Abstract
While numerous studies have implicated copy number variants (CNVs) in a range of neurological phenotypes, the impact relative to disease severity has been difficult to ascertain due to small sample sizes, lack of phenotypic details, and heterogeneity in platforms used for discovery. Using a customized microarray enriched for genomic hotspots, we assayed for large CNVs among 1,227 individuals with various neurological deficits including dyslexia (376), sporadic autism (350), and intellectual disability (ID) (501), as well as 337 controls. We show that the frequency of large CNVs (>1 Mbp) is significantly greater for ID–associated phenotypes compared to autism (p = 9.58×10−11, odds ratio = 4.59), dyslexia (p = 3.81×10−18, odds ratio = 14.45), or controls (p = 2.75×10−17, odds ratio = 13.71). There is a striking difference in the frequency of rare CNVs (>50 kbp) in autism (10%, p = 2.4×10−6, odds ratio = 6) or ID (16%, p = 3.55×10−12, odds ratio = 10) compared to dyslexia (2%) with essentially no difference in large CNV burden among dyslexia patients compared to controls. Rare CNVs were more likely to arise de novo (64%) in ID when compared to autism (40%) or dyslexia (0%). We observed a significantly increased large CNV burden in individuals with ID and multiple congenital anomalies (MCA) compared to ID alone (p = 0.001, odds ratio = 2.54). Our data suggest that large CNV burden positively correlates with the severity of childhood disability: ID with MCA being most severely affected and dyslexics being indistinguishable from controls. When autism without ID was considered separately, the increase in CNV burden was modest compared to controls (p = 0.07, odds ratio = 2.33).
Author Summary
Deletions and duplications, termed copy number variants (CNVs), have been implicated in a variety of neurodevelopmental disorders including intellectual disability (ID), autism, and schizophrenia. Our understanding of the relevance of large, rare CNVs in a range of neurodevelopmental phenotypes, varying in severity and prevalence, has been difficult because these studies were restricted to the analysis of one disorder at a time using different CNV detection platforms, insufficient sample sizes, and a lack of detailed clinical information. We tested 1,227 individuals with different neurological diseases including dyslexia, autism, and ID using the same CNV detection platform. We observed striking differences in CNV burden and inheritance characteristics among these cohorts and show that ID is the primary correlate of large CNV burden. This correlation is well illustrated by a comparison of autism patients with and without ID—where the latter show only modest increases in large CNV burden compared to controls. We also find significant depletion in the frequency of large CNVs in dyslexia compared to the other cohorts. Further studies on larger sets of individuals using high-resolution arrays and next-generation sequencing are warranted for a detailed understanding of the relative contribution of genetic variants to neurodevelopmental disorders.
Introduction
Recent studies have implicated large, rare CNVs in a range of neurodevelopmental disorders including intellectual disability (ID) [1], [2], autism [3], [4], schizophrenia [5], [6], bipolar disorder [7], [8], epilepsy [9], [10], and attention deficit hyperactivity disorder (ADHD) [11], [12]. Several themes have emerged from these studies: first, a significant enrichment for rare CNVs in individuals with the disease compared to unaffected controls was observed, independently, for each of these disorders; second, the same recurrent CNVs are associated with different neuropsychiatric phenotypes; and third, locus heterogeneity is substantial as many distinct variants can lead to similar phenotypes.
Our understanding of the relevance of rare CNVs across a broad spectrum of neurodevelopmental disorders, varying in severity and prevalence, is limited as previous studies were restricted to the analysis of one phenotype at a time and each of such studies was performed using different CNV genotyping methodologies with distinct platform-specific biases, making comparisons difficult. We undertook a systematic analysis of 1,227 cases and 337 controls to assess the relative contribution of CNVs in three phenotypically distinct neurodevelopmental disorders. We designed a whole-genome custom microarray targeted to genomic hotspots for comparative genomic hybridization (CGH) to identify potentially pathogenic CNVs that contribute to ID, autism, and dyslexia.
Results
We analyzed 1,227 individuals ascertained for three neurodevelopmental disorders: 376 dyslexic children with a verbal IQ (VIQ) ≥90 on the Wechsler Intelligence Scale for Children [13] and dyslexia defined as poor performance and IQ-performance discrepancy in one or more of a set of standardized reading measures, 350 cases with sporadic autism from the Simons Simplex Collection (SSC), and 501 cases with ID. We used 337 NIMH control individuals for comparison. Further, based on the presence or absence of ID (full-scale IQ score cutoff of 70), autism cases were divided into those with ID (n = 97) or without ID (n = 253) (see Materials and Methods). Based on the presence of multiple congenital anomalies (MCA), individuals with ID were divided into those with ID only—i.e. idiopathic ID (n = 428)—and those with ID and MCA (n = 73).
All copy number variation analyses were performed using a custom microarray with a high probe density (∼2.6 kbp) targeted to 107 genomic hotspot regions [14] (∼251 Mbp) and a median probe spacing of ∼36 kbp in the genomic backbone (see Materials and Methods, Table S1). We used a Hidden Markov Model (HMM)-based algorithm to identify deletions and duplications. We restricted our analysis to CNVs >50 kbp in size to reduce false positive calls and validated all relevant CNVs using a second custom designed high-density array. To empirically determine the validation rate of the array at different genomic regions, we examined 118 CNVs detected in 24 samples and confirmed 117 events (>99% accuracy, see Table S2). While we were easily able to detect smaller events in the hotspot regions, the specificity of the array restricted our CNV discovery to >50 kbp in hotspot-associated regions and to >300 kbp in regions not associated with genomic hotspots (Figure S1).
Analysis for large CNV burden in neurodevelopmental phenotypes
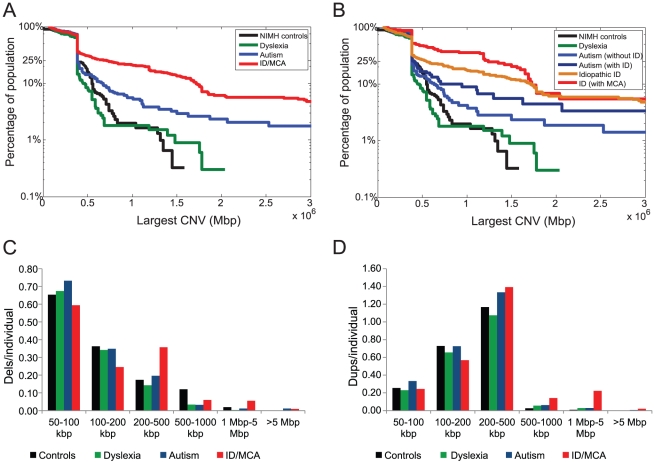
After quality control (QC) filtering and manual curation, we obtained 5,086 CNVs in 1,395 out of 1,564 individuals (89.2%) with high-quality array CGH data (Table 1; Datasets S1, S2, S3, S4). Using these data, we compared the CNV enrichment between the multiple cohorts tested. We found a significant excess of large CNVs (>1 Mbp) in individuals with ID (p = 2.75×10−17, odds ratio = 13.71) or autism (p = 0.012, odds ratio = 2.99) when compared to controls analyzed on the same microarray platform (Figure 1). The frequency of large CNVs among children with dyslexia was similar to controls (p = 0.64, odds ratio = 0.94), although this might indicate a lack of statistical power in our study to detect any subtle enrichment (power >0.8 to detect 4.2% increase in burden) for large CNVs in dyslexia.
Table 1. Summary of disease cohorts and CNV analysis.
| All | Total analyzed | Passed QC | Total CNVs | Average CNV size (bp) | Proportion of deletions | Proportion of CNVs disrupting genes | Average gene density |
| Controls | 337 | 306 | 1,074 | 229,701 | 0.38 | 0.33 | 3.70 |
| Dyslexia | 376 | 322 | 1,041 | 217,135 | 0.37 | 0.34 | 3.82 |
| Autism (no ID) | 253 | 246 | 923 | 249,996 | 0.33 | 0.36 | 4.15 |
| Autism (with ID) | 97 | 90 | 362 | 342,637 | 0.41 | 0.33 | 4.95 |
| Combined Autism | 350 | 336 | 1,285 | 276,094 | 0.35 | 0.35 | 4.55 |
| ID | 428 | 358 | 1,306 | 442,519 | 0.33 | 0.38 | 6.12 |
| ID/MCA | 73 | 73 | 380 | 637,004 | 0.36 | 0.39 | 8.20 |
| Combined ID cohort | 501 | 431 | 1,686 | 486,353 | 0.34 | 0.38 | 7.16 |
For breaking genes, one or both of the CNV breakpoints should traverse a gene. CNV: Copy Number Variant; ID: Intellectual Disability; MCA: Multiple Congenital Anomalies; QC: Quality Control.
Figure 1. CNV burden in neurodevelopmental disorders.
(A) The figure shows the population frequency of the largest CNV (as a survivor function) in individuals with ID, autism, dyslexia, and controls. (B) Population frequency of the largest CNV is shown for ID, ID with MCA, autism with ID, autism without ID, dyslexia, and NIMH control individuals. (C) Histograms depicting deletions per individual at each size range are shown. Note that 35 NIMH control samples carried an approximately 560 kbp deletion involving PRAME on distal 22q11.2. (D) Duplications per individual at each size range are shown. The hotspot chip has higher coverage over segmental duplication regions and therefore there is an expected abundance of duplications per individual compared to deletions.
Within the neurodevelopmental disorder cohorts, a comparison showed a significantly greater large CNV burden in individuals with ID compared to autism (p = 9.58×10−11, odds ratio = 4.58) or dyslexia (p = 3.81×10−18, odds ratio = 14.45). When we partitioned the ID cohort into subsets with and without MCA, we observed a significantly increased large CNV burden in individuals with ID/MCA compared to ID alone (p = 0.001, odds ratio = 2.54). This trend was also observed when individuals with autism were separated into those with ID and without ID (Figure 1), although not statistically significant (p = 0.102, odds ratio = 2.1). When compared to controls, we noted a trend for increase in large CNV burden for autism without ID (p = 0.07, odds ratio = 2.33) as well as autism with ID (p = 0.0048, odds ratio = 4.85). In addition, a gene-based analysis showed an incremental increase in the proportion of disrupted genes and average gene density per CNV with higher estimates for the ID/MCA cohort as compared to ID alone or autism (Table 1). We also note that within the cohorts no bias towards deletions or duplications was observed in relation to phenotypic severity or variability (Tables S3, S4, S5, S6, S7). Overall, our results suggest a positive correlation of the severity of the phenotype to the size and gene density of CNVs.
Rare CNVs in dyslexia, autism, and intellectual disability phenotypes
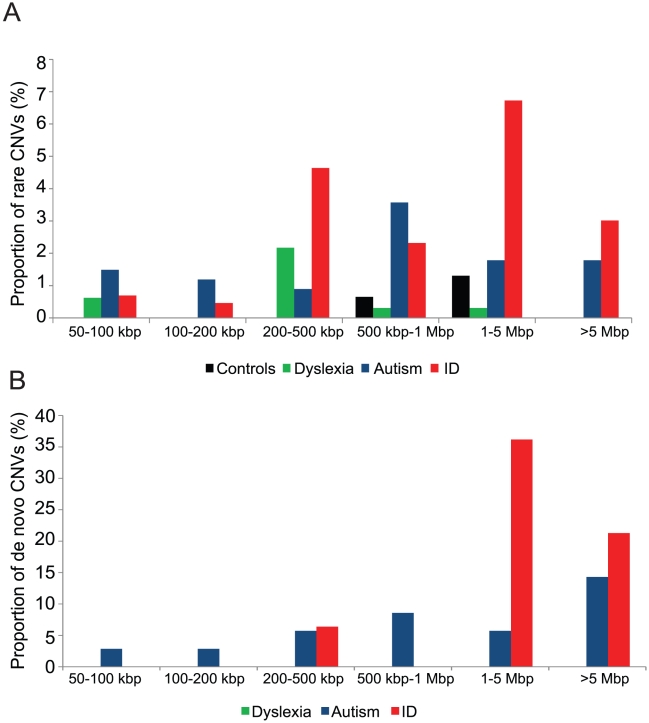
To identify rare CNVs of likely pathogenic significance, we compared the pattern of CNVs from dyslexia, autism, ID, and NIMH control cohorts to a map developed from an expanded set of 8,329 normal individuals genotyped with Illumina microarrays and to the publicly available Database of Genomic Variants [15] (see Materials and Methods). We eliminated common copy number polymorphisms and CNVs from our cases if they had a reciprocal overlap of 50% or more of their length with CNVs found in these 8,329 controls. After filtering, we compared the groups. We found a significant increase of rare CNVs in individuals with autism (35/336, 10%; p = 2.4×10−6, odds ratio = 6) or ID (69/431, 16%; p = 3.55×10−12, odds ratio = 10) compared to individuals with dyslexia (6/322, 2%) (Figure 2A, Table 2). In fact, when analyzed separately, the frequency of rare CNVs in NIMH controls (6/306, 2%) was not different compared to dyslexia (p = 0.57, odds ratio = 0.94) (Table S8).
Figure 2. Rare CNVs and de novo rates in neurodevelopmental disorders.
(A) The proportion of rare CNVs as a function of size is shown for NIMH controls and dyslexia, autism, and ID cohorts. To identify rare CNVs, we compared the pattern of CNVs from each of these cohorts to the CNV frequency map from 8,329 controls genotyped on Illumina arrays. (B) The proportion of de novo occurrence of CNVs among the three cohorts is shown for each size range. Note that the CNVs from the dyslexia cohort are all inherited. DNA from parents of NIMH controls was not available and hence not tested for de novo CNV frequency.
Table 2. Rare CNVs in neurodevelopmental disorders.
| Cohort | Total individuals analyzed | Number of individuals with rare CNVs | Total rare CNVs | Number of individuals with two rare CNVs | RefSeq genes | Median size of CNV | Hotspot CNVs | Genomic disorder CNVs |
| Dyslexia | 322 | 6 (1.9%) | 6 (1.9%) | 0 (0%) | 10 | 302 kbp | 3 (50.0%) | 0 |
| Autism (with ID) | 90 | 10 (11.1%) | 11 (12.2%) | 1 (10%) | 445 | 1.62 Mbp | 6 (54.5%) | 5 |
| Autism (no ID) | 246 | 25 (10.2%) | 25 (10.2%) | 0 (0%) | 235 | 633 kbp | 15 (60.0%) | 3 |
| Combined Autism | 336 | 35 (10.4%) | 36 (10.7%) | 1 (2.9%) | 680 | 662 kbp | 21 (58.3%) | 8 |
| Idiopathic ID | 358 | 60 (16.8%) | 64 (17.9%) | 4 (6.7%) | 1537 | 849 kbp | 20 (31.3%) | 15 |
| ID (with MCA) | 73 | 9 (12.3%) | 13 (17.8%) | 4 (44.4%) | 678 | 1.86 Mbp | 5 (38.5%) | 5 |
| Combined ID cohort | 431 | 69 (16.0%) | 77 (17.9%) | 8 (11.6%) | 2215 | 1.5 Mbp | 25 (32.5%) | 20 |
CNV: Copy Number Variant; ID: Intellectual Disability; MCA: Multiple Congenital Anomalies.
Given the high population prevalence of dyslexia [16], we then relaxed our selection to include events present at an allele frequency of <0.1% in controls (8/8,635) and identified four additional CNVs—i.e., a total of 10 CNV events (Table 3). The analysis of hotspot regions identified only one individual with dyslexia who carried a 15q11.2 BP1–BP2 deletion, which has previously been associated with ID [17], schizophrenia [18], [19], and epilepsy [20]; however, this deletion was also observed in 25/8,635 of our total control individuals. None of the seven deletions and three duplications detected in our dyslexia cohort mapped to candidate loci known to be associated with dyslexia [21].
Table 3. List of rare CNVs identified in neurodevelopmental disorders.
| Chr | Start | End | Size | Chr. Band | CNV | Sample | Cohort | Type | Gene count | Inheritance | Control count | DECIPHER count | DGV count |
| chr14 | 21533898 | 22239332 | 705434 | 14q11.2 | deletion | Si238 | Autism_ID | non HS | 12 | maternal | 0 | 2 | 6 |
| chr12 | 15036168 | 29977743 | 14941575 | 12p12.3p11.2 | deletion | Si159 | Autism_ID | non HS | 3 | de novo | 0 | 0 | 0 |
| chr15 | 66881730 | 71972563 | 5090833 | 15q23q24.1 | deletion | Si169 | Autism_ID | HS assoc | 2 | de novo | 0 | 0 | 0 |
| chr3 | 59681529 | 79199503 | 19517974 | 3p14.2p12.3 | deletion | Si163 | Autism_ID | non HS | 3 | de novo | 0 | 0 | 0 |
| chr5 | 175479593 | 175584441 | 104848 | 5q35.2 | deletion | Si118 | Autism_ID | HS | 1 | paternal | 0 | 0 | 4 |
| chr7 | 31583983 | 31702682 | 118699 | 7p15.3 | duplication | Si309 | Autism_ID | HS | 2 | maternal | 0 | 0 | 2 |
| chr16 | 21645311 | 22520339 | 875028 | 16p12.1 | duplication | Si247 | Autism_No ID | HS | 3 | NA | 0 | 2 | 0 |
| chr22 | 38790464 | 39138992 | 348528 | 22q13.1 | deletion | Si126 | Autism_No ID | non HS | 4 | de novo | 0 | 1 | 0 |
| chr3 | 67276200 | 72402720 | 5126520 | 3p14.1p13 | deletion | Si140 | Autism_No ID | non HS | 2 | de novo | 0 | 1 | 1 |
| chr1 | 145303997 | 145357746 | 53749 | 1q21.1 | homozyg deletion | Si192 | Autism_No ID | HS | 0 | both | 0 | 0 | 0 |
| chr1 | 145303997 | 145357746 | 53749 | 1q21.1 | deletion | Si85 | Autism_No ID | HS | 6 | paternal | 0 | 0 | 0 |
| chr11 | 21703096 | 26791696 | 5088600 | 11p14.5 | duplication | Si45 | Autism_No ID | non HS | 0 | maternal | 0 | 0 | 0 |
| chr16 | 72859686 | 72917454 | 57768 | 16p22.3 | deletion | Si99 | Autism_No ID | HS | 5 | maternal | 0 | 0 | 0 |
| chr17 | 15301836 | 16542913 | 1241077 | 17p11.2 | duplication | Si153 | Autism_No ID | HS | 147 | paternal | 0 | 0 | 0 |
| chr17 | 32250000 | 32400000 | 150000 | 17q12 | duplication | Si114 | Autism_No ID | HS | 1 | paternal | 0 | 0 | 0 |
| chr17 | 42115600 | 42437714 | 322114 | 17q21.32 | duplication | Si186 | Autism_No ID | HS | 53 | de novo | 0 | 0 | 0 |
| chr17 | 58889277 | 59561178 | 671901 | 17q23.3 | duplication | Si87 | Autism_No ID | non HS | 1 | maternal | 0 | 0 | 0 |
| chr18 | 69039514 | 69822140 | 782626 | 18q22.3 | deletion | Si173 | Autism_No ID | non HS | 2 | maternal | 0 | 0 | 0 |
| chr19 | 14760644 | 15064029 | 303385 | 19p13.12 | deletion | Si125 | Autism_No ID | non HS | 5 | maternal | 0 | 0 | 0 |
| chr2 | 95159338 | 95228560 | 69222 | 2q11.1 | duplication | Si226 | Autism_No ID | HS | 1 | de novo | 0 | 0 | 0 |
| chr20 | 12657983 | 13311383 | 653400 | 20p12.1 | deletion | Si20 | Autism_No ID | non HS | 17 | paternal | 0 | 0 | 0 |
| chr22 | 18562002 | 18748556 | 186554 | 22q11.21 | duplication | Si207 | Autism_No ID | HS | 5 | de novo | 0 | 0 | 0 |
| chr3 | 84868129 | 85439477 | 571348 | 3p12.1 | duplication | Si128 | Autism_No ID | non HS | 73 | paternal | 0 | 0 | 0 |
| chr5 | 370492 | 1003781 | 633289 | 5p15.33 | duplication | Si82 | Autism_No ID | HS | 17 | paternal | 0 | 0 | 0 |
| chr5 | 175504664 | 175584441 | 79777 | 5q35.2 | deletion | Si191 | Autism_No ID | HS | 3 | maternal | 0 | 0 | 4 |
| chr6 | 51096930 | 51899775 | 802845 | 6p12.2 | duplication | Si142 | Autism_No ID | non HS | 4 | paternal | 0 | 0 | 0 |
| chr7 | 152102637 | 153356944 | 1254307 | 7q36.2 | duplication | Si119 | Autism_No ID | HS | 123 | paternal | 0 | 0 | 0 |
| chr7 | 153451569 | 154285634 | 834065 | 7q36.2 | deletion | Si132 | Autism_No ID | non HS | 0 | maternal | 0 | 0 | 0 |
| chr1 | 144106777 | 144451305 | 344528 | 1q21.1 | deletion | 2602 | Dyslexia | HS | 1 | NA | 2 | 3 | 0 |
| chr3 | 139732796 | 140171095 | 438299 | 3q22.3 | duplication | 1806 | Dyslexia | non HS | 142 | NA | 1 | 1 | 0 |
| chr6 | 65179840 | 66364033 | 1184193 | 6q12 | deletion | 2803 | Dyslexia | non HS | 1 | paternal | 1 | 1 | 0 |
| chr4 | 123017758 | 123458923 | 441165 | 4q27 | duplication | 2286 | Dyslexia | non HS | 0 | maternal | 0 | 0 | 0 |
| chr7 | 40606348 | 40819984 | 213636 | 7p14.1 | deletion | 2244 | Dyslexia | non HS | 1 | maternal | 0 | 0 | 0 |
| chr7 | 68820751 | 68904999 | 84248 | 7q11.22 | deletion | 2867 | Dyslexia | HS | 2 | paternal | 0 | 0 | 0 |
| chr7 | 69876932 | 70546042 | 669110 | 7q11.22 | duplication | 1102 | Dyslexia | HS | 1 | paternal | 0 | 0 | 0 |
| chr7 | 110381300 | 110851860 | 470560 | 7q31.1 | deletion | 3437 | Dyslexia | non HS | 102 | paternal | 2 | 0 | 0 |
| chr8 | 11373083 | 11434911 | 61828 | 8p23.1 | deletion | 1012 | Dyslexia | HS | 16 | maternal | 0 | 0 | 0 |
| chr9 | 6348644 | 6740836 | 392192 | 9p24.1 | deletion | 1004 | Dyslexia | non HS | 11 | paternal | 0 | 0 | 0 |
| chr9 | 1 | 9098781 | 9098780 | 9p24 | deletion | 3381 | ID | non HS | 3 | de novo | 0 | 15 | 0 |
| chr11 | 121813520 | 134447248 | 12633728 | 11q24.1-q25 | deletion | 2597 | ID | non HS | 33 | de novo | 0 | 15 | 0 |
| chr18 | 1 | 15313807 | 15313806 | 18p11.21 | duplication | 2492 | ID | non HS | 1 | de novo | 0 | 4 | 0 |
| chr9 | 73827781 | 79830447 | 6002666 | 9q21.13 | deletion | 3413 | ID | non HS | 1 | de novo | 0 | 4 | 0 |
| chr3 | 196825112 | 197208742 | 383630 | 3q29 | deletion | 3331 | ID | HS | 0 | NA | 0 | 2 | 1 |
| chr6 | 161747330 | 162612669 | 865339 | 6q26 | deletion | 2562 | ID | non HS | 0 | maternal | 0 | 2 | 0 |
| chr6 | 162129914 | 162555946 | 426032 | 6q26 | deletion | 2548 | ID | non HS | 48 | paternal | 0 | 2 | 0 |
| chr9 | 218822 | 3742630 | 3523808 | 9p24 | deletion | 2615 | ID | non HS | 1 | de novo | 0 | 2 | 0 |
| chr3 | 71242809 | 77832202 | 6589393 | 3p13 | deletion | 2509 | ID | non HS | 0 | de novo | 0 | 1 | 0 |
| chr3 | 127000260 | 131353408 | 4353148 | 3q21.3 | duplication | 2237 | ID | non HS | 1 | NA | 0 | 1 | 0 |
| chr6 | 107959196 | 111971187 | 4011991 | 6q21 | deletion | 2644 | ID | non HS | 0 | de novo | 0 | 1 | 0 |
| chrX | 146437800 | 147110597 | 672797 | Xq27 | duplication | 2643 | ID | non HS | 6 | NA | 0 | 1 | 0 |
| chrY | 6895278 | 7233586 | 338308 | Yp11.2 | duplication | 2580 | ID | non HS | 2 | NA | 0 | 1 | 0 |
| chr1 | 76466419 | 77200494 | 734075 | 1p31.1 | duplication | 3399 | ID | non HS | 0 | maternal | 0 | 0 | 0 |
| chr1 | 90483825 | 90786224 | 302399 | 1p22.2 | duplication | 1799 | ID | non HS | 48 | NA | 0 | 0 | 0 |
| chr1 | 235537560 | 237086860 | 1549300 | 1q43 | deletion | 2518 | ID | non HS | 2 | NA | 0 | 0 | 0 |
| chr10 | 128662416 | 129042087 | 379671 | 10q26.2 | deletion | 1402 | ID | non HS | 23 | maternal | 0 | 0 | 0 |
| chr11 | 22232079 | 25091772 | 2859693 | 11p14.3 | deletion | 1613 | ID | non HS | 2 | de novo | 0 | 0 | 0 |
| chr13 | 95576502 | 96051348 | 474846 | 13q31.3q32.2 | deletion | 2175 | ID | non HS | 3 | NA | 0 | 0 | 0 |
| chr14 | 40428504 | 40755943 | 327439 | 14q21.1 | duplication | 3322 | ID | non HS | 89 | NA | 0 | 0 | 1 |
| chr15 | 80767738 | 100147041 | 19379303 | 15q25 | duplication | 2522 | ID | non HS | 36 | paternal or de novo | 0 | 0 | 0 |
| chr17 | 34089604 | 34566438 | 476834 | 17q12 | duplication | 72 | ID | HS | 4 | NA | 0 | 0 | 0 |
| chr18 | 50965716 | 52820402 | 1854686 | 18q21 | duplication | 1164 | ID | non HS | 37 | de novo | 0 | 0 | 0 |
| chr19 | 60032498 | 61147051 | 1114553 | 19q13.42 | deletion | 3262 | ID | non HS | 24 | de novo | 0 | 0 | 0 |
| chr2 | 153753287 | 183588035 | 29834748 | 2q24.3q32.1 | duplication | 2559 | ID | non HS | 5 | de novo | 0 | 0 | 0 |
| chr2 | 188179827 | 188853079 | 673252 | 2q32.1 | duplication | 2522 | ID | non HS | 1 | paternal or de novo | 0 | 0 | 0 |
| chr3 | 50846910 | 58424157 | 7577247 | 3p21.31p14.3 | duplication | 3448 | ID | non HS | 54 | de novo | 0 | 0 | 0 |
| chr3 | 62489781 | 63320060 | 832020 | 3p14.2 | duplication | 3445 | ID | non HS | 413 | paternal | 0 | 0 | 0 |
| chr3 | 137464270 | 137768886 | 304616 | 3q22.3 | deletion | 3349 | ID | non HS | 25 | NA | 0 | 0 | 0 |
| chr4 | 7148184 | 7818626 | 670442 | 4p16.1 | duplication | 2488 | ID | non HS | 2 | NA | 0 | 0 | 0 |
| chr4 | 43534966 | 45590689 | 2055723 | 4p13 | deletion | 1318 | ID | non HS | 0 | paternal | 0 | 0 | 0 |
| chr4 | 57346833 | 86106712 | 28759879 | 4q12q21.33 | duplication | 2154 | ID | non HS | 2 | de novo | 0 | 0 | 0 |
| chr4 | 152300259 | 152723977 | 423718 | 4q31.3 | deletion | 699 | ID | non HS | 9 | de novo | 0 | 0 | 0 |
| chr5 | 28427525 | 28630668 | 203143 | 5p14.1 | deletion | 2569 | ID | non HS | 4 | NA | 0 | 0 | 0 |
| chr5 | 98793016 | 98851760 | 58744 | 5q21.1 | deletion | 1519 | ID | HS | 1 | NA | 0 | 0 | 0 |
| chr5 | 156526018 | 164133824 | 7607806 | 5q33.3q34 | duplication | 3448 | ID | non HS | 1 | de novo | 0 | 0 | 0 |
| chr6 | 92767349 | 92988105 | 220756 | 6q16.1 | deletion | 3316 | ID | non HS | 15 | NA | 0 | 0 | 0 |
| chr7 | 45180992 | 45274014 | 93022 | 7p13 | deletion | 3296 | ID | HS | 1 | paternal or de novo | 0 | 0 | 1 |
| chr7 | 51216627 | 51313401 | 96774 | 7p12.1 | deletion | 2571 | ID | non HS | 24 | NA | 0 | 0 | 0 |
| chr7 | 68713709 | 69068502 | 354793 | 7q11.22 | deletion | 2433 | ID | HS | 66 | NA | 0 | 0 | 0 |
| chr7 | 72300576 | 72486542 | 185966 | 7q11.23 | duplication | 2424 | ID | HS | 47 | maternal | 0 | 0 | 0 |
| chr7 | 89028789 | 89548951 | 520162 | 7q21.13 | duplication | 3352 | ID | non HS | 42 | NA | 0 | 0 | 0 |
| chr8 | 123404368 | 123637074 | 232706 | 8q24.13 | deletion | 3247 | ID | non HS | 0 | de novo | 0 | 0 | 0 |
| chr9 | 21080948 | 21484861 | 403913 | 9p21.3 | deletion | 2462 | ID | non HS | 5 | NA | 0 | 0 | 0 |
| chr9 | 38634661 | 38791196 | 156535 | 9p13.1 | deletion | 3236 | ID | HS | 0 | paternal | 0 | 0 | 0 |
| chrX | 28400903 | 28659988 | 259085 | Xp21.3 | duplication | 3321 | ID | non HS | 0 | NA | 0 | 0 | 0 |
| chrX | 65611216 | 65934000 | 322784 | Xq12 | deletion | 2597 | ID | non HS | 2 | NA | 0 | 0 | 2 |
| chrX | 88508702 | 91291323 | 2782621 | Xq21.31q31.32 | deletion | 2511 | ID | non HS | 4 | NA | 0 | 0 | 0 |
| chrY | 3072083 | 6154525 | 3082442 | Yp11.2 | duplication | 699 | ID | HS | 2 | de novo | 0 | 0 | 0 |
| chr22 | 40103633 | 41458051 | 1354418 | 22q13.2 | deletion | GB43 | ID/MCA | non HS | 2 | NA | 0 | 2 | 0 |
| chr4 | 110560125 | 113895249 | 3335124 | 4q25 | deletion | GB6 | ID/MCA | non HS | 15 | de novo | 0 | 1 | 0 |
| chr1 | 174979260 | 238257861 | 63278601 | 1q24qter | duplication | GB88 | ID/MCA | non HS | 2 | 46,XX,t(1;5)(q23;p15) balanced | 0 | 0 | 0 |
| chr13 | 83679489 | 84385310 | 705821 | 13q31.1 dup | duplication | GB71 | ID/MCA | non HS | 9 | maternal | 0 | 0 | 0 |
| chr3 | 164241146 | 168622524 | 4381378 | 3q26.1 | deletion | GB42 | ID/MCA | non HS | 11 | maternal | 0 | 0 | 0 |
| chr5 | 90252 | 1630763 | 1540511 | 5p15.33 | deletion | GB88 | ID/MCA | HS | 0 | 46,XX,t(1;5)(q23;p15) balanced | 0 | 0 | 0 |
| chr7 | 27294680 | 28799546 | 1504866 | 7p15.3 | duplication | GB65 | ID/MCA | non HS | 23 | de novo | 0 | 0 | 0 |
| chr9 | 201336 | 16672312 | 16470976 | part trisomy 9 | duplication | GB71 | ID/MCA | HS assoc | 1 | 46,XX rcp (8;10)(q2.2;q21.2)+t (9;12)(p2.2;p1.3) | 0 | 0 | 0 |
This list does not contain known genomic disorders. Please refer to Table S9 for known genomic disorders identified in this study. CNV: Copy Number Variant; ID: Intellectual Disability; HS: Hotspot; MCA: Multiple Congenital Anomalies.
Analysis of 336 individuals from the SSC autism cohort showed that 35 individuals (10%) carried 36 rare CNVs (680 RefSeq genes, median size = 662 kbp) and about 58% (21/36) of these CNVs mapped to genomic hotspots (Table 2). Only eight of the events (all hotspot sites) associated with genomic disorders, including 22q11.2 deletion (TBX1, DiGeorge syndrome), 17p12 duplication (PMP22, Charcot-Marie-Tooth disease), and 15q11.2q13.1 duplication (UBE3A and SNRPN) (Table S9). In addition, as reported previously [3], [22], [23], the autism-associated proximal 16p11.2 deletion (TBX6) was observed in approximately 1% (3/336) of all autism cases analyzed. Interestingly, one case with a de novo 16p11.2 deletion also inherited a 2 Mbp duplication 22q11.2 (TBX1) from the mother.
Among 431 cases with ID (358 cases with ID only and 73 cases with ID plus MCA), 69 individuals carried 77 rare CNVs (2,215 RefSeq genes, median size = 1.5 Mbp) that were either of known pathogenic significance or not observed in a total set of 8,635 controls, and 32% (25/77, median size = 1.42 Mbp) of these variants localized to genomic hotspot regions (Table 2). This is a significant enrichment for rare CNVs in the ID cohort compared to autism (p = 0.019, odds ratio = 1.6) or dyslexia cohorts (p = 3.55×10−12, odds ratio = 10). Interestingly, 20/77 CNVs (16 hotspot and four non-hotspot sites) mapped to a known genomic disorder site, including those associated with variable phenotypes such as 15q13.1q13.3 (CHRNA7), 16p11.2 proximal (TBX6; two cases) and distal (SH2B1) hotspots, 16p13.11 (MYH11; three cases), 17q12 (TCF2), and 3q29 (DLG1) as well as syndromic regions such as 7q11.23 (Williams syndrome), 17q21.31 (MAPT), 5q35 (Sotos syndrome), 8p23.1, 22q13 (Phelan-McDermid syndrome) [24] and 1p36 [25].
We next sought to determine whether these rare CNVs were inherited or if they arose de novo in the probands. Parental DNA samples were available to investigate inheritance for 90 out of 123 rare CNVs detected in all three disease cohorts (Table S8). In four cases, only maternal DNA was available. We find that 44/90 CNVs arose de novo and a majority (77%, 34/44) of these de novo CNVs were large (>1 Mbp). Overall, we find a greater proportion of de novo events in ID (64%, 30/47) compared to autism (40%, 14/35; p = 0.027, odds ratio = 2.6) or dyslexia (0/8; p = 0.0009, odds ratio = infinity) (Figure 2B). These data are suggestive of a general trend of increased de novo rates and CNV size with increased severity of the disorder.
Novel, rare CNVs reveal potential candidate genes
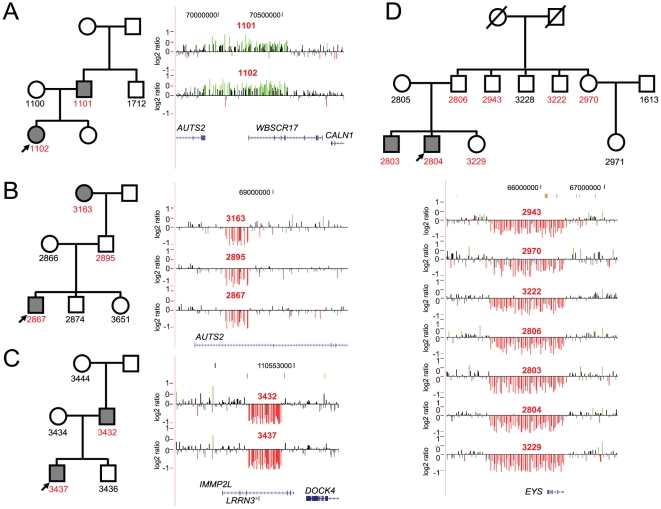
We then focused on rare CNVs involving single genes or regions of potential interest. In the dyslexia cohort, two unrelated families carried CNVs on chromosome 7q11.23 that involved the autism susceptibility candidate 2 (AUTS2, MIM# 607270). A 669 kbp duplication that included AUTS2 and WBSCR17 was transmitted from an affected father to the daughter and an approximately 84 kbp deletion was transmitted from the affected paternal grandmother through the unaffected father to the proband (Figure 3). In addition, we also identified a 354 kbp deletion encompassing AUTS2 in one individual with idiopathic ID, pervasive developmental delay, partial epilepsy, and left hemihypertrophy. An approximately 1.2 Mbp deletion encompassing the eyes shut drosophila homolog gene (EYS, MIM# 612424) on chromosome 6q12 was detected in an affected proband and several unaffected family members. Although autosomal recessive single-nucleotide mutations in EYS have been reported in patients with retinitis pigmentosa [26], [27], the role of heterozygous microdeletions involving this gene is unknown.
Figure 3. Pedigree shows the inheritance of a 669 kbp duplication encompassing AUTS2 and WBSCR17 from a father to the daughter.
(A) The father has features of dyslexia with a verbal IQ (VIQ) of 122, WATTa 93, WRAT3spb 83 and WIATspc 94. The daughter's scores are VIQ 122, WIDd 86, WATT 91, WRAT3sp 90, and WIATsp 94. (B) In this pedigree the 84 kbp deletion within AUTS2 is transmitted to the proband (VIQ 122, WATT 97, WRAT3sp 96, WIATsp 94) from the affected paternal grandmother (VIQ 118, WATT 88) through the unaffected father. (C) A deletion within IMMP2L is shown for this family. The deletion is transmitted to the proband (VIQ 111, WID 83, WATT 82, WRAT3sp 85, WIATsp 81) from his affected father (VIQ 84, WRAT3sp 68, WIAT-2sp 66). Interestingly, IMMP2L variants have been associated with Tourette syndrome, ADHD, and autism. (D) A 1.2 Mbp deletion within EYS is shown in several family members of this large pedigree. While the proband (VIQ 107, WID 56, WATT 81, WRAT3sp 87, WIATsp 87) and his affected brother (VIQ 101, WID 66, WATT 82, WRAT3sp 78, WIATsp 85) carried the deletion, so did many other unaffected relatives, including the father. Although no inference can be drawn for its role in dyslexia, as the deletion does not segregate with the phenotype, recessive mutations in EYS have been associated with retinitis pigmentosa. aWATT- WRMT-R Woodcock Reading Mastery Test – Revised; Word Attack subtest [67]. A measure of untimed reading of single non-words. bWRAT3sp - Wide Range Achievement Tests – Third Addition; Spelling subtest [68]. Spelling of single words from dictation in writing. cWIAT(2)sp - Wechsler Individual Achievement Test (2nd edition); Spelling subtest [69]. Spelling of single words from dictation in writing. dWID - WRMT-R Woodcock Reading Mastery Test – Revised; Word Identification subtest [67]. A measure of untimed reading of single words.
We also identified a 471 kbp deletion involving IMMP2L inherited by the proband from the affected mother (Figure 3). Deletions involving IMMP2L have been associated with ADHD [12], autism [28], and Tourette syndrome [29]. Recently, Pagnamenta and colleagues also reported a 594 kbp IMMP2L-DOCK4 deletion resulting in a fusion transcript and an intragenic DOCK4 deletion segregating with dyslexia [30]. Our results are best interpreted within the context of candidate gene identification in dyslexia. Although at least nine chromosomal loci are associated with dyslexia, for two of these loci the candidate genes were identified on the basis of a rare balanced chromosomal translocations disrupting ROBO1 [31] and DYX1C1/EKN1 [32], [33]. More recently, a Danish Cytogenetic Registry study of all cases with chromosomal translocations identified additional novel dyslexia candidate genes affirming the value of rare structural variants in understanding the genetics of dyslexia [34]. Our study is the first to systematically characterize rare CNVs in dyslexia and thus evaluate the contribution of rare deletions and duplications to this common genetic disorder.
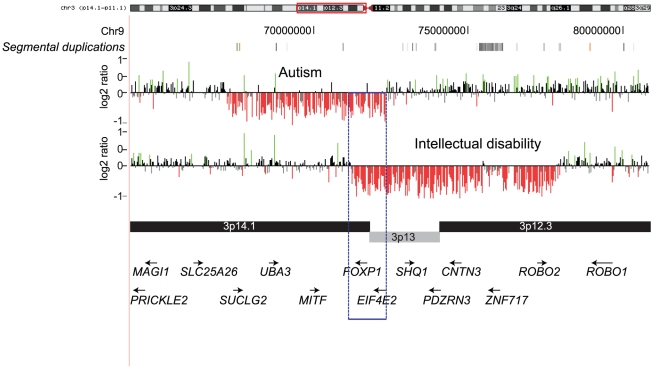
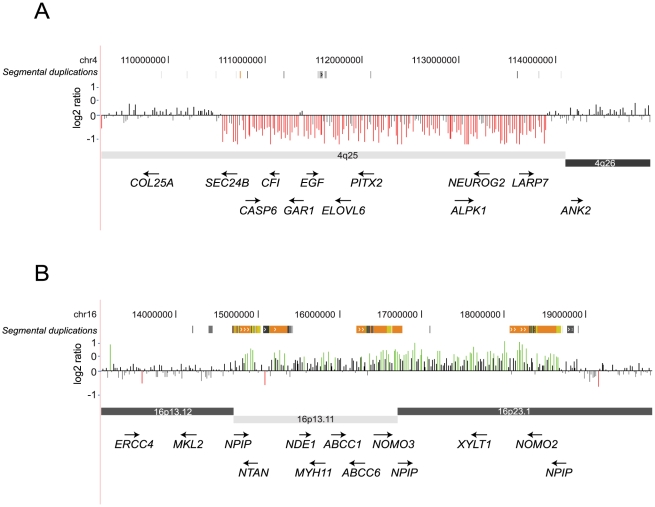
Within the autism cohort, several novel deletions and duplications involving neurologically-relevant genes were identified. A 5 Mbp de novo deletion involving FOXP1 on chromosome 3p14.1 was identified in an individual with features of idiopathic autism (full-scale IQ = 75). An additional 6.6 Mbp de novo deletion overlapping FOXP1 was also identified in an individual with idiopathic ID (Figure 4). A review of the DECIPHER database revealed a similar-sized deletion disrupting FOXP1 in an individual with developmental delay, sensorineural deafness, hypotonia, club foot, and dislocation of hip. Recently, FOXP1 was implicated in autism, ID, and language impairment [35], [36], [37]. It is believed that FOXP1interacts with FOXP2 and CNTNAP2, both implicated in speech disorders and autism [38], [39]. The overlapping 1.16 Mbp region of the deletion common to both autism and ID indicates a potential involvement of FOXP1 in pathways related to both of these disorders. Other variants involving functionally relevant genes include 7q36.2 deletion and duplication (DPP6), 17q23.3 duplication (SCN4A), and 17q21.32 duplication (WNT3 and WNT9B).
Figure 4. FOXP1 deletions in individuals with autism and ID.
Two deletions (5 Mbp and 6.6 Mbp) are shown intersecting at a common region of 1.16 Mbp containing FOXP1. Note that the deletion in the autism individual also covers ROBO2 and CNTN3.
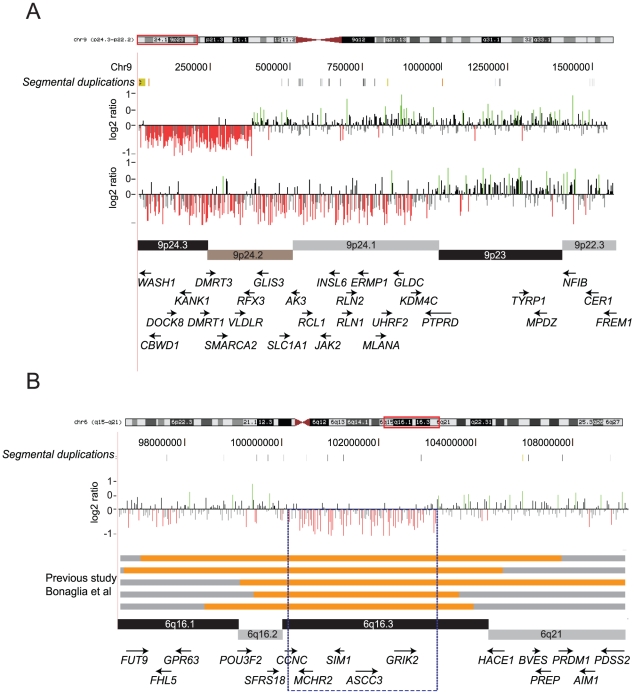
Our analysis of the ID cohort was enriched for singleton events often involving genes related to developmental or neurological functions including SYNPR, GABRA, AUTS2, FOXP1, FKBP6, COBL, and FMR1. However, within the same cohort we were also able to detect novel overlapping deletions (3.5 Mbp and 9 Mbp) on chromosome 9p24 in two unrelated individuals (Figure 5A). Both cases exhibited clinical features of ID and Pervasive Developmental Delay-Not Otherwise Specified. The distal breakpoints of these deletions map to segmental duplications while the proximal end maps within a high density of repeat elements. A survey of this region in the DECIPHER database [40] revealed about 15 cases with overlapping deletions. Variable clinical presentations and heterogeneity of deletion breakpoints preclude further genotype-phenotype correlation studies for this region (Figure S2). We also identified a nonrecurrent 6q16 deletion (chr6: 100,383,567-103,310,184) that potentially narrows the critical region for this recently described Prader-Willi-like syndrome [41] to approximately 2.9 Mbp. The refined critical region contains only five genes including the obesity-associated SIM1 [42] and the autism-associated GRIK2 [43] (Figure 5B). About 70% of children with 6q16 deletion manifest obesity [41]; however, our case with the smaller deletion, encompassing SIM1, showed no evidence of obesity at 10 years of age (Table S10).
Figure 5. Novel CNVs identified in the ID cohort.
(A) Overlapping deletions on chromosome 9p24 are shown. Deletions of this region containing DMRT1 and DMRT3 have also been associated with urogenital abnormalites and sex reversal. (B) A ∼3 Mbp deletion on 6q16 is shown encompassing SIM1. Larger deletions of this region were previously reported and have been associated with obesity. Orange bars denote deletions and gray regions are non-deleted regions from previous studies [41]. Taken together with other published studies, this deletion narrows down the critical region (dotted box) to about 2.9 Mbp.
Multiple large CNVs associate with phenotypic severity
We find that 8/69 (11.6%) cases in the ID cohort carried more than one large, rare CNV and all of these individuals presented with severe clinical features (Tables S6, S7, S8). A striking difference (p = 0.008, odds ratio = 11.2) in multiple CNV rates was also observed when the ID cohort was divided into those with severe MCA (44%) and those with idiopathic ID (6.7%). Notable examples are co-occurrences of a 3.4 Mbp 16p13.11 duplication and a 3.3 Mbp deletion on chromosome 4q25 involving PITX2 in a case with features of Rieger syndrome [44] (Figure 6) and a 17p13.3 deletion (YWHAE, Miller-Dieker syndrome) and 3q29 duplication (DLG1) in a child with cryptorchidism, ventricular septal defect, and seizures. The 3q29 duplication is a recurrent interstitial rearrangement [45], [46] potentially mediated by flanking segmental duplications of high sequence identity (27 kbp size, 96% identity). The 17p13.3 deletion is a previously reported nonrecurrent rearrangement associated with Miller-Dieker syndrome [47], [48]. In contrast, only 1/35 (2.9%) autism cases and none of dyslexia individuals carried another large CNV. This observation suggests that the severity of the phenotypes can be influenced by more than one large, rare CNV co-occurring in the same individual. During this analysis we considered the possibility of a derivative chromosome representing an unbalanced translocation possibly creating the impression of multiple CNVs in our cases. We carefully reviewed available chromosomal analysis data (G-banded karyotyping or FISH) for each of the individuals with two hits reported in our study. We did find one case with two hits where apparent CNVs represent a derivative chromosome inherited from a balanced translocation carrier parent (Table S8D).
Figure 6. Two large CNV hits in a case with ID/MCA.
A 3.3 Mbp deletion containing PITX2 as well as the 3.4 Mbp paternally inherited 16p13.33 duplication is shown for an individual with ID plus MCA. This individual has features of Rieger syndrome including visual defects, mild hypotonia, right congenital glaucoma, left microophalmia, and anterior segment dysgenesis. Other features include cleft uvula, hypodontia and conical teeth, hyperplasia of frenulum of tongue, midface hypoplasia, strabismus, and deafness.
Discussion
Initial discoveries of significant enrichment of rare CNVs for ID and autism led to testing the CNV basis for other behavioral and neurodevelopmental disorders of varying population frequency and severity, such as schizophrenia, ADHD, epilepsy, bipolar disorder, and Tourette syndrome. However, comparisons between these studies have been difficult due to differences in study design, insufficient sample sizes, and lack of detailed phenotype information. In this study, we compared 1,564 individuals (cases and controls) on a single platform of relatively modest density with the same type of detection bias. We utilized the duplication architecture of the human genome to custom design a DNA oligonucleotide microarray enriched for genomic hotspots, i.e., regions flanked by high-identity segmental duplications. This array has an advantage over several other commercial arrays in that there is a 25-fold enrichment for recurrent events in the genomic hotspots compared to the rest of the genome [49]. Therefore, fewer samples are required to identify several unrelated individuals with the same pathogenic mutation. We find that our array has a comparable diagnostic yield of 16% for the ID cohort compared to other clinical chromosomal microarray studies reported in the literature (Figure S3).
In strong agreement with previous studies, our data suggest that multiple, rare CNVs contribute to the etiology of autism and ID. In contrast, we find no increase in large pathogenic CNVs in individuals with dyslexia compared to controls. Notwithstanding, our analysis revealed novel regions of potential relevance to the etiology of dyslexia. Two unrelated children (2/322, 0.6%) with dyslexia carried CNVs encompassing AUTS2, both inherited from a parent. While the phenotype of dyslexia segregated with the AUTS2 duplication in the first family (Figure 3A), in the second family the deletion was inherited from affected grandmother through unaffected father (Figure 3B). This could be due to a phenomenon described as “compensation”, where some adults that reported difficulties with reading in childhood no longer evidence signs of dyslexia [50]. Previous studies of de novo chromosomal translocations and inversions identified breakpoints within AUTS2 in individuals with autism and/or ID phenotypes [51], [52], [53], [54], [55]. More recently, unique AUTS2 deletions and duplications were observed in Juvenile Myoclonic Epilepsy [10] and ADHD [11], [12]. It is interesting to note that ADHD and dyslexia are frequently comorbid and may have shared genetic risk factors [56], [57], [58]. When our study is taken together with recent CNV studies of ADHD [11], [12], AUTS2 CNVs were observed in 5/2,306 combined cases and 3/46,947 unscreened controls (p = 1.12×10−5, odds ratio = 33.9), indicating that AUTS2 might have an important role in pathways related to cognition. While the function of AUTS2 is still unclear, it is strongly expressed in fetal and adult brains, particularly in the frontal, parietal, and temporal lobes [59]. Interestingly, AUTS2 and the 7q11.2 region were identified as having the strongest statistical signal for positive selection in early modern humans as compared to the Neanderthal genome [60], indicating that AUTS2 might be important for a specialized human function such as cognition.
The CNV profile we observed in individuals with dyslexia was essentially the same as that in control individuals. This is not surprising if we take into consideration that all the subjects in our dyslexia sample had a VIQ above the 25%ile and the mean VIQ of the cohort was 110 (2/3 standard deviations above the general population mean), and given that we have shown that the CNV profile correlates with the severity of ID. The genes involved in dyslexia are likely to affect more specialized cognitive functions, may not adversely affect general intelligence, and may be more amenable to discovery with high-density arrays capable of detecting single gene or single exon CNVs or SNP microarrays that can leverage SNP allele frequency information in addition to signal intensity. In addition, all hybridization-based platforms fail to detect copy number neutral changes, such as balanced chromosomal rearrangements and inversions. This is particularly germane to dyslexia where a large number of candidate genes have been identified through mapping of translocation breakpoints [21], [34].
A comparison of rare de novo CNV rates for autism shows that our estimates (4%, 14/336) fall within a range of 4–10% reported previously by other large-scale, high-density array studies [4], [61], [62], [63]. This suggests that no platform-specific bias exists for large variants and also that the contribution of large CNVs is consistent across all studies for autism. We find a significantly greater enrichment for large CNVs, higher de novo rates, and a higher frequency of two rare CNV hits in individuals with ID-associated phenotypes compared to autism or dyslexia. This observation is exemplified by the fact that individuals with autism with ID have more large CNVs than those with autism only. We also find a significant difference between individuals with autism versus those with dyslexia. Sanders and colleagues recently analyzed 1,124 SSC families affected with autism spectrum disorder (ASD). Using stepwise linear models, they evaluated the relationship between intellectual functioning, sex, and the number of genes within rare, de novo CNVs. While the number of genes affected correlated with the size of the de novo CNV, the authors did not find a strong correlation of the Autism Diagnostic Observation Schedule (ADOS) combined severity score (p = 0.25, R2 = 0.005) or full-scale IQ (p = 0.02, R2 = 0.08) with the size of the CNV. In contrast, we considered all large CNVs (common and rare, de novo and transmitted) identified in a relatively smaller sample size and essentially bifurcated the autism cohort using a full-scale IQ score cutoff of 70. There was also a greater enrichment of two hits in the ID cohort (11.6%) compared to the autism cohort (2.8%). In fact, one individual carrying a 16p11.2 deletion with autism and features of ID also has a maternally inherited 22q11.2 duplication (TBX1) providing further evidence for the two-hit hypothesis we previously proposed for severe developmental delay [64]. Further, the frequency of two hits was even more striking when only individuals with ID/MCA were considered (44%), albeit the number of cases is few. We believe these data provide support for an incremental effect of CNV size and number on the severity of phenotypic outcome.
Our experimental design is biased towards interrogating hotspot regions in the human genome. A comparison to recently reported studies [61], [63] suggests that the majority of false-negative calls will reside within non-hotspot regions due to a lack of probe coverage (<10 probes). While the detection power of our array increases with the size of the variant, we would certainly miss smaller and intragenic CNVs, for example in autism candidate genes such as NRXN1 [65], [66], CACNA1C, SLC4A10, MAGI1 [63], SYNGAP1, DLGAP2 [62], NLGN1, ASTN2 [67], and exonic copy number variants in ASPM, DPP10, CNTNAP2, A2BP1, PCDH9 [68], and PTCHD1 [69]. While we find no excess of large CNVs in dyslexia, there is still the possibility that large CNVs are relevant in some familial cases of the disease as well as occasional sporadic cases. Further studies are warranted for a more detailed analysis of all the three neurodevelopmental cohorts using high-resolution arrays and next-generation exome and/or whole-genome sequencing. While it can be difficult to compare data derived from different microarrays, there is value to multiple array platforms and cross-platform validation. The depositing of the resulting data into publicly available databases will facilitate the continued elucidation of recurring clinically significant CNV and genotype-phenotype correlations.
Materials and Methods
Ethics statement
Patients from each of study cohort were recruited after appropriate human subjects approval and informed consent. Informed consent was also obtained to publish photographs.
Patient ascertainment based on severity of phenotypes
DNA samples were obtained from cases ascertained for three neurodevelopmental disorders of varying severity: (1) ID/developmental delay and MCA, (2) dyslexia or reading impairment, and (3) idiopathic autism. We defined severity of clinical features based on presence or absence of ID (IQ<70) for the autism group and congenital malformation for the ID group. Our dyslexia cohort had no ID or congenital malformation cases; as an IQ≤90 and the presence of congenital malformations were exclusion criteria. Individuals with idiopathic autism were partitioned into those with autism and ID (IQ<70) and those without ID (IQ>70). For the ID cohort, those individuals with brain malformations, gross craniofacial dysmorphology, cardiac defects, and neurological deficits were separated into an ID plus MCA (ID/MCA) group. Thus, in the order of severity, the ID/MCA cohort is considered the most severe, followed by ID only, autism with ID, autism without ID, dyslexia, and normal controls. However, we note that although the individuals with dyslexia do not have ID, they have severe impairments in core phonological measures leading to significantly reduced reading abilities despite normal IQ (IQ≥90). Detailed descriptions of each of the cohorts are given below.
Ascertainment of individuals with dyslexia or reading disability
For the dyslexia subject set, children were considered eligible for the study if they met researcher-defined criteria based on test scores from a standardized battery of tests. DNA samples were obtained from two cohorts. The first cohort included probands aged 6 to 16 from 198 families who were initially ascertained at the University of Washington (UW) multidisciplinary Learning Disability Center (UWLDC) under protocols approved by the UW Institutional Review Board. For the UWLDC cohort, probands were required to have a prorated VIQ at or above 90 (≥25%ile) on the Wechsler Intelligence Scale for Children – 3rd edition [13], with performance below the age-specific population mean and at least one standard deviation below the VIQ on one or more out of 10 research measures of reading, writing, or spelling. As a group, on average, probands met the impairment criteria between 6 and 7 measures. As expected by ascertainment requirements, the average VIQ of probands in this cohort was 110 (≥75%ile) [70], [71]. Siblings older than 6.5 years were invited to participate, and additional family members were added using a sequential sampling strategy to extend pedigrees through family members with the most extreme impairment values on the same 10 research measures. Detailed recruitment and evaluation procedures for the UWLDC cohort were described earlier [50], [72] (see Table S4A).
For the second cohort, 178 children aged 5 to 12 were recruited from a special K-6 school for students with dyslexia or via their direct relatives in the Atlanta area (The Schenck School, Atlanta, GA). For this cohort, children were required to have a psychological battery of tests completed by a licensed psychologist and usually have a diagnosis of a reading disability. Based on strong verbal comprehension score, perceptual reasoning score, Peabody picture vocabulary test, or other cognitive tests that measure intelligence, these children have average to above-average intelligence. Both cohorts were composed of individuals with >90% Caucasian ethnicity with an approximately equal number of males and females. Except for ADHD, children with other psychiatric and neurological disorders, moderate to severe receptive language disorders, developmental disabilities, or other conditions known to affect cognition were excluded based on parental questionnaire. Clinical details are shown in Table S4B.
Ascertainment of individuals with features of autism with or without ID
For the autism cohort, families were identified through the SSC (www.sfari.org) [73]. The Simons Foundation-funded SSC includes families with no more than one child with autism ascertained through 12 data collection sites across North America. Of the 350 individuals included in this study 297 (85%) are of Caucasian ethnicity. Inclusion criteria in the collection requires that the child with autism meet ASD criteria on the ADOS [74], on the Autism Diagnostic Interview, Revised (ADI-R) [75], and meet expert clinical judgment. Nonverbal IQ estimate must also be greater than 35. Children with significant hearing, vision, or motor problems, significant birth complications (e.g. extended NICU stay), or with a diagnosis of ASD-related disorders, such as Fragile X, were excluded. Children with a relative (up to third degree) with ASD or sibling who showed ASD-related symptoms were also excluded. Diagnostic evaluations, cognitive assessment, and phenotypic characterization were conducted at each site with data collection, data entry, and data validation methods standardized across sites to ensure reliability of sample collection. We further partitioned the autism cohort into those associated with ID (average full scale IQ = 49) consisting of 97 cases (73 males, 24 females; median age, 12 years) and those without ID (average full scale IQ = 98.9) comprising 253 cases (228 males, 25 females; median age, 11 years and 11 months). Clinical details are shown in Table S5.
Ascertainment of individuals with intellectual disability with or without congenital malformation
The idiopathic ID cohort was selected from individuals admitted to the IRCCS Associazione Oasi Maria Santissima and screened for ID according to the Diagnostic and Statistical Manual of Mental Disorders-IV-Text Revision (DSM-IV-TR) criteria. This cohort consists of 428 cases (153 females, 275 males; median age, 15 years) of Caucasian ethnicity with idiopathic ID and previously excluded for common causes of ID, including Fragile X syndrome, trisomies 21 and 13. In addition, classical genomic syndromes such as Smith-Magenis, DiGeorge, Prader-Willi/Angelman, and Williams syndromes, if recognized by clinical evaluation, were followed up for confirmation using targeted multiplex ligation-dependent probe amplification and excluded. We note that cases with phenotypic variability that escape clinical detection might not have been excluded. Typically, idiopathic cases of ID with no classical constellation of clinical features suggestive of a known disorder or those with mild to moderate ID without significant congenital malformation were included in this cohort. Clinical details are shown in Table S6.
Individuals with features of ID with MCA not necessarily assigned to a specific syndrome were evaluated and recruited at the University of Torino. This cohort consists of 73 individuals (32 females and 41 males) of Caucasian ethnicity with a median age of 2 years at diagnosis. Clinical features of these individuals included brain malformations, craniofacial dysmorphology, and neurological deficits along with variable ID (Table S7). Informed consent was obtained from all the subjects included in both the studies.
Ascertainment of normal controls
The control cohort consisted of 337 DNA samples obtained from the Rutgers University Cell and DNA Repository (www.rucdr.org). These individuals were ascertained by the NIMH Genetics Initiative [76] through an online self-report based on the Composite International Diagnostic Instrument Short-Form (CIDI-SF) [77] and screened specifically for eight mental health disorders, including major depression, bipolar disorder, and psychosis, but were not screened for dyslexia and therefore not ideal for such comparisons. Those who did not meet DSM-IV criteria for major depression, denied a history of bipolar disorder or psychosis, and reported exclusively European origins were included [78], [79].
Additionally, CNV data from 8,329 additional cell line and blood-derived controls were used to assess the frequency of our putative pathogenic CNVs in a larger population of neurologically normal individuals. These data were derived primarily from genome-wide association studies of non-neurological phenotypes. Although these data were not ascertained specifically for neurological disorders, they consist of adult individuals providing informed consent. Specifically, datasets from the following sources were included in our analysis: Human Genome Diversity Project [49], [80]; National Institute of Neurological Disorders and Stroke (NINDS) (dbGaP accession no. phs000089) [49], [81]; Pharmacogenomics and Risk of Cardiovascular Disease (PARC/PARC2) [82], [83]; parents of asthmatic children courtesy of Stephanie London [49]; Fred Hutchinson Cancer Research Center (prerelease data provided courtesy of Aaron Aragaki, Charles Kooperberg, and Rebecca Jackson as part of an ongoing genome-wide association study to identify genetic components of hip fracture in the Women's Health Initiative); InCHIANTI (data provided by InCHIANTI study of aging, www.inchiantistudy.net) [49], [84]; and the Wellcome Trust Case Control Consortium phase 2 (National Blood Service) [7]. All samples were genotyped on Illumina arrays using methodology described previously [49] [85] and either natively processed in hg18 or re-mapped after CNV calling (NINDS and PARC) to hg18 using the UCSC LiftOver tool (http://genome.ucsc.edu).
Array CGH and analysis
We designed custom targeted hotspot v1.0 arrays comprised of 135,000 probes (by Roche NimbleGen) with higher density probe coverage (median probe spacing 2.6 kbp) in the genomic hotspots (regions flanked by segmental duplications) and a lower probe density in the genomic backbone (median probe spacing 36 kbp). All microarray hybridization experiments were performed as described previously [86], using a single unaffected male (GM15724 from Coriell) as reference. All validation experiments were performed using two custom array designs: (1) a custom targeted 4×180 K Agilent chip with median probe spacing of 2 kbp in the genomic hotspots and whole-genome backbone coverage of one probe every 36 kbp (Agilent Technologies) and (2) a custom targeted 3×720 K NimbleGen or 2×400 K Agilent chip with median probe spacing of 500 bp in the genomic hotspots and probe spacing of 14 kbp in the genomic backbone.
All arrays were analyzed by mapping probe coordinates to the human genome assembly Build 36 (hg18). Using chromosome-specific means and standard deviations, normalized log intensity ratios for each sample were transformed into z-scores. These z-scores were then classified as “increased”, “normal”, or “decreased” in copy number using a three-state HMM. The HMM was applied using HMMSeg [87]. For each sample, HMM state assignments of probes were merged into segments if consecutive probes of the same state less than 50 kbp apart. If two segments of the same state were separated by an intervening sequence of ≤5 probes and ≤10 kbp, both segments and intervening sequence were called as a single variant. Further, we employed stringent QC measures and empirically estimated post-HMM filtering thresholds (absolute z-score >1.5 and >10 probes) to increase the specificity of our experiments. With these filtering criteria, we were able to thoroughly scan HMM outputs for CNV events and manually check the validity of each call by examining the normalized log intensity ratios across a chromosome. For the Agilent arrays, data analysis was performed following feature extraction using DNA analytics with ADM-2 setting according to the manufacturer's instructions. All CNVs calls were visually inspected in the UCSC genome browser.
First, we carried out validation on 24 samples from the developmental delay cohort and confirmed 117/118 HMM-inferred calls with a validation rate of 99.15%. Next, we validated 84 CNVs from an independent set of cases both validated using fluorescence in situ hybridization (FISH) and locus-specific custom high-density arrays [64] (Girirajan and Eichler, unpublished). We also validated all 44 calls from the autism cohort, 22 calls from the dyslexia cohort, and 78 calls from the developmental delay cohort. In addition, in an analysis of 517 individuals with epilepsy using this array design, 61/63 CNVs were validated on a different array platform [10].
Supporting Information
CNV calls in autism.
(XLSX)
CNV calls in ID.
(XLSX)
CNV calls in NIMH controls.
(XLSX)
CNV calls in dyslexia.
(XLSX)
Probe densities and CNV detection threshold of hotspot v1 chip. Although the median density of the chip was designed to be approximately 2.6 kbp in the genomic hotspots and 36 kbp in genomic backbone, the limitations of the chip design (probe assignment restricted to only up to five mismatches) precluded uniform distribution of the probes throughout the genome. Therefore, the actual probe density varied across regions of the human genome. (a) The plot shows the size-wise distribution of CNVs and the density of array probes targeted to the genomic hotspots and non-hotspot regions. Note that non-hotspot regions contain two different probe densities (probe spacing of 20,000 bp and about 30,000 bp labeled a & b) and the hotspots (shaded) are covered every 10,000 bp. (b) The histogram shows the CNV detection threshold for the hotspot v1 chip. The data represent all the CNV calls obtained from analyzing cases with intellectual disability (ID). The number of CNVs detected in the aggregate at different size thresholds is shown on the Y-axis. We utilized a threshold of >10 probes, >1.5 z-score, and >50 kbp for CNV detection analysis. While we were easily able to detect events >50 kbp in the hotspot regions, we were only able to call variants ranging from 150 kbp (hotspot-associated CNVs) and 300 kbp onwards (mostly non-hotspot CNVs) with confidence (i.e., able to validate) for the non-hotspot regions.
(PDF)
Comparison of 9p24 deletions identified in our study to DECIPHER database. The figure shows genome browser snapshot of the 9p24 region with red and blue bars denoting deletions and duplications respectively in the DECIPHER database. Major clinical features observed in these patients are also shown. Please note that the black bars at the top denote CNVs identified in our study. PDD-NOS, Pervasive developmental delay-not otherwise specified; ID/DD, intellectual disability/developmental delay.
(PDF)
Diagnostic yield of different microarray reports from literature. Histograms show the number of rare CNVs (usually disease-associated) observed under different diagnostic centers. Data is shown for sample sizes >50. Data obtained from Table 2 of Miller et al., AJHG.
(PDF)
Chromosomal regions and probes targeted in the hotspot chip. List of all regions targeted in the hotspot chip. The list is derived from the original curation of segmental duplication regions and genomic hotspots from Bailey et al, 2002 [14].
(PDF)
Confirmation of CNVs arrays using custom high-density arrays. Validation of CNVs identified using NimbleGen hotspotv1 arrays (12×135 K) using a higher density 3×720K NimbleGen or 2×400K Agilent arrays.
(PDF)
Global analysis for CNVs in neurodevelopmental disorders. Summary and characteristics of deletions and duplications identified in the four cohorts studied.
(PDF)
(A) Characteristics of dyslexia cases from UW. Dyslexia measurements and scores of children tested at the UW. WATT- WRMT-R Woodcock Reading Mastery Test – Revised; Word Attack subtest. A measure of untimed reading of single non-words. WRAT3sp - Wide Range Achievement Tests – Third Addition; Spelling subtest. Spelling of single words from dictation in writing. WIAT(2)sp - Wechsler Individual Achievement Test (2nd edition); Spelling subtest. Spelling of single words from dictation in writing. WID - WRMT-R Woodcock Reading Mastery Test – Revised; Word Identification subtest. A measure of untimed reading of single words. (B) Characteristics of dyslexia cases recruited from Atlanta. Gender, phenotype and age of children recruited from the Atlanta collection is shown.
(PDF)
Clinical details of cases with autism (both with and without ID). Clinical features of individuals recruited through the Simons Simplex Collection (n = 350) are shown.
(PDF)
Clinical details of cases with idiopathic ID. Clinical features of individuals with ID are shown. These individuals were recruited and evaluated at the IRCCS Associazione Oasi Maria Santissima, Troina.
(PDF)
Clinical features of cases with ID plus MCA. Presenting clinical features, additional malformations, and preliminary cytogenetic and genetic evaluations performed for these cases are shown.
(PDF)
(A) Comparison of rare CNV rates in the cohorts studied. (B) Rare CNVs in dyslexia, autism, and ID. (C) Inheritance of rare CNVs in the disease cohorts. (D) Individuals with two rare copy number variants (two hits).
(PDF)
Genomic disorders identified in 1,227 cases with ID, autism, and ID/MCA. Genomic disorders are defined as copy number variants that are previously identified to be associated significantly in individuals with disease compared to controls. These CNVs can either map within genomic hotspot (HS) or non-hotspot regions (non-HS).
(PDF)
Clinical features of a case with 6q16 deletion. Comparison of clinical features from published sources with those in the current study.
(PDF)
Acknowledgments
We thank Catarina Campbell, Mark Matsushita, John Wolff, Arthur Ko, Kenneth Mark, Tonia Brown, Nik Krumm, Noelani Laycock, and David A. Hughes for valuable discussions and technical assistance. A full list of the investigators who contributed to the generation of the data is available from www.wtccc.org.uk. EEE is an investigator with the Howard Hughes Medical Institute.
Footnotes
EEE is on the scientific advisory board for Pacific Biosciences.
This work was supported by grants from the National Institutes of Health 5R01 HD054562 to WHR and 1R01 HD065285 to EEE, a Simons Foundation grant to EEE, and in part by the Cancer Genomics Shared Resource at the Winship Cancer Institute, Emory University, to CSM. This study also makes use of data generated by the Wellcome Trust Case-Control Consortium. Funding for the project was provided by the Wellcome Trust under awards 076113 and 085475. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sharp AJ, Hansen S, Selzer RR, Cheng Z, Regan R, et al. Discovery of previously unidentified genomic disorders from the duplication architecture of the human genome. Nat Genet. 2006;38:1038–1042. doi: 10.1038/ng1862. [DOI] [PubMed] [Google Scholar]
- 2.de Vries BB, Pfundt R, Leisink M, Koolen DA, Vissers LE, et al. Diagnostic genome profiling in mental retardation. Am J Hum Genet. 2005;77:606–616. doi: 10.1086/491719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marshall CR, Noor A, Vincent JB, Lionel AC, Feuk L, et al. Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet. 2008;82:477–488. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Schizophrenia Consortium. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455:237–241. doi: 10.1038/nature07239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- 7.Craddock N, Hurles ME, Cardin N, Pearson RD, Plagnol V, et al. Genome-wide association study of CNVs in 16,000 cases of eight common diseases and 3,000 shared controls. Nature. 2010;464:713–720. doi: 10.1038/nature08979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grozeva D, Kirov G, Ivanov D, Jones IR, Jones L, et al. Rare copy number variants: a point of rarity in genetic risk for bipolar disorder and schizophrenia. Arch Gen Psychiatry. 2010;67:318–327. doi: 10.1001/archgenpsychiatry.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helbig I, Mefford HC, Sharp AJ, Guipponi M, Fichera M, et al. 15q13.3 microdeletions increase risk of idiopathic generalized epilepsy. Nat Genet. 2009;41:160–162. doi: 10.1038/ng.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mefford HC, Muhle H, Ostertag P, von Spiczak S, Buysse K, et al. Genome-wide copy number variation in epilepsy: novel susceptibility loci in idiopathic generalized and focal epilepsies. PLoS Genet. 2010;6:e1000962. doi: 10.1371/journal.pgen.1000962. doi: 10.1371/journal.pgen.1000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams NM, Zaharieva I, Martin A, Langley K, Mantripragada K, et al. Rare chromosomal deletions and duplications in attention-deficit hyperactivity disorder: a genome-wide analysis. Lancet. 2010;376:1401–1408. doi: 10.1016/S0140-6736(10)61109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elia J, Gai X, Xie HM, Perin JC, Geiger E, et al. Rare structural variants found in attention-deficit hyperactivity disorder are preferentially associated with neurodevelopmental genes. Mol Psychiatry. 2010;15:637–646. doi: 10.1038/mp.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wechsler D. Wechsler intelligence scale for children - third edition (WISC-III) San Antonio: The Psychological Corporation; 1991. [Google Scholar]
- 14.Bailey JA, Gu Z, Clark RA, Reinert K, Samonte RV, et al. Recent segmental duplications in the human genome. Science. 2002;297:1003–1007. doi: 10.1126/science.1072047. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Feuk L, Duggan GE, Khaja R, Scherer SW. Development of bioinformatics resources for display and analysis of copy number and other structural variants in the human genome. Cytogenet Genome Res. 2006;115:205–214. doi: 10.1159/000095916. [DOI] [PubMed] [Google Scholar]
- 16.Pennington BF. The genetics of dyslexia. J Child Psychol Psychiatry. 1990;31:193–201. doi: 10.1111/j.1469-7610.1990.tb01561.x. [DOI] [PubMed] [Google Scholar]
- 17.Mefford HC, Cooper GM, Zerr T, Smith JD, Baker C, et al. A method for rapid, targeted CNV genotyping identifies rare variants associated with neurocognitive disease. Genome Res. 2009 doi: 10.1101/gr.094987.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirov G, Grozeva D, Norton N, Ivanov D, Mantripragada KK, et al. Support for the involvement of large copy number variants in the pathogenesis of schizophrenia. Hum Mol Genet. 2009;18:1497–1503. doi: 10.1093/hmg/ddp043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stefansson H, Rujescu D, Cichon S, Pietilainen OP, Ingason A, et al. Large recurrent microdeletions associated with schizophrenia. Nature. 2008;455:232–236. doi: 10.1038/nature07229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Kovel CG, Trucks H, Helbig I, Mefford HC, Baker C, et al. Recurrent microdeletions at 15q11.2 and 16p13.11 predispose to idiopathic generalized epilepsies. Brain. 2010;133:23–32. doi: 10.1093/brain/awp262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paracchini S, Scerri T, Monaco AP. The genetic lexicon of dyslexia. Annu Rev Genomics Hum Genet. 2007;8:57–79. doi: 10.1146/annurev.genom.8.080706.092312. [DOI] [PubMed] [Google Scholar]
- 22.Kumar RA, KaraMohamed S, Sudi J, Conrad DF, Brune C, et al. Recurrent 16p11.2 microdeletions in autism. Hum Mol Genet. 2008;17:628–638. doi: 10.1093/hmg/ddm376. [DOI] [PubMed] [Google Scholar]
- 23.Weiss LA, Shen Y, Korn JM, Arking DE, Miller DT, et al. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med. 2008;358:667–675. doi: 10.1056/NEJMoa075974. [DOI] [PubMed] [Google Scholar]
- 24.Phelan MC, Rogers RC, Saul RA, Stapleton GA, Sweet K, et al. 22q13 deletion syndrome. Am J Med Genet. 2001;101:91–99. doi: 10.1002/1096-8628(20010615)101:2<91::aid-ajmg1340>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 25.Gajecka M, Mackay KL, Shaffer LG. Monosomy 1p36 deletion syndrome. Am J Med Genet C Semin Med Genet. 2007;145C:346–356. doi: 10.1002/ajmg.c.30154. [DOI] [PubMed] [Google Scholar]
- 26.Abd El-Aziz MM, Barragan I, O'Driscoll CA, Goodstadt L, Prigmore E, et al. EYS, encoding an ortholog of Drosophila spacemaker, is mutated in autosomal recessive retinitis pigmentosa. Nat Genet. 2008;40:1285–1287. doi: 10.1038/ng.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collin RW, Littink KW, Klevering BJ, van den Born LI, Koenekoop RK, et al. Identification of a 2 Mb human ortholog of Drosophila eyes shut/spacemaker that is mutated in patients with retinitis pigmentosa. Am J Hum Genet. 2008;83:594–603. doi: 10.1016/j.ajhg.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maestrini E, Pagnamenta AT, Lamb JA, Bacchelli E, Sykes NH, et al. High-density SNP association study and copy number variation analysis of the AUTS1 and AUTS5 loci implicate the IMMP2L-DOCK4 gene region in autism susceptibility. Mol Psychiatry. 2010;15:954–968. doi: 10.1038/mp.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petek E, Windpassinger C, Vincent JB, Cheung J, Boright AP, et al. Disruption of a novel gene (IMMP2L) by a breakpoint in 7q31 associated with Tourette syndrome. Am J Hum Genet. 2001;68:848–858. doi: 10.1086/319523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pagnamenta AT, Bacchelli E, de Jonge MV, Mirza G, Scerri TS, et al. Characterization of a family with rare deletions in CNTNAP5 and DOCK4 suggests novel risk loci for autism and dyslexia. Biol Psychiatry. 2010;68:320–328. doi: 10.1016/j.biopsych.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hannula-Jouppi K, Kaminen-Ahola N, Taipale M, Eklund R, Nopola-Hemmi J, et al. The axon guidance receptor gene ROBO1 is a candidate gene for developmental dyslexia. PLoS Genet. 2005;1:e50. doi: 10.1371/journal.pgen.0010050. doi: 10.1371/journal.pgen.0010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nopola-Hemmi J, Taipale M, Haltia T, Lehesjoki AE, Voutilainen A, et al. Two translocations of chromosome 15q associated with dyslexia. J Med Genet. 2000;37:771–775. doi: 10.1136/jmg.37.10.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taipale M, Kaminen N, Nopola-Hemmi J, Haltia T, Myllyluoma B, et al. A candidate gene for developmental dyslexia encodes a nuclear tetratricopeptide repeat domain protein dynamically regulated in brain. Proc Natl Acad Sci U S A. 2003;100:11553–11558. doi: 10.1073/pnas.1833911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buonincontri R, Bache I, Silahtaroglu A, Elbro C, Nielsen AM, et al. A cohort of balanced reciprocal translocations associated with dyslexia: identification of two putative candidate genes at DYX1. Behav Genet. 2011;41:125–133. doi: 10.1007/s10519-010-9389-2. [DOI] [PubMed] [Google Scholar]
- 35.O'Roak BJ, Deriziotis P, Lee C, Vives L, Schwartz JJ, et al. Exome sequencing in sporadic autism spectrum disorders identifies severe de novo mutations. Nat Genet. 2011;43:585–589. doi: 10.1038/ng.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamdan FF, Daoud H, Rochefort D, Piton A, Gauthier J, et al. De novo mutations in FOXP1 in cases with intellectual disability, autism, and language impairment. Am J Hum Genet. 2010;87:671–678. doi: 10.1016/j.ajhg.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horn D, Kapeller J, Rivera-Brugues N, Moog U, Lorenz-Depiereux B, et al. Identification of FOXP1 deletions in three unrelated patients with mental retardation and significant speech and language deficits. Hum Mutat. 2010;31:E1851–1860. doi: 10.1002/humu.21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teramitsu I, Kudo LC, London SE, Geschwind DH, White SA. Parallel FoxP1 and FoxP2 expression in songbird and human brain predicts functional interaction. J Neurosci. 2004;24:3152–3163. doi: 10.1523/JNEUROSCI.5589-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vernes SC, Newbury DF, Abrahams BS, Winchester L, Nicod J, et al. A functional genetic link between distinct developmental language disorders. N Engl J Med. 2008;359:2337–2345. doi: 10.1056/NEJMoa0802828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Firth HV, Richards SM, Bevan AP, Clayton S, Corpas M, et al. DECIPHER: Database of Chromosomal Imbalance and Phenotype in Humans Using Ensembl Resources. Am J Hum Genet. 2009;84:524–533. doi: 10.1016/j.ajhg.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonaglia MC, Ciccone R, Gimelli G, Gimelli S, Marelli S, et al. Detailed phenotype-genotype study in five patients with chromosome 6q16 deletion: narrowing the critical region for Prader-Willi-like phenotype. Eur J Hum Genet. 2008;16:1443–1449. doi: 10.1038/ejhg.2008.119. [DOI] [PubMed] [Google Scholar]
- 42.Holder JL, Jr, Butte NF, Zinn AR. Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Hum Mol Genet. 2000;9:101–108. doi: 10.1093/hmg/9.1.101. [DOI] [PubMed] [Google Scholar]
- 43.Jamain S, Betancur C, Quach H, Philippe A, Fellous M, et al. Linkage and association of the glutamate receptor 6 gene with autism. Mol Psychiatry. 2002;7:302–310. doi: 10.1038/sj.mp.4000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strungaru MH, Dinu I, Walter MA. Genotype-phenotype correlations in Axenfeld-Rieger malformation and glaucoma patients with FOXC1 and PITX2 mutations. Invest Ophthalmol Vis Sci. 2007;48:228–237. doi: 10.1167/iovs.06-0472. [DOI] [PubMed] [Google Scholar]
- 45.Ballif BC, Theisen A, Coppinger J, Gowans GC, Hersh JH, et al. Expanding the clinical phenotype of the 3q29 microdeletion syndrome and characterization of the reciprocal microduplication. Mol Cytogenet. 2008;1:8. doi: 10.1186/1755-8166-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lisi EC, Hamosh A, Doheny KF, Squibb E, Jackson B, et al. 3q29 interstitial microduplication: a new syndrome in a three-generation family. Am J Med Genet A. 2008;146A:601–609. doi: 10.1002/ajmg.a.32190. [DOI] [PubMed] [Google Scholar]
- 47.Cardoso C, Leventer RJ, Ward HL, Toyo-Oka K, Chung J, et al. Refinement of a 400-kb critical region allows genotypic differentiation between isolated lissencephaly, Miller-Dieker syndrome, and other phenotypes secondary to deletions of 17p13.3. Am J Hum Genet. 2003;72:918–930. doi: 10.1086/374320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagamani SC, Zhang F, Shchelochkov OA, Bi W, Ou Z, et al. Microdeletions including YWHAE in the Miller-Dieker syndrome region on chromosome 17p13.3 result in facial dysmorphisms, growth restriction, and cognitive impairment. J Med Genet. 2009;46:825–833. doi: 10.1136/jmg.2009.067637. [DOI] [PubMed] [Google Scholar]
- 49.Itsara A, Cooper GM, Baker C, Girirajan S, Li J, et al. Population analysis of large copy number variants and hotspots of human genetic disease. Am J Hum Genet. 2009;84:148–161. doi: 10.1016/j.ajhg.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raskind W. Current understanding of the genetic basis of reading and spelling disability. Learning Disability Quarterly. 2001;24:141–157. [Google Scholar]
- 51.Bakkaloglu B, O'Roak BJ, Louvi A, Gupta AR, Abelson JF, et al. Molecular cytogenetic analysis and resequencing of contactin associated protein-like 2 in autism spectrum disorders. Am J Hum Genet. 2008;82:165–173. doi: 10.1016/j.ajhg.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de la Barra F, Skoknic V, Alliende A, Raimann E, Cortes F, et al. [Twins with autism and mental retardation associated with balanced (7;20) chromosomal translocation]. Rev Chil Pediatr. 1986;57:549–554. [PubMed] [Google Scholar]
- 53.Huang XL, Zou YS, Maher TA, Newton S, Milunsky JM. A de novo balanced translocation breakpoint truncating the autism susceptibility candidate 2 (AUTS2) gene in a patient with autism. Am J Med Genet A. 2010;152A:2112–2114. doi: 10.1002/ajmg.a.33497. [DOI] [PubMed] [Google Scholar]
- 54.Kalscheuer VM, FitzPatrick D, Tommerup N, Bugge M, Niebuhr E, et al. Mutations in autism susceptibility candidate 2 (AUTS2) in patients with mental retardation. Hum Genet. 2007;121:501–509. doi: 10.1007/s00439-006-0284-0. [DOI] [PubMed] [Google Scholar]
- 55.Sultana R, Yu CE, Yu J, Munson J, Chen D, et al. Identification of a novel gene on chromosome 7q11.2 interrupted by a translocation breakpoint in a pair of autistic twins. Genomics. 2002;80:129–134. doi: 10.1006/geno.2002.6810. [DOI] [PubMed] [Google Scholar]
- 56.Fletcher J, Shaywitz S, Shaywitz B. Comorbidity of learning and attention disorders. Separate but equal. Pediatr Clin North Am. 1999;46:885–897. doi: 10.1016/s0031-3955(05)70161-9. [DOI] [PubMed] [Google Scholar]
- 57.Willcutt EG, Pennington BF, Olson RK, DeFries JC. Understanding comorbidity: a twin study of reading disability and attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:709–714. doi: 10.1002/ajmg.b.30310. [DOI] [PubMed] [Google Scholar]
- 58.Willcutt EG, Betjemann RS, McGrath LM, Chhabildas NA, Olson RK, et al. Etiology and neuropsychology of comorbidity between RD and ADHD: the case for multiple-deficit models. Cortex. 2010;46:1345–1361. doi: 10.1016/j.cortex.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bedogni F, Hodge RD, Nelson BR, Frederick EA, Shiba N, et al. Autism susceptibility candidate 2 (Auts2) encodes a nuclear protein expressed in developing brain regions implicated in autism neuropathology. Gene Expr Patterns. 2010;10:9–15. doi: 10.1016/j.gep.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Green RE, Krause J, Briggs AW, Maricic T, Stenzel U, et al. A draft sequence of the Neandertal genome. Science. 2010;328:710–722. doi: 10.1126/science.1188021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Levy D, Ronemus M, Yamrom B, Lee YH, Leotta A, et al. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron. 2011;70:886–897. doi: 10.1016/j.neuron.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 62.Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466:368–372. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, et al. Multiple Recurrent De Novo CNVs, Including Duplications of the 7q11.23 Williams Syndrome Region, Are Strongly Associated with Autism. Neuron. 2011;70:863–885. doi: 10.1016/j.neuron.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Girirajan S, Rosenfeld JA, Cooper GM, Antonacci F, Siswara P, et al. A recurrent 16p12.1 microdeletion supports a two-hit model for severe developmental delay. Nat Genet. 2010;42:203–209. doi: 10.1038/ng.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim HG, Kishikawa S, Higgins AW, Seong IS, Donovan DJ, et al. Disruption of neurexin 1 associated with autism spectrum disorder. Am J Hum Genet. 2008;82:199–207. doi: 10.1016/j.ajhg.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Szatmari P, Paterson AD, Zwaigenbaum L, Roberts W, Brian J, et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Glessner JT, Wang K, Cai G, Korvatska O, Kim CE, et al. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature. 2009;459:569–573. doi: 10.1038/nature07953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bucan M, Abrahams BS, Wang K, Glessner JT, Herman EI, et al. Genome-wide analyses of exonic copy number variants in a family-based study point to novel autism susceptibility genes. PLoS Genet. 2009;5:e1000536. doi: 10.1371/journal.pgen.1000536. doi: 10.1371/journal.pgen.1000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Noor A, Whibley A, Marshall CR, Gianakopoulos PJ, Piton A, et al. Disruption at the PTCHD1 Locus on Xp22.11 in Autism spectrum disorder and intellectual disability. Sci Transl Med. 2010;2:49ra68. doi: 10.1126/scitranslmed.3001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brkanac Z, Chapman NH, Igo RP, Jr, Matsushita MM, Nielsen K, et al. Genome Scan of a Nonword Repetition Phenotype in Families with Dyslexia: Evidence for Multiple Loci. Behav Genet. 2008 doi: 10.1007/s10519-008-9215-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rubenstein K, Matsushita M, Berninger VW, Raskind WH, Wijsman EM. Genome scan for spelling deficits: effects of verbal IQ on models of transmission and trait gene localization. Behav Genet. 2011;41:31–42. doi: 10.1007/s10519-010-9390-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Berninger V. A developmental approach to learning disabilities. In: Siegel I, Renninger A, editors. Handbook of Child Psychology, Vol IV, Child Psychology and Practice. New York: John Wiley and Sons; 2006. pp. 420–452. [Google Scholar]
- 73.Fischbach GD, Lord C. The Simons Simplex Collection: a resource for identification of autism genetic risk factors. Neuron. 2010;68:192–195. doi: 10.1016/j.neuron.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 74.Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- 75.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 76.Moldin SO. NIMH Human Genetics Initiative: 2003 update. Am J Psychiatry. 2003;160:621–622. doi: 10.1176/appi.ajp.160.4.621. [DOI] [PubMed] [Google Scholar]
- 77.Kessler RC, Ustun TB. The World Mental Health (WMH) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI). Int J Methods Psychiatr Res. 2004;13:93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Talati A, Fyer AJ, Weissman MM. A comparison between screened NIMH and clinically interviewed control samples on neuroticism and extraversion. Mol Psychiatry. 2008;13:122–130. doi: 10.1038/sj.mp.4002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baum AE, Akula N, Cabanero M, Cardona I, Corona W, et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol Psychiatry. 2008;13:197–207. doi: 10.1038/sj.mp.4002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li B, Leal SM. Methods for detecting associations with rare variants for common diseases: application to analysis of sequence data. Am J Hum Genet. 2008;83:311–321. doi: 10.1016/j.ajhg.2008.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Simon-Sanchez J, Scholz S, Fung HC, Matarin M, Hernandez D, et al. Genome-wide SNP assay reveals structural genomic variation, extended homozygosity and cell-line induced alterations in normal individuals. Hum Mol Genet. 2007;16:1–14. doi: 10.1093/hmg/ddl436. [DOI] [PubMed] [Google Scholar]
- 82.Albert MA, Danielson E, Rifai N, Ridker PM. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286:64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 83.Simon JA, Lin F, Hulley SB, Blanche PJ, Waters D, et al. Phenotypic predictors of response to simvastatin therapy among African-Americans and Caucasians: the Cholesterol and Pharmacogenetics (CAP) Study. Am J Cardiol. 2006;97:843–850. doi: 10.1016/j.amjcard.2005.09.134. [DOI] [PubMed] [Google Scholar]
- 84.Melzer D, Perry JR, Hernandez D, Corsi AM, Stevens K, et al. A genome-wide association study identifies protein quantitative trait loci (pQTLs). PLoS Genet. 2008;4:e1000072. doi: 10.1371/journal.pgen.1000072. doi: 10.1371/journal.pgen.1000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cooper GM, Coe BP, Girirajan S, Rosenfeld JA, Vu TH, et al. A copy number variation morbidity map of developmental delay. Nat Genet. 2011;43:838–846. doi: 10.1038/ng.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Selzer RR, Richmond TA, Pofahl NJ, Green RD, Eis PS, et al. Analysis of chromosome breakpoints in neuroblastoma at sub-kilobase resolution using fine-tiling oligonucleotide array CGH. Genes Chromosomes Cancer. 2005;44:305–319. doi: 10.1002/gcc.20243. [DOI] [PubMed] [Google Scholar]
- 87.Day N, Hemmaplardh A, Thurman RE, Stamatoyannopoulos JA, Noble WS. Unsupervised segmentation of continuous genomic data. Bioinformatics. 2007;23:1424–1426. doi: 10.1093/bioinformatics/btm096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CNV calls in autism.
(XLSX)
CNV calls in ID.
(XLSX)
CNV calls in NIMH controls.
(XLSX)
CNV calls in dyslexia.
(XLSX)
Probe densities and CNV detection threshold of hotspot v1 chip. Although the median density of the chip was designed to be approximately 2.6 kbp in the genomic hotspots and 36 kbp in genomic backbone, the limitations of the chip design (probe assignment restricted to only up to five mismatches) precluded uniform distribution of the probes throughout the genome. Therefore, the actual probe density varied across regions of the human genome. (a) The plot shows the size-wise distribution of CNVs and the density of array probes targeted to the genomic hotspots and non-hotspot regions. Note that non-hotspot regions contain two different probe densities (probe spacing of 20,000 bp and about 30,000 bp labeled a & b) and the hotspots (shaded) are covered every 10,000 bp. (b) The histogram shows the CNV detection threshold for the hotspot v1 chip. The data represent all the CNV calls obtained from analyzing cases with intellectual disability (ID). The number of CNVs detected in the aggregate at different size thresholds is shown on the Y-axis. We utilized a threshold of >10 probes, >1.5 z-score, and >50 kbp for CNV detection analysis. While we were easily able to detect events >50 kbp in the hotspot regions, we were only able to call variants ranging from 150 kbp (hotspot-associated CNVs) and 300 kbp onwards (mostly non-hotspot CNVs) with confidence (i.e., able to validate) for the non-hotspot regions.
(PDF)
Comparison of 9p24 deletions identified in our study to DECIPHER database. The figure shows genome browser snapshot of the 9p24 region with red and blue bars denoting deletions and duplications respectively in the DECIPHER database. Major clinical features observed in these patients are also shown. Please note that the black bars at the top denote CNVs identified in our study. PDD-NOS, Pervasive developmental delay-not otherwise specified; ID/DD, intellectual disability/developmental delay.
(PDF)
Diagnostic yield of different microarray reports from literature. Histograms show the number of rare CNVs (usually disease-associated) observed under different diagnostic centers. Data is shown for sample sizes >50. Data obtained from Table 2 of Miller et al., AJHG.
(PDF)
Chromosomal regions and probes targeted in the hotspot chip. List of all regions targeted in the hotspot chip. The list is derived from the original curation of segmental duplication regions and genomic hotspots from Bailey et al, 2002 [14].
(PDF)
Confirmation of CNVs arrays using custom high-density arrays. Validation of CNVs identified using NimbleGen hotspotv1 arrays (12×135 K) using a higher density 3×720K NimbleGen or 2×400K Agilent arrays.
(PDF)
Global analysis for CNVs in neurodevelopmental disorders. Summary and characteristics of deletions and duplications identified in the four cohorts studied.
(PDF)
(A) Characteristics of dyslexia cases from UW. Dyslexia measurements and scores of children tested at the UW. WATT- WRMT-R Woodcock Reading Mastery Test – Revised; Word Attack subtest. A measure of untimed reading of single non-words. WRAT3sp - Wide Range Achievement Tests – Third Addition; Spelling subtest. Spelling of single words from dictation in writing. WIAT(2)sp - Wechsler Individual Achievement Test (2nd edition); Spelling subtest. Spelling of single words from dictation in writing. WID - WRMT-R Woodcock Reading Mastery Test – Revised; Word Identification subtest. A measure of untimed reading of single words. (B) Characteristics of dyslexia cases recruited from Atlanta. Gender, phenotype and age of children recruited from the Atlanta collection is shown.
(PDF)
Clinical details of cases with autism (both with and without ID). Clinical features of individuals recruited through the Simons Simplex Collection (n = 350) are shown.
(PDF)
Clinical details of cases with idiopathic ID. Clinical features of individuals with ID are shown. These individuals were recruited and evaluated at the IRCCS Associazione Oasi Maria Santissima, Troina.
(PDF)
Clinical features of cases with ID plus MCA. Presenting clinical features, additional malformations, and preliminary cytogenetic and genetic evaluations performed for these cases are shown.
(PDF)
(A) Comparison of rare CNV rates in the cohorts studied. (B) Rare CNVs in dyslexia, autism, and ID. (C) Inheritance of rare CNVs in the disease cohorts. (D) Individuals with two rare copy number variants (two hits).
(PDF)
Genomic disorders identified in 1,227 cases with ID, autism, and ID/MCA. Genomic disorders are defined as copy number variants that are previously identified to be associated significantly in individuals with disease compared to controls. These CNVs can either map within genomic hotspot (HS) or non-hotspot regions (non-HS).
(PDF)
Clinical features of a case with 6q16 deletion. Comparison of clinical features from published sources with those in the current study.
(PDF)