Abstract
Tobacco use is a major health problem that is estimated to cause 4 million deaths a year worldwide. Nicotine is the main addictive component of tobacco. It acts as an agonist to activate and desensitize nicotinic acetylcholine receptors (nAChRs). A component of nicotine's addictive power is attributable to actions on the mesolimbic dopaminergic system, which serves a fundamental role in the acquisition of behaviors that are inappropriately reinforced by addictive drugs. Here we show that nicotine, in the same concentration and time ranges as obtained from tobacco, has three main actions that regulate the activity of midbrain dopamine (DA) neurons. Nicotine first activates and then desensitizes nAChRs on the DA neurons. This process directly excites the DA neurons for a short period of time before the nAChRs desensitize. Nicotine also enhances glutamatergic excitation and decreases GABAergic inhibition onto DA neurons. These events increase the probability for synaptic plasticity, such as long-term potentiation. The short-lived direct excitation of the DA neurons coupled with the enhanced glutamatergic afferent activity provides the presynaptic and postsynaptic coincidence necessary to initiate synaptic potentiation. In total, these synaptic events lead to a relatively long-lasting heightened activity of midbrain DA neurons. Consistent with other summarized studies, this work indicates that the synaptic changes normally associated with learning and memory can be influenced and commandeered during the nicotine addiction process.
About one-third of the adults in the world smoke tobacco. Most of them start as adolescents, and half of those that continue smoking die from smoking-related diseases (WHO 1997). Because tobacco usage is increasing in less developed countries, it is one of the few causes of death that is on the rise (Peto et al. 1996). In developed countries, smoking is estimated to be the largest single cause of premature death (Peto et al. 1992). From 1995 to 1999 in the United States alone, smoking annually caused ∼440,000 deaths and $157 billion in health-related economic losses (CDC 2002).
Nicotine is the main addictive component of tobacco (Karan et al. 2003). When studied under laboratory conditions, nicotine elicits drug-seeking behavior in animals (Corrigall and Coen 1989; Stolerman and Shoaib 1991; Corrigall 1999; Di Chiara 2000). It also produces additional effects commonly seen with addictive drugs such as amphetamines and cocaine. Nicotine reinforces self-administration, increases locomotor activity, enhances reward from brain stimulation, and reinforces place preference (Clarke 1990, 1991; Stolerman and Shoaib 1991; Stolerman and Jarvis 1995; Dani and Heinemann 1996; Corrigall 1999; Di Chiara 2000; Dani and De Biasi 2001; Mansvelder and McGehee 2002). Furthermore, nicotine cessation produces a withdrawal syndrome, and those symptoms can be relieved by nicotine replacement (Stolerman and Jarvis 1995).
Midbrain dopaminergic systems serve an important role in nicotine addiction because they reinforce acquisition of behaviors that are driven by salient environmental cues or by the inappropriate stimuli of addictive drugs (Wonnacott et al. 1990; Corrigall et al. 1992; Nestler 1992, 1993; Nisell et al. 1994; Pontieri et al. 1996; Spanagel and Weiss 1999; Balfour et al. 2000; Di Chiara 2000; Dani and De Biasi 2001; Dani et al. 2001; Karan et al. 2003). The role of the midbrain DA systems in nicotine addiction is supported by the findings that DA antagonists or lesions of DA neurons or of the nucleus accumbens (NAc) reduce self-administration (Corrigall and Coen 1989; Corrigall et al. 1992, 1994; Corrigall 1999; Di Chiara 2000). By acting at nicotinic acetylcholine receptors (nAChRs), nicotine can activate neurons of the ventral tegmental area (VTA) and the substantia nigra compacta (SNc; Clarke et al. 1985; Grenhoff and Johnson 1996; Calabresi et al. 1989; Pidoplichko et al. 1997; Picciotto et al. 1998; Dani et al. 2001; Mansvelder and McGehee 2002) and cause release of DA in the NAc of rats (Clarke 1991; Nisell et al. 1994, 1995; Pontieri et al. 1996). Furthermore, presynaptically located nAChRs can potently regulate DA release in the striatum, including the NAc (Wonnacott et al. 2000; Jones et al. 2001; Zhou et al. 2001).
Neuronal nAChRs are formed from combinations of five subunits arising from α2–α10 and β2–β4. Therefore, many compositionally and functionally different nAChR subtypes are possible (McGehee and Role 1995; Role and Berg 1996; Wonnacott 1997; Jones et al. 1999; Wooltorton et al. 2003). Five α subunits (α2–α6) and three β subunits (β2–β4) assemble in various combinations into the vast majority of neuronal hetero-oligomeric nAChRs. These various hetero-oligomeric receptors commonly share some functional and pharmacological properties. The other common neuronal subtype arises from nAChRs containing the α7 (α7*) subunit. The neuronal α7* nAChRs are functionally similar to homo-oligomeric α7 receptors studied in exogenous expression systems, but α7 also may more rarely form heterooligomeric nAChRs (Yu and Role 1998). The α7* nAChRs have rapid activation and desensitization kinetics, and are specifically inhibited by α-bungarotoxin (α-BTX) and by low concentrations of methyllycaconitine (MLA; Alkondon et al. 1992; Castro and Albuquerque 1995; Gray et al. 1996). Although α7* nAChRs are quickly desensitized by very high agonist concentrations (e.g., 500 μM ACh or nicotine), they have a lower affinity for nicotine. Consequently, the α7* nAChRs are not significantly desensitized by the low concentrations of nicotine obtained from tobacco (see Quick and Lester 2002; Wooltorton et al. 2003).
The predominant nAChR-mediated currents from VTA and SNc neurons have relatively slow kinetics and are inhibited by the β2-selective inhibitor, dihydro-β-erythroidine (DHβE; Pidoplichko et al. 1997; Picciotto et al. 1998; Klink et al. 2001; Wooltorton et al. 2003). Those characteristics indicate that the vast majority of the nicotinic currents on midbrain neurons are mediated by β2-containing (β2*) nAChRs. The β2 subunit is in combination with other nicotinic subunits that are expressed in these areas, particularly α4, α6, and β3 (Wada et al. 1989, 1990; Le Novère et al. 1996; Goldner et al. 1997; Charpantier et al. 1998; Klink et al. 2001). That conclusion was verified using β2-null mice in which nicotinic currents were dramatically decreased in the midbrain neurons (Picciotto et al. 1998; Wooltorton et al. 2003). In midbrain slices from the β2-null mice, the only consistent nicotinic current remaining was a small minority current mediated by α7* nAChRs. Therefore, the α7* nAChRs are present on VTA/SNc neurons, but at a much lower density than β2* nAChRs (Wooltorton et al. 2003).
The purpose of this report is to review and further examine nicotinic synaptic mechanisms in midbrain dopaminergic areas. New data are presented along with review material to show that the majority subtypes of nAChRs on DA neurons are directly activated by nicotine, but after a short time, they desensitize. Glutamatergic afferents into this region supply convergent excitation from a number of brain areas. The predominant subtype of nAChR located on the glutamatergic presynaptic terminals is not significantly desensitized by the concentration of nicotine obtained from smoking. Thus, nicotine causes a persistent enhancement of the afferent glutamatergic excitation onto DA neurons. The GABAergic inhibition in this midbrain region arises mainly from the NAc, the ventral pallidum, and local midbrain interneurons (Kalivas et al. 1993; Steffensen et al. 1998). The predominant nAChR subtypes on the GABAergic neurons desensitize after some exposure to nicotine, thereby decreasing the inherent inhibition onto DA neurons. The consequence of these synaptic events is a prolonged firing of DA neurons in response to nicotine. In summary, nicotine as obtained from tobacco interacts with multiple nAChR subtypes on DA neurons and on afferent neurons, fibers, and presynaptic terminals to produce synaptic events much like those that underlie the synaptic changes associated with learning and memory.
RESULTS
Nicotine Induces a Long-lasting DA Signal in the Nucleus Accumbens
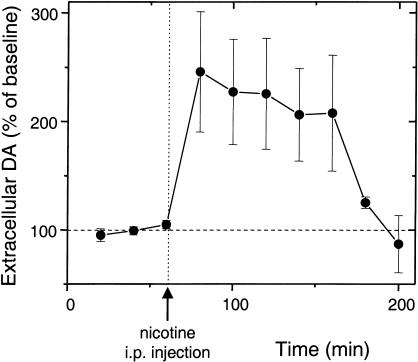
A common feature of addictive drugs such as cocaine, amphetamine, and nicotine is that at the doses they are self-administrated, those drugs increase the concentration of DA in the NAc (Di Chiara and Imperato 1988; Clarke 1991; Corrigall et al. 1992; Di Chiara 1999, 2000; Pontieri et al. 1996; Balfour et al. 2000; Dani and De Biasi 2001). Using microdialysis in the rat NAc shell, we found that nicotine causes more than a doubling in the background DA concentration (Fig. 1). Furthermore, the DA concentration remains elevated for well over an hour (Imperato et al. 1986). The main dopaminergic projections to the NAc arise from VTA neurons of the mesolimbic dopamine system. The long-lasting elevation of DA in the NAc presents a problem because the direct activation of nAChRs on VTA DA neurons is usually much shorter. There is significant (but not uniform) desensitization of nAChRs on DA neurons in a few minutes (Pidoplichko et al. 1997; Dani et al. 2000; Wooltorton et al. 2003).
Figure 1.
In vivo microdialysis indicates that nicotine boosts the DA concentration in the NAc shell for more than an hour. Samples were taken every 20 min from a wake, freely moving rat (n = 3) before and after i.p. injection of 0.6 mg/kg of nicotine. Separate injections of saline did not produce a DA signal above baseline in these microdialysis measurements.
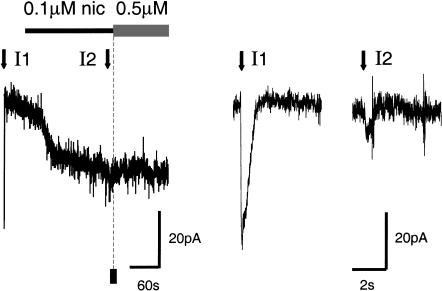
Cigarette smoking delivers ∼50 nM to 500 nM nicotine throughout the brain on a time scale of many seconds to minutes (Russell 1987; Benowitz et al. 1989; Henningfield et al. 1993; Gourlay and Benowitz 1997; Karan et al. 2003). Significantly lower concentrations of nicotine will linger in the human brain for hours. When 100 nM nicotine is applied to the bath of a midbrain slice, VTA DA neurons display inward-going (depolarizing or activating) current (Fig. 2; Pidoplichko et al. 1997). However, this relatively low concentration of nicotine also causes a great deal of nAChR desensitization because the subsequent addition of 500 nM nicotine causes no further current (Fig. 2). The desensitization is further documented by brief pressure-puff applications of ACh (1 mM for 30 msec; downward arrows in Fig. 2). Before nicotine is applied, the ACh puff activates a 50-pA current (I1 of Fig. 2), but after 3 min in 100 nM nicotine, the ACh puff activates a barely detectable current (I2 of Fig. 2; Pidoplichko et al. 1997). Despite the long-lasting DA signal detected by microdialysis (Fig. 1), the results of Figure 2 demonstrate that after a few minutes, many nAChRs on the surface of midbrain DA neurons are desensitized. The prolonged DA signal can be explained by considering the afferent drive onto the VTA neurons (Mansvelder and McGehee 2000, 2002; Dani et al. 2001; Mansvelder et al. 2002; Wooltorton et al. 2003).
Figure 2.
Nicotine, at the concentration experienced by smokers, activates and desensitizes nAChRs. (Left) Bath application of 0.1 μM nicotine activated a 17-pA current, and after 3 min application of 0.5 μM nicotine activated very little additional current. If there had been no desensitization, the solid square marks the average size of the current that would have been activated by 0.5 μM nicotine. ACh pressure injections (1 mM, 30 msec, downward arrows) were applied before (I1) and near the end (I2) of the 0.1 μM nicotine. Those ACh-induced currents are shown on an expanded time scale (right) to illustrate the extent of desensitization. (These data were adapted from Pidoplichko et al. 1997.)
Nicotine Increases Glutamatergic Excitation and Decreases GABAergic Inhibition Onto DA Neurons
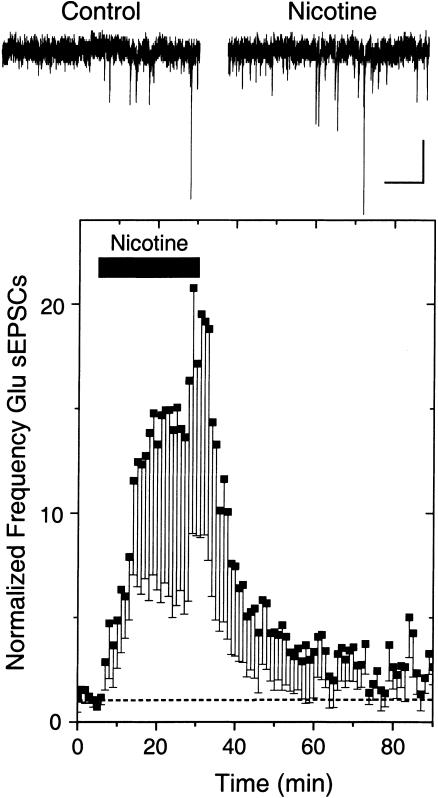
Upon smoking, it is estimated that the nicotine in the brain reaches ∼500 nM (possibly as high as 1 μM), and nicotine lingers in brain tissue longer than the time it takes to consume the tobacco (Russell 1987; Benowitz et al. 1989; Henningfield et al. 1993; Gourlay and Benowitz 1997). To mimic this situation in vitro, we bath-applied 500 nM nicotine for 25 min to midbrain slices while recording spontaneous glutamatergic afferent excitatory postsynaptic currents (sEPSCs) arriving at VTA DA neurons (Fig. 3). In eight out of 12 neurons (four showed no change), nicotine caused an increase in the frequency (but not the amplitude) of sEPSCs (Fig. 3; Mansvelder and McGehee 2000, 2002; Dani et al. 2001; Mansvelder et al. 2002). The frequency of the sEPSCs remained elevated throughout the 25-min period, indicating that desensitization did not terminate the nicotinic effect.
Figure 3.
Nicotine increases the frequency not the amplitude of spontaneous EPSCs recorded from VTA DA neurons. Example traces (upper) show that bath-applied nicotine (500 nM) increases the frequency of sEPSCs, and the average (eight of 12; four showed no change) shows the long-lasting effect of the frequency increase (lower). The recordings were at room temperature, at a holding potential of –60 mV. Scale bars represent 10 pA and 500 msec.
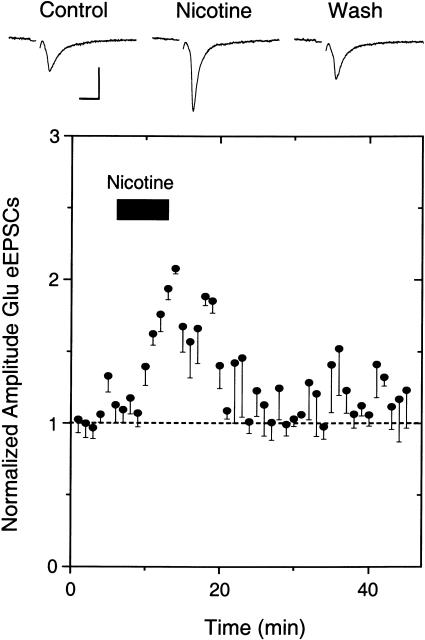
Nicotine also increased the amplitude of electrically evoked EPSCs on DA neurons (Fig. 4). The enhancement was favored by cutting sagittal slices and stimulating with a low stimulus strength (∼10% of the maximal response). In five out of 11 neurons (six showed no change), the amplitude of the eEPSCs increased (Mansvelder and McGehee 2000, 2002; Dani et al. 2001; Mansvelder et al. 2002). It is interesting to note that both the spontaneous and evoked EPSCs remain elevated after the nicotine was washed away, consistent with the induction of long-term potentiation of glutamatergic afferents (Mansvelder and McGehee 2000, 2002; Dani et al. 2001; Ji et al. 2001; Mansvelder et al. 2002). This result is consistent with presynaptic nAChRs that boost EPSC frequency without changing the amplitude, as has been demonstrated in the hippocampus and elsewhere (McGehee and Role 1995; McGehee et al. 1995; Gray et al 1996; Role and Berg 1996; Albuquerque et al 1997; Wonnacott 1997; Guo et al 1998; Li et al 1998; Radcliffe and Dani 1998; Jones et al 1999; Radcliffe et al 1999; Mansvelder and McGehee 2000, 2002; Dani et al. 2001; Mansvelder et al. 2002). The nicotine-induced long-lasting potentiation of glutamatergic afferent excitation onto DA neurons is similar to the synaptic plasticity that is normally thought to underlie learning and memory (Martin et al. 2000).
Figure 4.
Nicotine increases the amplitude of evoked EPSCs recorded from VTA DA neurons. Example traces (upper) show that bath-applied nicotine (1 μM) increases the amplitude of eEPSCs, and the average (five of 11; six showed no change) shows the long-lasting effect of the amplitude increase (lower). A low concentration of EGTA (0.4 μM) was used in the patch pipette to enhance the long-lasting increase in amplitude. The recordings were at room temperature at a holding potential of –65 mV, and a weak simulation strength was used. The scale bars represent 100 pA and 10 msec.
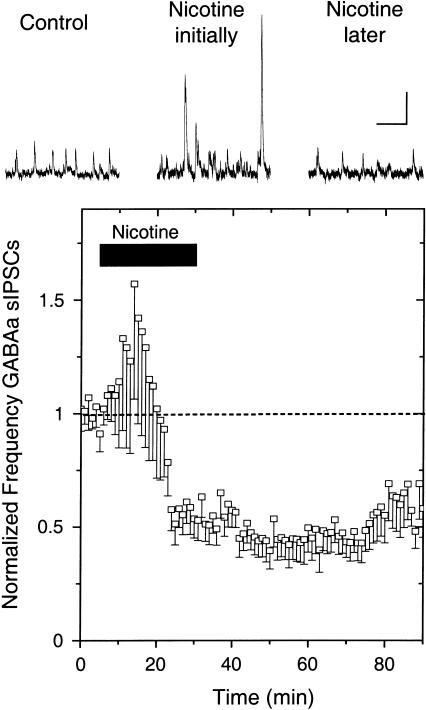
The response to nicotine by the spontaneous GABAergic afferent inhibitory postsynaptic currents (sIPSCs) was markedly different from the long-lasting boost of glutamatergic spontaneous or evoked EPSCs. Bath-applied 500 nM nicotine for 25 min briefly boosted sIPSCs, but that was followed by a strong long-lasting inhibition (n = 13 of 18; five showed no change; Fig. 5). The amplitudes of the sIPSCs also responded. During the increase in sIPSC frequency, there were larger sIPSCs and the average increase in amplitude was 16% ± 6% (Mansvelder et al. 2002). This amplitude increase is consistent with the interpretation that the nAChRs are located on preterminal and somal locations where nicotine can boost the fraction of action potential-dependent IPSCs (Lena et al 1993; McMahon et al 1994; Alkondon et al. 1997; Ji and Dani 2000; Mansvelder et al. 2002). Activation of nAChRs located on the soma or preterminally has been found to depolarize the membrane locally, leading to activation of voltage-dependent channels that directly mediate action potentials. Thus, adding nicotine to the bath briefly boosts the action potential firing of GABAergic neurons, decreasing the relative contribution from the smaller-amplitude miniature IPSCs, which arise from the stochastic release of a single quantum of neurotransmitter. After a short time, the nAChRs desensitize, removing the nicotine-derived excitation as well as removing any endogenous nicotinic cholinergic drive onto the GABAergic neurons.
Figure 5.
Nicotine increases then decreases the frequency of spontaneous IPSCs recorded from VTA DA neurons. Example traces (upper) show that bath-applied nicotine (0.5 μM) first increases the frequency (and amplitude) of sIPSCs, but the average (13 of 18; five showed no change) shows the more potent, long-lasting effect is an inhibition of sIPSC frequency (lower). The recordings were at room temperature at a holding potential of –20 mV. The scale bars represent 20 pA and 0.5 sec.
Endogenous Cholinergic Activity Influences GABAergic and Glutamatergic Afferents
Nicotine activates and desensitizes nAChRs, and in that way directly influences afferent activity into the midbrain and the firing of the DA neurons (Pidoplichko et al. 1997; Picciotto et al. 1998; Mansvelder and McGehee 2000, 2002; Dani et al. 2001; Mansvelder et al. 2002). Desensitization also can have a long-lasting effect on the normal nicotinic mechanisms driven by endogenous cholinergic activity. For example, the long-lasting decrease of sIPSC frequency (Fig. 5) is consistent with nicotine-desensitizing cholinergic afferents that partially drive the GABAergic activity, as has been seen in the hippocampus (Alkondon et al. 1998; Frazier et al. 1998; Hefft et al. 1999). Cholinergic innervation into the midbrain DA areas arises from neurons in the pedunculopontine tegmentum (PPT) and the laterodorsal pontine tegmentum (LDT), which provide widespread innervation mainly to the thalamus and midbrain areas and descending innervation that reaches to the brain stem.
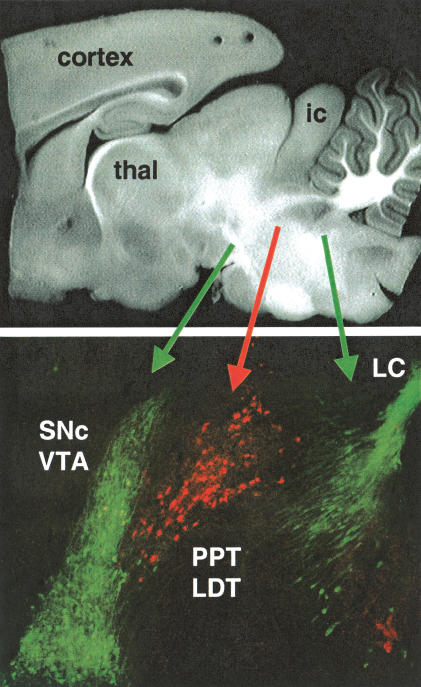
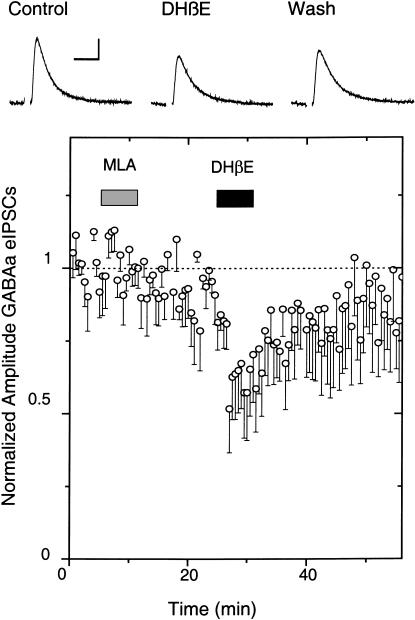
When we cut horizontal slices and electrically stimulated rostral inputs to the VTA, we found that inhibiting nAChRs had little or no effect on the amplitude of GABAergic-evoked IPSCs (Mansvelder et al. 2002; data not shown). Neither 1 μM DHβE (which is selective for non-α7, mainly β2*, nAChRs) nor 5 nM MLA (which is selective for α7* nAChRs) influenced the eIPSCs. Therefore, to better preserve the cholinergic inputs into the VTA from the PPT/LDT, we cut parasagittal slices and stimulated caudally to the VTA. Figure 6 shows that the cholinergic neurons of the PPT/LDT (labeled red for ChAT activity) are near to the DA neurons of the VTA/SNc (labeled green for TH activity). For our electrophysiological studies, we tried to select DA neurons near the interface with the cholinergic neurons, and stimulated near the border between the PPT/LDT and the VTA. Under those conditions, we found that the β2-selective inhibitor, 1 μM DHβE, inhibited the amplitude of the GABAergic eIPSCs (n = 4 out of 10 neurons; six showed no effect; Fig. 7). MLA (5 nM), which inhibits α7* nAChRs, did not influence the eIPSCs when it was added separately (data not shown) or when it was added prior to the DHβE (Fig. 7). The results indicate that non-α7, mainly β2* nAChRs, are activated by endogenous cholinergic activity that helps to drive GABAergic IPSCs onto DA neurons. Thus, ongoing endogenous nicotinic cholinergic activity contributes to the background GABAergic inhibition onto DA neurons.
Figure 6.
Cholinergic neurons project from the pedunculopontine tegmentum (PPT) and the laterodorsal pontine tegmentum (LDT) into the dopaminergic VTA/SNc. The border of these two regions can be seen in these sagittal sections of rat brain. A bright field photomicrograph shows the area at low resolution (top). Tyrosine hydroxylase (TH, indicating catecholamine synthesis, green) and choline acetyltransferase (ChAT, indicating ACh synthesis, red) immunohistochemistry is shown at higher resolution (bottom). The blending of the PPT/LDT and the VTA/SNc can be seen at their border. The TH region to the right is the noradrenergic locus ceruleus (LC) of the brain stem. (thal) Thalamus; (ic) inferior colliculus.
Figure 7.
The β2* antagonist, DHβE, decreased the amplitude of GABAa-evoked IPSCs onto DA neurons. Example traces (upper) show that bath-applied DHβE (1 μM) decreases the amplitude of eIPSCs, and the average (four out of 10 neurons; six showed no effect) shows the amplitude decrease (lower). The α7* nAChR antagonist, MLA, had no effect in separate experiments (data not shown) or when applied before DHβE. The recordings were at room temperature at a holding potential of –60 mV, and weak simulation strength was used. The scale bars represent 50 pA and 20 msec.
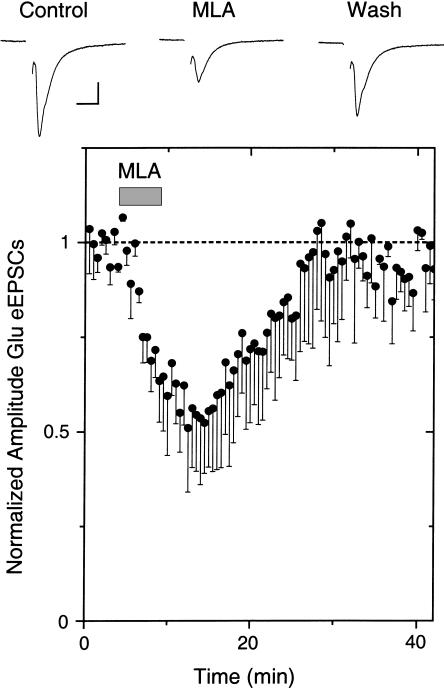
When we inhibited nAChRs while recording glutamatergic eEPSCs onto VTA DA neurons, we found that α7* nAChRs were important. Inhibition with DHβE had no effect on the eEPSC amplitude (data not shown), but inhibition of α7* nAChRs with 5 or 10 nM MLA decreased the eEPSC amplitude (n = 3 out of 10; seven showed no effect; Fig. 8). The results are consistent with sparse endogenous cholinergic innervation stimulating presynaptic α7* nAChRs on some glutamatergic terminals, and in that way boosting excitatory eEPSCs onto DA neurons.
Figure 8.
The α7* antagonist, MLA, decreased the amplitude of Glu-evoked EPSCs. Example traces (upper) show that bath-applied MLA (10 nM) decreases the amplitude of eEPSCs, and the average (three out of 10; seven showed no effect) shows the amplitude decrease (lower). The recordings were at room temperature at a holding potential of –65 mV, and a weak simulation strength was used. The scale bars represent 100 pA and 10 msec.
Nicotine Differentially Desensitizes the nAChR Subtypes
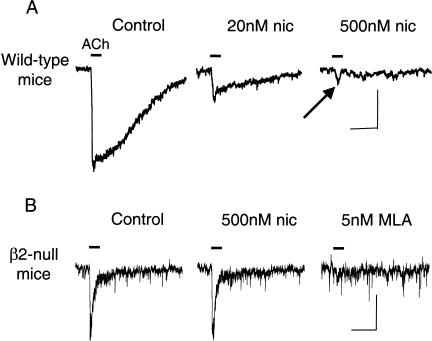
Midbrain DA neurons express different nAChR subunits, but the pharmacological and physiological characteristics of nAChRs currents indicate that β2* nAChRs are by far the predominant subtypes (Pidoplichko et al. 1997; Picciotto et al. 1998; Klink et al. 2001; Wooltorton et al. 2003). Nicotine at the concentration achieved by smokers desensitizes nAChR currents from VTA DA neurons (Pidoplichko et al. 1997; Dani et al. 2000; Wooltorton et al. 2003). An important characteristic, however, is the difference in desensitization of nAChR subtypes (Wooltorton et al. 2003). Nearly all VTA DA neurons display an ACh-induced current with slow kinetics that is consistent in most cases with α4β2*-type nAChRs, sometimes in combinations with α6 (Le Novère et al. 1996; Picciotto et al. 1998; Arroyo-Jimenez et al. 1999; Klink et al. 2001; Azam et al. 2002; Champtiaux et al. 2002; Wooltorton et al. 2003). Bath application of 20 nM nicotine for 20 min causes about 45% desensitization of this slow component of ACh-induced currents (Fig. 9A; Wooltorton et al. 2003). The slow component was nearly completely desensitized by 500 nM nicotine. This result is consistent with those from others who have estimated that α4β2* nAChRs have an IC50 for nicotine-induced desensitization of ∼1–60 nM (Lippiello et al. 1987; Wonnacott 1987; Peng et al. 1994; Rowell 1995; Fenster et al. 1999; Quick and Lester 2002). However, as indicated by the arrow in Figure 9A, a fast component of the ACh-induced current is not desensitized by 500 nM nicotine, but that current is inhibited by MLA (Wooltorton et al. 2003; data not shown), indicating that α7* nAChRs are not desensitized by the low concentrations of nicotine.
Figure 9.
Exposure to low concentrations of nicotine differentially desensitizes the fast and slow components of nicotinic currents from VTA DA neurons. (A) ACh-induced currents (1 mM ACh, 200 msec puff, horizontal line) in the absence (Control) and presence of bath-applied nicotine (20 nM or 500 nM for 20 min). Although the slow component of the current (mainly α4β2* nAChRs) was desensitized, the fast component (indicated by the notch, arrow) was not desensitized. The scale bars represent 100 pA and 0.5 sec. (B) Exposure to low concentrations of nicotine does not desensitize the fast, MLA-sensitive currents from VTA DA neurons of β2-null mice. ACh-induced currents (1 mM ACh, 200 msec puff, horizontal line) are shown from the same neuron. In the absence (Control) or presence of nicotine (500 nM for 20 min) the currents are the same. The ACh-induced currents were inhibited by 5 nM MLA, confirming that these are α7* nAChR currents. The ACh-induced currents were not inhibited by 1 μM DHβE (data not shown). The scale bars represent 50 pA and 0.5 sec. (These data are taken and adapted from Wooltorton et al. 2003.)
In most cases, the fast and slow components of the current are not easily separable because the small amplitude of the fast component is contaminated by the rising phase of the slow component, which is the predominant current. Using mutant mice lacking the β2 nAChR subunit almost always eliminates all the contributions to the slow component of the current, revealing the fast, α7 component. The fast, α7 component of the current is not significantly desensitized by bath-applied nicotine in the range experienced by smokers (20–500 nM; Fig. 9B). Although α7* nAChRs desensitize rapidly when exposed to high concentrations of nicotine or ACh (Alkondon and Albuquerque 1991; Bertrand et al. 1992; Dani et al. 2000; Papke et al. 2000; Wooltorton et al. 2003), they have a relatively low affinity for desensitization by nicotine. The results of Figure 9 are consistent with measurement of α7* nAChRs from rodent hippocampus or expressed in oocytes, where estimates of IC50 for nicotine-induced desensitization of α7* nAChRs range from about 0.5 to 7 μM (Fenster et al. 1997; Frazier et al. 1998; McQuiston and Madison 1999; Alkondon et al. 2000; Quick and Lester 2002). In summary, the β2* subtypes of nAChRs are strongly desensitized by the concentration of nicotine obtained from tobacco, but the α7* nAChRs are not.
DISCUSSION
Nicotine as Obtained From Tobacco
Nicotine obtained from tobacco is initially at a significant concentration in the arterial blood, lung, and brain (roughly 100–500 nM), and then it distributes to storage adipose and muscle tissue (Karan et al. 2003). The distribution half-life of ∼8 min determines the initial action of nicotine within the central nervous system. The elimination half-life is ∼2 h, which allows nicotine to accumulate with ongoing smoking and persist for many hours after the cessation of smoking. Although there is significant individual variability, the steady-state plasma concentration of nicotine plateaus in the early afternoon roughly in the range of 10–50 ng/mL (Karan et al. 2003). Thus, smokers often deliver a small pulse of nicotine with each episode of smoking, and nicotine accumulates and lingers in the body (and brain) as the day progresses. This situation will initially cause some activation of most nAChR subtypes, but then the prolonged low levels of nicotine will favor desensitization of most non-α7 nAChR subtypes.
Desensitization of nAChR Subtypes in the Ventral Tegmental Area
Midbrain DA regions contain many nAChR subunits: α3–α7 and β2–β4. Although α7* nAChRs are commonly expressed at a low density on the DA neurons, the vast majority of subtypes contain β2, often in combinations with α4 and α6 (Le Novère et al. 1996; Picciotto et al. 1998; Arroyo-Jimenez et al. 1999; Klink et al. 2001; Azam et al. 2002; Champtiaux et al. 2002; Wooltorton et al. 2003). These β2* nAChRs also are the predominant subtype on the midbrain GABAergic interneurons, but again, there are other minority subtypes. On the other hand, α7* nAChRs are the predominant subtype on the presynaptic terminals of glutamatergic afferents onto DA neurons. This difference in the distribution of nAChR subtypes is important because nicotine does not identically activate or desensitize the subtypes.
The β2* subtypes have a higher affinity for nicotine than the α7* subtypes. Therefore, the β2* subtypes are activated, but then they strongly proceed into desensitization. Wooltorton et al. (2003) showed that 80 nM nicotine caused an 80% desensitization of the β2* nAChRs in the midbrain. That result is consistent with previous estimates that β2* nAChRs have an IC50 for nicotine-induced desensitization of ∼1–60 nM (Lippiello et al. 1987; Wonnacott 1987; Peng et al. 1994; Rowell 1995; Fenster et al. 1999; Quick and Lester 2002). Thus, nicotine strongly desensitizes the majority subtypes of nAChRs (i.e., β2*) on midbrain DA and GABA neurons. On the other hand, α7* nAChRs are not strongly desensitized by the low concentrations of nicotine obtained from tobacco. Wooltorton et al. (2003) showed that up to 500 nM nicotine caused very little desensitization of VTA α7* nAChRs. That result is consistent with previous estimates that α7* nAChRs have an IC50 for nicotine-induced desensitization of up to 7 μM (Fenster et al. 1997; Frazier et al. 1998; McQuiston and Madison 1999; Alkondon et al. 2000; Quick and Lester 2002). This result may be surprising to some because high concentrations of agonist desensitize α7* nAChRs more rapidly than other nAChR types (Alkondon and Albuquerque 1991; Bertrand et al. 1992; Dani et al. 2000; Papke et al. 2000). Despite the rapid kinetics, the lower affinity of α7* nAChRs for nicotine underlies the lack of desensitization. At low nicotine concentrations, only a small proportion of the α7* nAChR population is occupied by nicotine, and that proportion proceeds toward desensitization. Because of the rapid kinetics, those α7* nAChRs rapidly recover from desensitization when nicotine unbinds. Consequently, only a very small portion of the overall α7 population is desensitized at any moment, leaving the remaining receptors available to activate.
Model of the Synaptic Action of Nicotine in the Ventral Tegmental Area
Although other minority subtypes are present, β2* nAChRs make up the vast majority of subtypes on VTA DA neurons and GABAergic interneurons. Those receptors underlie the initial direct activation of DA neurons by nicotine (see Fig. 10; Mansvelder and McGehee 2000, 2002; Dani et al. 2001; Mansvelder et al. 2002; Wooltorton et al. 2003). When nicotine first arrives, these β2* nAChRs are activated, causing direct excitation of the DA neurons and the GABAergic interneurons (indicated by Figs. 2 and 5; Calabresi et al. 1989; Pidoplichko et al. 1997; Picciotto et al. 1998; Dani et al. 2000, 2001; Mansvelder and McGehee 2002). In minutes, significant desensitization affects these (predominantly) β2* nAChRs (see Figs. 2, 5, and 9). The GABAergic activity declines more rapidly because desensitization removes the direct excitation caused by nicotine and decreases the endogenous cholinergic drive onto the GABAergic interneurons (Figs. 5 and 7). Whereas a small subset of DA neurons receives cholinergic inputs, there is greater innervation of GABAergic neurons in the VTA (Garzon et al. 1999).
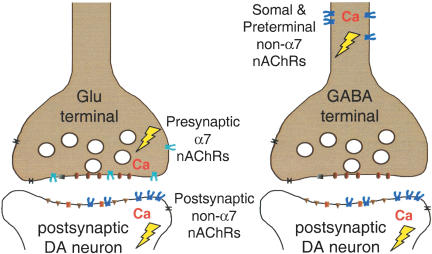
Figure 10.
The major sites of influence by nAChR subtypes at glutamatergic and GABAergic synapses onto VTA DA neurons are shown. Activation of nAChRs will induce a local depolarization (lightning bolt) than can activate voltage-dependent channels and in some cases an action potential. The activity also will initiate a direct and indirect Ca2+ signal. The Ca2+ signal can influence subsequent Ca2+ release from intracellular stores and initiate intracellular cascades. The size of the signals will depend on many factors, including the subtypes of nAChRs that are present and activation versus desensitization by agonists and modulators. The diagram is simplified to the major subtypes at each location, but other minority subtypes also can be present. Nicotine, as obtained from tobacco, will briefly activate then begin to significantly desensitize the non-α7 (usually β2*) nAChRs located on DA and GABA neurons. The α7* nAChRs will be activated somewhat, but will not be strongly desensitized by those levels of nicotine. Thus, the increased excitatory drive via presynaptic α7* nAChR on glutamatergic (Glu) terminals coupled with the short-lasting increase in DA neuron firing caused by nicotine's direct action creates the coincidence of presynaptic and postsynaptic activity that favors the initiation of synaptic plasticity, such as STP and LTP. Thus, multiple synaptic events contribute to the prolonged increased firing by DA neurons.
As the excitation of DA neurons by nicotine decreases owing to desensitization of the predominant nAChR subtypes, other synaptic factors have already provided further excitation to produce the prolonged DA signal observed in the NAc (Fig. 1; Imperato et al. 1986; Di Chiara and Imperato 1988; Clarke 1991; Corrigall et al. 1992; Pontieri et al. 1996; Di Chiara 1999, 2000; Dani and De Biasi 2001). The initial “pulse” of nicotine provided by smoking activates presynaptic α7* nAChRs located on glutamatergic terminals that synapse onto DA neurons (see Figs. 3, 4, and 8), and the α7* subtype does not desensitize even at the higher concentrations of nicotine obtained from smoking (Fig. 9). Because the α7* subtype is the most highly permeable nAChR to calcium, it often mediates a direct Ca2+ increase as well as initiating indirect Ca2+ influx caused by the local depolarization and via intracellular Ca2+ stores (Séguéla et al. 1993; McGehee and Role 1995; McGehee et al. 1995; Gray et al. 1996; Rathouz et al. 1996; Radcliffe and Dani 1998; Mansvelder and McGehee 2000, 2002; Dani et al. 2001; Ji et al. 2001; Mansvelder et al. 2002). Consequently, the activation of presynaptic α7* nAChRs initiates a calcium increase in the glutamatergic presynaptic terminals that increases glutamate release and excitation of DA neurons, even while the β2* nAChRs on the DA neurons are desensitizing (see Fig. 10).
The situation favors longer-term synaptic plasticity that potentiates the glutamatergic drive. Long-term potentiation (LTP) arises when presynaptic glutamatergic excitation is coincident with a large enough postsynaptic response to induce a Ca2+ signal through NMDA-type glutamate receptors (NMDARs). When nicotine initially arrives, it excites the DA neurons to increase their action potential firing rate. That postsynaptic DA neuron activity is coupled with a nicotine-induced increase in presynaptic glutamatergic afferent excitation (see Fig. 10). That combination produces the presynaptic and postsynaptic coincidence that boosts the production of LTP (see Mansvelder and McGehee 2000, 2002; Dani et al. 2001; Ji et al. 2001; Mansvelder et al. 2002). Subsequently, non-α7 subtypes desensitize, thereby, decreasing the inhibition onto DA neurons by GABAergic neurons. In addition, the α7* nAChRs on presynaptic glutamate terminals do not desensitize. Thus, they continue to enhance glutamatergic excitation as long as the nicotine signal is present. This seemingly choreographed complex of nicotinic synaptic mechanisms contributes to the prolonged DA signal in the NAc and elsewhere that is thought to be a critical component in the addiction process.
The synaptic changes that are induced by nicotine are much like the normal synaptic plasticity that underlies learning and memory: Presynaptic calcium signals enhance excitatory transmission coupled to a strong postsynaptic response, leading to short-term and long-term potentiation. Nicotine tips the normal balance, inappropriately favoring potentiation of synapses and, ultimately, favoring inappropriate behaviors. In this way, the addictive drug, nicotine, commandeers fundamental synaptic mechanisms that normally subserve learning and memory.
MATERIALS AND METHODS
Brain Slice Preparation and Electrophysiology
Midbrain horizontal or sagittal slices containing the VTA and SNc were prepared from 14- to 25-day-old Sprague Dawley rats that were anesthetized before decapitation (see Wooltorton et al. 2003). Slices (300–350 μm thick) were cut in ice-cold cutting solution, and all the solutions were saturated with 95% O2, 5% CO2 to achieve a pH near 7.4 during the experiments. The cutting solution was either of the following or a 50%/50% mixture of the two solutions. The sucrose cutting solution was 230 mM sucrose, 1 mM KCl, 1.25 mM NaH2PO4, 30 mM NaHCO3, 1 mM CaCl2, 7 mM MgCl2, and 25 mM D-glucose. The N-methyl-D-glucamine (NMDG) cutting solution was 144 mM NMDG, 1.5 mM KCl, 1.25 mM NaH2PO4, 30 mM NaHCO3, 2 mM CaCl2, 2 mM MgCl2, and 25 mM D-glucose, and the pH was adjusted to 7.4 with 50% D-gluconic acid and sodium bicarbonate. The slices were then transferred to a holding chamber containing the bath solution: 125 mM NaCl, 2.5 mM KCl, 1.25 mM NaH2PO4 (or 1.24 mM KH2PO4), 21 mM NaHCO3, 2.5 mM CaCl2 (or 2.1 mM CaCl2), 1 mM MgCl2 (or 0.3 mM MgCl2), and 25 mM D-glucose 25. Slices were held either for 1 h at room temperature or for 20 min at 34°C and, then, for a minimum of 20 min at room temperature. The experimental chamber (0.8 mL capacity) had continuously flowing bath solution (∼5 mL/min) at room temperature near 23°C. The osmolality of the external solution was adjusted to 320 mOsm with D-glucose. All the experiments studying evoked responses had 0.5 μM atropine added to the external bath solutions to block muscarinic AChRs. During the measurement of glutamatergic evoked EPSCs, the bath solution contained 20 μM bicuculline to block GABAergic transmission. During the measurement of evoked GABAergic IPSCs, the bath solution contained 20 μM CNQX to block non-NMDA Glu receptors. When used, nicotine and nAChR antagonists were applied via the continuously flowing bath solution.
Neurons were visualized under infrared light using Nomarski optics of an upright microscope (Zeiss Axioscope) equipped with a CCD camera (Hamamatsu Photonics K.K.). The patch electrodes had resistances of 3.5–4.5 MΩ for evoked responses and up to 7 MΩ for spontaneous events when filled with the internal solution: 60 mM CsCH3SO3, 60 mM KCH3SO3, 1 or 10 mM KCl, 0.4 mM EGTA, 10 mM HEPES, 5 mM Mg-ATP, 0.3 mM Na3GTP (pH 7.2), 290 mOsm osmolality; or 140 mM K-gluconate; 1 mM MgCl2; 1 mM CaCl2; 10 mM HEPES; 10 mM EGTA; 5 mM ATP-Mg; and 0.3 mM GTP-Na (pH 7.3 adjusted with Tris-base). The holding potential for voltage-clamp recordings was the following: for eEPSCs, –65 mV; for sEPSCs and eIPSCs, –60 mV; for sIPSCs, –20 mV. The spontaneous or evoked glutamatergic events were completely inhibited by CNQX (20 μM) and AP-5 (50 μM), and the spontaneous or evoked GABAergic events were completely inhibited by bicuculline (20 μM).
The stimulation arrangement we used attempted to optimize the stimulation of the sparse cholinergic fibers that range through the thickness of the slice. Nine Teflon-coated wires, with the isolation stripped at the very end of each wire, formed a V-shaped sink electrode array beneath the slice. The VTA-containing mesolimbic system was positioned inside the V-shaped area formed by the tips of nine wires. A monopolar tungsten stimulation electrode could be positioned near the top of the slice either on the rostral or caudal side close to the VTA. Switching between sink electrodes allowed choosing the best input. In other cases to favor rostral or caudal stimulation, two of the nine V-shaped electrodes serve as source and sink. EPSCs or IPSCs were evoked via a computer-controlled stimulus isolator model A-360 (WPI) applying minimal stimulation intensity. The intensity of the stimulus was adjusted to elicit amplitudes at 10%–20% of the maximum better to see the effect of nicotine (see Mansvelder and McGehee 2000). Stimulation currents ranged from 150 to 450 μA and usually lasted 0.7 msec. Currents were amplified and filtered (1 kHz) using Axopatch 200B or 1C amplifiers (Axon Instruments) with a four-pole low-pass Bessel filter and were digitally sampled (up to 5 kHz). Currents were recorded using pClamp software (Axon), and further analyzed using Origin (MicroCal Software). Additional off-line filtering and signal averaging were sometimes used in the figures. Neurons that were patch-clamped were identified as VTA DA based on large Ih currents (Pidoplichko et al. 1997; Bonci and Malenka 1999).
Immunohistochemistry
Tyrosine hydroxylase (TH, indicating catecholamine synthesis) and choline acetyltransferase (ChAT, indicating ACh synthesis) immunohistochemistry were adapted from published methods (Zhou et al. 2001). For ChAT and TH double immunofluorescence, brains were fixed in 0.1 M phosphate buffer containing 4% (w/v) paraformaldehyde and 14% (v/v) picric acid. Sections were cut on a cryostat, and were incubated with goat anti-ChAT and rabbit anti-TH antibodies. The secondary antibody mixture containing Cy2-conjugated donkey anti-rabbit and rhodamine-conjugated donkey anti-goat IgG antisera was used. All images were captured with a digital camera and processed in Adope PhotoShop.
Microdialysis
Long-Evans male rats (Harlan) were housed together on a 12-h light/dark cycle. Their body weight was from 280–300 g. The rats were anesthetized using a ketamine–xylazine combo injected intraperitoneally (1.8 μL per gram body weight) and subsequently maintained on an isoflurane-gas mixture. The microdialysis CMA/12 (CMA/Microdialysis) guide cannula was aimed at the NAc shell (1.7 mm AP; 0.8 mm L; 6.5 mm DV, with the probe at 7.5 mm) and was secured with bone wax reinforced with acrylic cement and three screws into the skull. The rat was allowed to recover fully for a minimum of 48 h.
CMA/12 probes (diameter, 0.5 mm; length, 1 mm; membrane, polycarbonate; cutoff, 20,000 D) were prepared, and filtered degassed artificial cerebrospinal fluid (aCSF; from ESA) was perfused through the probe at a flow rate of 1 μL/min using a CMA/100 pump. Following a 2-h recovery period, three 20-min fractions were collected to assess the basal output of dopamine in the dialysate. Subsequently, saline and nicotine (0.6 mg/kg i.p.) were injected, and samples were collected every 20 min for 3 h. After these experiments, rats were killed with an overdose of anesthetics and trans-cardially perfused with PBS and then 10% formalin. The brain was removed and fixed in 10% formalin. The accuracy of probe placement was later confirmed by histological sectioning.
Dopamine contents of microdialysates were determined using a high-performance liquid chromatography (HPLC) system (model 580 pump, Coulochem II electrochemical detector, model 5014B analytical cell; ESA, Inc.). Separation of dopamine was achieved on a 150 × 3 mm column with 3 μm particle size (ESA, Inc.; MD-150). An isocratic mobile phase (pH 4.0) containing 75 mM NaH2PO4, 2 mM 1-octane sulphonic acid-sodium salt, 20 mM EDTA, 100 μL/L triethylamine, and 18% methanol (from ESA) was used at a flow rate of 0.6 mL/min. This mobile phase and the protocols used produced clearly separable dopamine peaks with a retention time of ∼5.6 min. Chromatograms were analyzed with the ESA software. Freshly prepared standards ranging from 0–6 nM DA were used to calibrate the readings.
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke (NS21229) and the National Institute on Drug Abuse (DA09411 and DA12661).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.70004.
References
- Albuquerque, E.X., Alkondon, M., Pereira, E.F., Castro, N.G., Schrattenholz, A., Barbosa, C.T., Bonfante-Cabarcas, R., Aracava, Y., Eisenberg, H.M., and Maelicke, A. 1997. Properties of neuronal nicotinic acetylcholine receptors: Pharmacological characterization and modulation of synaptic function. J. Pharmacol. Exp. Ther. 280: 1117–1136. [PubMed] [Google Scholar]
- Alkondon, M. and Albuquerque, E.X. 1991. Initial characterization of the nicotinic acetylcholine receptors in rat hippocampal neurons. J. Recept. Res. 11: 1001–1021. [DOI] [PubMed] [Google Scholar]
- Alkondon, M., Pereira, E.F., Wonnacott, S., and Albuquerque, E.X. 1992. Blockade of nicotinic currents in hippocampal neurons defines methyllycaconitine as a potent and specific receptor antagonist. Mol. Pharmacol. 41: 802–808. [PubMed] [Google Scholar]
- Alkondon, M., Pereira, E.F., Barbosa, C.T., and Albuquerque, E.X. 1997. Neuronal nicotinic acetylcholine receptor activation modulates γ-aminobutyric acid release from CA1 neurons of rat hippocampal slices. J. Pharmacol. Exp. Ther. 283: 1396–1411. [PubMed] [Google Scholar]
- Alkondon, M., Pereira, E.F., and Albuquerque, E.X. 1998. α-Bungarotoxin- and methyllycaconitine-sensitive nicotinic receptors mediate fast synaptic transmission in interneurons of rat hippocampal slices. Brain Res. 810: 257–263. [DOI] [PubMed] [Google Scholar]
- Alkondon, M., Pereira, E.F., Almeida, L.E., Randall, W.R., and Albuquerque, E.X. 2000. Nicotine at concentrations found in cigarette smokers activates and desensitizes nicotinic acetylcholine receptors in CA1 interneurons of rat hippocampus. Neuropharmacology 39: 2726–2739. [DOI] [PubMed] [Google Scholar]
- Arroyo-Jimenez, M.M., Bourgeois, J.P., Marubio, L.M., Le Sourd, A.M., Ottersen, O.P., Rinvik, E., Fairen, A., and Changeux, J.P. 1999. Ultrastructural localization of the α4-subunit of the neuronal acetylcholine nicotinic receptor in the rat substantia nigra. J. Neurosci. 19: 6475–6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azam, L., Winzer-Serhan, U.H., Chen, Y., and Leslie, F.M. 2002. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J. Comp. Neurol. 444: 260–274. [DOI] [PubMed] [Google Scholar]
- Balfour, D.J., Wright, A.E., Benwell, M.E., and Birrell, C.E. 2000. The putative role of extra-synaptic mesolimbic dopamine in the neurobiology of nicotine dependence. Behav. Brain Res. 113: 73–83. [DOI] [PubMed] [Google Scholar]
- Benowitz, N.L., Porchet, H., and Jacob III, P. 1989. Nicotine dependence and tolerance in man: Pharmacokinetic and pharmacodynamic investigations. Prog. Brain Res. 79: 279–287. [DOI] [PubMed] [Google Scholar]
- Bertrand, D., Devillers-Thiery, A., Revah, F., Galzi, J.L., Hussy, N., Mulle, C., Bertrand, S., Ballivet, M., and Changeux, J.P. 1992. Unconventional pharmacology of a neuronal nicotinic receptor mutated in the channel domain. Proc. Natl. Acad. Sci. 89: 1261–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci, A. and Malenka, R.C. 1999. Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the ventral tegmental area. J. Neurosci. 19: 3723–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi, P., Lacey, M.G., and North, R.A. 1989. Nicotinic excitation of rat ventral tegmental neurones in vitro studied by intracellular recording. Br. J. Pharmacol. 98: 135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro, N.G. and Albuquerque, E.X. 1995. α-Bungarotoxin-sensitive hippocampal nicotinic receptor channel has a high calcium permeability. Biophys. J. 68: 516–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. 2002. Annual smoking-attributable mortality, years of potential life lost, and economic costs; United States, 1995–1999. Morbidity and Mortality Weekly Report, 51: 300–303. CDC. [PubMed] [Google Scholar]
- Champtiaux, N., Han, Z.Y., Bessis, A., Rossi, F.M., Zoli, M., Marubio, L., McIntosh, J.M., and Changeux, J.P. 2002. Distribution and pharmacology of α 6-containing nicotinic acetylcholine receptors analyzed with mutant mice. J. Neurosci. 22: 1208–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpantier, E., Barneoud, P., Moser, P., Besnard, F., and Sgard, F. 1998. Nicotinic acetylcholine subunit mRNA expression in dopaminergic neurons of the rat substantia nigra and ventral tegmental area. Neuroreport 9: 3097–3101. [DOI] [PubMed] [Google Scholar]
- Clarke, P.B. 1990. Dopaminergic mechanisms in the locomotor stimulant effects of nicotine. Biochem. Pharmacol. 40: 1427–1432. [DOI] [PubMed] [Google Scholar]
- Clarke, P.B. 1991. The mesolimbic dopamine system as a target for nicotine. In Effects of nicotine on biological systems (eds. F. Adlkofer and K. Thurau), pp. 285–294. Birkhäuser Verlag, Basel.
- Clarke, P.B., Schwartz, R.D., Paul, S.M., Pert, C.B., and Pert, A. 1985. Nicotinic binding in rat brain: Autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-α-bungarotoxin. J. Neurosci. 5: 1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall, W.A. 1999. Nicotine self-administration in animals as a dependence model. Nicotine Tob. Res. 1: 11–20. [DOI] [PubMed] [Google Scholar]
- Corrigall, W.A. and Coen, K.M. 1989. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology 99: 473–478. [DOI] [PubMed] [Google Scholar]
- Corrigall, W.A., Franklin, K.B., Coen, K.M., and Clarke, P.B. 1992. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology 107: 285–289. [DOI] [PubMed] [Google Scholar]
- Corrigall, W.A., Coen, K.M., and Adamson, K.L. 1994. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 653: 278–284. [DOI] [PubMed] [Google Scholar]
- Dani, J.A. and De Biasi, M. 2001. Cellular mechanisms of nicotine addiction. Pharmacol. Biochem. Behav. 70: 439–446. [DOI] [PubMed] [Google Scholar]
- Dani, J.A. and Heinemann, S. 1996. Molecular and cellular aspects of nicotine abuse. Neuron 16: 905–908. [DOI] [PubMed] [Google Scholar]
- Dani, J.A., Radcliffe, K.A., and Pidoplichko, V.I. 2000. Variations in desensitization of nicotinic acetylcholine receptors from hippocampus and midbrain dopamine areas. Eur. J. Pharmacol. 393: 31–38. [DOI] [PubMed] [Google Scholar]
- Dani, J.A., Ji, D., and Zhou, F.M. 2001. Synaptic plasticity and nicotine addiction. Neuron 31: 349–352. [DOI] [PubMed] [Google Scholar]
- Di Chiara, G. 1999. Drug addiction as dopamine-dependent associative learning disorder. Eur. J. Pharmacol. 375: 13–30. [DOI] [PubMed] [Google Scholar]
- Di Chiara, G. 2000. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur. J. Pharmacol. 393: 295–314. [DOI] [PubMed] [Google Scholar]
- Di Chiara, G. and Imperato, A. 1988. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. 85: 5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster, C.P., Rains, M.F., Noerager, B., Quick, M.W., and Lester, R.A. 1997. Influence of subunit composition on desensitization of neuronal acetylcholine receptors at low concentrations of nicotine. J. Neurosci. 17: 5747–5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenster, C.P., Whitworth, T.L., Sheffield, E.B., Quick, M.W., and Lester, R.A. 1999. Upregulation of surface α4β2 nicotinic receptors is initiated by receptor desensitization after chronic exposure to nicotine. J. Neurosci. 19: 4804–4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier, C.J., Buhler, A.V., Weiner, J.L., and Dunwiddie, T.V. 1998. Synaptic potentials mediated via α-bungarotoxin-sensitive nicotinic acetylcholine receptors in rat hippocampal interneurons. J. Neurosci. 18: 8228–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzon, M., Vaughan, R.A., Uhl, G.R., Kuhar, M.J., and Pickel, V.M. 1999. Cholinergic axon terminals in the ventral tegmental area target a subpopulation of neurons expressing low levels of the dopamine transporter. J. Comp. Neurol. 410: 197–210. [DOI] [PubMed] [Google Scholar]
- Goldner, F.M., Dineley, K.T., and Patrick, J.W. 1997. Immunohistochemical localization of the nicotinic acetylcholine receptor subunit α6 to dopaminergic neurons in the substantia nigra and ventral tegmental area. Neuroreport 8: 2739–2742. [DOI] [PubMed] [Google Scholar]
- Gourlay, S.G. and Benowitz, N.L. 1997. Arteriovenous differences in plasma concentration of nicotine and catecholamines and related cardiovascular effects after smoking, nicotine nasal spray, and intravenous nicotine. Clin. Pharmacol. Ther. 62: 453–463. [DOI] [PubMed] [Google Scholar]
- Gray, R., Rajan, A.S., Radcliffe, K.A., Yakehiro, M., and Dani, J.A. 1996. Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature 383: 713–716. [DOI] [PubMed] [Google Scholar]
- Grenhoff, J. and Johnson, S.W. 1996. Sulfonylureas enhance GABAA synaptic potentials in rat midbrain dopamine neurones. Acta Physiol. Scand. 156: 147–148. [DOI] [PubMed] [Google Scholar]
- Guo, J.Z., Tredway, T.L., and Chiappinelli, V.A. 1998. Glutamate and GABA release are enhanced by different subtypes of presynaptic nicotinic receptors in the lateral geniculate nucleus. J. Neurosci. 18: 1963–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefft, S., Hulo, S., Bertrand, D., and Muller, D. 1999. Synaptic transmission at nicotinic acetylcholine receptors in rat hippocampal organotypic cultures and slices. J. Physiol. 515: 769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henningfield, J.E., Stapleton, J.M., Benowitz, N.L., Grayson, R.F., and London, E.D. 1993. Higher levels of nicotine in arterial than in venous blood after cigarette smoking. Drug Alcohol Depend. 33: 23–29. [DOI] [PubMed] [Google Scholar]
- Imperato, A., Mulas, A., and Di Chiara, G. 1986. Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Eur. J. Pharmacol. 132: 337–338. [DOI] [PubMed] [Google Scholar]
- Ji, D. and Dani, J.A. 2000. Inhibition and disinhibition of pyramidal neurons by activation of nicotinic receptors on hippocampal interneurons. J. Neurophysiol. 83: 2682–2690. [DOI] [PubMed] [Google Scholar]
- Ji, D., Lape, R., and Dani, J.A. 2001. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron 31: 131–141. [DOI] [PubMed] [Google Scholar]
- Jones, S., Sudweeks, S., and Yakel, J.L. 1999. Nicotinic receptors in the brain: Correlating physiology with function. Trends. Neurosci. 22: 555–561. [DOI] [PubMed] [Google Scholar]
- Jones, I.W., Bolam, J.P., and Wonnacott, S. 2001. Presynaptic localization of the nicotinic acetylcholine receptor β2 subunit immunoreactivity in rat nigrostriatal dopaminergic neurones. J. Comp. Neurol. 439: 235–247. [DOI] [PubMed] [Google Scholar]
- Kalivas, P.W., Churchill, L., and Klitenick, M.A. 1993. GABA and enkephalin projection from the nucleus accumbens and ventral pallidum to the ventral tegmental area. Neuroscience 57: 1047–1060. [DOI] [PubMed] [Google Scholar]
- Karan, L.D., Dani, J.A., and Benowitz, N.L. 2003. The pharmacology of nicotine dependence. In Principles of addiction medicine, 3rd ed. (eds. A. Graham et al.), pp. 225–248. American Society of Addiction Medicine, Inc.
- Klink, R., de Kerchove d'Exaerde, A., Zoli, M., and Changeux, J.P. 2001. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J. Neurosci. 21: 1452–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lena, C., Changeux, J.P., and Mulle, C. 1993. Evidence for “preterminal” nicotinic receptors on GABAergic axons in the rat interpeduncular nucleus. J. Neurosci. 13: 2680–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Novère, N., Zoli, M., and Changeux, J.P. 1996. Neuronal nicotinic receptor α 6 subunit mRNA is selectively concentrated in catecholaminergic nuclei of the rat brain. Eur. J. Neurosci. 8: 2428–2439. [DOI] [PubMed] [Google Scholar]
- Li, X., Rainnie, D.G., McCarley, R.W., and Greene, R.W. 1998. Presynaptic nicotinic receptors facilitate monoaminergic transmission. J. Neurosci. 18: 1904–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippiello, P.M., Sears, S.B., and Fernandes, K.G. 1987. Kinetics and mechanism of L-[3H]nicotine binding to putative high affinity receptor sites in rat brain. Mol. Pharmacol. 31: 392–400. [PubMed] [Google Scholar]
- Mansvelder, H.D. and McGehee, D.S. 2000. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron 27: 349–357. [DOI] [PubMed] [Google Scholar]
- Mansvelder, H.D. and McGehee, D.S. 2002. Cellular and synaptic mechanisms of nicotine addiction. J. Neurobiol. 53: 606–617. [DOI] [PubMed] [Google Scholar]
- Mansvelder, H.D., Keath, J.R., and McGehee, D.S. 2002. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron 33: 905–919. [DOI] [PubMed] [Google Scholar]
- Martin, S.J., Grimwood, P.D., and Morris, R.G. 2000. Synaptic plasticity and memory: An evaluation of the hypothesis. Annu. Rev. Neurosci. 23: 649–711. [DOI] [PubMed] [Google Scholar]
- McGehee, D.S. and Role, L.W. 1995. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu. Rev. Physiol. 57: 521–546. [DOI] [PubMed] [Google Scholar]
- McGehee, D.S., Heath, M.J., Gelber, S., Devay, P., and Role, L.W. 1995. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science 269: 1692–1696. [DOI] [PubMed] [Google Scholar]
- McMahon, L.L., Yoon, K.W., and Chiappinelli, V.A. 1994. Nicotinic receptor activation facilitates GABAergic neurotransmission in the avian lateral spiriform nucleus. Neuroscience 59: 689–698. [DOI] [PubMed] [Google Scholar]
- McQuiston, A.R. and Madison, D.V. 1999. Nicotinic receptor activation excites distinct subtypes of interneurons in the rat hippocampus. J. Neurosci. 19: 2887–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler, E.J. 1992. Molecular mechanisms of drug addiction. J. Neurosci. 12: 2439–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler, E.J. 1993. Cellular responses to chronic treatment with drugs of abuse. Crit. Rev. Neurobiol. 7: 23–39. [PubMed] [Google Scholar]
- Nisell, M., Nomikos, G.G., and Svensson, T.H. 1994. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse 16: 36–44. [DOI] [PubMed] [Google Scholar]
- Nisell, M., Nomikos, G.G., and Svensson, T.H. 1995. Nicotine dependence, midbrain dopamine systems and psychiatric disorders. Pharmacol. Toxicol. 76: 157–162. [DOI] [PubMed] [Google Scholar]
- Papke, R.L., Meyer, E., Nutter, T., and Uteshev, V.V. 2000. α7 receptor-selective agonists and modes of α7 receptor activation. Eur. J. Pharmacol. 393: 179–195. [DOI] [PubMed] [Google Scholar]
- Peng, X., Gerzanich, V., Anand, R., Whiting, P.J., and Lindstrom, J. 1994. Nicotine-induced increase in neuronal nicotinic receptors results from a decrease in the rate of receptor turnover. Mol. Pharmacol. 46: 523–530. [PubMed] [Google Scholar]
- Peto, R., Lopez, A.D., Boreham, J., Thun, M., and Heath Jr., C. 1992. Mortality from tobacco in developed countries: Indirect estimation from national vital statistics. Lancet 339: 1268–1278. [DOI] [PubMed] [Google Scholar]
- Peto, R., Lopez, A.D., Boreham, J., Thun, M., Heath Jr., C., and Doll, R. 1996. Mortality from smoking worldwide. Br. Med. Bull. 52: 12–21. [DOI] [PubMed] [Google Scholar]
- Picciotto, M.R., Zoli, M., Rimondini, R., Lena, C., Marubio, L.M., Pich, E.M., Fuxe, K., and Changeux, J.P. 1998. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature 391: 173–177. [DOI] [PubMed] [Google Scholar]
- Pidoplichko, V.I., De Biasi, M., Williams, J.T., and Dani, J.A. 1997. Nicotine activates and desensitizes midbrain dopamine neurons. Nature 390: 401–404. [DOI] [PubMed] [Google Scholar]
- Pontieri, F.E., Tanda, G., Orzi, F., and Di Chiara, G. 1996. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature 382: 255–257. [DOI] [PubMed] [Google Scholar]
- Quick, M.W. and Lester, R.A. 2002. Desensitization of neuronal nicotinic receptors. J. Neurobiol. 53: 457–478. [DOI] [PubMed] [Google Scholar]
- Radcliffe, K.A. and Dani, J.A. 1998. Nicotinic stimulation produces multiple forms of increased glutamatergic synaptic transmission. J. Neurosci. 18: 7075–7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radcliffe, K.A., Fisher, J.L., Gray, R., and Dani, J.A. 1999. Nicotinic modulation of glutamate and GABA synaptic transmission of hippocampal neurons. Ann. NY Acad. Sci. 868: 591–610. [DOI] [PubMed] [Google Scholar]
- Rathouz, M.M., Vijayaraghavan, S., and Berg, D.K. 1996. Elevation of intracellular calcium levels in neurons by nicotinic acetylcholine receptors. Mol. Neurobiol. 12: 117–131. [DOI] [PubMed] [Google Scholar]
- Role, L.W. and Berg, D.K. 1996. Nicotinic receptors in the development and modulation of CNS synapses. Neuron 16: 1077–1085. [DOI] [PubMed] [Google Scholar]
- Rowell, P.P. 1995. Nanomolar concentrations of nicotine increase the release of [3H]dopamine from rat striatal synaptosomes. Neurosci. Lett. 189: 171–175.7624037 [Google Scholar]
- Russell, M.A. 1987. Estimation of smoke dosage and mortality of non-smokers from environmental tobacco smoke. Toxicol. Lett. 35: 9–18. [DOI] [PubMed] [Google Scholar]
- Séguéla, P., Wadiche, J., Dineley-Miller, K., Dani, J.A., and Patrick, J.W. 1993. Molecular cloning, functional properties, and distribution of rat brain α 7: A nicotinic cation channel highly permeable to calcium. J. Neurosci. 13: 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel, R. and Weiss, F. 1999. The dopamine hypothesis of reward: Past and current status. Trends Neurosci. 22: 521–527. [DOI] [PubMed] [Google Scholar]
- Steffensen, S.C., Svingos, A.L., Pickel, V.M., and Henriksen, S.J. 1998. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J. Neurosci. 18: 8003–8015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman, I.P. and Jarvis, M.J. 1995. The scientific case that nicotine is addictive. Psychopharmacology (Berl) 117: 2–20. [DOI] [PubMed] [Google Scholar]
- Stolerman, I.P. and Shoaib, M. 1991. The neurobiology of tobacco addiction. Trends Pharmacol. Sci. 12: 467–473. [DOI] [PubMed] [Google Scholar]
- Wada, E., Wada, K., Boulter, J., Deneris, E., Heinemann, S., Patrick, J., and Swanson, L.W. 1989. Distribution of α 2, α 3, α 4, and β 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: A hybridization histochemical study in the rat. J. Comp. Neurol. 284: 314–335. [DOI] [PubMed] [Google Scholar]
- Wada, E., McKinnon, D., Heinemann, S., Patrick, J., and Swanson, L.W. 1990. The distribution of mRNA encoded by a new member of the neuronal nicotinic acetylcholine receptor gene family (α 5) in the rat central nervous system. Brain Res. 526: 45–53. [DOI] [PubMed] [Google Scholar]
- WHO. 1997. Tobacco or health, a global status report, p. 495. World Health Organization Publications, Geneva.
- Wonnacott, S. 1987. Brain nicotine binding sites. Hum. Toxicol. 6: 343–353. [DOI] [PubMed] [Google Scholar]
- Wonnacott, S. 1997. Presynaptic nicotinic ACh receptors. Trends Neurosci. 20: 92–98. [DOI] [PubMed] [Google Scholar]
- Wonnacott, S., Drasdo, A., Sanderson, E., and Rowell, P. 1990. Presynaptic nicotinic receptors and the modulation of transmitter release. Ciba Found. Symp. 152: 87–101. [DOI] [PubMed] [Google Scholar]
- Wonnacott, S., Kaiser, S., Mogg, A., Soliakov, L., and Jones, I.W. 2000. Presynaptic nicotinic receptors modulating dopamine release in the rat striatum. Eur. J. Pharmacol. 393: 51–58. [DOI] [PubMed] [Google Scholar]
- Wooltorton, J.R., Pidoplichko, V.I., Broide, R.S., and Dani, J.A. 2003. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J. Neurosci. 23: 3176–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, C.R. and Role, L.W. 1998. Functional contribution of the α7 subunit to multiple subtypes of nicotinic receptors in embryonic chick sympathetic neurones. J. Physiol. 509: 651–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, F.M., Liang, Y., and Dani, J.A. 2001. Endogenous nicotinic cholinergic activity regulates dopamine release in the striatum. Nat. Neurosci. 4: 1224–1229. [DOI] [PubMed] [Google Scholar]