Abstract
Purpose
Micro-computed tomography (micro-CT) has been widely used in the evaluation of regenerated bone tissue but the reliability of micro-CT has not yet been established. This study evaluated the correlation between histomorphometric analysis and micro-CT analysis in performing new bone formation measurement.
Methods
Critical-size calvarial defects were created using a 8 mm trephine bur in a total of 24 Sprague-Dawley rats, and collagen gel mixed with autogenous rat bone marrow stromal cells (BMSCs) or autogenous rat BMSCs transduced by adenovirus containing bone morphogenic protein-2 (BMP-2) genes was loaded into the defect site. In the control group, collagen gel alone was loaded into the defect. After 2 and 4 weeks, the animals were euthanized and calvaria containing defects were harvested. Micro-CT analysis and histomorphometric analysis of each sample were accomplished and the statistical evaluation about the correlation between both analyses was performed.
Results
New bone formation of the BMP-2 group was greater than that of the other groups at 2 and 4 weeks in both histomorphometric analysis and micro-CT analysis (P=0.026, P=0.034). Histomorphometric analysis of representative sections showed similar results to histomorphometric analysis with a mean value of 3 sections. Measurement of new bone formation was highly correlated between histomorphometric analysis and micro-CT analysis, especially at the low lower threshold level at 2 weeks (adjusted r2=0.907, P<0.001). New bone formation of the BMP-2 group analyzed by micro-CT tended to decline sharply with an increasing lower threshold level, and it was statistically significant (P<0.001).
Conclusions
Both histomorphometric analysis and micro-CT analysis were valid methods for measurement of the new bone in rat calvarial defects and the ability to detect the new bone in micro-CT analysis was highly influenced by the threshold level in the BMP-2 group at early stage.
Keywords: Bone morphogenic protein-2, Gene therapy, Histology, X-Ray microtomography
INTRODUCTION
Bone morphogenic protein-2 (BMP-2) is a potent osteoblastic differentiation factor of mesenchymal progenitor cells and enhances new bone formation [1,2]. Regenerative therapy using BMP-2 has been widely studied in protein therapy with recombinant human BMP-2 as well as BMP-2 gene therapy [3-7]. Since BMP-2 gene therapy can induce the sustained expression of BMP-2 synthesized in vivo, BMP-2 gene therapy can produce positive results for unfavorable bone healing conditions such as a critically sized defects (CSDs) [8,9].
In the measurement of regenerated bone tissue in BMP-2 gene therapy, most studies have performed histological examination [10,11]. For histomorphometric analysis to measure the newly formed bone tissue, a representative histology of each sample was examined using a coronally sectioned slide at the center of the defect. Although the histological examination provides precise information about the regenerated bone, the information is limited to only one slide at the center of the defect. Therefore, histological examination alone could be insufficient to evaluate the status of entire defects sites.
Recently, micro-computed tomography (micro-CT) has been performed to observe the bone regeneration at the entire defect sites [12-14]. Using a computer program to convert micro-CT images to 3-dimensional form, all the regenerated bone in the defects can be observed. In addition, information about the newly formed bone such as the bone mineral density or percent of the bone volume could be obtained via a micro-CT analysis program [12,15]. While the validity and reliability of micro-CT has been reported in previous studies [16,17], no report has addressed which factor affects the correlation between micro-CT analysis and histomorphometric analysis. Moreover, the optimal threshold level for detection of the newly formed bone tissue at the calvarial defect was not determined.
This study aimed to evaluate the correlation between micro-CT analysis and histomorphometric analysis in rat calvarial defects treated by BMP-2 ex vivo gene delivery. The factors affecting the correlation between both analyses were examined and the optimal threshold level for detection of the newly formed bone was evaluated. With these objectives, 8-mm critically sized bone defects were made in the rat calvaria, and autogenous rat bone marrow stromal cells (BMSCs) transfected by adenovirus containing BMP-2 gene delivered at the defects.
MATERIALS AND METHODS
Animal models
A total of 24 male Spraque-Dawley rats (8 weeks old) were used in this study. The animal research protocol was approved by the Institute of Laboratory Animal Resources, Seoul National University. The animal experiments were conducted according to the guidelines established by the Seoul National University Institutional Animal Care and Use Committee.
Preparation of autogenous rat BMSCs
Two weeks before calvarial surgery, autogenous rat BMSCs were harvested from both tibia under general anesthesia. The harvested BMSCs were cultured in the media of α-minimum essential medium (MEM) supplemented 10% (v/v) fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA) and 1% (v/v) penicillin-streptomycin solution (5,000 U/mL penicillin and 50 µg/mL streptomycin) (Gibco) at 37℃ in a humidified atmosphere of 95% air and 5% CO2. Media changes were done twice a week. When the cells reached confluence, the cells were released with 0.25% trypsin/1 mM ethylenediaminetetraacetic acid (EDTA). The cells of two or three passages of cells were used for transduction.
Adenoviral vector construction and transduction
Replication-defective human Ad5 containing the cDNA for human BMP-2 (ATCC, Manassas, VA, USA) in the E1 region of the virus (AdBMP2) was constructed by in vivo homologous recombination. Briefly, an expression cassette encoding the BMP-2 gene was amplified in Escherichia coli and ligated to Adeno-X Viral DNA (BD Biosciences, San Jose, CA, USA). Recombinant adenoviruses were obtained from human embryonic kidney 293 cells transfected by recombinant Adeno-X vectors. The rat BMSCs were plated at a density of 1×106 cells/cm2 and transduced with AdBMP2 in α-MEM supplemented with 10% FBS and antibiotic-antimycotic solution. The virus was allowed to adsorb for 4 hours and the media was then added. Expression of BMP-2 by AdBMP2-transduced rat BMSCs was quantified by enzyme-linked immunosorbent assay (Quantikine BMP-2 microplate, R&D systems Inc., Minneapolis, MN, USA).
Surgical procedures
General anesthesia was induced by a combination of an inhalation of isoflurane and intramuscular injection of ketamine and xylazine. A midline incision was made over the calvarium and a full-thickness flap was elevated. An 8 mm critically sized calvarial defect was created using a trephine bur (3i Implant Innovation, Palm Beach Gardens, FL, USA) under sterile saline irrigation. The animals were divided into three groups as follows: 1) collagen gel (control group), 2) non-transduced autogenous rat BMSC mixed collagen gel group (BMSC group), 3) AdBMP-2 transduced autogenous rat BMSC mixed collagen gel group (BMP-2 group). Collagen gel was prepared with 1% collagen (MATRIXEN-PSP, Bioland, Cheongwon, Korea), 10-3 N HCl, 26 mM NaHCO3, 20 mM HEPES, 0.025 N NaOH with cells according to the experimental group before the surgery. The flaps were sutured by layers with 5-0 chromic gut and 4-0 silk. All the animals received a single intramuscular injection of cefazolin.
Micro-CT
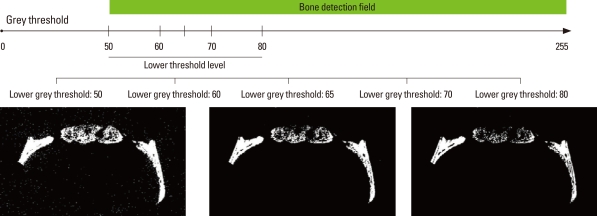
Four rats of each group were sacrificed at 2 and 4 weeks after surgery, respectively. The tissues including the surgical sites were harvested and fixed in 10% neutralized-buffered formalin solution and micro-CT was taken using Skyscan 1172 (SkyScan, Kontich, Belgium). Through a computer analysis program, the percent of bone volume in the whole defect site was calculated (CT analyzer, SkyScan). The measurement was repeated at each 50, 60, 65, 70, and 80 grayscale index of the lower grey threshold level for determination of the optimal threshold level. The automatically set threshold level in analyzing program was 65.
Histological procedure
After micro-CT taking, each sample was decalcified with 10% EDTA solution for 2 weeks and dehydrated through a series of ethanol solutions of increasing concentration and embedded in paraffin. Coronal sections 5 µm thickwere obtained at the three regions: the anterior 1/3 position, center, and posterior 2/3 position of the circular defects. Among them, the coronally sectioned slide at the center was defined as the representative histology. Each specimen was stained in hematoxylin and eosin. After microscopic examination, a photograph of each specimen was taken with a digital camera and was used for histomorphometric analysis using a computer analysis program (Tomoro Scope Eye 3.5 Image Analyzer, Techsan Digital Imaging, Seoul, Korea). Regeneration of bone was quantified by calculating the percentage of the new bone area out of the whole defect area. The whole defect area was measured arbitrarily by the area surrounded by two imaginary lines along the inner and outer calvarial bone contour and both sides of defect border.
Statistical analysis
Statistical analysis was performed using a computer program SPSS ver. 18 (SPSS Inc., Chicago, IL, USA). Two-way analysis of variance (ANOVA) was conducted to compare the new bone formation of each group in both histomorphometric analysis and micro-CT data analysis. A Student's t-test was performed to compare the new bone formation of each group between 2 and 4 weeks. Using a paired t-test, the result of histomorphometric analysis from one representative section was compared to the results from the 3 sections. To evaluate the correlation between histomorphometric analysis and micro-CT data analysis, a Pearson's correlation coefficient test was performed at first and high correlation coefficient values were obtained. Subsequently, a linear regression analysis between both analyses was performed to determine the optimal threshold level for the micro-CT data analysis. Additionally, a repeated measures ANOVA was performed to observe the change in the pattern of the bone volume measurements with increasing of the lower threshold level. The statistical significance level was P<0.05.
RESULTS
Histology and micro-CT images
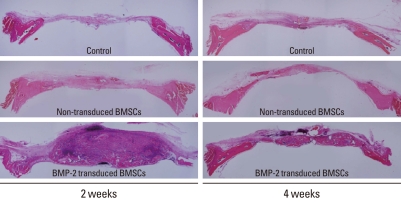
The representative histology of each group was examined, at first. As shown in Fig. 1, the BMP-2 group showed remarkable bone formation at 2 weeks although the newly formed bone was sponge-like woven bone. At 4 weeks, the BMP-2 group also produced lots of newly formed bone, which was more mature than at 2 weeks. The BMSC group showed slight new bone formation at 2 weeks and new bone formation had increased by 4 weeks. In the control group, a collagen remnant and slight new bone formation was observed at 2 and 4 weeks. From histomorphometric analysis, in the BMP-2 group, the new bone formation was found to be 76.21±26.24% at 2 weeks and 55.20±7.60% at 4 weeks, and it was significantly different from the other groups (P=0.026, P=0.034) (Table 1). There were no significant differences between the values at 2 and 4 weeks in any of the groups.
Figure 1.
Representative histology of each group at 2 and 4 weeks. The bone morphogenic protein-2 (BMP-2) group showed remarkable new bone formation at both 2 and 4 weeks. The new bone had a sponge-like immature woven bone morphology at 2 weeks, while more mature new bone with lamellar bone and marrow space had formed by 4 weeks. BMSCs, bone marrow stromal cells.
Table 1.
Histomorphometric analysis (%).
BMP-2, bone morphogenic protein-2.
a)Statistically significant from bone marrow stromal cell (BMSC) group (analysis of variance [ANOVA]).
b)Statistically significant from control group (ANOVA).
c)Statistically significant from control group and BMSC group (ANOVA).
d)Statistically significant from histomorphometric analysis with representative section (P=0.004) (paired t-test).
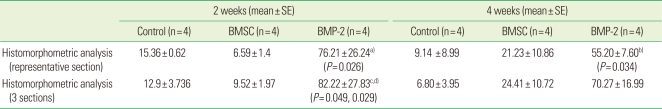
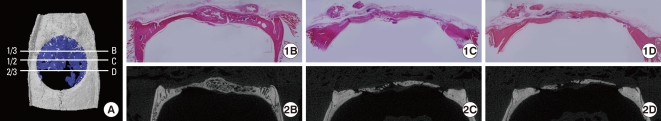
To compensate for the limited information from only one section, two additional histological sections at the anterior 1/3 and posterior 2/3 region, as shown in Fig. 2, were taken from each sample. A mean value of measurement from 3 sections was used as a representative value of each sample and it was highly correlated to the new bone area measured from a representative histology (adjusted r2=0.845, P<0.001). However, in the BMP-2 group, there was a significant difference at 2 weeks between measurement from 1 section and from 3 sections (P=0.004).
Figure 2.
Illustration of the position of the additional sections (A) and corresponding histology and micro computed tomography (micro-CT) image (B-2D). The histology at different positions showed different newly formed areas. This micro-CT image was obtained from the bone morphogenic protein-2 group at 4 weeks.
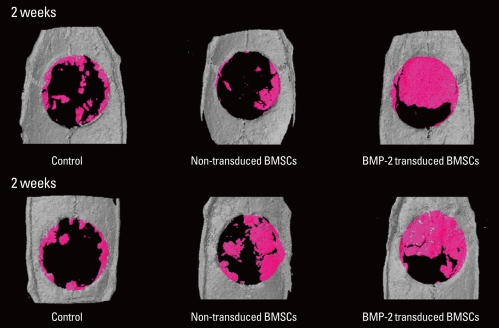
Micro-CT reconstructing a 3-dimensional view showed a result similar to that from the histomorphometric analysis (Fig. 3). A 3-dimensional image was obtained with the automatically set-up threshold level (lower grey threshold level=65). The defect fill of the BMP-2 group at 2 and 4 weeks was greater than that of the other groups. At 4 weeks, the BMSC group showed more bone fill than at 2 weeks.
Figure 3.
Micro-computed tomography images reconstructing each group 3-dimensionally at 2 and 4 weeks. These images were built with the lower threshold level of 65, the value set automatically. The bone morphogenic protein-2 (BMP-2) group showed remarkable bone fill of the defect at 2 and 4 weeks. New bone of the bone marrow stromal cell (BMSC) group was greater at 4 weeks than 2 weeks.
Correlation between Micro-CT analysis and histomorphometric analysis
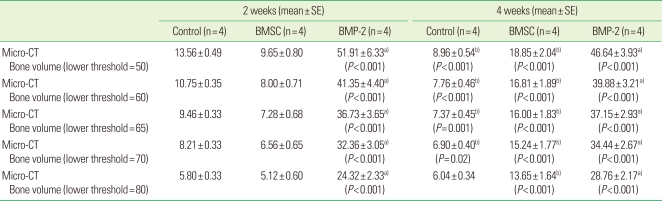
Since the threshold level influences the new bone detection, the bone volume was repeatedly measured with the lower threshold level set at 50, 60, 65, 70, and 80 (Fig. 4, Table 2). As the lower grey threshold level was increased, new bone volume generally decreased. The bone volume measured from micro-CT in the BMP-2 group was significantly greater than in the other groups at all threshold levels, similar to the histomorphometric analysis (P<0.001 for all cases).
Figure 4.
Illustration and micro-computed tomography images according to the lower threshold level. At a lower grey threshold level of 50, the noise on the background was more pronounced; however, more newly formed bone was detected. As the threshold level was increased, the new bone detected on the radiology decreased. The unit of the threshold level was the grayscale index.
Table 2.
The bone volume measurements from micro computed tomography (micro-CT) images according to the lower threshold level (%).
Bone volume was calculated from bone volume (pixel3)/tissue volume (pixel3). Pixel size was 17.29057 µm.
The unit of threshold level was the grayscale index.
BMP-2, bone morphogenic protein-2.
a)Statistically significant from control group and bone marrow stromal cell (BMSC) group (analysis of variance).
b)Statistically significant from 2 weeks (Student's t-test).
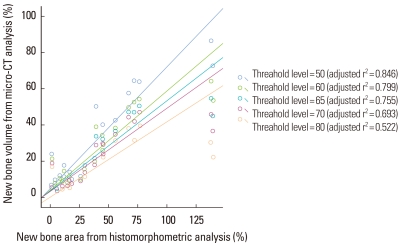
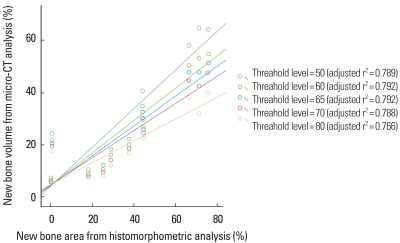
The volume (%) of new bone from micro-CT analysis was highly correlated with the new bone area (%) obtained from the histomorphometric analysis of the representative histology (P<0.001) (Fig. 5). As a result of a linear regression analysis, the bone volume of micro-CT measured at the threshold level of 50 was highly correlated to the new bone of histomorphometric analysis (adjusted r2=0.846, P<0.001). As the threshold level was increased, the correlation between the micro-CT analysis and histomorphometric analysis decreased.
Figure 5.
Scatter diagram and linear regression analysis between new bone area (%) of histomorphometric analysis of a representative section and bone volume (%) of micro computed tomography (micro-CT) analysis. The correlation between the two analyses was statistically significant (P<0.0001, all groups). As the threshold level was increased, the correlation between the two analyses decreased. At the threshold level of 50, the correlation was the most significant. The unit of the threshold level was the grayscale index.
The bone volume measured from micro-CT was also highly correlated with the new bone of the histomorphometric analysis of 3 sections (P<0.001), and the correlation was the highest at the threshold level of 50 (adjusted r2=0.824, P<0.001) (Fig. 6).
Figure 6.
Scatter diagram and linear regression analysis between new bone area (%) of histomorphometric analysis with 3 sections and bone volume (%) of micro computed tomography (micro-CT) analysis. The correlation between the two analyses was statistically significant (P<0.0001, all groups). As the threshold level was increased, the correlation between the two analyses decreased. At a threshold level of 50, the correlation was the most significant. The unit of threshold level was the grayscale index.
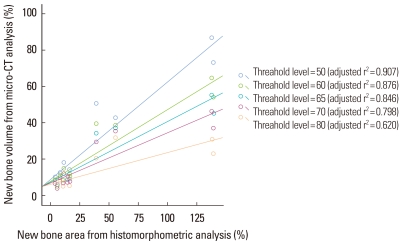
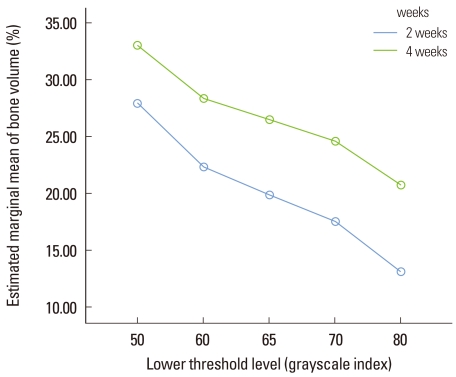
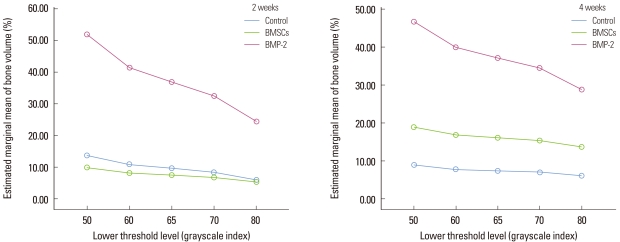
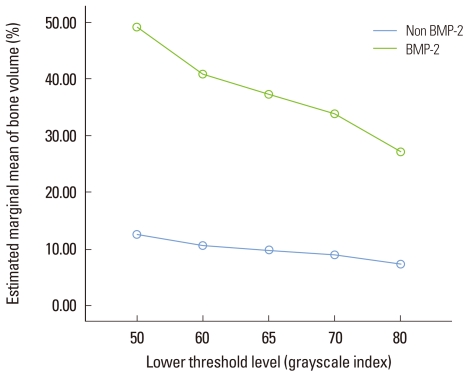
At 2 weeks, the histomorphometric analysis and micro-CT analysis were the most highly correlated at the lower threshold level of 50 with adjusted r2=0.907 (Fig. 7). At 4 weeks, the correlation between the two analyses was decreased than at 2 weeks and the influence of the lower threshold level on the measurement was disappeared (Fig. 8). The new bone volume of overall samples at 4 weeks was higher than at 2 weeks and it was decreased as the lower threshold level increased with a similar slope to 2 weeks (Fig. 9). At 2 weeks, the bone volume of the BMP-2 group was sharply decreased as the lower threshold level increased and the reduction pattern was significantly different from the other groups (P<0.001). The BMP-2 group at 4 weeks also showed a significantly steeper reduction pattern than the other groups (P<0.001). The BMSC group had a higher bone volume than the control group although the reduction pattern was similar to the control group (Fig. 10). Regardless of timing, as the threshold level increased, the BMP-2 group with a great deal of new bone formation also had a significantly steeper reduction pattern than the non-BMP-2 groups, which showed only slight bone formation (Fig. 11).
Figure 7.
Scatter diagram and linear regression analysis between new bone area (%) of histomorphometric analysis of a representative section and bone volume (%) of micro computed tomography (micro-CT) analysis at 2 weeks. The correlation between the two analyses was statistically significant (P<0.0001, all groups). As the threshold level was increased, the correlation between the two analyses decreased. At a threshold level of 50, the correlation was the most significant. The unit of the threshold level was the grayscale index.
Figure 8.
Scatter diagram and linear regression analysis between the new bone area (%) of histomorphometric analysis with a representative section and bone volume (%) of micro computed tomography (micro-CT) analysis at 4 weeks. The correlation between the two analyses was statistically significant (P<0.0001, all groups). The correlation between the two analyses was independent of the threshold level. The unit of the threshold level was the grayscale index.
Figure 9.
Illustration of the bone volume (%) measurement at 2 and 4 weeks according to the lower threshold level. The bone volume gradully decreased with the increase in the threshold level at 2 and 4 weeks and the slope at 2 weeks and 4 weeks was similar (repeated measures analysis of variance, not significant). The unit of the threshold level was the grayscale index.
Figure 10.
Illustration of the bone volume (%) measurement of each group at 2 and 4 weeks according to the lower threshold level. The bone morphogenic protein-2 (BMP-2) group showed a sharper decline as the threshold level increased at 2 weeks than the other groups, and the difference was significant (repeated measures analysis of variance [ANOVA], statistically significant, P<0.001). This pattern was similar at 4 weeks (repeated measures ANOVA, statistically significant, P<0.001). The unit of the threshold level was the grayscale index. BMSCs, bone marrow stromal cells.
Figure 11.
Illustration of the bone volume (%) measurement of the bone morphogenic protein-2 (BMP-2) group vs. the non-BMP-2 group according to the lower threshold level. The BMP-2 group showed a sharper decline than the non-BMP-2 group (repeated measures analysis of variance, statistically significant, P<0.001). The unit of the threshold level was the grayscale index.
DISCUSSION
For the evaluation of new bone formation in regenerative therapy, most studies have been performed using histomorphometric analysis, which is limited to 2-dimensional information from a representative slide. Therefore, recent reports have also performed micro-CT, observing the newly formed bone of the entire defect site using 3-dimensionally reconstructed images [8,18]. In the present study, the amount of new bone measured from histomorphometric analysis and from micro-CT analysis was highly correlated in regeneration therapy with BMP-2 gene therapy on rat calvarial defects. This result was similar to previous studies. Maréchal et al. [19] reported a strong correlation between micro-CT and serial histological sections of bone augmentation under a titanium barrier membrane with r2=0.072. Umoh et al. [20] compared the histological assessments and in vivo micro-CT images in a rat calvarial model and also observed a high correlation between both examinations with r2=0.70. Kochi et al. [21] also reported consistent patterns of bone formation in both analyses in bone augmentation with hydroxyapatite.
In the present study, the adjusted r2 value of linear regression analysis was greater than in other studies although the direct comparison to previous studies has been limited. This is related to the threshold level used in each experiment. The present study showed that the correlation between both analyses was highly dependent on the lower threshold level. At the lower threshold level of 50, the most significant correlation was observed, and the adjusted r2 value decreased as the lower threshold level increased. This phenomenon was maximized in the BMP-2 group. In the histology at 2 weeks, the new bone was the immature and sponge-like woven bone, contrasting with the matured bone with lamellar bone and marrow space at 4 weeks. Therefore, the bone volume measured from micro-CT was highly influenced by the threshold level at 2 weeks in BMP-2 group. At the lower threshold level of 50, the immature bone tissue with low radiopacity could be detected despite of an increase of the background noise. Since the newly formed bone at 4 weeks was mature enough to detect in the radiology, the new bone volume measured from micro-CT analysis was not sensitive at the change of the threshold level. Therefore, the optimal threshold level of the micro-CT analysis should be determined according to the purpose of the study. To observe the new bone formation in the early period, a low lower threshold level was advantageous for the measurement of immature new bone despite of possibility of false positives. On the other hand, to distinguish the mature bone, micro-CT analysis using a higher threshold level could be proper during the evaluation of the newly-formed bone.
Despite of the high correlation between two analyses, the difference in the new bone area from histomorphometric analysis between 2 and 4 weeks was greater than that from micro-CT analysis. Because some newly formed bone of the BMP-2 group was mixed with collagen gel, the border between them was not clear in histological examination and possibility of false positives existed. Since measurement using micro-CT analysis detects only the mineralized bone, the measurement the bone volume obtained from micro-CT is also helpful in judgment of the histomorphometric analysis.
Micro-CT has some advantages in the bone research; the sequential data through in vivo micro-CT could be obtained from individual animals during the research period and the number of animals could be reduced. Additionally, micro-CT data could be acquired rapidly without a destructive and time consuming process related to the histological examination. Micro-CT analysis has been compared to the alternative methods for histology, and it could be a good monitoring method highly correlated to histomorphometric analysis of bone regeneration during a study.
In this study, new bone formation at 2 and 4 weeks was analyzed using histomorphometric analysis and micro-CT analysis. Since this study was focused on the methodological aspect of new bone formation using BMP-2 gene therapy, this interval was effective for the comparison of the early woven bone and lamellar bone. However, this interval could be insufficient for evaluating the effect of genetically modified rat BMSCs overexpressing BMP-2 in new bone formation. In previous studies about the regenerative therapy, a longer time interval such as 4 and 8 weeks was used to compare bone regeneration therapy methods in rodent models and further study will be needed to evaluate the new bone formation in BMP-2 ex-vivo gene therapy.
In conclusion, the new bone measurement using histomorphometric analysis and micro-CT analysis was highly correlated, especially at the low threshold level in the early period. The bone volume measured from micro-CT of the BMP-2 group was more sensitive to the changing of the lower threshold level than the other groups.
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korean Health Technology R&D Projects, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A085056).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Ryoo HM, Lee MH, Kim YJ. Critical molecular switches involved in BMP-2-induced osteogenic differentiation of mesenchymal cells. Gene. 2006;366:51–57. doi: 10.1016/j.gene.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Seol YJ, Park YJ, Lee SC, Kim KH, Lee JY, Kim TI, et al. Enhanced osteogenic promotion around dental implants with synthetic binding motif mimicking bone morphogenetic protein (BMP)-2. J Biomed Mater Res A. 2006;77:599–607. doi: 10.1002/jbm.a.30639. [DOI] [PubMed] [Google Scholar]
- 3.Kofron MD, Laurencin CT. Bone tissue engineering by gene delivery. Adv Drug Deliv Rev. 2006;58:555–576. doi: 10.1016/j.addr.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Lutz R, Park J, Felszeghy E, Wiltfang J, Nkenke E, Schlegel KA. Bone regeneration after topical BMP-2-gene delivery in circumferential peri-implant bone defects. Clin Oral Implants Res. 2008;19:590–599. doi: 10.1111/j.1600-0501.2007.01526.x. [DOI] [PubMed] [Google Scholar]
- 5.Jiang XQ, Sun XJ, Lai HC, Zhao J, Wang SY, Zhang ZY. Maxillary sinus floor elevation using a tissue-engineered bone complex with beta-TCP and BMP-2 gene-modified bMSCs in rabbits. Clin Oral Implants Res. 2009;20:1333–1340. doi: 10.1111/j.1600-0501.2009.01755.x. [DOI] [PubMed] [Google Scholar]
- 6.Evans C. Gene therapy for the regeneration of bone. Injury. 2011;42:599–604. doi: 10.1016/j.injury.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer J, Kolk A, Wolfart S, Pautke C, Warnke PH, Plank C, et al. Future of local bone regeneration - Protein versus gene therapy. J Craniomaxillofac Surg. 2011;39:54–64. doi: 10.1016/j.jcms.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Koh JT, Zhao Z, Wang Z, Lewis IS, Krebsbach PH, Franceschi RT. Combinatorial gene therapy with BMP2/7 enhances cranial bone regeneration. J Dent Res. 2008;87:845–849. doi: 10.1177/154405910808700906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patterson J, Siew R, Herring SW, Lin AS, Guldberg R, Stayton PS. Hyaluronic acid hydrogels with controlled degradation properties for oriented bone regeneration. Biomaterials. 2010;31:6772–6781. doi: 10.1016/j.biomaterials.2010.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang YC, Simmons C, Kaigler D, Rice KG, Mooney DJ. Bone regeneration in a rat cranial defect with delivery of PEI-condensed plasmid DNA encoding for bone morphogenetic protein-4 (BMP-4) Gene Ther. 2005;12:418–426. doi: 10.1038/sj.gt.3302439. [DOI] [PubMed] [Google Scholar]
- 11.Chang PC, Seol YJ, Cirelli JA, Pellegrini G, Jin Q, Franco LM, et al. PDGF-B gene therapy accelerates bone engineering and oral implant osseointegration. Gene Ther. 2010;17:95–104. doi: 10.1038/gt.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young S, Patel ZS, Kretlow JD, Murphy MB, Mountziaris PM, Baggett LS, et al. Dose effect of dual delivery of vascular endothelial growth factor and bone morphogenetic protein-2 on bone regeneration in a rat critical-size defect model. Tissue Eng Part A. 2009;15:2347–2362. doi: 10.1089/ten.tea.2008.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel ZS, Young S, Tabata Y, Jansen JA, Wong ME, Mikos AG. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43:931–940. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang SC, Chuang H, Chen YR, Yang LC, Chen JK, Mardini S, et al. Cranial repair using BMP-2 gene engineered bone marrow stromal cells. J Surg Res. 2004;119:85–91. doi: 10.1016/j.jss.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Park EJ, Kim ES, Weber HP, Wright RF, Mooney DJ. Improved bone healing by angiogenic factor-enriched platelet-rich plasma and its synergistic enhancement by bone morphogenetic protein-2. Int J Oral Maxillofac Implants. 2008;23:818–826. [PMC free article] [PubMed] [Google Scholar]
- 16.Gielkens PF, Schortinghuis J, de Jong JR, Huysmans MC, Leeuwen MB, Raghoebar GM, et al. A comparison of micro-CT, microradiography and histomorphometry in bone research. Arch Oral Biol. 2008;53:558–566. doi: 10.1016/j.archoralbio.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Kochi G, Sato S, Fukuyama T, Morita C, Honda K, Arai Y, et al. Analysis on the guided bone augmentation in the rat calvarium using a microfocus computerized tomography analysis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:e42–e48. doi: 10.1016/j.tripleo.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 18.Shin JH, Kim KH, Kim SH, Koo KT, Kim TI, Seol YJ, et al. Ex vivo bone morphogenetic protein-2 gene delivery using gingival fibroblasts promotes bone regeneration in rats. J Clin Periodontol. 2010;37:305–311. doi: 10.1111/j.1600-051X.2009.01522.x. [DOI] [PubMed] [Google Scholar]
- 19.Maréchal M, Luyten F, Nijs J, Postnov A, Schepers E, van Steenberghe D. Histomorphometry and micro-computed tomography of bone augmentation under a titanium membrane. Clin Oral Implants Res. 2005;16:708–714. doi: 10.1111/j.1600-0501.2005.01205.x. [DOI] [PubMed] [Google Scholar]
- 20.Umoh JU, Sampaio AV, Welch I, Pitelka V, Goldberg HA, Underhill TM, et al. In vivo micro-CT analysis of bone remodeling in a rat calvarial defect model. Phys Med Biol. 2009;54:2147–2161. doi: 10.1088/0031-9155/54/7/020. [DOI] [PubMed] [Google Scholar]
- 21.Kochi G, Sato S, Ebihara H, Hirano J, Arai Y, Ito K. A comparative study of microfocus CT and histomorphometry in the evaluation of bone augmentation in rat calvarium. J Oral Sci. 2010;52:203–211. doi: 10.2334/josnusd.52.203. [DOI] [PubMed] [Google Scholar]