Abstract
Routine screening for infectious agents is critical in establishing and maintaining specific pathogen free (SPF) nonhuman primate (NHP) colonies. More efficient, higher throughput, less costly reagent, and reduced sample consumption multiplex microbead immunoassays (MMIAs) using purified viral lysates have been developed previously to address some disadvantages of the traditional individual enzyme-linked immunosorbent assay (ELISA) methods. To overcome some of the technical and biosafety difficulties in preparing antigens from live viruses for viral lysate protein based MMIAs, novel MMIAs using recombinant glycoprotein D precursor (gD) protein of herpesvirus B and four viral gag proteins of Simian Immunodeficiency Virus (SIV), Simian T Cell Lymphotropic Virus (STLV), Simian Foamy Virus (SFV) and Simian Betaretrovirus (SRV) as antigens have been developed in the current study. The data showed that the recombinant viral protein based MMIAs detected simultaneously antibodies to each of these five viruses with high sensitivity and specificity, and correlated well with viral lysate based MMIAs. Therefore, recombinant viral protein based MMIA is an effective and efficient routine screening method to determine the infection status of nonhuman primates.
Keywords: recombinant viral protein, nonhuman primate, specific pathogen free, simian virus, Multiplex Microbead ImmunoAssay
1. Introduction
Nonhuman primates (NHPs) have been utilized widely as animal models for human diseases in biomedical research because they are closer phylogenetically to humans than other animals (Boffelli et al., 2003; Sibal and Samson, 2001; Siepel, 2009). Identifying and eliminating bacterial and viral pathogens from nonhuman primates can increase their value as animal models (Mansfield and Kemnitz, 2008). In addition, removing pathogens which cause infectious diseases in the nonhuman primates can decrease the morbidity and mortality while improving the overall health of colony animals. Infection by simian immunodeficiency virus (SIV) results in loss of CD4+ T cells and an acquired immune deficiency syndrome (AIDS)-like disease in Asian macaques, although it is often asymptomatic in its natural hosts, African nonhuman primates (Hirsch and Johnson, 1994; Silvestri et al., 2007). Simian Betaretrovirus (SRV) also causes immunodeficiency disease in Asian macaques; and Simian T cell leukemia virus (STLV) has been reported to induce adult T-cell Leukemia-like disease in African green monkeys (Maul et al., 1986; Tsujimoto et al., 1987). In addition, these and other microbes can cause physiological dysfunction and abnormality in nonhuman primate hosts leading to erroneous data and failed experiments. Persistent infection with simian retroviruses such as SIV, SRV, STLV and Simian foamy virus (SFV), have impaired toxicological research in the nonhuman primates in many ways (Lerche, 2010; Lerche and Osborn, 2003). Another concern is the possibility that some pathogens could cause zoonotic diseases in humans who are exposed to blood, body fluids or tissues of infected nonhuman primates. This concern even applies to pathogens that are nonpathogenic in the nonhuman primates. Herpesvirus B (B virus; Cercopithecine Herpesvirus 1) can be fatal in humans, while only causing a virtually asymptomatic infection in its natural nonhuman primate carriers (Huff and Barry, 2003). SFV transmission to humans exposed occupationally has been found more frequently than other simian retroviruses (Lerche et al., 2001; Sandstrom et al., 2000; Switzer et al., 2004). Therefore, routine screening for SIV, SRV, STLV, SFV, and B virus is critical to identifying and maintaining specific pathogen free (SPF) nonhuman primates.
Traditionally, researchers have used enzyme-linked immunosorbent assays (ELISA), indirect immunofluorescence antibody assays (IFA) and Western blot assays (WB) to select and monitor the SPF nonhuman primates (Andrade et al., 2003; Lerche et al., 1994; Simmons, 2008; Wolf et al., 2010). However, multiple replicates of these conventional methods are not only expensive in terms of time, labor, and reagents; but also in their large sample (tissue, blood, or other body fluids) volume requirements. As the demand for the number of SPF agents as well as the number of nonhuman primates in research increases, a more efficient, higher throughput, and less costly reagent and sample consumption method will become an urgent preference. The multiplex microbead immunoassay (MMIA) based on the Luminex® xMAP® system is a method which meets these requirements (Mandy et al., 2001; Nolan and Mandy, 2001). The Luminex® xMAP® system incorporates 100 sets of 5.6 µm polystyrene microspheres which are filled by gradient ratio of two different fluorochromes, so that each microsphere set in the matrix can be identified by a different fluorescence signature (Dunbar, 2006). Each uniquely identifiable microsphere set can be conjugated to a unique antigen. The bead sets can be mixed and assayed simultaneously to detect antibodies to multiple infectious agents by the MMIAs, which is more efficient than multiple ELISAs. Furthermore, the high-speed laser scanner and digital signal processor can examine each sample in a few seconds, resulting in higher throughput, more replicates and increased efficiency compared to the traditional methods. Finally, the MMIAs simultaneous detection of multiple analytes can reduce the cost of reagents and required volume of samples.
The MMIA has become an established method for antibody detection in infectious diseases (Brown et al., 2011; Jones et al., 2002; Khan et al., 2006; Khan et al., 2008; Kuller et al., 2005; Smith et al., 2008). MMIAs reported previously which detected simultaneously antibodies to multiple viruses in nonhuman primates relied primarily on microbeads conjugated with antigens from purified preparations of specific viruses (Khan et al., 2006; Kuller et al., 2005). However, it may be difficult to obtain these purified lysates of viruses due to numerous regulatory, safety, stability, reproducibility, technical complexity, and commercial availability issues. The requirement of Biosafety Level 4 for B virus propagation and the recommendation of at least biosafety level 2 conditions for the others are often major obstacles. This study presents the development of MMIAs using purified preparations of E. coli-expressed gag proteins from highly conserved regions of four simian retroviruses (SIV, SRV, STLV-1 and SFV-1) and the glycoprotein D precursor of B virus as antigens to detect antibodies in nonhuman primates. The performances of these recombinant viral protein based MMIAs were compared with the results obtained using viral lysate based MMIAs and ELISAs used routinely for viral antibody screening in nonhuman primates (Khan et al., 2006; Lerche, 2010; Lerche and Osborn, 2003).
2. Materials and methods
2.1 Recombinant viral proteins
Sequences of all viral gag and glycoprotein D precursor (gD) proteins were cited from Genebank (SIV gag: AAA47632, from 1 to 510 aa; SRV 4/5 gag: BAD89356 (from 1 to 417 aa and from 627 to 659 aa) and AAF71355 (form 1 to 208 aa); STLV-1 gag: AAU34008, from 1 to 437 aa; SFV-1 gag: AAZ23611, from 1 to 643 aa; and B virus gD: AAL39132, from 1 to 394 aa). The SRV 4/5 sequence used was a combination of SRV-4 and SRV-5 gag gene sequences in which SRV-5 replaces the SRV-4 region from 418 to 626 aa. DNA sequences were designed by utilizing codons preferred by E. coli to express recombinant viral proteins in E. coli efficiently. All viral protein genes flanked by two enzyme digestion positions (Nde I and BamH I) were synthesized commercially and ligated into transfer plasmid pJ36 to construct five transfer plasmids (DNA 2.0 Inc., Menlo Park, CA, USA). 10 pg of each transfer plasmid were transformed and cultured separately in 50µl E. Coli TOPO 10 competent cells as specified by the manufacturer’s manual (Invitrogen, Carlsbad, CA, USA). Ten transformants of each transfer plasmid were chosen and cultured separately in 3 ml liquid LB media (Sigma, St. Louis, MO, USA) with 40 µg/ml kanamycin (Invitrogen, Carlsbad, CA, USA) at 37°C 150 rpm overnight, and kept at −80°C.
3 µl of TOPO 10 transformat storage liquid from each transfer plasmids was cultured in 3 ml liquid LB media (Sigma, St. Louis, MO, USA) with 40 µg/ml kanamycin (Invitrogen, Carlsbad, CA, USA) at 37°C, 150 rpm overnight. The five transfer plasmids were isolated by plasmid mini kit (Qiagen, Valencia, CA, USA). The five transfer plasmids and pET 15b vector (Novagen, Madison, WI, USA) were double digested by Nde I and BamH I restriction endonucleases (NEB, Ipswich, MA, USA) in a 50µl reaction system, respectively. Target fragments were recovered and purified by MinElute Gel Extraction Kit (Qiagen, Valencia, CA, USA). The five viral protein gene fragments were ligated with pET 15b fragments by T4 DNA ligase in 20µl reaction system following the manual (NEB, Ipswich, MA, USA) to generate five expression plasmids in which a start codon and a 6·His-tag sequence was inserted in the 5’ flanking region and in the same reading frame, respectively. As described previously, the five expression plasmids were also transformed into and cultured in E. Coli TOPO 10 competent cells (Invitrogen, Carlsbad, CA, USA) and the ten transformants of each expression plasmid were picked out and cultured separately, and kept at −80°C.
Before transforming into expressing host E. Coli ER2566, the five expression plasmids were isolated and the expressed regions were sequenced to proof with T7 promoter primer and T7 terminator primer by ABI 3100 (ABI, Carlsbad, CA, USA). After the five expression plasmids were transformed into E. Coli ER2566 independently, the five recombinant viral proteins with N-terminal His-tag were expressed in a Bio-Flow 2000 bench top bio-reactor (10L) (New Brunswick Scientific, Edison, NJ, USA) and were induced with isopropyl β-D-1-thiogalactopyranoside (IPTG) at 30°C and O.D.=25. Harvesting after 90 minutes inducing (O.D. > 32) at 4,000 ×g for 15 minutes, the cell pastes were suspended and washed with 600 ml buffer (40mM Sodium phosphate pH=7.0, 0.1M NaCl), and were homogenized in an APV1000 homogenizer (APV, Charlotte, NC, USA). The homogenized pastes were centrifuged at 15,000 ×g for 30 minutes. The pellets were suspended and washed with 200ml inclusion body wash buffer (8M urea, 2% NLS, 20mM triethanolamine, 500mM NaCl, 10mM imidazole, PH=8.0) and were stirred at 2,000 rpm overnight to dissolve adequately the inclusion body. The target proteins were purified by 6·His-tag in an Immobilized Metal Affinity Column (IMAC; GE Healthcare, Waukesha, WI, USA) as instructed by the manufacturer’s manual. The purified proteins were dissolved in solution buffer (20mM triethanolamine, 50mM NaCl, PH=8.0) and stored at −80°C. The purified proteins were examined by western blot using anti-His monoclonal antibodies (Invitrogen, Carlsbad, CA, USA) and specific simian virus positive sera as shown in Figure 1.
Figure 1.
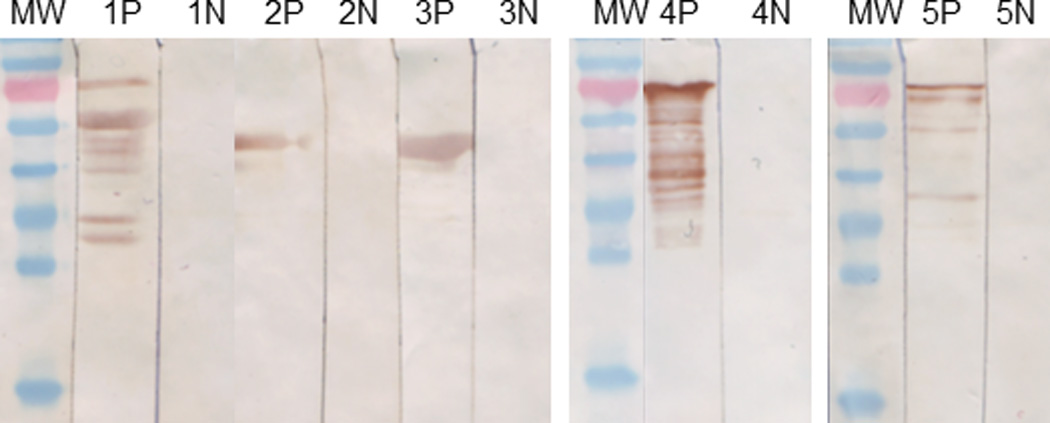
Western Blot of purified recombinant viral proteins reacted with specific simian virus positive (P) or negative (N) sera. 1: SIV gag (57kD); 2: B Virus gD (42 kD); 3: STLV gag (48kD); 4: SFV gag (69kD); 5: SRV4/5 gag (74kD). MW: PageRuler Prestained Protein Ladder 150kD, 95kD, 72kD(red), 55kD, 43kD, 34kD, 26kD, 17kD (from top to bottom) (Fermentas #SM0671, Shenzen, China).
2.2 Animal samples
The initial assay development and validation was performed using archived frozen sera or plasma from Pathogen Detection Laboratory (PDL) at the California National Primate Research Center. This group included uncharacterized samples from rhesus, cynomolgus, and pigtail macaques received for routine diagnostic screening as well as selected samples from monkeys infected experimentally with the target viruses. The California National Primate Research Center is accredited fully by the American Association for Accreditation of Laboratory Animal Care (AAALAC) and all animals were housed and handled in accordance with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Research CoLS, 1996). All procedures associated with collection of samples for this study were approved by the institutional animal care and use committee of the University of California, Davis.
The original antibody testing on these samples was performed using either commercial or in house prepared ELISA followed by IFA or WB to confirm any reactivity. HIV-2 ELISA (Biorad, Hercules, CA, USA) was validated to cross react with SIVmac and SIVmac WB strips (Zeptometrix, Buffalo, NY, USA) were used for SIV antibody. HTLV ELISA (BioMerieux, Durham, NC, USA) and WB strips (Zeptometrix, Buffalo, NY, USA) which had been validated to cross react with STLV were used for STLV. SRV and SFV testing was performed using ELISA and WB prepared by PDL (Andrade et al., 2003; Lerche et al., 1994). In addition, some SFV confirmatory testing was done using SFV IFA slides (Charles River Labs, Wilmington, MA, USA). Herpes virus papio 2 (HVP2) was used as a surrogate marker for B Virus (Yamamoto et al., 2005). HVP2 ELISA was prepared by PDL and HVP2 IFA was available commercially (Charles River Labs, Wilmington, MA, USA). All assays were performed according to manufacturer’s instructions and standard PDL protocols (Khan et al., 2006).
A 16 member challenge panel of characterized frozen sera or plasma whose aggregate consensus results from 12 other laboratories performing nonhuman primate antibody testing by various, unspecified methods was also utilized in the current study.
2.3 ELISA detection using recombinant protein coated plates
Purified recombinant proteins for SIV, SRV, STLV-1, SFV-1 and B virus were used to perform antibody detection by ELISA. Briefly, ELISA plates were prepared as follows: 96-microtiter plate wells (Nunc, Roskilde, Denmark) were coated with 100 µl of purified antigens at optimal protein concentrations of 2.5 µg/ml for SIV, STLV-1, SFV-1 and B virus, and 1µg/ml for SRV 4/5 diluted in coating buffer, 0.1 M sodium bicarbonate (pH 9.6). After being incubated overnight at 4°C, the plates were washed 250 µl/well of 0.01M PBS (PH=7.4) plus 0.05% Tween-20 (PBST) five times and blocked with 250 µl/well of PBS (pH 7.4) plus 2% bovine serum albumin (BSA; Sigma, St. Louis, MO, USA). To detect optimally antibody, 100 µl/well sera, titrated and optimized at 1:200 for SRV, STLV-1 and B virus, 1:400 for SIV, and 1:1000 for SFV-1 in PBS (pH 7.4) containing 1% BSA (Sigma, St. Louis, MO, USA), were loaded into the plates. Plates were incubated for 1 hour at 37°C and then wells were washed by PBST. 100 µl/well of the secondary antibody, goat anti-monkey conjugated to horseradish peroxidase (Sigma, St. Louis, MO, USA), was loaded into plate wells at a dilution of 1:15000 for SRV, STLV-1 and B virus, and 1:30000 for SIV and SFV-1. Plates were incubated for 30 minutes at 37°C and then washed with 250 µl/well of PBST five times. 100 µl/well of Tetramethylbenzidine (mixed, equal volumes of TMB A and B) (Kirkgaard and Perry, Gaithersburg, MD, USA) was loaded and reacted in the dark for 10 minutes at 37°C. The reaction was stopped by loading 100 µl/well of 2 M sulfuric acid. Color development was read at 450 nm, measured as optical density (O.D.) (Tecan Sunrise, Durham, NC, USA).
2.4 Recombinant viral protein based multiplex microbead immunoassays (MMIAs)
The five purified recombinant proteins were cross-linked chemically to uncoated microbeads (Luminex Corp. Austin, TX, USA) according to the manufacturer’s protocols. Since microbeads are light sensitive, as much as possible, all operations were carried out in the dark. Resuspension was by 20–30 second vortexing and 30 second treatment in a sonicator bath. 2.5 × 106 stock microbeads were required for each recombinant protein. The stock beads were resuspended and then centrifuged at 10,000 ×g for 2 minutes to remove the storage buffer supernatant. The pelleted microbeads were resuspended in 100 µl of dH2O and then centrifuged at 10,000 ×g for 1.5 minutes. To prepare for activation the microbeads were resuspended in 80 µl of 100 mM monobasic sodium phosphate (pH 6.3).
To activate the microbeads for cross-linking to the recombinant proteins, 10 µl of 50 mg/ml sulfo-N-hydroxysulfosuc-cinamide (NHS; Pierce, Rockford, IL, USA) and 10 µl of 50 mg/ml 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide (EDC; Pierce, Rockford, IL, USA) were loaded in turn, followed by mixing gently by vortex after each. The microbead mixture was incubated for 20 minutes at room temperature with gentle mixing by vortex at 10 minute intervals and then centrifuged at 10,000 ×g for 1.5 minutes. Microbeads were washed twice with 250 µl of 0.01M PBS (pH 7.4) buffer, followed by centrifugation at 10,000 ×g for 1.5 minutes after each washing. Finally, pelleted microbeads were resuspended in 100 µl of 0.01 M PBS (pH 7.4) buffer containing recombinant protein at the optimal concentration for each individual virus. The following total protein concentrations for purified recombinant proteins were used: 10 µg/ml for B virus gD and SIV gag; 4 µg/ml for STLV-1 gag and SRV gag; 2 µg/ml for SFV-1 gag. Microbead sets were also coated with Rhesus Monkey IgG (100 µg/ml) (Southern Biotech Corp., Birmingham, AL, USA), a positive control protein for reaction with R-phycoerythrin-conjugated goat anti-monkey, and with Bovine Serum Albumin (BSA) (100 µg/ml) (Sigma, St. Louis, MO, USA) or mock purified E-coli lysate as a negative control protein. For cross-linking, mixtures of activated microbeads and proteins were incubated by shaking on a rocker for 2 hours at room temperature. After being coated with proteins, beads were washed twice with 250 µl of PBST followed by centrifugation at 10,000 ×g for 1.5 minutes after each washing and then resuspended in 250 µl of PBS-BN (1% BSA in 0.01 M PBS (pH 7.4), 0.05% sodium azide) and shaken on a rocker at room temperature for 30 minutes. Microbeads coated with antigens were resuspended in 0.5 ml PBS-BN and kept at 2–8°C as recommended by the manufacturer. Repeat testing of positive and negative control sera against the recombinant SRV gag protein coated microbeads was performed after 6 months storage at 4°C to ensure reproducibility as compared to the results obtained immediately after coating.
96-well, filter-bottom plates (1.2-µm MultiScreen; Millipore Corporation, Bedford, MA, USA) were used for the immunoassay. Wells were loaded with 5000 beads from each individual bead set, coated with either specific recombinant viral proteins or a control protein in a total volume of 50 µl per well. An equal volume (50 µl) of monkey serum diluted 1:50 in PBS-BN, was added to the seven-plex bead mixture in each well. The plate was then incubated on a shaker at 400 rpm for 1 hour at room temperature. After incubation, the beads were washed by suctioning the liquid through the filter bottom of the plate on a vacuum manifold (Millipore Corporation, Bedford, MA, USA), followed by two additional washes with 100 µl/well of PBST. The detector antibody was R-phycoerythrin-conjugated goat anti-monkey. 100 µl of IgG-PE (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), diluted to a final concentration of 4 µg/ml in PBS-BN, was added to the beads in each well and incubated at room temperature for 30 minutes. Microbeads were washed twice with PBST as described previously, resuspended in 100 µl of PBS-BN per well, and then analyzed in a BioPlex (BioRad, Hercules, CA, USA) reader using the manufacturer’s default settings. The value of median fluorescence intensity (MFI) of antigen-coated microbead sets minus MFI of cell lysate coupled microbeads was calculated as real MFI of antigen-coated microbead sets. The MFI raw data were collected automatically by BioPlex Manager 4.1 (BioRad, Hercules, CA, USA) software.
2.5 Reference assays
A Luminex bead panel including purified viral lysate antigen coated beads for HVP2, STLV, SIV, SFV, and SRV purchased from the University of California, Davis Clinical Proteomics Core Laboratory (UCD; Davis, CA, USA) was used as the reference viral lysate based MMIAs (Khan et al., 2006).
In assaying the challenge panel, the viral lysate based MMIAs (UCD) and also a Simian Assessment Panel including viral lysate HVP2, STLV, SIV, SRV, SFV, as well as SIV and SRV recombinant envelope antigen bead sets purchased from Charles River Labs (CRL; Wilmington, MA, USA) were compared as reference assays.
2.6 Statistical analysis
The receiver operating characteristic (ROC) curve analysis was carried out with the MedCalc 11.6 software (free trial; MedCalc Software, Mariakerke, Belgium), in order to set the cutoff of recombinant protein based MMIAs and to compare recombinant and viral lysate protein based MMIAs. The area under the curve (AUC) of the two MMIAs were also calculated and compared using the same software. The suggested criteria published previously to interpret AUC values were applied in the current study (Swets, 1988). Student t-test was used to compare the difference between groups. Sensitivity, specificity, 95% Confidence Interval (CI) and Standard Error (SE) were calculated based on the standard, published equations (DeLong et al., 1988; Simmons, 2008). An exact binomial confidence interval for sensitivity, specificity, and difference between two AUC was calculated, respectively. The diagnostic performance of these MMIAs was also measured by calculating Youden’s index which is defined as sensitivity + specificity −1 (Youden, 1950).
3. Results
3.1. Sensitivity and specificity using recombinant viral proteins by ELISA
The recombinant antigens were evaluated first using frozen sera or plasma with known virus status based on historical ELISA and WB data from the Pathogen Detection Laboratory (PDL) archive. These samples were tested previously and demonstrated a broad range of positive and negative reactivity using purified viral lysate antigen based ELISA followed by infected cell IFA or purified viral lysate WB methods as validated previously (Lerche et al., 1994). The cutoff values were set for positivity at 2.5 times the average optical density (O.D.) of all historical negative samples for B virus, SIV, STLV-1 and SRV, and 3 times for SFV-1 in the recombinant viral protein based ELISAs. These cutoff values are similar to those used in the viral lysate antigen ELISAs and were chosen to maximize the sensitivity of detecting all true positive samples at the expense of specificity. As shown in table 1, the recombinant viral protein based ELISAs demonstrated high sensitivity and specificity. For seventy-one samples used for validation of B virus-gD, the data showed 100% (21/21) sensitivity and 96.0% (48/50) specificity. The fifty-seven samples used for validation of SIV-gag, demonstrated 100% (7/7) sensitivity and 98.0% (49/50) specificity. The sixty-six samples tested using STLV1-gag, yielded 100% (16/16) sensitivity and 96.0% (48/50) specificity. The seventy-eight samples tested using SFV1-gag, demonstrated 100% (28/28) sensitivity and 98.0% (49/50) specificity. Sixty-seven samples were tested using SRV4/5-gag, yielding 94.1% (16/17) sensitivity and 98.0% (49/50) specificity. The Youden’s indexes showed that all five recombinant viral proteins were highly sensitive and specific to relative virus positive or negative samples (γ>0.9).
Table 1.
Sensitivity, specificity and Youden’s index of ELISAs using recombinant viral proteins as compared to reference viral lysate ELISAs with WB or IFA confirmation of reactivity
| Sensitivity a | Specificity b | Youden’s index(γ) c | |
|---|---|---|---|
| B virus | 100% (21/21) | 96.0% (48/50) | 0.960 |
| SIV | 100% (7/7) | 98.0% (49/50) | 0.980 |
| STLV-1 | 100% (16/16) | 96.0% (48/50) | 0.960 |
| SFV-1 | 100% (28/28) | 98.0%(49/50) | 0.980 |
| SRV 4/5 | 94.1% (16/17) | 98.0% (49/50) | 0.921 |
Sensitivity= true positive/ (true positive + false negative);
Specificity= true negative/ (true negative+ false positive);
Youden’s index= sensitivity+specificity-1;
3.2. MMIAs comparison using recombinant viral proteins and viral lysates
A direct MMIA comparison was then performed in parallel using beads coated with viral recombinant proteins (from Vaccine Technology Inc. (VTI)) and viral lysate proteins (from University of California, Davis Clinical Proteomics Core Laboratory (UCD)). To set Median Fluorescence Index (MFI) cutoff values to prefer higher sensitivity over specificity for each analyte in the recombinant viral protein based MMIAs, the results were individually analyzed by receiver operating characteristic (ROC) curve (Table 2). Mean MFI values for a subset of samples was 91 for the E-coli negative control and 9176 for the Monkey IgG positive control. The supplier’s cutoff which were published previously were used for the viral lysate based MMIAs (Khan et al., 2006) (Table 2). For detection of B virus, SIV, STLV-1 and SFV-1 antibodies, both methods correlated well, ranging from 93.3%–100% (Table 2). For detection of SRV antibody, a higher sensitivity (80.0%, 50/70, 95%CI: 68.7 – 88.6) was obtained using SRV4/5-gag protein, while the sensitivity was only 55.7% (39/70, 95%CI: 43.3 – 67.6) using viral lysates (Table 2); but the specificity was lower using recombinant viral protein (81.0%, 171/211, 95%CI: 75.6 – 86.5) in comparison with using viral lysates (93.8%, 198/211, 95%CI: 89.7 – 96.7).
Table 2.
Cutoff, sensitivity and specificity of recombinant viral protein (R (VTI)) and viral lysate (VL (UCD)) based MMIAs
| Cutoff | Sensitivity a | Specificity b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R(VTI) (MFI)c |
VL(UCD) (MFI)d |
R(VTI) | 95% CI | VL(UCD) | 95%CI | R(VTI) | 95% CI | VL(UCD) | 95%CI | |
| B virus | 1000 | 95.7% (88/92) | 89.2 – 98.8 | 100.0% (92/92) | 96.1 – 100.0 | 93.3% (168/180) | 88.6 – 96.5 | 96.7% (174/180) | 92.9 – 98.8 | |
| SIV | 3500 | 97.8% (44/45) | 88.2 – 99.9 | 93.3% (42/45) | 81.7 – 98.6 | 95.4% (227/238) | 91.9 – 97.7 | 96.2% (229/238) | 93.5 – 98.5 | |
| STLV-1 | 2500 | 1000 | 95.8% (68/71) | 88.1 – 99.1 | 93.0% (66/71) | 84.3 – 97.7 | 94.1% (193/205) | 90.0 – 96.9 | 96.1% (197/205) | 92.5 – 98.3 |
| SFV-1 | 4600 | 99.4% (162/163) | 96.6 – 100.0 | 100.0% (163/163) | 97.8 – 100.0 | 97.5% (116/119) | 92.8 – 99.5 | 95.8% (114/119) | 90.5 – 98.6 | |
| SRV | 2700 | 80.0% (56/70) | 68.7 – 88.6 | 55.7% (39/70) | 43.3 – 67.6 | 81.0% (171/211) | 75.6 – 86.5 | 93.8% (198/211) | 89.7 – 96.7 | |
Sensitivity= true positive/ (true positive + false negative);
Specificity= true negative/(true negative+ false positive);
MMIAs using recombinant virus proteins from Vaccine Technology Inc. (VTI);
MMIAs using virus lysate preparation from University of California, Davis Clinical Proteomics Core Laboratory (UCD); MFI: median fluorescence intensity; 95% CI: 95% confidence interval;
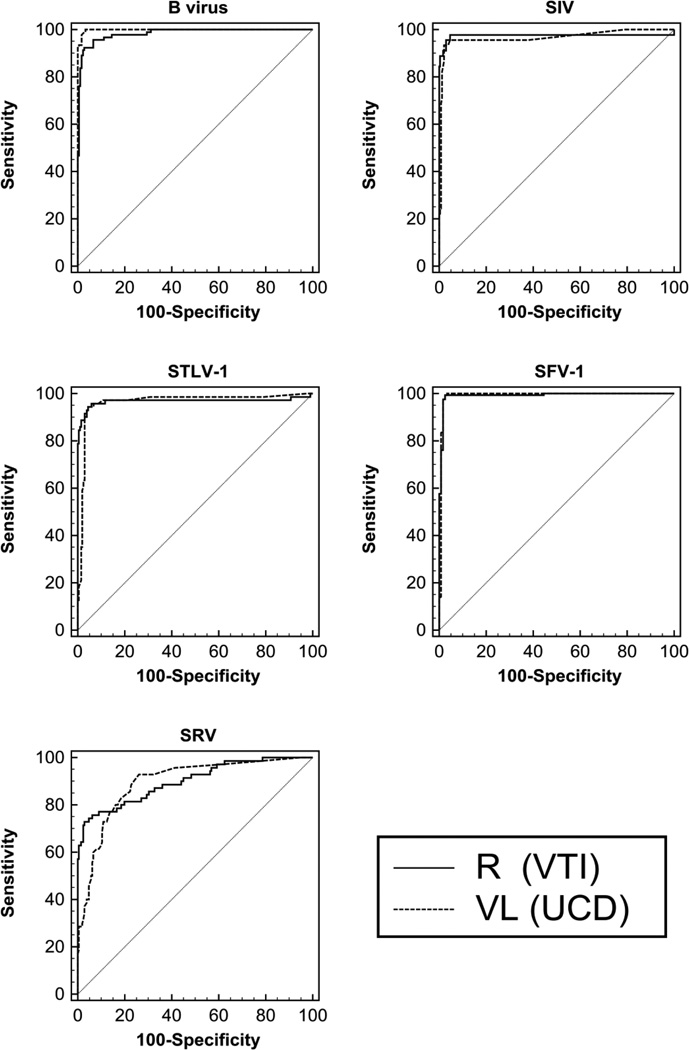
The ROC curves between recombinant viral protein and viral lysate based MMIAs for each of the five individual simian viruses were also compared (Figure 2 and Table 3). The area under curves (AUC) of all recombinant protein based MMIAs in the current study showed that these MMIAs were highly accurate in detecting specific viral antibodies in the nonhuman primate samples (AUC>=0.9). Except for the SRV (AUC=0.894; moderate), the viral lysate based MMIAs were also highly accurate in detecting viral antibodies. Figure 1 and Table 3 also show that the recombinant protein based MMIAs correlated well with viral lysate protein based MMIAs (p>0.05), although the difference between the two types of MMIAs for B virus approached significance (p=0.0155).
Figure 2.
comparison ROC curve between recombinant viral protein (R (VTI)) and viral lysate (VL (UCD)) based MMIAs. VTI: MMIAs using recombinant virus proteins from Vaccine Technology Inc. (VTI); UCD: MMIAs using virus lysate preparation from University of California, Davis Clinical Proteomics Core Laboratory (UCD).
Table 3.
Analyzing ROCs of recombinant viral protein (R (VTI)) and viral lysate (VL (UCD)) based MMIAs
| R (VTI) | VL(UCD) | Comparison AUC between R (VTI) and VL(UCD) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUC | SE | 95% CI | AUC | SE | 95% CI | Difference | SE | 95% CI | Significance level | |
| B virus | 0.984 | 0.00619 | 0.961 to 0.995 | 0.999 | 0.000872 | 0.984 to 1.000 | 0.0143 | 0.00592 | 0.00272 to 0.0259 | p = 0.0155 |
| SIV | 0.975 | 0.0222 | 0.949 to 0.990 | 0.965 | 0.0185 | 0.937 to 0.984 | 0.00938 | 0.0168 | −0.0236 to 0.0423 | p = 0.5768 |
| STLV-1 | 0.968 | 0.0189 | 0.940 to 0.985 | 0.964 | 0.0149 | 0.935 to 0.983 | 0.00395 | 0.0157 | −0.0267 to 0.0346 | p= 0.8008 |
| SFV-1 | 0.992 | 0.0049 | 0.973 to 0.999 | 0.991 | 0.00737 | 0.972 to 0.999 | 0.000516 | 0.00468 | −0.00866 to 0.00970 | p = 0.9124 |
| SRV | 0.9 | 0.0239 | 0.859 to 0.933 | 0.894 | 0.0217 | 0.852 to 0.928 | 0.00579 | 0.0226 | −0.0385 to 0.0500 | p= 0.7976 |
ROC curve: receiver operating characteristic curve; AUC: area under ROC curve; SE: Standard Error; 95% CI: 95% Confidence Interval; VTI: MMIAs using recombinant virus proteins from Vaccine Technology Inc. (VTI); UCD: MMIAs using virus lysate preparation from University of California, Davis Clinical Proteomics Core Laboratory (UCD);
By definition, the MMIAs test all antigens simultaneously. Thus multiple antigens for the same agent can be tested in parallel. Since false negatives are more detrimental than false positives (which can be corrected using confirmatory testing) it was decided to interpret any positive signal as positive to maximize sensitivity. This was illustrated in Table 4, which showed individual sensitivities of 55.7% for SRV viral lysate and 80% for SRV gag protein, but 84.3% for both in parallel.
Table 4.
Sensitivity and specificity of SRV recombinant viral protein (R) and virus lysate (VL) based MMIAs combination strategy
| Sensitivity | Specificity | ||
|---|---|---|---|
| Single | VL Screen | 39/70(55.7%) | 198/211(93.8%) |
| R Screen | 56/70(80.0%) | 171/211(81.0%) | |
| Parallel | VL or R Screen | 59/70(84.3%) | 164/211(77.7%) |
Single: two MMIAs were performed at the same time. The sensitivity and specificity of both MMIAs were calculated independently; Parallel: two MMIAs were performed at the same time. The samples negative in both MMIAs were counted as negative and the rest were counted as positive. And then the sensitivity and specificity of parallel combination were calculated.
3.3 Recombinant viral protein based MMIAs challenge against a panel examined by 12 different laboratories
The MMIAs were also tested against a challenge panel. This panel included 16 samples that were tested by 12 different laboratories. The MMIAs using the current study’s recombinant proteins, viral lysates proteins from University California, Davis Clinical Proteomics Core Laboratory (UCD), and antigens from Charles River Laboratories (CRL) were compared. For antibody detection of B virus, STLV, SIV, and SFV, the hit ratios were fairly close (from 14/16 to 16/16) (Table 5). For SRV, both viral lysate protein coated beads (one form UCD and the other from CRL) showed lower hit ratios (8/16 and 11/16) than the recombinant antigens hit ratios which were 15/16 for gag from VTI and 14/16 for env from CRL (Table 5).
Table 5.
Results of recombinant viral protein and viral lysate protein based MMIAs against a challenge panel of sera/plasma compared to consensus results from 12 different laboratories using an unspecified variety of reagents and methods
| Sample ID | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 187096 | 188397 | 188398 | 188399 | 188400 | 188402 | 188451 | 188452 | 184261 | 188722 | 188800 | 188801 | 188804 | 188807 | 188808 | 188809 | Hit ratio | ||
| B virus | Consensus Results a | N | P | P | P | N | P* | N | N | N | N | P | P | P* | N | N | N | |
| B virus gD (VTI) b | - | - | - | - | - | N | - | - | - | - | - | - | N | - | - | - | 14/16 | |
| HVP2 (CRL) c | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 16/16 | |
| HVP2 (UCD) d | - | - | - | - | - | N | - | - | - | - | - | - | - | - | - | - | 15/16 | |
| STLV | Consensus Results a | N | N | N | P | N | P | N | N | N | N | N | P | N | P | P | P | |
| STLV-1 gag (VTI) b | - | - | - | - | - | - | - | - | P | - | - | - | - | - | - | - | 15/16 | |
| STLV (CRL) c | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 16/16 | |
| STLV (UCD) d | - | - | - | - | - | - | - | - | - | - | - | - | P | - | - | - | 15/16 | |
| SIV | Consensus Results a | N | N | N | N | N | N | N | N | N | P | N | N | N | N | N | N | |
| SIV gag (VTI) b | - | - | - | - | - | - | - | - | P | - | - | - | - | - | - | - | 15/16 | |
| SIV (CRL) c | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 16/16 | |
| SIV env (CRL) e | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 16/16 | |
| SIV (UCD) d | - | - | - | - | - | - | - | - | - | - | - | - | P | - | - | - | 15/16 | |
| SFV | Consensus Results a | P | P | P | P | P | P | N | N | P | P | P | P | P | P | P | P | |
| SFV-1 gag (VTI) b | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 16/16 | |
| SFV (CRL) c | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 16/16 | |
| SFV (UCD) d | - | - | - | - | - | - | - | - | N | - | - | - | - | - | - | - | 15/16 | |
| SRV | Consensus Results a | P* | P | P | P* | P | P | N | N | N | N | N | N | N | N* | N | N | |
| SRV 4/5 gag (VTI) b | - | - | N | - | - | - | - | - | - | - | - | - | - | - | - | - | 15/16 | |
| SRV (CRL) c | N | N | N | N | N | - | - | - | - | - | - | - | - | - | - | - | 11/16 | |
| SRV env (CRL) e | - | - | - | N | - | - | - | - | - | - | - | - | - | P | - | - | 14/16 | |
| SRV (UCD) d | N | - | N | N | N | N | - | - | - | - | - | - | P | P | - | P | 8/16 | |
consensus results from 12 laboratories;
MMIAs using recombinant virus proteins from Vaccine Technology Inc. (VTI);
MMIAs using virus lysate preparation from Charles River Laboratory (CRL);
MMIAs using virus proteins from University of California, Davis Clinical Proteomics Core Laboratory (UCD);
MMIA using recombinant viral envelope proteins from Charles River Laboratory (CRL);
more than two laboratories did not agree with the consensus result; P: positive; N: negative; dash results agree with the consensus result.
4. Discussion
The five simian viruses in the current study are major concerns in building SPF nonhuman primate colonies, which can be established by detecting and removing infected monkeys (Morton et al., 2008). The accuracy of any serological assay used for this work depends largely on the quality of the target antigens used. Considering that the simian viruses have many serotypes and subtypes in the infected population, choosing the highly conserved gag proteins for the MMIAs allows them to be applied in more nonhuman primate species, while maintaining high accuracy (Chung et al., 2008; Hara et al., 2005; Lerche et al., 1994; Murray and Linial, 2006). In addition, gag proteins of the simian retroviruses and glycoprotein D (gD) of B virus stimulate robust humoral responses which are maintained for a long time and are the critical immunological biomarkers to show infection status in the nonhuman primates.(Hahn et al., 1994; Lairmore et al., 1990; Mahieux et al., 1997; Perelygina et al., 2002).
The positive /negative cutoff values for the recombinant viral protein based MMIAs were chosen to maximize sensitivity over specificity because the effect of a false negative result, not identifying an infected animal, would be more detrimental to the individual and colony SPF status and health than the effects of a false positive result. As has been the algorithm with ELISA, additional confirmatory testing can continue to be an appropriate option to improve specificity and correct any false positive result (Lerche et al., 1994). Given this priority, the results with recombinant viral protein antigens were optimized to correlate well with the reference testing in both ELISA and MMIA formats. The results using this recombinant viral protein based MMIAs demonstrated overall equivalent or increased sensitivity with only a relatively slight decrease in specificity as compared to viral lysate based MMIAs when testing for viral antibodies in the Pathogen Detection Laboratory (PDL) archived sera or plasma. In addition, results of recombinant viral protein based MMIAs against the Challenge Panel (Table 5) compared favorably with various other methods and reagents.
SIV results using recombinant gag protein were essentially equivalent to viral lysate results. The small number of positive samples is a potential concern; but non-experimental SIV infection in most monkey species used in biomedical research is extremely rare. STLV results using recombinant gag protein were slightly more sensitive and correspondingly slightly less specific than viral lysate results. SFV antibody detection was highly and equally accurate with either recombinant viral protein or viral lysate antigen. These results validate the use of these three recombinant gag proteins for antibody detection.
Herpes simplex virus type 1 (HSV1) and Herpes virus papio type 2 (HVP2) are surrogate markers which have been used successfully for B Virus detection with sensitivities of 96% and 98%, respectively (Ohsawa et al., 1999; Tanaka et al., 2004; Yamamoto et al., 2005). The recombinant viral protein sensitivities (ELISA 100%, MMIA 96%) in the current study were within this range. Confirmatory testing using B Virus antigen could not be performed to determine directly which antigen was more accurate.
Although the sensitivity of SRV antibody detection using recombinant gag protein was increased when compared with viral lysate, it was still much lower than that of the other agents. Another concern is the loss of specificity with the increased sensitivity. Since SRV is potentially an important SPF threat, additional improvement is warranted. An example of using two antigens for a single agent to increase sensitivity is illustrated in Table 4. Using results of SRV viral lysate and recombinant gag protein together in parallel improved the sensitivity with some loss of specificity, which could be corrected by confirmatory testing. In general, parallel testing improves sensitivity but is limited by the specificity of the least specific assay.
Having demonstrated the feasibility of using these recombinant antigens, further studies to develop additional recombinant antigens to add to the MMIAs can now be considered. The simultaneous, multiplex nature of the MMIA allows for the inclusion of additional antigens for minimal reagent and time costs with no increase in sample volume requirement.
The inclusion of additional antigens should improve the accuracy of testing for B Virus and SRV antibodies. Reports in the literature suggest the need for multiple epitopes to identify correctly B Virus infection in various individual host immune backgrounds over the course of time (Blewett et al., 1999; Perelygina et al., 2005; Ward and Hilliard, 2002). The panel results in table 5 showed that antibody against SRV recombinant proteins was detected in some samples which were not reactive to viral lysate antigens, particularly samples for which there was not good consensus. It has been reported previously that detection of SRV infection by analysis of blood samples is complicated additionally because antibody appears to be variable over time, often complementing the detection of virus by culture or DNA by PCR (Lerche and Osborn, 2003). Whether or not an antibody negative result in a sample from an infected monkey is a biological reality or a test method/sample limitation is an important question that has not been answered. The antigen in the ELISA used to original identify and characterize the samples in this study was SRV-5, which has been used successfully by the Pathogen Detection Laboratory for routine detection of all SRV serotypes. The five serotypes of SRV that have been found in macaques have been shown to be serologically cross-reactive, especially for gag gene proteins (Morton et al., 2008). However, multiplex technology will allow the opportunity to reconsider the efficient inclusion of additional serotype antigens in future studies.
Multiplexed recombinant antigens will be a practical, efficient and economical tool to not only improve antibody detection for the current agents but also to add additional SPF agents as they are needed (Lerche and Simmons, 2008). In addition to antibody detection for SPF surveillance, multiplexed additional antigens could provide an efficient tool for studying antibody profiles to virus components and perhaps defining the role of various epitopes in pathogenicity, disease progression, drug resistance, vaccine efficacy, and other applications.
5. Conclusion
Novel recombinant viral protein based MMIAs for the detection of B virus, SIV, STLV, SFV, and SRV antibodies have been developed and validated. These recombinant viral protein based MMIAs demonstrate at least equivalent accuracy as compared to corresponding viral lysate based MMIAs. The option to use recombinant virus proteins instead of viral lysate proteins overcomes many difficulties of working with potentially infectious and biologically variable materials. Future plans include development, incorporation, and validation of different or additional antigens to determine the best combination to further improve the accuracy of antibody testing, especially for B Virus and SRV. Additional antigens should also allow researchers to explore the possibility of combining screening and confirmatory testing into a single multiplex assay.
Highlights.
Detection of simian virus infection is important in nonhuman primates, especially in specific pathogen free colonies.
Assays using recombinant viral protein antigens are as accurate as viral lysate based assays.
Multiplex recombinant viral protein antigen based assays are potentially more efficient, less biohazardous, less variable, and less expensive than traditional assays.
Acknowledgements
This study was supported in part by NCRR/NIH grant RR00169 to the California National Primate Research Center. Viral lysate coated microbeads were prepared by Drs. Paul Luciw, Imran Khan, and Resmi Ravindran at the UCD Clinical Proteomics Core. The authors thank Haowei Zhang, Bisheng Lu, Ziyang Zhang of Haikou VTI Biological Institute and Amanda Carpenter and Ann Rosenthal of University of California Davis for their technical assistance.
Abbreviations
- SIV
Simian Immunodeficiency Virus
- STLV
Simian T Cell Lymphotropic Virus
- B Virus
herpesvirus B and Cercopithecine Herpesvirus 1
- SRV
Simian Betaretrovirus
- SFV
Simian Foamy Virus
- VL
viral lysate
- MMIA
multiplex microbead immunoassay
- NHP
nonhuman primate
- SPF
specific pathogen free
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrade MR, Yee J, Barry P, Spinner A, Roberts JA, Cabello PH, Leite JP, Lerche NW. Prevalence of antibodies to selected viruses in a long-term closed breeding colony of rhesus macaques (Macaca mulatta) in Brazil. Am. J. Primatol. 2003;59:123–128. doi: 10.1002/ajp.10069. [DOI] [PubMed] [Google Scholar]
- Blewett EL, Saliki JT, Eberle R. Development of a competitive ELISA for detection of primates infected with monkey B virus (Herpesvirus simiae) J. Virol. Methods. 1999;77:59–67. doi: 10.1016/s0166-0934(98)00134-7. [DOI] [PubMed] [Google Scholar]
- Boffelli D, McAuliffe J, Ovcharenko D, Lewis KD, Ovcharenko I, Pachter L, Rubin EM. Phylogenetic shadowing of primate sequences to find functional regions of the human genome. Science. 2003;299:1391–1394. doi: 10.1126/science.1081331. [DOI] [PubMed] [Google Scholar]
- Brown DR, Garland SM, Ferris DG, Joura E, Steben M, James M, Radley D, Vuocolo S, Garner EI, Haupt RM, Bryan JT. The humoral response to Gardasil(R) over four years as defined by Total IgG and competitive Luminex immunoassay. Hum. Vaccin. 2011;7:230–238. doi: 10.4161/hv.7.2.13948. [DOI] [PubMed] [Google Scholar]
- Chung HK, Unangst T, Treece J, Weiss D, Markham P. Development of real-time PCR assays for quantitation of simian betaretrovirus serotype-1, -2, -3, and -5 viral DNA in Asian monkeys. J. Virol. Methods. 2008;152:91–97. doi: 10.1016/j.jviromet.2008.05.021. [DOI] [PubMed] [Google Scholar]
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- Dunbar SA. Applications of Luminex xMAP technology for rapid, high-throughput multiplexed nucleic acid detection. Clin. Chim. Acta. 2006;363:71–82. doi: 10.1016/j.cccn.2005.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn H, Baunach G, Brautigam S, Mergia A, Neumann-Haefelin D, Daniel MD, McClure MO, Rethwilm A. Reactivity of primate sera to foamy virus Gag and Bet proteins. J. Gen. Virol. 1994;75:2635–2644. doi: 10.1099/0022-1317-75-10-2635. [DOI] [PubMed] [Google Scholar]
- Hara M, Sata T, Kikuchi T, Nakajima N, Uda A, Fujimoto K, Baba T, Mukai R. Isolation and characterization of a new simian retrovirus type D subtype from monkeys at the Tsukuba Primate Center, Japan. Microbes Infect. 2005;7:126–131. doi: 10.1016/j.micinf.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Hirsch VM, Johnson PR. Pathogenic diversity of simian immunodeficiency viruses. Virus Res. 1994;32:183–203. doi: 10.1016/0168-1702(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Huff JL, Barry PA. B-virus (Cercopithecine herpesvirus 1) infection in humans and macaques: potential for zoonotic disease. Emerg. Infect. Dis. 2003;9:246–250. doi: 10.3201/eid0902.020272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Research CoLS, N.R.C. Guide for the care and use of laboratory animals. Washington, D.C.: The National Academies Press; 1996. [Google Scholar]
- Jones LP, Zheng HQ, Karron RA, Peret TC, Tsou C, Anderson LJ. Multiplex assay for detection of strain-specific antibodies against the two variable regions of the G protein of respiratory syncytial virus. Clin. Diagn. Lab. Immunol. 2002;9:633–638. doi: 10.1128/CDLI.9.3.633-638.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan IH, Mendoza S, Yee J, Deane M, Venkateswaran K, Zhou SS, Barry PA, Lerche NW, Luciw PA. Simultaneous detection of antibodies to six nonhuman-primate viruses by multiplex microbead immunoassay. Clin. Vaccine Immunol. 2006;13:45–52. doi: 10.1128/CVI.13.1.45-52.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan IH, Ravindran R, Yee J, Ziman M, Lewinsohn DM, Gennaro ML, Flynn JL, Goulding CW, DeRiemer K, Lerche NW, Luciw PA. Profiling antibodies to Mycobacterium tuberculosis by multiplex microbead suspension arrays for serodiagnosis of tuberculosis. Clin. Vaccine Immunol. 2008;15:433–438. doi: 10.1128/CVI.00354-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuller L, Watanabe R, Anderson D, Grant R. Development of a whole-virus multiplex flow cytometric assay for antibody screening of a specific pathogen-free primate colony. Diagn. Microbiol. Infect. Dis. 2005;53:185–193. doi: 10.1016/j.diagmicrobio.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Lairmore MD, Lerche NW, Schultz KT, Stone CM, Brown BG, Hermann LM, Yee JA, Jennings M. SIV, STLV-I and type D retrovirus antibodies in captive rhesus macaques and immunoblot reactivity to SIV p27 in human and rhesus monkey sera. AIDS Res. Hum. Retrovir. 1990;6:1233–1238. doi: 10.1089/aid.1990.6.1233. [DOI] [PubMed] [Google Scholar]
- Lerche NW. Simian retroviruses: infection and disease--implications for immunotoxicology research in primates. J. Immunotoxicol. 2010;7:93–101. doi: 10.3109/15476911003657406. [DOI] [PubMed] [Google Scholar]
- Lerche NW, Osborn KG. Simian retrovirus infections: potential confounding variables in primate toxicology studies. Toxicol. Pathol. 2003;31:103–110. doi: 10.1080/01926230390174977. [DOI] [PubMed] [Google Scholar]
- Lerche NW, Simmons JH. Beyond specific pathogen-free: biology and effect of common viruses in macaques. Comp. Med. 2008;58:8–10. [PMC free article] [PubMed] [Google Scholar]
- Lerche NW, Switzer WM, Yee JL, Shanmugam V, Rosenthal AN, Chapman LE, Folks TM, Heneine W. Evidence of infection with simian type D retrovirus in persons occupationally exposed to nonhuman primates. J. Virol. 2001;75:1783–1789. doi: 10.1128/JVI.75.4.1783-1789.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerche NW, Yee JL, Jennings MB. Establishing specific retrovirus-free breeding colonies of macaques: an approach to primary screening and surveillance. Lab. Anim. Sci. 1994;44:217–221. [PubMed] [Google Scholar]
- Mahieux R, Pecon-Slattery J, Gessain A. Molecular characterization and phylogenetic analyses of a new, highly divergent simian T-cell lymphotropic virus type 1 (STLV-1marc1) in Macaca arctoides. J. Virol. 1997;71:6253–6258. doi: 10.1128/jvi.71.8.6253-6258.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandy FF, Nakamura T, Bergeron M, Sekiguchi K. Overview and application of suspension array technology. Clin. Lab. Med. 2001;21:713–729. [PubMed] [Google Scholar]
- Mansfield KG, Kemnitz JW. Introduction: challenges in microbial quality control for nonhuman primate. ILAR J. 2008;49:133–136. doi: 10.1093/ilar.49.2.133. [DOI] [PubMed] [Google Scholar]
- Maul DH, Lerche NW, Osborn KG, Marx PA, Zaiss C, Spinner A, Kluge JD, MacKenzie MR, Lowenstine LJ, Bryant ML. Pathogenesis of simian AIDS in rhesus macaques inoculated with the SRV-1 strain of type D retrovirus. Am. J. Vet. Res. 1986;47:863–868. [PubMed] [Google Scholar]
- Morton WR, Agy MB, Capuano SV, Grant RF. Specific pathogen-free macaques: definition, history, and current production. ILAR J. 2008;49:137–144. doi: 10.1093/ilar.49.2.137. [DOI] [PubMed] [Google Scholar]
- Murray SM, Linial ML. Foamy virus infection in primates. J. Med. Primatol. 2006;35:225–235. doi: 10.1111/j.1600-0684.2006.00171.x. [DOI] [PubMed] [Google Scholar]
- Nolan JP, Mandy FF. Suspension array technology: new tools for gene and protein analysis. Cell. Mol. Biol. (Noisy-Le-Grand, France) 2001;47:1241–1256. [PubMed] [Google Scholar]
- Ohsawa K, Lehenbauer TW, Eberle R. Herpesvirus papio 2: alternative antigen for use in monkey B virus diagnostic assays. Lab. Anim. Sci. 1999;49:605–616. [PubMed] [Google Scholar]
- Perelygina L, Patrusheva I, Hombaiah S, Zurkuhlen H, Wildes MJ, Patrushev N, Hilliard J. Production of herpes B virus recombinant glycoproteins and evaluation of their diagnostic potential. J. Clin. Microbiol. 2005;43:620–628. doi: 10.1128/JCM.43.2.620-628.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perelygina L, Zurkuhlen H, Patrusheva I, Hilliard JK. Identification of a herpes B virus-specific glycoprotein D immunodominant epitope recognized by natural and foreign hosts. J. Infect. Dis. 2002;186:453–461. doi: 10.1086/341834. [DOI] [PubMed] [Google Scholar]
- Sandstrom PA, Phan KO, Switzer WM, Fredeking T, Chapman L, Heneine W, Folks TM. Simian foamy virus infection among zoo keepers. Lancet. 2000;355:551–552. doi: 10.1016/S0140-6736(99)05292-7. [DOI] [PubMed] [Google Scholar]
- Sibal LR, Samson KJ. Nonhuman primates: a critical role in current disease research. ILAR J. 2001;42:74–84. doi: 10.1093/ilar.42.2.74. [DOI] [PubMed] [Google Scholar]
- Siepel A. Phylogenomics of primates and their ancestral populations. Genome Res. 2009;19:1929–1941. doi: 10.1101/gr.084228.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri G, Paiardini M, Pandrea I, Lederman MM, Sodora DL. Understanding the benign nature of SIV infection in natural hosts. J. Clin. Invest. 2007;117:3148–3154. doi: 10.1172/JCI33034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons JH. Development, application, and quality control of serology assays used for diagnostic monitoring of laboratory nonhuman primates. ILAR J. 2008;49:157–169. doi: 10.1093/ilar.49.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JF, Kowalski R, Esser MT, Brown MJ, Bryan JT. Evolution of type-specific immunoassays to evaluate the functional immune response to Gardasil: a vaccine for human papillomavirus types 16, 18, 6 and 11. Hum. Vaccin. 2008;4:134–142. doi: 10.4161/hv.4.2.5261. [DOI] [PubMed] [Google Scholar]
- Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285–1293. doi: 10.1126/science.3287615. [DOI] [PubMed] [Google Scholar]
- Switzer WM, Bhullar V, Shanmugam V, Cong ME, Parekh B, Lerche NW, Yee JL, Ely JJ, Boneva R, Chapman LE, Folks TM, Heneine W. Frequent simian foamy virus infection in persons occupationally exposed to nonhuman primates. J. Virol. 2004;78:2780–2789. doi: 10.1128/JVI.78.6.2780-2789.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Mannen K, Sato H. Use of herpesvirus papio 2 as an alternative antigen in immunoblotting assay for B virus diagnosis. J. Vet. Med. Sci. 2004;66:529–532. doi: 10.1292/jvms.66.529. [DOI] [PubMed] [Google Scholar]
- Tsujimoto H, Noda Y, Ishikawa K, Nakamura H, Fukasawa M, Sakakibara I, Sasagawa A, Honjo S, Hayami M. Development of adult T-cell leukemia-like disease in African green monkey associated with clonal integration of simian T-cell leukemia virus type I. Cancer Res. 1987;47:269–274. [PubMed] [Google Scholar]
- Ward JA, Hilliard JK. Herpes B virus-specific pathogen-free breeding colonies of macaques: serologic test results and the B-virus status of the macaque. Contemp. Top. Lab. Anim. Sci. 2002;41:36–41. [PubMed] [Google Scholar]
- Wolf RF, Eberle R, White GL. Generation of a specific-pathogen-free baboon colony. J. Am. Assoc. Lab. Anim. Sci. 2010;49:814–820. [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Ohsawa K, Walz SE, Mitchen JL, Watanabe Y, Eberle R, Origasa H, Sato H. Validation of an enzyme-linked immunosorbent assay kit using herpesvirus papio 2 (HVP2) antigen for detection of herpesvirus simiae (B virus) infection in rhesus monkeys. Comp. Med. 2005;55:244–248. [PubMed] [Google Scholar]
- Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]