Abstract
We investigated the role of acetylcholine (ACh) during encoding and retrieval of tone/shock-induced fear conditioning with the aim of testing Hasselmo's cholinergic modulation model of encoding and retrieval using a task sensitive to hippocampal disruption. Lesions of the hippocampus impair acquisition and retention of contextual conditioning with no effect on tone conditioning. Cholinergic antagonists also impair acquisition of contextual conditioning. Saline, scopolamine, or physostigmine was administered directly into the CA3 subregion of the hippocampus 10 min before rats were trained on a tone/shock-induced fear conditioning paradigm. Freezing behavior was used as the measure of learning. The scopolamine group froze significantly less during acquisition to the context relative to controls. The scopolamine group also froze less to the context test administered 24 h posttraining. A finer analysis of the data revealed that scopolamine disrupted encoding but not retrieval. The physostigmine group initially froze less during acquisition to the context, although this was not significantly different from controls. During the context test, the physostigmine group froze less initially but quickly matched the freezing levels of controls. A finer analysis of the data indicated that physostigmine disrupted retrieval but not encoding. These results suggest that increased ACh levels are necessary for encoding new spatial contexts, whereas decreased ACh levels are necessary for retrieving previously learned spatial contexts.
Cholinergic antagonists disrupt the acquisition of many spatial tasks that are also sensitive to hippocampal disruption, including, but not limited to, place navigation in a water-maze (Hagan et al. 1987), Hebb–Williams maze (Rogers and Kesner 2003), and contextual fear conditioning (Young et al. 1995; Wallenstein and Vago 2001). During tone/shock-induced fear conditioning, a conditioned stimulus (CS; tone) is paired with an unconditioned stimulus (US; shock). The unconditioned response (UR; freezing) is associated with the CS, producing a conditioned response (CR; freezing). Kim and Fanselow (1992) found that during tone/shock conditioning, rats also froze to the context in which the shock was administered. In addition, hippocampal lesions abolished this effect. Additional data from Philips and LeDoux (1992) demonstrated that this type of conditioning, called “contextual conditioning” requires an intact hippocampus, whereas tone/shock associations require an intact amygdala. Hippocampal function is required in order to associate a set of independent features, or “context” (Rudy and O'Reilly 2001), with an aversive stimulus (i.e., foot-shock).
Computational models of hippocampal function, namely those of Hasselmo and colleagues (Hasselmo and Bower 1993; Hasselmo 1995a,b, 1999; Hasselmo and McClelland 1999) and Rolls (1996, 1989), suggest that the hippocampus, in particular the CA3 subregion, participates in the processes of encoding and retrieval. According to Hasselmo (1995a,b, 1999), a match/mismatch operation occurs in CA1 based on entorhinal cortex (EC) inputs and Schaffer collateral inputs [via dentate gyrus (DG)-CA3 mossy fibers]. A mismatch between inputs results in excitatory input into the medial septum, resulting in high acetylcholine (ACh) release in CA3. The increased levels of ACh attenuate CA3 recurrent collaterals, establishing an encoding phase. In contrast, a match between EC and DG-CA3 inputs in CA1 results in little activation of the medial septum and therefore reduced release of ACh into CA3. The recurrent collaterals dominate CA3 activity in the absence of ACh, establishing retrieval dynamics (see Hasselmo 1999). Encoding and retrieval were defined using the procedures recently employed by Lee and Kesner (2004) in which within-day and between-day analyses of freezing behavior assessed encoding and retrieval, respectively. It is assumed that the encoding phase dominates as the animal learns within a day of testing, whereas the retrieval phase dominates as the animal is exposed to the context after a 24-h delay, during which it is assumed that some consolidation has occurred. The purpose of these experiments was to test computational models using behavioral paradigms; therefore, the terms “encoding” and “retrieval” are used.
In the present study, rats were administered saline, scopolamine, or physostigmine directly into the CA3 subregion of the hippocampus 10 min prior to tone/shock-induced fear conditioning. The contextual retention test was administered 24 h postconditioning, and the cue retention test was administered 48 h postconditioning. According to the model championed by Hasselmo, the cholinergic antagonist, scopolamine, should disrupt contextual conditioning by means of impaired encoding, while sparing contextual retrieval and cue conditioning. In addition, the acetylcholinesterase inhibitor, physostigmine, should disrupt contextual conditioning by means of impaired retrieval, while sparing contextual encoding and cue conditioning.
RESULTS
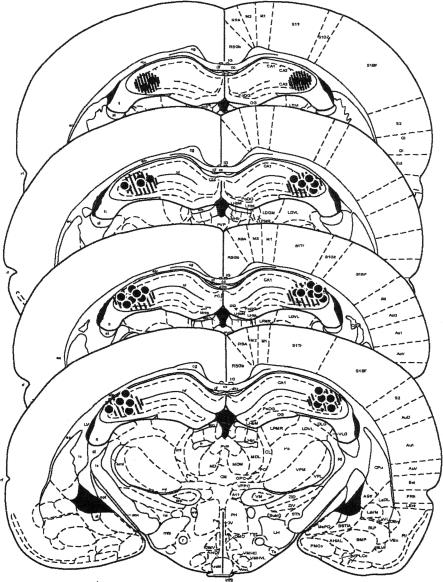
Figure 1 shows that the injection cannulae were located bilaterally within the CA3 subregion of the dorsal hippocampus (n = 20). The dye (Chicago blue) spread to the majority of the CA3 region and was limited to only some of the CA1 and hilar regions of the dorsal hippocampus. However, the spread of the dye may not reflect the spread of the drug accurately, due to the differences in molecular weights of each substance and viscosity of the solution.
Figure 1.
Histological verification of cannulae placement (n = 20) in the CA3 subregion and approximate spread of drug assessed by dye (Chicago blue) infusion indicated by circular cross-shaded area. Each dot represents the approximate point at which a cannula was positioned for each animal.
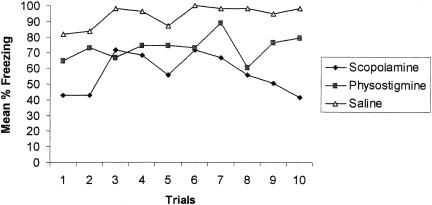
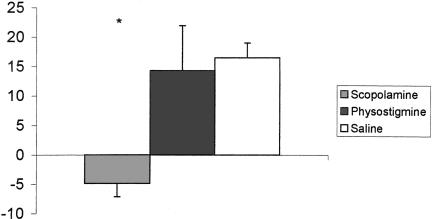
Once conditioning began, the saline group readily displayed freezing behavior to the intertrial interval (ITI). The physostigmine group froze less than the controls. In contrast, the scopolamine group displayed decreased freezing behavior relative to controls for the duration of conditioning, suggesting a failure to associate the context with the shock stimulus (Fig. 2). A repeated-measures ANOVA with groups as the between-factor and trials as the within-factor revealed a significant difference among the drug groups during ITI acquisition [F(2,17) = 4.59; P < 0.02]. A Duncan Multiple Range (DMR) test revealed that the scopolamine group froze significantly less than either the saline or the physostigmine group. Freezing behavior across trials was not significantly different [F(9,153) = 1.69; P > 0.05], although this could be due to a ceiling effect for the controls. There was no significant interaction between groups and trials [F(18,153) = 0.75; P > 0.05]. A finer analysis comparing the first and last trials of Day 1 (encoding index) revealed that the scopolamine group froze less at the end of testing, whereas the saline and physostigmine groups froze more at the end of testing (Fig. 3). An one-way ANOVA revealed that during encoding there was a significant group difference [F(2,17) = 3.81; P < 0.04]. A DMR test revealed that the scopolamine group froze significantly less than either saline or physostigmine groups (P < 0.05). The physostigmine group was not different from the controls during encoding.
Figure 2.
Percent freezing during the acquisition of contextual fear following the preacquisition period on Day 1. Data are presented as a function of trials. Note that the scopolamine group froze significantly less than the saline group.
Figure 3.
Encoding index for contextual conditioning. Note that the scopolamine group displayed little encoding compared to either the saline or physostigmine group. (*P < 0.05 in comparison to the control group).
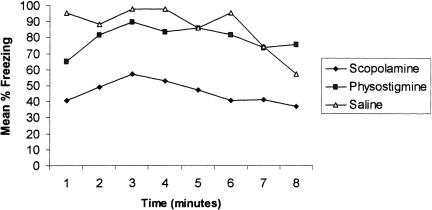
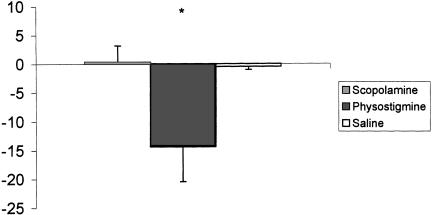
The scopolamine group also froze less than controls during the context test administered 24 h postconditioning (Fig. 4). A repeated-measures ANOVA with groups as the between-factor and trials as the within-factor revealed that there was a significant difference among drug groups [F(2,17) = 4.71; P < 0.02]. A DMR test indicated that the scopolamine group froze significantly less than either the saline or physostigmine group (P < 0.05). There was also a main effect for trials, suggesting that some extinction had occurred [F(7,119) = 2.10; P < 0.04]. Given that the scopolamine group froze less on trial 10 on Day 1 compared to trial 1 on Day 1, it is not surprising that this group froze significantly less than controls during the context test 24 h later. When a finer analysis (retrieval index) was performed on the data, however, the scopolamine group froze at similar levels of the first retention trial compared to the last conditioning trial. Interestingly, it was the physostigmine group that, at this point, froze less during retrieval (Fig. 5). An one-way ANOVA revealed that during retrieval there was a significant group difference [F(2,17) = 4.20; P < 0.03]. A DMR test indicated that the physostigmine group froze significantly less than either the saline or scopolamine group (P < 0.05).
Figure 4.
Percent freezing during the context retention test given 24 h postconditioning. Data are presented as a function of time. Note that the scopolamine group froze significantly less than the saline group.
Figure 5.
Retrieval index for contextual conditioning. Note that the physostigmine group was impaired during retrieval compared to either the saline or scopolamine group. (*P < 0.05 in comparison to the control group)
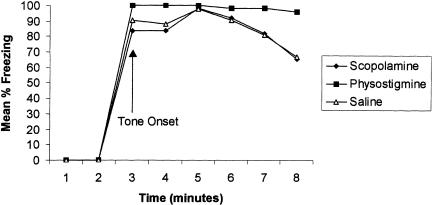
During the cue retention test administered 48 h posttraining, all groups displayed equal freezing levels during the onset of the tone stimulus (Fig. 6). A repeated-measures ANOVA revealed no group difference during cue testing [F(2,17) = 1.37; P > 0.28]. There was, however, a main effect for minutes, suggesting that some extinction occurred across time [F(5,85) = 5.01; P < 0.0004]. Interestingly, the physostigmine group did not indicate any signs of extinction, although this was not significantly different from either the saline or scopolamine group.
Figure 6.
Percent freezing during the cue retention test given 48 h postconditioning. Data are presented as a function of time. Note no significant difference among groups.
DISCUSSION
The present experiment demonstrates that the muscarinic antagonist, scopolamine, reduces the acquisition of tone/shock conditioning when administered before training. A finer analysis of the data indicated that scopolamine impairs the encoding of new contextual information when administered prior to conditioning on Day 1. The present findings also demonstrated that the acetylcholinesterase inhibitor, physostigmine, impairs the retrieval of previously learned contextual information when administered prior to conditioning. Rogers and Kesner (2003) recently observed very similar results when rats were tested on a spatial maze. Those authors also found that physostigmine impaired retrieval by disrupting the consolidation process.
Young and colleagues (1995), however, found that scopolamine impaired tone conditioning but had no effect on context conditioning. They also reported facilitation of consolidation when scopolamine is injected after conditioning. It should be noted that the injections of scopolamine were administered peripherally, therefore impacting the entire CNS. Wallenstein and Vago (2001) as well as Gale et al. (2001) found that scopolamine administration directly into dorsal hippocampus impaired acquisition to context conditioning, but spared conditioning to the tone. In the present study, scopolamine and physostigmine were administered directly in the CA3 subregion of the hippocampus. Computational models of hippocampal function suggest that the CA3 subregion participates in the processes of encoding and retrieval. Thus, the present experiments were created to test the role of ACh during the encoding and retrieval of context conditioning, subsequently blending computational models with data obtained from behavioral experiments.
The results indicate that scopolamine infusion prior to conditioning drastically reduces freezing behavior during context acquisition. A finer analysis of the data (encoding index) reveals that scopolamine impaired acquisition by disrupting encoding. During the context retention test given 24 h postconditioning, the scopolamine group also froze less compared to controls; however, given that the scopolamine group did not learn the task (Figs. 2, 3), it is not surprising that this group froze less than controls during the contextual retention test. A finer analysis of the data (retrieval index) revealed that the scopolamine group was not impaired during retrieval (Fig. 4). The results also indicate that physostigmine infusion prior to conditioning reduces freezing behavior during context acquisition, although these results were not significantly different from controls. Moreover, the physostigmine group had no impairment during encoding relative to the saline group. During the context retention test, the physostigmine group was not impaired compared to the saline group; however, a finer analysis of the data (retrieval index) revealed that physostigmine did in fact impair retrieval.
These data lend considerable evidence in support of Hasselmo's predictions regarding ACh during encoding and retrieval (1995a,b, 1999). Here, physostigmine impaired retrieval; however, the drug was given 10 min prior to conditioning on Day 1. It could be the case that physostigmine does not impair retrieval per se, but instead boosts ACh levels beyond the time of testing into a time when ACh levels need to drop, thereby interfering with the consolidation process (see Rogers and Kesner 2003).
A computational model offered by Lorincz and Buzsaki (2000) suggests that the EC may operate as a match/mismatch mechanism, rather than the CA1 as Hasselmo suggests. According to Lorincz and Buzsaki, the match/mismatch operation may lie in a comparison between layers II and V of the EC. Furthermore, the EC has been shown to generate oscillations that are thought to be important for inducing θ into the hippocampus (Dickson et al. 2000). The EC, therefore, could establish a θ oscillation from the septum to the hippocampus, causing an increase of ACh, at the same time activating the dentate gyrus to CA3 pathway to facilitate encoding. During retrieval, however, the EC may activate perforant path inputs directly to the CA3, with little or no ACh. A recent paper by Egorov et al. (2002) suggests that the EC is able to sustain activity over a time delay, further implicating layer V (the output layer from CA1) as a potential mechanism for a match/mismatch process. These data also support the computation model originally put forth by Treves and Rolls (1994), further expanded by Rolls (1996; 1984), who suggests that EC-DG mossy fiber inputs into CA3 are responsible for encoding, and that direct EC perforant path inputs are responsible for retrieval.
The findings obtained in the present study support the computational models suggested by Hasselmo and Lorincz and Buzsaki. These data do not allow for a comparison between these two models; however, taken with recent data from Lee and Kesner (2004) as well as Rogers and Kesner (2003), our findings suggest that the EC operates as a match/mismatch operation. Lee and Kesner (in press) reported that the mossy fiber inputs into CA3 are critical for encoding, whereas the direct EC-CA3 perforant path was critical for retrieval. Rogers and Kesner (2003) found that scopolamine injected directly into CA3 impaired maze acquisition by means of impaired encoding, whereas injections of physostigmine directly into CA3 impaired acquisition by means of impaired consolidation. Finally, those authors reported a consolidation gradient for physostigmine that is time-dependent. Consolidation was impaired greatest when physostigmine was administered before training each day. These data support Hasselmo's suggestion (1999) that decreased ACh action is necessary for consolidation; however, they do not support the computational model of encoding and retrieval (Hasselmo and McClelland 1999). Lee and Kesner (2004) found that CA1 lesions had no impact on encoding, which, according to Hasselmo and McClelland, should be critical for the match/mismatch operation. In conclusion, the data presented here, in addition with the data described above, suggest that the EC may subserve the match/mismatch operation, thus supporting the computational model championed by Lorincz and Buszaki (2000).
Based on the above findings, it is proposed that during encoding, layer II neurons activate DG and CA3, at the same time EC activates the medial septum, resulting in high levels of ACh into CA3. During retrieval, layer II neurons activate CA3 directly without enlisting the aid of the medial septum and ACh. In conclusion, the present findings provide a mechanism by which encoding and retrieval operate in the hippocampus, and some pharmacological understanding of that mechanism.
MATERIALS AND METHODS
Animals
Twenty Long-Evans rats (Simonsen Laboratories) approximately 4 mos of age at the start of the experiment, weighing ∼350 gm, served as subjects. The rats were housed individually in plastic tubs located in a colony with a 12-h light-dark cycle. All rats had free access to food and water. All testing was conducted during the light portion of a 12-h:12-h light-dark cycle. The rats used in the current study were previously used in a maze experiment. All experiments were conducted according to the NIH Guide for the Care and Use of Laboratory Animals and the University of Utah Institutional Animal Care and Use Committee.
Surgery
Rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (55 mg/kg) and placed in a stereotaxic apparatus (David Kopf Instruments). The scalp was incised and retracted to expose bregma and lambda in the same horizontal plane. Small burr holes (2.0 mm dia) were drilled bilaterally into the CA3 subregion of the dorsal hippocampus (3.3 mm posterior to bregma, 3.5 mm lateral to the midsagittal suture, and 1.8 mm ventral to the brain surface). Small skull screws (1 mm) were then positioned to anchor dental cement to the cannula guides. Once the dental cement dried, dummy inner cannulae were inserted to maintain guide cannulae reliability and prevent gliosis. Following surgery, the rats were allowed to recover on a heating pad before returning to their home cage.
Drug Infusion
After cannulae implantation, each rat was given bilateral injections of saline (0.035 μL; n = 6), 35 μg scopolamine hydrobromide (n = 7), or 10 μg physostigmine (n = 7). Scopolamine was dissolved in 1 μL of 0.9% saline solution and delivered in a volume of 0.35 μL. Both the concentration and volume of scopolamine were selected from previous studies in which scopolamine administration directly into the CA3 disrupted acquisition of a spatial task (Blockland et al. 1992; Wallenstein and Vago 2001). Physostigmine was dissolved in 1 μL of 0.9% saline solution and delivered in a volume of 0.10 μL. The concentration of physostigmine was selected from previous studies (Todd and Kesner 1978; DeGroot and Parent 2000), and the volume of physostigmine was selected on the basis of a pilot study. The criteria for selection were that there would be no major changes in activity level, no obvious side effects, but still an efficacy in producing memory problems (see Rogers and Kesner 2003).
Infusions were administered through an injection cannula (33 ga.) extending 1.0 mm below the guide cannula (28 ga.). The injection cannula was attached to a 10-μL Hamilton syringe with polyethylene tubing. The syringe was then mounted in an infusion pump (Harvard Apparatus), and substances were delivered via pressure injection with the tubing filled with water/air/drug at a constant rate of 0.5 μL/min for 30 sec. The two sides of the rat were infused sequentially, and injection cannulae were left in position for an additional 60 sec following the infusion to allow for diffusion of the drug or vehicle. To allow the drugs to take effect, there was a 10-min delay from the time of drug injection and testing on the maze.
Histology
After all behavioral testing commenced, rats were injected with a blue dye (Chicago blue) to test the diffusion of the drug throughout the hippocampus and patency (i.e., whether or not the drugs were injected into region CA3) of the drugs through the cannulae. It should be noted, however, that the exact degree of diffusion could not be ascertained from the dye due to the different molecular weights of the dye and drugs. Rats were then anesthetized with a lethal dose of sodium pentobarbital and perfused intracardially with saline solution followed by a 10% formalin solution. The brains were then extracted and stored in 30% sucrose formalin for 5 d before being frozen and sliced coronally into 40-μm sections with a freezing-stage microtome.
Behavioral Analysis
Two observation chambers were used during the three consecutive days of testing. The first chamber was used for conditioning and contextual retention. This chamber (28×21×22 cm; Coulbourn Instruments) consisted of two clear Plexiglas walls (rear wall and front door) and two aluminum sidewalls. The chamber floor contained 18 steel rods connected to a precision-regulated shocker (Coulbourn Instruments) delivering electric footshock stimuli. A speaker was inserted into one of the aluminum sidewalls of the conditioning chamber to deliver the tone. A computer program (Graphic State, Coulbourn Instruments) controlled all stimuli. The chamber was located in an isolated room lit with fluorescent and halogen lamps. Numerous visual cues such as toys and posters were located around the conditioning chamber. A video camera monitored and recorded the animal's behavior. The chamber was cleaned using a weakened cleaning solution (HDQ cleaner). A second observation chamber tested the retention of the tone stimulus in the absence of any contextual cues. This chamber (32×32×32 cm) was constructed from clear Plexiglas on all sides of the chamber. A speaker was attached to a hole (2.5 cm dia) made on one of the walls of the chamber to deliver auditory stimuli. The chamber was located in a different room surrounded by completely different visual cues. The chamber was cleansed using water.
Procedure
Day 1: Acquisition
Ten min after drug administration, rats were placed in the fear conditioning chamber for 2 min without a tone stimulus. After the 2-min baseline period, rats received 10 trials of tone/shock pairing. A tone (10 sec, 2 kHz, 85 dB) presented through a speaker initiated each trial. The tone coterminated with an electric foot-shock (2 sec 0.5mA) delivered through the shock-floor. A 64-sec intertrial interval (ITI) separated each successive trial. After the tenth and final tone/shock pairing, the rat remained in the chamber for an additional 2 min without tone or shock stimuli. A freezing response (e.g., absence of movement minus respiratory movement) was measured during each ITI (64 sec × 10). A blind observer scored freezing behavior every 8 sec, resulting in eight observations for each interval between tone and shock pair trials.
Day 2: Contextual Retention Test
Each rat was tested for retrieval of contextual conditioning 24 h after acquisition. The rat was placed in the same chamber used during the acquisition period for 8 min in the absence of the tone stimulus. Freezing behavior was measured every 8 sec by a blind observer.
Day 3: Cue Retention Test
The rat was placed in the clear Plexiglas chamber 48 h after acquisition. Rats were given a 2-min preexposure period followed by a 6-min continuous tone (the same tone presented in Day 1 acquisition). Freezing behavior was measured during the 2-min baseline and 6-min tone every 8 sec by a blind observer.
Data Analysis
Freezing scores were transformed to percent scores of total observations for data analysis throughout the experiment. A repeated-measures analysis of variance (ANOVA) was employed for testing group differences during acquisition and retention of the context and tone stimulus, and a post hoc comparison (Duncan Multiple Range test) was made when necessary. Acquisition data are presented as a function of trials shown as means. A finer analysis of the data was needed to assess the role of ACh during encoding and retrieval. It is assumed that the encoding and retrieval processes interact on a trial-by-trial basis in any behavioral task. This may be one of the reasons that encoding and retrieval are difficult to test behaviorally. Nonetheless, the control group displayed marked improvements in learning (more freezing) during the last trial than the first trial of tone/shock-induced fear conditioning. In addition, they continued to freeze when they were tested 24 h later, suggesting good retention from the previous day. Therefore, encoding and retrieval were operationally defined as follows. A within-day (W/D) encoding index was calculated within a day by subtracting D1A from D1B, where the first trial of the ITI during Day 1 was represented by D1A and the last trial of the ITI during Day 1 was represented by D1B. A between-day (B/D) retrieval index was calculated between 2 consecutive days by subtracting D1B from D2A, where D1B indicated the last trial of the ITI during Day 1 and D2A indicated the first 64 sec of the retention test administered 24-h postconditioning. It is feasible that the encoding phase is likely to dominate more during the learning of a new task within a day, whereas retrieval of encoded information is needed initially to perform the task 24 h later. Encoding and retrieval scores were generated only for contextual information; tone information may be acquired and retained without the use of the hippocampus (Philips and LeDoux 1992). Encoding and retrieval analyses are similar to analyses employed during the learning of a modified Hebb–Williams maze (see Lee and Kesner 2004; Rogers and Kesner 2003).
Acknowledgments
This research was supported by NSF Grant IBN-0135273 and NIH Grant 1R01MH065314. We thank Kelly Dalton, Michael Burton, Ryan Hota, and Kami Farnsworth for their assistance in data collection.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.learnmem.org/cgi/doi/10.1101/lm.64604.
References
- Blockland, A., Honig, W., and Raaijmakers, W. 1992. Effects of intrahippocampal scopolamine injections in a repeated spatial acquisition task in the rat. Psychopharmacology 109: 373–376. [DOI] [PubMed] [Google Scholar]
- DeGroot, A. and Parent, M. 2000. Increasing acetlycholine levels in the hippocampus or entorhinal cortex reverses the impairing effects of septal GABA receptor activation on spontaneous activation. Learn. Mem. 7: 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson, C., Magistretti, J., Shalinsky, M., Hamam, B., and Alonso, A. 2000. Oscillatory activity in entorhinal neurons and circuits. Mechanisms and function. Ann. NY Acad. Sci. 911: 127–150. [DOI] [PubMed] [Google Scholar]
- Egorov, V., Hamam, B., Fransen, E., Hasselmo, M., and Alonso, A. 2002. Graded persistent activity in entorhinal cortex neurons. Nature 420: 173–178. [DOI] [PubMed] [Google Scholar]
- Gale, G., Anagnostaras, G., and Fanselow, M. 2001. Cholinergic modulation of pavlovian fear conditioning: Effects of intrahippocampal scopolamine infusion. Hippocampus 11: 371–376. [DOI] [PubMed] [Google Scholar]
- Hagan, J., Jansen, J., and Broekkamp, C. 1987. Blockade of spatial learning by the M1 muscarinic antagonist pirenzepine. Psychopharmacology 93: 470–476. [DOI] [PubMed] [Google Scholar]
- Hasselmo, M. 1995a. Neuromodulation and cortical function: Modeling the physiological basis of behavior. Behav. Brain Res. 67: 1–27. [DOI] [PubMed] [Google Scholar]
- Hasselmo, M. 1995b. Dynamics of learning and recall at excitatory recurrent synapses and cholinergic modulation in rat hippocampal region CA3. J. Neurosci. 15: 5249–5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo, M. 1999. Neuromodulation: Acetlycholine and memory consolidation. Trends Cogn. Sci. 3: 351–359. [DOI] [PubMed] [Google Scholar]
- Hasselmo, M. and Bower, J. 1993. Acetylcholine and memory. Trends Neurosci. 16: 218–222. [DOI] [PubMed] [Google Scholar]
- Hasselmo, M. and McCleland, J. 1999. Neural models of memory. Curr. Opin. Neurobiol. 9: 184–188. [DOI] [PubMed] [Google Scholar]
- Kim, J. and Fanselow, M.S. 1992. Modality-specific retrograde amnesia of fear. Science 256: 675–677. [DOI] [PubMed] [Google Scholar]
- Lee, I. and Kesner, R. 2004. Encoding and retrieval of spatial memory: Dissociation between the dentate gyrus and perforant path inputs into CA3. Hippocampus (in press). [DOI] [PubMed]
- Lorincz, A. and Buzsaki G. 2000. Two-phase computational model training long-term memories in the entorhinal-hippocampal region. Ann. NY Acad. Sci. 911: 83–111. [DOI] [PubMed] [Google Scholar]
- Philips, R. and LeDoux, J. 1992. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 106: 274–285. [DOI] [PubMed] [Google Scholar]
- Rogers, J. and Kesner, R. 2003. Cholinergic modulation of the hippocampus during learning, encoding, and retrieval. Neurobiol. Learn. Mem 80: 332–342. [DOI] [PubMed] [Google Scholar]
- Rolls, E.T. 1989. Functions of neuronal networks in the hippocampus and neocortex in memory. In Neural models of plasticity: Theoretical and empirical approaches (eds. J.H. Byrne and W.O. Berry), pp. 240–265. Academic Press, New York.
- Rolls, E.T. 1996. The representation of space in the primate hippocampus, and its role in memory. In Brain processes and memory (eds. K. Ishikawa, J. McGaugh and H. Sakata), pp. 203–227. Elsevier Science, Amsterdam.
- Rudy, J. and O'Reilly, R. 2001. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cogn. Affect. Behav. Neurosci. 1: 66–82. [DOI] [PubMed] [Google Scholar]
- Todd, J. and Kesner, R. 1978. Effects of posttraining injection of cholinergic agonists and antagonists into the amygdala on retention of passive avoidance training in rats. J. Comp. Physiol. Psychol. 92: 958–968. [DOI] [PubMed] [Google Scholar]
- Treves, A. and Rolls, E.T. 1994. Computational analysis of the role of the hippocampus in memory. Hippocampus 2: 374–391. [DOI] [PubMed] [Google Scholar]
- Wallenstein, G. and Vago, D. 2001. Intrahippocampal scopolamine impairs both acquisition and consolidation of contextual fear conditioning. Neurobiol. Learn. Mem. 75: 245–252. [DOI] [PubMed] [Google Scholar]
- Young, S., Bohenek, D., and Fanselow, M.S. 1995. Scopolamine impairs acquisition and facilitates consolidation of fear conditioning: Differential effects for tone vs. context conditioning. Neurobiol. Learn. Mem. 63: 174–180. [DOI] [PubMed] [Google Scholar]