Abstract
This study was performed to evaluate the impact of pro- and anti-inflammatory molecules and human leukocyte antigen DR (HLA-DR) expression as markers of immune status for the final outcome of septic patients. The study included 30 patients with severe sepsis due to community-acquired infections. Concentrations of tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), IL-8, IL-10, and transforming growth factor β1 (TGF-β1) in serum, as well as monocyte HLA-DR expression, were determined on admission and on days 3, 10, 13, and 17 during hospitalization. Of the 30 patients enrolled, 13 survived, while 17 died during their hospital stay. All patients had significantly lower HLA-DR expression and higher pro- and anti-inflammatory cytokine levels than healthy individuals. HLA-DR expression was significantly decreased in nonsurvivors at almost all time points. In nonsurvivors, higher levels in serum of TNF-α on days 13 and 17; IL-6 levels on day 3; and IL-10 on days 3, 10, and 13 were found. Baseline levels of TGF-β1 were significantly higher in survivors. Independent risk factors of mortality were IL-10 levels on days 3 and 10, while monocyte HLA-DR expression on admission was a good predictor for survival. Several pro- and anti-inflammatory cytokines are oversynthesized during severe infections, especially in patients with a poor outcome. Monocyte HLA-DR expression is an early and constant predictive marker for survival in severe sepsis, while serum IL-10 levels on days 3 and 10 have negative prognostic value for the final outcome.
Sepsis is a leading cause of acute hospital admissions and often complicates the clinical course of patients hospitalized for other reasons. Despite the advent of innovative therapeutic strategies and a vast body of knowledge related to its pathophysiology, the mortality rate from severe sepsis remains high at 40% (25) and may exceed 50%, especially among patients who develop organ failure and septic shock.
The immune system plays a pivotal role in the pathogenesis of sepsis. Recent studies have shown that sepsis is a bimodal entity. The first phase is characterized by the systemic release of proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), IL-8, IL-12, gamma interferon (IFN-γ), and, possibly, IL-6 (24). Proinflammatory cytokines are necessary for initiating an effective inflammatory process against infection, whereas excess production of them has been associated with multiple-organ-system dysfunction and mortality (3). In the second phase, anti-inflammatory mediators such as transforming growth factor β (TGF-β), IL-10, IL-13, IL-4, and prostaglandin E2 may be released in an effort to counteract ongoing inflammation (4, 22, 31).
The expression of major histocompatibility complex (MHC) class II on monocytes is a prerequisite for effective antigen presentation to CD4 + T cells, an important component of the immune response to infection (13). Human leukocyte antigen DR (HLA-DR) expression on peripheral blood monocytes has been found to correlate highly with infection in many clinical scenarios (6). Several investigators have shown that low levels of HLA-DR expression in patients with infection and sepsis are linked to recovery and mortality rates (9, 14, 30, 34).
Recent evidence indicates that, during sepsis, enhanced production of proinflammatory cytokines is paralleled by increased activity of counterregulatory mechanisms. Indeed, elevated levels of several pro- and anti-inflammatory cytokines in plasma have been described in patients with sepsis. In a recent study, it was found that mainly immunoparalysis, as detected through an elevated serum IL-10/TNF-α ratio, characterized patients who succumbed to infection (12). So far, most studies had included only measurements upon the admission of patients, with no or limited follow-up (16, 18, 32, 33). In the present study, we sequentially measured the monocyte HLA-DR expression and the serum TNF-α, IL-6, IL-8, IL-10, and TGF-β1 concentrations in patients with severe sepsis for up to 17 days after admission. We also investigated their prognostic value for the final outcome and the interrelationships between cytokines and the monocyte immunophenotype.
The study protocol was approved by the University Hospital ethics committee, and all subjects gave written consent for participation.
MATERIALS AND METHODS
Patients.
Our study was performed at the Patras University Hospital, a 600-bed tertiary referral hospital in western Greece. During a period of 24 months, we included 40 patients (23 men and 17 women) with severe sepsis, admitted to the intermediate care unit of the Department of Medicine. Ten patients were excluded from the study because of prior antibiotic use, incomplete sampling, or because they were lost to follow-up, leaving 30 patients (15 males and 15 females, mean age of 64.6 ± 5.2 years; range, 23 to 86 years) assessable for response. All patients had recently acquired infections, with a mean duration of symptoms of 3.8 ± 1.4 days prior to admittance to the hospital. Subjects with trauma, human immunodeficiency virus disease, neutropenia, end-stage renal disease, or end-stage hepatic disease or subjects receiving immunosuppressive agents were excluded. Another group of 12 healthy individuals, matched for age and sex (7 males, 5 females, mean age of 62.8 ± 6.7 years), were also included in the study as controls for determination of monocyte HLA-DR expression and serum cytokine levels.
The definition of sepsis was based on the presence of at least two of the following criteria (28): fever or hypothermia (temperature, ≥38 or ≤36°C), tachycardia (heart rate, ≥90 beats/min), tachypnea (respiratory rate, ≥20 breaths/min) and leukocytosis or leukopenia (leukocyte count, ≥12,000 or ≤4,000 per μl or >10% immature forms), concurrently with the presence of confirmed infection.
Additional requirements for severe sepsis were at least one of the following (28): (i) hypotension (systolic blood pressure, ≤90 mm Hg; sustained drop in systolic blood pressure, ≤40 mm Hg; or mean arterial pressure, ≤65 mm Hg corrected within 1 h by fluid resuscitation), (ii) arterial hypoxemia (partial pressure of arterial oxygen, ≤75 mm Hg without evidence of primary lung disease), (iii) metabolic acidosis (pH ≤7.3 or base deficit ≥5 meq/liter), (iv) oliguria (urine output, ≤30 ml/h for at least 2 h despite adequate fluid replacement), (v) acute alteration of mental status, or (vi) coagulation abnormalities of recent onset (prothrombin time or activated partial thromboplastin time of ≥1.2 times the upper normal limit plus d-dimers at ≥500 or platelets at ≤100,000/μl).
Septic shock was defined as severe hypotension that lasts >1 h, despite adequate fluid resuscitation and pharmacologic intervention with vasopressor agents (1). The severity of the patients' condition was assessed according to the simplified acute physiology score II (SAPS II) (2), a reliable tool for measurement of illness in intermediate care units.
Blood cultures (three sets of aerobic and anaerobic broth) were done in all cases on admission and during follow-up. Additional specimens were obtained for bacteriologic culture and specific diagnostic procedures (e.g., ultrasound, computed tomography, or gallium scan) were done to identify the infection site. All patients were closely monitored during their hospital stay, and critically ill patients with septic shock were transferred to the intensive care unit.
Sampling and assays.
Sera for cytokine determination from patients and healthy subjects were obtained from whole blood on admission (day 0) and on days 3, 10, 13, and 17. They were clotted for 30 min at 37°C and stored at −70°C until analyzed. Determination of cytokine levels was performed by enzyme-linked immunosorbent assays (ELISAs) as instructed by the manufacturers. For TGF-β1, we used ELISA kits from R&D Systems (Quantikine, Minneapolis, Minn.); for TNF-α and IL-10, the ELISA kits were obtained from Endogen (Woburn, Mass.); and for IL-6 and IL-8, the ELISA kits were obtained from Diaclone (Besancon, France). The detection limits of the assays for TGF-β1, TNF-α, IL-10, IL-6, and IL-8 were 7, 1, 3, 2, and 25 pg/ml, respectively.
The monocyte HLA-DR expression was investigated on the same days. A double-immunofluorescent whole-blood technique was used. The following directly conjugated monoclonal antibodies (MAbs) were used: a CD14 MAb-fluorescein isothiocyanate (FITC) conjugate (clone MφP9) and an anti-HLA-DR-phycoerythrin (PE) conjugate (clone L243) from Becton Dickinson. After completion of the incubation, the erythrocytes were lysed, and the leukocytes were fixed with the Multi-Q-Prep system (Coulter). The appropriate isotype controls were used. At least 10,000 cells from each sample were analyzed on the EPICS XL (Coulter) flow cytometer, and the data were processed with XL2 software. The analysis was performed in a scattergram gate of cells with intermediate side and forward scatter. Results were expressed as percentages of HLA-DR-positive monocytes and as arbitrary units (mean fluorescent intensity [MFI]). The percentage of HLA-DR-positive monocytes was calculated by the coexpression of CD14 and HLA-DR antigens in the total CD14+ population. The arbitrary units were calculated as follows: the MFI of the isotype control was subtracted from the MFI of the sample, and the difference was divided by the MFI of the isotype control.
The serum lactate concentration was measured on admission by an enzymatic method (Sigma Diagnostics), while C-reactive protein (CRP) was determined quantitatively on days 0, 3, 10, 13, and 17 by rate nephelometry (Beckman IMMAGE Immunochemistry Systems).
Statistical analysis.
Values are expressed as prevalence rates or as the mean ± standard deviation, and in case of a skewed distribution, as the median and range. Differences in quantitative variables between healthy individuals and septic patients were analyzed by the unpaired t test or its nonparametric equivalent, the Mann-Whitney U test. Categorical data were analyzed with the χ2 test. Stepwise multiple regression analysis was utilized to explore relationships between noncategorical variables. Correlations between variables on septic patients were tested by Pearson's correlation coefficient or its nonparametric equivalent, the Spearman's rank correlation coefficient value. Logistic regression analysis was used to detect which variables were most predictive of final outcome in septic patients. Mortality odds ratio (ORs) were expressed with their 95% confidence intervals (CI). A P value of <0.05 was considered significant. All statistical analyses were performed with SPSS, version 9.0, software (SPSS, Chicago, Ill.).
RESULTS
Characteristics of the study population.
In the patients' group, severe sepsis was the consequence of pneumonia (n = 12), intra-abdominal infection (n = 9), urinary tract infection (n = 5), severe soft tissue infection (n = 2), or osteomyelitis (n = 2). Microorganisms were isolated in 18 patients (gram-negative bacteria, n = 10; gram-positive bacteria, n = 8). The most frequently isolated bacteria were Escherichia coli (n = 4), Streptococcus pneumoniae (n = 3), Proteus mirabilis (n = 2), methicillin-resistant Staphylococcus aureus (n = 2), Staphylococcus epidermidis (n = 2), and Enterococcus spp. (n = 1). Other gram-negative bacteria (Pseudomonas aeruginosa, Klebsiella pneumoniae, Enterobacter aerogenes, and Acinetobacter baumannii) were cultured from four patients. Candida was isolated from one patient.
Of the 30 patients enrolled, 13 survived, while 17 died during their hospital stay. The main patient characteristics are shown in Table 1. No difference was detected between survivors and nonsurvivors concerning age and sex distribution. The mean SAPS II, lactate concentration, and CRP values were not significantly different between these two groups. There was also no difference in the prevalence of bacteremia and gram-negative infection between survivors and nonsurvivors. No correlation between the types of organisms and the final patients' outcome was detected. Twelve (40%) of the patients developed septic shock within 48 h after admission, and 8 of them (66.7%) died, whereas the remaining 18 patients had no hemodynamic deterioration during their hospital stay.
TABLE 1.
Characteristics of survivors and nonsurvivors with severe sepsis
| Characteristica | Result for:
|
|
|---|---|---|
| Survivors (n = 13) | Nonsurvivors (n = 17) | |
| Age (yr) | 62.8 ± 4.1 | 65.7 ± 5.7 |
| Sex (no. of males/females) | 6/7 | 8/9 |
| SAPS IIb | 42.7 ± 14.6 | 48.6 ± 14.3 |
| No. (%) with gram-negative infection | 5 (38.5) | 5 (29.4) |
| No. (%) with bacteremia | 7 (53.8) | 8 (47) |
| Lactate concn (mg/dl) | 23.3 ± 13.7 | 28.4 ± 19 |
| CRP (mg/dl)c | 15.5 ± 14.6 | 23.3 ± 16.1 |
| No. (%) with hypoxemiad | 5 (38.5) | 9 (52.9) |
| No. (%) with renal failuree | 5 (38.5) | 8 (47) |
| No. (%) with DICf | 3 (23) | 6 (35.3) |
All comparisons (P) were nonsignificant.
Score on admission.
CRP on admission.
Ratio of partial pressure of arterial oxygen to forced inspiratory oxygen fraction of inspired oxygen: <100.
Creatinine concentration of >3.5 mg/dl or hemodialysis or oliguria of <250 ml/24 h.
DIC (disseminated intravascular coagulation) represents prothrombin time or partial thromboplastin time of ≥1.2 times the upper normal limit plus d-dimers ≥ 500 or platelet count of ≤100,000/μl.
Cytokine production.
Serum cytokine levels in healthy individuals were undetectable or ≤13 pg/ml for TNF-α, ≤2.6 pg/ml for IL-6, ≤2 pg/ml for IL-8, and ≤4 pg/ml for IL-10. Mean levels of serum TGF-β1 were 14,500 ± 3,500 pg/ml (range, 11 × 103 to 8 × 103 pg/ml).
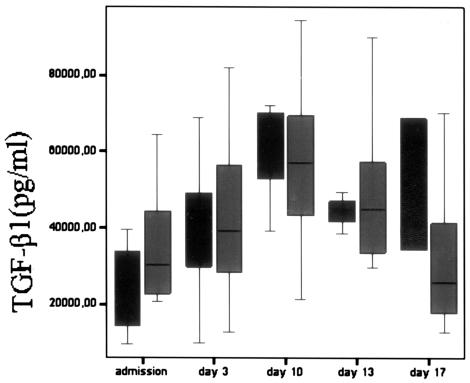
On admission, patients with severe sepsis had significantly higher levels of all measured cytokines (P < 0.001) than did controls. We found significantly higher levels of TNF-α on days 13 and 17 in nonsurvivors compared to survivors (P < 0.05), while there was no significant difference on admission and at days 3 and 10 (Table 2). Nonsurvivors had significantly higher serum IL-6 concentrations than survivors only on day 3 (P < 0.05). Serum IL-8 concentrations were similar in the two groups at all time points. IL-10 levels were significantly higher in nonsurvivors than in survivors on days 3 (P < 0.01) and 10 and 13 (P < 0.05). In surviving patients, IL-10 levels decreased steadily from admission to day 17. Baseline levels of TGF-β1 were significantly higher (P < 0.05) in survivors than in nonsurvivors (Fig. 1). On day 10, there was a peak serum TGF-β1 level in both groups, and then TGF-β1 concentrations decreased steadily in survivors, while they remained high in nonsurvivors. However, we didn't find significant differences between them at any time point after admission. Finally, no correlation was found between serum cytokine levels and the focus of infection.
TABLE 2.
Cytokine levels of survivors and nonsurvivors with severe sepsis from admission to day 17
| Day and group | Cytokine level (pg/ml)a
|
n | ||||
|---|---|---|---|---|---|---|
| TNF-α | IL-6 | IL-8 | IL-10 | TGF-β1 | ||
| 0 (Admission) | ||||||
| Survivors | 59.4 (33-157.4) | 179.3 (40-417.6) | 24.1 (0-297.5) | 55.6 (36.9-367.2) | 30,213 (20,643-64,419)* | 13 |
| Nonsurvivors | 115.9 (46.8-138.6) | 184.2 (6.9-408.8) | 4.3 (0-433.4) | 66.4 (41.5-143.2) | 25,144 (9,462-39,499) | 17 |
| 3 | ||||||
| Survivors | 54.3 (32.9-149.9) | 57.6 (13-274.9)* | 3.7 (0-49) | 40.2 (34.2-82.5)† | 39,357 (12,683-81,901) | 13 |
| Nonsurvivors | 117.8 (43-126) | 200.7 (10.8-407) | 0.8 (0-26.9) | 62.5 (40.2-102.8) | 40,541 (9,983-68,826) | 10 |
| 10 | ||||||
| Survivors | 48 (27.9-131) | 33.3 (6.8-154.3) | 0 (0-26.9) | 39.6 (35-62.7)* | 56,981 (21,307-94,551) | 13 |
| Nonsurvivors | 110.3 (40.5-117.2) | 83.2 (7.4-266.1) | 5.1 (0-17.6) | 54.8 (41.8-84.1) | 67,428 (39,263-72,047) | 4 |
| 13 | ||||||
| Survivors | 49.3 (17.6-119.7)* | 12.6 (5.4-66.7) | 0 (0-11.2) | 35.8 (11.3-54.5)* | 44,900 (29,560-90,003) | 13 |
| Nonsurvivors | 115.93 (56.8-131.2) | 74.6 (3.6-291.6) | 14.3 (0-35) | 54.5 (40.7-114.2) | 44,829 (38,457-49,354) | 4 |
| 17 | ||||||
| Survivors | 29.5 (11.8-128.5)* | 4.9 (1.3-76.6) | 0 (0-8.3) | 4.6 (0-50.7) | 25,671 (12,591-94,267) | 13 |
| Nonsurvivors | 122.8 (104.6-141) | 62.8 (5.7-120) | 0 (0-0) | 41.3 (40.2-42.3) | 51,580 (34,382-68,778) | 2 |
Shown are the median and range (in parentheses). *, P < 0.05; †, P < 0.01.
FIG. 1.
TGF-β1 levels in survivors (gray bars) and nonsurvivors (black bars) with severe sepsis on admission and at days 3, 10, 13, and 17. Data are presented as box plots with median lines, 25- and 75-percentile boxes, and 10- and 90-percentile error bars. A circle represents the outliers.
HLA-DR expression on CD14+ mononuclear cells.
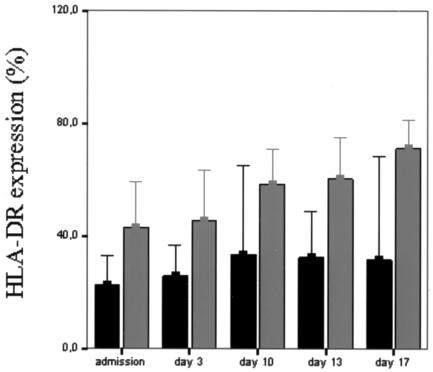
The percentage of peripheral blood monocytes expressing the HLA-DR antigen in healthy donors was ≥85%. Likewise, the mean level of HLA-DR per monocyte, expressed as MFI, was 52.3 ± 20 in healthy controls. On admission, the percentage of HLA-DR-bearing monocytes and the density of HLA-DR in patients with severe sepsis were significantly lower than those in controls (P < 0.01). As shown in Table 3, monitoring of monocyte HLA-DR expression revealed that nonsurvivors were characterized by significantly lower levels on admission and at days 3 and 13 (P < 0.05) and 17 (P < 0.01) compared to survivors, while on day 10, this difference did not achieve statistical significance (P = 0.057). It is noteworthy that all patients with monocyte HLA-DR expression of >40% survived, while those who died had <40% expression at all time points (Fig. 2). In surviving patients, the percentage of monocytes expressing HLA-DR (as well as MFI) increased progressively from admission to day 17.
TABLE 3.
Expression of HLA-DR on CD14+ monocytes in patients with severe sepsis from admission to day 17
| Day | HLA-DR expression ina:
|
|||
|---|---|---|---|---|
| Survivors
|
Nonsurvivors
|
|||
| % | MFI | % | MFI | |
| 0 (admission) | 43.1 ± 25.4 | 3.9 ± 3.3 | 23.4 ± 20.7* | 1.4 ± 1.5* |
| 3 | 45.6 ± 29.3 | 8.1 ± 9 | 25.7 ± 15.4* | 2.2 ± 2.1* |
| 10 | 58.4 ± 19.3 | 9.4 ± 7.7 | 33.4 ± 12.7 | 5.2 ± 3.7 |
| 13 | 60.3 ± 23.2 | 14.7 ± 13.8 | 32.4 ± 10.3* | 4.5 ± 4.6* |
| 17 | 71.2 ± 16.8 | 15.8 ± 9.1 | 31.6 ± 4.1† | 5 ± 0.3† |
*, P < 0.05; †, P < 0.01.
FIG. 2.
Percentage of CD14+ monocytes expressing HLA-DR in survivors (gray bars) and nonsurvivors (black bars) with severe sepsis on admission and at days 3, 10, 13, and 17 during hospitalization. Data are expressed as means and 95% CIs.
Correlation between HLA-DR expression and serum cytokine levels.
Stepwise multiple regression analysis was used to detect the underlying correlations between monocyte HLA-DR expression and cytokine levels at different time points. A significant negative effect of day 3 serum IL-10 levels on day 10 monocyte HLA-DR expression (P = 0.041) was demonstrated, while no other cytokine had any impact on HLA-DR. There were significant negative bivariate correlations between monocyte HLA-DR expression and concentrations of IL-10 (day 3, r = −0.479 and P = 0.031; day 13, r = −0.537 and P = 0.026; day 17, r = −0.822 and P < 0.0005), IL-8 (day 10, r = −0.533 and P = 0.028), and TGF-β1 (day 10, r = −0.518 and P = 0.04; day 17, r = −0.661 and P = 0.007) (Table 4). The IL-10/TNF-α ratio was inversely correlated with HLA-DR on days 3 (r = −0.479 and P = 0.021) and 17 (r = −0.683 and P = 0.005).
TABLE 4.
Bivariate correlations (Spearman rank coefficient) between various cytokines, SAPS II, CRP, and monocyte HLA-DR expression in patients with severe sepsis
| Data set | Correlation coefficient (P) for monocyte HLA-DR expression at daya:
|
||||
|---|---|---|---|---|---|
| 0b | 3 | 10 | 13 | 17 | |
| TNF-α | −0.035 (0.862) | −0.122 (0.590) | −0.037 (0.899) | −0.270 (0.295) | −0.700† (0.004) |
| IL-6 | −0.023 (0.911) | −0.361 (0.091) | −0.233 (0.368) | −0.396 (0.116) | −0.882† (0.000) |
| IL-8 | −0.334 (0.071) | −0.149 (0.499) | −0.533* (0.028) | −0.341 (0.180) | −0.355 (0.194) |
| IL-10 | −0.104 (0.598) | −0.471* (0.031) | −0.417 (0.108) | −0.537* (0.026) | −0.822† (0.000) |
| IL-10/TNF-α | −0.007 (0.973) | −0.479* (0.021) | −0.230 (0.374) | −0.256 (0.321) | −0.683† (0.005) |
| TGF-β1 | −0.118 (0.558) | −0.156 (0.477) | −0.518* (0.040) | −0.323 (0.207) | −0.661† (0.007) |
| SAPS II | −0.087 (0.648) | −0.533† (0.009) | −0.402 (0.110) | −0.514 (0.087) | −0.522 (0.288) |
| CRP | −0.163 (0.390) | −0.197 (0.368) | −0.370 (0.144) | −573 (0.051) | −0.100 (0.873) |
*, P < 0.05; †, P < 0.01.
Admission.
Concerning the severity of disease, the SAPS II in relation to monocyte HLA-DR expression and serum cytokine levels was evaluated. A significant inverse correlation of monocyte HLA-DR expression (r = −0.533, P = 0.009) on day 3 with the respective SAPS II was noted. The IL-10/TNF-α ratio was correlated with SAPS II on the same day (r = 0.536, P = 0.008). Regarding standard clinical variables, there was a significant inverse relationship between the baseline IL-10/TNF-α ratio and the serum lactate concentration (r = −0.687, P = 0.01).
Clinical significance of the immune parameters.
When we compared the monocyte HLA-DR expression and the serum cytokine levels as risk factors of mortality, we found that IL-10 levels on days 3 (OR, 0.93; 95% CI, 0.86 to 0.99; P = 0.035) and 10 (OR, 0.80; 95% CI, 0.65 to 0.98; P = 0.037) were independent predictors of poor outcome. On the contrary, we found that monocyte HLA-DR expression on admission (OR, 1.04; 95% CI, 1.00 to 1.08; P = 0.03) was a good predictor for survival in patients with severe sepsis. A most interesting finding of the present study was that all patients (100%) with serum TNF-α levels below the median value (≤89.52 pg/ml) and monocyte HLA-DR expression of ≥30% on admission survived (P = 0.037).
DISCUSSION
Mononuclear phagocytes are central elements in host defense against a variety of invading microorganisms and in the pathogenesis of sepsis. They play a central role in the immune response by presenting microbial antigens to T lymphocytes and producing cytokines, thus initiating and regulating both cellular and humoral immune responses. HLA-DR expression plays a central role in the processing of antigen by macrophages and helper T cells. Several previous studies demonstrated that low HLA-DR expression on monocytes was found in patients with sepsis (13, 17), trauma (9), and severe burns (26). Our study extends those observations and demonstrates significantly lower monocyte HLA-DR expression in all patients with severe sepsis compared to healthy controls. We also revealed that septic patients who survived had significantly higher levels of HLA-DR expression compared to nonsurvivors during a 17-day follow-up. This was found at almost all time points (except day 10), which means that HLA-DR expression may serve as a dynamic prognostic marker not only on admission but also during the whole septic episode in patients with severe infections.
On the other hand, during inflammatory syndromes, a number of proinflammatory molecules are released and serve as modulators of the immune response. Several studies have already been published that show increased concentrations of various cytokines in the serum of patients with sepsis, emphasizing their possible contribution to predicting the final outcome in these patients (5, 8). Elevated levels of TNF-α and IL-6 have been associated with increased mortality from sepsis (16, 29), and some investigators have indicated that IL-6 levels are a good prognostic parameter in the early phase of sepsis. However, sepsis is associated not only with the exacerbation of the production of proinflammatory cytokines but also with the release of many anti-inflammatory cytokines, such as IL-10 and TGF-β1. Previous investigations have indicated that TGF-β1 represses the production of inflammatory cytokines by activated macrophages and induces the release of soluble TNF receptor and IL-1 receptor α. It has been shown that mean plasma TGF-β1 levels were increased in patients with sepsis syndrome compared to healthy donors (19). Except for TGF-β1, IL-10 is one of the immunosuppressive mediators postulated to play an important role in down-regulation of the immune response. Elevated levels of IL-10 have been associated with increased mortality from septic shock (27, 32).
Our data indicated that all cytokine levels measured were significantly higher in septic patients than healthy individuals, as expected. Monitoring the serum cytokine levels was a good marker of the immune status and outcome, but at different time intervals, because TNF-α levels were significantly higher in nonsurvivors on days 13 and 17, IL-6 was higher only on day 3, and IL-10 was higher on days 3, 10, and 13. These findings are in accordance with previous studies of ours (12) and other investigators (33), who noted that TNF-α and IL-10 had a significant prognostic value for the final outcome.
On the contrary, HLA-DR expression on monocytes was significantly higher in survivors both at admission and at almost all time intervals. Therefore, according to our findings, monocyte HLA-DR expression seems to be an early and constant predictive marker for the prognosis of outcome in severe sepsis, while the main pro- and anti-inflammatory cytokine levels were significantly higher in a rather later stage. There seems to be a relationship between HLA-DR expression and cytokine synthesis, because we detected an inverse correlation between monocyte HLA-DR expression and IL-10 and IL-8 concentrations, as well as with the IL-10/TNF-α ratio. Interestingly, we detected that serum IL-10 levels on day 3 inversely affected monocyte HLA-DR expression on day 10 (P < 0.05). Similar findings have been reported by other investigators, who demonstrated that IL-10 induces in vitro the downregulation of HLA-DR surface expression of “normal” monocytes and of immature dendritic cells obtained from peripheral mononuclear cells, with accumulation of MHC II molecules in intracellular compartments (11, 15, 20).
Concerning TGF-β1, we found significantly higher levels of TGF-β1 in survivors with severe sepsis compared to nonsurvivors on admission. These results might be interpreted in terms of the beneficial effect of TGF-β1 on the hemodynamic compromise of septic shock in a previous murine model (23). In that study, it was shown that TGF-β1 inhibited inducible nitric oxide synthetase mRNA and NO production in vivo in vascular muscle cells, after its induction by cytokines critical in the sepsis cascade. TGF-β1 was shown in vitro to inhibit IFN-γ-induced HLA-DR surface expression of various cell types through a transcriptional effect (7, 21). TGF-β1 has also been described as downmodulating the monocyte surface expression of HLA-DR in association with IL-10 (10). In the present study, we found a significant inverse correlation between monocyte HLA-DR expression and TGF-β1 concentrations on days 10 and 17, which is in accordance with the previous observations.
On a clinical basis, it is noteworthy that there was no difference in the prevalence of bacteremia and gram-negative infection between survivors and nonsurvivors, while the frequencies of organ dysfunction were similar in both groups.
In conclusion, analysis of our data indicates that dynamic monitoring of the immune status of septic patients may contribute to the estimation of the death risk in relation to other clinical or laboratory markers. Pro- versus anti-inflammatory response may be sequentially identified at different time points by measuring certain serum cytokine levels in correlation with peripheral blood monocyte HLA-DR expression. The presence of immunoparalysis seems to be the main risk factor of poor outcome in patients with severe sepsis, as indicated by the diminished HLA-DR expression and the increased serum IL-10 levels. Monocyte HLA-DR expression seems to be an early prognostic marker for survival, while serum IL-10 levels may be a good marker for identification of patients with a guarded prognosis in a rather later stage.
REFERENCES
- 1.American College of Chest Physicians/Society of Critical Medicine Consensus Committee. 1992. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit. Care Med. 20:864-874. [PubMed] [Google Scholar]
- 2.Auriant, I., I. Vinatier, F. Thaler, M. Tourneur, and P. Loirat. 1998. Simplified acute physiology score II for measuring severity of illness in the intermediate care units. Crit. Care Med. 26:1368-1371. [DOI] [PubMed] [Google Scholar]
- 3.Balk, R. A. 2000. Pathogenesis and management of multiple organ dysfunction or failure in severe sepsis and septic shock. Crit. Care Clin. 16:337-352. [DOI] [PubMed] [Google Scholar]
- 4.Blackwell, T. S., and J. W. Christman. 1996. Sepsis and cytokines: current status. Br. J. Anaesth. 77:110-117. [DOI] [PubMed] [Google Scholar]
- 5.Casey, L. C., R. A. Balk, and R. C. Bone. 1993. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann. Intern. Med. 119:771-778. [DOI] [PubMed] [Google Scholar]
- 6.Cheadle, W. G. 1993. The human leukocyte antigens and their relationship to infection. Am. J. Surg. 165:S75-S81. [DOI] [PubMed] [Google Scholar]
- 7.Czarniecki, C. W., H. H. Chiu, G. H. Wong, S. M. McCabe, and M. A. Palladino. 1988. Transforming growth factor-beta 1 modulates the expression of class II histocompatibility antigens on human cells. J. Immunol. 140:4217-4223. [PubMed] [Google Scholar]
- 8.Damas, P., J. L. Canivet, D. de Groote, Y. Vrindts, A. Albert, P. Franchimont, and M. Lamy. 1997. Sepsis and serum cytokine concentrations. Crit. Care Med. 25:405-412. [DOI] [PubMed] [Google Scholar]
- 9.Ditschkowski, M., E. Kreuzfelder, V. Rebmann, S. Ferencik, M. Majetschak, E. N. Schmid, U. Obertacke, H. Hirche, U. F. Schade, and H. Grosse-Wilde. 1999. HLA-DR expression and soluble HLA-DR levels in septic patients after trauma. Ann. Surg. 229:246-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Döcke, W. D., F. Randow, U. Syrbe, D. Krausch, K. Asadullah, P. Reinke, H. D. Volk, and W. Kox. 1997. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat. Med. 3:678-681. [DOI] [PubMed] [Google Scholar]
- 11.Fumeaux, T., and J. Pugin. 2002. Role of interleukin-10 in the intracellular sequestration of human leukocyte antigen-DR in monocytes during septic shock. Am. J. Respir. Crit. Care Med. 166:1475-1482. [DOI] [PubMed] [Google Scholar]
- 12.Gogos, C. A., E. Drosou, H. P. Bassaris, and A. Skoutelis. 2000. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J. Infect. Dis. 181:176-180. [DOI] [PubMed] [Google Scholar]
- 13.Haveman, J. W., A. C. Muller Kobold, J. W. Cohen Tervaert, A. P. van den Berg, J. E. Tulleken, C. G. M. Kallenberg, and T. H. The. 1999. The central role of monocytes in the pathogenesis of sepsis: consequences for immunomonitoring and treatment. Neth. J. Med. 55:132-141. [DOI] [PubMed] [Google Scholar]
- 14.Hershman, M. J., W. G. Cheadle, S. R. Wellhausen, P. F. Davidson, and H. C. Polk, Jr. 1990. Monocyte HLA-DR antigen expression characterizes clinical outcome in the trauma patient. Br. J. Surg. 77:204-207. [DOI] [PubMed] [Google Scholar]
- 15.Koppelman, B., J. J. Neefjes, J. E. de Vries, and R. de Waal Malefyt. 1997. Interleukin-10 down-regulates MHC class II alphabeta peptide complexes at the plasma membrane of monocytes by affecting arrival and recycling. Immunity 7:861-871. [DOI] [PubMed] [Google Scholar]
- 16.Lin, R. Y., M. E. Astiz, J. C. Saxon, D. C. Saha, and E. C. Rackow. 1994. Relationships between plasma cytokine concentrations and leukocyte functional antigen expression in patients with sepsis. Crit. Care Med. 22:1595-1602. [PubMed] [Google Scholar]
- 17.Manjuck, J., D. C. Saha, M. Astiz, L.-J. Eales, and E. C. Rackow. 2000. Decreased response to recall antigens is associated with depressed costimulatory receptor expression in septic critically ill patients. J. Lab. Clin. Med. 135:153-160. [DOI] [PubMed] [Google Scholar]
- 18.Marchant, A., J. Deviere, B. Byl, D. de Groote, J. L. Vincent, and M. Goldman. 1994. Interleukin-10 production during septicaemia. Lancet 343:707-708. [DOI] [PubMed] [Google Scholar]
- 19.Marie, C., and M.-R. Losser. 1996. Elevated levels of circulating transforming growth factor-β1 in patients with the sepsis syndrome. Ann. Intern. Med. 125:520-521. [DOI] [PubMed] [Google Scholar]
- 20.Morel, A. S., G. Coulton, and M. Londei. 2002. Regulation of major histocompatibility complex class II synthesis by interleukin-10. Immunology 106:229-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nandan, D., and N. E. Reiner. 1997. TGF-beta attenuates the class II transactivator and reveals an accessory pathway of IFN-gamma action. J. Immunol. 158:1095-1101. [PubMed] [Google Scholar]
- 22.Parrillo, J. E., M. M. Parker, C. Natanson, A. F. Suffredini, R. L. Danner, R. E. Cunnion, and F. P. Ognibene. 1990. Septic shock in humans, advances in the understanding of pathogenesis, cardiovascular dysfunction and therapy. Ann. Intern. Med. 113:227-240. [DOI] [PubMed] [Google Scholar]
- 23.Perrella, M. A., C.-M. Hsieh, W.-S. Lee, S. Shieh, J.-C. Tsai, C. Patterson, C. Lowenstein, N. C. Long, E. Haber, S. Shore, and M.-E. Lee. 1996. Arrest of endotoxin-induced hypotension by transforming growth factor β1. Proc. Natl. Acad. Sci. USA 93:2054-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinsky, M. R. 2001. Sepsis: a pro- and anti-inflammatory disequilibrium syndrome. Contrib. Nephrol. 132:354-366. [DOI] [PubMed] [Google Scholar]
- 25.Rangel-Frausto, M. S., D. Pitter, M. Costigan, T. Hwang, C. S. Davis, and R. P. Wenzel. 1995. The natural history of the systemic inflammatory response syndrome (SIRS): a prospective study. JAMA 11:117-123. [PubMed] [Google Scholar]
- 26.Sachse, C., M. Prigge, G. Cramer, N. Pallua, and E. Henkel. 1999. Association between reduced human leukocyte antigen (HLA)-DR expression on blood monocytes and increased plasma level of interleukin-10 in patients with severe burns. Clin. Chem. Lab. Med. 37:193-198. [DOI] [PubMed] [Google Scholar]
- 27.Sfeir, T., D. C. Saha, M. Astiz, and E. C. Rackow. 2001. Role of interleukin-10 in monocyte hyporesponsiveness associated with septic shock. Crit. Care Med. 29:129-133. [DOI] [PubMed] [Google Scholar]
- 28.Sibbald, W. J., and J. L. Vincent. 1995. Round table conference on clinical trials for the treatment of sepsis. Crit. Care Med. 23:394-399. [DOI] [PubMed] [Google Scholar]
- 29.Spittler, A., M. Razenberger, H. Kupper, M. Kaul, W. Hackl, G. Boltz-Nitulescu, R. Függer, and E. Roth. 2000. Relationship between interleukin-6 plasma concentration in patients with sepsis, monocyte phenotype, monocyte phagocytic properties, and cytokine production. Clin. Infect. Dis. 31:1338-1342. [DOI] [PubMed] [Google Scholar]
- 30.van den Berk, J. M. M., R. H. J. Oldenburger, A. P. van den Berg, I. J. Klompmaker, G. Mesander, W. J. van Son, W. van der Bij, M. J. H. Slooff, and T. H. The. 1997. Low HLA-DR expression on monocytes as a prognostic marker for bacterial sepsis after liver transplantation. Transplantation 63:1846-1848. [DOI] [PubMed] [Google Scholar]
- 31.van der Poll, T., and S. J. H. van Deventer. 1999. Cytokines and anticytokines in the pathogenesis of sepsis. Infect. Dis. Clin. N. Am. 13:413-426. [DOI] [PubMed] [Google Scholar]
- 32.van der Poll, T., R. de Waal Malefyt, S. M. Coyle, and S. F. Lowry. 1997. Antiinflammatory cytokine responses during clinical sepsis and experimental endotoxemia: sequential measurements of plasma soluble interleukin-1 receptor type II, IL-10 and IL-13. J. Infect. Dis. 175:118-122. [DOI] [PubMed] [Google Scholar]
- 33.van Dissel, J. T., P. van Langevelde, R. G. J. Westendorp, K. Kwappenberg, and M. Frolich. 1998. Anti-inflammatory cytokine profile and mortality in febrile patients. Lancet 351:950-953. [DOI] [PubMed] [Google Scholar]
- 34.Wakefield, C. H., P. D. Carey, S. Foulds, J. R. T. Monson, and P. J. Guillou. 1993. Changes in major histocompatibility complex class II expression in monocytes and T cells of patients developing infection after surgery. Br. J. Surg. 80:205-209. [DOI] [PubMed] [Google Scholar]