Abstract
Genostem (acronym for “Adult mesenchymal stem cells engineering for connective tissue disorders. From the bench to the bed side”) has been an European consortium of 30 teams working together on human bone marrow Mesenchymal Stem Cell (MSC) biological properties and repair capacity. Part of Genostem activity has been dedicated to the study of basic issues on undifferentiated MSCs properties and on signalling pathways leading to the differentiation into 3 of the connective tissue lineages, osteoblastic, chondrocytic and tenocytic. We have evidenced that native bone marrow MSCs and stromal cells, forming the niche of hematopoietic stem cells, were the same cellular entity located abluminally from marrow sinus endothelial cells. We have also shown that culture-amplified, clonogenic and highly-proliferative MSCs were bona fide stem cells, sharing with other stem cell types the major attributes of self-renewal and of multipotential priming to the lineages to which they can differentiate (osteoblasts, chondrocytes, adipocytes and vascular smooth muscle cells/pericytes). Extensive transcription profiling and in vitro and in vivo assays were applied to identify genes involved in differentiation. Thus we have described novel factors implicated in osteogenesis (FHL2, ITGA5, Fgf18), chondrogenesis (FOXO1A) and tenogenesis (Smad8). Another part of Genostem activity has been devoted to studies of the repair capacity of MSCs in animal models, a prerequisite for future clinical trials. We have developed novel scaffolds (chitosan, pharmacologically active microcarriers) useful for the repair of both bone and cartilage. Finally and most importantly, we have shown that locally implanted MSCs effectively repair bone, cartilage and tendon.
Keywords: Animals, Bone Marrow Cells, cytology, Cell Culture Techniques, standards, Cell Differentiation, Cell Proliferation, Humans, Mesenchymal Stem Cells, cytology, Tissue Engineering
Keywords: differentiation, stem cell, bone, cartilage, tendon, smooth muscle, regenerative medicine
Genostem, acronym for “Adult mesenchymal stem cells engineering for connective tissue disorders. From the bench to the bed side” has been an European Integrated Project sponsored for 4 years by the European Community. It has included 30 teams, belonging to different European countries and to Israel, working together from the beginning of 2004 to the end of 2007.
This review highlights the essential scientific data provided by the consortium.
A. CELLULAR AND MOLECULAR ASPECTS
Approximately half of Genostem activity has been dedicated to the study of basic issues concerning bone marrow MSCs, the properties of undifferentiated MSCs and the signalling pathways leading to the differentiation into 3 of the connective tissue lineages, osteoblastic, chondrocytic and tenocytic.
1. Proliferating human bone marrow MSCs
Contrary to bone marrow native cells, that remain quiescent in vivo, MSCs actively proliferate once seeded in appropriate medium. Many attributes of the proliferating cells remain controversial to the point that the cell denomination varies from one author to the other (mesenchymal stem, or progenitor, or stromal cells, skeletal stem cells, stromal stem cells….). In Genostem, we have retained the term Mesenchymal Stem Cells for the population of human bone marrow cells culture amplified in standardized conditions and whose attributes are described below.
Standardization of the culture system
Standardisation of the culture system for ex vivo amplification was a pre-requisite to our work so that results could be compared between labs. Standards for the culture system included the use of alpha-MEM without nucleotides and of fetal calf serum selected for cell growth, and a cell seeding concentration of 5×104 cells/cm2 at culture initiation and of 103/cm2 at each passage (1). Fibroblast growth factor-2 (FGF-2) increased the growth of MSCs in elderly patients (> 60 years old), but not in children or younger adults (2). FGF-2 was therefore added at low concentration (1 ng/mL twice a week at medium renewal) in culture of elderly patients.
Proliferation potential
How primary layers are generated, to which extent do cells proliferate and which factors are implicated in MSC proliferation remained largely unknown and controversial. Studies in Genostem have addressed these issues.
Using large-scale Taqman Low-Density Array based on qRT-PCR, we have compared the expression level of 300 transcripts in passage 1 primary layers and in fast-growing clones developed at culture initiation and grown for a similar amount of time than primary layers. Gene expression levels were similar in intensity and in distribution among primary layers and most of the clones (3). This congruence of expression suggests that fast-growing clones can be taken as representative of the cell population found in primary layers. Whereas establishing primary cultures at non-clonal density results in an initially heterogeneous population of cells with variable potential for growth, it is likely that a subset of fast-growing cells becomes selected over time in culture and passaging, leading to a progressive increase in culture homogeneity.
Although MSCs actively proliferate in vitro, we have shown that their proliferation potential remains within the Hayflick’s limit of 50 population doublings (PDs). About 1/3 of the clones generated at culture inception reached more than 23–25 PDs, whereas clones developed later (at the end of P0 and P1) showed growth arrest after 18–20 PDs (3). The transcripts for telomerase reverse transcriptase (TERT) was not detected, which is in agreement with the limited, albeit large, proliferation potential.
Genostem teams have begun to unravel the network of cytokines and transcription factors controlling MSC proliferation. (3, 4). Part of this network is shown on Fig 1 ; it underscores the potential role of the cytokines IL6 and NRG1 and of the transcription factors GATA6, GATA2 and ZFMP2.
Figure 1. Gene network controlling bone marrow MSC proliferation.

We selected 64 transcripts that were downregulated after adipocytic, osteoblastic and chondrocytic differentiation (3). Ingenuity software allowed to determine the network with the highest score (score of 46, including 21 focus molecules effectively detected in the MSCs out of 30 molecules belonging to the theoretical network). Focus molecules are indicated by filled symbols.
We have also searched for factors selecting for highly proliferative clonogenic cells. Pre-treatment of cultures with antibodies neutralizing interferon-alpha (IFNA), or directed against its receptor, resulted in a marked increase in the number of very large and fast-growing colonies obtained in the presence of low, but necessary, concentrations of FGF-2 (4). Blockade of the interferon-alpha pathway may be a substitute for “competence growth factor”, with FGF-2 acting as “progression growth factor”. These data indicate that inhibition of the IFNA pathway is a way to increase the recruitment of clonogenic cells with high proliferative capacity.
Phenotype
In many publications MSCs are characterized mainly by their membrane phenotype. Studies in Genostem have shown that no single marker was specific for bone marrow MSCs, but that a large set of markers was required to characterize this cell population. MSC phenotype specificity has been defined by a set of 113 transcripts out of 1624 molecules coding for plasma membrane proteins inventoried in Affymetrix microarrays (5). This set includes 20 Clusters of Differentiation (CD), 17 of which were studied by flow cytometry at the protein level and were expressed at the plasma membrane. This set allows the identification of a mesenchymal phenotype clearly distinct from the hematopoietic/endothelial phenotypes (largely predominant in the bone marrow), and from the other skeletal mesenchymal cell populations (periosteal cells and synovial fibroblasts) (5, 6).
Another current issue was whether some of the markers characterizing human embryonic stem cells are also expressed by bone marrow MSCs. Our studies have shown that MSCs did not express the pluripotency gene trio OCT4/POU5F1, NANOG and SOX2 (3, 4, 7). The OCT4/POU5F1 transcripts that were detected corresponded to pseudogenes (7). Other embryonic stem cell markers were not detected, with the noticeable exception of the 2 stage-specific embryonic antigens 3 and 4 (SSEA-3, SSEA-4) resulting from the activity in MSCs of the sialyltransferase ST3GAL3 (4).
Differentiation potential
As expected, we have shown that MSC clones differentiate into the 3 mesenchymal lineages (osteoblastic, chondrocytic and adipocytic). We have also shown that they differentiate into the vascular smooth muscle cell lineage (3). To induce the full differentiation, cells were cultured for 21 days in the long-term culture medium described for the generation and maintenance of stromal cells associated to hematopoiesis, since previous experiments had shown that bone marrow stromal cells followed a vascular smooth muscle differentiation pathway (8).
Previous studies performed outside Genostem (9, 10) have shown that differentiation in the mesenchyme system is reversible since apparently differentiated mesenchymal cells are able to shift their differentiation pathway under modified external conditions. This plasticity was exemplified in clones where a switch was induced from the adipocytic to the osteoblastic lineage or from hypertrophic chondrocytes to osteoblasts. We have shown that this concept may be extended to the vascular smooth muscle lineage since MSC clones differentiated along this lineage can still differentiate into osteoblasts, chondrocytes and adipocytes when further cultured in osteo, chondro or adipogenic conditions (3).
Self-renewal
One of the key questions that have remained open for decades about the biology of MSCs is whether they are capable of self-renewal, thus qualifying as bona fide stem cells rather than simply multipotent cells. Identification of the in situ counterpart of bone marrow MSCs as CD146-expressing subendothelial (mural) cells in sinusoids has allowed to show that transplanted MSCs can reconstitute a compartment of mural cells with phenotype and clonogenic ability identical to those of the originally explanted cells. These data, and the possibility to secondarily passage single clonogenic CD146+ progenitors, represent direct evidence in support of the ability of bone marrow MSCs to self-renew in vivo, and therefore of their identity as bona fide stem cells (6).
Lineage priming
Lineage priming is a characteristic of stem cells whereby undifferentiated self-renewing stem cells express a subset of genes associated to the differentiation pathways to which they can commit. Lineage priming appears to be one major attribute of the paradigmal hematopoietic stem cell and is also suggested to be a property of embryonic stem cells. We have therefore evaluated whether lineage priming is also an attribute of bone marrow MSCs. We have shown that fast-growing clones initiated at culture inception are primed to the osteoblastic, chondrocytic, adipocytic and vascular smooth muscle lineages, but not to skeletal muscle, cardiac muscle, hematopoietic, hepatocytic or neural lineages (3).
Native bone marrow MSCs
Many hypotheses on the in situ original cells from which MSCs descend have been raised. Some investigators have suggested a non mesodermal, neuroectodermal origin (11); others have suggested that hematopoietic stem cells could generate mesenchymal cells (12). Our studies have shown that native bone marrow MSCs constitute a specialized, tissue-specific subset of subendothelial, mural cells (pericytes) essential for the establishment of the hematopoietic microenvironment (6, 13). Since pericytes belong to the vascular smooth muscle cell family, these data are in agreement with the vascular smooth muscle differentiation potential of the culture-amplified cells. Markers of bone marrow mural cells/pericytes can be used to prospectively isolate bone marrow MSCs, which can further be shown i) to contribute to the organization of microvascular structures in vivo and of their surrogates in vitro, ii) to express a broad range of mural cell markers in culture, and iii) to be regulated by known regulators of microvessel assembly and maturation (6). In addition to CD146 and CD105 (6), additional markers such as CD73, and CD200 (5) expressed in a minor population of bone marrow mononuclear cells (comprising 0,15–2% of the total number) can be used to isolate clonogenic bone marrow MSCs. Expression of these markers of uncultured cells is retained in non-differentiated cells in culture, and then is variably modulated upon induction of differentation.
Conclusions
The data collected during the Genostem project define bone marrow MSCs as adult tissue stem cells which are : 1) deriving from a subset of mural cells of bone marrow sinusoids, 2) self-renewing, 3) quadripotential and selectively primed to the mesenchymal and vascular smooth muscle lineages, 4) flexible in their differentiation options (mesenchyme plasticity), and 5) able to transfer and organize the hematopoietic microenvironment. The Genostem project has begun to unravel some of the key molecules that underly these specific properties.
2. Differentiation pathways
Genetic programs for osteo and chondrogenesis are partially deciphered. In particular, the master transcription factor RUNX2 is known to be the essential inducer of osteoblastic differentiation, and the Sox trio, SOX5, SOX6 and SOX9, appears to play a similar role in chondrogenesis. However, the description of the different molecules and pathways operative in these differentiations is far from complete. Little is known concerning the generation of tenocytes since these cells are obtained only after implantation in vivo of the cultured MSCs. Genostem teams have therefore searched for novel inducers of osteo, chondrogenesis and tenogenesis that may serve as bases for innovative therapies in regenerative medicine.
Osteogenesis
Recent advances have been made to isolate and expand MSCs from human bone marrow and to identify the mechanisms that are responsible for the osteogenic differentiation of these cells (14). A better understanding of the osteogenic differentiation program of MSCs is however required in order to develop optimal strategies to promote osteogenesis. The Genostem program offered the possibility to study the transcriptome of human bone marrow MSCs before and after osteoblastic differentiation. Transcription profiles were analyzed according to published standards (PMID: 19265543) and are available via www.bioretis.de. We have then evaluated the osteogenic potential of selected genes engineered in human primary MSCs in the preclinical model of long bone repair in immunodeficient mice. Major results of these studies are reported below.
FHL2 promotes the osteogenic potential of human bone marrow MSCs
In murine and human bone marrow MSCs, we have identified FHL2, a LIM-domain protein with four-and-a-half Lim domains, as an early transcriptional cofactor that is upregulated at early stages of osteoblastic differentiation induced by dexamethasone. We showed that over-expression of FHL2 increased osteoblastic marker gene expression as well as in vitro osteogenesis. We further showed that silencing of FHL2 abolished the stimulatory effect of dexamethasone on RUNX2 and type I collagen. To investigate how FHL2 may promote osteoblastic differentiation, we showed that FHL2 interacts with catenin beta1 and promotes catenin beta1 nuclear translocation and transcriptional activity, which indicates that Wnt/catenin signaling is a critical mechanism involved in the positive effect of FHL2 on osteoblastic differentiation in MSCs (15). Finally, we have shown that human MSCs overexpressing FHL2 produced 2 times more bone than control cells when implanted with a biomaterial in a standard ectopic subcutaneous implantation assay in immunodeficient mice (unpublished data). Overall, these findings suggest a strategy targeted to FHL2 to promote osteogenesis in human bone marrow MSCs.
FGF-18 is an essential positive regulator of the osteoblastic differentiation program in murine bone marrow MSCs
In murine bone marrow MSCs, we have found that fibroblast growth factor 18 (FGF-18) was upregulated by dexamethasone during osteoblastic differentiation. Overexpression of FGF-18 by lentiviral infection, or treatment of MSCs with recombinant human FGF-18 (rhFGF18), induced the expression of receptor 2 of FGF-2 (FGFR2), RUNX2 and downstream osteoblastic markers, and induced in vitro osteogenesis. Furthermore, FGF-18 appeared to promote the osteoblastic differentiation via activation of FGFR2 since downregulation of FGFR2 using lentiviral shRNAs blunted the osteoblastic gene expression induced by rhFGF18. Further biochemical and pharmacological analyses showed that rhFGF18-induced osteoblastic marker gene expression was mediated by mitogen-activated protein kinase (MAPK) and phosphatidylinositol kinase (PI3K) signaling pathways. Thus, FGF-18 is an essential positive regulator of the osteoblastic differentiation program in murine bone marrow MSCs. Demonstration of a similar role in human bone marrow MSCs awaits further studies.
Activated integrin alpha 5 promotes human bone marrow MSC osteoblastic differentiation and osteogenesis in vivo
In human bone marrow MSCs, we have found that that integrin alpha5 (ITGA5) was upregulated by dexamethasone during osteoblastic differentiation. Gain-of-function studies showed that ITGA5 promoted the expression of osteoblastic phenotypic markers as well as in vitro osteogenesis; in contrast, loss-of-function studies using shRNAs showed that downregulation of endogenous ITGA5 blunted the osteoblastic marker gene expression and the osteoblastic differentiation. Further molecular analyses showed that the enhanced osteoblastic differentiation induced by ITGA5 was mediated by activation of focal adhesion kinase (FAK), MAPK and PI3K signaling pathways. Remarkably, activation of endogenous ITGA5 using agonists that prime the integrin was sufficient to activate MAPK and PI3K signaling and to promote the osteoblastic differentiation and in vitro osteogenesis. Additionally, we demonstrated that MSCs engineered to overexpress ITGA5 exhibited a marked increase in their osteogenic potential in vivo (16). Taken together, these findings not only reveal that ITGA5 is required for osteoblastic differentiation of MSCs, but also provide a novel targeted strategy using ITGA5 agonists to promote the osteogenic capacity of these cells. This may be used for tissue regeneration in bone disorders where the recruitment or capacity of human bone marrow MSCs is compromised.
Conclusions
In summary, our studies led to significant advances in the mechanisms regulating bone marrow MSC osteoblastic differentiation (Fig 2). Specifically, progress has been made in the identification of novel factors that govern and promote human bone marrow MSC differentiation towards functional osteogenic cells. This knowledge may result in the development of innovative cell and gene therapeutic strategies to promote bone repair.
Fig 2. Major lineage-determining effectors in MSCs.
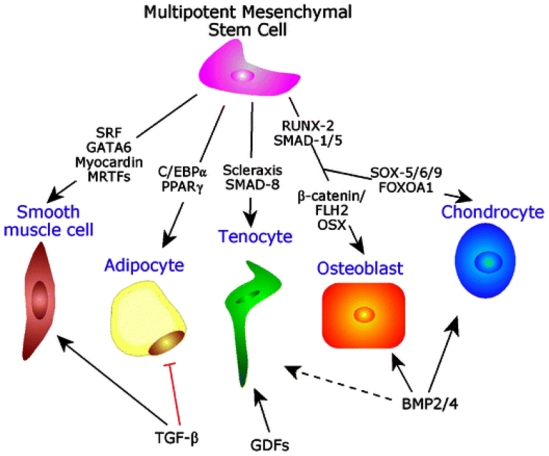
Lineages are regulated to a considerable extent by members of the TGF-β superfamily of growth factors activating downstream Smad signaling mediators. This is a modified diagram from (44).
Abbreviations: BMP: bone morphogenetic protein; C/EBP: CCAAT/enhancer binding protein; GDF: growth and differentiation factor; MRTF: myocardin-related transcription factor; Osx: Osterix; PPAR : peroxisome proliferator-activated receptor- ; Runx2: Runt-related transcription factor 2; Sox5/6/9: SRY (sex determining region Y)-box 5, -box 6, -box 9; SRF: serum response factor; TGF-β: Transforming growth factor-β.
Chondrogenesis
The major limitations of cell therapy applications of MSC differentiated to chondrocytes are due to the lack of specific differentiation factor and to the cell hypertrophy after implantation in vivo. Genostem has offered the opportunity to study on a large scale the factors involved in chondrocyte biology.
One of the major results has been the identification of new transcription factors involved in early stage chondrogenic differentiation (17). Among 1354 differentially regulated genes during chondrogenesis induced from human bone marrow MSCs, 705 were up-regulated. We first focused our attention on forkhead box protein O1 (FOXO1A) which was shown, using RT-PCR, to be increased by 6-fold as soon as day 2. We demonstrated that FOXO1A was sufficient to induce chondrogenesis. For this, we derived stable clones of the MSC murine embryonic line C3H10T1/2 over-expressing either wild-type or a constitutively active form of FOXO1A. After 21 days of culture in micropellet without any differentiation factor, we could show the up-regulation of aggrecan, collagen IIB and the down-regulation of collagen I. The engineered cells cultured in specific inducing conditions did not show higher osteogenic potential than naive cells and, even more interestingly, showed lower adipogenic potential. After injection of the engineered cells in the intra-articular space of knee joints, we could detect the formation of cartilage, staining positive for aggrecan and collagen II, in the areas of engineered cell injection, thus confirming their potential to differentiate into chondrocytes.
In another work (18), we studied the cartilaginous microenvironment generated by chondrocytes derived from human bone marrow MSCs. The data obtained through large-scale Taqman Low-Density Array based on qRT-PCR have been assembled into a biological process-oriented database that represents the first molecular profile of a cartilaginous MSC niche. It included secreted cysteine-rich regulatory proteins (CCNs), matrix metalloproteinases (MMPs), members of the disintegrin and metalloproteinase domain-containing protein family (ADAMs) and cell adhesion molecules (CAMs including cadherins). CCNs interact with growth factors and have important functions in cell proliferation and differentiation. CCN3, CCN4 and CCN5 were upregulated after differentiation whereas CCN1 and CCN6 were down-regulated. The timely degradation of the ECM is an important feature of development, morphogenesis and remodelling and is mainly mediated by MMPs. Only MMP2 and MMP9 were present in MSCs before and after differentiation, and MMP7, MMP3 and MMP28, which were not expressed in MSCs before differentiation, were highly up-regulated during chondrogenic differentiation. ADAMs interact with various partners such as integrins, syndecans and ECM proteins due to their role in cell-ECM interaction. ADAM8, ADAM9, ADAM19, ADAM23, ADAMTS4 and ADAMTS5 were expressed in MSCs. CAMs have important functions in development and tissue morphogenesis. CDH2 (N-cadherin), CDH4 and CDH13 were expressed in MSCs and all were decreased in chondrogenic differentiated cells. CDH11 (OB-cadherin), NRCAM and MCAM/CD146 were up-regulated for more than 30 fold by day 21 of chondrogenesis. Integrins act by transmitting signals from the ECM to the cellular machinery, resulting in changes in cell function. ITGA5, ITGA7, ITGA10 and ITGAE were expressed in non differentiated MSCs with high increase of ITGA6 by day 21 of chondrogenic differentiation.
Because chemokines and cytokines are thought to play an important role in cell activation, survival and differentiation, we analysed the data obtained from the transcriptome study and found that CCL2, CXCL12 and FLT3L were all down-regulated after chondrogenesis. In contrast we observed a significant increase of CCR1, CCR3, CCR4 and CXCR4 (18).
In a synthesis work on the transcriptome (19), we were able to describe a 3-step differentiation process. The first step corresponded to trancripts implicated in cell attachment and induction of apoptosis, the second step was characterized by transcripts implicated in proliferation/differentiation, and the third step was characterized by transcripts implicated in chondrocytic differentiation and/or hypertrophy.
In summary, our studies led to significant progress in the identification of the molecular microenvironment associated to the chondrocytic differentiation of MSCs, and in the molecular characterization of this differentiation (Fig 2).
Tenogenesis
Until the present time, therapeutic options used to repair tendon and ligament injuries have consisted in autografts, allografts or synthetic prostheses (20). None of these alternatives, however, has provided a successful long-term solution. In Genostem we developed the hypothesis that a potent inducer of tenocytic differentiation of MSCs might result in a novel and powerful modality for tendon repair. We have identified such an inducer in Smad8, a signaling mediator of the transforming growth factor beta/bone morphogenic protein (TGF-beta/BMP) family of growth factors (21). We characterized the role of Smad8 in the tendon differentiation pathway after forced expression of the biological active form of Smad8 in the well-studied murine MSC line C3H10T½ and in human bone marrow MSCs. A genome-wide analysis of gene expression during Smad8-dependent tenogenic differentiation has resulted in several candidate genes potentially involved in tenogenic differentiation program. Characterization of these factors is under investigation (Nuber, Häupl and Gross, in preparation).
In conclusion, we have pinpointed a pathway for tendon/ligament formation (Fig 2).
B. PRECLINICAL STUDIES IN ANIMAL MODELS
Another large part of Genostem activity has been devoted to studies of the repair capacity of MSCs in animal models, a prerequisite for future clinical trials. Work has been done to develop innovative biomaterials and to test the ability of MSC/biomaterial constructs to repair bone, cartilage and tendons.
2. Biomaterials
The Genostem consortium enabled developing various biomaterials tailored for specific applications in bone, cartilage and tendon repair. The biomaterials were developed and tested with bone marrow MSCs both in vitro and in vivo. The proposed biomaterials followed two major research lines: biomaterials with fast translation into the clinic and innovative biomaterials with a longer path to reach the clinical applications. Those strategies were pursued as complementary routes.
A new set of biodegradable biomaterials was developed by combining chitosan, a polysaccharide, with various biodegradable aliphatic polyesters. Those materials were intended to combine the good biological performance of chitosan with the melt processability of the polyesters. The materials were thoroughly characterized in terms of morphology, mechanical properties and kinetics of biodegradation showing excellent performance compatible with the application in bone and cartilage (22). The biological performance was evaluated in vitro using the mouse bone marrow MSC line BMC9 (23, 24). The combination of chitosan with poly(butylene succinate), in a equal fraction by weight (chitosan/PBS), showed high cell viability. Porous structures were shown to support viable cultures of BMC9. This biomaterial was compatible with the successful in vitro differentiation of BMC9 into the lineages of interest, expressing osteoblastic or chondrocytic genes depending on the medium used to differentiate the cells. Further work developed with primary human bone marrow MSCs confirmed the osteoinductive capacity of the scaffolds (25).
In vivo study of bone marrow MSC-scaffold combinations has been performed using non-invasive in vivo photonic imaging. Different scaffolds (PEG-RGD, gelatine-hydrogel, calcium alginate beads) were loaded with cells expressing luciferase gene reporter and were ectopically transplanted both subcutaneously and intramuscularly in animal models. Results have shown that intramuscular transplants were viable for up to 90 days, thus providing a safe method for monitoring localization and viability of transplanted cells following in vivo transplantation (26).
In summary, we have developed a set of novel scaffolds and procedures that will be useful for the repair of both bone and cartilage in the presence of MSCs.
2. Bone repair
For evaluation of the in vivo osteogenic functionality of MSCs, cell-scaffold constructs were transplanted in femoral bone defects in immunodeficient mice. Cells were isolated and expanded according to the Genostem protocol. Following osteogenic differentiation, cells were loaded onto fibrin/ceramic constructs and transplanted in athymic nude mice. After eight weeks tissue samples were processed for histology and immunohistochemistry. Previous results have shown that subcutaneous transplants of cell/ceramic constructs resulted in ectopic bone formation (27). When the same cell-scaffold constructs were implanted in femoral critical size defect we observed, 8 weeks after transplantation, bone formation in place of fibrous tissue, as shown in Fig 3A and 3B (Srouji et al. preliminary results).
Fig 3. Connective tissue repair by MSCs.

A, B: Orthotopic bone repair
Segmental critical size bone defect (2 mm) created in femoral midshaft of athymic nude mice; the defect was filled with MSC-ceramic transplant.
A: Defect in the absence of grafting; note the presence of fibrous tissue filling the gap
B: Bone reconstruction (8 weeks after engraftment) was apparent in place of the fibrous tissue (arrow).
C, D: Orthotopic cartilage repair
Large size defect was created in the patella of Merinos sheep. Autologous bone marrow MSCs were harvested and expanded in culture for 2 passages, before being seeded in fibrin clots or scaffolds of chitosan + TGFb3. The material was implanted in the patella defect. Animals were left in the field for 8 weeks before sacrifice.
C: lesions filled with ovine MSC in fibrin clot
D: Lesions filled with ovine MSCs embedded in chitosan scaffolds + TGFβ3. Arrows indicate the junction between endogenous and new tissues
E: Heterotopic tendon formation
The intramuscular transplantation of adenovirally modified MSCs (C3H10T½ embryonic cell line) expressing Smad8 and Bmp2 leads, 4 weeks after implantation, to the heterotopic formation of tendinous elements (hematoxylin and eosin staining). The tendinous element (shown within the black and white arrowheads) is characterized by a tendon-typical crimp pattern and flattened tenocyte-like cells. Abbreviations: B, bone; M, muscle; T, tendon
3. Cartilage repair
Clinical application of MSC-differentiated chondrocytes in rheumatic disease like osteoarthrirtis (OA) requires appropriate scaffolds that are chondro-inductive, bio-resorbable and non inflammatory, and are adapted for intra-articular injection. Genostem offered the opportunity to test in vivo different scaffolds combined with human bone marrow MSCs.
In order to deliver the growth factor TGF-beta3 (TGFb3) in a controlled manner we developed microparticles with a bio-mimetic surface of matrix molecules (Pharmacologically Active Microcarriers or PAM). We selected a combination of fibronectin (FN) and poly-D-lysine as the best bio-mimetic surface. The cell adhesion protocol has been completed by an overnight cell culture step necessary to obtain the formation of PAM and cell aggregates. When MSCs were cultured in presence of PAM-TGFb3, cells rapidly adhered onto the PAMs and progressively aggregated to form a unique pellet-like structure from day 7 to day 21. In PAM-TGFb3-induced aggregates, high expression of chondrogenic markers occurred in a time-dependent manner whereas expression of osteogenic and adipogenic markers was lower than those observed when PAM-FN were used. Intra-articular injection of MSCs mixed with PAM-TGFb3 confirmed their capacity to form a neotissue with characteristics of cartilage.
We used the ovine model of cartilage repair to demonstrate the capacity of bone marrow MSCs combined with fibrin clot ± chitosan/PBS scaffolds and TGFb3 to induce cartilage tissue in a preclinical model (28). Ovine MSCs were shown to display the three main characteristics of MSCs: adherence to plastic, characteristic phenotypic profile (positive for CD44, CD105 and vimentin, and negative for CD34 and CD45) and trilineage differentiation potential. Ovine MSCs, either in fibrin clot alone or with chitosan ± TGFβ3, were able to repair a partial-thickness defect in the cartilaginous tissue of sheep patella (Fig 3C and 3D).
4. Tendon repair
The strategies for MSC-mediated tendon repair was based on the Smad8-dependent tenogenic differentiation model described above. Tissue regeneration of a rat achilles tendon partial-defect model, using C3H10T½ MSCs expressing Smad8 and BMP-2 was demonstrated (21). We observed the formation of fibrous ligament-to-bone and tendon-to-bone interfaces (“entheses” or osteotendinous junctions) after heterotopic implantation of the genetically engineered MSC line in muscle tissue as shown of Fig 3E. Entheses serve to dissipate stress between soft tissue and bone and surgical reconstruction of these interfaces is an issue of considerable importance. Entheses are prone to injury and the integration of bone and tendon/ligament is in general not satisfactory. Our findings should eventually contribute to the establishment of MSC-dependent regenerative therapies for tendon-bone insertions (Shahab-Osterloh, et al, under revision).
Moreover, a novel method was devised to quantify in vivo tendon biomechanics by minimally invasive procedures establishing endoscopic fibered confocal fluorescence microscope images of externally loaded tendons. Through a series of image post-processing steps, cellular displacements may be reduced to tissue strains, giving a quantifiable estimate of the functional integrity of the tendon tissues (29–31). These methods may enable to assess the impact on normal tendon homeostasis and healing processes by minimally invasive procedures.
B. CONCLUSIONS AND PERSPECTIVES
Concerning bone marrow MSC biology, work performed in Genostem has has helped solve three major problems. We now know 1) where the native cells are located (on the abluminal side of endothelial cells of sinuses) and how to select them, 2) that stromal cells forming the niche of hematopoietic stem cells and bone marrow MSCs are the same entity, thus resolving a long-standing issue, and 3) that clonal highly proliferative culture-amplified cells are bona fide stem cells since sharing with the other paradigmal adult stem cells, the hematopoietic stem cells, two major properties, that of self-renewal and that of multipotential priming. Many issues remain to be solved. Are MSCs also primed to the tenogenic lineage ? Is it possible to describe for the MSC system a hierarchy among precursors that would discriminate between self-renewing multipotential MSCs and progenitors/transit amplifying cells devoid of self-renewal capacity, and more restricted in their differentiation ability (note that such ”classical” model for stem cell differentiation in other systems is presently under much debate (32, 33)) ? Is the self-renewal capacity of MSCs comparable to that of hematopoietic stem cells (sequential transplantations would solve this problem) ? Would cross-inhibitory loops between transcription factors account for multipotential lineage priming in MSCs, as suggested for hematopoietic or embryonic stem cell lineage priming (34) ? Does the reprogrammation of MSCs into non primed lineages (35, 36) implies reversion to pluripotent cell stage as described for skin fibroblasts (37), or is true transdifferentiation possible (38)? What is the influence of the surrounding matrix and biomechanical stress on lineage priming and programming/reprogramming of MSCs (39) ?
Genostem identified and developed a set of new biomaterials and scaffolds that showed adequate performance in vivo for the repair of bone and cartilage. The cohort of biomaterials and scaffolds proposed by Genostem continues being developed towards pre-clinical testing for the repair of connective tissues aiming at reaching the clinical testing stage.
Concerning bone repair, work performed in Genostem led to identify novel genes and factors that promote MSC osteogenic differentiation and osteogenesis in vitro and in vivo. Future studies, now ongoing, will determine whether some of these genes or factors can be used to promote bone repair in preclinical settings. Ongoing studies are also aimed at identifying other genes and proteins that are upregulated during MSC osteogenic differentiation and can be used to promote the osteogenic and bone repair processes.
Concerning cartilage repair, work performed in Genostem opens perspectives for the cell therapy of disorders including cartilage defect and cartilage damage related to arthritis/osteoarthitis. However, results in the long term evaluating integration of the newly formed tissue with the native cartilage need to be obtained before large application in clinical practice can be envisioned.
Concerning tendon repair, identification of the signalling molecules implicated in tenogenesis has been a major step forward. Future studies will determine how this newly-acquired knowledge may be applied to preclinical models using human bone marrow MSCs, before considering clinical application in cases of tendon rupture.
Whatever the site of repair, the mechanisms of repair still need to be elucidated. A traditional view would be that the transplanted donor MSCs migrate to the injured site where they proliferate and differentiate into appropriate cells (osteoblasts, chondrocytes or tenocytes pending on the injured tissue). An alternative view would be that MSCs provide growth factors helping in situ host MSCs to proliferate and differentiate. Such trophic effect has been recently shown in an animal model of fracture healing (40) and is suggested to be the major mechanism to explain the beneficial role of MSC administration in non-orthopedic-related disorders such as vascular repair (41).
A last important issue is whether bone marrow MSCs are identical to other connective-tissue forming cells not found in bone marrow (adipose tissue, umbilical cord vessel, Wharton’s jelly, placenta…). Many authors suggest this to be the case, the major arguments being the similarity of phenotype and of differentiation capacity (into osteoblasts, chondrocytes, adipocytes and even myocytes) between cells derived from bone marrow and other tissues (42). Data from Genostem contradict this hypothesis stressing that bone marrow MSCs present unique properties : specific expression of certain membrane antigens, unique ability to form bone and transfer the hematopoietic microenvironment in vivo after transplantation to ectopic sites, specific transcriptomic profile… (5–7, 43). Further studies should more closely discriminate the connective-tissue stem cell types with regard to their tissue of origin.
Acknowledgments
Work supported by the European Community (Key action 1.2.4-3 Integrated Project Genostem, contract N° 503161)
Footnotes
The authors declare no potential conflicts of interest.
References
- 1.Delorme B, Charbord P. Culture and characterization of human bone marrow mesenchymal stem cells. Methods Mol Med. 2007;140:67–81. doi: 10.1007/978-1-59745-443-8_4. [DOI] [PubMed] [Google Scholar]
- 2.Dimitriou H, Linardakis E, Martimianaki G, et al. Properties and potential of bone marrow mesenchymal stromal cells from children with hematologic diseases. Cytotherapy. 2008;10(2):125–33. doi: 10.1080/14653240701851332. [DOI] [PubMed] [Google Scholar]
- 3.Delorme B, Ringe J, Pontikoglou C, et al. Specific Lineage-Priming of Bone Marrow Mesenchymal Stem Cells Provides the Molecular Framework for Their Plasticity. Stem Cells. 2009;27(5):1142–51. doi: 10.1002/stem.34. [DOI] [PubMed] [Google Scholar]
- 4.Peiffer I, Eid P, Barbet R, et al. A sub-population of high proliferative potential-quiescent human mesenchymal stem cells is under the reversible control of interferon alpha/beta. Leukemia. 2007;21(4):714–24. doi: 10.1038/sj.leu.2404589. [DOI] [PubMed] [Google Scholar]
- 5.Delorme B, Ringe J, Gallay N, et al. Specific plasma membrane protein phenotype of culture-amplified and native human bone marrow mesenchymal stem cells. Blood. 2008;111(5):2631–5. doi: 10.1182/blood-2007-07-099622. [DOI] [PubMed] [Google Scholar]
- 6.Sacchetti B, Funari A, Michienzi S, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131(2):324–36. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 7.Kaltz N, Funari A, Hippauf S, et al. In vivo osteoprogenitor potency of human stromal cells from different tissues does not correlate with expression of POU5F1 or its pseudogenes. Stem Cells. 2008;26(9):2419–24. doi: 10.1634/stemcells.2008-0304. [DOI] [PubMed] [Google Scholar]
- 8.Galmiche MC, Koteliansky VE, Briere J, Herve P, Charbord P. Stromal cells from human long-term marrow cultures are mesenchymal cells that differentiate following a vascular smooth muscle differentiation pathway. Blood. 1993;82(1):66–76. [PubMed] [Google Scholar]
- 9.Bianco P, Gehron Robey P. Marrow stromal stem cells. J Clin Invest. 2000;105(12):1663–8. doi: 10.1172/JCI10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song L, Webb NE, Song Y, Tuan RS. Identification and functional analysis of candidate genes regulating mesenchymal stem cell self-renewal and multipotency. Stem Cells. 2006;24(7):1707–18. doi: 10.1634/stemcells.2005-0604. [DOI] [PubMed] [Google Scholar]
- 11.Takashima Y, Era T, Nakao K, et al. Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell. 2007;129(7):1377–88. doi: 10.1016/j.cell.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 12.Ogawa M, LaRue AC, Drake CJ. Hematopoietic origin of fibroblasts/myofibroblasts: Its pathophysiologic implications. Blood. 2006;108(9):2893–6. doi: 10.1182/blood-2006-04-016600. [DOI] [PubMed] [Google Scholar]
- 13.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2(4):313–9. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marie PJ, Fromigue O. Osteogenic differentiation of human marrow-derived mesenchymal stem cells. Regen Med. 2006;1(4):539–48. doi: 10.2217/17460751.1.4.539. [DOI] [PubMed] [Google Scholar]
- 15.Hamidouche Z, Hay E, Vaudin P, et al. FHL2 mediates dexamethasone-induced mesenchymal cell differentiation into osteoblasts by activating Wnt/beta-catenin signaling-dependent Runx2 expression. Faseb J. 2008;22(11):3813–22. doi: 10.1096/fj.08-106302. [DOI] [PubMed] [Google Scholar]
- 16.Hamidouche Z, Fromigue O, Ringe J, et al. Priming integrin {alpha}5 promotes human mesenchymal stromal cell osteoblast differentiation and osteogenesis. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0812334106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Djouad F, Bony C, Canovas F, et al. Transcriptomic analysis identifies Foxo3A as a novel transcription factor regulating mesenchymal stem cell chrondrogenic differentiation. Cloning Stem Cells. 2009;11(3):407–16. doi: 10.1089/clo.2009.0013. [DOI] [PubMed] [Google Scholar]
- 18.Djouad F, Delorme B, Maurice M, et al. Microenvironmental changes during differentiation of mesenchymal stem cells towards chondrocytes. Arthritis Res Ther. 2007;9(2):R33. doi: 10.1186/ar2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mrugala D, Dossat N, Ringe J, et al. Gene expression profile of multipotent mesenchymal stromal cells: Identification of pathways common to TGFbeta3/BMP2-induced chondrogenesis. Cloning Stem Cells. 2009;11(1):61–76. doi: 10.1089/clo.2008.0070. [DOI] [PubMed] [Google Scholar]
- 20.Boyer MI, Goldfarb CA, Gelberman RH. Recent progress in flexor tendon healing. The modulation of tendon healing with rehabilitation variables. J Hand Ther. 2005;18(2):80–5. doi: 10.1197/j.jht.2005.02.009. quiz 6. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann A, Pelled G, Turgeman G, et al. Neotendon formation induced by manipulation of the Smad8 signalling pathway in mesenchymal stem cells. J Clin Invest. 2006;116(4):940–52. doi: 10.1172/JCI22689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Correlo VM, Pinho ED, Pashkuleva I, Bhattacharya M, Neves NM, Reis RL. Water absorption and degradation characteristics of chitosan-based polyesters and hydroxyapatite composites. Macromol Biosci. 2007;7(3):354–63. doi: 10.1002/mabi.200600233. [DOI] [PubMed] [Google Scholar]
- 23.Costa-Pinto AR, Salgado AJ, Correlo VM, et al. Adhesion, proliferation, and osteogenic differentiation of a mouse mesenchymal stem cell line (BMC9) seeded on novel melt-based chitosan/polyester 3D porous scaffolds. Tissue Eng Part A. 2008;14(6):1049–57. doi: 10.1089/ten.tea.2007.0153. [DOI] [PubMed] [Google Scholar]
- 24.Oliveira JT, Correlo VM, Sol PC, et al. Assessment of the suitability of chitosan/polybutylene succinate scaffolds seeded with mouse mesenchymal progenitor cells for a cartilage tissue engineering approach. Tissue Eng Part A. 2008;14(10):1651–61. doi: 10.1089/ten.tea.2007.0307. [DOI] [PubMed] [Google Scholar]
- 25.Costa-Pinto AR, Correlo VM, Sol PC, et al. Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells Seeded on Melt Based Chitosan Scaffolds for Bone Tissue Engineering Applications. Biomacromolecules. 2009 doi: 10.1021/bm9000102. [DOI] [PubMed] [Google Scholar]
- 26.Roman I, Vilalta M, Rodriguez J, et al. Analysis of progenitor cell-scaffold combinations by in vivo non-invasive photonic imaging. Biomaterials. 2007;28(17):2718–28. doi: 10.1016/j.biomaterials.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Srouji S, Kizhner T, Ben David D, Riminucci M, Bianco P, Livne E. The Schneiderian membrane contains osteoprogenitor cells: in vivo and in vitro study. Calcif Tissue Int. 2009;84(2):138–45. doi: 10.1007/s00223-008-9202-x. [DOI] [PubMed] [Google Scholar]
- 28.Mrugala D, Bony C, Neves N, et al. Phenotypic and functional characterisation of ovine mesenchymal stem cells: application to a cartilage defect model. Ann Rheum Dis. 2008;67(3):288–95. doi: 10.1136/ard.2007.076620. [DOI] [PubMed] [Google Scholar]
- 29.Snedeker JG, Arav AB, Zilberman Y, Pelled G, Gazit D. Functional Fibered Confocal Microscopy: A Promising Tool for Assessing Tendon Regeneration. Tissue Eng Part C Methods. 2009 doi: 10.1089/ten.tec.2008.0612. [DOI] [PubMed] [Google Scholar]
- 30.Snedeker JG, Pelled G, Zilberman Y, et al. An Analytical Model for Elucidating Tendon Tissue Structure and Biomechanical Function from in vivo Cellular Confocal Microscopy Images. Cells Tissues Organs. 2008 doi: 10.1159/000189211. [DOI] [PubMed] [Google Scholar]
- 31.Snedeker JG, Pelled G, Zilberman Y, Gerhard F, Muller R, Gazit D. Endoscopic cellular microscopy for in vivo biomechanical assessment of tendon function. J Biomed Opt. 2006;11(6):064010. doi: 10.1117/1.2393153. [DOI] [PubMed] [Google Scholar]
- 32.Jones PH, Simons BD, Watt FM. Sic transit gloria: farewell to the epidermal transit amplifying cell? Cell Stem Cell. 2007;1(4):371–81. doi: 10.1016/j.stem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 33.Zipori D. The stem state: plasticity is essential, whereas self-renewal and hierarchy are optional. Stem Cells. 2005;23(6):719–26. doi: 10.1634/stemcells.2005-0030. [DOI] [PubMed] [Google Scholar]
- 34.Huang S. Reprogramming cell fates: reconciling rarity with robustness. Bioessays. 2009;31(5):546–60. doi: 10.1002/bies.200800189. [DOI] [PubMed] [Google Scholar]
- 35.Dezawa M, Ishikawa H, Itokazu Y, et al. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science. 2005;309(5732):314–7. doi: 10.1126/science.1110364. [DOI] [PubMed] [Google Scholar]
- 36.Dezawa M, Kanno H, Hoshino M, et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004;113(12):1701–10. doi: 10.1172/JCI20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1(1):39–49. doi: 10.1016/j.stem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 38.Slack JM. Metaplasia and transdifferentiation: from pure biology to the clinic. Nat Rev Mol Cell Biol. 2007;8(5):369–78. doi: 10.1038/nrm2146. [DOI] [PubMed] [Google Scholar]
- 39.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 40.Granero-Molto F, Weis JA, Miga MI, et al. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells. 2009;27(8):1887–98. doi: 10.1002/stem.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kinnaird T, Stabile E, Burnett MS, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109(12):1543–9. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 42.da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26(9):2287–99. doi: 10.1634/stemcells.2007-1122. [DOI] [PubMed] [Google Scholar]
- 43.Noel D, Caton D, Roche S, et al. Cell specific differences between human adipose-derived and mesenchymal-stromal cells despite similar differentiation potentials. Exp Cell Res. 2008;314(7):1575–84. doi: 10.1016/j.yexcr.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 44.Towler DA, Gelberman RH. The alchemy of tendon repair: a primer for the (S)mad scientist. J Clin Invest. 2006;116(4):863–6. doi: 10.1172/JCI28320. [DOI] [PMC free article] [PubMed] [Google Scholar]


