Abstract
Photosynthesis and photoinhibition in field-grown rice (Oryza sativa L.) were examined in relation to leaf age and orientation. Two varieties (IR72 and IR65598-112-2 [BSI206]) were grown in the field in the Philippines during the dry season under highly irrigated, well-fertilized conditions. Flag leaves were examined 60 and 100 d after transplanting. Because of the upright nature of 60-d-old rice leaves, patterns of photosynthesis were determined by solar movements: light falling on the exposed surface in the morning, a low incident angle of irradiance at midday, and light striking the opposite side of the leaf blade in the afternoon. There was an early morning burst of CO2 assimilation and high levels of saturation of photosystem II electron transfer as incident irradiance reached a maximum level. However, by midday the photochemical efficiency increased again almost to maximum. Leaves that were 100 d old possessed a more horizontal orientation and were found to suffer greater levels of photoinhibition than younger leaves, and this was accompanied by increases in the de-epoxidation state of the xanthophyll cycle. Older leaves had significantly lower chlorophyll content but only slightly diminished photosynthesis capacity.
Recent studies show that rice (Oryza sativa L.) yields need to increase by 70% of current levels by the year 2030 to meet the needs of a rapidly increasing human population, and this increase must arise almost exclusively from existing highly irrigated farmland (Khush and Peng, 1996). To achieve this increase in production, the yield potential needs to be increased and the rate of biomass production improved, particularly during the reproductive phase (Cassman, 1994). Of the numerous factors affecting crop yield, the efficiency with which solar radiation is transformed into biomass and the amount of radiation available are the most important (Russell et al., 1989).
Light saturation of photosynthesis leads to a decline in radiation-conversion efficiency. For rice this was estimated to be approximately 17% (Murata and Matsushima, 1975) but varies greatly according to variety and growth conditions. In the tropical dry season, high irradiance not only saturates photosynthesis but also subjects the exposed flag leaf to high-light stress. The response of rice leaves to high irradiance has not been characterized in the field. In laboratory and field studies of other plant species, mechanisms operate that down-regulate PSII via an increase in the dissipation of excess excitation energy (Demmig-Adams and Adams, 1996; Horton et al., 1996). Although the major fraction of this is controlled by the thylakoid ΔpH and the xanthophyll cycle, and therefore responds rapidly to changes in irradiance, some down-regulation is often long-lived and in extreme cases damage to thylakoid constituents can occur (Andersson and Barber, 1996). Both damage to and sustained down-regulation of PSII can be considered to be photoinhibitory (i.e. causing a decrease in the quantum efficiency of photosynthesis; Osmond, 1994) and potentially could impact the radiation-conversion efficiency of the crop. There is variation among rice cultivars in both the susceptibility to photoinhibition and the capacity for dissipation of excess absorbed light energy via the xanthophyll cycle (Black et al., 1995).
In the rice crop several factors influence the response of leaf photosynthesis to light. First, elevated leaf temperatures that accompany high irradiance have been shown to cause metabolic imbalances (Pastenes and Horton, 1996b), deleterious effects on thylakoid function (Pastenes and Horton, 1996a), enhanced photoinhibition (Fuse et al., 1993), and enhanced photorespiration (Leegood and Edwards, 1996).
Second, leaf angle has been identified as influencing the degree of light saturation of upper leaves (Yoshida, 1981b). At the IRRI in the Philippines, new rice varieties have been developed that possess a series of ideal traits, including rigid, upright leaves. These varieties are called NPT. Upright leaves were introduced to these new varieties to increase the penetration of sunlight through to lower leaves, thus optimizing light distribution throughout the canopy. Leaf orientation influences the amount of light absorbed by altering both the level of reflectance and the available cross-sectional area (He et al., 1996; Valladares and Pearcy, 1997). The upright leaf angle therefore profoundly influences the changes in light absorption occurring during the diurnal cycle. Light saturation of photosynthesis may not be reached, or may be short-lived, and the period of exposure to high-light stress reduced. An upright leaf angle is not expected to greatly alter the diurnal changes in leaf temperature, suggesting that there will be periods of low light absorption and high temperature.
Third, the light responses of leaves are predicted to be altered with growth of the crop. We have observed that, as the canopy matures and the grain-filling stage progresses, more than 50% of NPT flag leaves adopt a more horizontal orientation, predicting that there will be long periods of light saturation of photosynthesis. The grain-filling period also coincides with the onset of leaf senescence, the decline in photosynthesis capacity due to a breakdown of Rubisco and Chl-containing protein complexes (Makino et al., 1985; Kura-Hotta et al., 1987), again creating conditions in which light stress could be increased. In fact, there is some evidence to suggest that photoinhibition is enhanced during leaf senescence (Kar et al., 1993).
The filling capacity of the rice grain is often not attained, and this is particularly true in NPT rice (Khush and Peng, 1996). Since 60% to 100% of the carbon in mature rice grains originates from CO2 assimilation during the grain-filling period, with the flag leaf as the most photosynthetically active (Yoshida, 1981a), factors that lower the photosynthesis rate of the flag leaf during this period could potentially limit grain yield. The diminution of photosynthesis capacity arising from senescence and/or photoinhibition could limit the provision of photosynthate during this critical phase in crop development.
We report the results of an investigation of the light responses of leaf photosynthesis in a crop of both NPT and an older, established variety of rice. The objectives of this study were (a) to identify how leaf orientation may reduce photosynthesis but alleviate photoinhibition by decreasing the light absorbed at midday and by exposing each leaf blade surface to a maximum of a half-day of direct irradiance only, and (b) to test the hypothesis that photoinhibition of photosynthesis is enhanced in the flag leaf of rice during the grain-filling period, which coincides with the onset of flag leaf senescence and altered leaf angle.
MATERIALS AND METHODS
Growth of Plant Material
Experiments were carried out in the dry season of 1996 at the IRRI farm at Los Baños in the Philippines. The site was (14° 11′ N, 12° 15′ E, altitude 21 m). The rice (Oryza sativa L.) varieties used were IR72 and IR65598-112-2 (BSI206) (referred to hereafter as IR65). The former is a commonly used Indica variety and the latter a Tropical Japonica of the NPT class. Seedlings were transplanted in January and plants were highly irrigated throughout the study. A Maahas clay soil was used (Andaqueptic Haplaquoll). Nutrients were supplied so as to be plentiful throughout.
Analysis took place at two stages of growth: before flowering at approximately 60 d after transplanting, and approximately 100 d after transplanting, when grains were halfway through the filling stage (the yellow-ripe stage; Yoshida, 1981b) and the flag leaves had begun to senesce (as assessed by a decreasing Chl content).
Gas Exchange
Leaf gas-exchange measurements were made using a IR gas analyzer (model 6400, Li-Cor, Lincoln, NE) operating in the semi-open mode. External air was scrubbed of CO2 and mixed with a supply of pure CO2 to result in a reference concentration of 350 μL L−1. Flow rate was 500 μmol s−1 and external humidity (50%–60%) was used. The leaf chamber (2 × 3 cm) was constructed of two parts: the upper half could be replaced with the light-emitting diode light source, and the bottom half held the leaf-temperature thermocouple. Two GaAsP PAR sensors were fitted, one located inside the upper half of the leaf chamber and the other located externally, beside the leaf chamber.
Light-saturation curves were taken in the field by removing the chamber window fitting and attaching the light-emitting diode array. A range of light intensities between 0 and 2500 μmol m−2 s−1 were given, starting high and progressing toward 0, allowing 2 min at each light intensity. This was sufficient to achieve steady-state photosynthesis at each light intensity. This process took about 20 min, during which time the chamber was shaded to prevent overheating.
Chl Fluorescence
Chl fluorescence was measured using a portable fluorimeter (PAM 2000, Walz, Effeltrich, Germany) attached to a notebook computer (model T2130CS, Toshiba, Tokyo, Japan). Steady-state fluorescence during diurnal illumination was measured using a leaf clip (model 2030-B, Walz). Dark adaptation of leaves also took place with leaf clips (Walz). Clips were attached and left for 10 min before measurements. Nomenclature and equations for calculation of Chl fluorescence parameters were as described previously (Genty et al., 1989; Van Kooten and Snel, 1990). Electron-transport rates were calculated by the product of ΦPSII (ΔF/Fm′) and the incident photon flux density, 0.84/0.5. The latter two numbers represent estimates of the proportion of incident quanta absorbed by the leaf and a distribution of energy between PSII and PSI, respectively.
Carotenoid Analysis
Leaf samples were taken at various points during the day and immediately frozen in liquid N2. The average time between removal of leaf fragments and freezing was 5 s. Samples were kept at −80°C and transported to Sheffield in a dry shipping vessel (Biotrek III, Statebourne Cryogenics, Tyne & Wear, UK) for analysis by HPLC. Leaf samples were extracted and analyzed essentially as described by Johnson et al. (1993). DES was calculated as (zeaxanthin + ½ antheraxanthin)/(zeaxanthin + antheraxanthin + violaxanthin).
Chl Assay
The Chl content of leaves in the field was measured using a hand-held Chl meter (model SPAD-502, Minolta, Ramsey, NJ) as described previously (Markwell et al., 1995). Several measurements were taken on each leaf and averaged. Ten leaves were used to find an average value. Values from the Chl meter (SPAD values) were converted into Chl per unit leaf area using calibration curves developed for each variety. A calibration curve was constructed for SPAD-values versus Chl per unit leaf area (as estimated by extraction and assay in 80% acetone).
Experimental Protocols
It was important that photosynthesis be assessed in situ (i.e. that all conditions were kept as close as possible to those experienced by the leaf). Therefore, the leaf was kept at its natural angle of posture. For young leaves of IR65 this was a vertical position. For IR72 there was more variation in leaf angle, although flag leaves were mostly upright. It was also observed that the most common position for either variety was with the adaxial surface at 90° relative to the rising sun; we shall refer to this leaf surface as face 1. The opposite side of the blade, which received the afternoon sun, will be called face 2. Leaves were tagged before measurements were taken, and the same position on the leaf was measured each time (approximately 10 and 5 cm from the tip of the blade for IR65 and IR72, respectively).
The older flag leaves of both varieties had a more horizontal posture, which is a common feature of modern rice varieties (Rajaram and van Ginkel, 1996). Leaves of IR65, being generally heavier, reached an almost completely horizontal position, whereas there was more variation for leaves of IR72.
For the gas-exchange measurements, photosynthesis was measured with the leaf in two positions relative to the direction of sunlight. The first was with sunlight striking the leaf chamber window at 90° to the direction of sunlight. This was found to be saturating for photosynthesis for a large proportion of the day; therefore, we use the expressions “direct irradiance” and “direct photosynthesis.” For the second position, photosynthesis was measured as near as possible to the natural leaf angle by maintaining the leaf in its upright position. For this we use the expressions “in situ irradiance” and “in situ photosynthesis.” At midday it was not possible to use the leaf in its exact in situ posture because of shading by the sides of the leaf chamber; therefore, the minimum angle achievable was approximately 40°. There was a small difference between values of photon flux density for the internal and external sensors, but this was not affected by alteration of the chamber angle up to 40°. Therefore, this was a valid method for measurement of photosynthesis at different IARs. For Chl fluorescence measurements the in situ leaf angle was used throughout. Experiments were repeated two to three times, averaging values from 5 to 10 plants in each case. Values are given as means ± se.
RESULTS
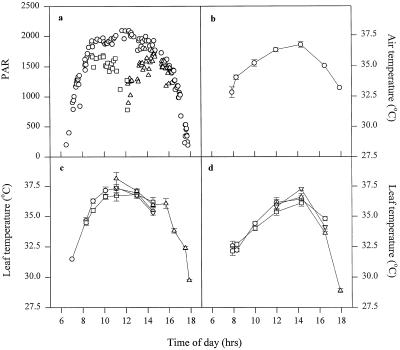
Figure 1a shows a diurnal irradiance profile for the dry season at the IRRI farm. Direct light represents the maximum possible irradiance. Also shown is the in situ irradiance, which was identical to the direct irradiance in the early morning until approximately 8:30 am, when the in situ irradiance remained at approximately 1500 μmol m−2 s−1 until 11 am. The direct irradiance was between 1900 and 2000 μmol m−2 s−1 for almost 7 h of the day (between 8 and 3 pm). At midday the in situ irradiance decreased to less than 1000 μmol m−2 s−1. At 3 pm the IAR was high enough to again eliminate the difference between direct and in situ irradiance.
Figure 1.
a, Typical daily irradiance profile for the dry season at the IRRI farm. ○, Direct (maximum) irradiance; □, in situ irradiance (face 1); ▵, in situ irradiance (face 2). b, Daily air temperature profile. c, Daily leaf temperature profile for IR65. d, Daily leaf temperature profile for IR72. ○, Face 1 direct; □, face 1 in situ; ▵, face 2 direct; ▿, face 2 in situ. Error bars represent se. Because of practical problems associated with measuring light at an IAR approaching 0°, the sensor was placed at an angle of approximately 10°; therefore, the values at 12:30 to 1 pm may be lower than those shown. See text for details.
Figure 1b shows air temperature measured using the external sensor attached to the IR gas analyzer leaf chamber and averaged over 3 successive days. Temperature increased steadily throughout the morning and peaked at an average of 37°C at midday. Figure 1, c and d, shows leaf temperature for the two cultivars used. These data show that the leaf temperature did not increase significantly above that of the external temperature during the time it took to take the measurement (approximately 1 min). In situ leaf temperature was slightly lower than that for leaves in a direct position, depending on the time of day.
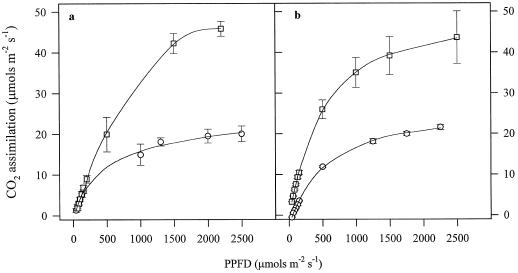
Figure 2 shows light-saturation curves for IR72 and IR65 measured in the field. At 350 μg mL−1 CO2, photosynthesis was saturated at 2000 μmol m−2 s−1 for both varieties (Fig. 2). This was the case whatever time during the day the light-saturation curves were taken. Therefore, by comparison with Figure 1a it may be concluded that photosynthesis was saturated for a significant part of the day (at least 4 h). At 900 μg mL−1 CO2, the photosynthesis rate was more than double that observed at ambient CO2 levels.
Figure 2.
Light-saturation curves taken in the field for IR65 (a) and IR72 (b) at 350 μg mL−1 (○) and 900 μg mL−1 (□) CO2. Measurements were made 60 d following transplanting.
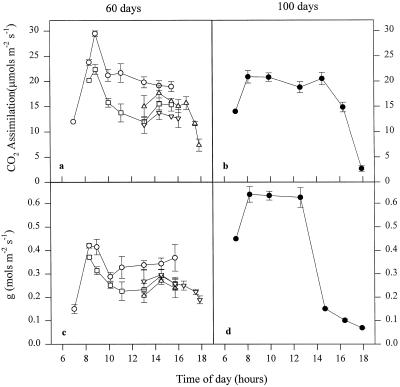
Figure 3 shows diurnal courses of CO2 exchange for 60- and 90-d leaves of IR65. Values for photosynthesis and stomatal conductance were comparable to those of previous experiments using the same varieties and carried out during the dry season at IRRI (S. Peng, unpublished data). For 60-d leaves of IR65, direct photosynthesis measured for face 1 increased dramatically from 7 to 9 am as photon flux density increased, and by this time photosynthesis was light saturated. However, between 9 and 10 am there was an unexpectedly early (Black et al., 1995) decline in photosynthesis capacity (Pmax) to 70% of this maximum rate and this was maintained for the rest of the morning. Values of Pmax for face 2 were at a similar level. Measurements of stomatal conductance indicated that this reduction in Pmax was associated with closure of stomata. Photosynthesis rates in situ were lower than direct rates after 8:30 am, and this disparity was further enhanced by midday. The decline of in situ photosynthesis was probably due to a combination of the decline in Pmax and a decreased IAR. At midday in situ photosynthesis was 63% of Pmax. Broadly similar behavior was shown by 60-d leaves of IR72 (data not shown).
Figure 3.
Daily profiles of photosynthesis (a and b) and stomatal conductance (c and d) for IR65 leaves 60 d (a and c) and 100 d (b and d) following transplanting. ○, •, Face 1 direct; □, face 1 in situ; ▵, face 2 direct; ▿, face 2 in situ. Error bars represent se of at least seven replicates. See text for details.
At the grain-filling stage only face 1 was measured in IR65 because of the loss of an erect leaf posture. Photosynthesis rates after 10 am were light saturated (compare with Fig. 2). In contrast to the young leaves, there was no midmorning decrease in Pmax, and, in fact, photosynthesis rates were the same as those found in the late morning and beyond in the younger leaves (Fig. 3). Photosynthesis remained constant for a large part of the day, declining as the irradiance decreased after 2 pm. At 1 pm a dramatic decline in stomatal conductance occurred, while photosynthesis rates remained high. A low internal CO2 concentration was also recorded at this time (data not shown). An almost identical pattern was noted for IR72 leaves (not shown).
With the exception of the early morning burst of CO2 assimilation, direct rates of photosynthesis for IR65 were similar at 60 and 100 d. In situ rates for the 100-d leaves were higher than for 60-d leaves because of the more favorable leaf angle, and total daily carbon gain, as assessed from the area under the curves in Figure 3, indicated approximately 30% more photosynthesis in the 100-d flag leaves. There was a small decrease in the light- and CO2-saturated Pmax (from 45 to 40 μmol CO2 m−2 s−1), suggesting that the onset of senescence and Chl content in the 100-d leaves was approximately 34% below the younger leaves (Table I). For IR72 there was no statistically significant difference in Pmax at 900 μg mL−1 CO2 between young and old leaves, although the Chl content had decreased by 26% in the older leaves.
Table I.
Photosynthetic capacity and Chl contents of leaves of IR65 and IR72
| Plant | Chl Content | Pmax |
|---|---|---|
| μmol m−2 | μmol m−2 s−1 | |
| IR65 | ||
| 60 d | 385.5 ± 25.1 | 45.9 ± 1.8 |
| 100 d | 253.1 ± 20.7 | 39.6 ± 3.3 |
| IR72 | ||
| 60 d | 334.9 ± 20.9 | 43.6 ± 6.4* |
| 100 d | 247.7 ± 13.9 | 32.7 ± 4.8* |
Data are shown for leaves 60 and 100 d following transplanting. For Chl the values represent the means ± se of between 7 and 10 leaves. Five measurements were taken from each leaf and averaged. Pmax represents light-saturated (>2000 μmol quanta m−3 s−1) and CO2-saturated (900 μg mL−1 CO2) photosynthesis measured in the field as in Figure 2. All values are significantly different to a 10% level when comparing 60- and 100-d plants, except those marked with an asterisk.
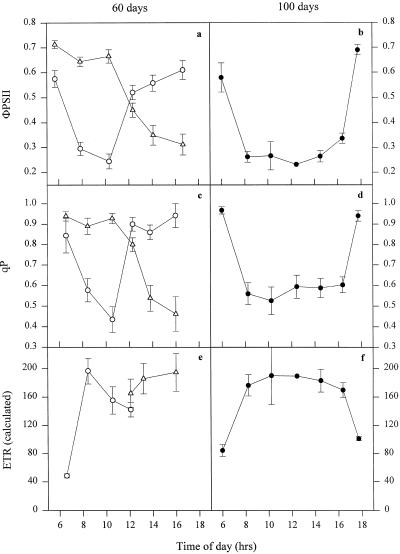
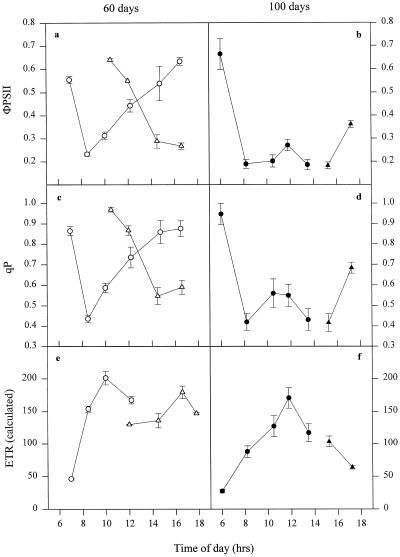
Chl fluorescence was used to more fully explore the effect of leaf posture and age on photosynthesis. For face 1 of young leaves of IR65, ΦPSII declined steeply as the irradiance level increased, reaching a minimum value of approximately 0.2 at 8 to 10 am (Fig. 4a). Such a low value for ΦPSII indicates a high degree of saturation of electron transport. Toward midday, as the IAR decreased, ΦPSII increased again to values close to those found in early morning, when the photon flux density was much lower. The reverse pattern was observed for face 2, with a high ΦPSII in the morning because of its shaded position but a steep decline after midday, when direct irradiance struck this surface of the leaf. The rate of electron transport was estimated from in situ photon flux density and ΦPSII. The estimated electron transport rate in young leaves followed the same pattern as above (Fig. 4e).
Figure 4.
Daily profiles of photochemical efficiency ΦPSII, qP, and estimated electron transport rate (ETR) for IR65 60 d (a, c, and e) and 100 d following transplanting (b, d, and f). ○, •, Face 1; ▵, face 2. Error bars represent se of at least 12 replicates. See text for further details.
The pattern of changes in qP on the two leaf faces was similar to that exhibited by ΦPSII (Fig. 4c). This parameter represents the proportion of PSII reaction centers in an open or oxidized state. At approximately midday, when direct photon flux density was highest but the IAR was lowest, the qP for both face 1 and face 2 was extremely high (approximately 0.9). The lowest value (0.4) was attained in the morning at approximately 8 am for face 1 and midafternoon for face 2, in both cases when the irradiance and the IAR were high.
For all of the above Chl fluorescence parameters the behavior of young leaves of IR72 matched closely that shown by IR65 (Fig. 5, a, c, and e). The main difference was that the increase in qP and ΦPSII following the midmorning minima was less steep than for IR65.
Figure 5.
Daily profiles of photochemical efficiency ΦPSII, qP, and estimated electron transport rate (ETR) for IR72 60 d (a, c, and e) and 100 d following transplanting (b, d, and f). ○, •, Face 1; ▵, ▴, face 2. Error bars represent se of the means of at least 12 replicates. See text for further details.
The data for flag leaves at 100 d shown in Figures 4 and 5 had a simpler pattern than that recorded for young leaves, as predicted from their horizontal orientation. For IR65 both ΦPSII and qP reached minimum values by 8 am, and this condition was maintained for the following 6 to 8 h before increasing toward the end of the photoperiod (Fig. 4). IR72 behaved similarly except that there was a midday increase in ΦPSII and qP, probably because senescing leaves of this variety maintained a slightly curved orientation (Fig. 5). In both varieties qP values of less than 0.6 were found for several hours.
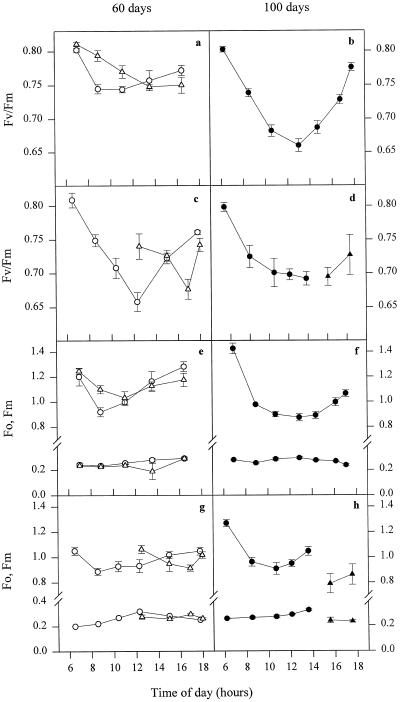
To determine whether the light saturation of photosynthetic electron transport (indicated by the low qP value) was giving rise to photoinhibition, the dark-adapted Fv/Fm was recorded (Fig. 6). In young leaves of IR65 the decline in Fv/Fm occurred first in face 1, reaching a minimum between 8 and 10 am (Fig. 6a). In the afternoon face 1 was shaded, allowing Fv/Fm to partially recover. Fv/Fm in face 2 also declined slightly throughout the morning (although to a much lesser degree than face 1) and reached a minimum at midafternoon. However, it should be emphasized that these decreases in Fv/Fm were small and indicate a less than 10% decrease in quantum yield. Measurement of F0 and Fm showed that this small decline was due mainly to quenching of Fm (Fig. 6e).
Figure 6.
Daily profiles of dark-adapted fluorescence parameters for IR65 (a, b, e, and f) and IR72 (c, d, g, and h) 60 d (a, c, e, and g) and 100 d (b, d, f, and h) following transplanting. Analyses were made following a 10-min dark adaptation. ○, •, Face 1; ▵, ▴, face 2. The first data point at approximately 6:30 am is the overnight dark-adapted state. Error bars represent se of at least seven replicates. See text for further details of methodology.
For young leaves of IR72 a similar pattern was shown, but the decline in Fv/Fm was much more pronounced than in IR65 (Fig. 6c). For face 1, Fv/Fm continued to decrease until midday, reaching minimum values of 0.65 (i.e. a nearly 20% decrease in quantum yield). For this variety an increase in F0 contributed substantially to the decrease in Fv/Fm (Fig. 6g).
At the grain-filling stage the pattern was also simple. In IR65, Fv/Fm declined progressively until midday, when irradiance was at its highest (Fig. 6b). The minimum value was 0.65, twice the level of photoinhibition compared with the younger leaves. This decrease in Fv/Fm partially recovered in the afternoon and overnight was fully recovered. The decline in Fv/Fm occurred because of quenching of Fm. For IR72 a similar pattern was found (Fig. 6d), although, in contrast to the younger leaves, the decrease in Fv/Fm was less than in IR65. Fv/Fm recovered more slowly during the afternoon compared with IR65, although full recovery was observed overnight.
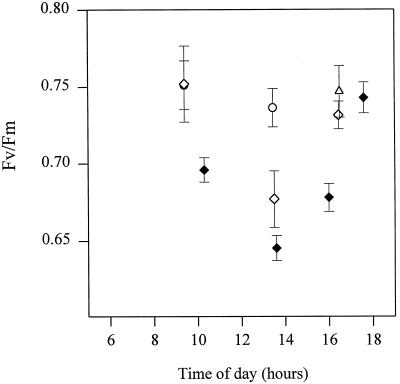
The leaves of 60- and 100-d plants differed not just in leaf orientation but also in Chl content and photosynthesis capacity (Table I). To determine the importance of leaf orientation in the differences in photoinhibition revealed in Figure 6, young leaves of IR65 were maintained in a horizontal position for 5 d. Figure 7 shows that the decline in Fv/Fm was dramatically increased by horizontal positioning of the leaves, even after 5 d. The data shown in Figure 7 for the horizontal young leaf strongly resembled the behavior of the 100-d leaf shown in Figure 6.
Figure 7.
Daily profiles of dark-adapted fluorescence parameters for IR65 leaves left in a vertical position (○) or placed in a horizontal position at 8 am and maintained there for several days thereafter. Measurements were taken on the day of changing leaf orientation (♦) or after 5 d in that position (⋄). Points represent means of at least eight replicates.
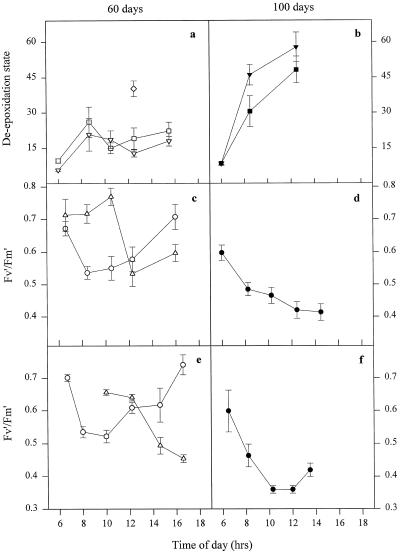
The results shown in Figures 4–6 provide evidence for substantial levels of saturation of the electron transport system and the presence of excess light. Under such conditions it would be predicted that the xanthophyll cycle would be activated and violaxanthin de-epoxidized to zeaxanthin. Figure 8 shows that for the 60-d leaves DES reached a maximum value of approximately 20 to 25 at approximately 8 am, after which time there was a decline before a further increase in the afternoon. These kinetics were the result of the contribution of the illumination of the two leaf surfaces. Leaves that had been maintained horizontally for 5 d had a much higher DES (approximately 40) than the maximum reached by vertical leaves.
Figure 8.
Daily profile of leaf xanthophyll cycle DES (a and b) for IR65 (□, ▪) and IR72 (▿, ▾). Also shown in a is DES for a leaf left in a horizontal position for 5 d (⋄). Fv′/Fm′ is shown for leaves of IR65 (c and d) and IR72 (e and f). ○, •, Face 1; ▵, face 2. Leaves are shown 60 d (a, c, and e) and 100 d (b, d, and f) following transplanting.
As predicted from the sustained light saturation of photosynthesis, the DES of 100-d leaves reached much higher values (45–60) than the 60-d leaves (Fig. 8b). These values are close to the maximum commonly observed for rice (data not shown) and many other plant species (60–65).
Also shown in Figure 8 are the values for Fv′/Fm′ at different times during the day. This parameter, which denotes the quantum efficiency of open PSII centers, declines as a result of NPQ. There were considerably greater overall levels of NPQ in the 100-d leaves compared with younger leaves, with Fv′/Fm′ reaching values of 0.3 to 0.4, which is consistent with the higher DES in these leaves. The kinetics of Fv′/Fm′ were different between old and young leaves. In younger leaves Fv′/Fm′ decreased rapidly on leaf face 1, as seen previously, but unlike qP (Fig. 4), it relaxed only slowly after 10 am (Fig. 8, c and d). In older leaves NPQ developed progressively between 7 am and midday (Fig. 8, d and f) and matched the increase in DES.
There were significant differences in the carotenoid content of young and older leaves. The main difference was the dramatic increase in the carotenoid to Chl ratio (Table II). The increase in this ratio was greater than the decrease in Chl content per unit leaf area (see Table I), indicating net synthesis of carotenoid on a leaf-area basis. The two cultivars behaved somewhat differently. In IR65 the carotenoid to Chl ratio doubled but there were no large differences in the relative content of different carotenoids. The xanthophyll cycle pool size was maintained at approximately 28% of total carotenoid. The content of xanthophyll cycle per unit of Chl increased from 0.08 mol/mol in 60-d leaves to 0.16 mol/mol in 100-d leaves. In IR72 the change in the carotenoid to Chl ratio was not as great, but the xanthophyll cycle pool size increased from approximately 26% of total carotenoid to 32%. The resultant increase in xanthophyll cycle carotenoid to Chl was from 0.08 to 0.15 mol/mol, which is similar to the change seen in IR65. In both varieties there were small decreases in the proportions of β-carotene.
Table II.
Carotenoid composition of leaves of IR65 and IR72
| Plant | Lutein | β-Carotene | Neoxanthin | Xanthophyll Cycle | Car/Chl |
|---|---|---|---|---|---|
| % | |||||
| IR65 | |||||
| 60 d | 33.2 ± 0.4 | 28.6 ± 0.5 | 9.6 ± 0.2 | 28.1 ± 1.1 | 0.29 ± 0.02 |
| 100 d | 37.3 ± 0.6 | 26.3 ± 0.7 | 9.8 ± 0.2 | 28.4 ± 1.0 | 0.58 ± 0.06 |
| 60 d (hor) | 31.6 ± 0.7 | 30.0 ± 1.3 | 9.4 ± 0.3 | 28.1 ± 0.9 | 0.31 ± 0.01 |
| IR72 | |||||
| 60 d | 33.4 ± 0.3 | 29.2 ± 0.3 | 10.5 ± 0.1 | 26.6 ± 0.4 | 0.29 ± 0.02 |
| 100 d | 35.0 ± 0.9 | 24.2 ± 0.5 | 10.0 ± 0.2 | 31.8 ± 1.1 | 0.46 ± 0.02 |
Data are shown for leaves 60 and 100 d following transplanting and for 60-d leaves that had been kept in a horizontal position for 5 d (hor). Values of lutein, β-carotene, and neoxanthin represent the amount of each carotenoid as a percentage of the total carotenoid pool. Xanthophyll cycle represents the total of all xanthophyll cycle constituents (violaxanthin + antheraxanthin + zeaxanthin) as a percentage of the total carotenoid pool. Car/Chl is the molar ratio of total carotenoid to total Chl. Values are means ± se of at least 12 replicates.
DISCUSSION
Leaf Temperature and Photorespiration
For the whole of the photoperiod leaf temperatures were in excess of 30°C, reaching peak values of approximately 37°C. Previous work suggests that this temperature profile should lead to large losses in carbon gain because of increased photorespiration (Leegood and Edwards, 1996). The increase in Pmax upon imposition of high CO2 was 55% (Table I). Data from Leegood and Edwards (1996) suggest that this difference was largely caused by photorespiration.
Midmorning Decrease in Photosynthesis Rate
In both rice varieties examined there was a substantial decline in photosynthesis when the leaf was exposed to maximum in situ photon flux density during early to midmorning. The decline in photosynthesis capacity was approximately 30% and coincided with the brief period when face 1 of the leaf was exposed to maximum irradiance. It is notable that the burst of CO2 fixation was not observed for face 2 and was not observed when the flag leaf was 100 d old. We also found that when plants were grown with reduced N fertilization this transient peak in photosynthesis was again lost (Y. Chen, unpublished data). Measurements of stomatal conductance show that stomatal closure accompanied the decline in photosynthesis, and the decline in the estimated electron transport rate was not as great as the decline in the in situ rate of photosynthesis, indicating the operation of alternative electron sinks such as the Mehler ascorbate-peroxidase reactions and Rubisco-dependent photorespiration (Osmond and Grace, 1995). It is impossible to ascribe cause and effect, but these observations suggest the occurrence of a water deficit, even though the crop was highly irrigated. Whatever the cause, the fact that the maximum rate of photosynthesis in young leaves could be expressed for only brief periods poses important questions concerning resource allocation in the rice plant: Could the extra level of Rubisco and other photosynthesis components needed to attain this rate be viewed as a wasted resource?
Light Saturation of Leaf Photosynthesis
The data presented in this paper show that in both young and old leaves photosynthesis is light saturated for varying periods throughout the day. This was reflected by the strong reduction of both Fv′/Fm′ and qP, resulting in a large decrease in ΦPSII. The recorded values for qP of approximately 0.4 were below the empirical threshold for chronic photoinhibition (Öquist et al., 1992). When light becomes saturating for photosynthesis, an increase occurs in the proportion of excitation energy that is quenched by thermal dissipation rather than through photochemical processes. The terms “dynamic” and “chronic” photoinhibition describe, respectively, quenching processes that relax within minutes and those that take hours (Osmond, 1994). Chronic photoinhibition, diagnosed by a sustained reduction in Fv/Fm and often an increased F0, is associated with a decline in the intrinsic quantum yield of CO2 assimilation. Although relatively well characterized empirically, the causes remain poorly understood. It is difficult to distinguish readily between components of sustained quenching that are regulatory and those that are associated with damage to the photosynthesis apparatus.
In the rice crop there are decreases in dark-adapted Fv/Fm and it is therefore concluded that rice plants undergo a form of chronic photoinhibition in the field, the extent of which is dependent on the variety under study. The Indica variety used (IR72) was more sensitive than the Tropical Japonica (IR65). Laboratory experiments carried out using growth conditions similar to those in the field have shown that the Fv/Fm reduction observed in these varieties takes hours to recover completely (Y. Chen and E.H. Murchie, unpublished data). The reduction in the quantum yield would therefore be important when the light level subsequently decreases and photosynthesis is no longer light saturated. This would happen upon the decrease in IAR toward midday and during shading caused by shifts in light-fleck patterns or by cloud cover. Therefore, the time taken to recover from sustained depressions in Fv/Fm is potentially important in determining daily carbon gain in an erect leaf because in situ fluctuations in PAR are more abrupt than in a horizontal leaf. The data indicate relatively small changes in Fv/Fm, particularly in IR65, but it should be pointed out that rice is frequently grown under conditions much less favorable than those used here; therefore, larger photoinhibitory responses are predicted.
Leaf Angle Determines in Situ Patterns of Photosynthesis and Photoinhibition
Leaf movement in high light is an established strategy for light-stress avoidance in higher plants (Björkman and Demmig-Adams, 1994). However, consideration of how a fixed vertical leaf angle is integrated into the photosynthesis performance of a cereal crop canopy is less well documented. The net result of an upright leaf position is that direct irradiation occurs on a given side of the leaf for a maximum of half a day, and during the hours surrounding midday, when the sun is overhead, both sides of the leaf have a reduced level of intercepted irradiance due to the low IAR.
Chl fluorescence measures only those chloroplasts that are positioned toward the surface of the leaf exposed to the fiberoptic probe of the fluorimeter and therefore provides the ideal technique for exploring the relationship between leaf angle and photosynthesis. We have established clearly that PSII is saturated on one surface of the leaf in the morning and the other surface in the afternoon. The values of qP recorded during these periods indicate a high level of light stress, but because the exposure was brief (a few hours), the amount of chronic photoinhibition was small. Photoinhibition is a time-dependent process that depends on accumulated photon dose (Park et al., 1995); it is not just the level of illumination but the period over which this occurs. This view was confirmed by the observation that leaves of IR65 forced into a horizontal position so that light saturation was maintained over the main part of the day exhibited greater photoinhibition than when in a vertical position.
It has been shown that leaves in a horizontal position receive a greater total daily irradiance (Duncan, 1971), but this has not previously been considered in terms of the extent of light stress. The advantageous effect of a vertical leaf posture is clearly shown in the observation of near maximum values of qP at midday: even though PAR is maximum, the potential for light stress is at a minimum. However, it is also clear that the rate of electron transport and carbon assimilation declined during this period as a result of the low-incident PAR. On an individual leaf basis, there is clearly a conflict between maximizing light absorption for carbon gain and minimizing light absorption to prevent photoinhibition. For the crop as a whole, the picture is more complex, since vertical leaves are considered beneficial for light distribution through the canopy. A detailed cost-benefit analysis of erect leaf orientation is required and needs to be carried out under different agronomic conditions. The conclusions of such an analysis may be different for the dry season and wet season because of differing irradiance levels.
Photosynthesis, Photoinhibition, and Chl Content during Leaf Senescence
The photosynthesis rate per unit leaf area of the leaves during grain filling remained high despite the large decrease in Chl content. Therefore, we conclude that Chl loss does not necessarily coincide with photosynthesis performance in rice, which is consistent with the results of Makino et al. (1985) but not with Kura-Hotta et al. (1987). Pmax at saturating CO2 also showed a much smaller difference between young and old leaves than the loss of Chl, suggesting that the loss of Rubisco protein was minimal. Rubisco amounts have been shown to limit Pmax throughout the life of the rice plant (Makino et al., 1985). The observation that a substantial decline in Chl content did not affect the photosynthesis rate substantially may be explained by suggesting that the Chl content of the young leaves is excessively high, particularly in IR65. It must be questioned whether the high Chl content of this variety confers any benefit in terms of photosynthesis performance under the dry season conditions, when irradiance is high and carbon assimilation is mostly light saturated. It is concluded that up to the 100-d period the senescence of the flag leaf does not contribute to a limitation in the provision of carbohydrate to the developing grain.
Photosynthesis was light saturated for several hours of the day in the senescing flag leaf. Chl fluorescence assays showed low values of qP from 8 am to 4 pm. This exposure to potentially photoinhibitory light was associated with a significant decrease in Fv/Fm, particularly in IR65. Although these decreases in quantum yield reversed overnight, the rate of recovery was slow and some effects persisted in late afternoon despite the decrease in photon flux density. The dramatic increase in DES in the older leaves also demonstrates that these leaves were absorbing light in excess of that which could be used in photosynthesis. Photosynthesis on a leaf-area basis was not changed by more than 10% in IR65, showing that the principal factor giving rise to the increase in DES was increased light absorption. Sustained periods of saturating illumination were needed for the buildup of the maximum DES. The gradual increase in DES in these leaves was associated with a similar progressive increase in NPQ, as assessed from Fv′/Fm′. For IR65 it is significant that the increase in NPQ meant that, although the ΦPSII in the 100-d leaves decreased to a value similar to younger leaves, qP was maintained at a slightly higher value.
Other evidence of sustained exposure to light stress comes from the doubling of the ratio of xanthophyll cycle carotenoid to Chl in the older leaves. In IR72 this was also associated with a change in carotenoid composition in favor of the xanthophyll cycle. Generally, plants exposed to light stress have a high xanthophyll cycle pool size (Demmig-Adams and Adams, 1996). Typically this will be 25% to 35% of total carotenoid; in IR72 and IR65 these values were 32% and 28%, respectively, in the 100-d leaves. For IR72 but not IR65, an increase in the total xanthophyll cycle pool in senescent leaves compared with young leaves was seen, demonstrating that a form of acclimation to enhance photoprotection was occurring in these leaves. Clearly, there are some differences in the acclimation of Tropical Japonica and Indica rice varieties to irradiance with regard to the xanthophyll cycle.
Chl loss from leaves can arise from a number of causes, including acclimation to high irradiance, oxidative stress, carbohydrate buildup, or hormonally regulated breakdown of the chloroplast to promote N recirculation to sinks. There is evidence to suggest that in rice a low source to sink ratio (i.e. a large sink size) induces senescence in the flag leaf (Wada et al., 1993; Nakano et al., 1995), but this may depend on the variety used (Wada and Wada, 1991). In the rice flag leaf under the field conditions used here each of these factors could be important. The observation that a younger leaf forced into a horizontal position did not show any Chl loss despite clear evidence of prolonged exposure to excess light stress suggests that oxidative stress and acclimation were not responsible. Although leaf senescence is principally controlled hormonally (Gan and Amasino, 1995), it may be triggered by environmental factors such as shading and high temperature and, once triggered, the rate of senescence may be altered by factors such as temperature (Thomas and Stoddart, 1980). Moreover, hormonal and metabolic factors also interact (Wingler et al., 1998). Clearly, further work is needed to elucidate the reasons for flag leaf senescence during the grain-filling period.
CONCLUSIONS
By measuring gas exchange, Chl fluorescence, and pigment content we have identified a number of possible limitations to photosynthesis in rice in the field. These limitations indicate for the most part a lack of adaptation of rice to the extremely high temperature and irradiance of the tropical environment. Limitation to photosynthesis as a result of high leaf temperature and a failure to sustain a maximum photosynthesis rate during periods of peak intercepted radiation have been identified. Photosynthesis of the flag leaf was light saturated for substantial parts of the day, giving rise to photoinhibition. Leaf orientation was identified as a key factor in determining both light utilization and the extent of photoinhibition. Leaf senescence, as determined by the decrease in Chl content, was found during the grain-filling period and this predisposed the leaf to sustained periods of light saturation of photosynthesis and greater photoinhibition. In qualitative terms, all of these characteristics were found in both the NPT and Indica rice varieties, although potentially important quantitative differences were found. When considering photosynthesis in relation to yield in crop plants, it is important to identify instances when the rate of carbon assimilation is less than expected. This “lost photosynthesis,” which has been clearly identified in studies of natural plant communities (Cheeseman et al., 1991), represents a resource to be exploited for increasing crop photosynthesis. Further work is needed to determine whether such photosynthesis losses are significant at the canopy level and whether they impact on carbohydrate supply to the grain.
Abbreviations:
- Chl
chlorophyll
- ΔF/Fm′
efficiency of PSII electron transfer
- DES
de-epoxidation stateF0, minimum fluoresence yield
- Fv/Fm
quantum yield of PSII centers in dark-adapted state
- Fv′/Fm′
ratio of variable portion of fluorescence yield to maximum
- IAR
incident angle of irradiation
- IRRI
International Rice Research Institute
- NPQ
non-qP
- NPT
New Plant Type
- Pmax
light-saturated rate of CO2 assimilation
- qP
photochemical quenching
- ΦPSII
efficiency of PSII electron transfer
Footnotes
This research was supported by contract no. ARP505H of the Department for International Development of the U.K.
LITERATURE CITED
- Andersson B, Barber J. Mechanisms of photodamage and protein degradation during photoinhibition of photosystem II. In: Baker NR, editor. Photosynthesis and the Environment. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 101–121. [Google Scholar]
- Björkman O, Demmig-Adams B. Regulation of photosynthetic energy capture, conversion and dissipation in leaves of higher plants. In: Schulze E-D, Caldwell MM, editors. Ecophysiology of Photosynthesis. Berlin: Springer-Verlag; 1994. pp. 17–47. [Google Scholar]
- Black CC, Tu Z-P, Counce PA, Yao P-F, Angelov MN. An integration of photosynthetic traits and mechanisms that can increase crop photosynthesis and grain production. Photosynth Res. 1995;46:169–175. doi: 10.1007/BF00020427. [DOI] [PubMed] [Google Scholar]
- Cassman KG (1994) Breaking the yield barrier. Proceedings of a workshop on rice yield potential in favorable environments. International Rice Research Institute, Los Baños, The Philippines
- Cheeseman JM, Clough BF, Carter DR, Lovelock CE, Eong OJ, Sim RG. The analysis of photosynthetic performance in leaves under field conditions—a case study using Bruguiera mangroves. Photosynth Res. 1991;29:11–22. doi: 10.1007/BF00035202. [DOI] [PubMed] [Google Scholar]
- Demmig-Adams B, Adams WW., III The role of the xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996;1:21–26. [Google Scholar]
- Duncan WG. Leaf angle, leaf area and canopy photosynthesis. Crop Sci. 1971;11:482–485. [Google Scholar]
- Fuse T, Iba K, Satoh H, Nishimura M. Characterisation of a rice mutant having an increased susceptibility to light stress at high temperature. Physiol Plant. 1993;89:799–804. [Google Scholar]
- Gan S, Amasino RM. Inhibition of leaf senescence by autoregulated production of cytokinin. Science. 1995;270:1986–1988. doi: 10.1126/science.270.5244.1986. [DOI] [PubMed] [Google Scholar]
- Genty B, Briantais J-M, Baker NR. The relationship between quantum yield of photosynthetic electron transport and quenching of Chl fluorescence. Biochim Biophys Acta. 1989;990:87–92. [Google Scholar]
- He J, Chee CW, Goh CJ. ‘Photoinhibition’ of Heliconia under natural tropical conditions: the importance of leaf orientation for light interception and leaf temperature. Plant Cell Environ. 1996;19:1238–1248. [Google Scholar]
- Horton P, Ruban AV, Walters RG. Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- Johnson GN, Scholes JD, Horton P, Young AJ. Relationships between carotenoid composition and growth habit in British plant species. Plant Cell Environ. 1993;16:681–686. [Google Scholar]
- Kar M, Streb P, Hertwig B, Feierabend J. Sensitivity to photodamage increases during senescence in excised leaves. J Plant Physiol. 1993;141:538–544. [Google Scholar]
- Khush GS, Peng S (1996) Breaking the yield frontier of rice. In MP Reynolds, S Rajaram, S McNab, eds, Increasing Yield Potential in Wheat: Breaking the Barriers. International Center for Development of Maize and Wheat (CIMMYT), Mexico City, pp 11–19
- Kura-Hotta M, Satoh K, Katoh S. Relationship between photosynthesis and Chl content during leaf senescence of rice seedlings. Plant Cell Physiol. 1987;28:1321–1329. [Google Scholar]
- Leegood RC, Edwards G. Carbon metabolism and photorespiration: temperature dependence in relation to other environmental factors. In: Baker NR, editor. Photosynthesis and the Environment. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. , 191–221. [Google Scholar]
- Makino A, Mae T, Ohira K. Photosynthesis and ribulose-1,5-bisphosphate carboxylase/oxygenase in rice leaves from emergence through senescence. Quantitative analysis by carboxylation/oxygenation and regeneration of ribulose-1,5-bisphosphate. Planta. 1985;166:414–420. doi: 10.1007/BF00401181. [DOI] [PubMed] [Google Scholar]
- Markwell J, Osterman JC, Mitchell JL. Calibration of the Minolta SPAD-502 leaf Chl meter. Photosynth Res. 1995;46:467–472. doi: 10.1007/BF00032301. [DOI] [PubMed] [Google Scholar]
- Murata Y, Matsushima S. Rice. In: Evans LT, editor. Crop Physiology: Some Case Histories. Cambridge, UK: Cambridge University Press; 1975. pp. 73–99. [Google Scholar]
- Nakano H, Makino A, Mae T. Effects of panicle removal on the photosynthetic characteristics of the flag leaf of rice plants during the ripening stage. Plant Cell Physiol. 1995;36:653–659. [Google Scholar]
- Osmond CB (1994) What is photoinhibition? Some insights from comparison of sun and shade plants. In NR Baker, JR Boyer, eds, Photoinhibition: Molecular Mechanisms to the Field. Bios Scientific Publications, Oxford, UK, pp 1–24
- Osmond CB, Grace SC. Perspectives on photoinhibition and photorespiration in the field: quintessential inefficiencies of the light and dark reactions of photosynthesis? J Exp Bot. 1995;46:1351–1362. [Google Scholar]
- Öquist G, Chow WS, Anderson JM. Photoinhibition of photosynthesis represents a long term mechanism for regulation of photosystem II. Planta. 1992;186:450–460. doi: 10.1007/BF00195327. [DOI] [PubMed] [Google Scholar]
- Park Y-I, Chow WS, Anderson J. Light inactivation of functional photosystem II in leaves of pea grown in moderate light depends on photon exposure. Planta. 1995;196:401–411. [Google Scholar]
- Pastenes C, Horton P. Effect of high temperature on photosynthesis in beans. I. Oxygen evolution and Chl fluorescence. Plant Physiol. 1996a;112:1245–1251. doi: 10.1104/pp.112.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastenes C, Horton P. Effect of high temperature on photosynthesis in beans. II. CO2 assimilation and metabolite contents. Plant Physiol. 1996b;112:1253–1260. doi: 10.1104/pp.112.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaram S, van Ginkel M (1996) Yield potential debate: germplasm versus methodology, or both. In MP Reynolds, S Rajaram, McNab, eds, Increasing Yield Potential in Wheat: Breaking the Barriers. International Center for Development of Maize and Wheat (CIMMYT), Mexico City, pp 11–19
- Russell G, Jarvis PG, Monteith JL. Absorption of radiation by canopies and stand growth. In: Russell G, Marshall B, Jarvis PG, editors. Plant Canopies: Their Growth, Form and Function. Cambridge, UK: Cambridge University Press; 1989. pp. 21–39. [Google Scholar]
- Thomas H, Stoddart JL. Leaf senescence. Annu Rev Plant Physiol. 1980;31:83–111. [Google Scholar]
- Valladares F, Pearcy RW. Interactions between water stress, sun-shade acclimation, heat tolerance and photoinhibtion in the sclerophyll Heteromeles arbutifolia. Plant Cell Environ. 1997;20:25–36. [Google Scholar]
- Van Kooten O, Snel JFH. The use of Chl fluorescence nomenclature in plant stress physiology. Photosynth Res. 1990;25:147–150. doi: 10.1007/BF00033156. [DOI] [PubMed] [Google Scholar]
- Wada Y, Miura K, Watanabe K. Effects of source to sink ratio on carbohydrate production and senescence of rice flag leaves during the ripening period. Jpn J Crop Sci. 1993;62:547–553. [Google Scholar]
- Wada Y, Wada G. Varietal difference in leaf senescence during ripening period of advanced Indica rice. Jpn J Crop Sci. 1991;60:529–553. [Google Scholar]
- Wingler A, von Schwaen A, Leegood R, Lea PJ, Quick WP. Regulation of leaf senescence by cytokinin, sugars and light. Effects on NADH-dependent hydroxypyruvate reductase. Plant Physiol. 1998;116:329–337. [Google Scholar]
- Yoshida S (1981a) Physiological analysis of rice yield. In Fundamentals of Rice Crop Science. The International Rice Research Institute, Los Baños, The Philippines, pp 231–251
- Yoshida S (1981b) Growth and development of the rice plant. In Fundamentals of Rice Crop Science. The International Rice Research Institute, Los Baños, The Philippines, pp 1–61