Abstract
Nonspecific antibodies, which are thought to be nonprotective, have been shown to contribute a substantial proportion of the measured concentration in the standardized immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA) for pneumococcal polysaccharide capsular antibodies. The presence of such antibodies in human immunodeficiency virus (HIV)-infected persons has not been evaluated. The amount of nonspecific antibodies is proportional to the reduction in IgG antibody concentration that occurs with serum absorption with the heterologous polysaccharide 22F. We measured the amount of nonspecific antibodies before and after vaccination with the pneumococcal conjugate vaccine (PCV; n = 33) or the pneumococcal polysaccharide vaccine (PPV; n = 34) in HIV-infected adults with CD4 counts of ≥200 cells/mm3. Blood was drawn before and 2 months after vaccination. For prevaccination sera, we found a substantial amount of nonspecific antibodies for serotypes 4, 6B, 9V, and 23F (23 to 47% of measured IgG concentration), but not for serotype 14. There tended to be proportionately less nonspecific antibodies in postvaccine sera than prevaccine sera for PCV, but not for PPV. Subjects with a low HIV viral load (≤400 copies/ml) had proportionately more nonspecific antibodies than those with higher viral load before and after both vaccines. After 22F absorption, the geometric mean concentrations of antibodies were significantly higher post-PCV than post-PPV for the high viral load group for all five serotypes, but for no serotypes in the low viral load group. These findings confirm that absorption with a heterologous pneumococcal polysaccharide (e.g., 22F) is necessary to remove nonspecific antibodies in a standardized IgG ELISA for pneumococcal capsular antibodies in HIV-infected adults.
The pneumococcal conjugate vaccine (PCV) has been found to be effective for preventing severe pneumococcal disease in infants and toddlers (2, 3, 9). Because of PCV's effectiveness in children, there is interest in determining the utility of this vaccine among adults who have high rates of pneumococcal disease (5). One such group is human immunodeficiency virus (HIV)-infected adults, who have an up to 50-fold increased risk of invasive pneumococcal disease (12, 19). As with infants, HIV-infected persons tend to have a diminished immune response to the pneumococcal polysaccharide vaccine (PPV) (1, 5, 10). Likewise, protection provided by PPV might be limited in HIV-infected persons, particularly in those with advanced disease (4, 8, 11).
Before considering efficacy studies in adults, the immunogenicity of PCV should be evaluated. Antibody concentration as measured in the immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA) may not correlate directly with vaccine efficacy in adults. Antibody concentrations do not always correlate with their functionality. This fact was shown in one study in the elderly, in which the antibodies produced to PPV had poor functional activity (23). An IgG ELISA measures not only serotype-specific antibodies but also nonspecific antibodies in adults (6, 7, 24, 27). Nonspecific antibodies, which are felt to be nonprotective, have been shown to contribute over half of the measured concentration in the ELISA for some serotypes in some immunocompetent adults (6). ELISAs can be made more specific for serotype-specific pneumococcal capsular antibodies by reducing nonspecific antibody binding through absorption with a heterologous polysaccharide (6, 24). It has been suggested that serotype 22F be used for this purpose because it has a substantial amount of the common epitopes of these nonspecific antibodies and it is not included in the new conjugate vaccines, although it is included in PPV (6).
In a previously published study, we reported the results of a randomized, placebo-controlled immunogenicity trial of PCV in HIV-infected adults (10). We found that PCV elicited higher antibody concentrations and functional antibody activity than did PPV to four of five serotypes tested (serotypes 4, 6B, 9V, and 23F, but not serotype 14), although the antibody responses were still lower than those in immunocompetent adults. A second vaccination with either PCV or PPV given 8 weeks after the first PCV dose produced no further increase in immune responses. In that study, we reported only antibody concentrations after absorption with 22F to remove the nonspecific antibodies. Here we report in greater detail on the effects of these nonspecific antibodies in measuring and interpreting the immune response to pneumococcal vaccines in HIV-infected adults.
MATERIALS AND METHODS
A double-blinded, randomized trial of pneumococcal vaccines in HIV-infected adults >17 years old with CD4 counts of ≥200 was conducted in infectious disease clinics at the VA Greater Los Angeles Healthcare System, Los Angeles, Calif., and at Grady Health Systems, Atlanta, Ga., from January 1998 to June 1999 (10). We obtained written informed consent from all study subjects in accordance with the Institutional Review Board guidelines of the Centers for Disease Control and Prevention (CDC), the VA Greater Los Angeles Healthcare System, and Emory University. Vaccines administered were the heptavalent conjugate pneumococcal vaccine (Wyeth-Lederle) containing 2 μg of capsular polysaccharide from each of six serotypes (4, 9V, 14, 18C, 19F, and 23F) and 4 μg of capsular polysaccharide from serotype 6B, covalently linked to a total of 20 to 25 μg of CRM197, a nontoxic mutant diphtheria toxin; the 23-valent pneumococcal polysaccharide vaccine (Wyeth-Lederle) containing 25 μg of each of 23 capsular polysaccharides; and a saline-alum phosphate placebo vaccine (Wyeth-Lederle). Subjects were randomly assigned to one of four study groups. The study groups received one of the following two-dose regimens, the doses being given 8 weeks apart: group a, conjugate-conjugate; group b, conjugate-polysaccharide; group c, placebo-polysaccharide; and group d, placebo-placebo. Eight weeks after the second vaccine dose, the study pharmacist unmasked the study groups and subjects who had not received PPV(groups a and d) were given PPV, because the Advisory Committee on Immunization Practices recommends PPV for HIV-infected adults (5). Blood for immunologic assays was drawn before and 8 weeks after vaccination. In this analysis, we compared the immune response 8 weeks after a single dose of PCV (groups a and b) with that after a single dose of PPV (groups c and d).
Sera were frozen at −70°C within 4 h of collection. Specimens were shipped on dry ice to the CDC in Atlanta for testing. Sera were tested for antibody concentrations to serotypes 4, 6B, 9V, 14, and 23F. Total IgG anticapsular antibody concentrations were measured by a previously described ELISA protocol that measures binding of serotype-specific IgG to antigen-coated ELISA wells (20). The assay included absorption of a 1:200 dilution of serum with cell wall polysaccharide (C-PS; 10 μg/ml). Serum samples were assayed a second time using a modification of the above protocol, which included absorption with a heterologous pneumococcal polysaccharide of serotype 22F at a final concentration of 16 μg/ml, optimized for the lot number and assay used in the CDC laboratory (10). The standard reference serum (89S-F) and quality control sera were absorbed with only C-PS. We analyzed optical density data using a previously defined method (20).
Sera were also tested for functional antibody activity to the same five serotypes by means of a previously described standardized opsonophagocytosis assay (22). This assay used viable bacteria as the target cells and differentiated HL-60 granulocytes as the effector cells. Opsonophagocytic titers were given as the reciprocal of the serum dilution yielding ≥50% killing of the bacterial inoculum compared with the complement controls. Sera with opsonophagocytic titers of less than 1:8 (lowest measurable titer) were reported as having a titer of 4.
Viral load testing was done on plasma using reverse transcriptase PCR that provided quantification of copies of HIV RNA (Amplicor; Roche Diagnostics, Nutley, N.J.). Viral load at the initial visit (range, <400 to 358,000 copies/ml) was used to dichotomize individuals as having low viral load (≤400 copies/ml) or high viral load (>400 copies/ml).
Total IgG was measured at the initial visit using a commercial nephelometric test kit (Minineph; The Binding Site Ltd., Birmingham, United Kingdom). Serum specimens were diluted and added to human IgG antiserum to form insoluble immune complexes (16). The scatter of light through the suspension measured the total protein concentration and was quantified against a standard curve. In another study using the same technique, the mean value in healthy adults was 9.9 g/liter (95% confidence interval, 6.4 to 13.5 g/liter) (16).
For the statistical analysis, antibody concentrations and opsonophagocytic titers were log-transformed to approximate normal distributions. The geometric mean concentrations and titers were obtained by taking the antilogarithm of the means of the log-transformed values. Baseline antibody concentrations and opsonophagocytic titers were compared using analysis of variance (ANOVA). Differences in the reduction in IgG concentrations with 22F absorption were determined by the Wilcoxon rank sum test for nonparametric continuous variables. Correlations between serotype-specific IgG concentrations and opsonophagocytic titers were calculated using the Pearson correlation coefficient. Differences between correlation coefficients were compared using a standard formula (Scientific tables; GEIGY.) We compared the postvaccination geometric mean concentrations (GMC) using a mixed ANOVA model, controlling for baseline IgG concentrations, race, and study site (PROC MIXED; SAS version 8.02).
RESULTS
Baseline demographic characteristics were similar among the 33 subjects who received a single dose of PCV and the 34 subjects who received a dose of PPV (10). The prevaccination geometric mean antibody concentrations were similar for both groups, both with and without 22F absorption (Table 1). Baseline opsonophagocytic titers were also similar between the two groups.
TABLE 1.
GMCs pre- and post-PPV and -PCV and reduction of IgG antibody binding by absorption with serotype 22F, stratified by HIV viral load
| Serotype | Viral loadc | Pre-PPVa
|
Post-PPVa
|
Pre-PCVb
|
Post-PCVb
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GMC − 22f | GMC + 22f | % Reductiond | GMC − 22f | GMC + 22f | % Reductiond | GMC − 22f | GMC + 22f | % Reductiond | GMC − 22f | GMC + 22f | % Reductiond | ||
| 4 | Low | 1.56 | 0.76 | 52 | 2.28 | 1.12 | 58 | 2.00 | 0.77 | 67 | 2.67 | 1.38 | 50 |
| High | 0.86 | 0.69 | 10e | 1.16 | 0.85 | 22e | 1.11h | 0.66 | 42 | 1.73 | 1.41f | 10e | |
| 6B | Low | 1.96 | 1.1 | 39 | 2.68 | 1.6 | 40 | 2.58 | 1.15 | 53 | 3.33 | 1.52 | 50 |
| High | 1.13 | 1.1 | 14e | 1.70 | 0.82 | 24 | 1.38 | 0.99 | 28 | 2.37 | 2.13f | 8e | |
| 9V | Low | 1.50 | 0.89 | 37 | 2.50 | 1.48 | 41 | 2.16 | 0.89 | 60 | 4.09 | 2.15 | 41 |
| High | 0.87 | 0.71 | 22e | 1.55 | 1.20 | 20e | 1.32 | 0.85 | 21e | 2.82 | 2.39f | 8e | |
| 14 | Low | 5.26 | 5.63 | −2 | 21.1 | 23.0 | −6 | 4.11 | 4.57 | −2 | 7.70 | 8.07f | −4 |
| High | 4.30 | 5.26 | −19 | 5.52g | 7.8g | −4 | 4.66 | 5.44 | −9 | 11.8 | 13.0f | −7 | |
| 23F | Low | 1.83 | 1.12 | 35 | 2.59 | 1.67 | 34 | 2.18 | 1.05 | 47 | 3.68 | 1.67 | 46 |
| High | 0.93 | 0.88 | 0e | 1.12 | 1.01 | 9 | 1.12 | 0.88 | 10e | 1.79 | 1.68f | 5e | |
For PPV, low viral load n = 12; high viral load n = 19.
For PCV, low viral load n = 18; high viral load n = 14.
Low load was ≤400 copies/ml; high load was >400 copies/ml.
Percent reduction was calculated by study subject, so it may differ slightly from the percent reduction in GMC.
P < 0.05 (comparing percent reduction between low and high viral local groups using Wilcoxon rank sum test).
P < 0.05 (comparing post-PCV7 and post-PPV GMCs using a mixed ANOVA model).
P < 0.01 (comparing GMCs using a mixed ANOVA model controlling for study site, race, and prevaccine GMC).
P < 0.05 (comparing GMCs using a mixed ANOVA model controlling for study site and race).
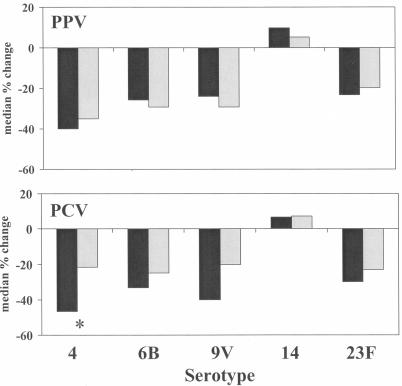
For the prevaccination sera for both vaccine groups, absorption with 22F significantly reduced the IgG binding for serotypes 4, 6B, 9V, and 23F, but not for serotype 14 (Fig. 1). Absorption with 22F caused a greater reduction in prevaccination IgG binding for these four serotypes in subjects with a low viral load than in those with a high viral load (Table 1).
FIG. 1.
The percent change in IgG antibody binding after absorption with serotype 22F in prevaccine sera (black bars) and postvaccine sera (gray bars) for PPV (n = 34) and PCV (n = 33). *, P < 0.05 for the difference between pre- and postvaccine sera (Wilcoxon rank sum test).
After a single dose of either vaccine, there continued to be a reduction in the IgG binding with 22F absorption for serotypes 4, 6B, 9V, and 23F (Fig. 1). However, the amount of reduction in IgG binding tended to be less post-PCV than pre-PCV (P < 0.05 for serotype 4); this difference was not found with PPV. As in the prevaccine sera, the amount of reduction in IgG binding with 22F postvaccine was greater in the low viral load group than in the high viral load group (Table 1). After 22F absorption, the post-PCV GMCs were significantly higher than the post-PPV GMCs for the high viral load group for all five serotypes, but for none of the serotypes in the low viral load group (Table 1). With 22F absorption, there were no significant differences in the post-PCV GMC between the low and high viral load groups. In contrast, the low viral load group tended to have higher post-PPV GMCs than did the high viral load group (P < 0.05 for type 14). When analyzing the vaccine response based on the use of highly active antiretroviral therapy (HAART) rather than viral load, we found that the post-PCV GMCs were also higher than the post-PPV GMCs in persons not on HAART, but not for persons on HAART, for all five serotypes (both with and without 22F absorption). However, HAART use was strongly associated with lower viral load (P < 0.0001).
There was no difference in the total IgG between the low and high viral load groups. The median IgG concentration for the low viral load group was 17.1 g/liter, and for the high viral load group it was 17.6 g/liter (P = 0.35, Wilcoxon rank sum test).
Serotype-specific IgG concentrations and opsonophagocytic titers were poorly correlated (r < 0.30) for prevaccination sera, both with and without 22F absorption, except for serotype 14 (Table 2). After PCV vaccination, the correlation improved significantly for serotypes 6B, 9V, and 23F and tended to be higher with 22F absorption than without, except for serotype 14. The post-PCV correlation tended to be higher in the high viral load group than in the low viral load group (data not shown). After PPV vaccination, the correlation improved significantly for serotype 4 but remained poor for serotypes 6B, 9V, and 23F. The correlation remained significant for serotype 14 after both vaccines. The post-PCV correlation, with 22F absorption, was significantly higher than the post-PPV correlation for serotype 9V and tended to be higher for serotypes 6B and 23F.
TABLE 2.
Correlation between pneumococcal capsular IgG ELISA (natural log) and opsonophagocytic assay (log 2) pre- and post-PPV and -PCV, with and without absorption with serotype 22F
| Serotype | Absorption condition | Pre-PPV (n = 33)
|
Post-PPV (n = 34)
|
Pre-PCV (n = 32)
|
Post-PCV (n = 33)
|
||||
|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | ||
| 4 | Without 22F | 0.17 | 0.33 | 0.35 | 0.053 | 0.053 | 0.93 | 0.085 | 0.64 |
| With 22F | 0.16 | 0.36 | 0.48 | 0.0058d | 0.015 | 0.93 | 0.25 | 0.17 | |
| 6B | Without 22F | 0.25 | 0.16 | 0.017 | 0.93 | 0.23 | 0.22 | 0.37 | 0.036 |
| With 22F | 0.21 | 0.24 | 0.085 | 0.64 | 0.13 | 0.49 | 0.51a | 0.003 | |
| 9V | Without 22F | 0.24 | 0.18 | 0.058 | 0.75 | 0.27 | 0.13 | 0.50 | 0.003 |
| With 22F | 0.24 | 0.18 | 0.08 | 0.66 | 0.29 | 0.11 | 0.57b | 0.0007 | |
| 14 | Without 22F | 0.79 | <0.0001 | 0.88 | <0.0001 | 0.50 | 0.004 | 0.75 | <0.0001 |
| With 22F | 0.68 | <0.0001 | 0.87 | <0.0001 | 0.48 | 0.005 | 0.74 | <0.0001 | |
| 23F | Without 22F | 0.037 | 0.84 | 0.10 | 0.57 | 0.084 | 0.65 | 0.28 | 0.12 |
| With 22F | 0.14 | 0.45 | 0.015 | 0.94 | 0.069 | 0.71 | 0.45c | 0.012 | |
P = 0.062, comparing post-PCV r value with post-PPV r value.
P = 0.026, comparing post-PCV r value with post-PPV r value.
P = 0.066, comparing post-PCV r value with post-PPV r value.
Values in bold indicate statistically significant correlation at a value of P < 0.05.
DISCUSSION
Our findings show that in HIV-infected adults a substantial amount of nonspecific antibodies are measured in the standardized IgG ELISA. This was true for four of the five serotypes tested, the exception being serotype 14. These findings confirm that absorption with a heterologous polysaccharide, such as serotype 22F, in order to remove these nonspecific antibodies is an important step in the ELISA for pneumococcal capsular antibodies in adults, including those who are HIV infected (5, 6, 24, 27).
Both children and adults have nonfunctional antibodies to pneumococcal C-PS. Because antibodies to C-PS can account for part of the measured antibody concentration in serotype-specific ELISAs, current ELISA protocols call for removal of these antibodies by absorption with C-PS prior to running the serotype-specific assay (20). However, more recent work has shown that even after absorption with C-PS, the ELISA often measures some quantity of nonfunctional, nonspecific antibodies (5, 6, 24, 27). It is unclear to what antigens these antibodies are targeted, but it has been hypothesized that they are directed towards common epitopes that link the C-PS with the polysaccharide capsule (6). There is conflicting evidence about whether nonspecific antibodies are present in infants as well as in adults. One study showed that these antibodies were not present in infants (6), while another showed that they were present, although in a lower concentration than in adults (24).
Other studies have shown that there is a decrease in the proportion of antibody that is nonspecific after vaccination with PPV in adults (6, 24) and PCV in infants (24), suggesting that the vaccines elicit more serotype-specific antibodies than nonspecific antibodies. In our study, there was some decrease in the relative amount of nonspecific antibodies after PCV, but not after PPV. In addition, the correlation between ELISA IgG concentrations and opsonophagocytic titers tended to improve after PCV but not PPV. Unlike in other studies (6, 25), in our study the removal of nonspecific antibodies with 22F absorption did not significantly improve the correlation between ELISA IgG concentrations and opsonophagocytic titers, although there was a trend towards improved correlation in the post-PCV sera. This lack of improvement in the correlation may be due to production of small amounts of functional serotype-specific capsular antibody or the diminished functional activity of these antibodies in HIV-infected individuals.
Individuals who had a low viral load had more nonspecific antibodies than did those with a high viral load, both before and after either vaccine. The amount of nonspecific antibodies in the low viral load group was similar to that found in non-HIV-infected immunocompetent adults (6). The reason for this difference based on viral load is unclear. We considered whether this finding might be related to differences in the degree of hypergammaglobulinemia that tends to develop fairly early in the course of HIV disease and then diminish as the disease progresses (14, 18). However, the finding that high and low viral load groups had similar total IgG concentrations would make this explanation unlikely. Both low and high viral load groups had total IgG concentrations higher than those reported in previous studies in immunocompetent adults (16).
The immune response varied by viral load more for PPV than for PCV. The low viral load group tended to have higher antibody concentrations after PPV than did the high viral load group. In contrast, the responses to PCV were similar between the two viral load groups, especially after 22F absorption. The response to PCV was significantly greater than that to PPV among the high viral load group, but not among the low viral load group. Other studies have also shown the immune response to PPV is better when a person's CD4 count is higher, when presumably the viral load is lower, except for soon after seroconversion (1, 13, 15, 21). Functional B-cell responses tend to show hyporesponsiveness in HIV disease independent of any T-cell defects, as shown by poor immunogenicity to T-cell-independent antigens (18). This finding may explain the poor response to PPV among HIV-infected persons, especially among those with worse virologic control. T-cell-dependent B-cell activation is also impaired in HIV disease (17, 18). However, the immune response to PCV, which because of conjugation with the CRM197 protein elicits a T-cell-dependent humoral response, did not vary by viral load group. This finding suggests that even in the high viral load group there might have been enough helper T-cell function to elicit an equivalent response. Our study did not include patients with CD4 counts of <200, and so we could not observe what happens with further depletion of CD4 T cells when T-cell-dependent B-cell responses might deteriorate more precipitously. This finding has been observed in other studies among HIV-infected adults, one with PCV and one with Haemophilus influenzae type b conjugate vaccine, in which the immune response to the conjugate vaccines in individuals with CD4 counts of <200 was poor, producing less antibodies than vaccination with the pneumococcal and H. influenzae type b polysaccharide vaccines, respectively (1, 26).
In conclusion, our study showed that nonspecific antipneumococcal antibodies are present in HIV-infected adults, although their presence may diminish in persons with higher viral loads. Future immunogenicity studies of the serotype-specific response to pneumococcal vaccines in HIV-infected adults should absorb out these antibodies with a heterologous polysaccharide, such as 22F, as part of the ELISA in order to better interpret the results.
Acknowledgments
We thank Sally Shupien and Barbara Rossman for study management at the Los Angeles site, Ericka Patrick and Marie Todd-Turner for study management at the Atlanta site, Steve McDougal and Trudy Dobbs for running the total IgG assays, Tajel Desai and Ian Lentnek for laboratory support, and John Walls for data entry.
This work was supported by The Opportunistic Infections Working Group, National Center for HIV, STD, and TB Prevention, the National Center for Infectious Diseases, and the National Vaccine Program Office, Centers for Disease Control and Prevention. We also thank Wyeth-Lederle for the donation of vaccines and provision of randomization codes.
REFERENCES
- 1.Ahmed, F., M. C. Steinhoff, M. C. Rodriguez-Barradas, R. G. Hamilton, D. M. Musher, and K. E. Nelson. 1999. Effect of human immunodeficiency virus type 1 infection on the antibody response to a glycoprotein conjugate pneumococcal vaccine: results from a randomized trial. J. Infect. Dis. 173:83-90. [DOI] [PubMed] [Google Scholar]
- 2.Black, S., H. Shinefield, B. Fireman, E. Lewis, P. Ray, J. R. Hansen, L. Elvin, K. M. Ensor, J. Hackell, G. Siber, F. Malinoski, D. Madore, I. Chang, R. Kohberger, W. Watson, R. Austrian, and K. Edwards. 2000. Efficacy, safety, and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr. Infect. Dis. J. 19:187-195. [DOI] [PubMed] [Google Scholar]
- 3.Black, S. B., H. R. Shinefield, S. Ling, J. Hansen, B. Fireman, D. Spring, J. Noyes, E. Lewis, P. Ray, J. Lee, and J. Hackell. 2002. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than five years of age for prevention of pneumonia. Pediatr. Infect. Dis. J. 21:810-815. [DOI] [PubMed] [Google Scholar]
- 4.Breiman, R. F., D. W. Keller, M. A. Phelan, D. H. Sniadack, D. S. Stephens, D. Rimland, M. M. Farley, A. Schuchat, and A. L. Reingold. 2000. Evaluation of effectiveness of the 23-valent pneumococcal capsular polysaccharide vaccine for HIV-infected patients. Arch. Intern. Med. 160:2633-2638. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 1997. Prevention of pneumococcal disease. Morb. Mortal. Wkly. Rep. 46:1-24. [Google Scholar]
- 6.Concepcion, N. F., and C. E. Frasch. 2001. Pneumococcal type 22f polysaccharide absorption improves the specificity of a pneumococcal-polysaccharide enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 8:266-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coughlin, R. T., A. C. White, C. A. Anderson, G. M. Carlone, D. L. Klein, and J. Treanor. 1998. Characterization of pneumococcal specific antibodies in healthy unvaccinated adults. Vaccine 16:1761-1767. [DOI] [PubMed] [Google Scholar]
- 8.Dworkin, M. S., J. W. Ward, D. L. Hanson, J. L. Jones, and J. E. Kaplan. 2001. Pneumococcal disease among human immunodeficiency virus-infected persons: incidence, risk factors, and impact of vaccination. Clin. Infect. Dis. 32:794-800. [DOI] [PubMed] [Google Scholar]
- 9.Eskola, J., T. Kilpi, A. Palmu, J. Jokinen, J. Haapakoski, E. Herva, A. Takala, H. Kayhty, P. Karma, R. Kohberger, G. Siber, and P. H. Makela. 2001. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 344:403-409. [DOI] [PubMed] [Google Scholar]
- 10.Feikin, D. R., C. M. Elie, M. B. Goetz, J. L. Lennox, G. M. Carlone, S. Romero-Steiner, P. F. Holder, W. A. O'Brien, C. G. Whitney, J. C. Butler, and R. F. Breiman. 2001. Randomized trial of the quantitative and functional antibody responses to a 7-valent pneumococcal conjugate vaccine and/or 23-valent polysaccharide vaccine among HIV-infected adults. Vaccine 20:545-553. [DOI] [PubMed] [Google Scholar]
- 11.French, N., J. Nakiyingi, L. M. Carpenter, E. Lugada, C. Watera, K. Moi, M. Moore, D. Antvelink, D. Mulder, E. N. Janoff, J. Whitworth, and C. F. Gilks. 2000. 23-Valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet 355:2106-2111. [DOI] [PubMed] [Google Scholar]
- 12.Gilks, C. F., S. A. Ojoo, J. C. Ojoo, R. J. Brindle, J. Paul, B. I. Batchelor, J. N. Kimari, R. Newnham, J. Bwayo, and F. A. Plummer. 1996. Invasive pneumococcal disease in a cohort of predominantly HIV-1 infected female sex-workers in Nairobi, Kenya. Lancet 347:718-723. [DOI] [PubMed] [Google Scholar]
- 13.Glaser, J. B., S. Volpe, A. Aguirre, H. Simpkins, and G. Schiffman. 1991. Zidovudine improves response to pneumococcal vaccine among persons with AIDS and AIDS-related complex. J. Infect. Dis. 164:761-764. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson, D. L., J. A. McCutchan, P. L. Spechko, I. Abramson, R. S. Smith, A. Bartok, G. R. Boss, D. Durand, S. A. Bozzette, S. A. Spector, and D. D. Richman. 1991. The evolution of lymphadenopathy and hypergammaglobulinemia are evidence for early and sustained polyclonal B lymphocyte activation during human immunodeficiency virus infection. J. Infect. Dis. 163:240-246. [DOI] [PubMed] [Google Scholar]
- 15.Janoff, E. N., J. M. Douglas, Jr., M. Gabriel, M. J. Blaser, A. J. Davidson, D. L. Cohn, and F. N. Judson. 1988. Class-specific antibody response to pneumococcal capsular polysaccharides in men infected with human immunodeficiency virus type 1. J. Infect. Dis. 158:983-990. [DOI] [PubMed] [Google Scholar]
- 16.Jolliff, C. R., K. M. Cost, P. C. Stivrins, P. P. Grossman, C. R. Nolte, S. M. Franco, K. J. Fijan, L. L. Fletcher, and H. C. Shriner. 1982. Reference intervals for serum IgG, IgA, IgM, C3, and C4 as determined by rate nephelometry. Clin. Chem. 28:126-128. [PubMed] [Google Scholar]
- 17.Lane, H. C., J. M. Depper, W. C. Greene, G. Whalen, T. A. Waldmann, and A. S. Fauci. 1985. Qualitative analysis of immune function in patients with the acquired immunodeficiency syndrome. Evidence for a selective defect in soluble antigen recognition. N. Engl. J. Med. 313:79-84. [DOI] [PubMed] [Google Scholar]
- 18.Lane, H. C., H. Masur, L. C. Edgar, G. Whalen, A. H. Rook, and A. S. Fauci. 1983. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 309:453-458. [DOI] [PubMed] [Google Scholar]
- 19.Nuorti, J., J. Butler, L. Gelling, J. L. Kool, A. L. Reingold, and D. J. Vugia. 2000. Epidemiologic relation between HIV and invasive pneumococcal disease in San Francisco County, California. Ann. Intern. Med. 132:182-190. [DOI] [PubMed] [Google Scholar]
- 20.Plikaytis, B. D., D. Goldblatt, C. E. Frasch, C. Blondeau, M. J. Bybel, G. S. Giebink, I. Jonsdottir, H. Kayhty, H. B. Konradsen, D. V. Madore, M. H. Nahm, C. A. Schulman, P. F. Holder, T. Lezhava, C. M. Elie, and G. M. Carlone. 2000. An analytical model applied to a multicenter pneumococcal enzyme-linked immunosorbent assay study. J. Clin. Microbiol. 38:2043-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodriguez-Barradas, M. C., D. M. Musher, C. Lahart, C. Lacke, J. Groover, D. Watson, R. Baughn, T. Cate, and G. Crofoot. 1992. Antibody to capsular polysaccharides of Streptococcus pneumoniae after vaccination of human immunodeficiency virus-infected subjects with 23-valent pneumococcal vaccine. J. Infect. Dis. 165:553-556. [DOI] [PubMed] [Google Scholar]
- 22.Romero-Steiner, S., D. Libutti, L. B. Pais, J. Dykes, P. Anderson, J. C. Whitin, H. L. Keyserling, and G. M. Carlone. 1997. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin. Diagn. Lab. Immunol. 4:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romero-Steiner, S., D. Musher, and M. Cetron. 1999. Reduction in functional antibody activity against Streptococcus pneumoniae in vaccinated elderly individuals highly correlates with decreased IgG antibody avidity. Clin. Infect. Dis. 29:281-288. [DOI] [PubMed] [Google Scholar]
- 24.Soininen, A., G. van den Dobbelsteen, L. Oomen, and H. Kayhty. 2000. Are the enzyme immunoassays for antibodies to pneumococcal capsular polysaccharides serotype specific? Clin. Diagn. Lab. Immunol. 7:468-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soininen, A., M. Karpala, S. Wahlman, H. Lehtonen, and H. Kayhty. 2002. Specificities and opsonophagocytic activities of antibodies to pneumococcal capsular polysaccharides in sera of unimmunized young children. Clin. Diagn. Lab. Immunol. 9:1032-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinhoff, M. C., B. S. Auerbach, K. E. Nelson, D. Vlahov, R. L. Becker, N. M. Graham, D. H. Schwartz, A. H. Lucas, and R. E. Chaisson. 1991. Antibody responses to Haemophilus influenzae type B vaccines in men with human immunodeficiency virus infection. N. Engl. J. Med. 325:1837-1842. [DOI] [PubMed] [Google Scholar]
- 27.Yu, X., Y. Sun, C. Frasch, N. Concepcion, and M. H. Nahm. 1999. Pneumococcal capsular polysaccharide preparations may contain non-C-polysaccharide contaminants that are immunogenic. Clin. Diagn. Lab. Immunol. 6:519-524. [DOI] [PMC free article] [PubMed] [Google Scholar]