Abstract
Background
A behavioral phenotype that characterizes nicotine dependence, the time to first cigarette after waking, is hypothesized to increase the risk of head and neck cancer.
Methods
A case-control study of histologically-confirmed head and neck cancer was conducted that included 1055 cases and 795 controls with a history of cigarette smoking.
Results
The pack-years -adjusted odds ratio was 1.42 (95% confidence intervals [CI] 1.02–1.99) for a 31–60 minute interval after waking and 1.59 (95% CI 1.19–2.11) for a 1–30 minute interval. The risk estimates were similar when smoking was modeled as total years, smoking status (current vs. former), cigarettes per day, years since quitting, and excess odds ratio. Findings were consistent for cancers of the floor of the mouth, palate and pharynx.
Conclusion
Time to first cigarette is an indicator of increased nicotine dependence, smoke uptake and head and neck cancer risk. This high risk group would benefit from targeted smoking interventions.
Keywords: Nicotine, addiction, dependence, head and neck cancer, smoking, cotinine, case-control
INTRODUCTION
The risk of head and neck cancer increases with the frequency and duration of cigarette smoking (1–4). The physiological dependence to nicotine determines the degree of nicotine uptake and associated tobacco toxins. This is not readily quantifiable in population-based studies. Smoke uptake has traditionally been characterized by the age of smoking onset, frequency, duration and years since quitting. These measures have satisfactorily documented the high rates and risk of many cancers and cardiovascular disease from cigarette smoking despite the moderate association between self-reported measures of cigarette frequency and biochemical exposure markers (5–6).
The time to first cigarette (TTFC), an item of the Fagerstrom Test for Nicotine Dependence (FTND) (7), is a objective measure of nicotine dependence (8–9) and is associated with the many behavioral traits of nicotine addiction including smoking amount (10), inability to quit (11–12), smoking relapse (13) and tolerance (14). Nicotine dependence can also be measured biochemically, by quantifying the blood levels of cotinine, the major nicotine metabolite. The levels of cotinine are associated with the number of cigarettes smoked per day, and to a lesser extent the nicotine content of cigarettes. A shorter time elapsed between waking up and the first cigarette smoked was recently associated with significantly higher levels of cotinine in several hundred current smokers who smoked five or more cigarettes per day (15). Two nicotine dependence phenotypes were found. The “low” dependent phenotype was characterized by smoking >30 minutes after waking and smoking < or =20 cigarettes per day. In this group, cotinine levels were relatively low but increased linearly with cigarette consumption. The “high” dependent phenotype was characterized by smoking < or =30 minutes after waking but having a wide range in the frequency of cigarettes smoked (e.g. 6–70 cigarettes per day). In this group, cotinine levels were much higher but there were little differences in cotinine by cigarette frequency. If the time to first cigarette is an independent marker of nicotine dependence and tobacco smoke exposure, subjects with early TTFC might have an increased risk of smoking-related cancers. The current study examined whether the time to first cigarette is a predictor of oral and pharyngeal cancer risk.
MATERIALS AND METHODS
The methods were previously described in an analysis that shows increased risks of oral cancer with smoking and alcohol consumption (16). In brief, the study was conducted in large academic medical centers in the New York Metropolitan area from 1985–1991. Case patients were identified on a daily basis from surgery schedule logs. Eligibility criteria included speaking English and free of any mental impairment. All newly diagnosed patients with histologically-confirmed cancer of the oral cavity, pharynx or nasal cavity were asked to participate and sign an Institutional Review Board-approved consent form. All subjects were interviewed in person by a trained interviewer using the same structured questionnaire. Controls were consented patients admitted to the same hospital for conditions unrelated to tobacco smoke exposure and frequency matched to cases by sex, age (within five years), race and month of diagnosis. Controls were selected from daily admission rosters and interviewed using the same structured questionnaire. There was a wide range of control diagnoses including acute conditions, fractures and injuries, nonmalignant diagnosis such as benign prostatic hypertrophy and cancers not known to be caused by tobacco smoking including breast and prostate. Information on the subsite of the lesion was obtained from the pathology report, and ICD-9 codes were abstracted. The response rate for both cases and controls, which was the rate of participation for eligible subjects who were approached and asked to participate, was over 90%. Reasons for not participating included not feeling well or lack of interest.
The current analysis included only subjects with a history of smoking at least one cigarette per day for one or more years. Never smokers were excluded, leaving 1,850 subjects including 1055 cases and 795 controls. The data were analyzed using R (R Foundation for Statistical Computing, Austria) and SAS (Cary, NC) statistical software packages. All tests were two-sided. Unconditional logistic regression procedures were used to calculate odds ratios (OR) and 95% confidence intervals (CI).
The patient smoking history was obtained directly from the patient interview. A subject was considered as having ever smoked cigarettes if they smoked cigarettes at least once a day for one or more years. A subject was defined as a current smoker if they smoked within the last year and a former smoker if they quit one or more years ago. The time to first cigarette information was also obtained directly from the structured questionnaire. It included the following categories of responses: 1–30 minutes; 31–60 minutes; > 1 hour (reference category). All subjects were asked about the frequency and amount of beer, wine and hard liquor consumed. Subjects were defined as current drinkers of beer if they drank at least one glass in the past month. Similar questions were used to define wine and liquor drinkers.
Adjustment for cigarette smoking was performed in several ways. Models were fitted that controlled for pack-years, intensity (e.g. cigarettes per day), smoking status (current vs. former), years since quitting (0 years [current smoker], 1–5 years, 6–10 years and >10 years) and the excess OR (EOR) where pack-years is linear and the logarithm of cigarettes per day and its square is exponential (17 As the risk for oral cancer varies by smoking intensity the risk associated with time to first cigarette adjusted for EOR was stratified by categories of smoking intensity. The following covariates were included in the models: age (≤50, 51–60, 61–70 and >70), sex (male vs. female), race (blacks vs. whites), education (≤12 years, 12 years, 13–15 years and ≥ 16 years), alcohol consumption (current beer drinker vs. not current beer drinker; current wine drinker vs. not current wine drinker; and current hard liquor drinker vs. not current hard liquor drinker) and body mass index (Weight[lbs]*703/(height[in.])2). ). A multiplicative interaction for ever alcohol use and pack-years was estimated by using a product term of those two variables in the logistic regression model. Odds ratios were calculated for specific subsites within the oral cavity or for pharyngeal cancer using the entire control series as the comparison group. Statistical significance was set at P < 0.05, and all tests were 2-sided. There were no missing values in this analysis. A goodness of fit test for every model was performed using the Hosmer and Lemeshow chi-square statistic (18).
RESULTS
Table 1 shows the basic characteristics of the study subjects. After excluding never smokers, there were a larger number of cancer cases than controls (1055 vs. 795). Head and neck cancer was more frequent in men than in women. About 91% of all subjects were white and 9% were black. The crude odds ratio associated with TTFC was 1.71 (95% CI 1.26–2.34) for 31–60 minutes after waking and 2.54 (95% CI 1.98–3.25) for 1–30 minutes after waking compared to waiting one hour after waking.
Table 1.
Characteristics of head and neck cancer cases and controls
| Characteristic | Cases N=1055 (%) | Controls N=795 (%) |
|---|---|---|
| Mean Age | 58 | 58 |
| Sex | ||
| Men | 754 (71.5) | 616 (77.5) |
| Women | 301 (28.5) | 179 (22.5) |
| Race | ||
| White | 945 (89.6) | 732 (92.1) |
| Black | 103 (9.8) | 62 (7.8) |
| Other | 7 (0.6) | 1 (0.1) |
| Smoking Status | ||
| Current | 759 (71.9) | 342 (43.0) |
| Former | 296 (28.1) | 453 (57.0) |
| Time to first cigarette | ||
| 1–30 min. | 751 (71.2) | 444 (55.9) |
| 31–60 min. | 168 (15.9) | 147 ()18.5 |
| >60 min. | 136 (12.9) | 204 (25.7) |
| Site | ||
| Floor of mouth | 259 (24.6) | |
| Tongue | 294 (27.9) | |
| Pharynx | 133 (12.6) | |
| Palate | 101 (9.6) | |
| Other | 307 (25.3) |
A current smoker was defined as having smoked at least one cigarette per day for one or more years (ever smoked) and within the last year. A former smoker was defined as an ever smoker who quit one or more years ago.
The pack-year adjusted risk of cancer was 1.42 (95% CI: 1.02–1.99) for 31–60 minutes after waking, and 1.59 (95% CI: 1.19–2.11) for smoking within 30 minutes after waking (Table 2). The total-years of smoking adjusted odds ratio was 1.43 (95% CI: 1.02–2.0) for 31–60 minutes after waking, and 1.69 (95% CI: 1.45–1.98) for smoking within 30 minutes after waking. The inclusion of the product term of pack-years and current alcohol consumption had no effect on the odds ratios for TTFC. Consequently a product term for smoking and alcohol consumption was excluded from subsequent models. The odds ratio in a model that adjusted for cigarettes per day was 1.63 (95% CI: 1.18–2.26) for 31–60 minutes after waking, and 2.11 (95% CI: 1.61–2.77) for within 30 minutes after waking. Findings were similar when smoking was adjusted for by smoking status and years since quitting (Table 2).
Table 2.
Odds ratios and 95% confidence intervals for head and neck cancer and time to first cigarette in ever smokers, adjusting for different measures of smoking history.
| Time to first cigarette* | OR adjusted for pack- years of smoking | 95% CI | OR adjusted for total years of smoking | 95% CI | OR adjusted for cigarettes per day | 95% CI | OR adjusted for smoking status | 95% CI | OR adjusted for years since quitting | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|
| >60 min. | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |||||
| 31–60 min. | 1.42 | 1.02–1.99 | 1.43 | 1.02–2.0 | 2.11 | 1.61–2.77 | 1.50 | 1.07–2.09 | 1.47 | 1.05–2.06 |
| 1–30 min. | 1.59 | 1.19–2.11 | 1.69 | 1.45–1.98 | 1.63 | 1.18–2.26 | 1.77 | 1.34–2.32 | 1.69 | 1.28–2.23 |
| Trend X2 | P<0.01 | P<0.01 | P<0.01 | P<0.01 | P<0.01 |
Odds ratios adjusted for age, sex, race, education, alcohol consumption, and body mass index. The odds ratio adjusted for pack-years of smoking included a product term for pack-years and alcohol consumption. The odds ratios adjusted for years since quitting included current smokers.
Figure 1 shows the risk of cancer associated with TTFC adjusted for the EORs stratified by seven categories of smoking intensity (cigarettes per day). Compared to the referent group, the risks associated with smoking 31–60 minutes after waking and 1–30 minutes after waking were elevated for each smoking intensity category, except for the heaviest smokers (>30 cigarettes per day).
Figure 1.
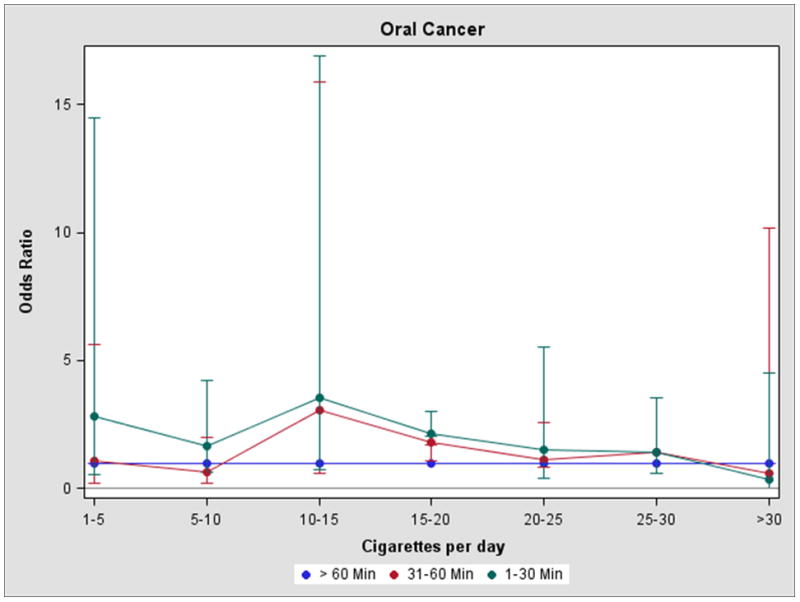
Time to first cigarette and head and neck cancer risk in ever smokers adjusting for the excess odds ratio per pack-year, by cigarette intensity (cigarettes per day)
Early TTFC was associated with an increased cancer risk for most subsites (Table 3). Findings are presented for cancers of the floor of mouth, palate, base and anterior tongue, and palate which comprise 75% of all the cases. The strongest association was observed between smoking within 30 minutes and cancer of the pharynx (OR = 2.19, 95% CI 0.99–4.83). Increased risks were observed for cancers of the floor of mouth and palate. The effect was smallest for cancers of the tongue.
Table 3.
Odds ratios and 95% confidence intervals for head and neck cancer and time to first cigarette in ever smokers by tumor site, adjusting for pack-years of smoking
| Time to first cigarette* | OR for floor of mouth | 95% CI | OR for palate | 95% CI | OR for anterior and base of tongue | 95% CI | OR for pharynx | 95% CI |
|---|---|---|---|---|---|---|---|---|
| >60 min. | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| 31–60 min. | 1.19 | 0.63–2.27 | 1.43 | 0.68–3.02 | 1.01 | 0.67–1.54 | 2.07 | 1.02–4.18 |
| 1–30 min. | 1.76 | 1.0–3.0 | 1.87 | 0.82–4.23 | 1.21 | 0.75–1.94 | 2.19 | 0.99–4.83 |
| Trend X2 | P<0.05 | P=0.45 | P=0.96 | P=0.06 |
Odds ratios adjusted for age, sex, race, education, alcohol consumption, and body mass index.
DISCUSSION
The time to first cigarette is a distinct nicotine dependence phenotype associated with the uptake of nicotine and tobacco smoke. The “high” dependent phenotype is associated with significantly higher cotinine levels than the low dependent phenotype per cigarette smoked. The TTFC is biochemically validated and can be considered a risk factor for smoking-related diseases. The current study shows that an early TTFC is significantly associated with an increased risk of head and neck cancer. The association was consistent for most cancer sites. The highest risk was found for pharyngeal cancer, which has the highest smoking-related risk of these sites (19).
Cotinine has a 24 hour half-life and has little value as a biomarker of smoke uptake in case-control studies. The dose of smoke uptake in epidemiologic studies has traditionally been measured by proxy measures such as the frequency and duration of cigarette smoking. However, the effect of inter-individual variability in nicotine dependence on cancer risk has not been determined. One study of 55 smoking lung cancer cases and 49 smoking controls did find a significant trend in the risk associated with the FTND score, although this association reflects to a certain extent just the association with smoking frequency, which is the single biggest contributor to the FTND index (20). The current analysis shows that the time to first cigarette after waking is a strong and independent predictor of head and neck cancer risk in a large study of ever smokers. A 1.6 pack-year adjusted risk was associated with smoking within 30 minutes after waking. The risk may be even higher among subjects who smoked within the first 15 minutes.
Limitations in this study include those that are known to be common in case-control studies including bias, measurement error and confounding. Cases and controls were hospitalized patients whose lifestyles might be different than the general population. Responses on smoking and alcohol consumption are subject to recall biases. While it is not possible to completely validate lifetime lifestyle habits, repeat interviews on a random sample of subjects showed high internal consistency for smoking and alcohol consumption. Little is known about smoking behaviors such as time to first cigarette over the course of a lifetime. This behavior was assessed by a single question, and it is possible that this particular smoking behavior may change over time or change in relation to smoking habits. Subjects may have switched from high yield cigarettes to low yield cigarettes which could potentially affect symptoms of nicotine dependence and smoking behaviors. However, the associations were similar for current and former smokers, indicating that recall bias was unlikely to have affected the findings. The association might have been confounded by smoking, although the study did carefully control for smoking dose in a number of different ways and the results were fairly consistent. The large sample size suggests the findings are generalizeable to the larger population of white smokers. However, there were few blacks or other minorities in this study and the results may not be applicable to these groups. Finally, the association might have been confounded by other factors. Human papilloma virus causes oropharyngeal carcinoma. It is unlikely that this was a major confounder for the cases with oropharyngeal cancer in the current study. The incidence of this cancer has increased dramatically since 2000, which was after the data collection period of our study.
A shorter time between waking and the first cigarette is associated with higher blood cotinine levels. Early morning smokers might have a greater craving for nicotine. The temporal effect is dose-dependent, where prolonging smoking abstinence after waking is associated with lower cotinine levels. If the TTFC-cotinine association is due to cravings, it likely reflects greater cravings throughout the day since intensive smoking of just the first cigarette only would not appreciable raise cotinine levels. We did not collect information on nicotine cravings throughout the day as this is not possible in case-control studies, especially for subjects who have quit smoking. There is little data on the relationship between TTFC and the urge to smoke or cravings. In a clinical smoking cessation trial of 207 smokers treated with buproprion, craving and withdrawal symptoms were lessened after smoking the first cigarette of the day. The TTFC was not correlated with a 10-item questionnaire that assessed the urge to smoke (21).
The cancer risk associated with time to first cigarette might reflect differences in smoking topography associated with nicotine cravings. There is also little data on this topic. The time to first cigarette after waking was unrelated to puffing intensity in a British study (22). Variation in the time to first cigarette could be due to genetic differences in nicotine dependence, non-genetic behavioral and socioeconomic factors, or both genetic and non-genetic factors interacting together.
To validate the current findings, prospective cohort studies with nicotine metabolite determinations are needed. Such studies would need to show the relationship between TTFC and cotinine, and determine the incidence rate of cancer according to TTFC groups. In conclusion, the significance of these findings is that TTFC may be a behavioral phenotype that identifies smokers who are at high risk of head and neck cancer. The risk of head and neck cancer was substantially elevated even among ever smokers. This underscores the need to recognize nicotine dependence rather than smoking habits as the major risk factor in smoking research since it the physiological dependence to nicotine that affects the dose of exposure. The study indicates that smokers who smoke soon after waking may require special efforts to make aware their increased risk and need for smoking cessation therapies.
Acknowledgments
Support: This study was supported by research grants (PO1 CA68384 and K07 CA104231) from the National Cancer Institute and (PA-DOH 4100038714) from the Pennsylvania Department of Health.
References
- 1.Wynder EL, Bross IJ. Aetiological factors in mouth cancer; an approach to its prevention. Br Med J. 1957;1:1137–1143. doi: 10.1136/bmj.1.5028.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wynder EL, Mushinski MH, Spivak JC. Tobacco and alcohol consumption in relation to the development of multiple primary cancers. Cancer. 1977;40:1872–1878. doi: 10.1002/1097-0142(197710)40:4+<1872::aid-cncr2820400817>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 3.Lubin JH, Purdue M, Kelsey K, Zhang ZF, Winn D, Wei Q, et al. Total exposure and exposure rate effects for alcohol and smoking and risk of head and neck cancer: a pooled analysis of case-control studies. Am J Epidemiol. 2009;170:937–947. doi: 10.1093/aje/kwp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston-Martin S, et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48:3282–3287. [PubMed] [Google Scholar]
- 5.Joseph AM, Hecht SS, Murphy SE, Carmella SG, Le CT, Zhang Y, et al. Relationships between cigarette consumption and biomarkers of tobacco toxin exposure. Cancer Epidemiol Biomarkers Prev. 2005;14:2963–2968. doi: 10.1158/1055-9965.EPI-04-0768. [DOI] [PubMed] [Google Scholar]
- 6.Carmella SG, Akerkar SA, Richie JP, Hecht SS. Intraindividual and interindividual differences in metabolites of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in smokers’ urine. Cancer Epidemiol Biomarkers Prev. 1995;4:635–642. [PubMed] [Google Scholar]
- 7.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 8.Haberstick BC, Timberlake D, Ehringer MA, Lessem JM, Hopfer CJ, Smolen A, et al. Genes, time to first cigarette and nicotine dependence in a general population sample of young adults. Addiction. 2007;102:655–665. doi: 10.1111/j.1360-0443.2007.01746.x. [DOI] [PubMed] [Google Scholar]
- 9.Fagerström K. Time to first cigarette; the best single indicator of tobacco dependence? Monaldi Arch Chest Dis. 2003;59:91–94. [PubMed] [Google Scholar]
- 10.Heatherton TF, Kozlowski LT, Frecker RC, Rickert WJR. Measuring the Heaviness of Smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addictc. 1989;84:791–800. doi: 10.1111/j.1360-0443.1989.tb03059.x. [DOI] [PubMed] [Google Scholar]
- 11.Kozlowski LT, Porter CQ, Orleans CT, Pope MA, Heatherton T. Predicting smoking cessation with self-reported measures of nicotine dependence: FTQ, FTND, and HSI. Drug Alcohol Depend. 1994;34:211–216. doi: 10.1016/0376-8716(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 12.Baker TB, Piper ME, McCarthy DE, Bolt DM, Smith SS, Kim S-Y, et al. Time to first cigarette in the morning as an Index of ability to quit smoking: Implications for nicotine dependence. Nicotine Tob Res. 2007;9:S555–570. doi: 10.1080/14622200701673480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toll BA, Schepis TS, O’Malley SS, McKee SA, Krishnan-Sarin S. Subjective reactivity to the first cigarette of the day as a predictor of smoking relapse: A preliminary study. Drug Alcohol Depend. 2007;89:302–305. doi: 10.1016/j.drugalcdep.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pillitteri JL, Kozlowski LT, Sweeney CT, Heatherton TF. Individual Differences in the Subjective Effects of the First Cigarette of the Day: A Self-Report Method for Studying Tolerance. Exp Clin Psychopharmacol. 1997;5:83–90. doi: 10.1037//1064-1297.5.1.83. [DOI] [PubMed] [Google Scholar]
- 15.Muscat JE, Stellman SD, Caraballo RS, Richie JP. Time to first cigarette after waking predicts cotinine levels. Cancer Epidemiol Biomarkers Prev. 2009;18:3415–3420. doi: 10.1158/1055-9965.EPI-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muscat JE, Richie JP, Jr, Thompson S, Wynder EL. Gender differences in smoking and risk for oral cancer. Cancer Research. 1996;56:5192–5197. [PubMed] [Google Scholar]
- 17.Lubin JH, Gaudet MM, Olshan AF, Kelsey K, Boffetta P, Brennan P, et al. Body mass index, cigarette smoking, and alcohol consumption and cancers of the oral cavity, pharynx, and larynx: modeling odds ratios in pooled case-control data. Am J Epidemiol. 2010;171:1250–1261. doi: 10.1093/aje/kwq088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hosmer DW, Lemeshow S. Applied Logistic Regression. Wiley; New York: 1989. [Google Scholar]
- 19.Gandini S, Botteri E, Iodice S, Boniol M, Lowenfels AB, Maisonneuve P, Boyle P. Tobacco smoking and cancer: a meta-analysis. Int J Cancer. 2008;122:155–164. doi: 10.1002/ijc.23033. [DOI] [PubMed] [Google Scholar]
- 20.Kubíka AN, Petr Z, Peter B, Chris R, Sara G, Tomášekc L, et al. A case-control study of lung cancer among Czech women. Lung cancer. 2001;31:111–122. doi: 10.1016/s0169-5002(00)00178-1. [DOI] [PubMed] [Google Scholar]
- 21.Toll BA, Schepis TS, O’Malley SS, McKee SA, Krishnan-Sarin S. Subjective reactivity to the first cigarette of the day as a predictor of smoking relaspse: A preliminary study. Drug and Alcohol Dependence. 2007;89:302–305. doi: 10.1016/j.drugalcdep.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grainge MJ, Shahab L, Hammond D, O’Connor RJ, McNeill A. First cigarette on waking and time of day as predictors of puffing behaviour in UK adult smokers. Drug Alcohol Depend. 2009;101:191–195. doi: 10.1016/j.drugalcdep.2009.01.013. [DOI] [PubMed] [Google Scholar]


