Abstract
Current therapies for pancreatic ductal adenocarcinoma (PDA) target individual tumor cells. Focal adhesion kinase (FAK) is activated in PDA and levels are inversely associated with survival. We investigated the effects of PF-562,271 (a small molecule inhibitor of FAK/PYK2) on a) in vitro migration, invasion and proliferation, b) tumor proliferation, invasion and metastasis in a murine model, and c) stromal cell composition in the PDA microenvironment. Migration assays were performed to assess tumor and stromal cell migration in response to cellular factors, collagen and the effects of PF-562,271. An orthotopic murine model was used to assess the effects of PF-562,271 on tumor growth, invasion and metastasis. Proliferation assays measured PF-562,271 effects on in vitro growth. Immunohistochemistry was used to examine the effects of FAK inhibition on the cellular composition of the tumor microenvironment. FAK and PYK2 are activated and expressed in patient-derived PDA tumors, stromal components and human PDA cell lines. PF-562,271 blocked phosphorylation of FAK Y397 in a dose-dependent manner. PF-562,271 inhibited migration of tumor cells, cancer associated fibroblasts, and macrophages. Treatment of mice with PF-562,271 resulted in reduced tumor growth, invasion, and metastases. PF-562,271 had no effect on tumor necrosis, angiogenesis or apoptosis, but did decrease tumor cell proliferation and resulted in fewer tumor-associated macrophages and fibroblasts compared to control or gemcitabine. These data support a role for FAK in PDA and suggest that inhibitors of FAK may contribute to efficacious treatment of patients with PDA.
Keywords: pancreatic cancer, focal adhesion kinase, tumor associated fibroblasts, tumor microenvironment
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDA) remains the 4th leading cause of cancer-related deaths in the United States (1). Current cytotoxic therapies (gemcitabine, 5-fluorouracil, and oxaliplatin) and anti-EGF therapy (erlotinib) provide only modest improvement in overall survival (2–6).
PDA is characterized by aggressive locoregional invasion and early metastasis, indicating a central role for signaling pathways that regulate cell migration. Additionally, PDA typically evokes a desmoplastic reaction characterized by significant invasion and proliferation of stromal cancer-associated fibroblasts (myofibroblasts-stellate cells) and deposition of extracellular matrix (ECM) proteins (7).
Focal adhesion kinase (FAK), a nonreceptor cytoplasmic protein tyrosine kinase, is a key regulator of signals from the ECM mediated by integrins and growth factor receptors (8). FAK has been implicated in the regulation of a variety of cellular signaling pathways that control cell proliferation, cell cycle progression, migration, apoptosis, and cell survival (9–12). Expression of FAK is reported to be upregulated in many tumor types including colon, breast, prostate, thyroid, head and neck, liver, and esophageal (12–17), and elevated expression of FAK correlates with poor survival rates (17, 18). In PDA, it has been reported that increased FAK expression correlates with tumor size (19). Gene silencing of FAK by RNA interference resulted in suppression of anoikis resistance and metastasis in a preclinical model of PDA (20). Several small molecule inhibitors of FAK have been described, most notably PF-562,271 a potent small molecule inhibitor of FAK and the related tyrosine kinase, PYK2 (21). PF-562,271 is reported to be efficacious in inhibiting the proliferation of tumors in both xenograft and transgenic mouse models (22, 23)
Because of the prominent role of the microenvironment in the progression and metastasis of PDA, we undertook to determine the role of FAK/PYK2 catalytic activity in the proliferation and migration of tumor cells, and stroma-associated cells (macrophages and pancreatic stellate cells), the latter cells that populate the tumor microenvironment and contribute to the desmoplastic response of PDA. We show that chemotactic (IGF-I and EGF) and haptotactic (Col-I) migration and invasion of human PDA cells were inhibited by concentrations of PF-562,271 that effectively block FAK catalytic activity. In addition, PF-562,271 inhibited the migration of primary mouse macrophages and both immortalized human stellate cells and tumor associated fibroblasts to tumor cell conditioned media. In a preclinical mouse model, in which tumor-derived or established cancer cells are implanted into the pancreas of immunocompromised mice, treatment with PF-562,271 or gemcitabine significantly reduced the rate of tumor growth, local tumor invasion, and metastasis. Interestingly, only PF-562,271 led to a significant decrease in tumor associated macrophages and cancer-associated fibroblasts. The decrease in these cells correlated with the onset of treatment with PF-562,271 and correlated with a decrease in tumor cell proliferation and an observed reduction in tumor volume. These observations suggest that in the setting of an orthotopic tumor xenograft, treatment with PF-562,271 inhibits PDA cell growth either directly or through a mechanism that disrupts the recruitment of cancer-associated fibroblasts and monocytic cells to the tumor.
MATERIALS AND METHODS
Cell lines and culture conditions
The human PDA cell line L3.6pl was kindly provided by I.J. Fidler (The University of Texas M.D. Anderson Cancer Center, Houston, TX; August 2005); MDACC). (24) Human Pancreatic Stellate Cells (HPSC) (25) were obtained from Rosa Hwang (MDACC, July 2009). MPanc-96 and BxPC-3 were obtained from the American Type Culture Collection in (Rockville, MD, August 2005) and maintained in DMEM (HPSC, MPanc-96) or RPMI (BxPC3) supplemented with 10% FBS and antibiotics. Mouse macrophages were obtained in 2007 and prepared as described previously (26). All cell lines were expanded, aliquoted and frozen upon initial receipt, then new cells were thawed, propagated and used for experiments every six months. MPanc-96 and BxPC-3 were authenticated prior to purchase by the ATCC with cytochrome c oxidase subunit 1 ( COI) analysis, DNA profiling, cytogenetic analysis, flow Cytometry, and immunocytochemistry. L3.6pl cells, HPSCs, and MAD08-608 cells and mouse fibroblasts (described below) were authenticated in 2010 by the University of Virginia Biomolecular Research Facility with DNA profiling, cytogenetic analysis, flow Cytometry and immunocytochemistry.
MAD08-608 tumors and cell lines
The MAD08-608 tumor was derived from remnant tissue from a surgical pathology specimen of a patient with PDA in 2008 which was obtained under an institutional review board (IRB)-approved protocol. Tumor tissue (1mm3 cubes) was affixed to the pancreas of athymic nude mice. After 8 weeks, tumors were passaged into a second generation (F2) of mice. Tumors from F2 mice were then either digested with 0.8 mg/mL collagenase to establish a cell line or passaged into a third generation (F3) of mice for further experiments. The MAD08-608 fibroblasts were isolated by preferential trypsinization from an in vitro co-culture of tumor cells and fibroblasts. Fibroblast cultures were greater that 90% pure as determined by staining with anti-vimentin.
PF-562,271 and gemcitabine treatment
PF-562,271 (N-methyl-N-{3-[({2-[(2-oxo-2,3-dihydro-1H-indol-5-yl)amino]-5-(trifluoromethyl)pyrimidin-4-yl}amino)methyl]pyridin-2-yl}methanesulfonamide), generously provided by Pfizer, Inc. (La Jolla, CA), is a potent ATP-competitive reversible inhibitor selective for recombinant FAK and PYK2 kinase with an IC50 of 1.5 nM and 14 nM, respectively (21, 22). Animals were treated with PF-562,271 at 33 mg/kg body weight twice per day as described previously (22, 23). Gemcitabine (2′,2′-difluoro-2′-deoxycytidine) was given 10 mg/kg i.p. twice per week as described previously (27).
Western blot and receptor tyrosine kinase arrays
Cultured cells were lysed and subjected to Western blotting for FAK, PYK2, phospho-FAK (pY397), and phospho-PYK2 (pY402) as described previously (28). Human Phospho-Receptor Tyrosine Kinase (pRTK) Arrays (R&D Systems, Minneapolis, MN) were used to evaluate the pRTK signature in PDA cell lines per manufacturer’s protocol.
Transwell migration, invasion and proliferation assays
Cell migration and invasion assays were performed using transwell migration chambers (BD Biosciences, Bedford, MA) as previously described (26–28). Conditioned media was prepared from subconfluent MPanc-96 or MAD08-608 cells by harvesting growth medium (containing 0.5% FBS) for two 24 h periods. The conditioned media was centrifuged to remove cellular debris and concentrated 3 fold using Amicon Ultra-15 3K centrifugal filter devices (Millipore, Billerica, MA). Mock conditioned media was prepared by concentrating 0.5% FBS in similar fashion. For proliferation assays, cells (1.5 × 105) were plated in 96-well plates in 1% FBS-containing media and cell number was measured using the CyQuant assay (Invitrogen, Grand Island, NY).
Orthotopic tumor model
Six-week old male athymic nude mice (NIC, Fredericksburg, MD) were maintained in accordance with IACUC standards. Mice were anesthetized, a skin incision was made on the left flank and the pancreas was exteriorized. For MPanc-96 experiments, 50 μL of cell suspension was inoculated into the pancreatic parenchyma. For MAD08-608 experiments, 1 mm3 fragments were affixed to the pancreas using a 6-0 polypropylene suture (Ethicon, Cincinnati, OH). Mice were randomized into one of four treatment groups: PF-562,271 (33 mg/kg in 200μL vehicle by gastric lavage twice/day) and gemcitabine (10 mg/kg i.p. twice/week) beginning on day 7, and treatment continued until the mice were sacrificed on day 21 (MPanc-96) or day 35 (MAD08-608). At necropsy, retroperitoneal tumor invasion was assessed (confirmed on H&E), tumors were excised, measured and tumor volume was calculated. Livers were excised and examined, along with the peritoneal and mesenteric surfaces, for the presence of surface metastases.
Magnetic resonance imaging
Small animal magnetic resonance imaging (MRI) was performed on a 7.0T Clinscan MRI system (Siemens/Bruker, Siemens Corp, New York, NY). MRI data were collected using a True FISP sequence with a 30 mm mouse whole body coil and respiratory gating (SA instruments, Stony Point, NY). Images were processed and reviewed on PACS (Kodak Carestream, Rochester, NY). Volumetric analysis was performed by multiplying the slice thickness (0.5 mm) by the sum of the area of the tumor visible (mm2) in each slice.
Tissue staining and immunohistochemistry
Paraffin-embedded sections of tumors were stained with H&E using standard methods. Immunohistochemical staining was performed using antibodies to human vimentin (Epitomics, Burlingame, CA), human and mouse vimentin (Abcam, Cambridge, MA), FAK (Santa Cruz, Santa Cruz, CA), Ki67 (Cell Signaling, Davens, MA), F4/80 (AbD Serotec, Raleigh, NC), alpha smooth muscle actin [α-SMA] (Abcam), and PARP (Santa Cruz). CD31 (BD Pharmingen, San Jose, CA) staining was performed using frozen sections of tissue embedded in OCT. Negative controls were performed by omitting the primary antibody. Colon tissue from normal mice was used as a positive control.
Statistical analysis
Statistical analyses were performed using GraphPad Prism (GraphPad Software, Inc., San Diego, CA.) Nonlinear regression was used to evaluate the inhibitory effect of PF-562,271. One-way ANOVA was used to determine significance of difference among multiple arms of treatment in vivo and Bonferroni’s Multiple Comparison Test defined significance between groups. Student’s t test was used to determine the significance of difference in the mean values and Fisher’s exact test to calculate the significance of difference between categorical variables. Significance was determined with 95% confidence.
RESULTS
FAK/PYK2 in pancreatic cancers
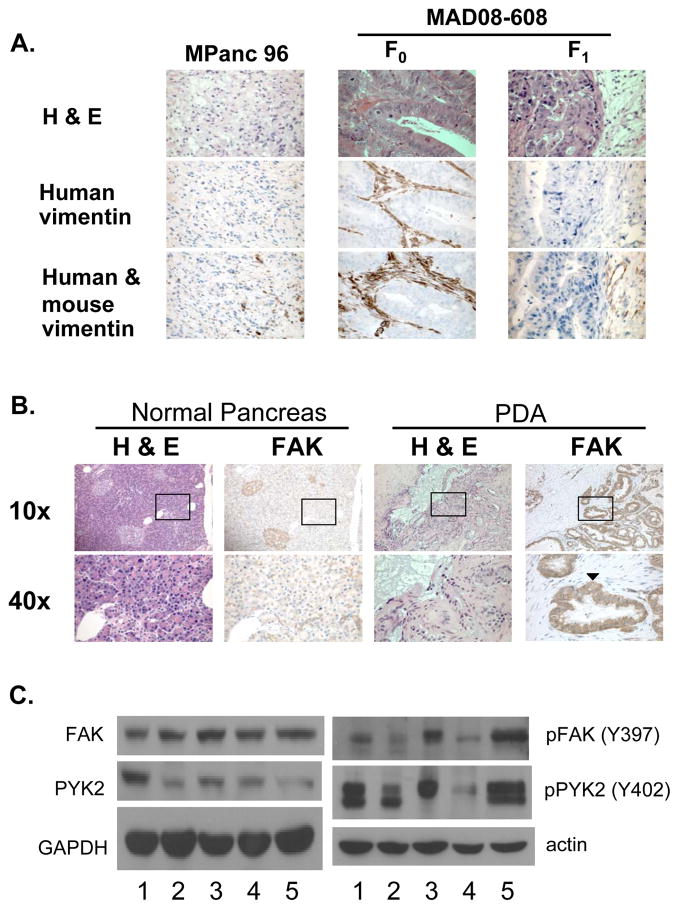
FAK and the related protein PYK2 are well studied regulators of cell adhesion signaling and migration (29). To better understand the role of FAK/PYK2 signaling in both PDA and stromal cell migration we have examined MPanc-96 and MAD08-608 PDA cells, macrophages and HPSCs (25). Orthotopic implantation of either MPanc-96 or MAD08-608 cells in the mouse pancreas resulted in the rapid growth of tumors and the maintenance of pancreatic cancer architecture (Fig. 1A). The original patient MAD08-608 tumor (F0) readily stained with a human-specific vimentin antibody confirming presence of human-derived stromal tissue. F1 tumors propagated in mice failed to stain with a human-specific anti-vimentin antibody but demonstrated robust staining with an antibody that recognized both mouse and human vimentin (Fig. 1A), indicating that the microenvironment of human tumors growing orthotopically in the mouse pancreas was reconstituted by stroma of mouse origin. Immunohistochemical staining of normal pancreas and PDA with an antibody to FAK revealed strong staining for FAK throughout the PDA sections with staining most intense in the malignant ductal cells (Fig. 1B). In contrast, weaker staining of normal pancreatic tissue for FAK expression was observed (Fig. 1B). Using Western immunoblotting, FAK and PYK2 expression were readily observed in both MPanc-96 and MAD08-608 cells as well as several other PDA cell lines and cultured HPSCs (Fig. 1C). In all cases, FAK and PYK2 were activated as evidenced by tyrosine phosphorylation of FAK397Y and PYK402Y (Fig. 1C).
Figure 1.
FAK expression in PDA. A, Representative photomicrographs are shown from tumor sections of mice bearing MPanc-96 or MAD08-608 F1 tumors and from the original patient tumor, designated MAD08-608 F0. Human-specific vimentin antibody (middle panel) demonstrates human-derived stromal tissue. Staining of tumors with an antibody recognizing both mouse and human-derived vimentin (bottom panel) demonstrated staining of stromal tissue in each of the tumor samples. B, The staining for FAK observed throughout the PDA sections was most intense in the malignant ductal cells (arrowhead). C, The level of FAK and PYK2 was determined by Western blotting of human PDA cell lines (1, BxPC-3; 2, L3.6pl; and 3, MPanc-96) the patient-derived tumor, MAD08-608 (4) and HPSCs (5). Parallel analysis was carried out to detect phosphotyrosine containing FAK and PYK2.
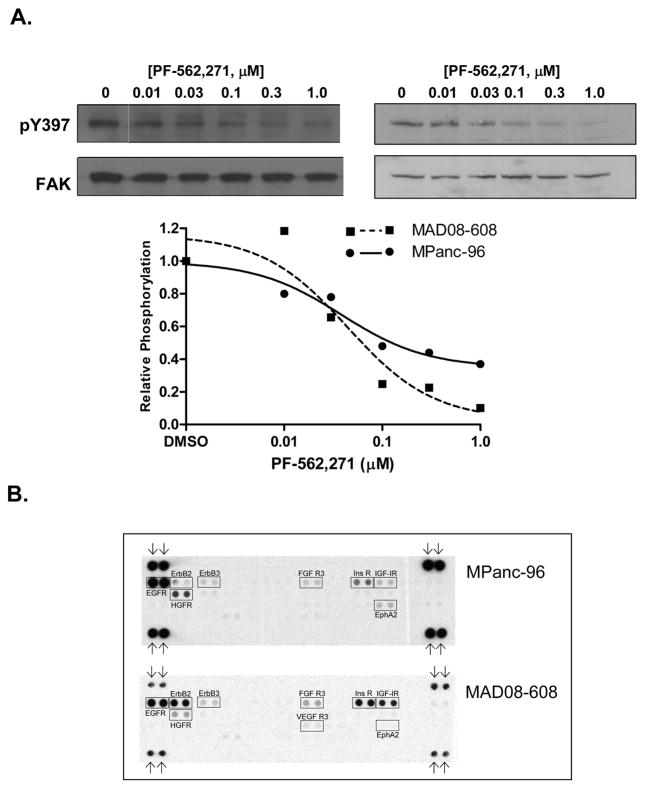
To evaluate the effects of PF-562,271 on FAK activity, MPanc-96 and MAD08-608 cells were incubated PF-562,271, and FAK activity was assessed by immunoblotting extracts with an antibody to FAK phospho-Y397. As shown in Fig. 2A, addition of PF-562,271 reduced the phosphorylation of FAK in both cell lines. Maximal inhibition of FAK Y397 phosphorylation was observed at 0.1 to 0.3 μM PF-562,271, consistent with earlier published observations (22, 28). These experiments confirm that FAK is active in PDA and is inhibited by treatment with PF-562,271.
Figure 2.
Effects of PF-562,271 on FAK phosphorylation. A, Maximal inhibition of FAK Y397 phosphorylation was observed at 0.1 to 0.3 μM PF-562,271 (left, MPanc-96; right, MAD08-608). B, Phospho-RTK arrays of MPanc-96 and MAD08-608 cell lines. Arrows denote the position of anti-pY controls.
PDA cell receptor signaling, migration and invasion
The activation of growth factor receptor (GFR) signaling pathways plays an important role in PDA growth, migration, invasion and metastasis. Both MPanc-96 and MAD08-608 cells express multiple activated GFRs as detected using phospho-RTK arrays. As shown in Fig. 2B, 9 of the 42 selected RTKs exhibited signals above background, strongest of which were EGF-R, ErbB2 (MAD08-608), FGFR3, Insulin-R, IGF-1R, and HGFR (c-Met) and EphA2. Western blots of extracts prepared from MAD08-608 and MPanc-96 cells confirmed the expression of EGFR, ErbB2, FGFR and HGFR (data not shown).
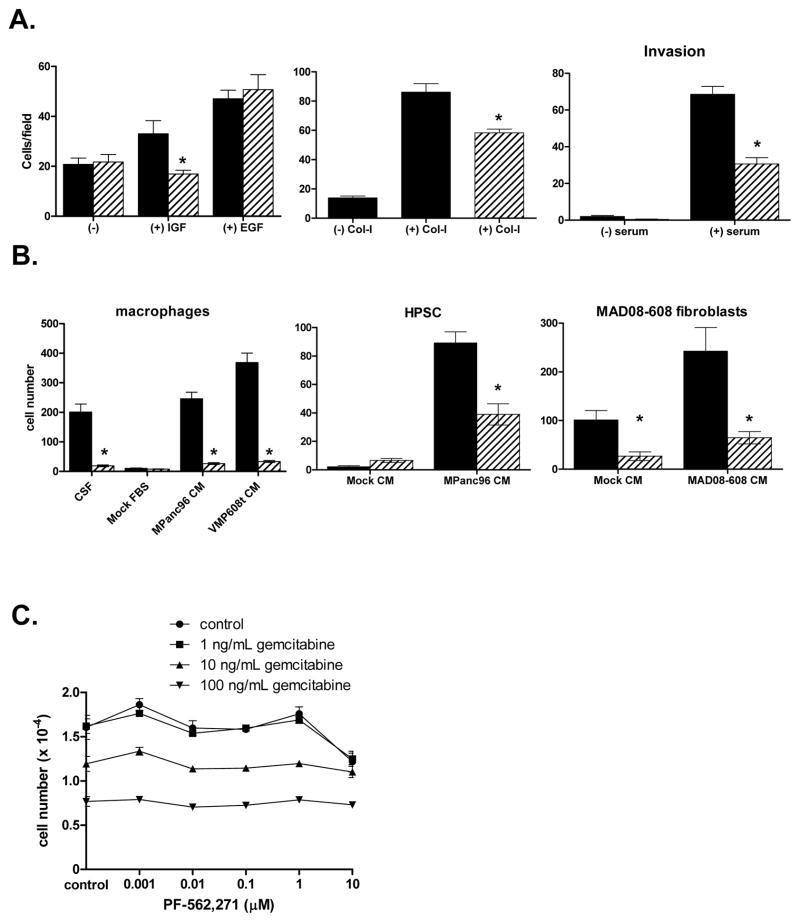
Since activated EGF and insulin/IGF family receptors were expressed in both MPanc-96 and MAD08-608 cells, we evaluated the effects of PF-562,271 on growth factor-mediated cell migration. In MPanc-96 cells, IGF-I (100 ng/mL) significantly stimulated cell migration (Fig. 3A) and treatment of MPanc-96 cells with 0.1 μM PF-562,271 resulted in a significant decrease in migration compared to IGF-I stimulation alone (Fig. 3A). EGF also stimulated migration, but interestingly, the observed migration was insensitive to addition of PF-562,271.
Figure 3.
Effects of PF-562,271 on tumor and stromal cell migration and proliferation. A, Left panel: Cell migration of MPanc-96 cells was measured in response to PBS (“−”), IGF-I (100 ng/mL) and EGF (25 ng/mL) in the presence (hatched bars) or absence (black bars) of 0.1μM PF-562,271. Middle panel: Haptotactic migration of MPanc-96 cells in uncoated (−) or Collagen-I-coated (+ Col-I) transwells in the presence (hatched bars) or absence (black bars) of 0.1μM PF-562,271. Right panel: Serum-stimulated invasion in the presence (hatched bars) or absence (black bars) of 0.1μM PF-562,271. * denotes p<0.05; bars denote standard error. B, The migration of stromal cells to either CSF (macrophages) or conditioned media (CM) from MPanc-96 or MAD08-608 cells Black bars denote cells cultured in the presence of ligand or conditioned medium, hatched bars denote cells cultured with the addition of 0.1μM PF-562,271. * denotes p<0.05 vs. control; bars denote standard error. C, Effects of PF-562,271 and gemcitabine on in vitro cell proliferation. Bars denote standard error.
Because of the increased deposition of ECM proteins (e.g. Col-I) in PDA, we evaluated the ability of PF-562,271 to inhibit haptotactic migration. Col-I stimulated migration of both MPanc-96 (Fig. 3A) and MAD08-608 cells (supplemental data). Pretreatment of either cell line with PF-562,271 (0.1 μM) led to a significant reduction in haptotactic migration (Fig. 3A). Perineural, lymphovascular, and extrapancreatic invasion are common histologic characteristics of PDA and associated with worse prognosis. To assess the invasive activities of MPanc-96 and MAD08-608 cells, assays were performed as above, but with Matrigel inserts. Serum stimulated invasion in both cell types and pretreatment with 0.1 μM of PF-562,271 significantly inhibited invasion (Fig. 3A and supplemental data). These data illustrate that migration and invasion of MPanc-96 and MAD08-608 cells are dependent to some degree on the catalytic activity of FAK and/or PYK2.
Tumor cells often produce cellular factors that contribute to the recruitment of host monocytes/macrophages and fibroblasts/myofibroblasts. To test whether MPanc-96 and MAD08-608 cells produced factors chemotactic for stromal cells, conditioned medium from tumor cells was used to stimulate the migration of mouse macrophages and either HPSCs or tumor-associated mouse fibroblasts (Fig. 3B). Tumor cell conditioned medium readily stimulated the migration of both macrophages and HPSCs, and this migration was significantly inhibited by treatment with PF-562,271. These observations support a role of FAK and PYK2 in cell migration of both tumor cells and cells that comprise the tumor microenvironment.
We evaluated the effects of PF-562,271 on in vitro cell proliferation. Growth assays were performed in the presence of serum-containing media supplemented with either PF-562,271, gemcitabine (a known inhibitor of PDA cell proliferation) or both. Treatment of both MPanc-96 and MAD08-608 cells with increasing concentrations of gemcitabine (1, 10, 100 ng/mL) resulted in the dose-dependent inhibition of cell growth with approximately 50% inhibition achieved at 100 ng/mL (Fig. 3C and supplemental data). Addition of PF-562,271 at concentrations up to 10 times the dose required to inhibit catalytic activity and migration/invasion (1 μM) had no effect on cell proliferation, either alone or in combination with gemcitabine. These results clearly indicated that PF-562,271 does not effectively block the growth of PDA cells under the culture conditions used in these experiments and does not enhance the inhibition of growth by gemcitabine.
PF-562,271 Inhibition of Orthotopic Growth, Invasion, and Metastasis
Because PF-562,271 inhibited tumor and stromal cell migration in vitro, we tested the efficacy of this inhibitor in an orthotopic mouse model. Following injection (MPanc-96) or implantation (MAD08-608) into the mouse pancreas, mice were treated with vehicle, PF-562,271 [33 mg/kg, twice daily, a dose that inhibited growth of several different tumor types in subcutaneous models (22)], gemcitabine [10 mg/kg, twice weekly, a dose selected to reduce tumor growth by approximately 50% (27)], or PF-562,271 plus gemcitabine. Tumor growth was assessed using MRI at weekly intervals (Fig. 4). As shown in Fig. 4B, two weeks post-treatment tumor volumes in mice treated PF-562,271 were 46 ± 8% smaller than control tumors, and gemcitabine treated mice were 53 ± 7% smaller than control mice. Tumors in mice treated with gemcitabine plus PF-562,271 were significantly smaller than control tumors; however, were not significantly different than tumors receiving either drug alone.
Figure 4.
Effects of PF-562,271 on pancreatic tumor growth in an orthotopic murine model. A, Representative MRI images of mice bearing MPanc-96 tumors after 2 weeks of treatment. B, Tumor volumes of MPanc-96 mice. C, Tumor volumes of MAD08-608 mice. * denotes p<0.05 vs. control; bars denote standard error.
Parallel analysis of mice bearing MAD08-608 tumors (Fig. 4C) showed that treatment with PF-562,271 or gemcitabine significantly reduced tumor size relative to control mice (59 ± 15%, and 60 ± 11% reduction, respectively). Tumors in mice treated with gemcitabine plus PF-562,271 were significantly smaller than control tumors; however, were not significantly different than tumors receiving either drug alone.
At necropsy, tumors were evaluated for invasion into the retroperitoneum and mouse livers and peritoneum were excised and evaluated for the presence of surface metastases. Treatment with PF-562,271 reduced the number of MPanc-96 tumor-bearing mice with detectable retroperitoneal invasion and liver and peritoneal metastasis (Table 1). Mice bearing MAD08-608 tumors showed a reduced incidence of peritoneal metastasis upon treatment with PF-562,271.
Table 1.
Effect of PF-562,271 on Metastasis and Invasion in Orthotopic Tumor-bearing Mice
| Control | PF-562,271 | |||
|---|---|---|---|---|
| MPanc-96 | ||||
| Retroperitoneal Invasion | 17/19 (89%) | 4/19 (21%)* | ||
| Liver Metastasis | 17/19 (89%) | 8/19 (42%)* | ||
| Peritoneal Metastasis | 19/19 (100%) | 11/19 (58%)* | ||
| MAD08-608 | ||||
|
| ||||
| Retroperitoneal Invasion | 14/16 (88%) | 7/13 (54%) | ||
| Liver Metastasis | 9/16 (56%) | 3/13 (23%) | ||
| Peritoneal Metastasis | 16/16 (100%) | 5/13 (38%)* | ||
P < 0.05 versus control
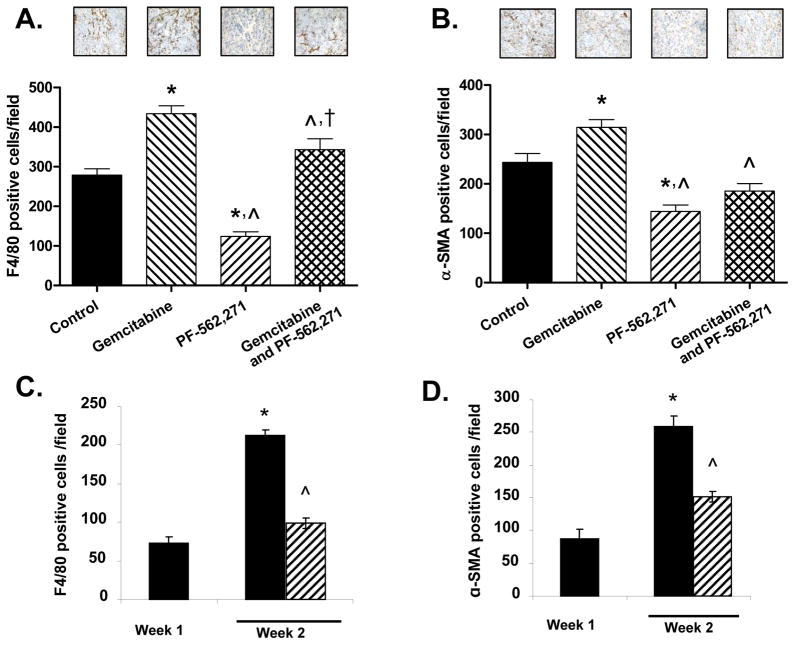
To evaluate the effects of PF-562,271, gemcitabine, and combination treatment on tumor cell proliferation, apoptosis, angiogenesis, macrophage infiltration, and fibroblast infiltration, representative sections from individual tumors from mice treated for 2 weeks (MPanc-96) or 4 weeks (MAD08-608) were stained with antibodies for Ki67, cleaved PARP, CD31, F4/80, and α-SMA, respectively. Analysis of sections from MPanc-96 and MAD08-608 tumors revealed no significant differences in apoptosis (cleaved PARP staining) or angiogenesis (CD31 staining) among any of the treatment groups (data not shown). Treatment of mice with PF-562,271 +/− gemcitabine led to a significant decrease in tumor cell proliferation (Ki67-positive cells) in MPanc-96 (29%, p<0.01 and 23%, p<0.05, respectively) and MAD08-608 (47%, p<0.001 and 41%, p<0.01, respectively) tumors relative to control (data not shown). In mice tumored with MPanc-96 (Fig. 5A) and MAD08-608 (supplemental data), significantly fewer F4/80-staining cells (macrophages) were observed in tumors from mice treated with PF-562,271 compared to control mice or mice treated with gemcitabine. Interestingly, mice treated with gemcitabine had the same (MAD08-608) or significantly more (MPanc-96) macrophages present in their tumors compared to control, although this difference was blunted in mice treated with both agents.
Figure 5.
Effects of treatment with PF-562,271 or gemcitabine on macrophage and myofibroblast infiltration. MPanc-96 tumor sections stained for macrophages with an F4/80 antibody (A) or fibroblasts with anti-α-SMA antibody (B). * denotes p<0.05 vs. control; ^ vs. gemcitabine; † vs. PF-562,271; bars denote standard error. C, D, Animals were orthotopically injected with MPanc-96 cells and one week later treated with PF-562,271. Tumors were harvested at one or two weeks post-implantation and sections stained with antibodies to F4/80 (C) or α-SMA (D). * denotes p<0.05 vs. week 1; ^denotes p<0.05 vs. week 2 control; bars denote standard error. The data in A–D represent the number of positively stained cells per high-powered field assessed in 7 sections from 3 individual tumors.
The presence of α-SMA-positive cells (cancer-associated fibroblasts) was also significantly reduced in mice treated with PF-562,271 in a pattern similar to that seen with the macrophages (Fig. 5B and supplemental data). Treatment of mice with gemcitabine also led to no change or a slight increase in fibroblasts within the tumor compared to control, while treatment with PF-562,271 yielded significantly fewer fibroblasts as a single agent compared to control. (Fig. 5B and supplemental data).
To further evaluate the effects of PF-562,271 on timing of macrophage and fibroblast infiltration during the early phase of treatment, MPanc-96 mice were sacrificed at weeks 1 and 2 and tumor sections stained for F4/80 and α-SMA. Normal murine pancreas had no detectable F4/80 or α-SMA staining (week 0; data not shown). The appearance of F4/80-positive cells (macrophages, Fig. 5C) and α-SMA-positive cells (fibroblasts, Fig. 5D) was readily observed after one week of tumor growth. The number of F4/80- and α-SMA-positive cells increased between weeks 1 and 2 in control mice harboring either MPanc-96 or MAD08-608 tumors and in MAD08-608 mice an additional increase was observed after 3 weeks of tumor growth (Fig 5C, D and supplemental data). Treatment of mice with PF-562,271 resulted in a reduction in the number of both F4/80 and α-SMA positive cells (Fig. 5C, D and supplemental data) compared to control mice. These observations support the idea that treatment with the PF-562,271 influenced the recruitment or persistence of macrophages and fibroblasts within the tumor microenvironment.
DISCUSSION
PDA is a dismal disease with a poor prognosis and limited therapeutic options. Tumor cell migration and invasion are critical steps in tumor progression and metastasis, both occurring at early stages in PDA. An additional hallmark of PDA is the intense desmoplastic, fibrotic response, characterized by copious collagen deposition and abundant infiltrating macrophages and CAFs (7). In this study we utilize the FAK/PYK2 inhibitor, PF-562,271, to examine the efficacy of PF-562,271 treatment in chemotactic and haptotactic migration of PDA cells in vitro, the migration of macrophages and stromal cells to factors produced by tumor cells, and tumor formation and metastasis following orthotopic implantation of tumor cells in immunocompromised mice. We show that treatment of MPanc-96 cells, a well characterized PDA cell line, or MAD08-608, a recently derived tumor, with PF-562,271 inhibited cell migration to IGF-I and collagen, but failed to block cell proliferation in culture. Stromal cell migration and migration of macrophages and fibroblasts to tumor cell conditioned medium were also blocked by PF-562,271. Treatment of mice bearing MPanc-96 or MAD08-608 tumors with PF-562,271 inhibited tumor growth and reduced retroperitoneal invasion and peritoneal and liver metastasis. Importantly, we demonstrate that tumors from animals treated with PF-562,271 showed a significant decrease in the number of tumor associated F4/80-staining (macrophages) and α-SMA-staining (CAFs) cells and a significant decrease in tumor cell proliferation. In contrast, we observed no difference in the number of F4/80 or α-SMA staining cells in tumors from mice treated with tumor-inhibitory doses of gemcitabine. These data lead us to propose that PF-562,271 is acting to inhibit tumor proliferation either directly by inhibiting tumor cell proliferation or indirectly by blocking the recruitment and/or proliferation of stromal cells to the tumor microenvironment.
FAK upregulation in solid organ malignancies and the inverse correlation between FAK levels and overall survival have been demonstrated previously (12–17). Using immunohistochemistry, FAK was readily detectable in the malignant ductal cells, and to a lesser degree the surrounding stromal cells. Activated FAK and PYK2 were present in both human tumor cells and immortalized human stellate cells as well as in mouse macrophages (26). FAK plays a significant role in the regulation of adhesion turnover and migration (10, 12, 30). Consistent with this function of FAK, treatment of PDA cells with PF-562,271 blocked migration mediated by IGF-I and collagen and invasion in response to serum. Paradoxically, PF-562,271 failed to block the migration of MPanc-96 cells to EGF, indicating that these cells may express an alternative pathway(s) to regulate cell migration. This latter observation is consistent with recent findings that PF-562,271 fails to block the migration of a number of other non-PDA cell lines (JSD and JTP, unpublished observations) underscoring the variability of pathways available to cancer cells to mediate migration. Of note is the observation that PF-562,271 efficiently blocked the serum-induced invasion of both MPanc-96 and MAD08-608 cells. Indeed, other studies have shown that PF-562,271 consistently inhibits the invasive properties of non-PDA cell lines (JSD and JTP, unpublished observations), supporting the notion that FAK/PYK2 are important for events leading to the invasive properties of tumor cells. The observations that PF-562,271 failed to inhibit the growth in culture of MPanc-96 and MAD08-608 cells are consistent with a growing body of data indicating that PF-562,271 is not an effective inhibitor of cancer cell proliferation when cells are grown in vitro on rigid substrates such as plastic (28) (JSD, RWT and JTP, unpublished observations). These observations are in contrast to published reports, as well as data presented herein that show that in vivo PF-562,271 mediates significant tumor inhibition, suggesting that the importance of the FAK/PYK2 pathway(s) in tumor cell proliferation in vitro and in vivo is distinct.
A role of FAK in macrophage and fibroblast migration is well established (26, 31). Deletion of FAK in primary bone marrow macrophages leads to altered adhesion dynamics and impaired chemotaxis. Deletion of FAK impairs the recruitment of monocytes to sites of inflammation. In addition, knockdown of PYK2 expression in primary macrophages also resulted in a diminution of invasive capacity, indicating a possible role of this FAK-related tyrosine kinase in the inflammatory response. Similarly, either deletion of FAK, knockdown of FAK or inhibition of FAK catalytic activity results in the impaired migration of fibroblasts (29, 31). Given these observations it is not surprising that treatment with PF-562,271 could possibly restrict the recruitment of macrophages and fibroblasts to sites of tumor growth in the pancreas.
Using an orthotopic model of tumor growth that closely mimics the natural progression of PDA, we showed that treatment of mice with PF-562,271 inhibited tumor growth. Interestingly, the addition of gemcitabine revealed little increase in tumor inhibition. The lack of inhibition of in vitro proliferation by PF-562,271, but significant inhibition of tumor cell proliferation in vivo suggested to us that PF-562,271 may inhibit tumor growth in mice via a direct effect on the tumor as well as indirect mechanisms affecting the microenvironment. Using IHC, we found that CD31 staining of tumors was not affected by PF-562,271, suggesting reduced tumor growth was not the result of inhibition of angiogenesis. In contrast, F4/80 and α-SMA staining was significantly reduced after PF-562,271 treatment, but not in mice treated with gemcitabine. The decreased staining is consistent with the possibility that PF-562,271 is either directly inhibiting the recruitment of monocytic cells and CAFs or is inhibiting the tumor-dependent production of cytokines necessary for the recruitment of such cells. The fact that tumors from mice treated with gemcitabine (and having tumors similar in size to PF-562,271 treated animals) have F4/80 and α-SMA staining similar to control tumors argues that the reduction in staining is not due simply to a decrease in tumor size. Thus, we conclude from these observations that PF-562,271 treatment has an effect on the tumor microenvironment that suppresses tumor growth and metastasis. Interestingly, recent data demonstrate the importance of CAFs in pancreatic tumor progression and chemotherapy resistance (32).
The efficacy of PF-562,271 has been demonstrated in a number of preclinical studies. Roberts, et al. showed that administration of PF-562,271 inhibited the growth of several tumor cell lines engrafted subcutaneously in immunocompromised mice (22). Slack-Davis, et al. reported that PF-562,271 inhibited the outgrowth of castrate resistant prostate cancer in a genetically engineered murine model of prostate cancer (23). Recently, Bagi, et al. reported that the combination of sunitinib and PF-562,271 inhibited both angiogenesis and tumor aggressiveness, exhibiting greater effects than the relevant single agent (21). This treatment not only blocked tumor growth, but also impacted the ability of the tumor to recover upon withdrawal of the therapy.
Targeting the tumor cell-microenvironment interaction represents a novel approach to cancer therapy. Given recent evidence that CAFs promote tumor progression and our results in this study, further investigation of FAK inhibition and other therapies targeting the tumor microenvironment are warranted in the treatment of PDA.
Supplementary Material
Acknowledgments
Financial Support: NIH T32 HL007849 (JBS), American Cancer Society MRSG-0700201CCE (TWB), UVA Cancer Center Grant P30 CA44579 (TWB), CA 40042 (JTP), Pfizer (JTP)
Abbreviation list and notes
- PDA
pancreatic ductal adenocarcinoma
- FAK
focal adhesion kinase
- EGF
epidermal growth factor
- IGF
insulin-like growth factor
- ECM
extracellular matrix
- HPSC
human pancreatic stellate cell
- Col-I
collagen-I
- RTK
receptor tyrosine kinase
- GFR
growth factor receptor
Footnotes
Conflicts of Interest: none
Presented in part at the 2009 AACR Annual Meeting
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA: a cancer journal for clinicians. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DG, Benson AB., 3rd Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma: Eastern Cooperative Oncology Group Trial E2297. J Clin Oncol. 2002;20:3270–5. doi: 10.1200/JCO.2002.11.149. [DOI] [PubMed] [Google Scholar]
- 3.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 4.Di Costanzo F, Carlini P, Doni L, Massidda B, Mattioli R, Iop A, et al. Gemcitabine with or without continuous infusion 5-FU in advanced pancreatic cancer: a randomised phase II trial of the Italian oncology group for clinical research (GOIRC) Br J Cancer. 2005;93:185–9. doi: 10.1038/sj.bjc.6602640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louvet C, Labianca R, Hammel P, Lledo G, Zampino MG, Andre T, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23:3509–16. doi: 10.1200/JCO.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 6.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib Plus Gemcitabine Compared With Gemcitabine Alone in Patients With Advanced Pancreatic Cancer: A Phase III Trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 7.Mahadevan D, Von Hoff DD. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007;6:1186–97. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- 8.Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992;89:5192–6. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golubovskaya V, Beviglia L, Xu LH, Earp HS, 3rd, Craven R, Cance W. Dual inhibition of focal adhesion kinase and epidermal growth factor receptor pathways cooperatively induces death receptor-mediated apoptosis in human breast cancer cells. J Biol Chem. 2002;277:38978–87. doi: 10.1074/jbc.M205002200. [DOI] [PubMed] [Google Scholar]
- 10.Schaller MD. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophys Acta. 2001;1540:1–21. doi: 10.1016/s0167-4889(01)00123-9. [DOI] [PubMed] [Google Scholar]
- 11.Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71:435–78. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 12.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–16. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 13.Cance WG, Harris JE, Iacocca MV, Roche E, Yang X, Chang J, et al. Immunohistochemical analyses of focal adhesion kinase expression in benign and malignant human breast and colon tissues: correlation with preinvasive and invasive phenotypes. Clin Cancer Res. 2000;6:2417–23. [PubMed] [Google Scholar]
- 14.Owens LV, Xu L, Dent GA, Yang X, Sturge GC, Craven RJ, et al. Focal adhesion kinase as a marker of invasive potential in differentiated human thyroid cancer. Ann Surg Oncol. 1996;3:100–5. doi: 10.1007/BF02409059. [DOI] [PubMed] [Google Scholar]
- 15.Agochiya M, Brunton VG, Owens DW, Parkinson EK, Paraskeva C, Keith WN, et al. Increased dosage and amplification of the focal adhesion kinase gene in human cancer cells. Oncogene. 1999;18:5646–53. doi: 10.1038/sj.onc.1202957. [DOI] [PubMed] [Google Scholar]
- 16.Judson PL, He X, Cance WG, Van Le L. Overexpression of focal adhesion kinase, a protein tyrosine kinase, in ovarian carcinoma. Cancer. 1999;86:1551–6. doi: 10.1002/(sici)1097-0142(19991015)86:6<1551::aid-cncr23>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 17.Miyazaki T, Kato H, Nakajima M, Sohda M, Fukai Y, Masuda N, et al. FAK overexpression is correlated with tumour invasiveness and lymph node metastasis in oesophageal squamous cell carcinoma. Br J Cancer. 2003;89:140–5. doi: 10.1038/sj.bjc.6601050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itoh S, Maeda T, Shimada M, Aishima S, Shirabe K, Tanaka S, et al. Role of expression of focal adhesion kinase in progression of hepatocellular carcinoma. Clin Cancer Res. 2004;10:2812–7. doi: 10.1158/1078-0432.ccr-1046-03. [DOI] [PubMed] [Google Scholar]
- 19.Furuyama K, Doi R, Mori T, Toyoda E, Ito D, Kami K, et al. Clinical significance of focal adhesion kinase in resectable pancreatic cancer. World J Surg. 2006;30:219–26. doi: 10.1007/s00268-005-0165-z. [DOI] [PubMed] [Google Scholar]
- 20.Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. Focal adhesion kinase gene silencing promotes anoikis and suppresses metastasis of human pancreatic adenocarcinoma cells. Surgery. 2004;135:555–62. doi: 10.1016/j.surg.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 21.Bagi CM, Roberts GW, Andresen CJ. Dual focal adhesion kinase/Pyk2 inhibitor has positive effects on bone tumors: implications for bone metastases. Cancer. 2008;112:2313–21. doi: 10.1002/cncr.23429. [DOI] [PubMed] [Google Scholar]
- 22.Roberts WG, Ung E, Whalen P, Cooper B, Hulford C, Autry C, et al. Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res. 2008;68:1935–44. doi: 10.1158/0008-5472.CAN-07-5155. [DOI] [PubMed] [Google Scholar]
- 23.Slack-Davis JK, Hershey ED, Theodorescu D, Frierson HF, Parsons JT. Differential requirement for focal adhesion kinase signaling in cancer progression in the transgenic adenocarcinoma of mouse prostate model. Mol Cancer Ther. 2009;8:2470–7. doi: 10.1158/1535-7163.MCT-09-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruns CJ, Harbison MT, Kuniyasu H, Eue I, Fidler IJ. In vivo selection and characterization of metastatic variants from human pancreatic adenocarcinoma by using orthotopic implantation in nude mice. Neoplasia. 1999;1:50–62. doi: 10.1038/sj.neo.7900005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwang RF, Moore T, Arumugam T, Ramachandran V, Amos KD, Rivera A, et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918–26. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Owen KA, Pixley FJ, Thomas KS, Vicente-Manzanares M, Ray BJ, Horwitz AF, et al. Regulation of lamellipodial persistence, adhesion turnover, and motility in macrophages by focal adhesion kinase. The Journal of cell biology. 2007;179:1275–87. doi: 10.1083/jcb.200708093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bauer TW, Liu W, Fan F, Camp ER, Yang A, Somcio RJ, et al. Targeting of urokinase plasminogen activator receptor in human pancreatic carcinoma cells inhibits c-Met- and insulin-like growth factor-I receptor-mediated migration and invasion and orthotopic tumor growth in mice. Cancer Res. 2005;65:7775–81. doi: 10.1158/0008-5472.CAN-05-0946. [DOI] [PubMed] [Google Scholar]
- 28.Slack-Davis JK, Martin KH, Tilghman RW, Iwanicki M, Ung EJ, Autry C, et al. Cellular characterization of a novel focal adhesion kinase inhibitor. J Biol Chem. 2007;282:14845–52. doi: 10.1074/jbc.M606695200. [DOI] [PubMed] [Google Scholar]
- 29.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nature reviews Molecular cell biology. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 30.Tilghman RW, Parsons JT. Focal adhesion kinase as a regulator of cell tension in the progression of cancer. Semin Cancer Biol. 2008;18:45–52. doi: 10.1016/j.semcancer.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tilghman RW, Slack-Davis JK, Sergina N, Martin KH, Iwanicki M, Hershey ED, et al. Focal adhesion kinase is required for the spatial organization of the leading edge in migrating cells. J Cell Sci. 2005;118:2613–23. doi: 10.1242/jcs.02380. [DOI] [PubMed] [Google Scholar]
- 32.Muerkoster SS, Werbing V, Koch D, Sipos B, Ammerpohl O, Kalthoff H, et al. Role of myofibroblasts in innate chemoresistance of pancreatic carcinoma--epigenetic downregulation of caspases. Int J Cancer. 2008;123:1751–60. doi: 10.1002/ijc.23703. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.