensis via the P2X7 receptor is independent of nitric oxide; instead, it is associated with host cell apoptosis [52]. However, when P2X7 receptor gene knockout mice and parental C57BL/6J mice are infected intradermally with Leishmania major, no difference in resolution of lesions is observed, suggesting that, in the absence of P2X7 receptors, other anti-parasitic defence mechanisms compensate to control infection in vivo (C. Miller, A. Zakrzewski, M. Katrib, N. Smith, unpublished observations).
Recently, the P2X7 receptor has been implicated in the immune response to another intracellular protozoan, Toxoplasma gondii, a parasite that is able to infect and survive in cells of the monocyte/macrophage lineage. However, the role of the P2X7 receptor in the response to T. gondii is an intriguing and complex one. Activation of “wild-type” human or murine macrophages with ATP induces killing of tachyzoites of both virulent and avirulent strains of the parasite, but the same treatment of macrophages from humans with the 1513 A>C loss-of-function polymorphism or macrophages from P2X7 receptor knockout mice has no effect on parasite viability [53], [54]. Interestingly, P2X7 receptor–mediated killing of T. gondii is not linked to generation of nitric oxide but is, rather, associated with either phagolysosome formation accompanied by production of free oxygen radicals [53] or host cell apoptosis [54]. Thus, P2X7 receptor–mediated killing of T. gondii has much in common with similarly mediated killing of Mycobacteria, Chlamydia, and Leishmania.
However, susceptibility to either congenital or ocular toxoplasmosis in humans is not associated significantly with the 1513 A>C loss-of-function polymorphism [55]. Intriguingly, though, resistance to both congenital and ocular toxoplasmosis, in the United States and Brazil, respectively, is associated positively with the 1068 T>C polymorphism [55]. This polymorphism has recently been revealed to code for a gain-of-function phenotype in P2X7 receptor activity [27] and may be the true ancestral primate sequence [55]. Thus, the American and Brazilian association studies could be interpreted to indicate that reduced inflammatory disease of the foetus or the eye is a consequence of efficient control of parasite load in the presence of a fully functional, ancestral P2X7 receptor [55]. Larger scale association studies may be needed to truly resolve the association, or otherwise, of various polymorphisms in the P2X7 receptor and congenital or acquired toxoplasmosis.
In vivo murine evidence for a significant role of the P2X7 receptor in controlling T. gondii is also inconsistent. In one study, where mice were infected intraperitoneally with tachyzoites of the type 2, avirulent ME49 strain of T. gondii, splenic parasite burdens in different mouse strains were in proportions consistent with their relative P2X7 receptor activity [54]; thus, P2X7 receptor knockout mice harboured more parasites than the parental C57BL/6J mice, whereas, in another study, using the same parasites and route of infection, no difference in parasite burden between receptor knockout mice and the parental strain was observed in vivo [56]. C57BL/6J mice are known to possess a proline-to-leucine polymorphism at amino acid 453 in their P2X7 receptor that affects some [22], [57]–[59], but not all [22], [60], [61], functions of the receptor and so, C57BL/6J mice, in turn, had higher parasite burdens than resistant BALB/c mice with fully functioning P2X7 receptors. It should be noted, however, that BALB/c and C57BL/6J mice display differing susceptibilities to T. gondii infection and multiple genes are associated with this [62]. This may help explain the discrepancy seen in the in vivo studies.
The apparent inconsistency between P2X7 receptor–mediated killing of T. gondii in vitro versus in vivo may simply reflect the fact that type 2 strains of T. gondii, like ME49, induce a wide variety of pro-inflammatory responses that can control the reproduction of this parasite [63]. Put another way, P2X7 receptor–mediated killing is just one means of control of T. gondii, and others remain operative in knockout mice so that little effect on parasite burden is observed in vivo. This does, however, indicate that the P2X7 receptor plays a contributory rather than major role in controlling T. gondii, which is underscored by the fact that production of several cytokines known to be important in the control of T. gondii, including gamma-interferon and IL-12, are not inhibited in T. gondii–infected P2X7 receptor knockout mice [56].
Most intriguingly, P2X7 receptor knockout mice are very susceptible to acute toxoplasmosis, losing weight faster and more dramatically than either C57BL/6J or BALB/c mice [56]. This susceptibility is not related to relative parasite burdens or a general alteration in cytokine responses but is associated with an inability of the knockout mice to limit nitric oxide production [56], a well-known cause of immunopathology in parasitic infections, including T. gondii [63]. P2X7 receptor knockout mice infected with T. gondii also display a delayed—but not abrogated—production of IL-10 [56], which may partially explain their inability to control nitric oxide production. However, it must be stressed that these observations are correlative rather than definitive evidence that the susceptibility to toxoplasmosis apparent in P2X7 receptor–deficient mice is due to an over-exuberant inflammatory response and, as this immunoregulatory role for the P2X7 receptor has not been noted previously, it does require in-depth follow-up.
It is tempting to speculate that extracellular ATP is an effective danger signal for the immune system, produced as a result of damage caused by factors like nitric oxide. Since nitric oxide is potentially so damaging, its production is tightly controlled, possibly through a negative feedback mechanism. In this scenario, perhaps cells lacking the P2X7 receptor to sense extracellular ATP continue producing nitric oxide, whereas cells with functional receptors are able to regulate nitric oxide production. Figure 1 presents a possible molecular explanation. CREB [64] is a transcription factor that has been linked to the suppression of inducible nitric oxide synthase. Extracellular ATP induces activation of CREB as well as suppressing LPS-induced nitric oxide production [65]. Although the exact mechanism has not been elucidated, it is thought that activated CREB sequesters a transcriptional co-activator, CBP, preventing it from interacting with the nuclear factor kappa B (NFκB) subunit p65, thus inhibiting expression of inducible nitric oxide synthase [65]. Hence, during infection with T. gondii, extracellular ATP, operating through the P2X7 receptor, may activate CREB and down-regulate nitric oxide production. Cells lacking the P2X7 receptor would, therefore, be less responsive to any build-up in extracellular ATP. The downstream effect of this would be a lack of phosphorylation, allowing CBP to remain bound to NFκB, and inducible nitric oxide synthase transcription and nitric oxide production to continue indefinitely. Pathology would result.
Conclusion
Clearly, the P2X7 receptor comprises an important part of the host arsenal against invading pathogens. However, there is growing evidence of bacteria and parasites subverting the P2X7 receptor pathways for their own advantage, be that through P2X7 receptor–mediated apoptosis, phospholipase D production, or inhibition of these, as highlighted above.
We speculate that many other pathogens also modulate the host P2X7 receptor. For example, intracellular parasites of various genera—including Theileria [66], Leishmania [67], [68], Cryptosporidium [69], [70], and Microsporidia [71], and bacteria such as Brucella [72], [73]—all prevent apoptosis of host cells to aid their own survival. Other pathogens, such as Plasmodium [74], [75], Tritrichomonas [76], [77], Streptococcus [78], [79], and Legionella [80], actually increase apoptosis of host cells, thereby reducing the numbers of circulating immune cells and increasing their chances for survival. Intriguingly, T. gondii is able to both induce and suppress apoptosis in a cell type–dependent manner that allows it to establish a persistent infection [81]. Could some, or all of these, be affecting host cell apotosis via the P2X7 receptor? Legionella [82], Francisella [83], [84], and Leishmania [85] prevent phagolysosome fusion—perhaps this is in a P2X7 receptor–dependent manner analogous to Chlamydia? And, although no definitive link with the P2X7 receptor exists, infection of peritoneal macrophages with Trypanosoma cruzi down-regulates expression of P2X7 receptors [86], which may reduce the immune pressure on these parasites. It would be interesting to investigate whether the P2X7 receptor is a common target in the immune evasion strategies of these diverse pathogens. It should also be noted that many of these studies have been conducted in vitro, and it will be important to confirm that the P2X7 receptor is playing a non-redundant role in controlling these pathogens in vivo using P2X7 receptor knockout mice.
Perhaps surprisingly, extracellular pathogens may also be affected by, or react to, P2X7 receptor activity. For example, Pseudomonas aeruginosa, a major pathogen in the lungs of cystic fibrosis patients, and Vibrio cholerae, the causative agent of cholera, have been shown to secrete multiple enzymes with ATP-modifying activities, such as adenylate kinase, ATPase, and 5′-nucleotidase [87]–[89]. It is believed that these enzymes are used to modulate external ATP levels and, thereby, increase P2X7 receptor function, mediating apoptosis of macrophages. Extracellular bacteria, such as Staphylococcus aureus and Escherichia coli, have been linked to P2X7 receptor dependence in a study of caspase-1 activation and subsequent IL-1β secretion [90]. It is unclear in these studies, however, how this stimulation of apoptosis affects the survival and growth of the bacteria. Thus, future work may well reveal that the P2X7 receptor has—sometimes quite unexpected—roles in modulating a variety of infectious diseases, not just those that parasitise macrophages.
Footnotes
The authors have declared that no competing interests exist.
Professors Smith and Wiley gratefully acknowledge the support of the Australian Research Council (ARC) for funding through its Discovery Project Scheme (DP0666515). The ARC played no role in the literature review, writing, editing or decision to publish this manuscript.
References
- 1.Crane JK, Naeher TM, Choudhari SS, Giroux EM. Two pathways for ATP release from host cells in enteropathogenic Escherichia coli infection. Am J Physiol Gastrointest Liver Physiol. 2005;289:G407–G417. doi: 10.1152/ajpgi.00137.2005. [DOI] [PubMed] [Google Scholar]
- 2.Jarvis MF, Khakh BS. ATP-gated ion channels. Neuropharmacol. 2009;56:208–215. doi: 10.1016/j.neuropharm.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 3.Young MT. P2X receptors: dawn of the post-structure era. Trends Biochem Sci. 2010;35:83–90. doi: 10.1016/j.tibs.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.North RA, Barnard EA. Nucleotide receptors. Curr Opin Neurobiol. 1997;7:346–357. doi: 10.1016/s0959-4388(97)80062-1. [DOI] [PubMed] [Google Scholar]
- 7.Jiang L-H. Inhibition of P2X7 receptors by divalent cations: old action and new insight. Eur Biophys J. 2009;38:339–346. doi: 10.1007/s00249-008-0315-y. [DOI] [PubMed] [Google Scholar]
- 8.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- 9.Wiley JS, Gargett CE, Zhang W, Snook MB, Jamieson GP. Partial agonists and antagonists reveal a second permeability state of human P2Z/P2X7 channel. Am J Physiol. 1998;275:C1224–C1231. doi: 10.1152/ajpcell.1998.275.5.C1224. [DOI] [PubMed] [Google Scholar]
- 10.Faria RX, Cascabulho CM, Reis RA, Alves LA. Large-conductance channel formation mediated by P2X(7) receptor activation is regulated through distinct intracellular signaling pathways in peritoneal macrophages and 2BH4 cells. Naunyn Schmiedebergs Arch Pharmacol. 2010;382:73–87. doi: 10.1007/s00210-010-0523-8. [DOI] [PubMed] [Google Scholar]
- 11.Gavala ML, Pfeiffer ZA, Bertics PJ. The nucleotide receptor P2RX7 mediates ATP-induced CREB activation in human and murine monocytic cells. J Leukoc Biol. 2008;84:1159–1171. doi: 10.1189/jlb.0907612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bulanova E, Budagian V, Orinska Z, Koch-Nolte F, Haag F, et al. ATP induces P2X7 receptor-independent cytokine and chemokine expression through P2X1 and P2X3 receptors in murine mast cells. J Leukoc Biol. 2009;85:692–702. doi: 10.1189/jlb.0808470. [DOI] [PubMed] [Google Scholar]
- 13.Gudipaty L, Humphreys BD, Buell G, Dubyak GR. Regulation of P2X7 nucleotide receptor function in human monocytes by extracellular ions and receptor density. Am J Physiol Cell Physiol. 2001;280:943–953. doi: 10.1152/ajpcell.2001.280.4.C943. [DOI] [PubMed] [Google Scholar]
- 14.Humphreys BD, Dubyak GR. Modulation of P2X7 nucleotide receptor expression by pro- and anti-inflammatory stimuli in THP-1 monocytes. J Leukoc Biol. 1998;64:256–273. doi: 10.1002/jlb.64.2.265. [DOI] [PubMed] [Google Scholar]
- 15.Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, et al. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity. 2007;26:433–443. doi: 10.1016/j.immuni.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Pétrilli V, Papin S, Dostert C, Mayor A, Martinon F, et al. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 17.Yu HB, Finlay BB. The caspase-1 inflammasome: A pilot of innate immune responses. Cell Host and Microbe. 2008;4:198–208. doi: 10.1016/j.chom.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 18.Humphreys BD, Rice J, Kertsey SB, Dubyak GR. Stress-activated protein kinase/JNK activation and apoptotic induction by the macrophage P2X7 nucleotide receptor. J Biol Chem. 2000;275:26791–26798. doi: 10.1074/jbc.M002770200. [DOI] [PubMed] [Google Scholar]
- 19.Guerra AN, Gavala ML, Chung HS, Bertics PJ. Nucleotide receptor signalling and the generation of reactive oxygen species. Purinergic Signalling. 2007;3:39–51. doi: 10.1007/s11302-006-9035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfeiffer ZA, Guerra AN, Hill LM, Gavala ML, Prabhu U, et al. Nucleotide receptor signalling in murine macrophages is linked to reactive oxygen species generation. Free Radic Biol Med. 2007;42:1506–1516. doi: 10.1016/j.freeradbiomed.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruffell D, Mourkioti F, Gambardella A, Kirstetter P, Lopez RG, et al. A CREB-C/EBPβ cascade induces M2 macrophage specific gene expression and promotes muscle injury repair. Proc Natl Acad Sci U S A. 2009;106:17475–17480. doi: 10.1073/pnas.0908641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Stunff H, Auger R, Kanellopoulos J, Raymond M-N. The Pro-451 to Leu polymorphism within the C-terminal tail of the P2X7 receptor impairs cell death but not phospholipase D activation in murine thymocytes. J Biol Chem. 2004;279:16918–16926. doi: 10.1074/jbc.M313064200. [DOI] [PubMed] [Google Scholar]
- 23.Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, et al. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168:6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, Brosnan CF. Exacerbation of experimental autoimmune encephalomyelitis in P2X7R-/- mice: evidence for loss of apoptotic activity in lymphocytes. J Immunol. 2006;176:3115–3126. doi: 10.4049/jimmunol.176.5.3115. [DOI] [PubMed] [Google Scholar]
- 25.Shemon AN, Sluyter R, Fernando SL, Clarke AL, Dao-Ung LP, et al. A Thr357 to Ser polymorphism in homozygous and compound heterozygous subjects causes absent or reduced P2X7 function and impairs ATP-induced mycobacterial killing by macrophages. J Biol Chem. 2006;81:2079–2086. doi: 10.1074/jbc.M507816200. [DOI] [PubMed] [Google Scholar]
- 26.Fuller SJ, Stokes L, Skarratt KK, Gu BJ, Wiley JS. Genetics of the P2X7 receptor and human disease. Purinergic Signalling. 2009;5:257–262. doi: 10.1007/s11302-009-9136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stokes L, Fuller SJ, Sluyter R, Skarratt KK, Gu BJ, et al. Two haplotypes of the P2X7 receptor containing the Ala-348 to Thr polymorphism exhibit a gain-of-function effect and enhanced interleukin-1β secretion. FASEB J. 2010;24:2916–2927. doi: 10.1096/fj.09-150862. [DOI] [PubMed] [Google Scholar]
- 28.Denlinger LC, Sorkness CA, Chinchilli VM, Lemanske RF Human P2X7 pore function predicts allele linkage disequilibrium. Clin Chem. 2006;52:995–1004. doi: 10.1373/clinchem.2005.065425. [DOI] [PubMed] [Google Scholar]
- 29.Cabrini G, Falzoni S, Forchap SL, Pellegatti P, Balboni A, et al. A His-155 to Tyr polymorphism confers gain-of-function to the human P2X7 receptor of human leukemia lymphocytes. J Immunol. 2005;175:82–89. doi: 10.4049/jimmunol.175.1.82. [DOI] [PubMed] [Google Scholar]
- 30.Gu BJ, Sluyter R, Skarratt KK, Shemon AN, Dao-Ung LP, et al. An Arg307 to Gln polymorphism within the ATP-binding site causes loss of function of the human P2X7 receptor. J Biol Chem. 2004;23:31287–95. doi: 10.1074/jbc.M313902200. [DOI] [PubMed] [Google Scholar]
- 31.Gu BJ, Zhang W, Worthington RA, Slutyer R, Dao-Ung P, et al. A Glu-496 to Ala polymorphism leads to loss of function of the human P2X7 receptor. J Biol Chem. 2001;276:11135–11142. doi: 10.1074/jbc.M010353200. [DOI] [PubMed] [Google Scholar]
- 32.Wiley JS, Dao-Ung LP, Li C, Shemon AN, Gu BJ, et al. An Ile-568 to Asn polymorphism prevents normal trafficking and function of the human P2X7 receptor. J Biol Chem. 2003;278:17108–17113. doi: 10.1074/jbc.M212759200. [DOI] [PubMed] [Google Scholar]
- 33.Vergne I, Chua J, Lee HH, Lucas M, Belisle J, Deretic V. Mechanism of phagolysosome biogenesis block by viable Mycobacterium tuberculosis. . Proc Natl Acad Sci U S A. 2005;102:4033–4038. doi: 10.1073/pnas.0409716102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fairbairn IP, Stober CB, Kumararatne DS, Lammas DA. ATP-mediated killing of intracellular mycobacteria is a P2X7-dependent process inducing bacterial death by phagosome-lysosome fusion. J Immunol. 2001;167:3300–3307. doi: 10.4049/jimmunol.167.6.3300. [DOI] [PubMed] [Google Scholar]
- 35.Biswas D, Qureshi OS, Lee WY, Croudace JE, Mura M, et al. ATP-induced autophagy is associated with rapid killing of intracellular mycobacteria within human monocytes/macrophages. BMC Immunology. 2008;9:35. doi: 10.1186/1471-2172-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fernando SL, Saunders BM, Sluyter R, Skarratt KK, Wiley JS, et al. A polymorphism in the P2X7 gene increases susceptibility to extrapulmonary tuberculosis. Am J Resp Crit Care Med. 2007;175:360–366. doi: 10.1164/rccm.200607-970OC. [DOI] [PubMed] [Google Scholar]
- 37.Niño-Moreno P, Portales-Pérez D, Hernández-Castro B, Portales-Cervantes L, Flores-Meraz V, et al. P2X7 and NRAMP1/SLC11 A1 gene polymorphisms in Mexican mestizo subjects with pulmonary tuberculosis. Clin Exp Immunol. 2007;148:469–477. doi: 10.1111/j.1365-2249.2007.03359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mokrousov I, Sapozhnikova N, Narvskaya O. Mycobacterium tuberculosis co-existence with humans: making an imprint on the macrophage P2X7 receptor gene? J Med Microbiol. 2008;57:581–584. doi: 10.1099/jmm.0.47455-0. [DOI] [PubMed] [Google Scholar]
- 39.Sharma S, Kumar V, Khosla R, Kajal N, Sarin B, et al. Association of P2X7 receptor +1513 (A>C) polymorphism with tuberculosis in a Punjabi population. Int J Tuberc Lung Dis. 2010;14:1159–1163. [PubMed] [Google Scholar]
- 40.Tekin D, Kayaalti Z, Dalgic N, Cakir E, Soylemezoglu T, et al. Polymorphism in the p2x7 gene increases susceptibility to extrapulmonary tuberculosis in Turkish children. Pediatr Infect Dis. 2010;29:779–782. doi: 10.1097/INF.0b013e3181d9932e. [DOI] [PubMed] [Google Scholar]
- 41.Li CM, Campbell SJ, Kumararatne DS, Bellamy R, Ruwende C, et al. Association of a polymorphism in the P2X7 gene with tuberculosis in a Gambian population. J Infect Dis. 2002;186:1458–1462. doi: 10.1086/344351. [DOI] [PubMed] [Google Scholar]
- 42.Sambasivan V, Murthy KJ, Reddy R, Vijayakshimi V, Hasan Q. P2X7 gene polymorphisms and risk assessment for pulmonary tuberculosis in Asian Indian. Dis Markers. 2010;28:43–48. doi: 10.3233/DMA-2010-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xiao J, Sun L, Jiao W, Li Z, Zhao S, et al. Lack of association between polymorphisms in the P2X7 gene and tuberculosis in a Chinese Han population. FEMS Immunol Med Microbiol. 2009;55:107–111. doi: 10.1111/j.1574-695X.2008.00508.x. [DOI] [PubMed] [Google Scholar]
- 44.Xiao J, Sun L, Yan H, Jiao W, Miao Q, et al. Metaanalysis of P2X7 gene polymorphisms and tuberculosis susceptibility. FEMS Immunol Med Microbiol. 2010;60:165–170. doi: 10.1111/j.1574-695X.2010.00735.x. [DOI] [PubMed] [Google Scholar]
- 45.Saunders BM, Fernando SL, Sluyter R, Britton WJ, Wiley JS. A loss-of-function polymorphism in the human P2X7 receptor abolishes ATP-mediated killing of mycobacteria. J Immunol. 2003;171:5442–5446. doi: 10.4049/jimmunol.171.10.5442. [DOI] [PubMed] [Google Scholar]
- 46.Fernando SL, Saunders BM, Sluyter R, Skarratt KK, Wiley JS, et al. Gene dosage determines the negative effects of polymorphic alleles of the P2X7 receptor on adenosine triphosphate-mediated killing of mycobacteria by human macrophages. J Infect Dis. 2005;192:149–155. doi: 10.1086/430622. [DOI] [PubMed] [Google Scholar]
- 47.Koehler L, Nettelnbreker E, Hudson AP, Ott N, Gérard HC, et al. Ultrastructural and molecular analysis of the persistence of Chlamydia trachomatis (serovar K) in human monocytes. Microb Pathog. 1997;22:133–142. doi: 10.1006/mpat.1996.0103. [DOI] [PubMed] [Google Scholar]
- 48.Gérard HC, Branigan PJ, Schumacher HR, Hudson AP. Synovial Chlamydia trachomatis in patients with reactive arthritis/Reiters's syndrome are viable but show aberrant gene expression. J Rheumatol. 1998;25:734–742. [PubMed] [Google Scholar]
- 49.Coutinho-Silva R, Perfettini JL, Persechini PM, Dautry-Varsat A, Ojcius DM. Modulation of P2Z/P2X7 receptor activity in macrophages infected with Chlamydia psittaci. Am J Physiol Cell Physiol. 2001;280:C81–C89. doi: 10.1152/ajpcell.2001.280.1.C81. [DOI] [PubMed] [Google Scholar]
- 50.Coutinho-Silva R, Stahl L, Raymond MN, Jungas T, Verbeke P, et al. Inhibition of chlamydial infectious activity due to P2X7R-dependent phospholipase D activation. Immunity. 2003;19:403–412. doi: 10.1016/s1074-7613(03)00235-8. [DOI] [PubMed] [Google Scholar]
- 51.Darville T, Welter-Stahl L, Cruz C, Sater AA, Andrews CW, Jr, et al. Effect of the purinergic receptor P2X7 on Chlamydia infection in cervical epithelial cells and vaginally infected mice. J Immunol. 2007;179:3707–3714. doi: 10.4049/jimmunol.179.6.3707. [DOI] [PubMed] [Google Scholar]
- 52.Chaves SP, Torres-Santos EC, Marques C, Figliuolo VR, Persechini PM, et al. Modulation of P2X7 purinergic receptor in macrophages by Leishmania amazonensis and its role in parasite elimination. Microbes Infect. 2009;11:842–849. doi: 10.1016/j.micinf.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 53.Correa G, da Silva C M, Moreira-Souza AC, Vommaro RC, Coutinho-Silva, R Activation of the P2X7 receptor triggers the elimination of Toxoplasma gondii tachyzoites from infected macrophages. Microbes Infect. 2010;12:497–504. doi: 10.1016/j.micinf.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 54.Lees MP, Fuller SJ, McLeod R, Boulter NR, Miller CM, et al. P2X7 receptor-mediated killing of an intracellular parasite, Toxoplasma gondii by human and mouse macrophages. J Immunol. 2010;184:7040–7046. doi: 10.4049/jimmunol.1000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jamieson SE, Peixoto-Rangel AL, Hargrave AC, Roubaix L-A, Mui EJ, et al. Evidence for associations between the purinergic receptor P2X7 (P2RX7) and toxoplasmosis. Genes and Immunity. 2010;11:374–383. doi: 10.1038/gene.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller CM, Zakrzewski AM, Ikin RJ, Boulter NR, Katrib M, et al. Dysregulation of the inflammatory response to the parasite Toxoplasma gondii in P2X7 receptor-deficient mice. Int J Parasitol. 2011;41:301–308. doi: 10.1016/j.ijpara.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 57.Adriouch S, Dox C, Welge V, Seman M, Koch-Noble F, et al. A natural P451L mutation in the cytoplasmic domain impairs the function of the mouse P2X7 receptor. J Immunol. 2002;169:4108–4112. doi: 10.4049/jimmunol.169.8.4108. [DOI] [PubMed] [Google Scholar]
- 58.Yip L, Woehrle T, Corriden R, Hirsh M, Chen Y, et al. Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. FASEB J. 2009;23:1685–1693. doi: 10.1096/fj.08-126458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Suadicani SO, Iglesias R, Spray DC, Scemes E. ASN Neuro Apr . 1. 14 1. 2009. Point mutation in the mouse P2X7 receptor affects intercellular calcium waves in astrocytes. p. pii: e00005. doi: 10.1042/AN20090001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Asward F, Dennert G. P2X7 receptor expression levels determine lethal effects of a purine based danger signal in T lymphocytes. Cell Immunol. 2006;243:58–65. doi: 10.1016/j.cellimm.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hong S, Schwarz N, Brass A, Seman M, Haag F, et al. Differential regulation of P2X7 receptor activation by extracellular nicotinamide adenine dinucleotide and ecto-ADP-ribosyltransferases in murine macrophages and T cells. J Immunol. 2009;183:578–592. doi: 10.4049/jimmunol.0900120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suzuki Y, Sher A, Yap G, Park D, Neyer LE, et al. IL-10 is required for prevention of necrosis in the small intestine and mortality in both genetically resistant BALB/c and susceptible C57BL/6 mice following peroral infection with Toxoplasma gondii. . J Immunol. 2000;164:5375–5382. doi: 10.4049/jimmunol.164.10.5375. [DOI] [PubMed] [Google Scholar]
- 63.Miller CM, Boulter NR, Ikin RJ, Smith NC. The immunobiology of the innate response to Toxoplasma gondii. Int J Parasitol. 2009;39:23–39. doi: 10.1016/j.ijpara.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 64.Wen AY, Sakamoto KM, Miller LS. The role of the transcription factor CREB in immune function. J Immunol. 2010;185:6413–6419. doi: 10.4049/jimmunol.1001829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brautigam VM, Frasier C, Nikodemova M, Watters JJ. Purinergic receptor modulation of BV-2 microglial cell activity: Potential involvement of p38 MAP kinase and CREB. J Neuroimmunol. 2005;166:113–125. doi: 10.1016/j.jneuroim.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 66.Heussler VT, Machado J, Jr, Fernandez PC, Botteron C, Chen CG, et al. The intracellular parasite Theileria parva protects infected T cells from apoptosis. Proc Natl Acad Sci USA. 1999;96:7312–7317. doi: 10.1073/pnas.96.13.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moore KJ, Matlashewski G. Intracellular infection by Leishmania donovani inhibits macrophage apoptosis. J Immunol. 1994;152:2930–2937. [PubMed] [Google Scholar]
- 68.Aga E, Katschinski DM, van Zandbergen G, Laufs H, Hansen B, et al. Inhibition of the spontaneous apoptosis of neutrophil granulocytes by the intracellular parasite Leishmania major. J Immunol. 2002;169:898–905. doi: 10.4049/jimmunol.169.2.898. [DOI] [PubMed] [Google Scholar]
- 69.Chen XM, Levine SA, Splinter PL, Tietz PS, Ganong AL, et al. Cryptosporidium parvum activates nuclear factor kappa B in biliary epithelia preventing epithelial cell apoptosis. Gastroenterology. 2001;120:1774–1783. doi: 10.1053/gast.2001.24850. [DOI] [PubMed] [Google Scholar]
- 70.Liu J, Enomoto S, Lancto CA, Abrahamsen MS, Rutherford MS. Inhibition of apoptosis in Cryptosporidium parvum-infected intestinal epithelial cells is dependent on surviving. Infect Immun. 2008;76:3784–3792. doi: 10.1128/IAI.00308-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.del Aguila C, Izquierdo F, Granja AG, Hurtado C, Fenoy S, et al. Encephalitozoon microsporidia modulates p53-mediated apoptosis in infected cells. Int J Parasitol. 2006;36:869–876. doi: 10.1016/j.ijpara.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 72.Eskra L, Mathison A, Splitter G. Microarray analysis of mRNA levels from RAW 264.7 macrophages infected with Brucella abortus. Infect Immun. 2003;71:1125–1133. doi: 10.1128/IAI.71.3.1125-1133.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gross A, Terraza A, Ouahrani-Bettache S, Liautard JP, Dornand J. In vitro Brucella suis infection prevents the programmed cell death of human monocytic cells. Infect Immun. 2000;68:342–351. doi: 10.1128/iai.68.1.342-351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Toure-Balde A, Sarthou JL, Aribot G, Michel P, Trape JF, et al. Plasmodium falciparum induces apoptosis in human mononuclear cells. Infect Immun. 1996;64:744–750. doi: 10.1128/iai.64.3.744-750.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pino P, Vouldoukis I, Kolb JP, Mahmoudi N, Desportes-Livage I, et al. Plasmodium falciparum-infected erythrocyte adhesion induces caspase activation and apoptosis in human endothelial cells. J Infect Dis. 2003;187:1283–1290. doi: 10.1086/373992. [DOI] [PubMed] [Google Scholar]
- 76.Singh BN, Lucas JJ, Hayes GR, Kumar I, Beach DH, et al. Tritrichomonas foetus induces apoptotic cell death in bovine vaginal epithelial cells. Infect Immun. 2004;72:4151–4158. doi: 10.1128/IAI.72.7.4151-4158.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lucas JJ, Hayes GR, Kalsi HK, Gilbert RO, Choe Y, et al. Characterization of a cysteine protease from Tritrichomonas foetus that induces host-cell apoptosis. Arch Biochem Biophys. 2008;477:239–243. doi: 10.1016/j.abb.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 78.Braun JS, Novak R, Murray PJ, Eischen CM, Susin SA, et al. Apoptosis-inducing factor mediates microglial and neuronal apoptosis caused by Pneumococcus. J Infect Dis. 2001;184:1300–1309. doi: 10.1086/324013. [DOI] [PubMed] [Google Scholar]
- 79.Colino J, Snapper CM. Two distinct mechanisms for induction of dendritic cell apoptosis in response to intact Streptococcus pneumoniae. . J Immunol. 2003;171:2354–2365. doi: 10.4049/jimmunol.171.5.2354. [DOI] [PubMed] [Google Scholar]
- 80.Takamatsu R, Takeshima E, Ishikawa C, Yamamoto K, Teruya H, et al. Inhibition of Akt/GSK3beta signalling pathway by Legionella pneumophila is involved in induction of T-cell apoptosis. Biochem J. 2010;427:57–67. doi: 10.1042/BJ20091768. [DOI] [PubMed] [Google Scholar]
- 81.Lüder CG, Gross U. Apoptosis and its modulation during infection with Toxoplasma gondii: molecular mechanisms and role in pathogenesis. Curr Top Microbiol Immunol. 2005;289:219–237. doi: 10.1007/3-540-27320-4_10. [DOI] [PubMed] [Google Scholar]
- 82.Roy CR, Berger KH, Isberg RR. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol Microbiol. 1998;28:663–674. doi: 10.1046/j.1365-2958.1998.00841.x. [DOI] [PubMed] [Google Scholar]
- 83.Clemens DL, Lee BY, Horwitz MA. Virulent and avirulent strains of Francisella tularensis prevent acidification and maturation of their phagosomes and escape into the cytoplasm in human macrophages. Infect Immun. 2004;72:3204–3217. doi: 10.1128/IAI.72.6.3204-3217.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clemens DL, Lee BY, Horwitz MA. Francisella tularensis phagosomal escape does not require acidification of the phagosome. Infect Immun. 2009;77:1757–1773. doi: 10.1128/IAI.01485-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Desjardins M, Descoteaux, A Inhibition of phagolysosomal biogenesis by the Leishmania lipophosphoglycan. J Exp Med. 1997;185:2061–2068. doi: 10.1084/jem.185.12.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Casabulho CM, Menna-Barreto RF, Coutinho-Silva R, Persechini PM, Henriques-Pons A. P2X7 modulatory web in Trypanosoma cruzi infection. Parasitol Res. 2008;103:829–838. doi: 10.1007/s00436-008-1063-8. [DOI] [PubMed] [Google Scholar]
- 87.Zaborina O, Misra N, Kostal J, Kamath S, Kapatral V, et al. P2Z-independent and P2Z receptor-mediated macrophage killing by Pseudomonas aeruginosa isolated from cystic fibrosis patients. Infect Immun. 1999;67:5231–5242. doi: 10.1128/iai.67.10.5231-5242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zaborina O, Dhiman N, Ling Chen M, Kostal J, Holder IA, et al. Secreted products of a nonmucoid Pseudomonas aeruginosa strain induce two modes of macrophage killing: external-ATP-dependent, P2Z-receptor-mediated necrosis and ATP-independent, caspase-mediated apoptosis. Microbiol. 2000;146:2521–2530. doi: 10.1099/00221287-146-10-2521. [DOI] [PubMed] [Google Scholar]
- 89.Punj V, Zaborina O, Dhiman N, Falzari K, Bagdasarian M, et al. Phagocytic cell killing mediated by secreted cytotoxic factors of Vibrio cholerae. Infect Immun. 2000;68:4930–4937. doi: 10.1128/iai.68.9.4930-4937.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Franchi L, Kanneganti TD, Dubyak GR, Núñez G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem. 2007;282:18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]