Abstract
Dysregulated Notch signaling plays an important role in the progression of cancer. Notch signaling affects tumor growth and angiogenesis through the actions of its ligand Jagged1. In this study, we have developed a novel compound 3, 5-bis (2, 4-difluorobenzylidene)-4-piperidone (DiFiD) and determined that it inhibits cancer cell growth and its effects on Notch signaling. Intraperitoneal administration of DiFiD significantly suppressed growth of pancreatic cancer tumor xenografts. There was a reduction in CD31 positive blood vessels suggesting that there was an effect on angiogenesis. In vitro, DiFiD inhibited the proliferation of various human and mouse pancreatic cancer cells while increasing activated caspase-3. Cell cycle analyses demonstrated that DiFiD induced G2/M arrest and decreased the expression of cell cycle related proteins cyclin A1 and D1, while upregulating cyclin-dependent kinase inhibitor p21WAF1. We next determined the mechanism of action. DiFiD reduced Notch-1 activation, resulting in reduced expression of its downstream target protein Hes-1. We further determined that the reduced Notch-1 activation was due to reduction in the ligand Jagged1, and two critical components of the γ-secretase enzyme complex Presenilin-1 and Nicastrin. Ectopic expression of the Notch Intracellular domain (NICD) rescued the cells from DiFiD-mediated growth suppression. DiFiD treated tumor xenografts also showed reduced levels of Jagged1, and the γ-secretase complex proteins Presenilin-1 and Nicastrin. Taken together, these data suggest that DiFiD is a novel potent therapeutic agent that can target different aspects of the Notch signaling pathway to inhibit both tumor growth and angiogenesis.
Keywords: Notch signaling, DiFiD, tumor xenograft, Pancreatic cancer, γ-secretase complex
Introduction
Pancreatic cancer is the fourth leading cause of adult cancer related death associated with a high mortality rate (1). The American Cancer Society have estimated that 43,140 new cases and 36,800 deaths would occur during 2010 (2). Despite the advances in molecular pathogenesis, pancreatic cancer remains a major unsolved health problem in the United States (3, 4). Pancreatic cancer is a rapidly invasive, metastatic tumor which is resistant to standard therapies (5, 6). At present, single agent based chemotherapy (e.g. Gemcitabine) is the mainstay treatment for metastatic adenocarcinoma of pancreas. Gemcitabine treatment has a tumor response rate of below 10%; similarly none of the available current chemotherapeutic agents has objective response rate of over 10% (3, 5). The magnitude of this problem mandates the need for novel therapeutic agents.
Curcumin, an active ingredient of the spice turmeric, has been used to treat a number of ailments. Recent preclinical and clinical studies have demonstrated the anti-tumor, anti-angiogenic properties of curcumin (7–9). Pilot clinical trials have shown that curcumin is safe when consumed at a daily dose of 12 g for 3 months (10–12). However, poor intestinal absorption and bioavailability has limited its use (12, 13). Consequently, analogs of curcumin with similar safety profiles but increased anticancer activity and solubility are being developed. We and others have demonstrated that EF24, a fluorinated curcumin analog had greater biological activity and better bioavailability, but no increased toxicity (14–16). More importantly, EF24 had better pharmacokinetic profile when compared to curcumin (14, 15). Based on this compound, we further developed a novel derivative DiFiD and have determined the effect of this compound on tumor growth.
Notch signaling plays a critical role in maintaining the balance between cell proliferation and apoptosis and in the development of pancreatic cancer (17). Interaction of Jagged-1/2 with the Notch-1 receptor promotes a γ-secretase-dependent cleavage of the receptor and release of the intracellular domain (NICD), which translocates to the nucleus and activates transcription of target genes such as Hes-1and Hey1 (18). Increased expression of Notch genes and their ligands has been detected in human pancreatic cancer tissues (19). Overexpression of NICD accelerates the formation of oncogenic KRas-induced PanIN lesions (20). Oral administration of γ-secretase inhibitor in mice blocks the progression of PanIN to ductal adenocarcinoma (21). γ-secretase is a multiprotein intramembrane-cleaving protease with a growing list of protein substrates, including the Notch receptors. The four components of γ-secretase complex-presenilin, nicastrin, Pen2, and Aph1-are all thought to be essential for activity (19). The catalytic domain resides within presenilin; nicastrin has been suggested to be critical for substrate recognition. In this article, we have determined the effect of DiFiD on pancreatic cancer cells and identified one mechanism of action to be inhibition of the Notch signaling pathway.
Materials and Methods
Cells and Reagents
AsPC-1, MiaPaCa-2, PanC-1, BxPC-3 human and mouse Embryonic Fibroblast cell lines were obtained from American Type Culture Collection, Manassas, VA at Passage 4. Pan02 mouse pancreatic cancer cell line was obtained from the NCI DCTD tumor repository and previously published (9). All cells were grown in RPMI 1640 or DMEM containing 10% heat inactivated fetal bovine serum (Sigma Alrich, St. Louis, MO) and 1% antibiotic-antimycotic solution (Mediatech Inc, Herndon, VA) at 37°C in a humidified atmosphere of 5% CO2. All cells used in this study were within 20 passages after receipt or resuscitation (~3 months of noncontinuous culturing). The cell lines were not authenticated as they came from national repositories. 3,5-bis (2,4-difluorobenzylidene)-4-piperidone (DiFiD) was synthesized by Dr. Awasthi. N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT) was purchased (Sigma Alrich, St. Louis, MO).
Proliferation and Apoptosis assays
To assess proliferation, cells were seeded on to 96 well plates and grown overnight before treatment with increasing doses of DiFiD. Cell proliferation was determined by enzymatic hexoseaminidase assay as described previously (22). For apoptosis, caspase 3/7 activity was measured using the Apo-one Homogeneous Caspase-3/7 Assay kit (Promega, Madison, WI).
Colony formation assay
Briefly, 500 cells were incubated with DiFiD for 24 h, and then incubated for an additional 10 d in complete medium to allow colonies to form. The colonies were fixed in 10% formalin, followed by staining with hematoxylin. Experiments were done in triplicate.
Cell cycle analyses
Cells were treated with DiFiD for 24 h, and subsequently trypsinized and suspended in phosphate buffered saline (PBS). Single-cell suspensions were fixed using 70% ethanol for 2 h, and subsequently permeabilized with PBS containing 1 mg/ml propidium iodide (Sigma-Aldrich), 0.1% Triton X-100 (Sigma-Aldrich) and 2 µg DNase-free RNase (Sigma-Aldrich) at room temperature. Flow cytometry was done with a FACSCalibur analyzer (Becton Dickinson, Mountain, View, CA), capturing 50,000 events for each sample. Results were analyzed with ModFit LT™ software (Verity Software House, Topsham, ME).
Real Time Reverse-Transcription Polymerase Chain Reaction Analysis
Total RNA isolated from MiaPaCa-2 or Pan02 cells and tumor xenograft using TRIZOL reagent was reverse transcribed with Superscript II reverse transcriptase in the presence of random hexanucleotide primers (Invitrogen, Carlsbad, CA). Real Time PCR was performed using Jumpstart Tag DNA polymerase (Sigma Alrich, St.Louis, MO) and SYBR Green nucleic acid stain (Molecular Probes, Eugene, OR). Crossing threshold values for individual genes were normalized to β-Actin. Changes in mRNA expression were expressed as fold change relative to control. Primers used in this study were as follows: β-Actin: 5 ’-GCTGATCCACATCTGCTGG-3’ and 5’-ATCATTGCTCCTCCTCAGCG-3’; COX-2 : 5 ’-GAATCATTCACCAGGCAAATTG-3 ’ and 5 ’-TCTGTACTGCGGGTGGAACA-3’; VEGF: 5’-AGCGCAAGAAATCCCGGTA-3 ’ and 5 ’-TGCTTTCTCCGCTCTGAGC-3’; IL-8, 5′-CTCTTGGCAGCCTTCCTGATT-3′ and 5′-TATGCACTGACATCTAAGTTCCTTTAGCA-3′; Cyclin D1: 5’-AATGACCCCGCACGATTTC-3’ and 5’-TCAGGTTCAGGCCTTGCAC-3’; Notch-1: 5’-CACTGTGGGCGGGTCC-3’and 5’-GTTGTATTGGTTCGGCACCAT-3’; Hes-1 5’-AGGCGGACATTCTGGAAATG-3 ’ and 5 ’-CGGTACTTCCCCAGCACACTT-3’; Presenilin-1 5’ ATCATGCTCTTTGTCC-3’ and 5’- TCTTCTGTGAATGGG-3’; Nicastrin 5’-CAGATTGGCTGCCAGT-3’ and 5’-CTCCAGCAGAACCAT-3’.
Western Blot Analysis
Cell lysates were subjected to polyacrylamide gel electrophoresis and blotted onto Immobilion polyvinylidene difluoride membranes (Millipore, Bedford, MA). Antibodies were purchased from Cell Signaling Technology (Beverly, MA), Abcam Inc (Cambridge, MA) and Santa Cruz Biotechnology Inc (Santa Cruz, CA) and specific proteins were detected by the enhanced chemiluminescence system (GE Healthcare, Piscataway, NJ).
Immunofluorescence staining
The cells were grown on coverslips and treated with DiFiD for 24 h. After formalin fixing, the cells were then incubated with anti-Notch-1, anti-Jagged-1 and anti-Hes-1 antibody followed by FITC-conjugated secondary antibody. Cell images were observed under a fluorescent microscope.
Plasmids and Transfections
MiaPaCa-2 cells were transfected with plasmid EF.hICN1.CMV.GFP encoding the Notch-1 intracellular domain (NICD) or the empty vector EF.v-CMV.GFP (Addgene Inc, Cambridge, MA) and subsequently treated with 1 µM DiFiD for 24 h. Cell proliferation and apoptosis were detected using hexoaminadase assay and Apo-one Homogeneous Caspase-3/7 Assay kit, respectively.
Pan02 xenograft tumors in mice
Five-week-old male athymic nude mice (Jackson Labs) were utilized for in vivo experiments; they were maintained with water and standard mouse chow ad libidum as per the approved protocol by the KU Institutional Animal Care and Use Committee. Animals were injected with 1×106 Pan02 cells in the left and right flank and allowed to form tumors. One week following planting the cells and after observing the presence of a palpable tumor, DiFiD (200 µg/kg body weight) in 5% Na2 HCO3 buffer was administered intraperitoneally daily for 23 d. Tumor size was measured weekly. At the end of treatment the animals were euthanized, and the tumors were removed and weighed for use in histology and gene expression studies.
Immunohistochemistry
Tissues embedded in paraffin were cut to a section of 4 µM, deparaffinized and blocked with Avidin/Biotin for 20 min. The slides were then incubated with anti-COX-2, VEGF, CD31,Cyclin D1 or Notch-1 antibodies followed by secondary antibody such as HRP-goat anti rabbit antibody (for COX-2, VEGF, Cyclin D1 and Notch-1) and goat anti-rat (for CD31), and then developed with DAB (Sigma Aldrich). Finally, the slides were counterstained with hematoxylin.
Statistical analysis
All values are expressed as the mean ± SEM. Data was analyzed using an unpaired 2-tailed t test. P value of less than 0.05 was considered statistically significant.
Results
DiFiD inhibits pancreatic cancer cell proliferation
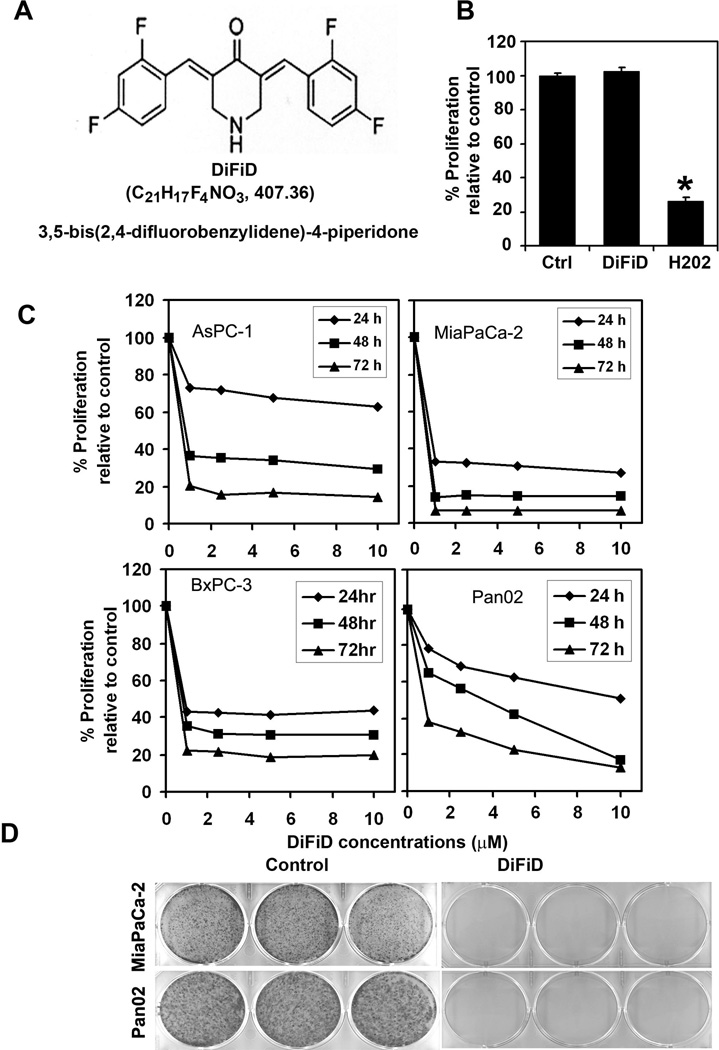
Curcumin is known to induce apoptosis of cancer cells, but the need for the high dose raises the question of in vivo bioavailability (23). Accordingly, we generated a novel compound, DiFiD. We first determined the effect of DiFiD on proliferation of four pancreatic cancer cell lines (Fig. 1A). DiFiD significantly suppressed the proliferation of these pancreatic cancer cells in a dose and time dependent manner. This anti-proliferation effect on tumor cells was seen within 24 h at a dose of 1 µM, which continued to significantly increase over the next 72 h (Fig. 1B). Similar results were obtained with colon, breast, lung, esophageal and gastric cancer cells (data not shown). Furthermore, the compound had much higher potency in inhibiting proliferation when compared to the parent In contrast, DiFiD did not affect the proliferation of normal mouse embryonic fibroblasts even when treated at 5 µM (Fig. 1C). As a positive control, hydrogen peroxide a known inducer of apoptotic cell death was used. Treatment with hydrogen peroxide significantly affected the proliferation of the MEFs (Fig. 1 C). These data suggest that DiFiD is not toxic to normal cells. To determine the long-term effect of DiFiD treatment, cells were treated with DiFiD for 24 h, following which they were allowed to grow in normal medium. DiFiD treatment suppressed colony formation in all the pancreatic cancer cells (Fig. 1D), suggesting that DiFiD-mediated effects on the tumor cells are irreversible.
Figure 1. DiFiD inhibits pancreatic cancer cell proliferation.
A, Molecular structure of DiFiD. B, Proliferation of normal mouse embryonic fibroblast cells is not affected by 5 µM DiFiD treatment for 48 h. C, DiFiD inhibits proliferation of pancreatic cancer cells. Cells were incubated with increasing doses of DiFiD (1–10 µM) for up to 72 h and analyzed for cell proliferation. DiFiD treatment resulted in a significant dose- and time-dependent decrease in cell proliferation in all four cells when compared with untreated controls. D, DiFiD inhibits colony formation. Pancreatic cancer cells were incubated with 1 µM DiFiD for 24 h and allowed to grow into colonies for 10 d. Incubation with DiFiD inhibits colony formation. Results are representative of three independent experiments.
DiFiD induces cell cycle arrest and apoptosis
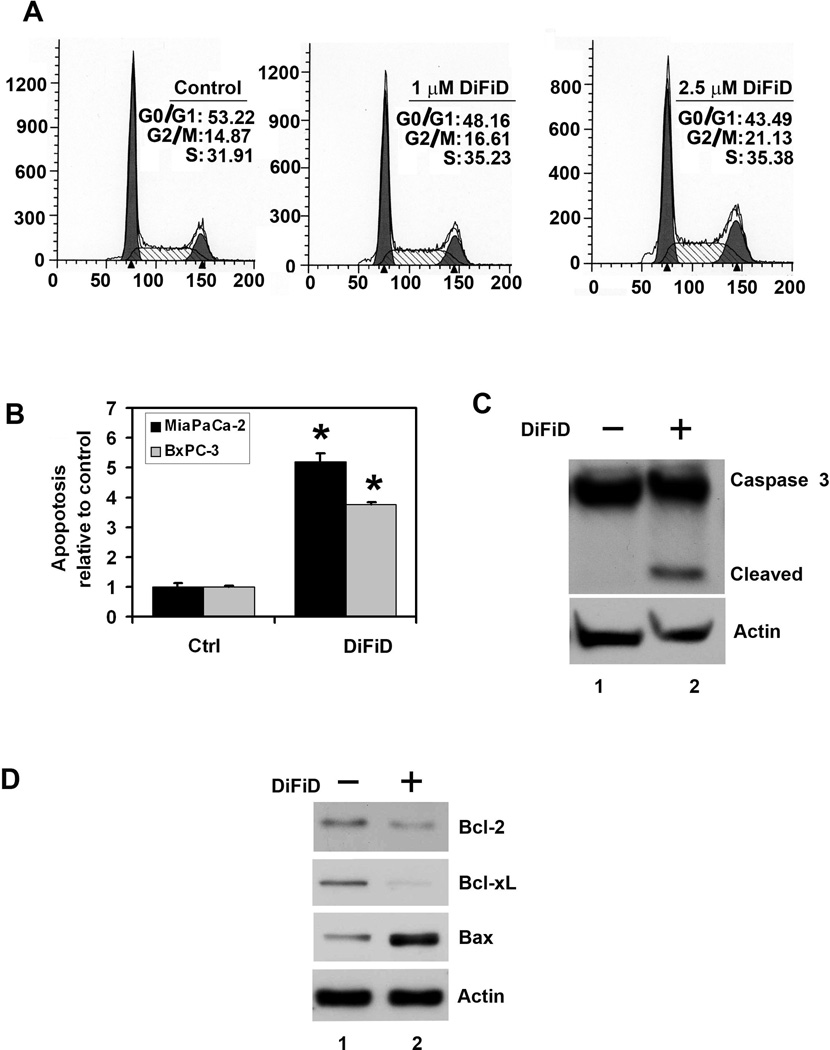
Given its effects on cell proliferation, we next performed cell cycle analysis to further characterize DiFiD’s effects. At 24 h, DiFiD (1 and 2.5 µM) induced growth arrest of MiaPaCa-2 cells at the G2/M and S-phase (Fig. 2A). At 48 h, there was a significant increase of cells in the G0 hypodiploid/fragmented DNA stage (data not shown). Similar results were observed in Pan02 cells (data not shown). Suppression of colon formation following treatment suggested that the compound was killing the cells. We therefore determined whether cell death was occurring through the apoptotic pathway. Caspase-3 and caspase-7 are key effector proteins in the apoptosis pathway involved in amplifying the signal from initiator caspases, such as caspase-8 and caspase-9 (24, 25). Increased activation of caspase-3 and caspase-7 was observed within 24 h in BxPC-3 and MiaPaCa-2 cells treated with 1 µM DiFiD (Fig. 2B). This was further confirmed by western blot analyses of MiaPaCa-2 cell lysates, which showed a significant increase in activated caspase-3 in cells treated with 1µM DiFiD (Fig. 2C). In addition, 1µM DiFiD inhibited the expression of anti-apoptotic genes Bcl-2 and Bcl-xL protein while increasing the expression of apoptosis-promoting Bax protein (Fig. 2D). These data suggest that even at a dose of 1 µM, DiFiD is a potent inducer of apoptosis of pancreatic cancer cells.
Figure 2. DiFiD induces cancer cell apoptosis.
A, Cell cycle analysis of DiFiD treated cells. MiaPaCa-2 cells were treated with up to 2.5 µM DiFiD for 24 h and examined by flow cytometry following propidium iodide staining for DNA content. DiFiD treatment leads to increased number of cells in the G2/M phase. Graphs are representative of data collected from three experiments. B, DiFiD treatment induces apoptosis in MiaPaCa-2 and BxPC-3 cells. The cells incubated with 1 µM DiFiD for 24 h and analyzed for apoptosis by caspase 3/7 activation. DiFiD treatment increased the number of apoptotic cells compared to untreated controls (*P <0.05). Results are from three independent experiments. C, DiFiD induces caspase 3, an apoptosis mediator. Lysates from MiaPaCa-2 cells incubated with 1 µM DiFiD were analyzed by western blotting for caspase 3 protein levels using rabbit anti-caspase 3 antibody. DiFiD treated cells shows cleaved (activated) caspase 3 while untreated cells have no cleaved caspase-3. D, DiFiD reduces expression of anti-apoptotic proteins Bcl-2 and Bcl-xL in treated cells when compared to untreated cells. Lysates from MiaPaCa-2 cells incubated with 1 µM DiFiD were analyzed by western blotting for Bcl-2, Bcl-xL, and Bax proteins. Both Bcl-2 and Bcl-xL were reduced while Bax expression was increased following DiFiD treatment.
DiFiD affects cell cycle related proteins
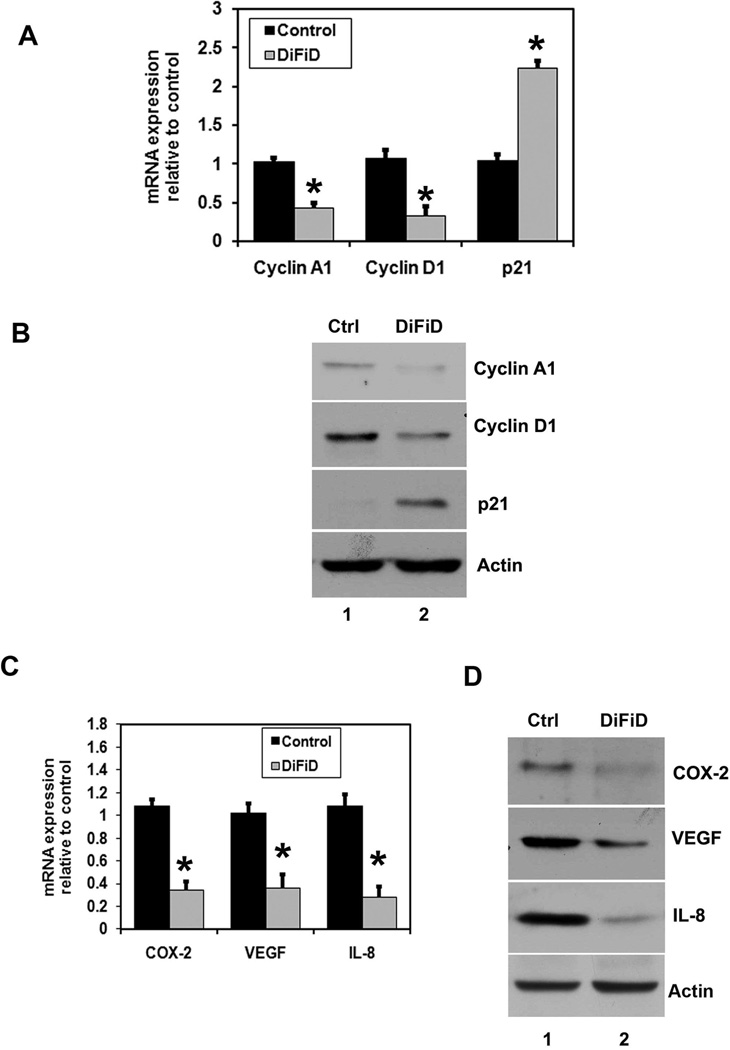
To further characterize the S-phase arrest, we examined the level of expression of several known S-phase cell cycle regulatory factors. Consistent with cell cycle arrest, the expression of cyclin A and D1 was found to be decreased, whereas p21 expression was increased (Fig. 3A, B), suggesting the mechanistic roles of these molecules during DiFiD induced cell cycle progression and cell cycle arrest by DiFiD. This observation suggests that the S-phase arrest by DiFiD is in part due to profound alterations in the expression of positive and negative regulatory cell cycle related proteins. Cyclin D1 overexpression has been linked to the development and progression of cancer. It is a cell cycle regulatory protein that regulates the G1 to S-phase transition of the cell cycle and functions as a cofactor for several transcription factors (26). However, MiaPaCa-2 cells treated with DiFiD resulted in reduced cyclin D1 expression at 24 h (Fig. 3A, B). Furthermore, Cyclin A2, which regulates S/G2 progression, was also down-regulated potentially slowing progression of cells out of S-phase.
Figure 3. DiFiD affects expression of cell cycle and cancer promoting genes.
A, MiaPaCa-2 cells were incubated with 1 µM of DiFiD for 24 h and mRNA levels of several cell cycle regulatory factors were analyzed by real-time PCR. DiFiD treatment showed downregulation in cyclin A1 and cyclin D1 mRNA and upregulation of p21 mRNA (*P <0.05). Data from three independent experiments. B, Lysates were analyzed by western blotting for cyclin A1, cyclin D1 and p21WAF1. DiFiD treatment caused significant reduction in cyclin A1 and cyclin D1 proteins while increasing expression of p21WAF1 protein. C, Real Time PCR analysis of total RNA from MiaPaCa-2 cells treated with 1 µM DiFiD for 24 h. DiFiD treatment showed reduction in the expression of COX-2, VEGF, and IL-8 mRNA (*P <0.05). Data from three independent experiments. D, Lysates from MiaPaCa-2 cells were analyzed by western blotting for COX-2, VEGF and IL-8 expression levels. DiFiD treatment caused significant reduction of the three proteins in the cells.
DiFiD inhibits the expression of cancer-promoting genes
COX-2, a key rate-limiting enzyme in prostaglandin synthesis is over expressed in many cancers. COX-2 plays a significant role in carcinogenesis, including increased invasiveness, promotion of angiogenesis and resistance to apoptosis (27). Previous studies have demonstrated increased COX-2 levels in pancreatic adenocarcinomas (28). Therefore, we next determined the effects of DiFiD treatment on COX-2 expression. DiFiD treatment significantly reduced COX-2 mRNA and protein levels in MiaPaCa-2 cells (Fig. 3A). Prostaglandins and the other tumor promoters are known to induce the expression of vascular endothelial growth factor (VEGF) and interleukin-8 (IL-8) in epithelial cells, thereby promoting angiogenesis and hence tumor growth (29). VEGF and IL-8 are known potent inducers of capillary growth into the tumor, and without angiogenesis, tumor growth normally stops at a diameter of about 1 to 2 mm (30). Previous studies have demonstrated that prostaglandins and the other tumor-promoting mediators are known to induce the expression of VEGF and IL-8 in epithelial cells (31). Hence, we also determined the effect of DiFiD on the expression of these two genes. Both VEGF and IL-8 mRNA and protein expression were significantly reduced in MiaPaCa-2 cells (Fig. 3C, D). Similar results were obtained with other pancreatic cancer cells (data not shown).
DiFiD inhibits Notch activation by downregulating the γ-secretase complex
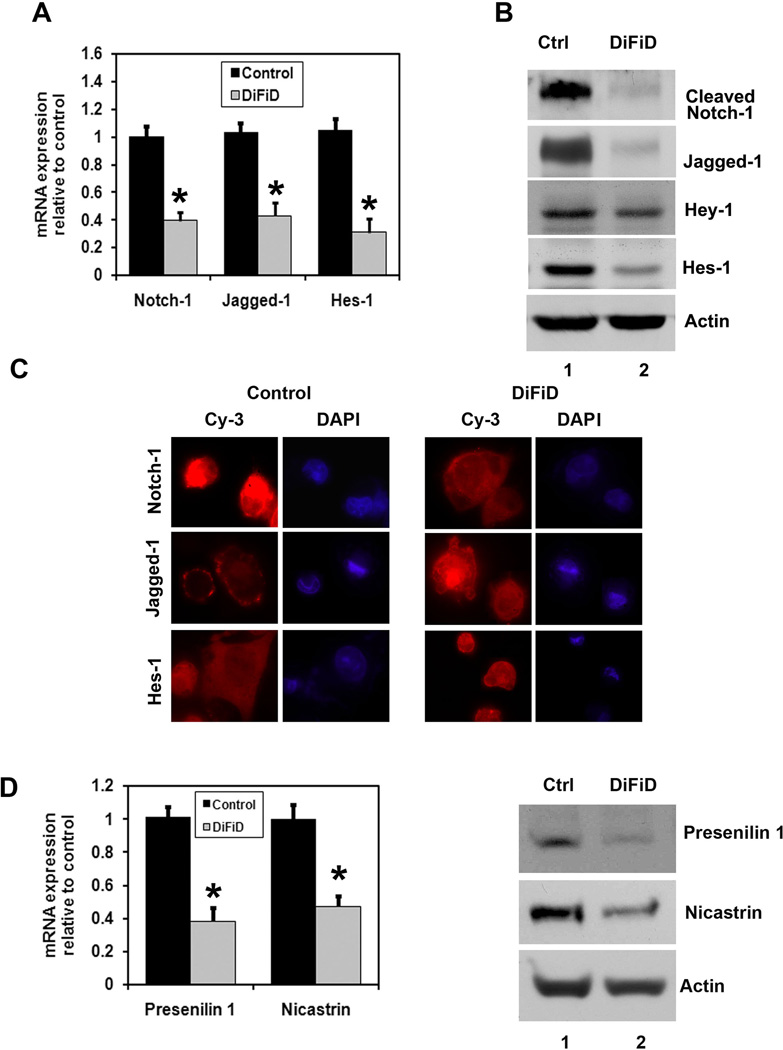
Notch-1 is a cell membrane associated protein. Ligand engagement causes the intracellular domain of the transmembrane receptor Notch (NICD) to be cleaved from the membrane through the action of the γ-secretase complex. NICD translocates to the nucleus, where it associates with a family of DNA binding proteins to activate transcription of Notch target genes such as hairy and enhancer-of-split 1 (Hes1) (32). We determined the effect of DiFiD on Notch-1, its ligand Jagged-1 and Hes-1 in MiaPaCa-2 cells. Both Notch-1 and Jagged-1 were down-regulated by DiFiD in MiaPaCa-2 cells within 24 h at the mRNA and protein levels (Fig. 4A, B). Protein levels were confirmed by immunofluoresence staining, where significantly lower levels of nuclear Notch-1 and cytoplasmic Jagged-1 were observed in the DiFiD treated cells (Fig. 4C). Moreover, there was a downregulation in Jagged-1 mRNA after DiFiD treatment in MiaPaCa-2 cells, suggesting the transcriptional inactivation of Jagged-1 gene expression in pancreatic cancer cells. To conform that Notch-1 activation was inhibited, we determined the effect of DiFiD on Hes-1 expression. There was a significant downregulation of Hes-1 at both the RNA and protein levels following DiFiD treatment in MiaPaCa-2 cells (Fig. 4 A, B, C). We next determined the mechanism by which DiFiD affects Notch-1 activation. γ-secretase is a multiprotein complex containing an intra-membrane cleaving protease. The complex has a growing list of proteins substrates, including the Notch receptors. The four components of γ-secretase complex, Presenilin, Nicastrin, Pen2, and Aph1 are all thought to be essential for activity (19, 33, 34). The catalytic domain resides within presenilin, while nicastrin has been suggested to be critical for substrate recognition (35). DiFiD treatment resulted in significant downregulation in the expression of two γ-secretase complex proteins, Presenilin and Nicastrin (Fig. 4D). This was at both the mRNA and protein levels. In addition, treatment with combination of DiFiD with a γ-secretase complex inhibitor DAPT further inhibits proliferation and induce apoptosis (Supplementary Fig. 1B). Effect of the combination on the inhibition of Notch activity was confirmed by the reduced expression of Hes-1 protein (Supplementary Fig. 1A). These data suggest that DiFiD-mediated downregulation of the Notch signaling pathway occurs in part through the inhibition of the γ-secretase complex.
Figure 4. DiFiD inhibits Notch-1 activation and the γ-secretase complex.
A, Real Time PCR analysis of total RNA from MiaPaCa-2 cells treated with 1 µM DiFiD treatment for 24 h. DiFiD treatment showed reduction in the expression of Notch-1, its ligand Jagged-1and the downstream target gene Hes-1 (*P <0.05). Data from three independent experiments. B, Lysates were also analyzed by western blotting. DiFiD treatment showed significant reduction in the expression of cleaved Notch-1, its ligand Jagged-1 and its target genes Hes-1 and Hey-1. C, MiaPaCa-2 cells treated with 1 µM of DiFiD for 24 h were subjected to immunoflurescence staining using anti-Notch-1, anti-Jagged-1 and anti-Hes-1 antibodies. DiFiD treatment resulted in reduced levels of Notch-1 protein in the nucleus, reduced levels of membrane bound Jagged-1 and inhibited Hes-1 expression (600X). D, γ-secretase complex proteins such as Presenilin-1 and Nicastrin were measured after treatment with 1 µM DiFiD for 24 h. Both the mRNA and protein levels were reduced with DiFiD treatment (*P <0.05). Data from three independent experiments.
Ectopic expression of NICD protects DiFiD-mediated inhibition of proliferation and induction of apoptosis
We next determined whether lack of Notch-1 activation is the reason for reduced growth of pancreatic cells, we expressed the intracellular domain NICD in MiaPaCa-2 and BxPC-3 cells. Western blot analyses of extracts from MiaPaCa-2 cells demonstrated increased expression of Hes-1 following ectopic expression of NICD (Supplementary Fig. 1C). Furthermore, while DiFiD alone inhibited the basal levels of Hes-1 expression, NICD rescued this inhibition resulting in increased Hes-1 expression. Moreover, ectopic expression of NICD reversed DiFiD-mediated inhibition of MiaPaCa-2 cell proliferation and induction of apoptosis (Supplementary Fig. 1D). Similar results were obtained in BxPC-3 cells (data not shown).
DiFiD inhibits tumor growth and angiogenesis
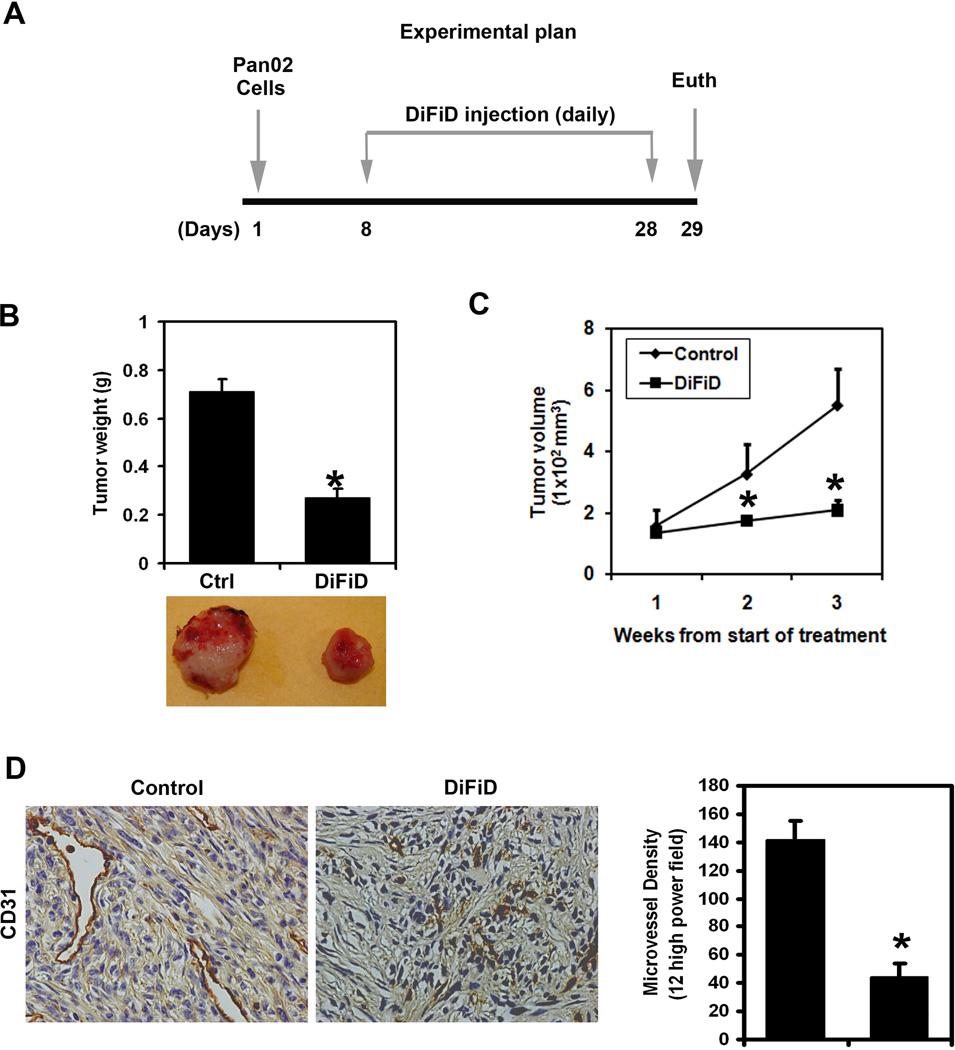
To evaluate the role of DiFiD on tumor growth in vivo, we next examined the ability of the compound in suppressing the growth of mouse pancreatic cancer cell xenografts. Pancreatic cancer cell xenograft tumors were allowed to develop and grow for one week following which DiFiD was administered intraperitoneally daily for three weeks. Treatment with DiFiD significantly inhibited the growth of the tumor xenografts (Fig. 5A). The excised tumors from control animals ranged from 700–800 mg, while those treated with DiFiD weighed <300 mg (Fig. 5B). In addition, tumor volume was significantly decreased (Fig. 5C). There was no apparent change in liver weight, spleen weight, or body weight in the animals (data not shown). These data imply that DiFiD is a potential therapeutic agent for treating pancreatic cancers but is relatively non-toxic to the mice. We also determined the effect of DiFiD on tumor vascularization by staining for the endothelial-specific antigen CD31. As shown in Fig. 5D, DiFiD treatment leads to a significant reduction in CD31 staining and to the obliteration of the normal vasculature that is associated with tumor angiogenesis. We also calculated the microvessel density and found it to be significantly decreased following DiFiD treatment (Fig. 5D).
Figure 5. DiFiD inhibits growth of Pan02 tumor xenografts.
A, Experimental Plan: Pan02 cells were injected in to the flanks of nude mice and palpable tumors were allowed to develop for 7 d. Subsequently DiFiD was injected daily intraperitoneally for up to 21 d. Tumor size was measured every week. On d 22, mice were euthanized, tumors were excised. B, DiFiD treatment resulted in significantly lower tumor weight when compared to controls (*P <0.05). C, Tumor volumes in DiFiD administered mice were smaller than that of control mice (*P <0.05). D, Tumor sections were stained for CD31, an endothelial cell specific surface marker and the vessel areas were counted. A representative figure is presented showing significant reduction in microvessels (400X). Data shows that microvessel density was significantly reduced in the xenografts of DiFiD treated animals (*P <0.05).
DiFiD inhibits the expression of cancer and angiogenesis-related genes and Notch-1
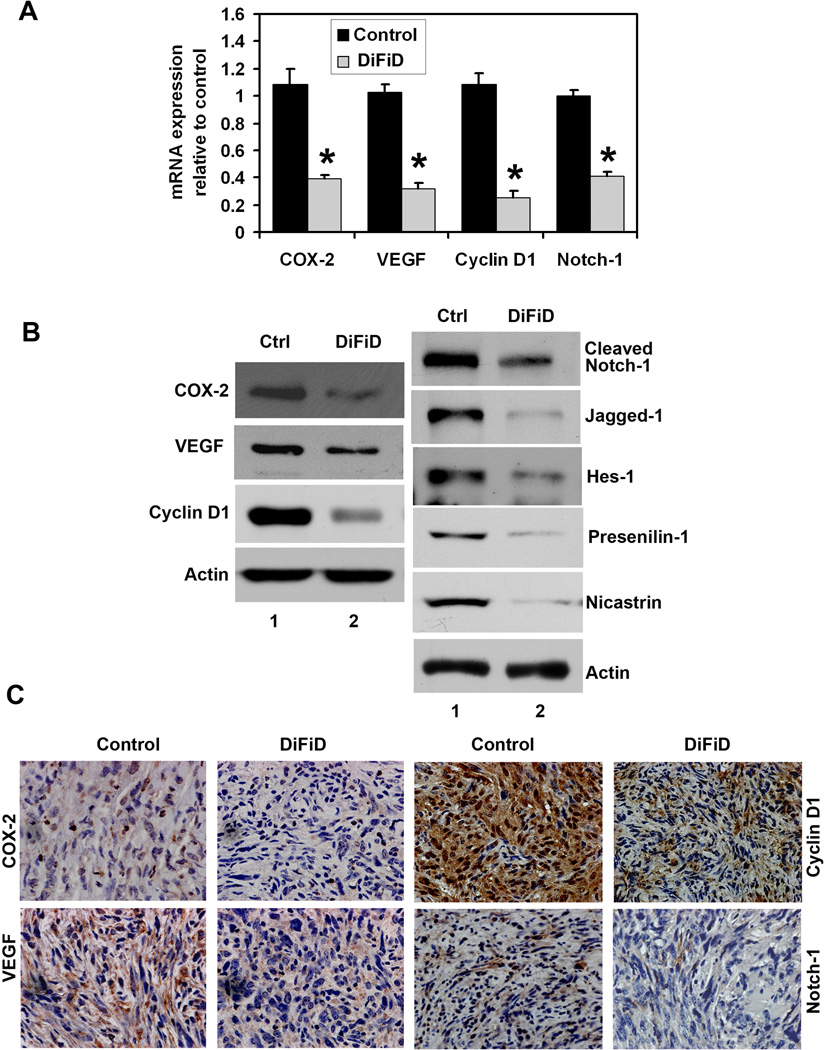
Given that DiFiD affected COX-2 expression in cells in culture, we next determined the effects of treatment on COX-2 expression. Both COX-2 mRNA and protein expression were significantly lower in DiFiD treated tumor xenografts when compared to the control tumors (Fig. 6A, B). Immunohistochemistry demonstrated diffuse cytoplasmic staining for COX-2 in the epithelial cells of the control tumors, with accentuated staining in subepithelial myofibroblasts (Fig. 6C). COX-2 staining was significantly reduced in both the epithelial cells and myofibroblasts in DiFiD -treated tumors.
Figure 6. DiFiD suppresses cancer promoting genes and Notch-1.
A, Total RNA from Pan02 tumor xenografts were subjected to Real Time PCR. DiFiD treatment resulted in reduced expression of COX-2, VEGF, cyclin D1 and Notch-1 mRNA levels when compared to control Pan02 tumor xenografts (*P <0.05). B, Western blot analysis showed that DiFiD treated animals have significantly lower levels of COX-2, VEGF, cyclin D1, cleaved Notch-1, Jagged-1, Hes-1, Presenilin 1 and Nicastrin proteins. C, Immunohistochemistry demonstrated that DiFiD treatment results in significantly reduced expression of COX-2, VEGF, cyclin D1 and Notch-1 in the tumor xenografts. Representative photographs are the magnification of × 400.
We also determined the effect of DiFiD on VEGF and cyclin D1 expression. Both VEGF mRNA and protein expression were significantly lower in DiFiD treated tumor xenografts when compared to controls (Fig. 6A, B). Immunohistochemistry further demonstrated that DiFiD treatment significantly reduced VEGF staining (Fig. 6C). DiFiD treatment also resulted in decreased cyclin D1 expression in the tumor xenografts (Fig. 6A–C). To further investigate whether DiFiD could down-regulate Notch-1 in vivo, we examined the Notch-1 expression in tumor tissues obtained from control and DiFID treated tumor mice. DiFiD treatment resulted in significantly lower levels of Notch-1 when compared to control untreated tumors suggesting that DiFiD could down-regulate Notch-1 in vivo (Fig. 6A–C). There was also a significant reduction in the expression of γ-secretase complex proteins, Presenilin and Nicastrin, as well as the downstream target gene Hes-1 (Fig. 6B).
Discussion
Our results indicate that DiFiD possesses great potential as a promising anti-pancreatic cancer therapeutic agent. Pancreatic cancer is one of the most lethal cancers and has emerged as a leading cause of cancer-related deaths in the western world, with most patients dying within one year of diagnosis. The significant morbidity, apparent toxicity and poor response rates of current chemotherapy regimens have led to searches for less toxic alternative therapies. The data presented in the article show that DiFiD inhibits the proliferation of pancreatic cancer cells, induces cell cycle arrest and apoptosis, resulting in reduced colony formation. These results were also replicated in vivo, where DiFiD decreased tumor growth and microvessel formation. Consistent with these findings, we observed reduced expression of the angiogenesis-inducing proteins VEGF and IL-8.
DiFiD treatment resulted in downregulation of Notch signaling through the inhibition of the γ-secretase complex. DiFiD also inhibited the expression of a downstream target for Notch-1, the Hes-1 gene. Recently, it has been reported that the Notch pathway plays a critical role in the processes of tumor cell proliferation and apoptosis in pancreatic cancer (18). Therefore, DiFiD mediated cell growth inhibition could be partly mediated via inactivation of Notch-1 activity. This was further confirmed by the combination of a GSI and DiFiD, which further inhibited proliferation and induced apoptosis. However, ectopic expression of NICD reversed the effects of DiFiD, and partially restored cell growth. Similarly, while the combination of DiFiD with a GSI further inhibited Hes-1 expression, the ectopic NICD partially rescued Hes-1 expression. However, Notch-1 is not the only pathway active in pancreatic cancer as many other cellular pathways are activated (36, 37). It would be interesting to determine whether DiFiD is equally potent in inhibiting these other signal transduction pathways.
Although curcumin can effectively inhibit the growth of pancreatic cancer cells, its low bioavailability in vivo means higher doses are required for effective treatment or prevention (38, 39). Hence, more potent and soluble curcumin analogs are being developed (14–16). DiFiD is a novel derivative that interferes with the progression of cancer by disrupting many of the characteristic cancer-promoting events. Moreover, in cell cycle analyses, DiFiD was observed to increase the number of cells in the G2/M phase after 24 h. At the same time, there are significantly higher levels of apoptosis. These data imply that DiFiD treatment leads to mitotic catastrophe in which the proliferating cancer cells undergo cell death but not necessarily an arrest in the G2/M phase of the cell cycle. This was further supported through our observation that the majority of cells were present in the sub-G0 phase at 48 h following treatment. Additional studies are of course necessary to determine if indeed there is mitotic catastrophe happening as a result of DiFiD treatment. This would include effects on cyclin B1 and cdc2, proteins that are abnormally activated during mitotic catastrophe (40). The role of checkpoint kinases Chk1/Chk2 and microtubule assembly also needs to be determined, and these studies are in progress.
In our studies, we observed marked suppression of tumor growth in mouse xenografts with DiFiD treatment. Further studies are needed to extend these findings prior to initiating clinical trials for pancreatic cancer. Specifically, absorption and pharmacokinetic activity is needed; nevertheless, preliminary studies in this manuscript suggest that DiFiD does not have any toxicity in liver, kidney, and spleen at the levels tested and allows the mice to maintain normal weight gain (data not shown). In addition, DiFiD seems to mediate its actions through multiple molecular targets, including COX-2, VEGF, IL-8 and Notch-1. Because COX-2 overexpression during pancreatic carcinogenesis causes resistance to apoptosis (41), treatment of pancreatic cancer cells with DiFiD may potentially restore susceptibility to apoptosis. Furthermore, overexpression of IL-8 plays an important role in tumor angiogenesis and contributes significantly to the aggressive biology of human pancreatic cancer (42, 43), so treatment with DiFiD may also potentially inhibit angiogenesis and decrease the aggressive behavior of the pancreatic cancer. Finally, VEGF is important in angiogenesis and promotion of tumor growth in many cancers including pancreatic cancer. VEGF and its receptors are overexpressed in pancreatic cancer (44). The ability of DiFiD to inhibit VEGF expression is yet another molecular mechanism by which DiFiD may function to prevent pancreatic cancer. In fact, previous studies have demonstrated that down-regulation of Notch-1 or Jagged-1 lead to decreased expression and the activity of NF-κB transcription factor (37). Indeed, COX-2, VEGF and IL-8 are targets for NF-κB–mediated transcription. Furthermore, Notch ligand Jagged1 has been demonstrated to be a proangiogenic regulator (45, 46). DiFiD treatment reduced Jagged1 expression. Therefore, DiFiD-mediated suppression of cell growth may in part be due to loss of Notch-1 mediated activation of COX-2, VEGF and IL-8 expression through NF-κB–mediated transcriptional activity or through downregulation of Jagged1 expression. Further studies in these directions are currently being explored.
In conclusion, our studies show that DiFiD treatment of pancreatic cancer cells results in growth inhibition in vitro and in vivo. It should be noted however, that the drug seems to do multiple things and is not clear which one is key for the antitumor effects. While Notch is a target, there are also cell cycle blockade and inhibition of angiogenesis. Clearly, more detailed mechanistic work is needed. Given the broad effects, this may be an agent for which system biology approaches such as gene expression profiling before and after treatment with pathway analyses may provide clues, which is a focus of our future studies. Nevertheless, given the observation that DiFiD does not affect proliferation of normal cells strongly suggests that DiFiD has promising potential for use as a therapeutic or chemopreventive agent for pancreatic cancer as well as other cancers and inflammatory disease states.
Supplementary Material
Acknowledgements
The authors thank Lauren Larsen for editing the manuscript.
Grant Support
This work was supported by grants from the University of Kansas Cancer Center pilot project and Thomas O’Sullivan Foundation (D. Subramaniam), and DK062265, CA109269 and CA135559 (S. Anant) from NIH. The work was also funded, in part, by the Kansas Biosciences Authority (SA, RAJ and DRW) and the National Foundation for Cancer Research (DRW).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest are disclosed.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 3.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 4.Nieto J, Grossbard ML, Kozuch P. Metastatic pancreatic cancer 2008: is the glass less empty? The oncologist. 2008;13:562–576. doi: 10.1634/theoncologist.2007-0181. [DOI] [PubMed] [Google Scholar]
- 5.Burris H, Storniolo AM. Assessing clinical benefit in the treatment of pancreas cancer: gemcitabine compared to 5-fluorouracil. Eur J Cancer. 1997;33 Suppl 1:S18–S22. doi: 10.1016/s0959-8049(96)00324-3. [DOI] [PubMed] [Google Scholar]
- 6.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–2900. [PubMed] [Google Scholar]
- 7.Kuo ML, Huang TS, Lin JK. Curcumin, an antioxidant and anti-tumor promoter, induces apoptosis in human leukemia cells. Biochim Biophys Acta. 1996;1317:95–100. doi: 10.1016/s0925-4439(96)00032-4. [DOI] [PubMed] [Google Scholar]
- 8.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23:363–398. [PubMed] [Google Scholar]
- 9.Subramaniam D, Ramalingam S, Linehan DC, Dieckgraefe BK, Postier RG, Houchen CW, et al. RNA Binding Protein CUGBP2/CELF2 Mediates Curcumin-Induced Mitotic Catastrophe of Pancreatic Cancer Cells. PLoS One. 2011;6:e16958. doi: 10.1371/journal.pone.0016958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shehzad A, Wahid F, Lee YS. Curcumin in cancer chemoprevention: molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch Pharm (Weinheim) 2010;343:489–499. doi: 10.1002/ardp.200900319. [DOI] [PubMed] [Google Scholar]
- 11.Shehzad A, Khan S, Shehzad O, Lee YS. Curcumin therapeutic promises and bioavailability in colorectal cancer. Drugs Today (Barc) 2010;46:523–532. doi: 10.1358/dot.2010.46.7.1509560. [DOI] [PubMed] [Google Scholar]
- 12.Goel A, Jhurani S, Aggarwal BB. Multi-targeted therapy by curcumin: how spicy is it? Mol Nutr Food Res. 2008;52:1010–1030. doi: 10.1002/mnfr.200700354. [DOI] [PubMed] [Google Scholar]
- 13.Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as "Curecumin": from kitchen to clinic. Biochem Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Adams BK, Ferstl EM, Davis MC, Herold M, Kurtkaya S, Camalier RF, et al. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorg Med Chem. 2004;12:3871–3883. doi: 10.1016/j.bmc.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Adams BK, Cai J, Armstrong J, Herold M, Lu YJ, Sun A, et al. EF24, a novel synthetic curcumin analog, induces apoptosis in cancer cells via a redox-dependent mechanism. Anticancer Drugs. 2005;16:263–275. doi: 10.1097/00001813-200503000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Subramaniam D, May R, Sureban SM, Lee KB, George R, Kuppusamy P, et al. Diphenyl difluoroketone: a curcumin derivative with potent in vivo anticancer activity. Cancer Res. 2008;68:1962–1969. doi: 10.1158/0008-5472.CAN-07-6011. [DOI] [PubMed] [Google Scholar]
- 17.Miele L. Notch signaling. Clin Cancer Res. 2006;12:1074–1079. doi: 10.1158/1078-0432.CCR-05-2570. [DOI] [PubMed] [Google Scholar]
- 18.Miele L, Golde T, Osborne B. Notch signaling in cancer. Curr Mol Med. 2006;6:905–918. doi: 10.2174/156652406779010830. [DOI] [PubMed] [Google Scholar]
- 19.Miele L, Miao H, Nickoloff BJ. NOTCH signaling as a novel cancer therapeutic target. Curr Cancer Drug Targets. 2006;6:313–323. doi: 10.2174/156800906777441771. [DOI] [PubMed] [Google Scholar]
- 20.Habbe N, Shi G, Meguid RA, Fendrich V, Esni F, Chen H, et al. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc Natl Acad Sci U S A. 2008;105:18913–18918. doi: 10.1073/pnas.0810097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plentz R, Park JS, Rhim AD, Abravanel D, Hezel AF, Sharma SV, et al. Inhibition of γ-secretase activity inhibits tumor progression in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology. 2009;136:1741–1749. doi: 10.1053/j.gastro.2009.01.008. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landegren U. Measurement of cell numbers by means of the endogenous enzyme hexosaminidase. Applications to detection of lymphokines and cell surface antigens. Journal of immunological methods. 1984;67:379–388. doi: 10.1016/0022-1759(84)90477-0. [DOI] [PubMed] [Google Scholar]
- 23.Ponnurangam S, Mondalek FG, Govind J, Subramaniam D, Houchen CW, Anant S, et al. Urine and Serum Analysis of Consumed Curcuminoids Using an I{kappa}B-Luciferase Surrogate Marker Assay. In Vivo. 2010;24:861–864. [PMC free article] [PubMed] [Google Scholar]
- 24.Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725–731. doi: 10.1016/j.ceb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 25.Blanchard H, Donepudi M, Tschopp M, Kodandapani L, Wu JC, Grutter MG. Caspase-8 specificity probed at subsite S(4): crystal structure of the caspase-8-Z-DEVD-cho complex. J Mol Biol. 2000;302:9–16. doi: 10.1006/jmbi.2000.4041. [DOI] [PubMed] [Google Scholar]
- 26.Alao JP. The regulation of cyclin D1 degradation: roles in cancer development and the potential for therapeutic invention. Mol Cancer. 2007;6:24. doi: 10.1186/1476-4598-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molina MA, Sitja-Arnau M, Lemoine MG, Frazier ML, Sinicrope FA. Increased cyclooxygenase-2 expression in human pancreatic carcinomas and cell lines: growth inhibition by nonsteroidal anti-inflammatory drugs. Cancer Res. 1999;59:4356–4362. [PubMed] [Google Scholar]
- 28.Matsubayashi H, Infante JR, Winter J, Klein AP, Schulick R, Hruban R, et al. Tumor COX-2 expression and prognosis of patients with resectable pancreatic cancer. Cancer Biol Ther. 2007;6:1569–1575. doi: 10.4161/cbt.6.10.4711. [DOI] [PubMed] [Google Scholar]
- 29.Grau R, Iniguez MA, Fresno M. Inhibition of activator protein 1 activation, vascular endothelial growth factor, and cyclooxygenase-2 expression by 15-deoxy-Delta12,14-prostaglandin J2 in colon carcinoma cells: evidence for a redox-sensitive peroxisome proliferator-activated receptor-gamma-independent mechanism. Cancer Res. 2004;64:5162–5171. doi: 10.1158/0008-5472.CAN-04-0849. [DOI] [PubMed] [Google Scholar]
- 30.Pavlakovic H, Havers W, Schweigerer L. Multiple angiogenesis stimulators in a single malignancy: implications for anti-angiogenic tumour therapy. Angiogenesis. 2001;4:259–262. doi: 10.1023/a:1016045012466. [DOI] [PubMed] [Google Scholar]
- 31.Sureban SM, Ramalingam S, Natarajan G, May R, Subramaniam D, Bishnupuri KS, et al. Translation regulatory factor RBM3 is a proto-oncogene that prevents mitotic catastrophe. Oncogene. 2008;27:4544–4556. doi: 10.1038/onc.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, Azmi AS, Ahmad A, Banerjee S, Wang S, Sarkar FH, et al. TW-37, a small-molecule inhibitor of Bcl-2, inhibits cell growth and induces apoptosis in pancreatic cancer: involvement of Notch-1 signaling pathway. Cancer Res. 2009;69:2757–2765. doi: 10.1158/0008-5472.CAN-08-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li H, Wolfe MS, Selkoe DJ. Toward structural elucidation of the γ-secretase complex. Structure. 2009;17:326–334. doi: 10.1016/j.str.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolfe MS. γ-secretase in biology and medicine. Semin Cell Dev Biol. 2009;20:219–224. doi: 10.1016/j.semcdb.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Gong P, Vetrivel KS, Nguyen PD, Meckler X, Cheng H, Kounnas MZ, et al. Mutation analysis of the presenilin 1 N-terminal domain reveals a broad spectrum of γ-secretase activity toward amyloid precursor protein and other substrates. J Biol Chem. 2010;285:38042–38052. doi: 10.1074/jbc.M110.132613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Banerjee S, Li Y, Rahman KM, Zhang Y, Sarkar FH. Down-regulation of notch-1 inhibits invasion by inactivation of nuclear factor-kappaB, vascular endothelial growth factor, and matrix metalloproteinase-9 in pancreatic cancer cells. Cancer Res. 2006;66:2778–2784. doi: 10.1158/0008-5472.CAN-05-4281. [DOI] [PubMed] [Google Scholar]
- 38.Bachmeier BE, Killian P, Pfeffer U, Nerlich AG. Novel aspects for the application of Curcumin in chemoprevention of various cancers. Front Biosci (Schol Ed) 2010;2:697–717. doi: 10.2741/s95. [DOI] [PubMed] [Google Scholar]
- 39.Surh YJ, Chun KS. Cancer chemopreventive effects of curcumin. Adv Exp Med Biol. 2007;595:149–172. doi: 10.1007/978-0-387-46401-5_5. [DOI] [PubMed] [Google Scholar]
- 40.Castedo M, Perfettini JL, Roumier T, Kroemer G. Cyclin-dependent kinase-1: linking apoptosis to cell cycle and mitotic catastrophe. Cell Death Differ. 2002;9:1287–1293. doi: 10.1038/sj.cdd.4401130. [DOI] [PubMed] [Google Scholar]
- 41.Cascinu S, Scartozzi M, Carbonari G, Pierantoni C, Verdecchia L, Mariani C, et al. COX-2 and NF-KB overexpression is common in pancreatic cancer but does not predict for COX-2 inhibitors activity in combination with gemcitabine and oxaliplatin. Am J Clin Oncol. 2007;30:526–530. doi: 10.1097/COC.0b013e318054675c. [DOI] [PubMed] [Google Scholar]
- 42.Shi Q, Abbruzzese JL, Huang S, Fidler IJ, Xiong Q, Xie K. Constitutive and inducible interleukin 8 expression by hypoxia and acidosis renders human pancreatic cancer cells more tumorigenic and metastatic. Clin Cancer Res. 1999;5:3711–3721. [PubMed] [Google Scholar]
- 43.Le X, Shi Q, Wang B, Xiong Q, Qian C, Peng Z, et al. Molecular regulation of constitutive expression of interleukin-8 in human pancreatic adenocarcinoma. J Interferon Cytokine Res. 2000;20:935–946. doi: 10.1089/10799900050198372. [DOI] [PubMed] [Google Scholar]
- 44.Baker CH, Solorzano CC, Fidler IJ. Blockade of vascular endothelial growth factor receptor and epidermal growth factor receptor signaling for therapy of metastatic human pancreatic cancer. Cancer Res. 2002;62:1996–2003. [PubMed] [Google Scholar]
- 45.Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benedit R, Roca C, Sorenson I, Adams S, Gossler A, Fruttiger M, et al. The Notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.