Abstract
We disrupted the rtxC gene on the chromosome of Bradyrhizobium elkanii USDA94 by insertion of a nonpolar aph cartridge. The rtxC mutant, designated ΔrtxC, produced serinol and dihydrorhizobitoxine but no rhizobitoxine, both in culture and in planta. The introduction of cosmids harboring the rtxC gene into the ΔrtxC mutant complemented rhizobitoxine production, suggesting that rtxC is involved in the final step of rhizobitoxine biosynthesis in B. elkanii USDA94. Glycine max cv. Lee inoculated with ΔrtxC or with a null mutant, Δrtx::Ω1, showed no foliar chlorosis, whereas the wild-type strain USDA94 caused severe foliar chlorosis. The two mutants showed significantly less nodulation competitiveness than the wild-type strain on Macroptilium atropurpureum. These results indicate that dihydrorhizobitoxine, the immediate precursor of the oxidative form of rhizobitoxine, has no distinct effect on nodulation phenotype in these legumes. Thus, desaturation of dihydrorhizobitoxine by rtxC-encoded protein is essential for the bacterium to show rhizobitoxine phenotypes in planta. In addition, complementation analysis of rtxC by cosmids differing in rtxC transcription levels suggested that rhizobitoxine production correlates with the amount of rtxC transcript.
Recent studies have suggested that rhizobia adopt at least two strategies to reduce the amount of ethylene synthesized by their host legumes and thus decrease the negative effect of ethylene on nodulation (15). 1-Aminocyclopropane-1-carboxylate (ACC) deaminase, which degrades ACC (an immediate precursor of ethylene) into ammonia and α-ketobutyrate, has been found and well characterized in plant growth-promoting rhizobacteria (9, 36). The ACC deaminase of plant growth-promoting rhizobacteria reduces the amount of ACC in associated roots and promotes root elongation via the reduction of ethylene production (8). Genes encoding ACC deaminase have also been found in some rhizobia, including Rhizobium leguminosarum, Mesorhizobium loti, and Bradyrhizobium japonicum (17). In R. leguminosarum bv. viciae, ACC deaminase has been confirmed to enhance nodulation of Pisum sativum (16).
Another strategy used by some strains of rhizobia to lower ethylene is the production of rhizobitoxine [2-amino-4-(2-amino-3-hydropropoxy)-trans-but-3-enoic acid] (27). Thus far, Bradyrhizobium elkanii and the plant pathogen Burkholderia andropogonis are known as rhizobitoxine-producing bacteria (18, 22). The biochemical action of rhizobitoxine is to inhibit ACC synthase (39) and β-cystathionase (31). Rhizobitoxine is regarded as a phytotoxin because it induces chlorosis in a variety of plants (13, 25). However, recent studies have shown that it has a positive role in the symbiosis between B. elkanii strains and their host legumes. Enhanced nodulation and competitiveness by rhizobitoxine production in B. elkanii have been reported so far for three legumes: Amphicarpaea edgeworthii (28), Vigna radiata (4), and Macroptilium atropurpureum (41).
The biosynthetic genes of rhizobitoxine have been identified for B. elkanii USDA61 (32-34) and USDA94 (38). For a previous paper (38), members of our laboratory cloned and sequenced the genetic locus involved in rhizobitoxine biosynthesis and suggested that at least rtxA and rtxC are necessary for rhizobitoxine production in B. elkanii USDA94. A large-deletion mutant of B. elkanii, USDA94Δrtx::Ω1, which lacks rtxA, rtxC, and two other open reading frames (ORFs), did not produce rhizobitoxine, dihydrorhizobitoxine, or serinol. A cosmid containing a kanamycin resistance cassette within the rtxC gene complemented the production of serinol and dihydrorhizobitoxine but not of rhizobitoxine. The deduced amino acid sequence of rtxC showed 31% similarity to Pseudomonas fatty acid desaturase. From these results, we proposed that rtxC protein is involved in desaturation of dihydrorhizobitoxine, although possible polar effects of the kanamycin resistance cassette and cosmid instability may have complicated this interpretation.
A large amount of dihydrorhizobitoxine, an oxidative form of rhizobitoxine, is generally coproduced with rhizobitoxine (20, 26). Biochemical analysis indicates that the ability of dihydrorhizobitoxine to inhibit ACC synthase and β-cystathionase is approximately 99% less than that of rhizobitoxine (39). However, no conclusive data have been reported on the biological effects of dihydrorhizobitoxine.
The first objective of this work was to verify the involvement of rtxC in rhizobitoxine biosynthesis by chromosomal nonpolar mutation and complementation analysis. Our second objective was to assess the biological effect of dihydrorhizobitoxine on host legumes, separately from that of rhizobitoxine, in terms of enhancement of nodulation competitiveness and development of chlorosis symptoms, by using wild-type and rtxC mutants of B. elkanii USDA94.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. B. elkanii cultures were grown aerobically at 30°C in HM medium (3) supplemented with 0.1% arabinose and 0.025% yeast extract (Difco, Detroit, Mich.) or in Tris-YMRT medium (21). Escherichia coli cells were grown at 37°C in Luria-Bertani medium (35). The following antibiotics were added to media at the indicated concentrations: for B. elkanii, kanamycin at 150 mg liter−1 and spectinomycin and streptomycin at 250 mg liter−1; for E. coli, tetracycline at 12.5 mg liter−1, ampicillin at 100 mg liter−1, and kanamycin at 50 mg liter−1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Reference or Source |
|---|---|---|
| Bacterial strains | ||
| B. elkanii | ||
| USDA94 | Wild-type strain producing high concentrations of rhizobitoxine in culture, Tcr Kms | Keyserb |
| MA941 | gusA-marked USDA94 | 40 |
| ΔrtxC | rtxC::aph derivative of USDA94, Smr Spr | This study |
| Δrtx::Ω1 | USDA94, nodPQ rtxA rtxC (ORF1), ORF2, ORF3::del/ins Ω cassette, Smr Spr | 38 |
| USDA61 | Wild-type strain producing rhizobitoxine in culture, Kms | 32 |
| RX18E | Tn5 mutant derivative of USDA61, rhizobitoxine production deficient, Kmr | 32 |
| E. coli | ||
| JM109 | recA, cloning strain | Toyoboc |
| DH5α | recA, cloning strain | Toyoboc |
| Plasmids | ||
| pRK2013 | ColE1 replicon carrying RK2 transfer genes, Kmr | 6 |
| pBluescript II (SK+) | Cloning vector, Apr | Takarad |
| pSUP202 | pBR325 carrying oriT from RP4, Apr Cmr Tcr | 37 |
| pUC4-KIXX | Plasmid carrying 1.6-kb aph cassette, Apr Kmr | Pharmacia |
| pCR2.1 | Cloning vector, Apr Kmr | Invitrogen |
| pHP45Ω | Plasmid carrying 2.1-kb aph cassette, Smr Spr Apr | 5 |
| pBS-3.4 | pBluescript II SK(+) containing 3.4-kb BglII fragment | This study |
| pSUP-rtxC::aph | pSUP202 carrying a 4-kb fragment, rtxC::aph | This study |
| pLAFR1 | Broad-host-range cosmid, IncP, Tcr | 7 |
| pRTF1 | pLAFR1 containing rtxA and flanking regions from B. elkanii USDA94 | 38 |
| pLAFRSS | pLAFR1 carrying 1.1-kb aad, Smr Spr Tcr | This study |
| pLAFRSS-rtxC | pLAFRSS containing 1.9-kb rtxC and flanking regions from B. elkanii USDA94 | This study |
| pLAFRSS-PahprtxC | pLAFRSS carrying aph promoter-fused rtxC | This study |
Apr, ampicillin resistant; Tcr, tetracycline resistant; Kmr, kanamycin resistant; Kms, kanamycin sensitive; Smr, streptomycin resistant; Spr, spectinomycin resistant; Cmr, chloramphenicol resistant; aph, aminoglycosidase-3′- O-phosphotransferase gene; aad, aminoglycoside adenyltransferase gene.
H. H. Keyser, U.S. Department of Agriculture, Beltsville, Md.
Toyobo Inc., Tokyo, Japan.
Takara Shuzo Co., Kusatsu, Japan.
DNA manipulations.
Isolation of plasmid DNA, restriction enzyme digestion, DNA ligation, bacterial transformation of E. coli, and Southern blot hybridization were performed as described by Sambrook et al. (35). DNA sequencing was performed by using dye primer technology and a model 373A sequencer (Perkin-Elmer Applied Biosystems, Warrington, United Kingdom).
Construction of nonpolar rtxC mutants of B. elkanii.
For the construction of the nonpolar rtxC mutant, a 3.4-kb BglII fragment carrying the rtxC ORF and flanking region from cosmid pRTF1 (38) was cloned into the BamHI site of pBluescript II SK(+). The resultant plasmid, pBS-3.4, was digested by BamHI to remove the 0.5-kb fragment within the rtxC ORF, and a 5.9-kb fragment was extracted from an agarose gel. The resulting fragment was ligated with a nonpolar aminoglycoside 3′-phosphotransferase (aph) cartridge generated by PCR as follows. Two primers derived from the aph sequences aph-1 (5′-TTCATAGGATCCAAGCCAGTCCGAAGAAACG-3′) and aph-2 (5′-TTATGGATCCTCAGAAGAACTCGTCAAG-3′), containing the newly created BamHI sites (underlined), were used to amplify the aph gene without the transcription terminator by PCR from template pUC4-KIXX (Amersham-Pharmacia Biotech, Uppsala, Sweden). The amplified fragment (nonpolar aph cartridge) was digested by BamHI and then ligated with the 5.9-kb fragment described above, yielding pBS-rtxC::aph. Plasmid pBS-rtxC::aph was digested with SpeI and HindIII, and a 4-kb fragment containing rtxC::aph was blunted and recloned into the EcoRV site of a pSUP202 suicide vector (37), yielding pSUP-rtxC::aph (Fig. 1). Plasmid pSUP-rtxC::aph was introduced into B. elkanii USDA94 by triparental mating, with pRK2013 as a helper plasmid (6). Crossover mutants were selected on HM medium containing 150 mg of kanamycin per liter, and a double-crossover mutant was identified by Southern blot hybridization with the 3.4-kb BglII fragment as a probe.
FIG. 1.
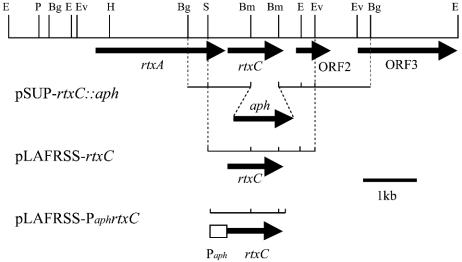
Physical and restriction enzyme map of the rtxC gene and its flanking region in B. elkanii USDA94. Arrows denote ORFs. The internal 396-bp BamHI fragment of rtxC was removed and replaced with the aph gene in pSUP-rtxC::aph. For complementation analysis, pLAFRSS-rtxC and pLAFRSS-PaphrtxC were constructed. About 350 bp of the aph promoter region was fused with rtxC in pLAFRSS-PaphrtxC. Abbreviations: Bm, BamHI; Bg, BglII; E, EcoRI; Ev, EcoRV; P, PstI; S, SmaI.
Construction of cosmids for complementation of rtxC.
Because B. elkanii strains possess high levels of resistance to tetracycline, a pLAFR1 cosmid (7) with a tetracycline selection marker was not available for transconjugation. Therefore, we conferred streptomycin and spectinomycin resistance on pLAFR1 by introducing the aminoglycoside adenyltransferase (aad) gene. The aad gene was amplified by PCR from plasmid pHP45Ω (5) with the primers aad-F (5′-TCATAGGATCCGAATTCATAAGCCTGTTC-3′; the restriction sites of BamHI and EcoRI are underlined and double underlined, respectively) and aad-R (5′-TTATGGATCCTTATTTGCCG-3′; the BamHI restriction site is underlined). The 1.1-kb PCR products obtained were digested with BamHI, blunted, and cloned into the EcoRI site of pLAFR1, yielding cosmid pLAFRSS.
For complementation analysis of rtxC, two cosmids were constructed as follows. pBS3.4 was digested with SmaI and EcoRV, and a 2.0-kb fragment containing the rtxC ORF was isolated, blunted with the Klenow fragment, and ligated with pLAFRSS, which was then digested with EcoRI followed by blunting with the Klenow fragment, generating pLAFRSS-rtxC (Fig. 1).
For assembly of aph promoter-fused rtxC, we used the overlap-extension PCR technique (2). First, the aph promoter region (fragment 1) and rtxC gene (fragment 2) were separately amplified with the following primers, which were designed to create complementarity between the 3′ end of fragment 1 and the 5′ end of fragment 2. Fragment 1 was amplified with the primers prtxC-1 (5′-GCCCTTCGAGTCCATCTAGAAAGCCAGTCCGC-3′; the XbaI restriction site is underlined) and prtxC-2 (5′-GCGTCTGCCTGATTCATGCGAAACGATCC-3′), using pUC4-KIXX as a template. Fragment 2 was amplified with the primers prtxC-3 (5′-GAGGATCGTTTCGCATGAATCAGGCAGACGC-3′) and prtxC-4 (5′-CAATCTAGTTATCACTGCAGTCACGCCACGTGCCCC-3′; the PstI restriction site is underlined), using pBS-3.4 as a template. Both fragments 1 and 2 were purified from an agarose gel with a QIAEX II gel extraction kit (Qiagen, Hilden, Germany). Second, fragments 1 and 2 were mixed and subjected to template annealing and extension reactions, as described elsewhere (2). Finally, hybrid DNA of fragments 1 and 2 (aph promoter-fused rtxC) was amplified by using the products of the second step as a template, with primers prtxC-1 and prtxC-4. The amplified fragments were cloned and sequenced in pBluescript II SK(+), followed by cloning into the EcoRI site of pLAFRSS, generating pLAFRSS-PaphrtxC (Fig. 1).
RNA isolation and reverse transcription (RT)-PCR analysis.
For RNA isolation, B. elkanii strains were cultured in 15 ml of Tris-YMRT medium at 30°C for 7 days, yielding late-log-phase cells (optical density at 600 nm, 0.5 to 0.6). Total RNA of B. elkanii cells was prepared by use of RNAWIZ (Ambion Inc., Austin, Texas) according to the manufacturer's instructions. To remove intact DNA completely, the resultant total RNA extract was treated with DNase I (Takara, Tokyo, Japan) for 30 min at 37°C, followed by purification with an RNeasy mini kit (Qiagen). The resultant total RNA was resuspended in RNase-free water and then quantified by its A260 with a spectrophotometer.
RT-PCR was performed with a One-Step RT-PCR kit (Qiagen) according to the manufacturer's instructions. We used 50 ng of total RNA as the template in a total reaction volume of 50 μl. The primers rtxC-7 (5′-GCGGTACGCCTTCAATATCA-3′) and rtxC-8 (5′-TCCGATTCCTGGTTAGATA-3′) were used in the PCR to amplify a 331-bp product from the rtxC transcript. As controls, aad-1 (5′-GCGAGATTCTCCGCGCTGTA-3′) and aad-2 (5′-AGCTTCAAGTATGACGGG-3′) were used to amplify a 445-bp product from the aad gene in the vector pLAFRSS.
Plant assays.
The host legumes used were Glycine max cv. Lee, V. radiata (mung bean), and A. edgeworthii. Mung bean and soybean seeds were surface sterilized with 0.5% hydrogen peroxide for 1 min and then washed 10 times with sterile distilled water. The surface-sterilized seeds were sown in a 300-ml plant box (CUL-JAR300; Iwaki, Tokyo, Japan) containing sterile vermiculite and watered with a nitrogen-free plant nutrient solution (1). Seeds of A. edgeworthii were scarified, surface sterilized by immersion in concentrated sulfuric acid for 38 min, rinsed 10 times with sterile water, and then planted in sterile growth pouches (Northrup King, Minneapolis, Minn.). Rhizobial inocula were cultured for 7 days at 30°C in HM medium containing 0.1% arabinose, 0.025% yeast extract, and either 150 mg of kanamycin per liter (for ΔrtxC) or 250 mg of spectinomycin per liter (for Δrtx::Ω1). The cells were collected by centrifugation at 5,000 × g for 10 min at room temperature and then were washed twice with sterile water. The number of cells was adjusted to 107 ml−1 in sterile water by direct counting with a Thoma hemocytometer (Kayagaki Irika Kogyo Co. Ltd., Tokyo, Japan), and the cells were inoculated onto the seeds at 107 cells per seed. Plants were grown in a plant growth cabinet (LH300; NK Systems Co. Ltd., Osaka, Japan) under a light-dark cycle of 16 h of light and 8 h of dark at 25°C.
Competitive nodulation assay.
The nodulation competitiveness of B. elkanii mutants was evaluated on Macroptilium atropurpureum Urb. cv. Siratro by using B. elkanii MA941, a gusA-marked strain of USDA94, as a reference strain. Seeds obtained from Yukijirushi Shubyo Co. (Hokkaido, Japan) were surface sterilized with 70% ethanol for 5 min and then with 3% hydrogen peroxide for 1 min; they were washed 10 times with sterile distilled water after each treatment. The surface-sterilized seeds were sown in a 300-ml plant box (CUL-JAR300; Iwaki) containing sterile vermiculite and were watered with a nitrogen-free plant nutrient solution (1). Strain USDA94, ΔrtxC, or Δrtx::Ω1 was mixed with strain MA941 at a concentration of 107 cells ml−1 for each strain (cell ratio, 1:1). We then inoculated 1 ml of each mixture onto the Siratro seeds. Plants were grown in a plant growth cabinet as described above. After 21 days of cultivation, all nodules of >1 mm in diameter were collected, and the nodule occupancy ratios of USDA94, ΔrtxC, and Δrtx::Ω1 to MA941 were determined by a β-glucuronidase assay, with X-Gluc (5-bromo-4-chloro-3-indolyl-β-d-glucuronide cyclohexylammonium salt; Wako Pure Chemical Industries Ltd., Osaka, Japan) as a substrate, as described previously (40). Approximately 100 nodules from three or four plants were examined by β-glucuronidase assay for each inoculation treatment. The chi-square test was used for statistical analysis at a confidence level of 0.05.
Determination of serinol, dihydrorhizobitoxine, and rhizobitoxine contents in culture and plants.
Serinol, dihydrorhizobitoxine, and rhizobitoxine were isolated from the cultures as described previously (38). B. elkanii strains were grown in 15 ml of Tris-YMRT medium at 30°C for 21 days. Culture supernatants were collected by centrifugation and loaded on a Dowex 50 column (H+ type; resin size, 50/100-mesh; column volume, 5 ml) (Muromachi Chemicals, Tokyo, Japan). The column was washed with 10 column volumes of deionized water. Serinol, dihydrorhizobitoxine, and rhizobitoxine were eluted with three column volumes of 2 M NH4OH and dried in a vacuum. Pellets were dissolved in 500-μl aliquots of deionized water.
Serinol, dihydrorhizobitoxine, and rhizobitoxine contents in G. max cv. Lee inoculated with B. elkanii strains were determined. These compounds were extracted from plant materials as described by Minamisawa and Watanabe (19). Thirty days after sowing and inoculation, nodules and upper shoots (from the third trifoliate leaves, where the chlorosis symptoms were severe) were harvested and weighed, and then the extracts were purified through a Dowex 50 column (20, 21). Purified serinol, dihydrorhizobitoxine, and rhizobitoxine were assayed by liquid chromatography-mass spectrometry as described previously (38).
RESULTS
Construction and characterization of nonpolar rtxC mutant of B. elkanii USDA94.
Members of our laboratory had proposed previously that the rtxC gene was responsible for desaturation of dihydrorhizobitoxine, on the basis of sequence homology to fatty acid desaturase and insertional mutagenesis achieved by inserting a cosmid with a kanamycin resistance cassette into rtxC (38). However, cosmid pLAFR1 is not always stable in rhizobial cells in planta, and the possible polar effects of the kanamycin resistance cassette restricted the interpretation. To verify the function of rtxC more directly, we chromosomally inserted a nonpolar aph cartridge into the BglII site of rtxC in the same transcriptional orientation (Fig. 1), generating a nonpolar rtxC mutant.
The nonpolar rtxC mutant, designated ΔrtxC, was tested for production of rhizobitoxine, dihydrorhizobitoxine, and serinol in Tris-YMRT culture supernatants (Table 2). As expected, ΔrtxC produced serinol and dihydrorhizobitoxine, but no rhizobitoxine. This result clearly demonstrates that rtxC encodes dihydrorhizobitoxine desaturase as a final step of rhizobitoxine biosynthesis.
TABLE 2.
Serinol, dihydrorhizobitoxine, and rhizobitoxine production by B. elkanii USDA94 and rhizobitoxine-deficient mutants in culture and in nodules of G. max cv. Lee
| B. elkanii strain | Production in culture supernatant (μM)b
|
Production in nodule extract (μmol/g of fresh wt)b
|
||||
|---|---|---|---|---|---|---|
| Serinol | DRTa | RTa | Serinol | DRT | RT | |
| USDA94 | 221 ± 15 | 106 ± 11 | 9.1 ± 3.7 | 14.9 ± 1.7 | 15.8 ± 0.8 | 1.3 ± 0.3 |
| ΔrtxC | 182 ± 21 | 48 ± 6 | <0.1 | 17.6 ± 1.8 | 11.2 ± 0.6 | <0.05 |
| Δrtx::Ω1 | <3.2 | <2.7 | <0.1 | <0.07 | <0.5 | <0.05 |
| ΔrtxC(pLAFRSS) | 88 ± 28 | 62 ± 11 | <0.1 | ND | ND | ND |
| ΔrtxC(pLAFRSS-rtxC) | 107 ± 29 | 33 ± 9 | 1.6 ± 1.1 | ND | ND | ND |
| ΔrtxC(pLAFRSS-PaphrtxC) | 134 ± 34 | 27 ± 13 | 3.5 ± 1.5 | ND | ND | ND |
DRT, dihydrorhizobitoxine; RT, rhizobitoxine.
Data are means ± SD; ND, not determined.
Complementation analysis of rtxC.
The newly constructed cosmid pLAFRSS, derived from pLAFR1, was introduced into B. elkanii USDA94 and mutant ΔrtxC by triparental mating, followed by selection with 250 mg of spectinomycin and streptomycin per liter. The resultant bacterial colonies were grown in HM liquid medium with 250 mg of spectinomycin per liter at 30°C for 7 days, and cell lysates were prepared for PCR amplification of the aad gene with aad-F and aad-R primers (data not shown). As a result, all transconjugants tested carried cosmid pLAFRSS, indicating that this cosmid could be maintained in B. elkanii cells and that transconjugants could be selected by spectinomycin and streptomycin.
Two cosmids, pLAFRSS-rtxC and pLAFRSS-PaphrtxC, were constructed for rtxC complementation and were introduced into ΔrtxC. The validity of cosmid constructs was checked by PCR, and rtxC transcripts were detected by RT-PCR (Fig. 2; see below). After complementation with either cosmid, transcripts of rtxC were recovered from cells cultured for 7 days in Tris-YMRT medium.
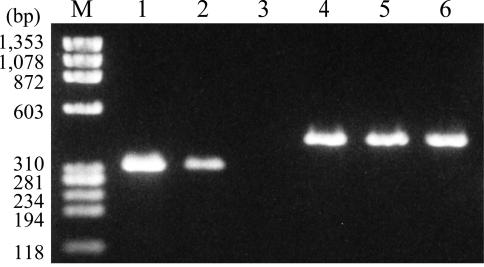
FIG. 2.
Determination of rtxC gene expression by RT-PCR. RNA was isolated from the rtxC-disrupted mutant ΔrtxC complemented with pLAFRSS-PaphrtxC (lanes 1 and 4), pLAFRSS-rtxC (lanes 2 and 5), or pLAFRSS (lanes 3 and 6). RT-PCR was carried out with gene-specific primers for rtxC (lanes 1 to 3) and aad (lanes 4 to 6), as described in Materials and Methods. M, size marker. Three independent experiments showed similar results.
The rhizobitoxine content of Tris-YMRT culture supernatants was examined after 21 days of cultivation. Rhizobitoxine production was restored in ΔrtxC strains complemented with pLAFRSS-rtxC or pLAFRSS-PaphrtxC (Table 2). The amounts of rhizobitoxine produced in the supernatants differed between the complemented cosmids (pLAFRSS-rtxC, 1.6 μM; pLAFRSS-PaphrtxC, 3.5 μM), and both amounts were lower than that of the wild-type strain.
Transcriptional analysis of complemented strains.
Total RNA was isolated from ΔrtxC strains complemented with pLAFRSS, pLAFRSS-rtxC, and pLAFRSS-PaphrtxC, and the expression of rtxC in these strains was tested by RT-PCR using the rtxC-specific primers rtxC-7 and rtxC-8. The same template RNA samples were also subjected to RT-PCR using primers for the aad gene to confirm the integrity of the RNA samples used. The amount of rtxC transcript in ΔrtxC(pLAFRSS-PaphrtxC) was higher than that in ΔrtxC(pLAFRSS-rtxC), whereas transcription levels of aad were similar in both strains (Fig. 2). The lower level of rtxC transcripts presumably reflected intrinsic transcription from the cosmid vector.
Chlorosis and rhizobitoxine productivity on G. max cv. Lee.
G. max cv. Lee is a soybean cultivar that is susceptible to chlorosis caused by rhizobitoxine (12). Plants inoculated with USDA94 showed foliar chlorosis on newly forming leaves from 20 days after inoculation, and by 30 days after inoculation these symptoms had worsened (Fig. 3). Inoculation with the null mutant Δrtx::Ω1, which does not produce serinol, dihydrorhizobitoxine, or rhizobitoxine (Table 2), produced no symptoms of chlorosis 30 days after inoculation. Similarly, plants inoculated with ΔrtxC showed no chlorosis symptoms. Other parameters, such as number of nodules, nodule weight, and plant fresh weight, were not significantly different between plants inoculated with Δrtx::Ω1 and ΔrtxC (data not shown).
FIG. 3.
Foliar chlorosis of G. max cv. Lee inoculated with B. elkanii USDA94 (A), ΔrtxC (B), and Δrtx::Ω1 (C), photographed 30 days after inoculation. The contents of serinol in upper shoots were as follows: with USDA94, 4.5; with ΔrtxC, 2.8; with Δrtx::Ω1, <0.1 (nmol/g of fresh weight). The contents of dihydrorhizobitoxine in upper shoots were as follows: with USDA94, 8.4; with ΔrtxC, 5.1; with Δrtx::Ω1, <0.2 (nmol/g of fresh weight). The contents of rhizobitoxine in upper shoots were as follows: with USDA94, 3.6; with ΔrtxC, <0.1; with Δrtx::Ω1, <0.1 (nmol/g of fresh weight).
We determined the rhizobitoxine, dihydrorhizobitoxine, and serinol contents of nodules and upper shoots of plants grown for 30 days after inoculation. As observed in the culture supernatants, ΔrtxC did not accumulate rhizobitoxine in the nodules or upper shoots, whereas USDA94 accumulated large amounts of rhizobitoxine in both the nodules and upper shoots (Table 2; also, see legend to Fig. 3). Plants inoculated with ΔrtxC accumulated similar amounts of dihydrorhizobitoxine to those in plants inoculated with USDA94. These results clearly indicated that chlorosis symptoms in G. max cv. Lee are caused, not by dihydrorhizobitoxine, but by rhizobitoxine.
Nodulation competitiveness of B. elkanii rhizobitoxine mutants on Macroptilium atropurpureum.
It was recently found that rhizobitoxine is a strong positive promoter of competitive nodulation in association with Macroptilium atropurpureum (24, 41). Thus, we compared the nodulation competitiveness of wild-type B. elkanii USDA94, ΔrtxC, and Δrtx::Ω1 on Macroptilium atropurpureum cv. Siratro by using MA941 as a reference strain (Fig. 4). When B. elkanii USDA94 and MA941 were mixed in the inoculum at a 1:1 ratio (107 cells of each), both strains formed similar numbers of nodules (USDA94, 47.3%; MA941, 52.7%) (Fig. 4). This indicates that gusA insertion in MA941 did not affect the nodulation competitiveness of the parent strain, USDA94. However, when ΔrtxC was coinoculated with reference strain MA941 onto Siratro roots at a 1:1 ratio, the nodule occupancy rate of ΔrtxC decreased to 25.7% (Fig. 4). Similarly, the null mutant Δrtx::Ω1 occupied 24.2% of the nodules. These nodule occupancy rates of rhizobitoxine mutants were significantly different from that of the wild-type strain, at a confidence level of 0.05. ΔrtxC produced serinol and dihydrorhizobitoxine at similar levels to those of USDA94 in soybean nodules (Table 2); therefore, the lower nodule occupancy rate of ΔrtxC was probably due to lack of rhizobitoxine productivity.
FIG. 4.
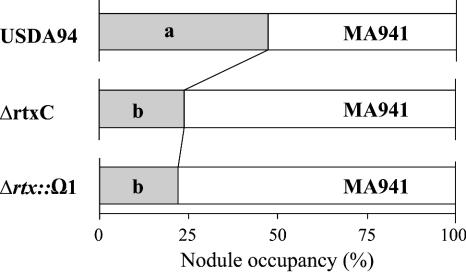
Nodule occupancy rates of B. elkanii strains USDA94, ΔrtxC, and Δrtx::Ω1 compared with that of a reference strain, MA941 (gusA-marked USDA94), on Macroptilium atropurpureum cv. Siratro. Nodule numbers per plant coinoculated with MA941 or USDA94, ΔrtxC, or Δrtx::Ω1 were 15.7 ± 4.8, 14.3 ± 4.5, and 15.2 ± 4.9 (mean ± SD), respectively. Different letters denote significant differences in nodule occupancy values between MA941 and USDA94, ΔrtxC, or Δrtx::Ω1 by the chi-square test (P = 0.05).
Nodulation profiles of B. elkanii rhizobitoxine-deficient mutants on V. radiata and A. edgeworthii.
We also examined the nodulation phenotypes of ΔrtxC and Δrtx::Ω1 on two other host legumes, V. radiata (mung bean) and A. edgeworthii. Nodulation enhancement by rhizobitoxine production has been reported for these species (4, 28). In our experiments, however, no significant differences were observed between inoculation with the wild-type strain USDA94 and inoculation with rhizobitoxine-deficient mutants in terms of nodule numbers, nodule weight, plant growth, or plant fresh weight (data not shown). Nodule numbers per plant on mung beans inoculated with USDA61, RX18E (a rhizobitoxine-deficient mutant of USDA61), and Δrtx::Ω1 (a rhizobitoxine-deficient mutant of USDA94) were 13.4 ± 1.7, 3.0 ± 1.8, and 27.3 ± 1.1 (mean ± standard deviation [SD]), respectively. This suggests that strain USDA94 lacking the ability to produce rhizobitoxine is already compatible with mung bean nodulation, which is probably due to the different strain background from that of strain USDA61 in B. elkanii.
DISCUSSION
We confirmed the function of the rtxC gene of B. elkanii in rhizobitoxine biosynthesis by nonpolar gene disruption on the chromosome to exclude possible polar effects and the effect of cosmid curing. As expected, the resulting mutant, ΔrtxC, produced serinol and dihydrorhizobitoxine, but no rhizobitoxine, and introduction of cosmids harboring rtxC complemented rhizobitoxine production in this mutant. Additionally, rhizobitoxine production in the complemented strains correlated somewhat with the amount of rtxC transcript. Therefore, we conclude that dihydrorhizobitoxine is an immediate precursor of rhizobitoxine and that rtxC is involved in the final step of rhizobitoxine biosynthesis (Fig. 5).
FIG. 5.
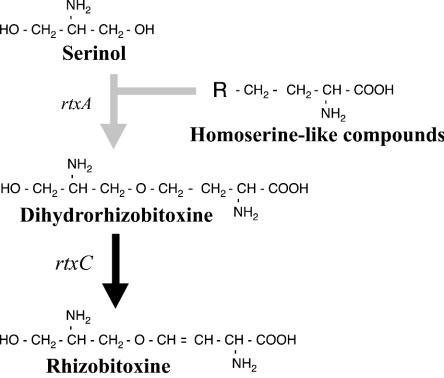
Structures of rhizobitoxine and relevant compounds in the rhizobitoxine biosynthetic pathway.
Dihydrorhizobitoxine is usually coproduced with rhizobitoxine in culture and in planta at concentrations that are >10 times higher than those of rhizobitoxine (20), but the biological effects of the two substances have not been characterized separately. Use of ΔrtxC and the null mutant Δrtx::Ω1 enabled us to separate the biological effects of dihydrorhizobitoxine and rhizobitoxine in symbiosis without the effects of cosmid curing. The accumulation of rhizobitoxine, dihydrorhizobitoxine, and serinol in plants, and the resulting nodulation profiles, provided clear insight into the fact that enhancement of nodulation competitiveness on Macroptilium atropurpureum and chlorosis induction on G. max were completely dependent on the presence of rhizobitoxine, not dihydrorhizobitoxine. Although the mechanism by which rhizobitoxine induces foliar chlorosis on soybean plants has not been elucidated fully, enhancement of nodulation competitiveness on Macroptilium atropurpureum by rhizobitoxine is known to be mediated by the reduction of ethylene evolution from the roots of the host legume (41). An in vitro enzymatic assay has shown dihydrorhizobitoxine to be approximately 99% less potent than rhizobitoxine as an inhibitor of ACC synthase (a key enzyme in plant ethylene biosynthesis) (39). This reduction in the inhibitory effect on ethylene biosynthesis resulted in the lower nodulation competitiveness of ΔrtxC. These results indicate that dihydrorhizobitoxine, the oxidative form of rhizobitoxine, has no distinct effect on nodulation phenotype in these legumes, and that introduction of a carbon double bond between C-3 and C-4 by the rtxC product is essential for the bacterium to show the rhizobitoxine phenotype in planta.
In contrast, we did not observe a significant difference between the nodulation profiles of rhizobitoxine mutants and the wild-type strain of B. elkanii USDA94 when V. radiata and A. edgeworthii were used as host plants. Other workers have suggested that rhizobitoxine plays a positive role in the establishment of compatible symbiosis between B. elkanii USDA61 and these legumes (4, 28). Our results indicate that rhizobitoxine production is not essential for the association between these legumes and USDA94. One possible explanation is that the nodulation ability of USDA94 makes it compatible with these legumes, whereas USDA61 is a less compatible partner; therefore, rhizobitoxine is probably required for effective nodulation by USDA61.
A practical aspect of rhizobitoxine production by B. elkanii is its application in the development of rhizobial inoculants. To attain a practical inoculation effect, the rhizobial inoculant has to be superior not only in the ability to fix nitrogen, but also in nodulation competitiveness with indigenous strains. Rhizobitoxine production could improve the inoculant in terms of both nodulation and competitiveness. We estimated previously that rhizobitoxine production by B. elkanii USDA94 gave the bacterium a nodulation competitiveness about 10 times higher than that of a non-rhizobitoxine-producing mutant strain on Macroptilium atropurpureum (24). Conferring the ability to produce rhizobitoxine and ACC deaminase into rhizobia would be one strategy to improve nodulation competitiveness of inoculants, because ethylene regulates nodulation negatively in various legumes, including P. sativum (10, 14), Trifolium repens (10), Medicago sativa (23, 30), Vicia sativa (11), Medicago truncatula (29), and Lotus japonicus (23). Furthering our understanding of rhizobitoxine biosynthesis will contribute to the construction of novel rhizobitoxine-producing rhizobia by genetic engineering. This in turn should be a very promising strategy for overcoming the competition problem and furthering progress towards sustainable agriculture.
Acknowledgments
This work was supported in part by a grant to K.M. from the Ministry of Education, Science, Sports and Culture of Japan (no. 11556012). We thank PROBRAIN (Japan) for supporting the research of K.M.
We are grateful to H. Ezura (Tsukuba University, Tsukuba, Japan) for his continuing interest and encouragement. We also acknowledge Matthew A. Parker (State University of New York), Nantakorn Boonkerd (Suranaree University of Technology, Nakhon Rachasima, Thailand), and N. Kent Peters (Agricultural University of Norway, Ås, Norway) for providing the seeds of A. edgeworthii, V. radiata (mung bean), and B. elkanii USDA61 derivatives, respectively.
REFERENCES
- 1.Akao, S., and H. Kouchi. 1989. Light microscopic observation of root hair curling of soybean induced by Rhizobium infection. Jpn. J. Soil Sci. Plant Nutr. 60:53-55. (In Japanese.) [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 3.Cole, M. A., and G. H. Elkan. 1973. Transmissible resistance to penicillin G, neomycin, and chloramphenicol in Rhizobium japonicum. Antimicrob. Agents Chemother. 4:248-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duodu, S., T. V. Bhuvaneswari, T. J. W. Stokkermans, and N. K. Peters. 1999. A positive role for rhizobitoxine in Rhizobium-legume symbiosis. Mol. Plant Microbe Interact. 12:1082-1089. [Google Scholar]
- 5.Fallay, R., J. Frey, and H. Kirsch. 1987. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertion mutagenesis of gram-negative bacteria. Gene 52:147-154. [DOI] [PubMed] [Google Scholar]
- 6.Figurski, D. H., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman, A. M., R. L. Sharon, S. E. Brown, W. J. Buikema, and F. M. Ausubel. 1982. Construction of broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene 18:289-296. [DOI] [PubMed] [Google Scholar]
- 8.Glick, B. R., C. B. Jacobson, and M. M. K. Schwarze. 1994. 1-Aminocyclopropane-1-carboxylic acid deaminase mutants of the plant growth promoting rhizobacterium Pseudomonas putida GR12-2 do not stimulate canola root elongation. Can. J. Microbiol. 40:911-915. [Google Scholar]
- 9.Glick, B. R., D. M. Karaturovic, and P. C. Newell. 1995. A novel procedure for rapid isolation of plant growth-promoting pseudomonads. Can. J. Microbiol. 41:533-536. [Google Scholar]
- 10.Goodlass, G., and K. A. Smith. 1979. Effects of ethylene on root extension and nodulation of pea (Pisum sativum L.) and white clover (Trifolium repens L.). Plant Soil 51:387-395. [Google Scholar]
- 11.Heidstra, R., W. C. Yang, Y. Yalcin, S. Peck, A. M. Emons, A. van Kammen, and T. Bisseling. 1997. Ethylene provides positional information on cortical cell division but is not involved in Nod factor-induced root hair tip growth in Rhizobium-legume interaction. Development 124:1781-1787. [DOI] [PubMed] [Google Scholar]
- 12.Johnson, H. W., and U. M. Means. 1960. Interactions between genotypes of soybeans and genotypes of nodulating bacteria. Agric. J. 52:651-654. [Google Scholar]
- 13.Johnson, H. W., U. M. Means, and F. E. Clark. 1959. Responses of seedlings to extracts of soybean nodules bearing selected strains of Rhizobium japonicum. Nature 183:308-309. [Google Scholar]
- 14.Lee, K. H., and T. A. LaRue. 1992. Exogenous ethylene inhibits nodulation of Pisum sativum L. cv. Sparkle. Plant Physiol. 100:11759-11763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma, W., D. M. Penrose, and B. R. Glick. 2002. Strategies used by rhizobia to lower plant ethylene levels and increase nodulation. Can. J. Microbiol. 48:947-954. [DOI] [PubMed] [Google Scholar]
- 16.Ma, W., F. C. Guinel, and B. R. Glick. 2003. Rhizobium leguminosarum biovar viciae 1-aminocyclopropane-carboxylate deaminase promotes nodulation of pea plants. Appl. Environ. Microbiol. 69:4396-4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma, W., S. B. Sebestianova, J. Sebestian, G. I. Burd, F. C. Guinel, and B. R. Glick. 2003. Prevalence of 1-aminocyclopropane-carboxylate deaminase in Rhizobium spp. Antonie Leeuwenhoek 83:285-291. [DOI] [PubMed] [Google Scholar]
- 18.Minamisawa, K. 1990. Division of rhizobitoxine-producing and hydrogen-uptake positive strains of Bradyrhizobium japonicum by nifDKE sequence. Plant Cell Physiol. 31:81-89. [Google Scholar]
- 19.Minamisawa, K., and H. Watanabe. 1986. Serinol (2-amino-1,3-propanediol) and 3-amino-1,2-propanediol in soybean nodules. Plant Cell Physiol. 27:1109-1116. [Google Scholar]
- 20.Minamisawa, K., and N. Kume. 1987. Determination of rhizobitoxine and dihydrorhizobitoxine in soybean plants by amino acid analyzer. Soil Sci. Plant Nutr. 33:645-649. [Google Scholar]
- 21.Minamisawa, K., K. Fukui, and T. Asami. 1990. Rhizobitoxine inhibition of hydrogenase synthesis in free-living Bradyrhizobium japonicum. J. Bacteriol. 172:4505-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell, R. E., E. J. Frey, and M. K. Benn. 1986. Rhizobitoxine and 1-threo-hydroxythrionine production by the plant pathogen Pseudomonas andropogonis. Phytochemistry 25:2711-2715. [Google Scholar]
- 23.Nukui, N., H. Ezura, K.-I. Yuhashi, T. Yasuta, and K. Minamisawa. 2000. Effect of ethylene precursor and inhibitors for ethylene biosynthesis and perception on nodulation in Lotus japonicus and Macroptilium atropurpureum. Plant Cell Physiol. 41:893-987. [DOI] [PubMed] [Google Scholar]
- 24.Okazaki, S., K. Yuhashi, and K. Minamisawa. 2003. Quantitative and time-course evaluation of nodulation competitiveness of rhizobitoxine-producing Bradyrhizobium elkanii. FEMS Microbiol. Ecol. 45:155-160. [DOI] [PubMed] [Google Scholar]
- 25.Owens, L. D., and D. A. Wright. 1965. Rhizobial-induced chlorosis in soybeans: isolation, production in nodules, and varietal specificity of the toxin. Plant Physiol. 40:927-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owens, L. D., J. F. Thompson, and P. V. Fennessy. 1972. Dihydrorhizobitoxine, a new ether amino-acid from Rhizobium japonicum. J. Chem. Soc. Chem. Commun. 1972:715. [Google Scholar]
- 27.Owens, L. D., J. F. Thompson, R. G. Pitcher, and T. Williams. 1972. Structure of rhizobitoxine, an antimetabolic enol-ether amino-acid from Rhizobium japonicum. J. Chem. Soc. Chem. Commun. 1972:714. [Google Scholar]
- 28.Parker, M. A., and N. K. Peters. 2001. Rhizobitoxine production and symbiotic compatibility of Bradyrhizobium from Asian and North American lineages of Amphicarpaea. Can. J. Microbiol. 47:1-6. [DOI] [PubMed] [Google Scholar]
- 29.Penmetsa, R. V., and D. R. Cook. 1997. A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science 275:527-530. [DOI] [PubMed] [Google Scholar]
- 30.Peters, N. K., and D. K. Crist-Estes. 1989. Nodule formation is stimulated by the ethylene inhibitor aminoethoxyvinylglycine. Plant Physiol. 91:690-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruan, X., and N. K. Peters. 1991. Rapid and sensitive assay for the phytotoxin rhizobitoxine. Appl. Environ. Microbiol. 57:2097-2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruan, X., and N. K. Peters. 1992. Isolation and characterization of rhizobitoxine mutants of Bradyrhizobium japonicum. J. Bacteriol. 174:3467-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruan, X., C. Zhang, and N. K. Peters. 1993. Bradyrhizobium japonicum rhizobitoxine genes and putative enzyme functions: expression requires a translational frameshift. Proc. Natl. Acad. Sci. USA 90:2641-2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruan, X., C. Zhang, and N. K. Peters. 1993. Authors' collection. Proc. Natl. Acad. Sci. USA 90:12055. [Google Scholar]
- 35.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 36.Shah, S., J. Li, B. A. Maffatt, and B. R. Glick. 1998. Isolation and characterization of ACC deaminase genes from two different plant growth-promoting bacteria. Can. J. Microbiol. 44:833-843. [PubMed] [Google Scholar]
- 37.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vitro genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology 1:789-791. [Google Scholar]
- 38.Yasuta, T., S. Okazaki, H. Mitsui, K. Yuhashi, H. Ezura, and K. Minamisawa. 2001. DNA sequence and mutational analysis of rhizobitoxine biosynthesis gene in Bradyrhizobium elkanii. Appl. Environ. Microbiol. 67:4999-5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yasuta, T., S. Satoh, and K. Minamisawa. 1999. New assay for rhizobitoxine based on inhibition of 1-aminocyclopropane-1-carboxylate synthase. Appl. Environ. Microbiol. 65:849-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuhashi, K., K. Minamisawa, Y. Minakawa, D. J. Tobis, M. Kubota, and S. Akao. 1997. Nodulation and competitiveness of gusA-marked Bradyrhizobium japonicum A1017 in soybean. Soil Sci. Plant Nutr. 43:473-478. [Google Scholar]
- 41.Yuhashi, K., N. Ichikawa, H. Ezura, S. Akao, Y. Minakawa, N. Nukui, T. Yasuta, and K. Minamisawa. 2000. Rhizobitoxine production by Bradyrhizobium elkanii enhances nodulation and competitiveness on Macroptilium atropurpureum. Appl. Environ. Microbiol. 66:2658-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]