Abstract
Leukocyte recruitment in the liver includes a two-step procedure in which selectin-dependent leukocyte rolling is a prerequisite for subsequent CD18-dependent leukocyte firm adhesion in postsinusoidal venules. However, the roles of the individual selectins in leukocyte rolling and adhesion, hepatocellular injury, and apoptosis remain elusive. Therefore, we examined the pathophysiological role of P-, E-, and L-selectin in male C57BL/6 mice challenged with lipopolysaccharide (LPS) and d-galactosamine (Gal) by use of intravital microscopy of the liver microcirculation. In control animals, administration of LPS-Gal provoked reproducible hepatic damage, including marked increases of leukocyte recruitment, liver enzymes, and hepatocyte apoptosis and reduced sinusoidal perfusion. Interestingly, pretreatment with an anti-P-selectin antibody (RB40.34) markedly reduced leukocyte rolling and firm adhesion by 65 and 71%, respectively. Moreover, interference with P-selectin function significantly improved sinusoidal perfusion and reduced the increase in liver enzymes by 49 to 84% in endotoxemic mice. Moreover, the activity of caspase-3 and the number of apoptotic hepatocytes were significantly reduced by 55 and 54%, respectively, in RB40.34-treated animals. In contrast, administration of an anti-E-selectin antibody (10E9.6) and an anti-L-selectin antibody (Mel-14) did not protect against endotoxin-induced leukocyte responses or hepatic injury. In conclusion, our novel findings document a principal role of P-selectin in mediating leukocyte rolling, a precondition to the subsequent firm adhesion of leukocytes in liver injury. Furthermore, our novel data demonstrate that inhibition of P-selectin function reduces hepatocellular injury and apoptosis, suggesting a causal relationship between leukocyte recruitment on one hand and hepatocellular injury and apoptosis on the other hand. Based on these findings, it is suggested that P-selectin may be an important therapeutic target in endotoxin-induced liver injury.
Activation and recruitment of leukocytes are features associated with liver injury in, e.g., ischemia-reperfusion and endotoxemia (10, 12, 23). In general, studies of various tissues have shown that infiltration of leukocytes involves a multistep cascade in which the initial rolling interaction between leukocytes and the endothelium is predominately mediated by the selectin family of adhesion molecules, including P-, E-, and L-selectin (1, 15, 20, 31). Subsequent firm adhesion of leukocytes to the endothelium is dependent on the function of β1- and β2-integrins (CD11/18) on leukocytes, interacting with members of the immunoglobulin supergene family expressed on activated endothelial cells, such as ICAM-1 and -2 and VCAM-1 (1, 12, 15, 16, 20, 21, 31).
However, the literature on the specific roles of P-, E-, and L-selectin and the relationship between leukocyte recruitment, cellular injury, and apoptosis is contradictory. For example, when blood flow velocity and shear rates are reduced, it has been suggested that leukocytes may bypass the selectins and roll on β2-integrins (27). In fact, a recent study forwarded the possibility that leukocyte recruitment in the liver, which is a low-flow organ, may be selectin independent (34). Nonetheless, we have recently demonstrated that leukocyte rolling is selectin dependent and a precondition for the subsequent firm adhesion in tumor necrosis factor alpha (TNF-α)-galactosamine-challenged rats by using fucoidan, a polysaccharide which blocks the function of both L- and P-selectin (15). On the other hand, E-selectin is expressed on hepatic endothelial cells in inflammation (3), and blocking of E-selectin has been suggested to protect against liver injury by reducing neutrophil accumulation (17). Thus, the role of the individual selectins in leukocyte-endothelium interactions in hepatic microcirculation is not fully understood.
The sequential relationship between hepatocellular apoptosis and leukocyte recruitment has also been debated. In hepatic ischemia-reperfusion, it has been demonstrated that leukocyte recruitment precedes apoptosis, whereas studies with endotoxemic mice have suggested that apoptosis per se triggers leukocyte recruitment (18).
Based on these considerations, the objective of the present study was to examine the pathophysiological roles of P-, E-, and L-selectin in leukocyte-endothelium interactions induced by lipopolysaccharide (LPS) and d-galactosamine (Gal). Moreover, we wanted to define the relationship between three features of endotoxemia, namely leukocyte recruitment, hepatocellular injury, and apoptosis. For this purpose, we used intravital microscopy to visualize leukocyte-endothelium interactions in the liver microcirculation of mice challenged with LPS-Gal after pretreatment with monoclonal antibodies against P-, E-, or L-selectin.
MATERIALS AND METHODS
Animals.
Adult male C57BL/6 mice weighing 23 to 27 g were kept on a 12-h light-dark cycle with free access to food and tap water. Animals were anesthetized by intraperitoneal (i.p.) administration of 7.5 mg of ketamine hydrochloride and 2.5 mg of xylazine per 100 mg of body weight. The right jugular vein was cannulated with a polyethylene catheter for intravenous administration of test substances, fluorescent dyes, and additional anesthesia. The local ethics committee approved all of the experiments in this study.
Experimental protocol.
Six hours prior to surgery and intravital observation, mice were pretreated with phosphate-buffered saline (PBS; 0.2 ml) or anti-P-selectin antibody (RB40.34; 40 μg/mouse), anti-E-selectin antibody (10E9.6; 40 μg/mouse), or anti-L-selectin antibody (Mel-14; 40 μg/mouse) dissolved in 0.2 ml of PBS intravenous by injection into a lateral tail vein immediately followed by i.p. administration of 0.25 ml of PBS (control animals) or a combination of LPS (10 μg/mouse) and Gal (18 mg/mouse) dissolved in PBS to a total volume of 0.25 ml.
Surgical procedure.
In anesthetized animals, a transverse subcostal incision was performed and the ligamentous attachments from the liver to the diaphragm and abdominal wall were gently released. The animals were positioned on their left sides and the left liver lobes were carefully exteriorized onto an adjustable stage for analysis of hepatic microcirculation by intravital fluorescence microscopy. The liver surface was covered with a circular glass to avoid tissue drying and exposure to ambient oxygen. An equilibration period of 5 min was allowed before microscopic observation started.
After intravital observations, animals were killed by exsanguination and blood drawn from the heart was used for analysis of liver function tests by standard spectrophotometric procedures. Systemic leukocyte counts, including polymorphonuclear leukocytes (PMNL) and monomorphonuclear leukocytes (MNL) were determined with a hematocytometer. After gently rinsing a piece of the right liver lobe in PBS, it was frozen for subsequent analysis of caspase-3 protease activity.
Intravital microscopy.
For observations of liver microcirculation, we used a modified Olympus microscope (BX50WI; Olympus Optical Co. GmbH, Hamburg, Germany) equipped with different water immersion lenses (×40 objective, numeric aperture 0.75; ×63 objective, numeric aperture 0.9). The image was televised (Sony Trinitron) with a charge-coupled device video camera (FK 6990 Cohu; Pieper GmbH, Schwerte, Germany) and recorded on videotape (Panasonic SVT-S3000 S-VHS recorder) for subsequent off-line evaluation. Blood perfusion within individual microvessels was studied after contrast enhancement by fluorescein isothiocyanate-dextran (0.1 ml, 0.1 mg/ml). In vivo labeling of leukocytes with rhodamine-6G (0.1 ml, 0.05 mg/ml) enabled quantitative analysis of leukocyte flow behavior in both sinusoids and postsinusoidal venules. Quantification of microcirculatory parameters was performed off-line by frame-to-frame analysis of the videotaped images. Five postsinusoidal venules with connecting sinusoids were evaluated in each animal. Microcirculatory analysis included determination of the number of perfused sinusoids as a percentage of the total number of sinusoids observed (named sinusoidal perfusion). Within postsinusoidal venules, leukocyte rolling was measured by counting the number of cells rolling in the venule during a 30-s period and is expressed as a number of cells per minute. Leukocyte adhesion was measured by counting the number of cells that adhered along the venular endothelium and remained stationary during the observation period of 30 s and is expressed as the number cells per millimeter of venule length. The diameters of the venules were not different between the experimental groups. Hepatocyte apoptosis was measured in the same microscopic setup as above. For this purpose, the DNA fluorochrome Hoechst 33342 (0.02 ml, 0.2 μg/ml) was topically applied onto the liver surface for staining. Hoechst 33342 is a fluorescent dye that has been widely used for analysis of nuclear morphology and features of apoptosis, e.g., nuclear condensation and fragmentation in cultured hepatocytes and endothelial cells (29). After exsanguination and 10 min of incubation, six microscopic fields (using a ×63 objective water immersion lens) were recorded for off-line quantification of hepatocyte nuclei showing signs of apoptosis (chromatin condensation and fragmentation). Hepatocyte apoptosis is given as a percentage determined from the number of hepatocyte nuclei showing apoptotic features divided by the total number of hepatocyte nuclei observed.
Measurement of caspase-3 protease activity.
Caspase-3 is an intracellular cysteine protease that becomes activated during the cascade of events associated with and required for the execution of apoptosis (6). Caspase-3 protease activity in the liver tissue was measured by using a caspase-3 colorimetric assay kit according to the manufacturer's instructions. Briefly, after homogenization of whole-liver tissue in cell lysis buffer, homogenates were centrifuged for 1 min at 10,000 × g, and the supernatant was incubated with DEVD-pNA and reaction buffer for 1.5 h at 37°C. Levels of the chromophore pNA released by caspase-3 activity were quantified spectrophotometrically. The data are given as increases (n-fold) in caspase-3 activity of test livers relative to PBS-treated control livers.
RT-PCR.
Three hours after challenge with PBS or LPS-Gal, total RNA was extracted from whole-liver tissue by using an acid guanidinium-phenol-chloroform method (TRIzol reagent) and treated with RNase-free DNase (DNase I) according to the manufacturer's protocol to remove potential contamination from genomic DNA. RNA concentrations were determined by measuring the absorbance spectrophotometrically at 260 nm. Reverse transcriptase PCR (RT-PCR) was performed with the SuperScript one-step RT-PCR system. Each reaction contained 500 ng of total liver RNA as a template and 0.2 μM consentrations of each primer in a final volume of 50 μl. Mouse β-actin served as an internal control gene. The RT-PCR profile was 1 cycle of DNA synthesis at 50°C for 30 min followed by 40 cycles of denaturation at 94°C for 30 s, annealing at 55°C, and extension at 72°C for 1 min and 1 cycle of final extension at 72°C for 10 min. After RT-PCR, aliquots of the RT-PCR products were separated on a 2% agarose gel containing ethidium bromide and photographed. The primer sequences for TNF-α, P-selectin, E-selectin, and β-actin were as follows: TNF-α forward (f), 5′-GGC AGG TCT ACT TTG GAG TCA TTG C-3′; TNF-α reverse (r), 5′-ACA TTC GAG GCT CCA GTG AAT TCG G-3′; P-selectin f, 5′-ACG AGC TGG ACG GAC CCG-3′; P-selectin r, 5′-GGC TGG CAC TCA AAT TTA CAG-3′; E-selectin f, 5′-GGT AGT TGC ACT TTC TGC GG-3′; E-selectin r, 5′-CCT TCT GTG GCA TGT TC-3′; β-actin f, 5′-ATG TTT GAG ACC TTC AAC ACC-3′; β-actin r, 5′-TCT CCA GGG AGG AAG AGG AT-3′.
Materials.
Fluorescein isothiocyanate-dextran, Gal, LPS (from Escherichia coli), and rhodamine-6G were purchased from Sigma Chemical Co., St. Louis, Mo. Ketamine hydrochloride was from Hoffman-La Roche, Basel, Switzerland. Xylazine was from Janssen Pharmaceutica, Beerse, Belgium. Monoclonal antibodies directed against mouse P-selectin (RB40.34), E-selectin (10E9.6), and L-selectin (Mel-14) were from Pharmingen, San Diego, Calif. Hoechst 33342 was from Molecular Probes, Leiden, The Netherlands. The caspase-3 colorimetric assay kit was from R & D Systems Europe, Ltd., Abingdon, Oxon, United Kingdom. TRIzol reagent and SuperScript one-step RT-PCR system were from GIBCO-BRL Life Technologies, Grand Island, N.Y. DNase I was from Amersham Pharmacia Biotech, Uppsala, Sweden.
Statistical analyses.
Data are presented as mean values ± standard errors of the means (SEM). Statistical evaluations were performed by using Kruskal-Wallis one-way analysis of variance on ranks followed by multiple comparisons versus the control group (Dunn's method). P values of <0.05 were considered significant, and n represents the number of animals.
RESULTS
Expression of TNF-α and P- and E-selectin in the liver.
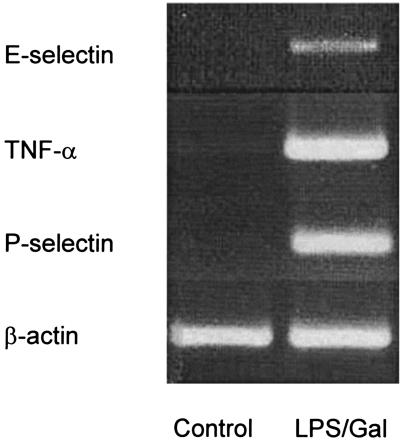
To investigate the effect of LPS-Gal challenge on the expression of TNF-α and P- and E-selectin in the liver, total RNA was isolated from livers of PBS-treated controls and LPS-Gal-treated animals. The RNA was reversely transcribed into cDNA and PCR amplified with specific primers. As shown in Fig. 1, TNF-α or P- or E-selectin mRNA was not detectable in PBS-treated control animals. Indeed, the mRNA expression of P-selectin was stronger than that of E-selectin (Fig. 1). In contrast, treatment with LPS-Gal induced a clear-cut expression of TNF-α, P-selectin, and E-selectin mRNA in the liver. β-Actin was similarly expressed in control and endotoxemic animals.
FIG. 1.
Expression of TNF-α and P- and E-selectin mRNA in the liver 3 h after i.p. administration of PBS (control) or LPS-Gal. β-Actin served as a housekeeping gene. The results presented are representative of four experiments performed.
Leukocyte-endothelium interactions in the liver.
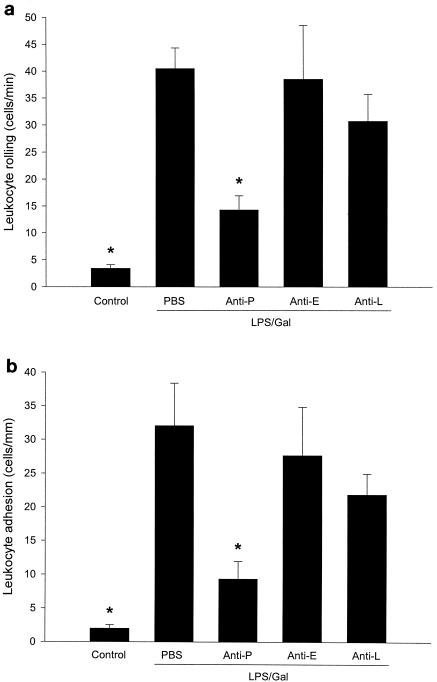
In PBS-treated control animals, it was found that the number of rolling and adherent leukocytes was 3.4 ± 1 cells per min and 2 ± 0.5 cells per mm of venule length, respectively (Fig. 2). Challenge with the combination of LPS (10 μg) and Gal (18 mg) for 6 h markedly increased leukocyte responses in the postsinusoidal venules. In fact, challenge with LPS-Gal increased venular leukocyte rolling by 12-fold, i.e., to 40.4 ± 3.9 cells per min. Moreover, this treatment enhanced firm leukocyte adhesion in venules by 16-fold, i.e., to 32 ± 6.3 cells per mm of venule length (Fig. 2) (P < 0.05 versus PBS, n = 5 to 7). Interestingly, administration of the anti-P-selectin antibody (RB40.34) significantly reduced leukocyte rolling by 65%, i.e., the number of rolling leukocytes decreased to 14.2 ± 2.7 cells per min (Fig. 2a) (P < 0.05 versus LPS-Gal plus PBS, n = 7 to 9), whereas the anti-E-selectin antibody and the anti-L-selectin antibody had no effect (Fig. 2a) (P > 0.05 versus LPS-Gal plus PBS, n = 5). These results suggest that LPS-induced leukocyte rolling in the liver is predominately mediated by P-selectin. Moreover, anti-P-selectin antibody pretreatment significantly reduced LPS-induced leukocyte adhesion by 71%, i.e., to 9.3 ± 2.6 cells per mm of venule length (Fig. 2b) (P < 0.05 versus LPS-Gal plus PBS, n = 7 to 9). Again, neither the anti-E- nor the anti-L-selectin antibody altered leukocyte adhesion. Importantly, administration of LPS-Gal, the anti-P-selectin antibody, and the anti-E-selectin antibody had no effect on systemic leukocyte counts (Table 1) (P > 0.05 versus PBS). However, injection of the anti-L-selectin antibody induced leukopenia at 6 h, reducing mononuclear leukocytes from 3.5 × 106 ± 0.5 × 106 to 1.4 × 106 ± 0.3 × 106 cells/ml and polymorphonuclear leukocytes from 1.9 × 106 ± 0.5 × 106 to 0.9 × 106 ± 0.4 × 106 cells/ml (Table 1) (P < 0.05 versus PBS, n = 5).
FIG. 2.
Leukocyte rolling (a) and firm adhesion (b) in hepatic postsinusoidal venules 6 h after treatment with a combination of LPS (10 μg) and Gal (18 mg) and monoclonal antibodies against P-selectin (RB40.34, 40 μg; anti-P), E-selectin (10E9.6, 40 μg; anti-E), or L-selectin (Mel-14, 40 μg; anti-L) or PBS. Control animals received PBS. Data represent means ± SEM. Asterisks indicate significant differences versus challenge with LPS-Gal plus PBS (P < 0.05).
TABLE 1.
Systemic leukocyte countsa
| Challenge | No. of:
|
||
|---|---|---|---|
| MNL | PMNL | Total cells | |
| PBS (control) | 3.5 ± 0.5 | 1.9 ± 0.4 | 5.4 ± 0.8 |
| LPS-Gal + PBS | 3.1 ± 0.4 | 1.5 ± 0.3 | 4.5 ± 0.5 |
| Anti-P + LPS-Gal | 3.7 ± 0.3 | 1.7 ± 0.5 | 5.4 ± 0.7 |
| Anti-E + LPS-Gal | 4.2 ± 0.7 | 2.1 ± 0.5 | 6.3 ± 0.8 |
| Anti-L + LPS-Gal | 1.4 ± 0.3* | 0.9 ± 0.4* | 2.3 ± 0.5* |
Leukocytes were counted in a hematocytometer and were defined as MNL or PMNL. Animals were challenged with PBS (control), LPS-Gal plus PBS, or LPS-Gal plus antibodies against P-, E-, or L-selectin (anti-P, -E, and -L, respectively). Data are given as means ± SEM and represent 106 cells/milliliter. Asterisks indicate significant differences versus challenge with LPS-Gal plus PBS.
Sinusoidal perfusion and sequestration of leukocytes.
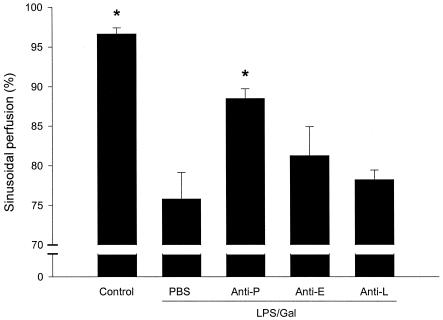
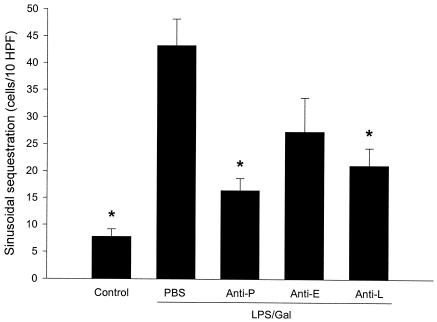
LPS-Gal induced a hepatic damage that markedly reduced sinusoidal perfusion from 96.6% ± 1% to 76% ± 3% (Fig. 3) (P < 0.05 versus PBS, n = 5). Administration of the anti-P-selectin antibody improved sinusoidal perfusion to 88% ± 1% (Fig. 3) (P < 0.05 versus LPS-Gal plus PBS, n = 9). In line with this, the number of leukocytes sequestered in sinusoids increased from 7.8 ± 1.4 cells/10 high-power fields (HPF) at the baseline to 43.2 ± 4.9 cells/10 HPF 6 h after LPS-Gal challenge. Pretreatment with the anti-P-selectin antibody reduced LPS-Gal-induced sinusoidal leukocyte sequestration down to 16.4 ± 2 cells/10 HPF (Fig. 4) (P < 0.05 versus LPS-Gal plus PBS, n = 10). Interestingly, pretreatment with the anti-L-selectin antibody also significantly reduced sinusoidal sequestration of leukocytes to 21 ± 3.2 cells/10 HPF (Fig. 4) (P < 0.05 versus LPS-Gal, n = 5), which was probably due to the concomitant reduction of circulating leukocytes. The anti-E-selectin antibody had no effect on LPS-Gal-induced perfusion failure or leukocyte sequestration in the liver sinusoids (Fig. 3 and 4) (P > 0.05 versus LPS-Gal, n = 5).
FIG. 3.
Sinusoidal perfusion in the murine liver 6 h after treatment with a combination of LPS (10 μg) and Gal (18 mg) and monoclonal antibodies against P-selectin (RB40.34, 40 μg; anti-P), E-selectin (10E9.6, 40 μg; anti-E), or L-selectin (Mel-14, 40 μg; anti-L) or PBS. Control animals received PBS. Sinusoidal perfusion is given as the percentage of observed sinusoids with functional perfusion. Data represent means ± SEM. Asterisks indicate significant differences versus challenge with LPS-Gal plus PBS (P < 0.05).
FIG. 4.
Leukocyte sequestration in hepatic sinusoids 6 h after treatment with a combination of LPS (10 μg) and Gal (18 mg) and monoclonal antibodies against P-selectin (RB40.34, 40 μg; anti-P), E-selectin (10E9.6, 40 μg; anti-E), or L-selectin (Mel-14, 40 μg; anti-L) or PBS. Control animals received PBS. Leukocyte sequestration is given as the number of leukocytes sequestered in sinusoids within 10 HPF. Data represent means ± SEM. Asterisks indicate significant differences versus challenge with LPS-Gal plus PBS (P < 0.05).
Liver enzymes, hepatocyte apoptosis, and caspase-3 protease activity.
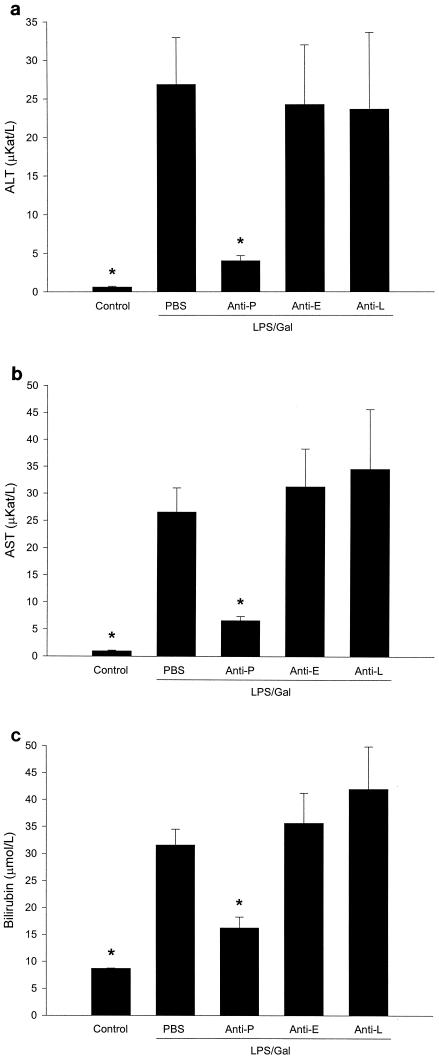
We found that challenge with LPS-Gal provoked a serious injury to the liver, illustrated by the increase in liver enzymes and apoptosis. As shown in Fig. 5, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were increased by more than 42- and 27-fold, respectively, and bilirubin was increased by almost 3-fold. Interestingly, the anti-P-selectin antibody protected against the increase in liver enzymes induced by LPS-Gal, i.e., ALT was significantly reduced by 85% (from 26.8 ± 6.1 to 4.1 ± 0.7 μkat/liter), AST was reduced by 75% (from 26.5 ± 4.4 to 6.6 ± 0.8 μkat/liter), and bilirubin was reduced by 49% (from 31.5 ± 2.9 to 16.2 ± 2 μmol/liter) (Fig. 5) (P < 0.05 versus LPS-Gal plus PBS, n = 6 to 15). In contrast, immunoneutralization of E- and L-selectin had no effect on liver enzymes in animals treated with LPS-Gal (Fig. 5).
FIG. 5.
Liver enzymes 6 h after treatment with a combination of LPS (10 μg) and Gal (18 mg) and monoclonal antibodies against P-selectin (RB40.34, 40 μg; anti-P), E-selectin (10E9.6, 40 μg; anti-E), or L-selectin (Mel-14, 40 μg; anti-L) or PBS. Control animals received PBS. The levels of ALT (a), AST (b), and bilirubin (c) were determined spectrophotometrically. Data represent means ± SEM. Asterisks indicate significant differences versus challenge with LPS-Gal plus PBS (P < 0.05).
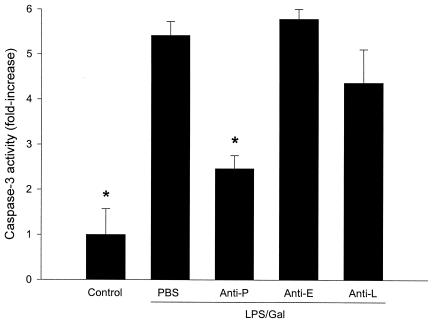
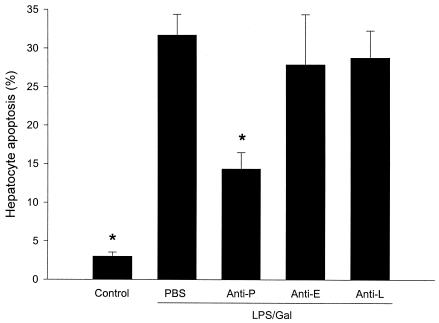
Hepatocyte apoptosis may play a major role in the development of liver failure associated with endotoxin shock (11). Herein, we evaluated apoptosis by two different methods. Activation of the caspase-3 protease is an important step in hepatic parenchymal cell apoptosis. Indeed, we found that caspase-3 activity in the liver increased by more than fivefold in animals treated with LPS-Gal (Fig. 6) (P < 0.05 versus PBS, n = 5). Interestingly, this increase in caspase-3 activity was reduced by 54% in animals pretreated with the anti-P-selectin antibody (Fig. 6) (P < 0.05 versus LPS-Gal plus PBS, n = 5). Neither administration of anti-E-selectin nor anti-L-selectin antibodies reduced LPS-Gal-induced caspase-3 activity (Fig. 6). A similar pattern was found when hepatocyte nuclei were stained with the fluorescent dye Hoechst 33342. In control animals, 3.0 ± 1.2% of the nuclei observed showed apoptotic features (Fig. 7). Challenge with LPS-Gal increased the percentage of apoptotic hepatocytes to 31 ± 2.7%. Importantly, administration of the anti-P-selectin antibody reduced LPS-Gal-induced apoptosis to 14.3 ± 2.1% (Fig. 7) (P < 0.05 versus LPS-Gal plus PBS, n = 10 to 11). Pretreatment with the anti-E- or anti-L-selectin antibody had no effect on LPS-Gal-induced hepatocyte apoptosis (Fig. 7).
FIG. 6.
Activity of the protease caspase-3 in the liver 6 h after treatment with a combination of LPS (10 μg) and Gal (18 mg) and monoclonal antibodies against P-selectin (RB40.34, 40 μg; anti-P), E-selectin (10E9.6, 40 μg; anti-E), or L-selectin (Mel-14, 40 μg; anti-L) or PBS. Control animals received PBS. Caspase-3 activity is given as the increase (n-fold) compared to PBS-treated controls. Data represent means ± SEM. Asterisks indicate significant differences versus challenge with LPS-Gal plus PBS (P < 0.05).
FIG. 7.
Apoptosis of hepatocytes 6 h after treatment with a combination of LPS (10 μg) and Gal (18 mg) and monoclonal antibodies against P-selectin (RB40.34, 40 μg; anti-P), E-selectin (10E9.6, 40 μg; anti-E), or L-selectin (Mel-14, 40 μg; anti-L) or PBS. Control animals received PBS. Hepatocyte apoptosis is given as the percentage of observed hepatocyte nuclei with morphological signs of apoptosis, i.e., chromatin condensation and fragmentation, after administration of the fluorochrome Hoechst 33342. Data represent means ± SEM. Asterisks indicate significant differences versus challenge with LPS-Gal plus PBS (P < 0.05).
DISCUSSION
This study demonstrates an instrumental role of leukocytes in endotoxemia-induced liver injury and provides evidence that hepatic recruitment of leukocytes is predominately mediated by P-selectin. We found that inhibition of P-selectin-dependent leukocyte rolling abolished not only subsequent firm adhesion of leukocytes but also the increase in liver enzymes and hepatocellular apoptosis in endotoxemic liver damage. Thus, these findings contribute to the understanding of the regulatory mechanisms in endotoxemia-induced leukocyte recruitment and cellular injury, which may be important for the development of specific therapies directed against pathological inflammation in the liver.
Leukocyte rolling is the initial step in the leukocyte recruitment process, which is mediated by the selectin family of adhesion molecules (1, 15, 16, 20, 31). The interplay between selectins and their high-affinity ligands results in the capture of free-flowing leukocytes followed by a rolling adhesive interaction along the microvascular endothelium (33). By use of the polysaccharide fucoidan, which blocks the function of P- and L-selectin, it was recently shown that leukocyte rolling is predominately mediated by the selectin family of adhesion molecules in postsinusoidal venules in response to endotoxemia (15). Herein, we expand on these findings by defining the individual roles of P-, E- and L-selectin in endotoxin-induced leukocyte rolling in postsinusoidal venules of the liver. We found that functional interference with P-selectin reduced the number of rolling leukocytes by more than 65%, suggesting that P-selectin is a dominant adhesion molecule in supporting venular leukocyte rolling in the liver provoked by LPS. Moreover, we observed that inhibition of P-selectin function not only abolished leukocyte rolling but also concomitantly completely inhibited endotoxemia-induced firm adhesion of leukocytes, indicating that P-selectin-dependent rolling is indeed a precondition for LPS-induced leukocyte adhesion in postsinusoidal venules. This notion is in line with previous findings showing that pretreatment with fucoidan inhibits both leukocyte rolling and firm adhesion, whereas late treatment with fucoidan only inhibits leukocyte rolling (15, 22).
On the other hand, we found that immunoneutralization of E- and L-selectin had no effect on leukocyte-endothelium interactions in the liver in response to LPS-Gal, indicating that E- and/or L-selectin does not play a redundant role endotoxemia-induced leukocyte rolling in the liver. Nonetheless, we observed, herein, that the anti-P-selectin antibody did not completely abolish leukocyte recruitment, which correlates well with our recent data on fucoidan and TNF-α-induced leukocyte rolling and recruitment (15). In the absence of a pathophysiological importance for E- and L-selectin as noted in the present study, it may be suggested that other pathways are involved in the leukocyte recruitment process in the liver. Indeed, a previous study indicated a functional importance of the α4-integrin and its receptor VCAM-1 (7). Thus, Fox-Robichaud and Kubes (7) suggested that leukocytes use α4-integrins for rolling and adhesion in hepatic venules independently of selectins. Furthermore, it has been reported that functional blocking of VCAM-1 may protect hepatocytes against neutrophil-induced liver injury in endotoxemia (4). Thus, it is possible that an α4-VCAM-1-mediated pathway may support the residual level (30 to 35%) of leukocyte recruitment, as observed herein after immunoneutralization of P-selectin.
Leukocytes accumulate not only in postsinusoidal venules but also in the sinusoids in the liver (2, 14). In fact, Chosay et al. (2) have forwarded the hypothesis that sinusoidal sequestration of leukocytes significantly contributes to endotoxemic liver injury. Indeed, we found an increased number of leukocytes arrested in the liver sinusoids in response to LPS. Interestingly, immunoneutralization of P-selectin markedly attenuated sinusoidal sequestration of leukocytes (62% reduction). However, this inhibitory impact of the anti-P-selectin antibody is not likely attributable to an anti-adhesive effect against leukocyte-endothelium interactions in the sinusoids, knowing that P-selectin is not expressed on the sinusoidal endothelium (5). Instead, leukocytes may be mechanically trapped in the sinusoids due to the fact that systemic activation, such as endotoxemia, reduces the deformability of circulating leukocytes and that the parenchymal injury and associated edema may increase the extravascular pressure and thereby reduce sinusoidal perfusion and diameters in the liver (24). Thus, the inhibitory effect exerted by the anti-P-selectin antibody on sinusoidal sequestration of leukocytes may be explained as a secondary phenomenon to the overall attenuation of the inflammatory response in endotoxemia as shown in the present study. Nonetheless, our data indicate that leukocyte recruitment in postsinusoidal venules is the initial and pivotal event causing an escalated hepatic injury, including leukocyte sequestration and decreased microvascular perfusion in sinusoids.
Endotoxin-induced liver damage may be mediated by leukocyte recruitment and by direct actions of soluble mediators (i.e., TNF-α). In the present study, we found that inhibition of P-selectin not only reduced leukocyte rolling and recruitment by more than 65 to 70% but also reduced liver injury in endotoxemic mice. In fact, administration of the anti-P-selectin antibody decreased liver enzymes by 49 to 85%, which corresponds well with the inhibitory effect on leukocyte accumulation. These findings suggest that leukocytes constitute the predominant effector arm in endotoxin-provoked liver injury, although potent compounds, such as TNF-α, are expressed in the liver and secreted into the circulation (3, 26) with the capacity to induce hepatocellular apoptosis. This notion is supported by a previous study by Hewett and coworkers (9), showing that neutrophil depletion markedly attenuates LPS-Gal-provoked liver injury. In line with the lack of effect of E- and L-selectin immunoneutralization on leukocyte rolling and adhesion, no protection from liver injury was observed after administration of antibodies against E- and L-selectin. Thus, our data demonstrate for the first time that functional interference with P-selectin protects against endotoxin-induced liver damage via inhibition of leukocyte rolling. It is also noteworthy that several studies have shown that nitric oxide exerts a protective effect in endotoxin-induced liver injury (8, 25) and that nitric oxide has been reported to inhibit P-selectin-dependent leukocyte-endothelial cell interactions (11). In this context, it should be mentioned that our findings are in contrast to those of a previous study, which reported that P-selectin plays no major role in liver injury in endotoxin shock (5). The exact reason for this discrepancy is not known at present but may be related to the fact that Essani et al. (5) used a Salmonella toxin in C3Heb/FeJ mice, whereas we used LPS derived from E. coli in C57/BL6 mice. In fact, it has been shown that the effectiveness of a specific antibody may be completely dependent on the type of mouse species used (28). Our data, showing that inhibition of P-selectin protects against LPS-Gal-induced liver injury, add endotoxemia to the list of conditions being protected by immunoneutralization of P-selectin, including models of hepatic injury induced by hemorrhage-reinfusion and ischemia-reperfusion (30, 35). Based on the experiments with anti-P-selectin antibody, the relative importance of sinusoidal and postsinusoidal leukocytes cannot be determined because immunoneutralization of P-selectin inhibited leukocyte accumulation in both microvascular sections. On the other hand, injection of the anti-L-selectin antibody reduced leukocyte sequestration in sinusoids by 51% but exerted no influence on leukocyte adhesion in postsinusoidal venules. This reduction was likely due to the significant neutropenia induced by this antibody (32). The lack of effect on liver damage of the anti-L-selectin treatment observed herein is in line with a previous study with an endotoxin shock model (17). Nonetheless, it is interesting that this anti-L-selectin antibody-mediated reduction of sinusoidal sequestration of leukocytes did not ameliorate the hepatic injury, indicating that it may be necessary to inhibit leukocyte accumulation in postsinusoidal venules to protect the liver in endotoxemia.
Numerous studies have demonstrated that, Gal-sensitized mice, LPS induces hepatocyte apoptosis, which is characterized by chromatin condensation, DNA fragmentation, and formation of intracellular apoptotic bodies (13, 18, 19). In this study, we used a DNA-binding fluorochrome, Hoechst 33342, which has been validated for determining morphological features of apoptosis in hepatocytes in vitro (29). We found that topical application of Hoechst 33342 onto the liver surface enabled quantitative analysis of apoptosis (i.e., chromatin condensation and fragmentation) by intravital microscopy in vivo. This approach based on Hoechst 33342 was validated by measurement of the liver content of caspase-3 protease activity, which demonstrated excellent correspondence. Using these two methods, we observed that the level of hepatic apoptosis increased markedly in endotoxemic animals. Interestingly, administration of the anti-P-selectin antibody reduced endotoxin-induced apoptosis by more than 54%, suggesting that P-selectin-dependent recruitment of leukocytes significantly contributes to apoptosis in this model of endotoxin shock.
Taken together, our novel data demonstrate that leukocyte rolling in postsinusoidal venules is a predominately P-selectin-dependent event and a precondition for the subsequent recruitment of leukocytes in response to LPS-Gal. Moreover, this investigation shows that functional interference with P-selectin not only abolishes leukocyte accumulation but also attenuates hepatocellular injury, apoptosis, and microvascular dysfunction in endotoxemic mice. These findings help explain the adhesive pathways supporting leukocyte recruitment in the liver and elucidate the important role of the leukocyte response in endotoxin-induced liver injury. Thus, it may be suggested that functional targeting of P-selectin may constitute a therapeutic approach against leukocyte-mediated hepatic injury.
Acknowledgments
We thank Yusheng Wang for excellent technical assistance.
This work was supported by grants from the Swedish Medical Research Council (K2000-4P-13411-01A and K2002-73-X-14273-01A), Cancerfonden (4265-B99-01XAB), Crafoordska stiftelsen (20010968), Blaceflors stiftelse, Einar och Inga Nilssons stiftelse, Harald och Greta Jaenssons stiftelse, Greta och Johan Kocks stiftelser, Fröken Agnes Nilssons stiftelse, Franke and Margareta Bergqvists stiftelse för främjande av cancerforskning, Nanna Svartz stiftelse, Ruth och Richard Julins stiftelse, Svenska Läkaresällskapet (2001-907), Teggers stiftelse, Allmäna sjukhusets i Malmö stiftelse för bekämpande av cancer, MAS fonder, Malmö University Hospital, and Lund University.
REFERENCES
- 1.Carlos, T. M., and J. M. Harlan. 1994. Leukocyte-endothelial adhesion molecules. Blood 84:2068-2101. [PubMed] [Google Scholar]
- 2.Chosay, J. G., N. A. Essani, C. J. Dunn, and H. Jaeschke. 1997. Neutrophil margination and extravasation in sinusoids and venules of liver during endotoxin-induced injury. Am. J. Physiol. 272:G1195-G1200. [DOI] [PubMed] [Google Scholar]
- 3.Eppihimer, M. J., J. Russell, R. Langley, M. Gerritsen, and D. N. Granger. 1999. Role of tumor necrosis factor and interferon gamma in endotoxin-induced E-selectin expression. Shock 11:93-97. [DOI] [PubMed] [Google Scholar]
- 4.Essani, N. A., M. L. Bajt, A. Farhood, S. L. Vonderfecht, and H. Jaeschke. 1997. Transcriptional activation of vascular cell adhesion molecule-1 gene in vivo and its role in the pathophysiology of neutrophil-induced liver injury in murine endotoxin shock. J. Immunol. 158:5941-5948. [PubMed] [Google Scholar]
- 5.Essani, N. A., M. A. Fisher, C. A. Simmons, J. L. Hoover, A. Farhood, and H. Jaeschke. 1998. Increased P-selectin gene expression in the liver vasculature and its role in the pathophysiology of neutrophil-induced liver injury in murine endotoxin shock. J. Leukoc. Biol. 63:288-296. [DOI] [PubMed] [Google Scholar]
- 6.Fernandes-Alnemri, T., G. Litwack, and E. S. Alnemri. 1994. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J. Biol. Chem. 269:30761-30764. [PubMed] [Google Scholar]
- 7.Fox-Robichaud, A., and P. Kubes. 2000. Molecular mechanisms of tumor necrosis factor alpha-stimulated leukocyte recruitment into the murine hepatic circulation. Hepatology 31:1123-1127. [DOI] [PubMed] [Google Scholar]
- 8.Harbrecht, B. G., T. R. Billiar, J. Stadler, A. J. Demetris, J. B. Ochoa, R. D. Curran, and R. L. Simmons. 1992. Nitric oxide synthesis serves to reduce hepatic damage during acute murine endotoxemia. Crit. Care Med. 20:1568-1574. [DOI] [PubMed] [Google Scholar]
- 9.Hewett, J. A., P. A. Jean, S. L. Kunkel, and R. A. Roth. 1993. Relationship between tumor necrosis factor-alpha and neutrophils in endotoxin-induced liver injury. Am. J. Physiol. 265:G1011-G1015. [DOI] [PubMed] [Google Scholar]
- 10.Holman, J. M., Jr., and T. M. Saba. 1988. Hepatocyte injury during post-operative sepsis: activated neutrophils as potential mediators. J. Leukoc. Biol. 43:193-203. [DOI] [PubMed] [Google Scholar]
- 11.Horie, Y., R. Wolf, D. C. Anderson, and D. N. Granger. 1998. Nitric oxide modulates gut ischemia-reperfusion-induced P-selectin expression in murine liver. Am. J. Physiol. 275:H520-H526. [DOI] [PubMed] [Google Scholar]
- 12.Jaeschke, H., A. Farhood, and C. W. Smith. 1991. Neutrophil-induced liver cell injury in endotoxin shock is a CD11b/CD18-dependent mechanism. Am. J. Physiol. 261:G1051-G1056. [DOI] [PubMed] [Google Scholar]
- 13.Jaeschke, H., M. A. Fisher, J. A. Lawson, C. A. Simmons, A. Farhood, and D. A. Jones. 1998. Activation of caspase 3 (CPP32)-like proteases is essential for TNF-alpha-induced hepatic parenchymal cell apoptosis and neutrophil-mediated necrosis in a murine endotoxin shock model. J. Immunol. 160:3480-3486. [PubMed] [Google Scholar]
- 14.Klintman, D., G. Hedlund, and H. Thorlacius. 2002. Protective effect of Linomide on TNF-alpha-induced hepatic injury. J. Hepatol. 36:226-232. [DOI] [PubMed] [Google Scholar]
- 15.Klintman, D., R. Schramm, M. D. Menger, and H. Thorlacius. 2002. Leukocyte recruitment in hepatic injury: selectin-mediated leukocyte rolling is a prerequisite for CD18-dependent firm adhesion. J. Hepatol. 36:53-59. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence, M. B., and T. A. Springer. 1991. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell 65:859-873. [DOI] [PubMed] [Google Scholar]
- 17.Lawson, J. A., A. R. Burns, A. Farhood, B. M. Lynn, R. G. Collins, C. W. Smith, and H. Jaeschke. 2000. Pathophysiologic importance. Hepatology 32:990-998. [DOI] [PubMed] [Google Scholar]
- 18.Lawson, J. A., M. A. Fisher, C. A. Simmons, A. Farhood, and H. Jaeschke. 1998. Parenchymal cell apoptosis as a signal for sinusoidal sequestration and transendothelial migration of neutrophils in murine models of endotoxin and Fas-antibody-induced liver injury. Hepatology 28:761-767. [DOI] [PubMed] [Google Scholar]
- 19.Leist, M., F. Gantner, I. Bohlinger, G. Tiegs, P. G. Germann, and A. Wendel. 1995. Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. Am. J. Pathol. 146:1220-1234. [PMC free article] [PubMed] [Google Scholar]
- 20.Lindbom, L., X. Xie, J. Raud, and P. Hedqvist. 1992. Chemoattractant-induced firm adhesion of leukocytes to vascular endothelium in vivo is critically dependent on initial leukocyte rolling. Acta Physiol. Scand. 146:415-421. [DOI] [PubMed] [Google Scholar]
- 21.Luscinskas, F. W., H. Ding, and A. H. Lichtman. 1995. P-selectin and vascular cell adhesion molecule 1 mediate rolling and arrest, respectively, of CD4+ T lymphocytes on tumor necrosis factor alpha-activated vascular endothelium under flow. J. Exp. Med. 181:1179-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mansson, P., X. W. Zhang, B. Jeppsson, O. Johnell, and H. Thorlacius. 2000. Critical role of P-selectin-dependent rolling in tumor necrosis factor-alpha-induced leukocyte adhesion and extravascular recruitment in vivo. Naunyn Schmiedebergs Arch. Pharmacol. 362:190-196. [DOI] [PubMed] [Google Scholar]
- 23.Marubayashi, S., Y. Oshiro, T. Maeda, K. Fukuma, K. Okada, T. Hinoi, M. Ikeda, K. Yamada, H. Itoh, and K. Dohi. 1997. Protective effect of monoclonal antibodies to adhesion molecules on rat liver ischemia-reperfusion injury. Surgery 122:45-52. [DOI] [PubMed] [Google Scholar]
- 24.McCuskey, R. S., R. Urbaschek, and B. Urbaschek. 1996. The microcirculation during endotoxemia. Cardiovasc. Res. 32:752-763. [PubMed] [Google Scholar]
- 25.Nishida, J., R. S. McCuskey, D. McDonnell, and E. S. Fox. 1994. Protective role of NO in hepatic microcirculatory dysfunction during endotoxemia. Am. J. Physiol. 267:G1135-G1141. [DOI] [PubMed] [Google Scholar]
- 26.Ohira, H., T. Ueno, T. Torimura, K. Tanikawa, and R. Kasukawa. 1995. Leukocyte adhesion molecules in the liver and plasma cytokine levels in endotoxin-induced rat liver injury. Scand. J. Gastroenterol. 30:1027-1035. [DOI] [PubMed] [Google Scholar]
- 27.Perry, M. A., and D. N. Granger. 1991. Role of CD11/CD18 in shear rate-dependent leukocyte-endothelial cell interactions in cat mesenteric venules. J. Clin. Investig. 87:1798-1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramos, C. L., E. J. Kunkel, M. B. Lawrence, U. Jung, D. Vestweber, R. Bosse, K. W. McIntyre, K. M. Gillooly, C. R. Norton, B. A. Wolitzky, and K. Ley. 1997. Differential effect of E-selectin antibodies on neutrophil rolling and recruitment to inflammatory sites. Blood 89:3009-3018. [PubMed] [Google Scholar]
- 29.Rauen, U., B. Polzar, H. Stephan, H. G. Mannherz, and H. de Groot. 1999. Cold-induced apoptosis in cultured hepatocytes and liver endothelial cells: mediation by reactive oxygen species. FASEB J. 13:155-168. [DOI] [PubMed] [Google Scholar]
- 30.Scalia, R., V. E. Armstead, A. G. Minchenko, and A. M. Lefer. 1999. Essential role of P-selectin in the initiation of the inflammatory response induced by hemorrhage and reinfusion. J. Exp. Med. 189:931-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Springer, T. A. 1994. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76:301-314. [DOI] [PubMed] [Google Scholar]
- 32.Sriramarao, P., U. H. von Andrian, E. C. Butcher, M. A. Bourdon, and D. H. Broide. 1994. L-selectin and very late antigen-4 integrin promote eosinophil rolling at physiological shear rates in vivo. J. Immunol. 153:4238-4246. [PubMed] [Google Scholar]
- 33.Tedder, T. F., D. A. Steeber, A. Chen, and P. Engel. 1995. The selectins: vascular adhesion molecules. FASEB J. 9:866-873. [PubMed] [Google Scholar]
- 34.Wong, J., B. Johnston, S. S. Lee, D. C. Bullard, C. W. Smith, A. L. Beaudet, and P. Kubes. 1997. A minimal role for selectins in the recruitment of leukocytes into the inflamed liver microvasculature. J. Clin. Investig. 99:2782-2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yadav, S. S., D. N. Howell, D. A. Steeber, R. C. Harland, T. F. Tedder, and P. A. Clavien. 1999. P-Selectin mediates reperfusion injury through neutrophil and platelet sequestration in the warm ischemic mouse liver. Hepatology 29:1494-1502. [DOI] [PubMed] [Google Scholar]