Abstract
The mechanism of resistance to macrolides, lincosamides, and streptogramins B was studied in four Bacillus clausii strains that are mixed in a probiotic administered to humans for prevention of gastrointestinal side effects due to oral antibiotic chemotherapy and in three reference strains of B. clausii, DSM8716, ATCC 21536, and ATCC 21537. An 846-bp gene called erm(34), which is related to the erm genes conferring resistance to these antibiotics by ribosomal methylation, was cloned from total DNA of B. clausii DSM8716 into Escherichia coli. The deduced amino acid sequence presented 61% identity with that of Erm(D) from B. licheniformis, B. halodurans, and B. anthracis. Pulsed-field gel electrophoresis of total DNA digested by I-CeuI, followed by hybridization with an erm(34)-specific probe, indicated a chromosomal location of the gene in all B. clausii strains. Repeated attempts to transfer resistance to macrolides by conjugation from B. clausii strains to Enterococcus faecalis JH2-2, E. faecium HM1070, and B. subtilis UCN19 were unsuccessful.
Spores of Bacillus sp. are administered to humans for prevention of gastrointestinal side effects due to oral antibiotic therapy. The potential effects of spores are to restore an intestinal flora following destruction of commensals by antibiotics, immunostimulation, and increased secretion of immunoglobulins A (22, 23). It has been shown in a murine model that Bacillus spores can germinate in significant numbers in the jejunum and ileum (5). Enterogermina is a mixture of antibiotic-resistant Bacillus strains NR, OC, SIN, and T (7, 23). These strains have been recently identified as belonging to the species Bacillus clausii (30). Since administration of the probiotic is often combined with oral antibiotic treatment, the strains of Bacillus Enterogermina were antibiotic resistant (7, 22). Little is known about the origin of the Enterogermina strains, and each has a specific pattern of antibiotic resistance (7, 22). They are supposed to be mutants from a parental Bacillus following multiple-step selection. The low genetic diversity among these strains is consistent with the notion that they derive from closely related strains or from an unknown common ancestor (30). Erythromycin resistance is one of the reported characteristics of B. clausii strains (7). Oral administration of high numbers of multiply drug-resistant microorganisms might be a cause for concern if clinically important resistance determinants happened to be located on transferable genetic elements. A potential hazard is transfer of resistance to microorganisms pathogenic for humans. The risk that this event will occur and the consequences in terms of morbidity and mortality have not been evaluated. Parameters required for risk assessment include studies on the nature and mobility of the resistance genes of probiotics.
The aim of this work was to identify the mechanism of macrolide resistance in the B. clausii probiotic strains and to characterize the genetic support for the resistance determinant.
MATERIALS AND METHODS
Bacterial strains.
The four B. clausii strains used for production of Enterogermina, OC, NR, SIN, and T, were obtained from Sanofi-Synthelabo OTC SpA (Milan, Italy) as separate spore suspensions. B. clausii DSM8716, ATCC 21536, and ATCC 21537 were used as reference strains.
Antibiotic susceptibility.
The disk diffusion method was used to determine bacterial susceptibility to antibiotics as recommended by the Comité de l'Antibiogramme de la Société Française de Microbiologie (8). Disks impregnated with 40 μg of pristinamycin I were prepared in the laboratory. Interpretive criteria for susceptibility or resistance were those recommended by the Comité de l'Antibiogramme de la Société Française de Microbiologie (8). MICs were determined by agar dilution in accordance with the NCCLS (26, 27).
Plasmid analysis.
Plasmid DNA was extracted from Bacillus strains as described by Ehrenfeld and Clewell (11). Briefly, bacterial cells were lysed with lysozyme and sodium dodecyl sulfate-NaOH. After treatment with potassium acetate, plasmid DNA was extracted with phenol-chloroform. Enterococcus faecalis JH2-2 containing plasmid pAD1 (59.6 kb) was used as a control (11). Plasmid size was estimated by comparison with a standard after digestion with EcoRI and electrophoretic migration.
Mating experiments.
E. faecalis JH2-2 (16), E. faecium HM1070 (resistant to rifampin and fusidic acid) (4), and B. subtilis UCN19 (resistant to ciprofloxacin) (3) were used as recipients in mating experiments. In every transfer experiment, E. faecalis BM4110 or B. subtilis BM450 containing the conjugative plasmid pAMβ1 (10, 21) was used as a control. Agar plates for selection of transconjugants contained rifampin (50 μg/ml) plus fusidic acid (20 μg/ml) or ciprofloxacin (8 μg/ml) combined with erythromycin (20 μg/ml). All mating experiments were repeated a minimum of three times.
PCR.
Deoxyoligonucleotide primers specific for the erm(A), erm(B), erm(C), and erm(TR) genes were those designed previously (1, 31). PCR experiments were carried out with a Perkin-Elmer 4600 thermal cycler with a denaturation step (94°C, 5 min), followed by 35 cycles of amplification (30 s of denaturation at 94°C, 45 s of annealing at 47°C, and 45 s of elongation at 72°C) and a final elongation step (72°C for 10 min). Primers 5′-GAGCTTAAAAAAATGAAAAA and 5′-TTTCTTTAACATTCTCTC were used to amplify the entire erm(34) gene.
Cloning experiments and gene analysis.
Extraction of total DNA from B. clausii and cloning were performed by standard techniques (29). DNA from B. clausii was digested with various restriction enzymes, including HindIII and EcoRI. The fragments were cloned into plasmid pUC18 and introduced by electrotransformation into E. coli DH10B, and transformants were selected on agar containing ampicillin (200 μg/ml) and erythromycin (50 μg/ml). Subcloning in E. faecalis JH2-2 was done by using the shuttle plasmid pAT28 as a vector (32). Nucleotide and amino acid sequences were analyzed by using the BLAST and FASTA softwares available over the Internet at the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov/). Multiple-sequence alignment and phylogenetic tree preparation were performed with the ClustalX and PHYLIP programs available at the Centre de Ressources Infobiogen website (http://www.infobiogen.fr/). Secondary structures of the attenuator mRNA were analyzed by using the Mu-fold software (34).
Southern hybridization.
DNA from B. clausii was digested with SmaI or I-CeuI, separated by pulsed-field gel electrophoresis by a technique similar to that used for enterococci (2), transferred onto a nylon membrane, and hybridized to a probe specific for erm(34) of B. clausii. The probe consisted in the entire gene amplified by PCR and labeled with digoxigenin (Boehringer Mannheim France, Meylan, France). Similar hybridization experiments were performed with plasmid DNAs from B. clausii OC and T digested with EcoRI.
Nucleotide sequence accession number.
The nucleotide sequence of the erm(34) gene from B. clausii DSM8716 has been deposited in the GenBank nucleotide sequence database under accession number AY234334.
RESULTS
Macrolide resistance in B. clausii.
All of the B. clausii strains studied, including the three reference strains, displayed similar phenotypes of resistance to macrolides. By the disk diffusion technique, no inhibition zone was visible around disks of erythromycin (14-membered ring macrolide), azithromycin (15-membered ring macrolide), spiramycin (16-membered ring macrolide), lincomycin, clindamycin (lincosamides), and pristinamycin I (streptogramin B). MICs of erythromycin, spiramycin, lincomycin, clindamycin, and pristinamycin I were greater than 128 μg/ml. All strains were susceptible to pristinamycin (a combination of oral streptogramins A and B). This pattern of resistance defines an MLSB phenotype generally due to the presence of an erm gene encoding a ribosomal methylase (19).
Identification of the erm(34) gene from B. clausii DSM8716.
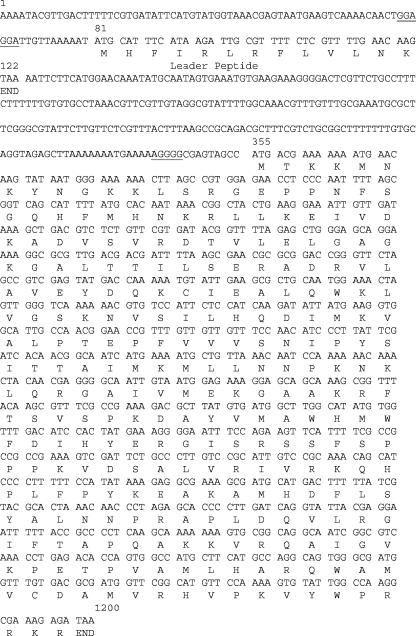
No DNA could be amplified with primers specific for the erm(A), erm(B), erm(C), and erm(TR) genes responsible for acquired MLSB resistance in gram-positive organisms pathogenic for humans and animals and total DNA of B. clausii strains as a template. Total DNAs from B. clausii DSM8716 and the probiotic strains were used to clone the determinant responsible for macrolide resistance. Three DNA fragments that conferred erythromycin resistance on E. coli DH10B were cloned, a 10-kb HindIII fragment from B. clausii DSM8716, a 4-kb HindIII fragment from B. clausii T, and a 6-kb EcoRI fragment from B. clausii SIN. A 1.4-kb EcoRI-HindIII fragment from strain DSM8716 was then subcloned and sequenced on both strands. Analysis of the sequence revealed an open reading frame of 846 bp preceded at 10 bp by an AGGGG sequence similar to the ribosome-binding site consensus sequence. This open reading frame could possibly code for a 281-amino-acid protein (Fig. 1). Comparison of the deduced sequence with proteins showed homology with various Erm proteins. These proteins are ribosomal methylases that monomethylate or dimethylate adenine at position 2058 (E. coli numbering) in 23S rRNA, which binds macrolides. The methylation confers cross-resistance to macrolides, lincosamides, and streptogramins B, the so-called MLSB resistance phenotype, because these molecules all have A2058 in their ribosomal binding site. The closest homology for the Erm sequence of B. clausii was with Erm(D) from B. licheniformis, B. halodurans, and B. anthracis (61% identity and 71% homology) and with Erm(W) from Micromonospora griseorubida (13, 14, 15, 17, 18) (Fig. 2). Although to a lesser extent, homology was also found with the other Erm proteins. erm genes with deduced amino acid sequences with less than 79% identity are given different letter or number designations (28). The erm-related gene of B. clausii DSM 8716 was thus designated erm(34). The 1.4-kb EcoRI-HindIII fragment containing the erm(34) gene was subcloned into shuttle plasmid pAT28 and introduced into E. faecalis JH2-2, where it conferred an MLSB phenotype characterized by cross-resistance between erythromycin and lincomycin (MIC, >128 μg/ml), showing that this gene could also be expressed in a heterologous gram-positive background.
FIG. 1.
erm(34) DNA sequence and deduced amino acid sequence. The nucleotide sequence of erm(34) is shown together with the deduced amino acid sequence of Erm34 methylase and its leader peptide. Putative ribosome-binding sites are underlined.
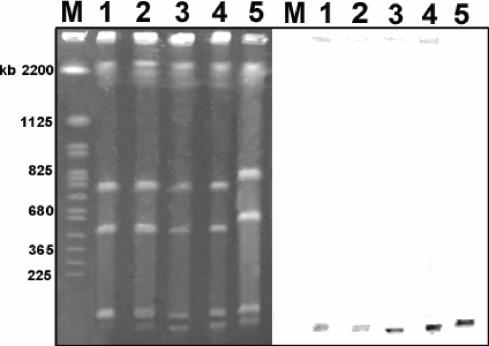
FIG. 2.
Localization of the erm(34) gene in B. clausii NR, OC, SIN, T, and DSM8716. (Left) Total DNAs from B. clausii strains NR (lane 1), OC (lane 2), SIN (lane 3), T (lane 4), and DSM8716 (lane 5) were digested with the I-CeuI restriction enzyme and submitted to pulsed-field gel electrophoresis. M, molecular size standard (Saccharomyces cerevisiae chromosomal DNA). (Right) DNA was transferred to a nylon membrane and hybridized with an erm(34) probe labeled with digoxigenin.
The structural gene for the putative methylase was preceded by a 68-nucleotide leader sequence, together with a ribosome-binding site, which could encode a 13-amino-acid peptide (MHFIRLRFLVLNK). In addition, series of inverted repeats that extended from the sequence of the leader peptide to the initiation sequences for the methylase (ribosome-binding site and initiation codon) were identified that could form stem-loops by base pairing. Computer analysis of the secondary structure of the mRNA proposed several alternative structural conformations. A final set of inverted repeats would sequester both the methylase ribosome-binding site and the codons for the first four amino acids of the methylase (data not shown). This structure resembles that involved in the expression of inducible erm genes, including erm(C), erm(A), and erm(D), which have been reported to function as translational or transcriptional attenuators (33).
Distribution and localization of the erm(34) gene.
An 856-bp fragment could be amplified by PCR from the DNAs of all B. clausii strains. The sequences of all of the amplified DNA fragments were nearly identical. The total DNAs of the reference B. clausii strains and the four probiotic strains were digested with I-CeuI or SmaI, submitted to pulsed-field gel electrophoresis, transferred to a nylon membrane, and hybridized successively with erm(34) and 16S rRNA probes. The I-CeuI enzyme cuts in a 26-bp DNA sequence that is specific for rRNA operons (20). After digestion with this enzyme, the DNA from the B. clausii strains yielded seven fragments that hybridized with the rRNA probe, indicating that this species contained a minimum of seven rRNA operons (data not shown). The erm(34) probe hybridized to a single low-molecular-weight fragment in all of the strains studied. The erm(34) probe also hybridized to an approximately 20-kb SmaI fragment in all of the strains tested (data not shown).
The B. clausii probiotic strains were analyzed for their plasmid content. A large plasmid could be visualized only in B. clausii T and OC, confirming a previous report (22). After digestion with EcoRI and electrophoretic migration, the two plasmids yielded similar restriction patterns composed of four fragments. The size of the plasmid was estimated to be approximately 30 kb. The DNA fragments were transferred to a nylon membrane and hybridized with the erm(34) probe. No signal was detected. We therefore concluded that the erm(34) gene was chromosomally located.
In vitro transfer of resistance to macrolides.
Repeated attempts to transfer resistance to macrolides by conjugation from B. clausii probiotic strains to E. faecalis JH2-2, E. faecium HM1070, and B. subtilis UCN19 were unsuccessful (frequencies inferior to the limit of detection, 10−9 per donor colony for B. subtilis and 5 × 10−10 for enterococci). By contrast, the 35-kb erythromycin resistance plasmid pAMβ1 could be transferred from E. faecalis BM4110/pAMβ1 or B. subtilis BM450/pAMβ1 to all recipient strains at frequencies approximately equal to 10−3 per donor colony for E. faecalis JH2-2 and E. faecium HM1070 and 10−4 per donor colony for B. subtilis UCN19.
DISCUSSION
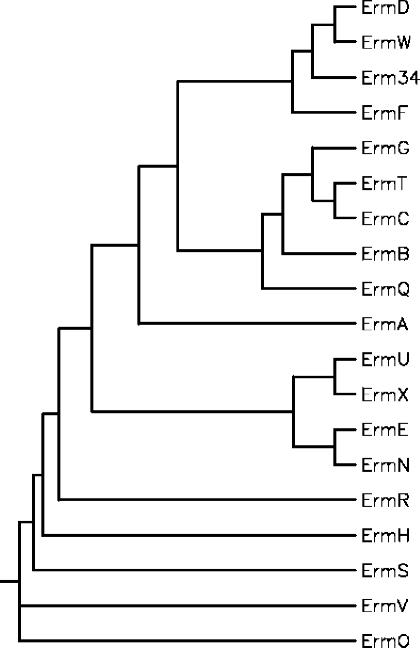
The B. clausii probiotic strains are resistant to clinically important antibiotics, including macrolides and aminoglycosides (3, 22). We have recently shown that resistance to aminoglycosides was due to the synthesis of an aminoglycoside-inactivating enzyme encoded by an aadD2 chromosomal gene (3). In this study, we have shown that resistance to macrolides was associated with the presence of an erm(34) gene that has not been characterized or found in other bacteria so far. A minimum of 21 erm gene classes have been reported, which are distinguished on the basis of sequence comparison (28). Some of the erm genes are found in the chromosome of microorganisms that produce antibiotics or in soil bacteria; others are found on plasmids and transposons in microorganisms pathogenic for humans and animals. The erm(34) gene differed from the other erm genes in Bacillus spp. As already mentioned, erm(D) genes, previously called ermD, ermK, and ermJ, were characterized in B. licheniformis, B. halodurans, and B. anthracis, respectively (13, 14, 17). The ermD and ermK genes are localized on the chromosome of the Bacillus strains, but the intrinsic or acquired nature of these determinants has not been established. By contrast, ermJ is probably acquired since B. anthracis strains are usually susceptible to macrolides. Since the sequences of ErmD, ErmK, and ErmJ are nearly identical, they were reclassified recently in a unique Erm(D) class (28). Another gene, erm(G), presumed to be chromosomal, has been characterized in B. sphaericus (25). A closely related gene (99.7% identity) borne by a conjugative transposon was found in Bacteroides sp. (9). Finally, a staphylococcal gene, erm(C), was detected in B. subtilis, where it is plasmid borne (24). Alignment of Erm methylases was used to construct a phylogenetic tree (12). The methylases from the antibiotic producers and those from pathogenic bacteria form two distinct groups, and Erm(34), although closely related to Erm(D) and Erm(W), was placed on a separate branch (Fig. 3).
FIG. 3.
Phylogenetic relationships among Erm methylases. The tree was constructed by using the neighbor-joining method. Sequences are from S. aureus [Erm(A) (accession no. X03216) and Erm(C) (accession no. J01755)], Streptococcus pneumoniae [Erm(B) (accession no. X52632)], B. licheniformis [Erm(D) (accession no. M29832)], Saccharopolyspora erythraea [Erm(E) (accession no. X51891)], Bacteroides fragilis [Erm(F) (accession no. M14730)], B. sphaericus [Erm(G) (accession no. M15332)], Streptomyces thermotolerans [Erm(H) (accession no. P13079)], Streptomyces fradiae [Erm(N) (accession no. X97721) and [Erm(S)], Streptomyces lividans [Erm(O) (accession no. M74717)], Clostridium perfringens [Erm(R) (accession no. L22689)], Arthrobacter sp. [Erm(R) (accession no. M11276)], Lactobacillus reuteri [Erm(T) (accession no. M64090)], Streptomyces lincolnensis [Erm(U) (accession no. X62867)], Corynebacterium diphtheriae [Erm(X) (accession no. M36726)], M. griseorubida [Erm(W) (accession no. D14532)], and B. clausii [Erm34].
An attenuator structure with a leader peptide and a set of inverted repeats similar to those regulating inducible expression of MLSB resistance in several erm genes was identified upstream of erm(34). The induction mechanism has been intensively studied in the case of erm(C) from Staphylococcus aureus. It has been shown that erm(C) mRNA exists in a stable conformation in which the initiation sequences for the methylase are sequestered by base pairing and thus rendered inaccessible for ribosome binding (33). Binding of erythromycin to a ribosome during translation of the leader peptide yields ribosomal stalling. This stalling event results in opening of the structure, exposing the initiation sequences and allowing translation to occur. Translational regulation has also been proposed for the regulation of resistance to MLSB antibiotics encoded by the erm(A) and erm(B) genes. In the case of ermK from B. licheniformis, both translational attenuation and transcriptional attenuation seem to contribute to the regulation of the gene (6, 18). Close similarities between the attenuators of ermK and erm(34) suggest that the same mechanisms might modulate the expression of macrolide resistance in B. clausii.
Although we could study only a few B. clausii strains, the erm(34) gene, which is chromosomal, is probably species specific and the MLSB resistance is inherent to B. clausii. The stability of the macrolide resistance and the high level of resistance conferred by the erm(34) gene constitute an advantage, allowing the probiotic to be maintained in the gut when it is coadministered with oral macrolides.
Acknowledgments
This study was supported in part by a grant from Sanofi-Synthelabo OTC SpA, Milan, Italy.
REFERENCES
- 1.Angot, P., M. Vergnaud, M. Auzou, R. Leclercq, and Observatoire de Normandie du Pneumocoque. 2000. Macrolide resistance phenotypes and genotypes in French clinical isolates of Streptococcus pneumoniae. Eur. J. Clin. Microbiol. Infect. Dis. 19:755-758. [DOI] [PubMed] [Google Scholar]
- 2.Bozdogan, B., L. Berrezouga, M. S. Kuo, D. A. Yurek, K. A. Farley, B. J. Stockman, and R. Leclercq. 1999. A new resistance gene, linB, conferring resistance to lincosamides by nucleotidylation in Enterococcus faecium HM1025. Antimicrob. Agents Chemother. 43:925-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bozdogan, B., S. Galopin, G. Gerbaud, P. Courvalin, and R. Leclercq. 2003. Chromosomal aadD2 gene encodes an aminoglycoside nucleotidyltransferase in Bacillus clausii. Antimicrob. Agents Chemother. 47:1343-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bozdogan, B., and R. Leclercq. 1999. Effects of genes encoding resistance to streptogramins A and B on the activity of quinupristin-dalfopristin against Enterococcus faecium. Antimicrob. Agents Chemother. 43:2720-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casula, G., and S. M. Cutting. 2002. Bacillus probiotics: spore germination in the gastrointestinal tract. Appl. Environ. Microbiol. 68:2344-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi, S.-S., S.-K. Kim, T.-G. Oh, and E.-C. Choi. 1997. Role of mRNA termination in regulation of ermK. J. Bacteriol. 179:2065-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciffo, F. 1984. Determination of the spectrum of antibiotic resistance of the “Bacillus subtilis” strains of Enterogermina. Chemioterapia 3:45-52. [PubMed] [Google Scholar]
- 8.Comité de l'Antibiogramme de la Société Française de Microbiologie. 1996. Report of the Comité de l'Antibiogramme de la Société Française de Microbiologie. Technical recommendations for in vitro susceptibility testing. Clin. Microbiol. Infect. 2S1:11-25. [Google Scholar]
- 9.Cooper, A. J., N. B. Shoemaker, and A. A. Salyers. 1996. The erythromycin resistance gene from the Bacteroides conjugal transposon Tcr Emr 7853 is nearly identical to ermG from Bacillus sphaericus. Antimicrob. Agents Chemother. 40:506-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Courvalin, P., and C. Carlier. 1986. Transposable multiple antibiotic resistance in Streptococcus pneumoniae. Mol. Gen. Genet. 205:291-297. [DOI] [PubMed] [Google Scholar]
- 11.Ehrenfeld, E. E., and D. B. Clewell. 1987. Transfer functions of the Streptococcus faecalis plasmid pAD1: organization of plasmid DNA encoding response to sex pheromone. J. Bacteriol. 169:3473-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felsenstein, J. 1988. Phylogenies from molecular sequences: inference and reliability. Annu. Rev. Genet. 22:521-565. [DOI] [PubMed] [Google Scholar]
- 13.Gryczan, T., M. Israeli-Reches, M. Del Bue, and D. Dubnau. 1984. DNA sequence and regulation of ermD, a macrolide-lincosamide-streptogramin B resistance element from Bacillus licheniformis. Mol. Gen. Genet. 194:349-356. [DOI] [PubMed] [Google Scholar]
- 14.Hue, K. K., and D. H. Bechhofer. 1992. Regulation of the macrolide-lincosamide-streptogramin B resistance gene ermD. J. Bacteriol. 174:5860-5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inouye, M., T. Morohoshi, S. Horinouchi, and T. Beppu. 1994. Cloning and sequences of two macrolides-resistance-encoding genes from mycinamicin-producing Micromonospora griseorubida. Gene 141:39-46. [DOI] [PubMed] [Google Scholar]
- 16.Jacob, A. E., and S. J. Hobbs. 1974. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J. Bacteriol. 117:360-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim, H. S., E. C. Choi, and B. K. Kim. 1993. A macrolide-lincosamide-streptogramin B resistance determinant from Bacillus anthracis 590: cloning and expression of ermJ. J. Gen. Microbiol. 139:601-607. [DOI] [PubMed] [Google Scholar]
- 18.Kwak, J. H., E. C. Choi, and B. Weisblum. 1991. Transcriptional attenuation control of ermK, a macrolide-lincosamide-streptogramin B resistance determinant from Bacillus licheniformis. J. Bacteriol. 173:4725-4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leclercq, R. 2002. Mechanisms of resistance to macrolides and lincosamides: nature of the resistance elements and their clinical implications. Clin. Infect. Dis. 15:482-492. [DOI] [PubMed] [Google Scholar]
- 20.Liu, S. L., A. Hessel, and K. E. Sanderson. 1993. Genomic mapping with I-CeuI, an intron-encoded endonuclease specific for genes for ribosomal RNA, in Salmonella spp., Escherichia coli, and other bacteria. Proc. Natl. Acad. Sci. USA 90:6874-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin, B., G. Alloing, V. Mejean, and J. P. Claverys. 1987. Constitutive expression of erythromycin resistance mediated by the ermAM determinant of plasmid pAM beta 1 results from deletion of 5′ leader peptide sequences. Plasmid 18:250-253. [DOI] [PubMed] [Google Scholar]
- 22.Mazza, P., F. Zani, and P. Martelli. 1992. Studies on the antibiotic resistance of Bacillus subtilis strains used in oral bacteriotherapy. Boll. Chim. Farm. 131:401-408. [PubMed] [Google Scholar]
- 23.Mazza, P. 1994. The use of Bacillus subtilis as an antidiarrhoeal microorganism. Boll. Chim. Farm. 133:3-18. [PubMed] [Google Scholar]
- 24.Monod, M., C. Denoya, and D. Dubnau. 1986. Sequence and properties of pIM13, a macrolide-lincosamide-streptogramin B resistance plasmid from Bacillus subtilis. J. Bacteriol. 167:138-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monod, M., S. Mohan, and D. Dubnau. 1987. Cloning and analysis of ermG, a new macrolide-lincosamide-streptogramin B resistance element from Bacillus sphaericus. J. Bacteriol. 169:340-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, fourth edition. Approved standard, document M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 27.National Committee for Clinical Laboratory Standards. 1997. Performance standards for antimicrobial susceptibility testing. Sixth information supplement M100-S7. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 28.Roberts, M. C., J. Sutcliffe, P. Courvalin, L. B. Jensen, J. Rood, and H. Seppälä. 1999. Nomenclature for macrolide and macrolide-lincosamide-streptogramin B resistance determinants. Antimicrob. Agents Chemother. 43:2823-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 30.Senesi, S., F. Celandroni, A. Tavanti, and E. Ghelardi. 2001. Molecular characterization and identification of Bacillus clausii strains marketed for use in oral bacteriotherapy. Appl. Environ. Microbiol. 67:834-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutcliffe, J., T. Grebe, A. Tait-Kamradt, and L. Wondrack. 1996. Detection of erythromycin-resistant determinants by PCR. Antimicrob. Agents Chemother. 40:2562-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trieu-Cuot, P., C. Carlier, C. Poyart-Salmeron, and P. Courvalin. 1990. A pair of mobilizable shuttle vectors conferring resistance to spectinomycin for molecular cloning in Escherichia coli and in gram-positive bacteria. Nucleic Acids Res. 18:4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weisblum, B. 1995. Insights into erythromycin action from studies of its activity as inducer of resistance. Antimicrob. Agents Chemother. 39:797-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuker, M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31:3406-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]