Abstract
Background
The etiology of carpal tunnel syndrome (CTS) remains idiopathic in many cases. Noninflammatory fibrosis of the subsynovial connective tissue (SSCT) within the carpal tunnel is common in CTS, and some clinicians have hypothesized that this fibrosis might be a cause rather than an effect of CTS. An animal model in which to test this hypothesis would be useful. The principal objective of this study was to investigate the effect of a surgical injury on SSCT fibrosis and median nerve function within the carpal tunnel in an in vivo rabbit model.
Methods
Rabbits were sacrificed 12 weeks after surgery and were evaluated by mechanical testing, histology, transmission and scanning electron microscopy, and electrophysiology.
Results
SSCT fibroblast density (p < 0.0001) and collagen fiber size (p = 0.0004) were significantly higher, and the median nerve distal motor amplitude was significantly lower (p = 0.0018), in the tendon injury group SSCT than in either the sham or control groups.
Conclusions
Our findings are similar to those seen in patients with carpal tunnel syndrome and suggest that the tendon injury procedure may be the basis of a new animal model of SSCT injury and, possibly, CTS.
Keywords: Animal model, Carpal tunnel syndrome, Subsynovial connective tissue
Introduction
Both clinical and animal studies have shown convincingly that carpal tunnel syndrome (CTS) is a compression neuropathy of the median nerve [7, 10, 11, 15, 17], but these studies have not identified how the compression is generated or maintained clinically. Animal models of CTS have focused on nerve pathology rather than changes in the surrounding tissues and create the compression by applying direct pressure to the nerve with a balloon, constricting band, or other artificial device [7, 10, 11]. While these studies are helpful in characterizing the effect of compression on a nerve, they do nothing to identify potential mechanisms by which compression might be created or maintained in vivo, in the absence of such devices.
The synovial tissues in the carpal tunnel are unique in containing a substantial, multilayered subsynovial connective tissue (SSCT). Normally, the SSCT loosely connects the finger flexor tendons and median nerve to the visceral synovial membrane, which in turn encloses the tendons and nerve within the ulnar tenosynovial bursa. This sliding system is grossly disrupted in patients with idiopathic CTS [1, 2].
Lluch hypothesized that activity-related damage may occur to the SSCT, resulting in CTS [8]. If this hypothesis regarding the etiology of CTS is correct, it would open the horizon to many new therapies and prevention strategies for CTS, based on an evaluation of SSCT material properties, including job modifications to avoid activities likely to damage the synovial gliding system and possibly interventions to abort or reverse the SSCT fibrosis, even before nerve dysfunction develops.
This study tested the basic premise of our hypothesis, i.e., SSCT injury leads to progressive SSCT fibrosis and subsequent median neuropathy, by investigating the effect of a surgically induced tendon injury, designed to create SSCT stretch, on the development of SSCT fibrosis and median nerve function in a rabbit model in vivo.
Material and Methods
Surgical Procedure
After approval of our Institutional Animal Care and Use Committee, 42 female New Zealand white rabbits weighing 3.6–6.2 kg were used for this study. Thirty animals were part of the experimental group and 12 served as normal controls.
In the experimental animals, following the induction of anesthesia, electrophysiological (EP) testing of the median nerve was performed in each forepaw as described below. After EP testing, both forepaws were scrubbed with povidone–iodine and sterilely draped. A rubber belt above the elbow was used as a tourniquet. A volar longitudinal incision was made 1 cm proximal from the proximal edge of the wrist cartilage, an easily palpable structure within the flexor retinaculum, marking the level of the rabbit carpal tunnel. The flexor digitorum superficialis (FDS) tendon of the middle digit was then exposed. In one randomly selected digit, after this exposure and a more distal exposure, described below, were made, and the wounds were closed. This was considered a “sham” surgical procedure, since no intervention other than a skin incision was actually performed.
In the contralateral paw, after identifying the FDS muscle–tendon junction and marking the adjacent flexor carpi ulnaris tendon using a 6–0 Prolene suture (Ethicon, Somerville, NJ, USA) to identify the relative position of the middle digit FDS tendon, the middle digit FDS tendon was cut at the muscle–tendon junction and the distal end of the middle digit FDS was marked using 6–0 Prolene. Then, a volar longitudinal incision was made on the third digit distal to the carpal tunnel, centered at the metacarpophalangeal joint, level to expose the flexor tendons and proximal annular pulley. Two marks were made, separated by a distance of 5 mm, on the surface of the middle digit FDS tendon proximal to the flexor sheath with the digit held in extension. Then, the two marks were brought together by pulling the distal tendon stump distally, resulting in a 5-mm separation of the tendon laceration which had previously been made proximal to the carpal tunnel. Once this separation was confirmed, the distal tendon loop was fixed with a single suture of 5–0 Ethibond (Ethicon). In effect, the middle digit FDS tendon was distally shifted by 5 mm (Fig. 1). The incisions were then closed with continuous subcuticular sutures of 4–0 Vicryl (Ethicon).
Fig. 1.
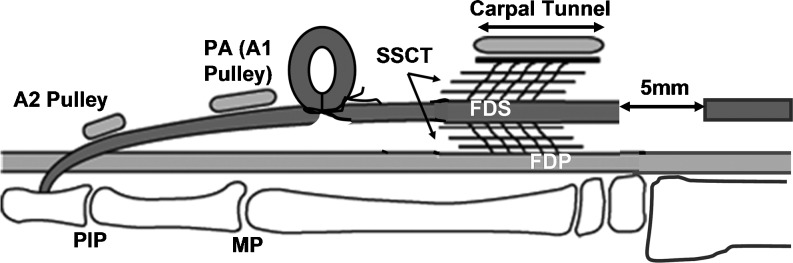
Schematic of the surgical procedure
After surgery, a sterile dressing was applied to each paw. After the rabbits were resuscitated, an Elizabethan collar was applied for 2 weeks to prevent chewing of the incisions. The rabbits were allowed full cage activity until the time of sacrifice. After the wounds had healed, the rabbits were allowed 30 min of exercise outside their cage twice a week until sacrifice.
All rabbits were sacrificed 12 weeks after the initial procedure. Before sacrifice, EP testing was performed on the median nerve of each forepaw under general anesthesia. Immediately after sacrifice, the forepaws of all rabbits were harvested and the total contents of the carpal tunnel were prepared either for median nerve histology or SSCT histology or SSCT mechanical evaluation, as described below.
Twelve normal female New Zealand white rabbits weighting 4.4–6.2 kg were used as a normal control group for histological evaluation of the median nerve and the SSCT and to test mechanical properties as the control group. In each of these rabbits, one paw was used for mechanical testing and the other for histology, either of the nerve (3 paws) or SSCT (9 paws).
Evaluation of Subsynovial Connective Tissue
Mechanical Testing
The method for mechanical testing has been described previously [22]. In brief, the FDS tendons were exposed at the antebrachial level, and if it had not been divided already, the middle digit FDS tendon was divided at a level 5 mm proximal to the proximal edge of the flexor retinaculum. The proximal end of the middle digit FDS tendon was sutured with 5–0 Vicryl as an anchor cable. The specimen was then mounted in a custom fixture. The fixture was mounted on a custom-made microtester, which was composed of a linear servo motor (MX 80 Daedal, Irwin, PA, USA) and a load cell with the accuracy of 0.01 N (MDB-5, Transducer Techniques, Temecula, CA, USA). The anchor cable was connected to the load cell. The middle digit FDS tendon was then sharply cut 5 mm distal to the distal edge of the carpal tunnel.
Under displacement control, the middle digit FDS tendon was moved proximally through the carpal tunnel at a rate of 0.5 mm/s until the tendon was fully pulled through the carpal tunnel. The load cell measurement and displacement were recorded through a LabVIEW program (National Instruments, Austin, TX, USA). Throughout testing, the specimen was kept moist by spraying saline solution. The ultimate tensile load was measured, and the energy absorption and the stiffness were calculated [23]. The ultimate tensile load and energy absorption were defined as the maximum load and the area under the load/displacement curve, respectively. The stiffness was defined as the slope of the load/displacement curve. For the stiffness measurement, the displacement at the ultimate tensile load was defined as 100% (the ultimate load displacement). The stiffness was then measured for each 10% increment of displacement until the ultimate load displacement. All testing was carried out at room temperature.
Histological Analysis
Immediately after sacrifice and removing the skin, the forepaws selected for routine histology were placed in Trump’s fixative (1% glutaraldehyde and 4% formaldehyde in 0.1 M phosphate buffer, pH 7.2) [12] for 48 h for primary fixation. The tissues were then sharply cut 5 mm proximal to the distal edge of the wrist cartilage.
Light Microscopy
The proximal half of the fixed specimen, including the proximal half, roughly 5 mm, of the carpal tunnel, was immersed into formalin and embedded in paraffin. Five-micrometer cross sections were made through the carpal tunnel and stained with standard hematoxylin and eosin (HE). Sections were evaluated under light microscopy (BLX51, Olympus Co., Tokyo, Japan) by the first author (TM), who was blinded to the identity of the specimens at the time of evaluation.
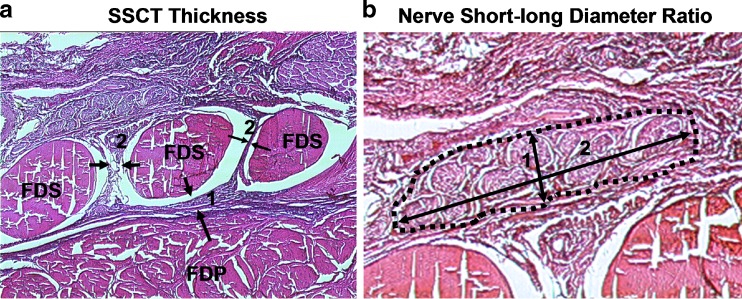
To measure SSCT thickness, the shortest distance between the middle digit FDS tendon and flexor digitorum profundus (FDP) tendon and the average of the shortest distance between index and middle FDS and between middle and ring finger FDS tendons were measured at the mid-carpal tunnel level with image J software (National Institute of Mental Health, MD, USA) (Fig. 2a). To measure SSCT cellularity, fibroblast density was measured by counting the number of fibroblast nuclei in four randomly chosen locations around the middle digit FDS tendon on each specimen, two palmar and two dorsal. A custom grating system was used to randomly count the number of fibroblasts on the photographic images of the histology sections. The SSCT thickness was assessed at low magnification (×90), while cellularity was assessed at high magnification (×920).
Fig. 2.
Histological measurement. a Subsynovial connective tissue thickness: 1 FDP–FDS thickness, 2 FDS–FDS thickness. b Nerve short–long diameter ratio
Transmission Electron Microscopy (TEM)
A cryostat was used to take a 0.5-mm section from the proximal end of the distal specimen to sample the distal half of the carpal tunnel and prepared for TEM. Tissues were rinsed in 0.1 M phosphate buffer and postfixed in phosphate-buffered 1% osmium tetroxide. After rinsing in three changes of distilled water, the tissue was stained en bloc with 2% uranyl acetate at 60°C. The tissues were then rinsed in distilled water, dehydrated in progressive concentrations of ethanol and propylene oxide, and embedded in Spurr’s resin [18, 21]. Resin was polymerized at 65°C and thin sections were mounted on copper grids for routine evaluation with a transmission electron microscope (JEOL 1200, JEOL Ltd., Tokyo, Japan). Images of cross sections of collagen fibers were captured. The mean size of the collagen fibers was measured with image J software (National Institute of Mental Health). A grating system was used to measure the mean size of randomly selected collagen fibers. The mean size of collagen fibers was assessed at high magnification (×120 k).
Scanning Electron Microscopy (SEM)
The remaining portion of the distal carpal tunnel was used for SEM. SEM imaging was used to determine the ultrastructural morphology of the SSCT in two specimens each group. The biopsies were dehydrated through a graded series of ethanol solutions in a critical point dryer. Tissue was then rinsed for 30 min in two changes of 0.1 phosphate buffer (pH = 7.2). The tissue was dehydrated in progressive concentrations of ethanol to 100% and critical point-dried. The specimens were then mounted on aluminum stubs and sputter-coated with gold–palladium. Images in one randomly chosen SSCT area around the third FDS tendon on each specimen were captured on a cold-field emission scanning electron microscope operation at 2 kV (Hitachi S-4700, Hitachi High Technologies America, Inc., Pleasanton, CA, USA). The structural morphology of the SSCT was assessed at high magnification (×2.00 k).
Evaluation of Median Nerve
Electrophysiological Evaluation
EP testing was performed on the median nerve of each forepaw under general anesthesia, administered as described above. For this procedure, two small pins (equal in size to a 30-g needle) were inserted into the forearm and forepaw, respectively, to measure median nerve conduction. The compound muscle action potential (CMAP) was recorded from the thenar muscle with stimulation of the median nerve 3.0 cm proximal to the recording point. Stimulation was carried out until a supramaximal response was visualized on the monitor. Recording was performed before the surgical procedure and 12 weeks after the surgical procedure, immediately prior to sacrifice. The distal motor latency and amplitude of CMAP were measured and compared between the initial and 12 weeks value in each paw.
Statistical Analysis
Based on a pilot study, which showed that the standard deviation of FDP–FDS thickness was 132.3 μm and the standard deviation of energy absorption was 2.41 mJ, we calculated that we could detect a difference of 52.4 μm in FDP–FDS thickness and 1.39 mJ in energy absorption, which we felt would be relevant differences, with a sample size of 9 for thickness and 12 for mechanical testing, using a two-sample t test with 80% power at a significance level of 0.05. With regard to EP testing, a previous study [23] had shown that the normal rabbit median nerve motor latency was 1.74 ± 0.25 ms and the normal motor amplitude was 1.71 ± 0.6 mV. Based on these data, a sample size of 12 would be more than sufficient to detect a difference of 25% in either value, which we thought would be a clinically important difference. We had no way to estimate sample size for histology, but recognized that the histology and mechanical testing methods were mutually exclusive. After balancing a desire to minimize animal use and acknowledging that some animals might have complications that would exclude them from further analysis, we chose to select only the animals with the most severe EP changes for nerve histology, accept some variability in the number allocated to SSCT histology, and thus randomized 30 rabbits in the surgical group, while another 12 rabbits were assigned to the normal control group.
A paired t test was used to compare EP variables before and 12 weeks after surgery. One-way and three-factor analyses of variance followed by the least standard difference (LSD) post hoc test were used for analyzing parameters of mechanical test and collagen size measured by TEM with variables among three groups, i.e., normal, sham, and tendon injury, where individual and left/right side were considered as blocking factors when applicable. The results were expressed as mean ± standard deviation. The overall difference was considered significant if p < 0.05. The post hoc pairwise comparisons were considered significant if p < 0.016, using the LSD rule. All statistical analyses were performed by SAS/STAT version 9.1.3 software (SAS Institute Inc.).
Results
Postoperatively, all the rabbits recovered without difficulty and the wounds healed uneventfully. The rabbits then resumed normal behavior and skin wound healing proceeded uneventfully until the time of sacrifice.
Five rabbits were excluded from mechanical and histological assessment because the distal end of the middle digit FDS was found to be inside the carpal tunnel when the paws were dissected after sacrifice. The EP results in these animals were similar to those in the included animals, but were not included in the EP analysis. The gaps in the middle digit FDS at 12 weeks after surgery in these animals were 17–26 mm, compared to an average final gap of 7 mm in the others.
Evaluation of Subsynovial Connective Tissue
Mechanical Testing
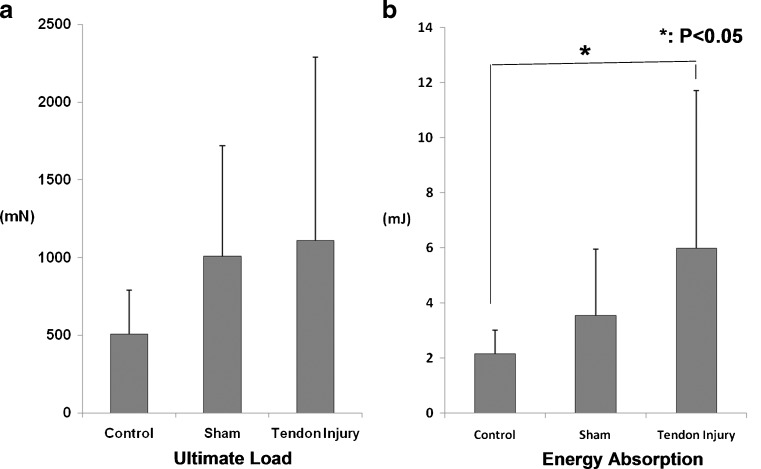
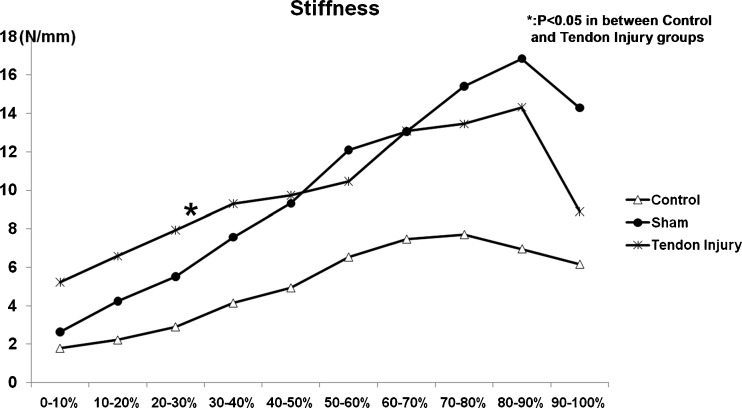
There was no significant difference in the ultimate load for any group (Fig. 3a). There were significant increases in energy absorption (Fig. 3b, p < 0.05) and stiffness, but only in the portion of the load/displacement curve between 20% and 30% of ultimate displacement (Fig. 4, p < 0.05) in the tendon injury group compared with the control group. There was no significant difference between the sham and control groups.
Fig. 3.
Mechanical testing results. a Ultimate load, b energy absorption
Fig. 4.
Mechanical testing (stiffness)
Light Microscopy
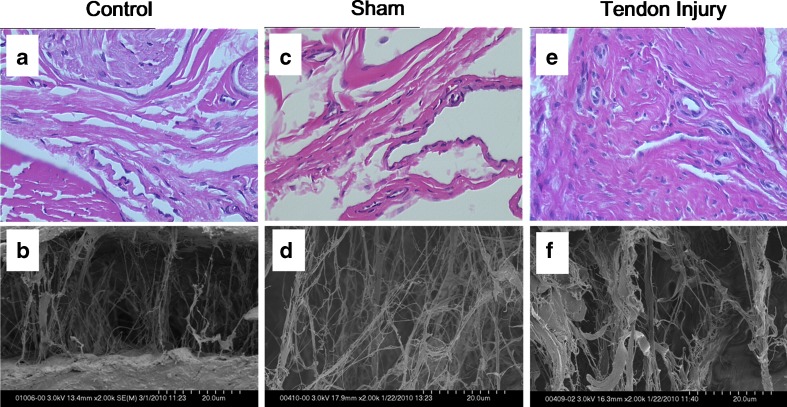
Twelve weeks after the surgery, we observed hypercellularity, vascular proliferation, thicker collagen bundles, and higher fibroblast density in the SSCT of the tendon injury group (Fig. 5e). In contrast, the sham group (Fig. 5c) appeared to be similar histologically to the control group (Fig. 5a). The mean fibroblast count in the measured area (0.005472 mm2) was 50.58 ± 6.63 in the specimens from the tendon injury group, 27.67 ± 5.15 in the specimens from the sham group, and 21.92 ± 4.15 in the control specimens. The mean fibroblast density was 9,244.03 ± 1,968.44/mm2 in the specimens from the tendon injury group, 5,002.74 ± 1,436.53/mm2 in the specimens from the sham group, and 4,020.47 ± 1,631.25/mm2 in the control specimens. The fibroblast density of the tendon injury group was significantly higher than both the sham (p < 0.0001) and normal control groups (p < 0.0001). There was no significant difference between the sham and control groups (p = 0.019).
Fig. 5.
Histology and structure of SSCT 12 weeks after surgery. a Control HE (×920), b control under SEM (×2,000), c sham HE (×920), d sham SEM (×2,000), e tendon injury HE (×920), f tendon injury SEM (×2,000)
Transmission Electron Microscopy
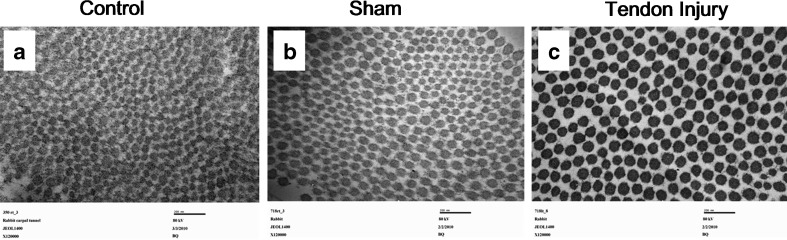
The mean size of the collagen fibers in the SSCT was estimated to be 55.55 ± 8.61 nm in the control specimens, 58.91 ± 10.80 nm in the tendon injury specimens, and 54.13 ± 7.72 nm in the sham specimens (Fig. 6a–c). The mean size of the collagen fibers of the SSCT of the tendon injury group was significantly higher than either the sham (p = 0.0004) or control groups (p = 0.0006). There was no significant difference between the sham and control groups (p = 0.36).
Fig. 6.
Cross-section of collagen fibers of SSCT under TEM 12 weeks after surgery (×120,000). a Control, b sham, c tendon injury
Scanning Electron Microscopy
Twelve weeks after the surgery, we observed thicker collagen bundles in the SSCT of the tendon injury group (Fig. 5f). In contrast, the sham group histology (Fig. 5d) appeared to be similar to the normal control group (Fig. 5b).
Evaluation of Median Nerve
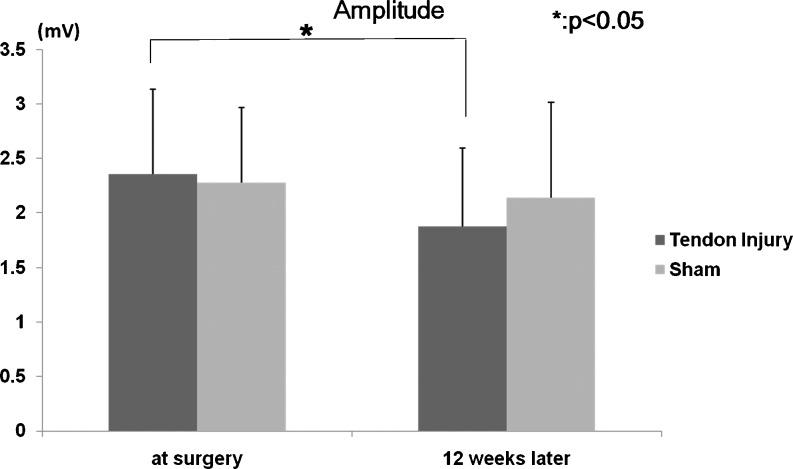
The distal motor amplitude showed a significant decrease at 12 weeks in the tendon injury group compared to the amplitude that was measured before surgery (p = 0.0027). There was no significant difference in distal motor amplitude before and after surgery in the sham surgery group (p = 0.43) (Fig. 7).
Fig. 7.
Motor amplitude
Discussion
The rabbit model is frequently used to study CTS [3, 7, 14, 22, 23] because the anatomy of the carpal tunnel is similar in humans and rabbits: all the digital flexors and the median nerve are within a well-defined tunnel, with a ligament bridging radial and ulnar carpal bones to form a canal, and there is a well-developed SSCT. Rats, while also commonly used [10], do not have an SSCT [3]; in dogs, the FDS tendons are outside the tunnel [3]. While primate species would of course be even closer to humans and thus would be appropriate as a CTS model [11], they were not available to us.
The use of hypertonic dextrose to induce SSCT fibrosis in an animal model of CTS has been previously reported [14, 23]. This model is based on the concept of proliferative therapy or prolotherapy, in which an irritant, in this case a hypertonic solution, is injected in order to initiate neovascularization and to stimulate collagen synthesis. However, the hypertonic solution can also have an osmotic effect on the median nerve, and thus, in this study, we sought to create the fibrosis by an intervention that would not injure the nerve and which might also be relevant to the hypothesis that CTS is caused by an injury to the SSCT.
In this current study, we designed the surgical intervention so that the treatment would be outside the carpal tunnel and not directly acting on the median nerve because we were attempting to create a model in which a non-neural injury occurs first, after which nerve changes are detected. We used only female rabbits, since clinically, carpal tunnel syndrome is far more common in women than in men [13, 19]. We chose a single time frame, 12 weeks, because we believed that, if there were an effect on the nerve, it would be apparent by that time; if such an effect was observed, we would then plan to investigate other time frames. Now that we have seen such a result, we do plan to examine both earlier and later time points to better understand the mechanism and longer-term effects of injury on the SSCT and median nerve.
By cutting the FDS tendon proximally, pulling it 5 mm distally through the carpal tunnel and then fixing the displaced tendon in that position, we hoped to at least pre-tension the SSCT, or possibly induce an SSCT injury. While there are data to support the idea that 5 mm of lengthening does apply some tension to the rabbit SSCT [22], we also hypothesized that such pre-tensioning would predispose the SSCT to further injury with continued activity postoperatively. The results here support that hypothesis, although we did not inspect or test the SSCT immediately post-tendon injury, to see if there was any measurable or observable injury. We plan that for a future study.
Our results do show that there were significant increases in SSCT energy absorption (p < 0.05) and stiffness (p < 0.05) in the tendon injury group compared with the control group, suggestive of injury and a healing response. However, there was no significant difference between the tendon injury and sham groups in this regard, suggesting that the sham procedure may also have some effect on the SSCT and that this effect can be detected 12 weeks postoperatively. Such data further suggest that the SSCT may be highly sensitive to local trauma, but that, too, require further study, especially to investigate the effects of trauma at earlier time points. Similar to the mechanical data, the SSCT histology data showed that both surgical interventions resulted in a significant change compared to controls, but there was no significant difference when comparing the tendon injury and sham surgery groups. The fibroblast density of the tendon injury group was significantly higher than either the sham (p < 0.0001) or control groups (p < 0.0001), but there was no significant difference between the sham and control groups (p = 0.019). A previous study reported that the mean fibroblast density in the specimens from patients with carpal tunnel syndrome (1,195 ± 374/mm2) was significantly higher than in unaffected individuals (721 ± 215/mm2) [1]. That study also reported that the mean size of the collagen fibers in the SSCT was 6.59 ± 3.43 μm in the specimens from the patients with carpal tunnel syndrome and 3.80 ± 1.51 μm in the unaffected individuals (p < 0.0001) [1]. In our study, we also observed thicker collagen bundles in the SSCT of the tendon injury group on SEM; these results are similar to the SEM results reported by Ettema et al. in specimens from patients with carpal tunnel syndrome [2]. Thus, our data suggest that a tendon injury can produce, at 12 weeks in a rabbit model, SSCT changes similar to those seen in human CTS patients. It is interesting to note that we found an increase in collagen fibril diameter in this study, as has also been observed in human CTS, when injury is most commonly associated with decreased fibril diameter [4–6]. We believe that the difference lies in the substrate. The SSCT normally has thin filaments of type VI collagen [1, 2], which thicken in CTS; tendons and ligaments in contrast normally have thick fibrils of type I collagen, which are larger than the diameters reported here, both before and after injury.
While the distal motor latency did not change significantly in our study, the distal motor amplitude did show a significant reduction at 12 weeks in the shear injury group (p = 0.0018). These findings are similar to those seen in mild cases of CTS, where a drop in amplitude has been shown to be an earlier finding than a change in latency, both clinically and experimentally [9, 16]. We did not see any evidence of demyelination, but demyelination is associated with decreased nerve conduction velocity, increased temporal dispersion, and in the most severe cases, conduction block, whereas a reduction in amplitude is more consistent with axonal loss [20]. Longer follow-up may be necessary to detect delayed distal motor latency and nerve demyelination.
With regard to testing, because the middle digit FDS tendon was divided proximally and pulled distally, we assumed that any resistance to FDS motion during our mechanical testing would come from a combination of friction between the tendon and pulley and the material properties of the tissue joining the middle digit FDS to the surrounding tendons and median nerve. The tendon pulley friction force would, presumably, be constant between all three test groups, while the latter force would be more variable as it would represent the sum of the material properties of the normal SSCT, which surrounds all the rabbit flexor tendons [3] and the properties of any adhesions that might have formed as a result of manually pulling this one tendon distally, which could have stretched or mechanically disrupted some SSCT fibers. We sutured the tendon in the distally displaced position so that any stretched SSCT fibers would remain under stretch and thus perhaps be liable to subsequent injury, in the postoperative period when the rabbit resumed normal activity, thus possibly amplifying the ongoing effect of the injury, while minimizing its immediate effect and thereby also minimizing the risk of any injury to the median nerve. We plan to study the effect of such SSCT tensioning in a future study. Because we were not certain if the sham surgery might also induce some degree of SSCT injury, we also included a normal control group.
This study has several limitations, in addition to those noted above. First, we did not assess the mechanisms of fibrosis by detecting specific collagen or cytokine expression in the initial days and weeks after surgery. As noted above, our choice of a single, medium term time point was based on a desire to limit animal use. If no changes were found in nerve and SSCT function at this time point, we considered that looking at earlier or later times, for details of early SSCT changes and more severe nerve changes, respectively, would not be warranted. Now that we have demonstrated that both the SSCT and median nerve are affected by this single intervention, we plan to study both short-term (initially days to weeks) and long-term (6 months or more) effects in this model. Second, since we only evaluated a single time point, we have no data on the time course of the changes we observed. It is possible that data collected at earlier or later time points could be quite different, both in terms of the results at 12 weeks in this rabbit model and in comparison to the situation in cases of clinical CTS. However, the finding from the current study will be useful in designing future short- and long-term studies. Third, we did not study hormonal effects on SSCT fibrosis. Menopause, pregnancy, hypothyroidism, and diabetes have all been considered to be risk factors for carpal tunnel syndrome. These studies should be conducted in the future.
In conclusion, cutting and displacing the FDS tendon distally results in SSCT fibrosis, changes in SSCT histology and material properties similar to those seen in patients with CTS, and changes in nerve physiology similar to those seen in mild carpal tunnel syndrome. Unlike other models, the tendon injury approach does not violate the carpal tunnel, does not introduce a foreign object within the carpal tunnel, and does not directly injure the median nerve. These features suggest that this SSCT injury model may provide a useful and clinically relevant tool with which to study the etiology and early treatment of CTS.
Acknowledgments
This study was funded by a grant from NIH (NIAMS AR 49823). The authors sincerely thank Ramona L. Kirk for her assistance with animal care.
Conflicts of Interest
The authors declare they have no conflict of interest.
References
- 1.Ettema AM, Amadio PC, Zhao C, et al. A histological and immunohistochemical study of the subsynovial connective tissue in idiopathic carpal tunnel syndrome. J Bone Joint Surg Am. 2004;86-A:1458–66. doi: 10.2106/00004623-200407000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Ettema AM, Amadio PC, Zhao C, et al. Changes in the functional structure of the tenosynovium in idiopathic carpal tunnel syndrome: a scanning electron microscope study. Plast Reconstr Surg. 2006;118:1413–22. doi: 10.1097/01.prs.0000239593.55293.c7. [DOI] [PubMed] [Google Scholar]
- 3.Ettema AM, Zhao C, An K-N, et al. Comparative anatomy of the subsynovial connective tissue in the carpal tunnel of the rat, rabbit, dog, baboon, and human. Hand. 2006;1:78–84. doi: 10.1007/s11552-006-9009-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frank C, Bray D, Rademaker A, et al. Electron microscopic quantification of collagen fibril diameters in the rabbit medial collateral ligament: a baseline for comparison. Connect Tissue Res. 1989;19:11–25. doi: 10.3109/03008208909016811. [DOI] [PubMed] [Google Scholar]
- 5.Frank C, McDonald D, Bray D, et al. Collagen fibril diameters in the healing adult rabbit medial collateral ligament. Connect Tissue Res. 1992;27:251–63. doi: 10.3109/03008209209007000. [DOI] [PubMed] [Google Scholar]
- 6.Frank C, McDonald D, Wilson J, et al. Rabbit medial collateral ligament scar weakness is associated with decreased collagen pyridinoline crosslink density. J Orthop Res. 1995;13:157–65. doi: 10.1002/jor.1100130203. [DOI] [PubMed] [Google Scholar]
- 7.Lim JY, Cho SH, Han TR, et al. Dose-responsiveness of electrophysiologic change in a new model of acute carpal tunnel syndrome. Clin Orthop Relat Res. 2004;120–6. [DOI] [PubMed]
- 8.Lluch AL. Thickening of the synovium of the digital flexor tendons: cause or consequence of the carpal tunnel syndrome? J Hand Surg Br. 1992;17:209–12. doi: 10.1016/0266-7681(92)90091-F. [DOI] [PubMed] [Google Scholar]
- 9.Loong SC. The carpal tunnel syndrome: a clinical and electrophysiological study of 250 patients. Clin Exp Neurol. 1977;14:51–65. [PubMed] [Google Scholar]
- 10.Mackinnon SE, Dellon AL, Hudson AR, et al. Chronic nerve compression—an experimental model in the rat. Ann Plast Surg. 1984;13:112–20. doi: 10.1097/00000637-198408000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Mackinnon SE, Dellon AL, Hudson AR, et al. A primate model for chronic nerve compression. J Reconstr Microsurg. 1985;1:185–95. doi: 10.1055/s-2007-1007073. [DOI] [PubMed] [Google Scholar]
- 12.McDowell EM, Trump BF. Histologic fixatives suitable for diagnostic light and electron microscopy. Arch Pathol Lab Med. 1976;100:405–14. [PubMed] [Google Scholar]
- 13.Nordstrom DL, DeStefano F, Vierkant RA, et al. Incidence of diagnosed carpal tunnel syndrome in a general population. Epidemiology. 1998;9:342–5. doi: 10.1097/00001648-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 14.Oh S, Ettema AM, Zhao C, et al. Dextrose-induced subsynovial connective tissue fibrosis in the rabbit carpal tunnel: a potential model to study carpal tunnel syndrome? Hand. 2008;3:34–40. doi: 10.1007/s11552-007-9058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okutsu I, Ninomiya S, Yoshida A, et al. Measurement of carpal canal and median nerve pressure in patients with carpal tunnel syndrome. Tech Hand Up Extrem Surg. 2004;8:124–8. doi: 10.1097/01.bth.0000127362.60294.92. [DOI] [PubMed] [Google Scholar]
- 16.Pavesi G, Olivieri MF, Misk A, et al. Clinical–electrophysiological correlations in the carpal tunnel syndrome. Ital J Neurol Sci. 1986;7:93–6. doi: 10.1007/BF02230424. [DOI] [PubMed] [Google Scholar]
- 17.Seradge H, Jia YC, Owens W. In vivo measurement of carpal tunnel pressure in the functioning hand. J Hand Surg Am. 1995;20:855–9. doi: 10.1016/S0363-5023(05)80443-5. [DOI] [PubMed] [Google Scholar]
- 18.Spurr AR. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969;26:31–43. doi: 10.1016/S0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- 19.Stevens JC, Sun S, Beard CM, et al. Carpal tunnel syndrome in Rochester, Minnesota, 1961 to 1980. Neurology. 1988;38:134–8. doi: 10.1212/WNL.38.1.134. [DOI] [PubMed] [Google Scholar]
- 20.Tankisi H, Pugdahl K, Johnsen B, et al. Correlations of nerve conduction measures in axonal and demyelinating polyneuropathies. Clin Neurophysiol. 2007;118:2383–92. doi: 10.1016/j.clinph.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 21.Wallis MA, Griffin RL. A routine method for embedding animal tissues in Spurr resin for electron microscopy. J Clin Pathol. 1973;26:77–8. doi: 10.1136/jcp.26.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamaguchi T, Osamura N, Zhao C, et al. The mechanical properties of the rabbit carpal tunnel subsynovial connective tissue. J Biomech. 2008;41:3519–22. doi: 10.1016/j.jbiomech.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshii Y, Zhao C, Schmelzer JD, et al. The effects of hypertonic dextrose injection on connective tissue and nerve conduction through the rabbit carpal tunnel. Arch Phys Med Rehabil. 2009;90:333–9. doi: 10.1016/j.apmr.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]